1. Introduction
The plasma membrane plays an extremely important role in the existence of any cell, as it separates its contents from the outer environment, determines the regulation of the transfer of substances into and out of the cell to ensure its integrity, and regulates the exchange between the cell and the environment. Disturbance of the membrane’s barrier function can lead to the death of target cells [
1]. Pathogenic bacteria produce substances that directly damage of structures or kill cells of the macroorganism, and facilitate their penetration into the eukaryotic cell. These agents play major roles in the development of diseases caused by bacteria. An important role in pathogenesis of such substances is played by cytolytic pore-forming toxins (PFTs). Almost all pathogenic bacteria are capable of disrupting the permeability of cell membranes by specialized PFTs to form oligomeric pores in the membrane. These toxins are the most common bacterial cytotoxic proteins necessary for the virulence of a variety of bacterial pathogens and promoting the survival of pathogenic bacteria in transition of these bacteria to the eukaryotic environment [
2]. Formation of pores causes swelling of cells followed by their death. One or two pores per cell cause complete destruction of eukaryotic cells [
3]. PFTs are usually synthesized as water-soluble molecules that penetrate the membrane in monomeric form [
4] and are formed into oligomeric pores within the membranes at the interaction with target cells’ membranes. Despite the slight similarity of the PFTs’ primary sequences, one of the families of these proteins, namely the β-barrel PFTs, demonstrate not only a conformational similarity but also the universality of the functional mechanisms of action of these toxins [
5]. Comparison of the amino acid sequences of β-PFTs with the
Bacillus cereus Lys171-Gly250 hemolysin II (HlyII) sequence [
6] revealed in their primary structures a peptide region with a high degree of homology to this peptide that we called “homologous peptide”, which contains an identical sequence YGNQLFM. One of the effective ways to suppress cytolytic activity is to use monoclonal antibodies (mAbs) against certain regions of the PFTs’ amino acid sequences [
7]. This has been demonstrated for
Staphylococcus aureus α-toxin (Hla) [
8],
Streptococcus pneumoniae pneumolysin [
9],
B. cereus HlyII [
10] and some others. The aim of this work was to obtain mAbs against homologous peptide of
B. cereus HlyII as a tool for simultaneous identification and assessment of the level of expression of various β-PFTs. We believe that the obtained mAbs against homologous peptide will enable identification of a number β-barrel PFTs representatives from various species of pathogenic bacteria. They may also be able to specifically detect the presence of water-soluble forms and inhibit the cytolytic activity of certain β-barrel PFTs.
2. Results
2.1. Selection of a Peptide Region to Produce mAbs against a Number of Bacterial PFTs
Using the HMMER tool [
11], we analyzed the sequence of homologous peptide for the presence of groups of identical and similar amino acid residues among the proteins using the UniProtKB database [
12]. The results of the analysis showed regions similar to homologous peptide to be found among representatives of different families of microorganisms.
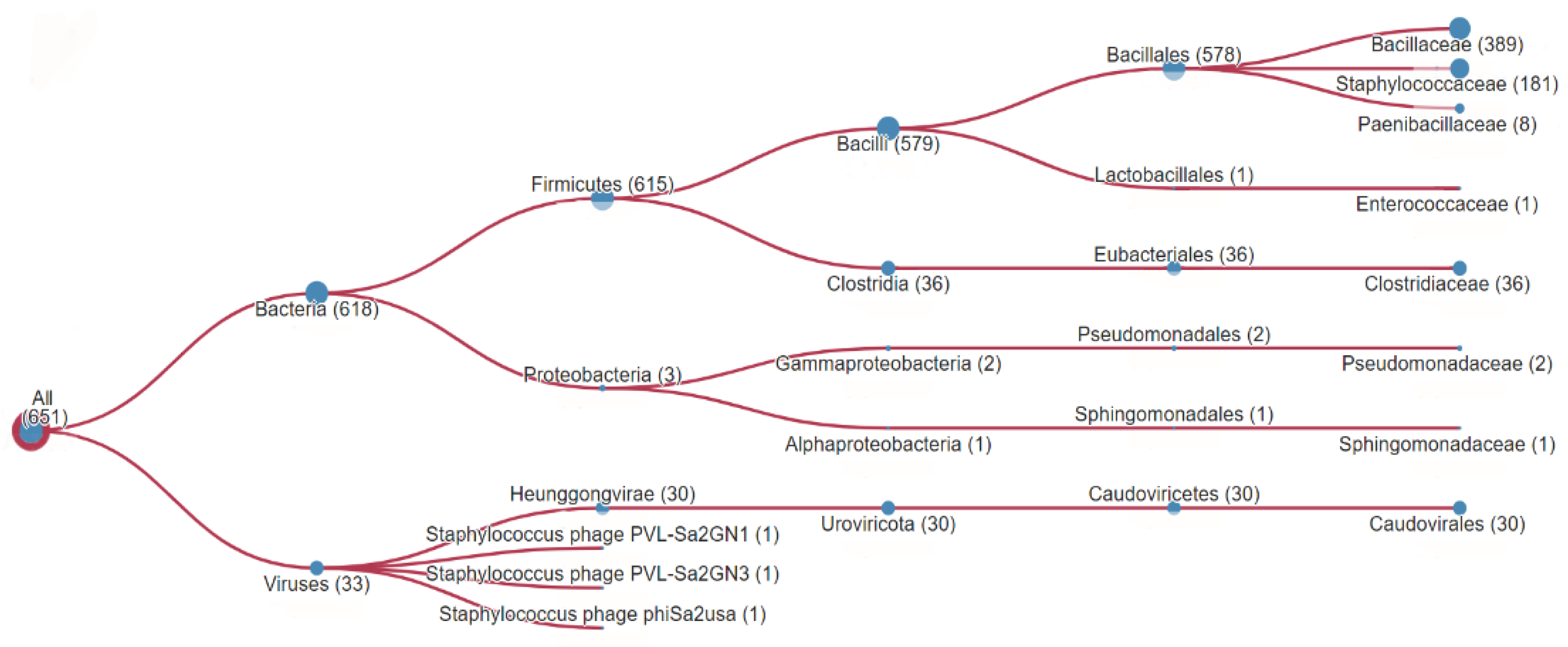
Figure 1 shows the representativeness of microorganisms having β-barrel PFTs that contain homologous peptide with varying degrees of homology. This group includes representatives of the following families:
Bacillaceae (389),
Staphilococcaceae (181),
Paenibacillaceae (8),
Enterococcaceae (1),
Clostridiaceae (36),
Pseudomonadaceae (2),
Sphingomonadaceae (1). The number of hits for the homologuos peptide among representatives of these families is listed in parentheses. Besides, homologous peptide was found in 30 representatives of
Caudovirales viruses and three streptococcal phages. Thus, bioinformatic analysis of the UniProtKB amino acid sequences revealed more than 600 β-barrel PFTs with a homologous peptide in their composition.
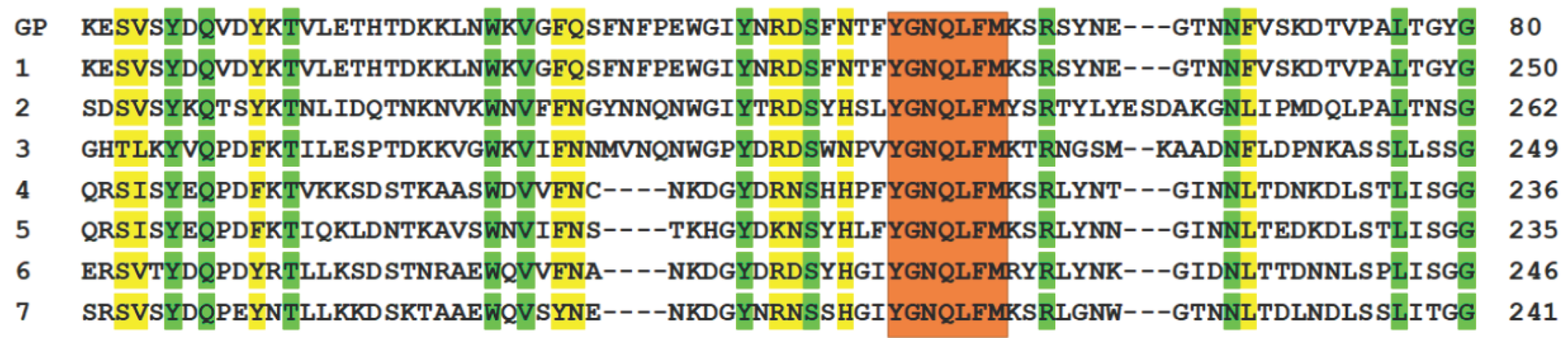
Figure 2 shows that homologous peptide with a significant homology is present in HlyII and cytotoxin K2 (CytK2) of
B. cereus, in Hla of
S. aureus, necrotic enterotoxins of
Clostridium perfringens, β-pore-forming cytolysins of
C. septicum and
C. botulinum, and several others.
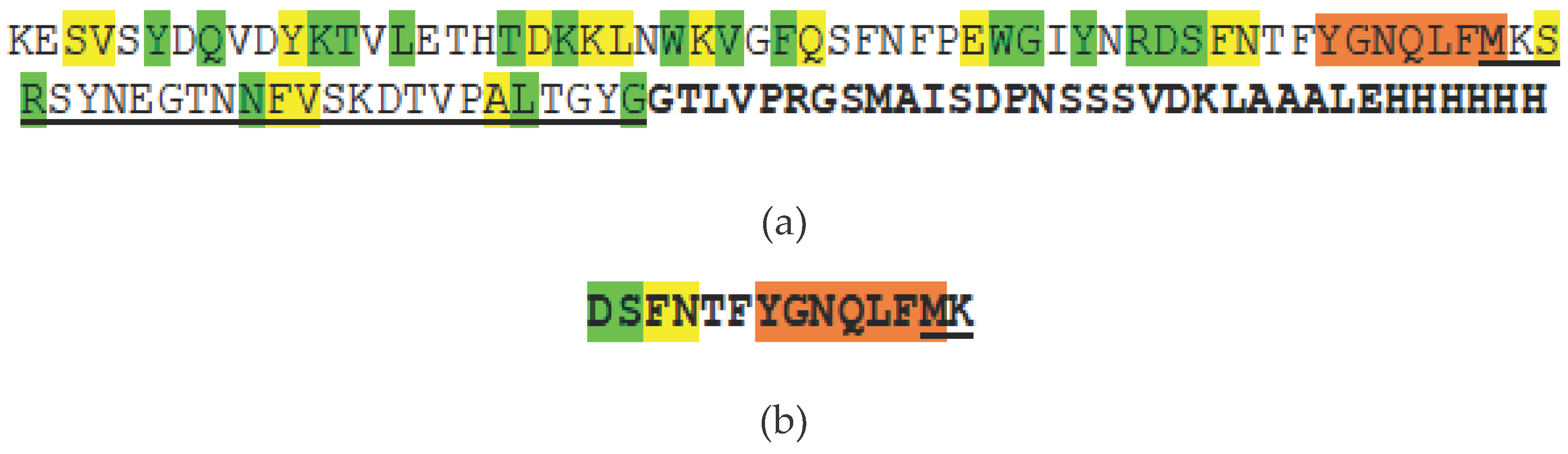
Production and purification of homologous peptide were achieved by cloning this region into an expression vector (see Materials and Methods). To increase immunogenicity, the sequence of homologous peptide was expanded in the N-terminal part to the region involved in the formation of the oligomeric spatial structure of the stem or transmembrane channel and the triangle region corresponding to the PFT region inserted into the membrane [
13]. Homologous peptide at the C-terminus used to obtain mAbs includes the HlyIILCTD region described previously [
14] (
Figure 3a). Besides, to obtain another series of mAbs, we used the synthesized oligopeptide presented in
Figure 3b. This 14-mer synthetic peptide included a primary sequence of seven amino acid residues YGNQLFM, identical to several PFTs, that are part of the homologous peptide.
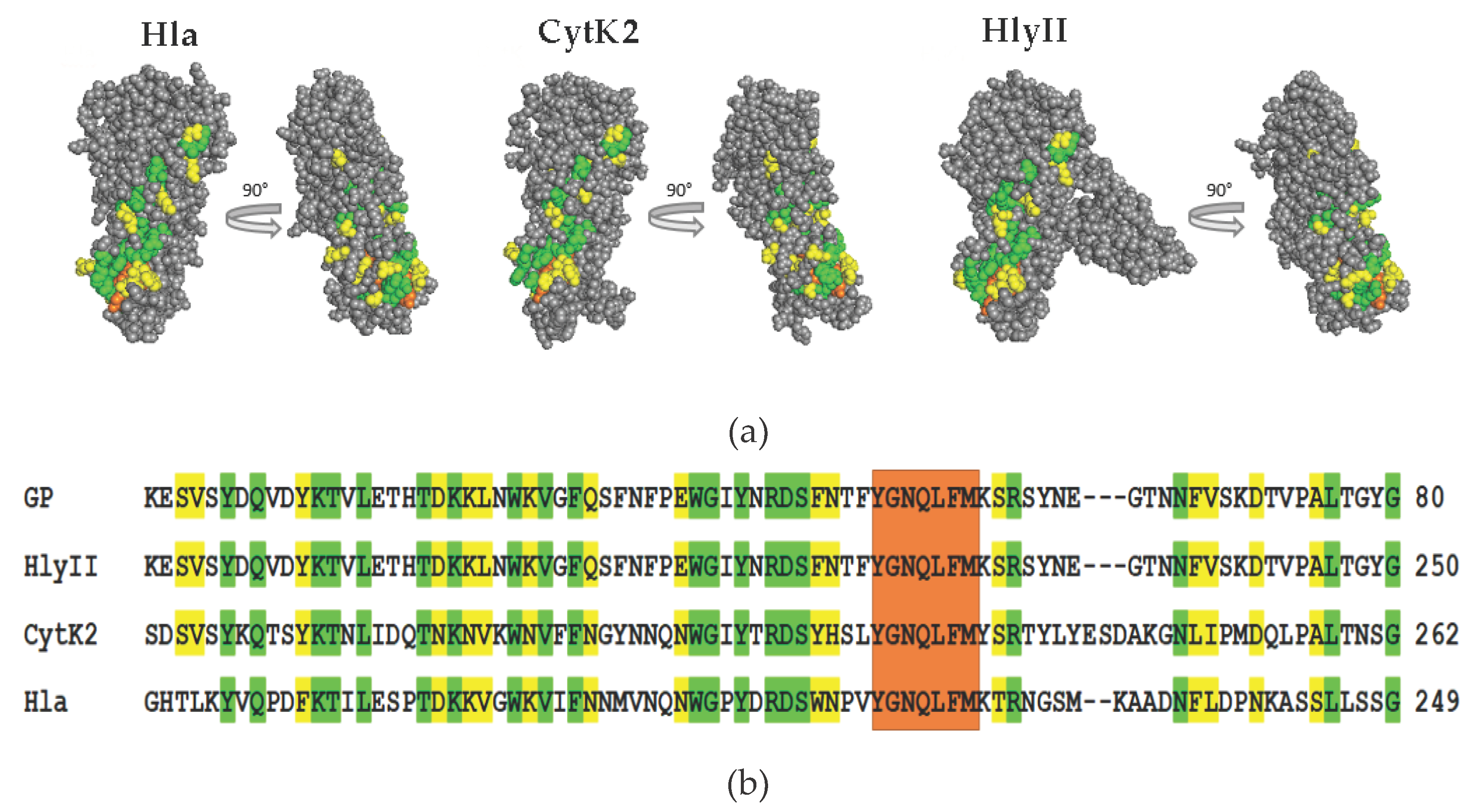
The amino acid sequence of homologous peptide contains more than 40% amino acid residues identical to a number of proteins of the β-PFT family. The degree of homology in this region with Hla of
S. aureus and CytK2 of
B. cereus is 47%. For comparison, we selected three β-barrel PFTs, two of which are synthesized in
B. cereus sensu lato cells, HlyII and CytK2; the third protein chosen for analysis is the most studied representative –Hla from
S. aureus. Protein modeling shows that some of the amino acids that make up homologous peptide (
Figure 3a and
Figure 4) are found on the surface of the monomeric forms of these PFTs (
Figure 4). The triangle region and the stem strands are formed by the most conserved sections of the amino acid sequence that are characterized by high hydrophobicity and smaller side radicals. These regions play an important role in conformational rearrangements during oligomerization and are also involved in interactions between monomers in transition of the water-soluble form of the toxin to the membrane-bound form [
15,
16].
2.2. Production of mAbs against Homologous Peptide and Synthetic Peptide DSFNTFYGNQLFMK
A preparation of recombinant homologous peptide was used as an antigen to produce GP-series mAbs. During immunization, the immune response of animals was assessed by solid-phase ELISA for interaction with immobilized homologous peptide and recombinant
B. cereus HlyII and
S. aureus Hla preparations. The maximum dilution of immune serum at a dose of 20 μg/mouse administered antigen at the interaction with HlyII was 1/128,000 (titer); with Hla, 1/16,000. Splenocytes from this animal were used as a source of lymphocytes for hybridomas secreting mAbs against homologous peptide according to the method of Keller and Milstein [
17].
To obtain the HP-series mAbs, synthetic peptide was conjugated with keyhole limpet hemocyanin (KLH) using glutaraldehyde as a cross-linking agent. To produce hybridomas secreting mAbs against synthetic peptide, we used mouse splenocytes, the serum of which interacted with the immobilized antigen to the greatest extent, up to a dilution of 1/64,000.
Based on the assessment of proliferative activity and stability of antibody production, we selected 5 stable hybridoma clones, secreting anti-HlyII GP-series mAbs, obtained after immunization with homologous peptide. GP-1, GP-3, GP-4 and GP-7 contained κ light chain and γ1 heavy chain; GP-5, Igµλ. We also obtained 5 stable hybridoma clones secreting anti-HlyII mAbs of the HP-series after immunization with synthetic peptide: HP-1, HP-2, HP-3, HP-4 and HP-5, each of which contained the µ heavy chain. HP-1, HP-4 and HP-5 contained the κ light chain, HP-2 and HP-3, the λ light chain.
2.3. Antigen-Binding Activity of the Resulting mAbs
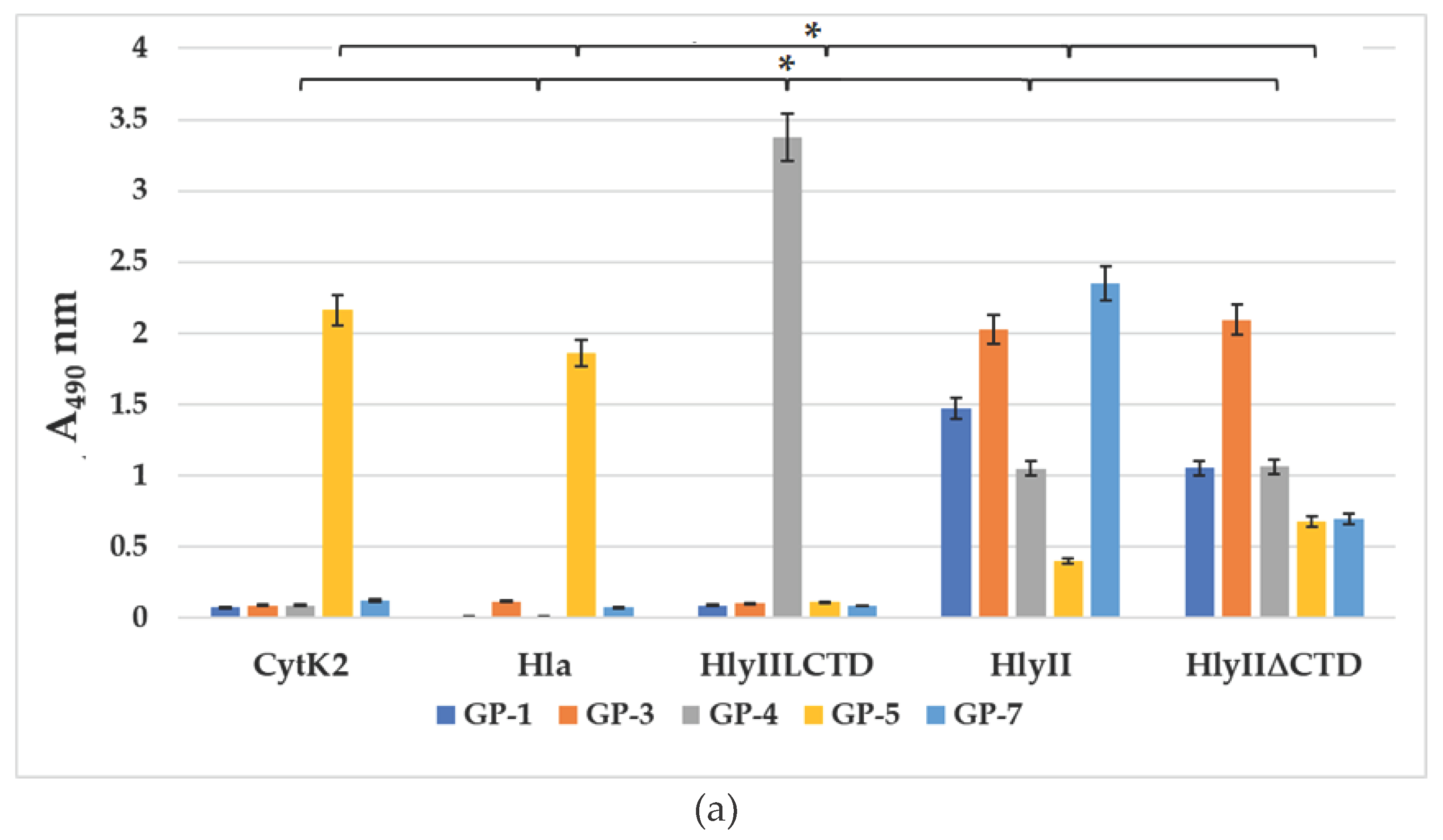
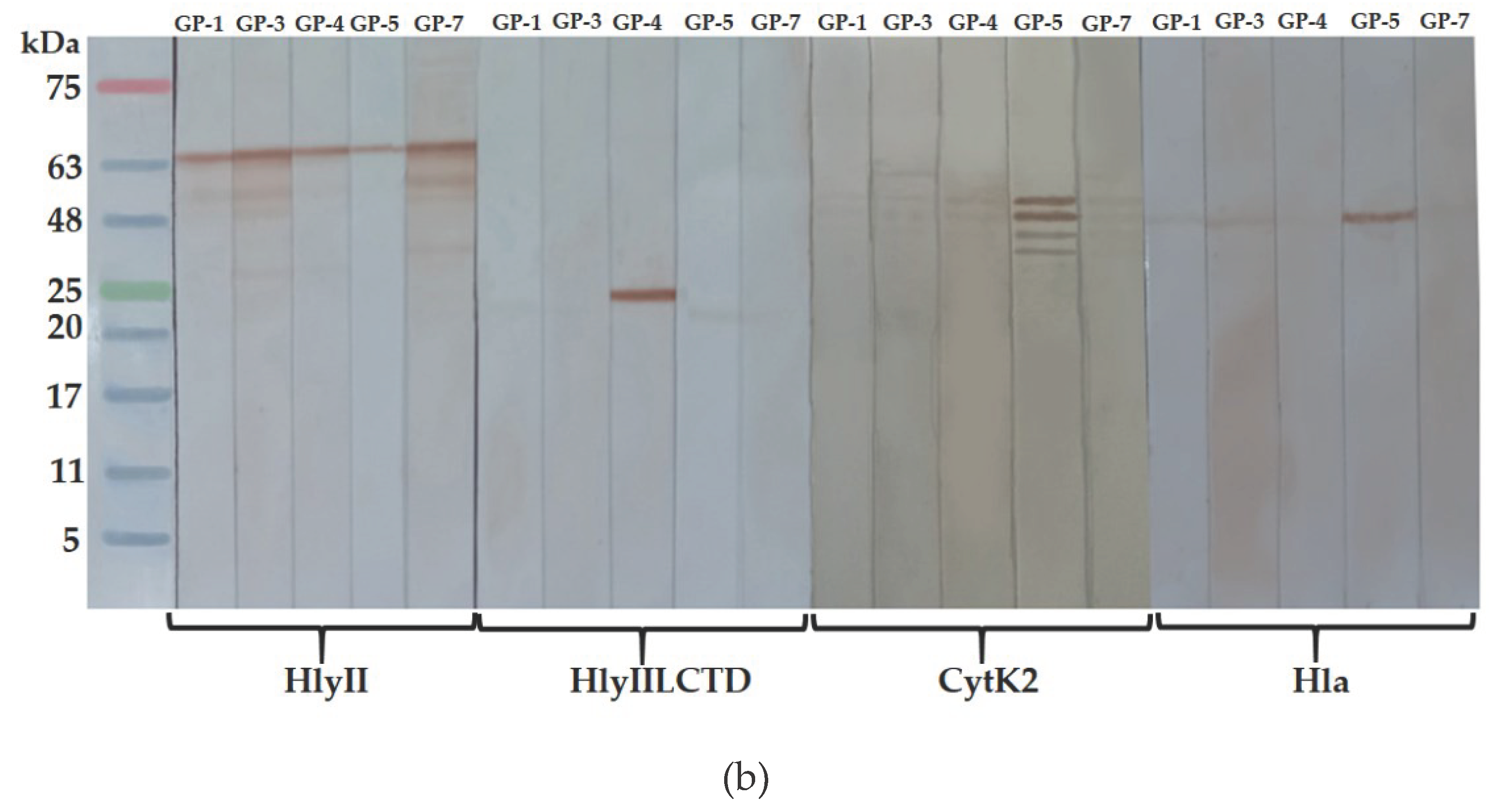
The antigen-binding activity of the resulting mAbs was studied by solid-phase ELISA under equal antigen sorption conditions and by immunoblotting. GP-1, GP-3 and GP-7 antibodies were found among the GP-series antibodies that can effectively recognize
B. cereus HlyII, but cannot interact with HlyIILCTD, as well as with
S. aureus Hla and
B. cereus CytK2 (
Figure 5a). These antibodies recognize the HlyII region within Lys171-Arg212, which does not contain a sequence of amino acid residues identical between PFTs. This conclusion is confirmed by immunoblotting (
Figure 5b) and the results presented below.
Figure 5a shows that antibody GP-4 interacts effectively with HlyIILCTD, which indicates that its binding region is at the C-terminal part of homologous peptide within the HlyII Met225-Gly250 sequence.
ELISA demonstrated that all the resulting GP-series mAbs (
Figure 5a) recognized homologous peptide in the full-length HlyII of
B. cereus. All HP-series mAbs obtained effectively recognized HlyII, CytK2 of
B. cereus and Hla of
S. aureus (data not shown). These results confirm that the DSFNTFYGNQLFMK sequence from
B. cereus HlyII, containing the primary YGNQLFM sequence identical in these PFTs, provides a tool (mAbs) for the simultaneous identification of at least three β-pore-forming toxins.
GP-5 recognized Hla from
S. aureus and CytK2 from
B. cereus with high efficiency (
Figure 5a,b), but HlyII was recognized less efficiently. Apparently, this is due to differences in the accessibility of the epitope for this antibody in the 3D structures of the compared PFTs. Besides,
B. cereus HlyII contains a C-terminal domain, which can also reduce the availability of epitopes for antibodies against the homologous peptide. This assumption was tested using the HlyII∆CTD protein [
18], in which the C-terminal domain was deleted. All GP-series mAbs recognized this protein (
Figure 5a). GP-5 recognized the HlyII∆CTD protein better than full-size HlyII protein in ELISA.
2.4. Suppression of Cytolytic Activity of GP-Series mAbs
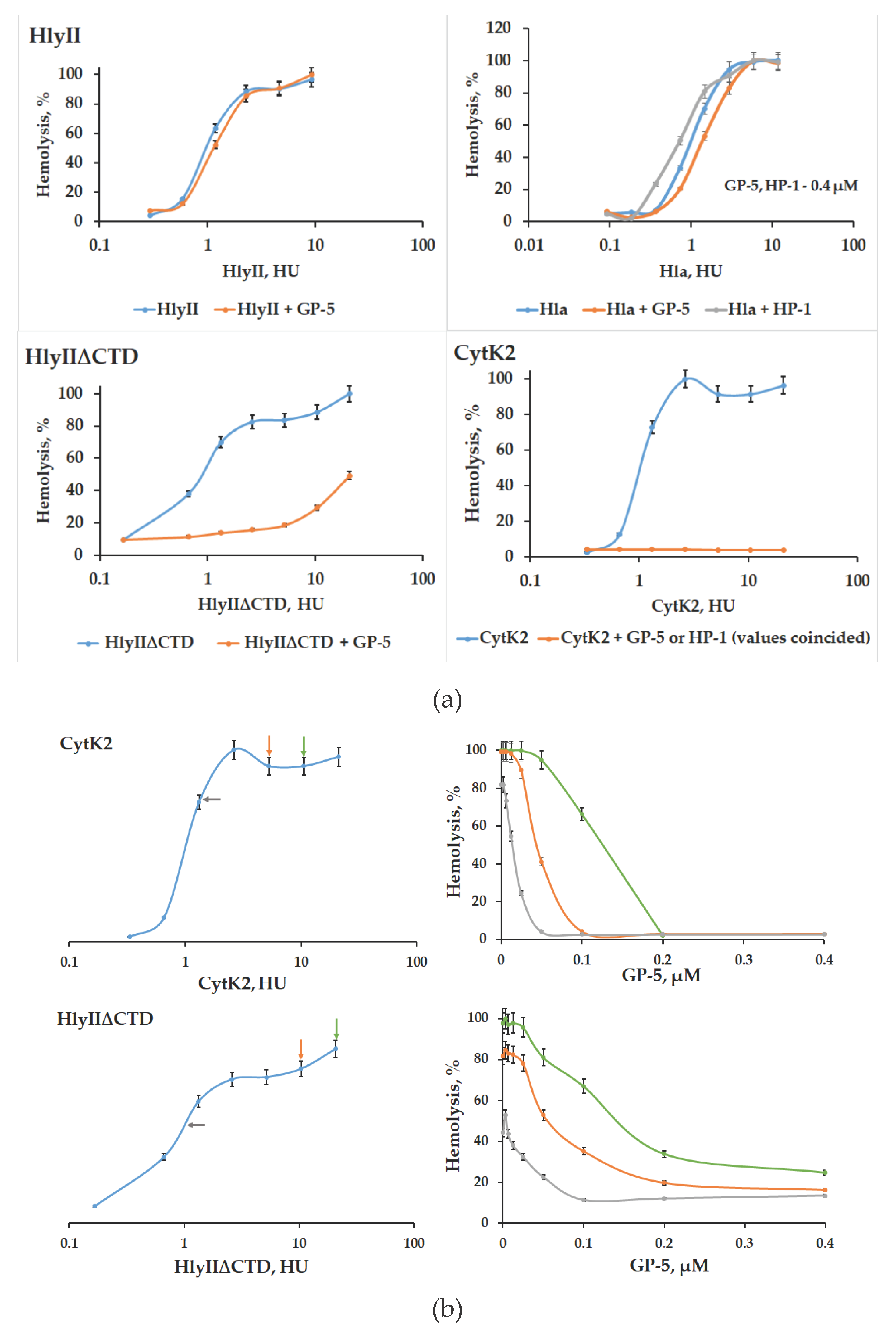
GP-5 mAb is capable of recognizing homologous peptide in HlyII, CytK2 of
B. cereus and Hla of
S. aureus. This antibody was used to investigate the possibility to inhibit their cytolytic activity. Analysis showed GP-5 significantly reduce the cytolytic activity of CytK2 and not to affect Hla or HlyII activity (
Figure 6a). A three-dimensional structure model of CytK2 and HlyII was obtained based on X-ray diffraction analysis of Hla (
Figure 4a). Possibly, slight variations in the three-dimensional structures are enough to alter the effectiveness of the PFTs cytolytic activity suppression. In addition, HlyII contains a C-terminal domain, which can change the availability of individual amino acids for mAbs. Using HlyII∆CTD, lacking the C-terminal domain [
18], we confirmed the effect of HlyIICTD on suppression of HlyII hemolytic activity by GP-5 antibodies. GP-5 suppressed the cytolytic activity of HlyII∆CTD (
Figure 6b). The kinetics of protection of the hemolysis of rabbit erythrocytes exposed to CytK2 and HlyII∆CTD depending on the number of PFTs and the amount of GP-5 is shown in
Figure 6.
3. Discussion
Bioinformatic analysis of β-PFTs revealed in their amino acid sequences a high homology region that corresponds to the Lys171-Gly250 region of
B. cereus HlyII [
6]. This homologous peptide contains the linear sequence YGNQLF, identical for certain PFTs.
Production of mAbs against homologous and synthetic (DSFNTFYGNQLFMK) peptides confirmed the conclusion made in the bioinformatic analysis regarding a high-homology region (including an identical section) in the primary sequences of β-pore-forming toxins. All obtained antibodies that simultaneously recognized various toxins belonged to class M – early immune response antibodies – which may indicate a high level of adaptation of pathogens to overcome the immune defense of the “host” and to weaken the formation of long-term immune memory.
All antibodies that simultaneously recognized the toxins under study recognized monomeric water-soluble forms of toxins; therefore, their close epitopes are located on the surface of the proteins.
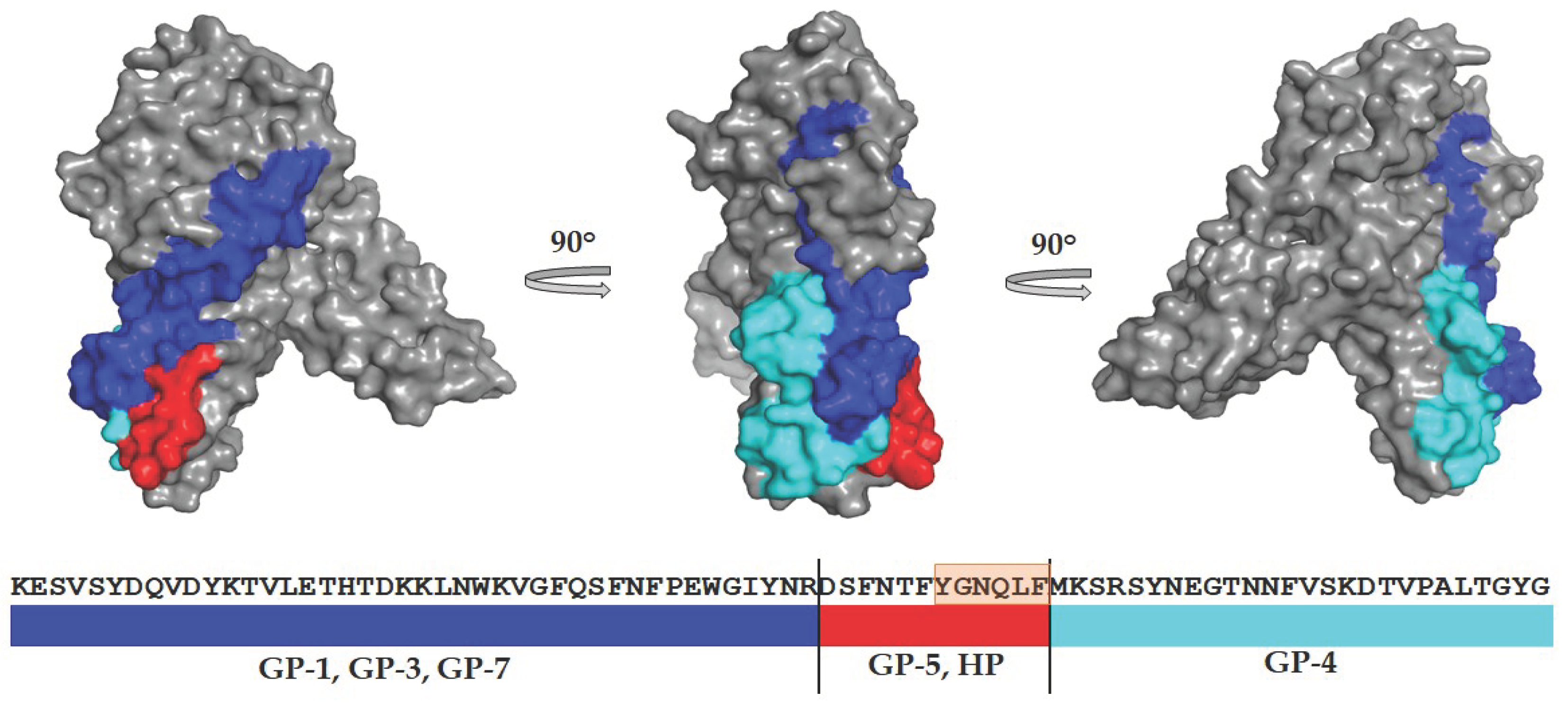
Figure 7 shows the location of the binding regions of mAbs obtained against homologous peptide and synthetic peptide on the 3D model of the HlyII monomer. GP-4 recognizes the region including HlyIILCTD [
14]. GP-1, GP-3 and GP-7 mAbs recognize HlyII regions located upstream from the identical section. Experimental data demonstrate that GP-5 recognizes a region containing the identical section, as confirmed by the production of mAbs against the synthetic peptide DSFNTFYGNQLFMK.
The amino acid sequence of the CytK2 protein is 37% identical to the sequence of
B. cereus hemolysin II described in [
6], and 30% identical to the sequence of
S. aureus α-hemolysin [
19], which suggests that from the structural point of view CytK2, as HlyII, belongs to the family of oligomeric β-barrel PFTs [
20]. It should be borne in mind that there are no experimental data on the 3D structure model for both HlyII and CytK2, and the model of their spatial structure [
21] was obtained on the basis of a previously constructed model based on X-ray diffraction analysis of Hla [
16]. In this regard, it can be assumed that the accessibility of individual amino acids on the surface of the three PFTs studied may be different for mAbs. These differences, probably, determine the efficiency of interaction with mAbs. The suppression of the cytolytic activity of CytK2, as well as the inability to suppress the cytolytic activity of HlyII and Hla by antibody GP-5 is, possibly, determined by some differences in the 3D structure of these PFTs, which are significant for the effect of GP-5 on the hemolytic activity of the toxins. HlyII, unlike Hla and CytK2, contains a C-terminal domain. The use of a deletion variant of HlyII lacking HlyIICTD revealed a marked suppression of the cytolytic activity of HlyII∆CTD by antibody GP-5.
A decrease in the efficiency of recognition and suppression of cytolytic activity may indicate that the epitopes located on HlyII for all antibodies obtained in monomeric water-soluble form may be partially altered under the HlyIICTD influence. Besides, the ability to recognize all three PFTs analyzed suggests that GP-5- and HP-series mAbs will be able to recognize other β-barrel PFTs.
The HP-series mAbs to the synthesized DSFNTFYGNQLFMK peptide are able to recognize CytK2, Hla and HlyII, as is the GP-5 monoclonal antibody. In addition, they show a similar effect on toxins: they suppress CytK2 activity and do not affect Hla. These data allow us to conclude that the epitope for GP-5 and the epitopes for HP-series mAbs are located at the same site of the homologous peptide. Thus, the localization of epitopes for the analyzed monoclonal antibodies in the sequence of the homologous peptide is seen as follows: GP-1, GP-3, GP-7 bind to HlyII within Lys171-Arg212, GP-4 binds to the epitope within Met225-Gly250; GP-5, as well as HP-series antibodies, bind to the epitope within 213-DSFNTFYGNQLF-224, while apparently the Phe215-Phe218 site is not essential for binding these antibodies – since FNTF is located in this place in HlyII; YHSL, in CytK2; and WNPV, in Hla – but can affect the function of suppressing hemolytic activity by antibodies. All three PFTs examined in this work were recognized by mAb GP-5. However, this antibody suppressed the hemolytic activity of only CytK2 and HlyIIΔCTD, in contrast to Hla. Comparison of the amino acid sequences of the homologous peptide region of three PFTs revealed that Hla was enriched in proline residues (4 residues in the homologous peptide section). One of them is located in the GP-5 recognize region sequence instead of Tre217 in HlyII and Ser236 in CytK2, while CytK2 and HlyIIΔCTD each have one proline residue outside this region. We have previously demonstrated the role of proline in the accessibility of mAbs amino acid sequences [
10,
22]. The presence of proline residues can significantly change the 3D structure of proteins and can modulate the suppression of PFTs activity.
4. Materials and Methods
4.1. Strains, Plasmid and Enzymes
The following E. coli strains were used: XL1-Blue recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F'proAB lacIqZΔM15 Tn10 (Tetr)] for gene cloning; BL21(DE3) F– ompT hsdSB (rB–, mB–) gal dcm (DE3) for high-level T7 expression of recombinant proteins.
Restriction endonucleases KpnI and NdeI (Thermo Scientific, Waltham, MA, USA), T4-DNA ligase (NEB, Ipswich, MA, USA), protein markers and DNA electrophoresis markers (Thermo Scientific, Vilnius, Lithuania), Q5 High-Fidelity DNA polymerase (NEB, Ipswich, MA, USA), TaqSE-DNA polymerase (SibEnzyme, Moscow, Russia), and dNTP mix (Thermo Scientific, Waltham, MA, USA) were used. The PCR product was cloned using the pET29b (+) vector plasmid.
4.2. Molecular Cloning
Plasmids pET29-hlyIIΔSP14579, pET29-hlyIIΔSPΔCTD14579 and pET28-hlaΔSP encoding intracellular HlyII, HlyIIΔCTD and Hla, respectively, have been were obtained earlier [
10,
18].
To create the pET29-GP plasmid, the gene encoding the homologous peptide (Lys171-Gly250 region of B. cereus HlyII) was amplified from genomic DNA using the following primers:
HlyIIfrgomF: 5'-CCTCTAGACATATGAAAGAAAGTGTATCTTATGATC
HlyIIfrgomR: 5'-CTCGAGGGTACCACCATATCCTGTTAAAGC
Similarly, to create the pET29-cytK2ΔSP plasmid, the gene encoding intracellular CytK2 was amplified from genomic DNA using the following primers:
F-CytK2ΔSP: 5'-TTATAGGATCCCATATGCAAACGACGTCACAAG
R-CytK2ΔSP: 5'-TTACTCGAGGGTACCTTTTTTCTCTACCAATTTCTTATTC
The PCR products were cloned into the pET29b vector using NdeI and KpnI restriction enzymes. This ensured that the final product included six histidine residues and a thrombin recognition site. This allows for the removal of the six histidines if necessary using the thrombin enzyme.
To produce proteins, the E. coli BL21(DE3) strain was transformed with recombinant plasmid.
4.3. Protein Expression and Purification
The expression of all protein products followed the same procedure. The culture was grown at 37°C in LB medium in two flasks (200 mL each), containing kanamycin at a concentration of 20 μg/mL, until the optical density at 600 nm (A
600) reached 0.7 - 0.9. Protein expression was then induced by adding isopropyl β-D-thiogalactoside (IPTG) to a final concentration of 0.1 mM, and the cultivation was continued at 20°C for 12 h. The biomass was harvested by centrifugation at 6,000 × g for 15 min. The pellet was resuspended in 20 mL of buffer A (50 mM sodium hydrogen phosphate, 500 mM sodium chloride, 5% glycerol, pH 8.0), and treated with 1 mM Fphenylmethylsulfonyl fluoride (PMSF) and 0.1% lysozyme. The resulting cell suspension was sonicated using a QSonica Q700 ultrasonic homogenizer for 7 cycles of 20 seconds each, with 2-min breaks between cycles at an amplitude of 40%. After sonication, the disrupted cells were centrifuged using a Beckman Coulter Avanti JXN-26 centrifuge at 12,000 rpm for 60 min at 6°C. The clarified lysate and remaining cell debris were then analyzed by SDS-PAGE. For further purification, the cell debris was washed with 10 mL of buffer B (100 mM NaH
2PO
4, 10 mM Tris-HCl, pH 8) containing 2 M urea. The debris was then dissolved in another solution of buffer B with 8 M urea and purified using Ni-NTA metal chelate affinity chromatography (Qiagen, Germantown, TN, USA) according to the manufacturer's protocol. The purified denatured homologous peptide was used to immunize mice. Other proteins were refolded according to the protocol described in [
23] and used for ELISA and suppression of hemolytic activity by mAbs.
4.4. Preparation and Purification of mAbs
For immunization in production of mAb-secreting hybridomas, we used animals kept under standard conditions in accordance with the Decree of the Chief State Sanitary Doctor of the Russian Federation dated August 29, 2014 No. 51 “On approval of SR 2.2.1 “Maintenance of experimental biological clinics (vivariums)”. For immunization, a homogeneous preparation of homologous peptide in 8 M urea was used.
Two groups of BALB/c mice (5 females, 2–3 months of age) were immunized. One batch of mice was immunized with a dose of 15 μg homologous peptide/mouse; the other, of 30 μg/mouse in Freund’s complete adjuvant. Animals of the first group were further immunized with a dose of 10 μg/mL in Freund’s incomplete adjuvant. The second group was further immunized with a dose of 20 μg/mL, also in Freund’s incomplete adjuvant. Five immunizations were carried out at two-week intervals.
Conjugation of the synthetic peptide DSFNTFYGNQLFMK (IQChem, Russia) with KLH was carried out as described [
24]. Immunization of experimental animals was performed with a dose of 10 μg of peptide per 100 μg of KLH in Freund’s adjuvant according to the scheme used for immunization with homologous peptide.
mAb-Secreting hybridomas were obtained using hybridoma technology [
17] with modifications from [
24]. Selection of hybridomas secreting specific antibodies was carried out by indirect ELISA by interaction of supracellular supernatants with HlyII and Hla immobilized on immunoplates.
The mAbs obtained were preparatively produced in the culture liquid during the cultivation of mAb-secreting hybridomas. Class G mAbs were purified by protein A-sepharose affinity chromatography [
25]. IgM was purified by 3-fold precipitation with 50% ammonium sulfate saturation.
Types of immunoglobulin heavy and light chains were determined by ELISA using the Rapid ELISA Mouse mAb Isotyping Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions.
4.5. The Enzyme-Linked Immunosorbent Assay
The enzyme-linked immunosorbent assay was performed as described in [
26].
4.6. Immunoblotting
Immunoblotting was performed as described in [
18].
4.7. Suppression of PFT Hemolytic Activity Suppression with mAbs
To evaluate the potential for suppression of hemolytic activity, different concentrations of the investigated PFTs were prepared by two-fold serial dilutions and incubated with mAbs at a concentration of 0.4 μM for 15 min at 37°C. To determine the effect of the concentration of mAbs on suppression of hemolytic activity, concentrations of mAbs ranging from 0.4 μM to 0.003 μM, obtained by two-fold serial dilutions, were incubated with a specific amount of toxin (see
Figure 6). The incubation was carried out at 37°C for 15 min. An equal volume of a 1% rabbit erythrocyte suspension was then added, and the mixture was further incubated for 30 min more at the same temperature. Following the incubation, the erythrocytes were pelleted by centrifugation, and the supernatant was used for spectrophotometric analysis of hemolysis levels via optical density measurement at a wavelength of 541 nm.
To construct normalized hemolytic activity curves, all PFT concentrations were converted to hemolytic units (HU). A HU is defined as the minimum quantity of toxin that causes hemolysis in 50% of a 1% suspension of rabbit erythrocytes after a 30-min incubation at 37°C. The hemolysis level was expressed as a percentage of hemolysis, where the maximum value of the optical density of the supernatant after the hemolysis reaction, measured at a wavelength of 541 nm, was taken as 100%.
Author Contributions
A.N., N.R., A.S. designed the research and wrote the paper; A.N., O.V., N.R., A.K., A.Z., Z.A.-K., V.S., N.E., A.S., T.I. and Kh.B. and performed the research; F.B. data curation.
Funding
This work was supported by the Russian Science Foundation (project no. 22-74-10026).
Conflicts of Interest
We declare no conflict of interest.
References
- Verma, P.; Gandhi, S.; Lata, K. Chattopadhyay K. Pore-forming toxins in infection and immunity. Biochem Soc Trans. 2021, 1, 455–465. [Google Scholar] [CrossRef]
- Los, F.C.; Randis, T.M.; Aroian, R.V.; Ratner, A.J. Role of pore-forming toxins in bacterial infectious diseases. Microbiol Mol Biol Rev. 2013, 2, 173–207. [Google Scholar] [CrossRef] [PubMed]
- Bhakdi, S.; Tranum-Jensen, J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991, 4, 733–751. [Google Scholar] [CrossRef]
- Kataev, A.A.; Andreeva-Kovalevskaya, Z.I.; Solonin, A.S.; Ternovsky, V.I. Bacillus cereus can attack the cell membranes of the alga Chara corallina by means of HlyII. Biochim Biophys Acta. 2012, 5, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.K.; Chattopadhyay, K. Structures and functions of the membrane-damaging pore-forming proteins. Adv Protein Chem Struct Biol 2022, 128, 241–288. [Google Scholar] [CrossRef]
- Baida, G.; Budarina, Z.I.; Kuzmin, N.P.; Solonin, A.S. Complete nucleotide sequence and molecular characterization of hemolysin II gene from Bacillus cereus. FEMS Microbiol Lett. 1999, 1, 7–14. [Google Scholar] [CrossRef]
- Omersam, N.; Podobnik, M.; Anderluh, G. Inhibition of Pore-Forming Proteins. Toxins (Basel). 2019, 9, 545. [Google Scholar] [CrossRef]
- Harshman, S.; Alouf, J.E.; Siffert, O.; Baleux, F. Reaction of staphylococcal alpha-toxin with peptide-induced antibodies. Infect. Immun. 1989, 57, 3856–3862. [Google Scholar] [CrossRef] [PubMed]
- Kucinskaite-Kodze, I.; Simanavicius, M.; Dapkunas, J.; Pleckaityte, M.; Zvirbliene, A. Mapping of Recognition Sites of Monoclonal Antibodies Responsible for the Inhibition of Pneumolysin Functional Activity. Biomolecules. 2020, 7, 1009. [Google Scholar] [CrossRef]
- Rudenko, N.; Nagel, A.; Zamyatina, A.; Karatovskaya, A.; Salyamov, V.; Andreeva-Kovalevskaya, Z.; Siunov, A.; Kolesnikov, A.; Shepelyakovskaya, A.; Boziev, K.; Melnik, B.; Brovko, F.; Solonin, A. A Monoclonal Antibody against the C-Terminal Domain of Bacillus cereus Hemolysin II Inhibits HlyII Cytolytic Activity. Toxins (Basel). 2020, 12, 806. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, W1, W200–W204. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium. UniProt: the Universal Protein Knowledgebase in 2023. Nucleic Acids Research D523, D531. [Google Scholar] [CrossRef]
- Ghanem, N.; Kanagami, N.; Matsui, T.; Takeda, K.; Kaneko, J.; Shiraishi, Y.; Choe, C.A.; Uchikubo-Kamo, T.; Shirouzu, M.; Hashimoto, T.; Ogawa, T.; Matsuura, T.; Huang, P.S.; Yokoyama, T.; Tanaka, Y. Chimeric mutants of staphylococcal hemolysin, which act as both one-component and two-component hemolysin, created by grafting the stem domain. FEBS J. 2022, 12, 3505–3520. [Google Scholar] [CrossRef] [PubMed]
- Nagel, A.S.; Rudenko, N.V.; Luchkina, P.N.; Karatovskaya, A.P.; Zamyatina, A.V.; Andreeva-Kovalevskaya, Z.I.; Siunov, A.V.; Brovko, F.A.; Solonin, A.S. Region Met225 to Ile412 of Bacillus cereus Hemolysin II Is Capable to Agglutinate Red Blood Cells. Molecules. 2023, 8, 3581. [Google Scholar] [CrossRef] [PubMed]
- Menestrina, G.; Serra, M.D.; Prevost, G. Mode of action of beta-barrel pore-forming toxins of the staphylococcal alpha-hemolysin family. Toxicon. 2001, 39, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Hobaugh, M.R.; Shustak, C.; Cheley, S.; Bayley, H.; Gouaux, J.E. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science. 1996, 14, 1859–1866. [Google Scholar] [CrossRef]
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975, 5517, 495–497. [Google Scholar] [CrossRef]
- Zamyatina, A.V.; Rudenko, N.V.; Karatovskaya, A.P.; Shepelyakovskaya, A.O.; Siunov, A.V.; Andreeva-Kovalevskaya, Z.I.; Nagel, A.S.; Salyamov, V.I.; Kolesnikov, A.S.; Brovko, F.A.; Solonin, A.S. Monoclonal Antibody HlyIIC-15 to C-End Domain HlyII B. cereus Interacts with the Trombin Recognition Site. Russ J Bioorg Chem. 2020, 6, 1214–1220. [Google Scholar] [CrossRef]
- Ramarao, N.; Sanchis, V. The pore-forming haemolysins of Bacillus cereus: a review. Toxins (Basel). 2013, 6, 1119–1139. [Google Scholar] [CrossRef]
- Lund, T.; de Buyser, M.L.; Granum, P.E. A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol. Microbiol. 2000, 38, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Miles, G.; Bayley, H.; Cheley, S. Properties of Bacillus cereus hemolysin II: a heptameric transmembrane pore. Protein Sci. 2002, 7, 1813–1824. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, N.V.; Nagel, A.S.; Melnik, B.S.; Karatovskaya, A.P.; Vetrova, O.S.; Zamyatina, A.V.; Andreeva-Kovalevskaya, Z.I.; Siunov, A.V.; Shlyapnikov, M.G.; Brovko, F.A.; Solonin, A.S. Utilizing Extraepitopic Amino Acid Substitutions to Define Changes in the Accessibility of Conformational Epitopes of the Bacillus cereus HlyII C-Terminal Domain. Int J Mol Sci. 2023, 22, 16437. [Google Scholar] [CrossRef] [PubMed]
- Fursova, K.K.; Laman, A.G.; Melnik, B.S.; Semisotnov, G.V.; Kopylov, P.K.; Kiseleva, N.V.; Nesmeyanov, V.A.; Brovko, F.A. Refolding of scFv mini-antibodies using size-exclusion chromatography via arginine solution layer. J Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2009, 877, 2045–2051. [Google Scholar] [CrossRef]
- Karatovskaya, A.P.; Rudenko, N.V.; Tsfasman, I.M.; Guseva, K.A.; Laman, A.G.; Brovko, F.A.; Vasilyeva, N.V. Development of a method for the quantitation of homologous endopeptidases AlpA and AlpB from Lysobacter sp. XL1. Process Biochem. 2016, 51, 1521–1526. [Google Scholar] [CrossRef]
- Mole, S.E.; Lane, D.P. DNA Cloning, v. III. //A Practical Approach; Glover, D.M.P., Ed.; IRL Press: Oxford, UK: Washington, DC, USA, 1989; pp. 197–198. [Google Scholar]
- Rudenko, N.V.; Karatovskaya, A.P.; Zamyatina, A.V.; Siunov, A.V.; Andreeva-Kovalevskaya, Zh.I.; Nagel, A.S.; Brovko, F.A.; Solonin, A.S. C-Terminal Domain of Bacillus cereus Hemolysin II Is Able to Interact with Erythrocytes. Russ. J. Bioorg. Chem. 2020, 46, 321–326. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).