Submitted:
18 March 2024
Posted:
19 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methodology
2.1. Inclusion and Exclusion Criteria
2.2. Search Strategy
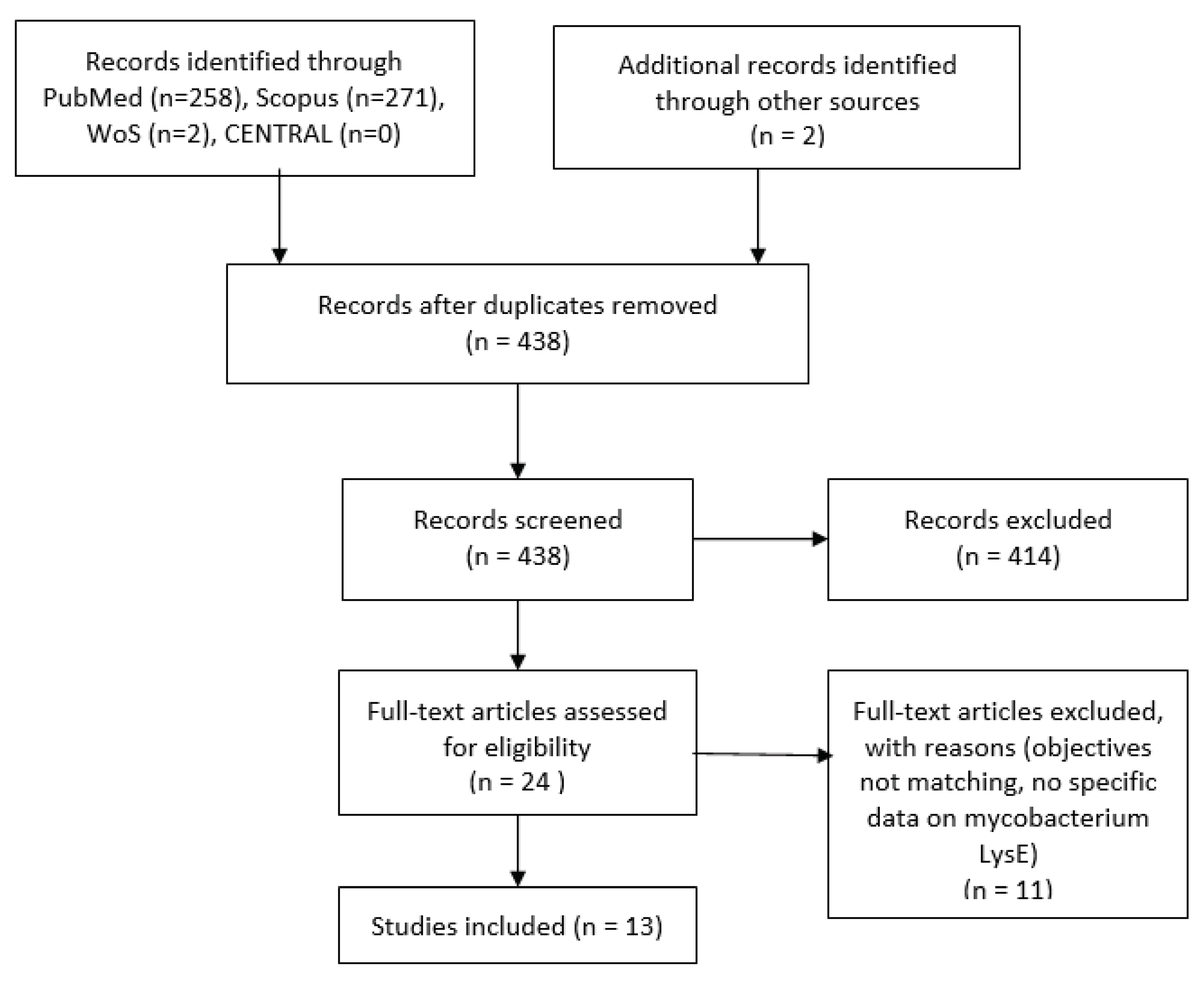
3. Result
3.1. Details of Included Studies
4. Discussion
4.1. Strength and Limitations
5. Conclusion
Author Contributions
Funding
Acknowledgements
Conflicts of Interests
References
- World Health Organization. GLOBAL TUBERCULOSIS REPORT 2021. geneva; 2021.
- World Health Organization. Global tuberculosis report 2022 [Internet]. 2022. Available from: https://iris.who.int/bitstream/handle/10665/363752/9789240061729-eng.pdf?sequence=1.
- World Health Organization. Tuberculosis: Key Facts [Internet]. 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/tuberculosis.
- WHO key facts. Tuberculosis: Key facts 2021 [Internet]. 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/tuberculosis#:~:text=In%202020%2C%20an%20estimated%2010,all%20countries%20and%20age%20groups.
- WHO. Tuberculosis: Fact sheets [Internet]. 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/tuberculosis.
- Pereira SM, Dantas OMS, Ximenes R, Barreto ML. Vacina BCG contra tuberculose: efeito protetor e políticas de vacinação. Rev Saúde Pública. 2007, 41 (suppl 1), 59–66. [Google Scholar] [CrossRef]
- Chen F, Shi T, Zhu Q, Xiao L. The Role of Mycobacterium tuberculosis Rv1986 and Rv3823c in Stimulating Humoral and Cell-Mediated Immune Responses. 2018, 1, 6.
- Dwivedi, M.; Mukhopadhyay, S.; Yadav, S.; Dubey, K.D. A multidrug efflux protein in Mycobacterium tuberculosis; tap as a potential drug target for drug repurposing. Comput. Biol. Med. 2022, 146, 105607. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, E.R.; Fanouraki, C.; Borbat, P.P. Expression, purification and initial characterization of LysE membrane exporter from Mycobacterium tuberculosis: Towards comprehensive functional and structural study. FASEB J. 2020, 34, 1–1. [Google Scholar] [CrossRef]
- Chen, J.; Ruan, Q.; Shen, Y.; Wang, S.; Shao, L.; Zhang, W. Assessing and screening for T-cell epitopes from Mycobacterium tuberculosis RD2 proteins for the diagnosis of active tuberculosis. Braz. J. Infect. Dis. 2018, 22, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Cockle, P.J.; Gordon, S.V.; Lalvani, A.; Buddle, B.M.; Hewinson, R.G.; Vordermeier, H.M. Identification of Novel Mycobacterium tuberculosis Antigens with Potential as Diagnostic Reagents or Subunit Vaccine Candidates by Comparative Genomics. Infect. Immun. 2002, 70, 6996–7003. [Google Scholar] [CrossRef] [PubMed]
- Gideon, H.P.; Wilkinson, K.A.; Rustad, T.R.; Oni, T.; Guio, H.; Kozak, R.A.; Sherman, D.R.; Meintjes, G.; Behr, M.A.; Vordermeier, H.M.; et al. Hypoxia Induces an Immunodominant Target of Tuberculosis Specific T Cells Absent from Common BCG Vaccines. PLOS Pathog. 2010, 6, e1001237. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, H.; Wang, X.; Xiao, S.; Li, M.; Li, G.; Zhao, L.; Zhao, X.; Dou, X.; Wan, K. Genetic diversity of immune-related antigens in Region of Difference 2 of Mycobacterium tuberculosis strains. Tuberculosis 2017, 104, 1–7. [Google Scholar] [CrossRef]
- Tsu, B.V.; Saier, M.H. The LysE Superfamily of Transport Proteins Involved in Cell Physiology and Pathogenesis. PLOS ONE 2015, 10, e0137184–e0137184. [Google Scholar] [CrossRef]
- Georgieva, E.R.; Fanouraki, C.; Borbat, P.P. Purification and Biophysical Characterization Of LysE Membrane Exporter From Mycobacterium tuberculosis in Lipodiscs Made of Native E. coli Membranes and Detergent. Biophys. J. 2020, 118, 503a–504a. [Google Scholar] [CrossRef]
- Hasenoehrl, E.J.; Sajorda, D.R.; Berney-Meyer, L.; Johnson, S.; Tufariello, J.M.; Fuhrer, T.; Cook, G.M.; Jacobs, W.R., Jr.; Berney, M. Derailing the aspartate pathway of Mycobacterium tuberculosis to eradicate persistent infection. Nat. Commun. 2019, 10, 4215. [Google Scholar] [CrossRef]
- Vrljic, M.; Garg, J.; Bellmann, A.; Wachi, S.; Freudl, R.; Malecki, M.J.; Sahm, H.; Kozina, V.J.; Eggeling, L.; Saier, M.H.; et al. The LysE superfamily: topology of the lysine exporter LysE of Corynebacterium glutamicum, a paradyme for a novel superfamily of transmembrane solute translocators. . 1999, 1, 327–336. [Google Scholar]
- Schneefeld, M.; Busche, T.; Geffers, R.; Kalinowski, J.; Bange, F.-C. The transcriptional regulator LysG (Rv1985c) of Mycobacterium tuberculosis activates lysE (Rv1986) in a lysine-dependent manner. PLOS ONE 2017, 12, e0186505–e0186505. [Google Scholar] [CrossRef]
- Torres, M.; García-García, L.; Cruz-Hervert, P.; Guio, H.; Carranza, C.; Ferreyra-Reyes, L.; Canizales, S.; Molina, S.; Ferreira-Guerrero, E.; Téllez, N.; et al. Effect of isoniazid on antigen-specific interferon-γ secretion in latent tuberculosis. Eur. Respir. J. 2014, 45, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Majumder, P.; Penmatsa, A.; Sardesai, A.A. Topological analyses of the L-lysine exporter LysO reveal a critical role for a conserved pair of intramembrane solvent-exposed acidic residues. J. Biol. Chem. 2021, 297, 101168. [Google Scholar] [CrossRef] [PubMed]
- Yelamanchi, S.D.; Surolia, A. Targeting amino acid metabolism of Mycobacterium tuberculosis for developing inhibitors to curtail its survival. IUBMB Life 2021, 73, 643–658. [Google Scholar] [CrossRef] [PubMed]
- Pavelka, M.S.; Jacobs, W.R. Biosynthesis of diaminopimelate, the precursor of lysine and a component of peptidoglycan, is an essential function of Mycobacterium smegmatis. J. Bacteriol. 1996, 178, 6496–6507. [Google Scholar] [CrossRef] [PubMed]
- Sahm, H.; Eggeling, L. New ubiquitous translocators: amino acid export by Corynebacterium glutamicum and Escherichia coli. Arch. Microbiol. 2003, 180, 155–160. [Google Scholar] [CrossRef]
- Eggeling L, Sahm H. The cell wall barrier of Corynebacterium glutamicum and amino acid efflux. J Biosci Bioeng. 2001 Jan;92(3):201–13.
- Krämer AB, R. Bacterial amino acid transport proteins: occurrence, functions, and significance for biotechnological applications. Appl Microbiol Biotechnol. 2002, 58, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Saier, Jr MH. Families of transmembrane transporters selective for amino acids and their derivatives The information presented in this review was initially prepared for presentation at the FASEB meeting on amino acid transport held in Copper Mountain, Colorado, June 26–July 1, 1999 and was updated in January 2000 following the meeting of the Transport Nomenclature Panel of the International Union of Biochemistry and Molecular Biology (IUBMB) in Geneva, November 28–30, 1999. The system of classification described in this review reflects the recommendations of that panel. Microbiology. 2000, 146, 1775–1795. [Google Scholar]
- Kozak, R.A.; Alexander, D.C.; Liao, R.; Sherman, D.R.; Behr, M.A. Region of Difference 2 Contributes to Virulence ofMycobacterium tuberculosis. Infect. Immun. 2011, 79, 59–66. [Google Scholar] [CrossRef] [PubMed]
| S.No | Study ID | Findings of the study | Conclusion |
|---|---|---|---|
| 1. | Georgieva et al. 2020 [15] United States of America (USA) |
LysE deficiency causes increased cellular levels of L-lysine, which inhibits bacterial growth. Hence, LysE emerges as potential targets for drug inhibition, requiring a thorough examination of their structures and the relationships between structure and function. The study involved protein purification in Lipodisc made up of native E.coli membrane and detergents. |
This study claimed to be the first study on highly pure LysE conducted in a controlled environment. It sets the groundwork for more in-depth investigations into how these proteins function. |
| 2. | Georgieva et al. 2020 [9] United States of America (USA) |
The Rv1986 (LysE) protein of M.tb is the primary lysine exporter. Its expression is increased during the early stages of M.tb infection under hypoxia conditions. Hence, LysE can be a probable vaccine candidate. There is a need to have detailed knowledge about the less studied M.tb membrane transport system. |
This study represented the initial production of highly pure LysE, providing a platform for in-depth investigations into the functional mechanisms of this protein. |
| 3. | Hasenoehrl et al., 2019 [16] United States of America (USA) |
In M.tb, lysine permease LysE, encoded by Rv1986, catalyses lysine export. A deficiency of LysE contributes to bacteria’s poor growth. LysE is a primary target for memory T cells secreting IL-2 and is believed to contribute to human protective immunity. Resting macrophage and lysosomal exposure models revealed that LysE is involved in human latent TB infection. The absence of LysE in eukaryotic cells makes it a probable target for drug discovery. |
These results demonstrate that the aspartate pathway depends on a combination of metabolic control mechanisms in M.tb. This pathway is essential for persistence and a promising target for developing anti-tuberculosis drugs. |
| 4. | Chen et al. 2018 [7] China |
Currently available BCG vaccine is less effective in M.tb prevention and transmission. Therefore, it is crucial to investigate M. tb proteins absent in BCG that can induce specific humoral, cellular, and innate responses in the host. Rv1986 (LysE) shows diagnostic promise and is present in virulent M. bovis but not in the BCG strain. Studies highlight its role as an immunodominant target for memory T cells, containing numerous T and B cell epitopes. |
The findings indicated that Rv1986 is a highly immunogenic antigen capable of eliciting both humoral and cell-mediated immune responses. |
| 5. | Jiazhen et al., 2018 [10] China |
In infected cattle, Rv1983, Rv1986, Rv1987, and Rv1989c within RD2 were identified as immunodominant. This study assessed their diagnostic potential in humans. Out of 87 screened peptides from RD2 proteins, only ten were recognized by over 10% of active TB patients. The active TB group exhibited significantly higher IFN-γ responses to Rv1986 compared to the control groups (p < 0.05). |
This study concluded that six epitopes from RD2, including Rv1986, have potential diagnostic value in active TB. |
| 6. | Jiang et al., 2017 [13] China |
Region of Deletion 2 (RD2) plays a role in mycobacterial virulence, and its deletion from M.tb leads to a decrease in bacterial growth. The amplification and comparison of the five genes in RD2 indicate the presence of T and B cell epitopic regions. The study proposes that Rv1980c, Rv1985, and Rv1986 RD2 proteins belong to RD2. These proteins exhibit antigenic variation in response to host immune pressure, suggesting their potential involvement in ongoing immune evasion. |
The data confirmed that RD2 regions, aiming to evade host immunity, exhibit unexpected variability, particularly in immune-related antigens and T-cell epitope regions. |
| 7. | Schneefeld er al., 2017 [18] Germany |
The study showed that the Rv1985c (LysG) protein binds to its own gene and its promoter region of Rv1986 (LysE). Additionally, the Rv1985c protein induces the transcription of Rv1986 in a lysine-dependent manner and autonomously regulates the expression of its own gene. While the function of the Rv1986 protein remains elusive, studies have demonstrated its regulation and recognition by memory T cells in human TB. |
This study delineated the regulatory network of Rv1985c in M.tb. Given the resemblance to an orthologous gene pair in C. glutamicum, the study proposed renaming Rv1985c to lysG(Mt) and Rv1986 to lysE(Mt). |
| 8. | Tsu and Saier, 2015 [14] China |
The LysE superfamily, comprising transmembrane transport proteins vital for exporting amino acids, lipids, and heavy metal ions, plays pivotal roles in maintaining ionic balance, cell envelope assembly, and shielding against excessive cytoplasmic heavy metal/metabolite concentrations. | Consequently, they influence the physiology and pathogenesis of microbes and present potential drug targets. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





