Submitted:
18 March 2024
Posted:
18 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Dietary Fibre and Its Importance in the Diet
2.1. Dietary Fibre Consumption
2.2. Properties of Dietary Fibre
2.3. Dietary Fibre in Diet and Its Effect on a Health
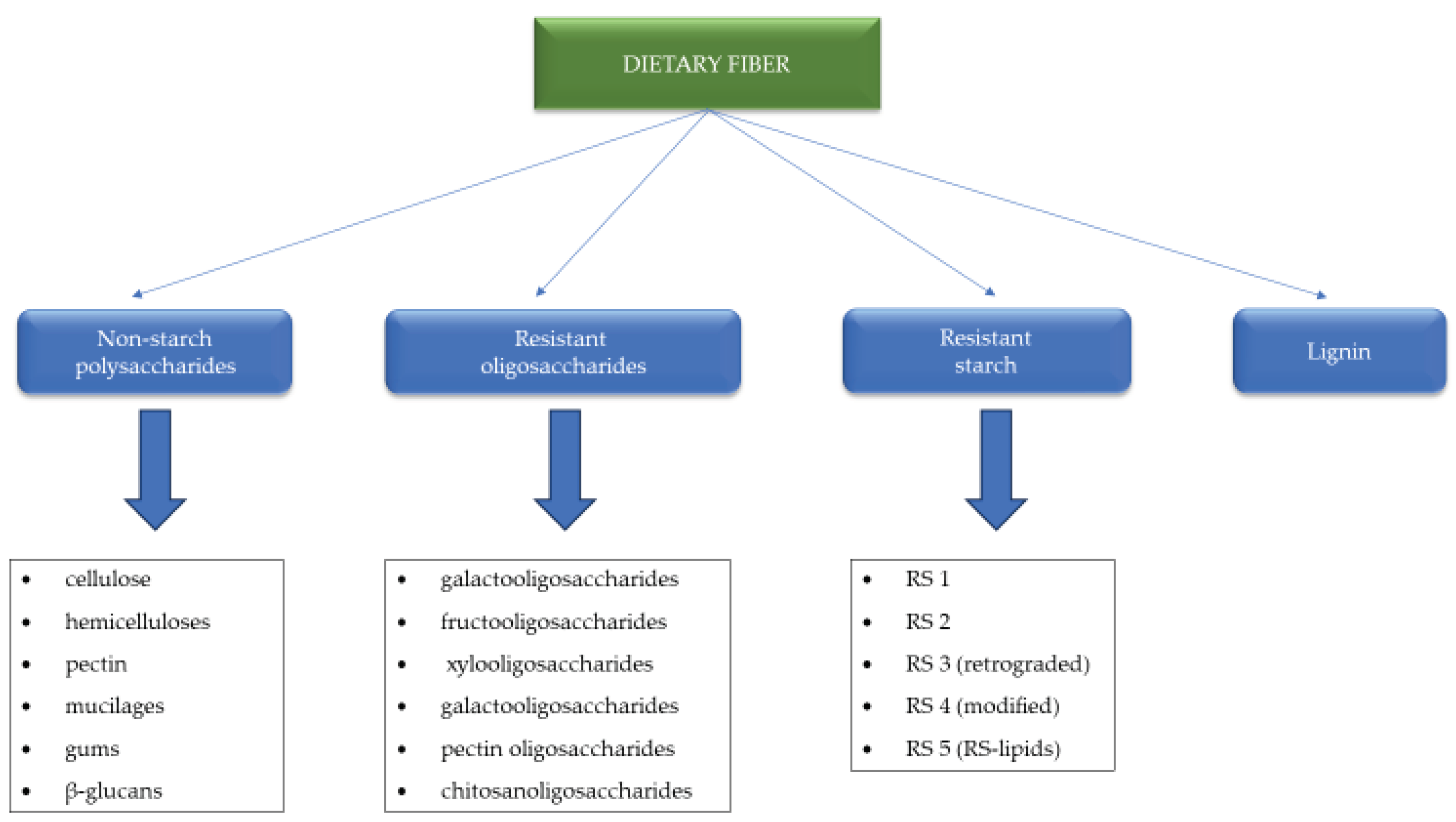
3. Types of Dietary Fibre and the Specificity of Their Action
3.1. Dietary Fibre Types and Its Action in Gastrointestinal Tract
3.2. Soluble Dietary Fibre and Its Action in Hindgut
3.3. SDF and Changes in Microbiome of Hindgut
4. Pectin Structure and Their Properties
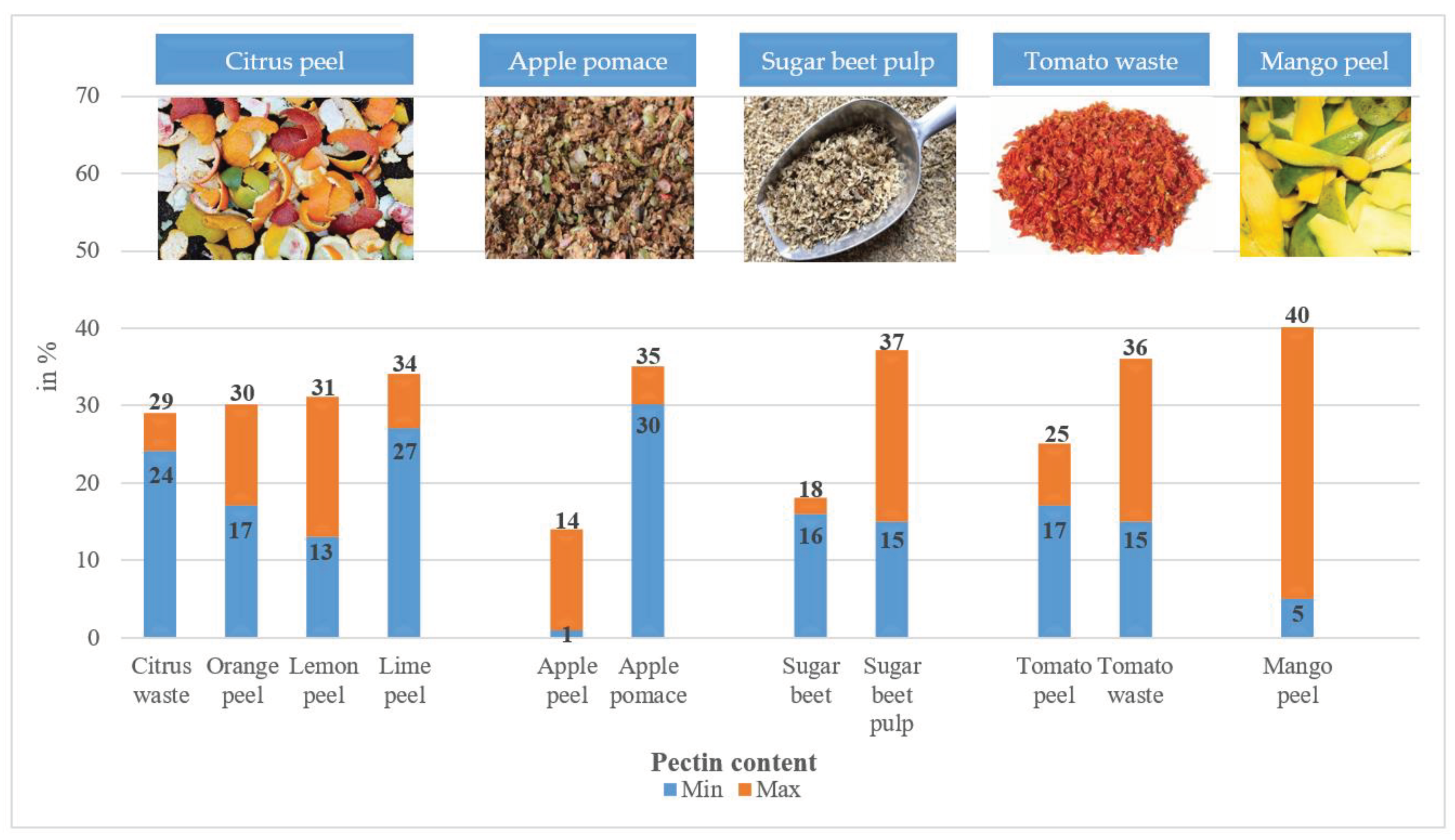
4.1. Occurrence of Pectin and Its Amount in Various Fruits and By-Products
4.2. Pectin in Cell Wall
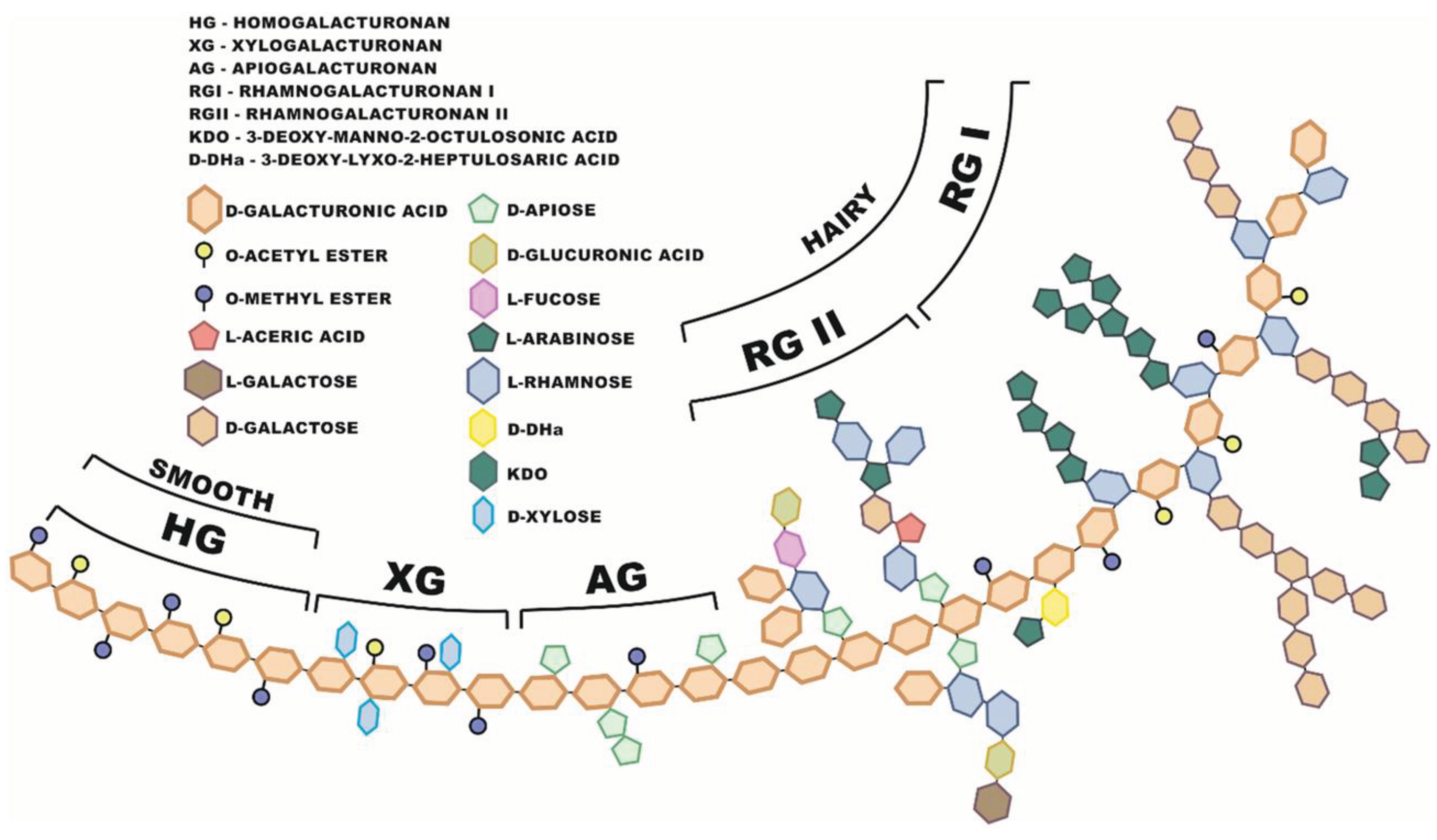
4.3. Structure of Pectin
- polygalactouronases and polymethylgalactouronases hydrolyzing α-1,4-glycosidic bonds of pectin molecules,
- polygalactouronate lyases and polymethylgalactouronase Lyases break down α-1,4-glycosidic bonds by a trans-elimination mechanism
5. Prebiotics
5.1. Oligosaccharides
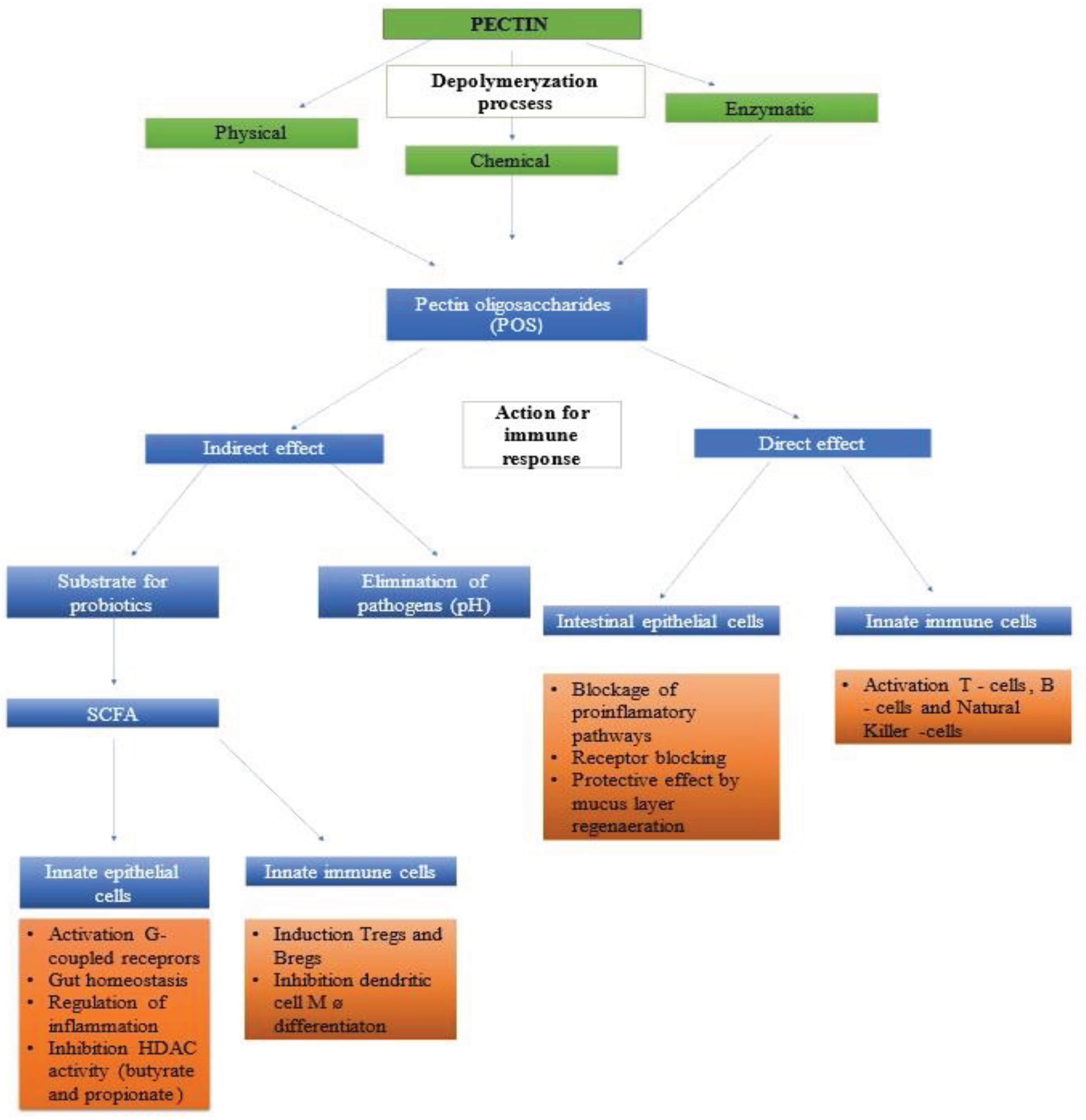
5.2. Pectin Oligosaccharides
6. Pectin and POS - Prebiotic Effect on Intestinal Microbiome
6.1. Prebiotics
6.2. Pectin and Its Effect on Microbiome
6.3. Pectin Oligosaccharides - Effect on Microbiome
6.4. Antibacterial and Immunomodulatory Effect of POS
7. Interaction of Pectin and POS with GALT
7.1. Dietary Fibre and Its Effect on GALT
7.2. Pectin Effect on GALT
7.3. POS Effect on GALT
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Y.; Wang, B.; Wen, L.; Wang, F.; Yu, H.; Chen, D.; Su, X.; Zhang, C. Effects of dietary fiber on human health. Food Sci. Hum. Wellness. 2022, 11, 1–10. [Google Scholar] [CrossRef]
- Maina, N.H.; Rieder, A.; de Bondt, Y.D.; Mäkelä-Salmi, N.; Sahlstrøm, S.; Mattila, O.; Lamothe, L.M.; Nyström, L.; Courtin, C.M.; Katina, K.; Poutanen, K. Process-induced changes in the quality and characteristics of grain dietary fiber. Foods 2021, 10, 2566. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Folkerts, J.; Folkerts, G.; Maurer, M.; Braber, S. Microbiota-dependent and -independent effects of dietary fibre on human health. Br. J. Pharmacol. 2020, 177, 1363–1381. [Google Scholar] [CrossRef] [PubMed]
- Pathania, S.; Kaur, N. Utilization of fruits and vegetable by-products for isolation of dietary fibres and its potential application as a functional ingredients. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100295. [Google Scholar] [CrossRef]
- Buttriss, J.L.; Stokes, C.S. Dietary fibre health: An overwiew. Nutr. Bull. 2008, 33, 186–200. [Google Scholar] [CrossRef]
- Chawla, R.; Patil, G.R. Soluble dietary fiber. Compr. Rev. Food Sci. Food Saf. 2010, 9, 178–196. [Google Scholar] [CrossRef]
- Widaningrum; Flanagan, B.M.; Williams, B.A.; Sonni, F.; Mikkelsen D.; Gidley, M.J. Fruit and vegetable insoluble dietary fibre in vitro fermentation characteristics depend on cell wall type. Bioact. Carbohydr. Diet. Fibre 2020, 23, 100223. [Google Scholar] [CrossRef]
- Liao, A.-M.; Zhang, J.; Yang, Z.L.; Huang, J.-H.; Pan, L.; Hou, Y.C.; Li, X.-X.; Zhao, P.H.; Dong, Y.Q.; Hu, Z. Y.; Hui, M. Structural, physiochemical and functional properties of wheat bran insoluble dietary fiber modified with probiotic fermentation. Front. Nutr. 2022, 9, 803440. [Google Scholar] [CrossRef]
- Ge, Q.; Li, H.-G.; Zheng, Z.-Y.; Yang, K.; Li, P.; Xiao, Z.-G.; Xiao, G.-M.; Mao, J.W. In vitro fecal fermentation characteristics of bamboo insoluble dietary fiber and its impacts on human gut microbiota. Food Res. Int. 2022, 156, 111173. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bai, Y.; Zhang, G.; Liu, L.; Lai, L. Relationship between dietary fiber fermentation and volatile fatty acids concentration in growing pigs. Animals 2020, 10, 263. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and prebiotics: Mechanism and health benefits. Nutrients, 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients, 2010, 2, 1266–1289. [Google Scholar] [CrossRef]
- Dalzenne, N.M. Oligosaccharides: State of the art. Proc. Nutr. Soc. 2003, 62, 177–182. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S. Composition properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. Biol. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef]
- Combo, A.M.M.; Aguedo, M.; Quiévy, N.; Danthine, S.; Goffin, D.; Jacquet, N.; Blecker, C.; Devaux, J.; Paquot, M. Characterization of sugar beet pectic-derived oligosaccharides obtained by enzymatic hydrolysis. Int. J. Biol. Macromol. 2013, 52, 148–156. [Google Scholar] [CrossRef] [PubMed]
- La Torre, D.; Verbeke, K.; Dalile, B. Dietary fibre and gut-brain axis: Microbiota-dependent and independent mechanism of action. Gut Microbiome 2021, 2, 1–26. [Google Scholar] [CrossRef]
- Raninen, K.; Lappi, J.; Mykkänen, H.; Poutanen, K. Dietary fiber type reflects physiological functionality: Comparison of grain fiber, inulin, and polydextrose. Nutr. Rev. 2011, 69, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Flanagan, B.M.; Williams, B.A.; Mikkelsen, D.; Gidley, M.J. Particle size of dietary fibre has diverse effects on in vitro gut fermentation rate and end-products depending on food source. Food Hydrocoll. 2023, 134, 108096. [Google Scholar] [CrossRef]
- McRorie Jr., J. W.; McKeown, N.M. Understanding the physics of functional fibers in the gastrointestinal tract: An evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef]
- Gidley, M.J.; Yakubov, G.E. Functional categorization of dietary fibre in foods: Beyond ‘soluble’ vs ‘insoluble’. Trends Sci. Food Technol. 2019, 86, 563–568. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E. The nutritional significance of “dietary fibre” analysis. Anim. Feed Sci. Tech. 2001, 90, 3–20. [Google Scholar] [CrossRef]
- Singh, R.P.; Tingirikari, J.M.R. Agro waste derived pectin poly and oligosaccharides: Synthesis and functional characterization. Biocatal. Agric. Biotechnol. 2021, 31, 101910. [Google Scholar] [CrossRef]
- Pop, C.; Suharoschi, R.; Pop, O.L. Dietary fiber and prebiotic compounds in fruits and vegetables for waste. Sustainability, 2021, 13, 7219. [Google Scholar] [CrossRef]
- Saber, W.I.A.; El-Naggar, N.E. Optimization of fermentation conditions for the biosynthesis of inulinase by the new source; Aspergillus tamari and hydrolysis of some inulin containing agro-wastes. Biotechnology 2009, 8, 425–433. [Google Scholar] [CrossRef]
- Sabater, C.; Calvette-Torre, I.; Villamiel, M.; Javier Moreno, F.; Margolles, A.; Ruiz, L. Vegetable waste and by-products to feed a healthy gut microbiota: Current evidence, machine learning and computational tools to design novel microbiome-targeted foods. Trends Food Sci. Technol. 2021, 118 (Part A), 399–417. [Google Scholar] [CrossRef]
- Elshahed, M.S.; Miron, A.; Aprotosoaie, A.C.; Farag, M.A. Pectin in diet: Interactions with the human microbiome, role in gut homeostasis, and nutrient-drug interactions. Carbohydr. Polym. 2021, 255, 117388. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liao, C.; Wu,L. ; Tang, J.; Chen, J.; Lei, C.; Zheng, L.; Zhang, C.; Lin, Y.-Y.; Xavier, J.; Dai, L. Ecological dynamics of the gut microbiome in response to dietary fiber. ISME J. 2022, 16, 2040–2055. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Goptenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; Biddinger, S.B.; Dutton, R.J.; Turnbaugh, P.J. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Feng, N.; Li, Q.; Wang, H.; Su, Y.; Zhu, W. Short-term supplementation of pectin alters substrate dynamics and modulates microbial carbohydrate metabolism in the gut of a pig model. J. Agric. Food Chem. 2023, 71, 10470–10482. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Peng, C.; Mao, A.; Zhong, M.; Hu, Z. An overview of microbial enzymatic approaches for pectin degradation. Int. J. Biol. Macromol. 2024, 254, 127804. [Google Scholar] [CrossRef] [PubMed]
- Lordan, C.; Thapa, D.; Ross, R.P.; Cotter, P.D. Potential for enriching next generation health-promoting gut bacteria through prebiotics and other dietary components. Gut Microbes 2020, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wilkowska, A.; Nowak, A.; Antczak-Chrobot, A.; Motyl, I.; Czyżowska, A.; Paliwoda, A. Structurally different pectic oligosaccharides produced from apple pomace and their biological activity in vitro. Foods 2019, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-C.; Chiang, H.-H.; Liu, C.-Y.; Li, Y.-J.; Lu, C.-L.; Lee, Y.-P.; Huang, C.-J.; Lai, C.-L. Intestinal mucosal barrier improvement with prebiotics: Histological evaluation of longish glucomannan hydrolysates-induced innate T-lymphocyte activities in mice. Nutrients 2022, 14, 2220. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Ding, X.; Zeng, Q.; Bai, S.; Zhang, K.; Chen, D.; Yu, B.; He, J.; Yu, J.; Luo, J.; Luo, Y.; Wang, J. The effect of dietary pectic oligosaccharide supplementation on intestinal health of broiler breeders with different egg-laying rates. Poultry Science 2021, 100, 100938. [Google Scholar] [CrossRef]
- Chudzik, A.; Orzyłowska, A.; Rola, R.; Stanisz, G.J. Probiotics, prebiotics and postbiotics on mitigation of depression symptoms: Modulation of the brain-gut-microbiome axis. Biomolecules 2021, 11, 1000. [Google Scholar] [CrossRef]
- Chen, H.; Hu, H.; Chen, D.; Tang, J.; Yu, J.; Luo, J.; He, J.; Luo, Y.; Yu, J.; Mao, X. Dietary pectic oligosaccharide administration improves growth performance and immunity in weaned pigs infected by rotavirus. J. Agric. Food. Chem. 2017, 65, 65,2923–2929. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The health benefits of dietary fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- O’Keffe, S.J. The association between dietary fibre deficiency and high-income lifestyle- associated diseases: Burkitt’s hypothesis revisited. Lancet Gastroenterol. Hepatol. 2019, 4, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Bach Knudsen, K.E.; Nørskov, N.P.; Bolvig, A.K.; Hedemann, M.S.; Lærke, H.N. Dietary fibers and associated phytochemicals in cereals. Mol. Nutr. Food Res. 2017, 61, 1600518. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Prado, T.R.; Mazzonetto, A.C.; Botelho, A.M.; Fiates, G.M.R. Home availability of ultraprocessed foods in families who prepare meals at home. Rev. Nutr. 2022, 35, e210249. [Google Scholar] [CrossRef]
- Farmer, N.; Lee, L.J.; Powell-Wiley, T.M.; Wallen, G.R. Cooking frequency and perception of diet among US adults are associated with US healthy and healthy Mediterranean-style dietary related classes: A latent class profile analysis. Nutrients 2020, 12, 3268. [Google Scholar] [CrossRef]
- Kopp, W. How western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221–2236. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffman, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; Sinha, R.; Gilroy, E.; Gupta, K.; Baldassano, R.; Nessel, L.; Li, H.; Bushman, F.D.; Lewis, J.D. Linking long-term dietary patterns with gut microbial enterotypes. Science, 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.A.; Mikkelsen, D.; Flangan, B.M.; Gidley, M.J. “Dietary fibre”: Moving beyond the “soluble/insoluble” classification for monogastric nutrition, with an emphasis on humans and pigs. J. Anim. Sci. Biotechnol. 2019, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Tungland, B.C.; Meyer, D. Nondigestible oligo- and polysaccharides (dietary fiber): Their physiology and role in human health and food. Comp. Rev. Food Sci. Food Saf., 2002, 3, 90–109. [Google Scholar] [CrossRef]
- Deehan, E.C.; Walter, J. The fiber gap and the disappearing gut microbiome: Implications for human nutrition. Trends Endocrinol. Metab. 2016, 27, P239–P242. [Google Scholar] [CrossRef]
- Beukema, M.; Faas, M.M.; de Vos, P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: Impact via gut microbiota and direct effects on immune cells. Exp. Mol. Med. 2020, 52, 1364–1376. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis Jr., R. H.; Ferreri, S,; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Ötles, S.; Ozgoz, S. Health effects of dietary fiber. Acta Sci. Pol. Technol. Aliment. 2014, 13, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.V.; Bailey, M.A.; Taylor, A.M.; Kaczmarek, J.L.; Mysonhimer, A.R.; Edwards, C.G.; Reeser, G.E.; Burd, N.A.; Khan, N.A.; Holscher, H.D. Avocado consumption alters gastrointestinal bacteria abundance and microbial metabolic concentrations among adults with overweight or obesity: A randomized controlled trial. J. Nutr. 2021, 151, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Bach Knudsen, K.E.; Lærke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Gundelund Nielsen, D.S.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G.; Marco, M.L.; Gregersen, S.; Hermasen, K. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients, 2018, 10, 1499. [Google Scholar] [CrossRef]
- Wang, G.; Zhong, D.; Lin, H.; Yang, T.; Liang, Q.; Wang, J.; Zhang, R.; Zhang, Y. Water soluble dietary fibre from walnut meal as a prebiotic in preventing metabolic syndrome. J. Funct. Foods, 2021, 78, 104358. [Google Scholar] [CrossRef]
- Holscher, H.D.; Gutterman, H.M.; Swanson, K.S.; An, R.; Matthan, N.R.; Lichtenstein, A.H.; Novotny, J.A.; Baer, D.J. Walnut consumption alters the gastrointestinal microbiota, microbially derived secondary bile acids, and health markers in healthy adults: A randomized controlled trial. J. Nutr. 2018, 148, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Holscher, H.D.; Maslov, S.; Hu, F.B.; Weiss, S.T.; Lin, Y.-Y. Predicting metabolic response to dietary intervention using deep learning. bioRxiv 2023. [Google Scholar] [CrossRef]
- Wang, H.; He, C.; Lin, Y.; Zhao, H.; Long, L.; Gai, X.; Zhao, H. Soluble dietary fiber protects intestinal mucosa barrier by improving intestinal flora in a murine model of sepsis. Biomed. Pharmacother. 2020, 129, 110343. [Google Scholar] [CrossRef] [PubMed]
- Nyström, E.E.L.; Martinez-Abad, B.; Arike, L.; Birchenough, G.M.H.; Nonnecke, E.B.; Castillo, P.A.; Svensson, F.; Bevins, C.L.; Hansson, G.C.; Johansson, M.E.V. An intercrypt subpopulation of goblet cells is essential for colonic mucus barrier function. Science 2021, 372, eaabb1590. [Google Scholar] [CrossRef] [PubMed]
- Sauvaitre, T. Etienne-Mesmin, L.; Sivignon, A.; Mosoni, P.; Courtin, C.M.; van de Wiele, T.; Blanquet-Diot, S. Tripartite relationship between gut microbiota, intestinal mucus and dietary fibers: Towards preventive strategies against enteric infections. FEMS Microbiol. Rev. 2020, 45, 1–36. [Google Scholar] [CrossRef]
- Fernández-Tomé, S.; Ortega Moreno, L.; Chaparro, M.; Gisbert, J.P. Gut microbiota and dietary factors as modulators of the mucus layer in inflammatory bowel disease. Int. J. Mol. Sci. 2021, 22, 10224. [Google Scholar] [CrossRef]
- Maguire, M.; Maguire, G. Gut dysbiosis, leaky gut, and intestinal epithelial proliferation in neurological disorders: Towards the development of a new therapeutic using amino acids, prebiotics, probiotics, and postbiotics. Rev. Neurosci., 2019, 30, 179–201. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Microbiota-brain-gut ais and neurodegenerative diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Bedford, M.R.; Wu, S.-B.; Morgan, N.K. Soluble non-starch polysaccharide modulates broiler gastrointestinal tract environment. Poultry Science 2021, 100, 101183. [Google Scholar] [CrossRef]
- Cameron-Smith, D.; Collier, G.R.; O’Dea, K. Effect of soluble dietary fibre on the viscosity of gastrointestinal contents and the acute glycaemic response in the rat. Br. J. Nutr. 1994, 71, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Tejeda, O.J.; Kim, W.K. Role of dietary fiber in poultry nutrition. Animals 2021, 11, 461. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Shah, B.R.; Li, J.; Liang, H.; Zhan, F.; Geng, F.; Li, B. A critical review on interplay between dietary fibres and gut microbiota. Trends Food Sci. Technol. 2022, 124, 237–249. [Google Scholar] [CrossRef]
- Baker, R.A. Reassessment of some fruit and vegetable pectin levels. Food Sci., 1997, 62, 225–229. [Google Scholar] [CrossRef]
- Khanum, F.; Sidallinga Swany, M.; Sudarshana Krishna, K.R.; Santhanam, K.; Viswanathan, K.R. Dietary fiber content at commonly fresh and cooked vegetables consumed in India. Plants Foods Hum. Nutr. 2000, 55, 207–218. [Google Scholar] [CrossRef]
- Schakel, S.F.; Dennis, B.H.; Wold, A.C.; Conway, R.; Zhao, L.; Okuda, N.; Okayama, A.; Moag-Stahlberg, A.; Robertson, C.; van Heel, N.; Buzzard, I.M.; Stamler, J. Enhancing data on nutrient composition of food eaten by participants in the INTERMAP study in China, Japan, the United Kingdom, and the United States. J. Food Compos. Anal. 2003, 16, 395–408. [Google Scholar] [CrossRef]
- Bailoni, L.; Schiavon, S.; Pagnin, G.; Tagliapietra, F.; Bousembiante, M. Quanti-qualitative evaluation of pectins in the dietary fibre of 24 foods. Ital. J. Anim. Sci. 2005, 4, 49–58. [Google Scholar] [CrossRef]
- Choi, H.; Sung, J.Y.; Kim, B.G. Neutral detergent fiber rather than other dietary fiber types as an independent variable increases the accuracy of prediction equation for digestible energy in feeds for growing pigs. Asian-Australas. J. Anim. Sci. 2020, 33, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Tonarelli, S.; Barracca, F.; Rettura, F.; Pancetti, A.; Cecarelli, L.; Ricchiuti, A.; Costa, F. , de Bortoli N., Marchi S., Rossi A. Chronic constipation: Is a nutritional approach reasonable. Nutrients, 2021, 13, 3386. [Google Scholar] [CrossRef] [PubMed]
- McRae, M.P. Effectiveness of fiber supplementation for constipation, weight loss and supporting gastrointestinal function: A narrative review of meta-analyses. J. Chiropr. Med. 2020, 19, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Prasadi, V.P.; Joye, I.J. Dietary fibre from whole grains and their benefits on metabolic health. Nutrients, 2020, 12, 3045. [Google Scholar] [CrossRef]
- Chutkan, R.; Fahey, G.; Wright, W.L.; McRorie, J. Viscous versus nonviscous soluble fiber supplements: Mechanism and evidence for fiber – specific health benefits. J. Am. Assoc. Nurse Pract. 2012, 24, 476–487. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products Nutrition, and Allergies (NDA). Scientific opinion on dietary reference values for carbohydrates and dietary fibre . 2010, 8, 1462.
- Cione, E.; Fazio, A.; Curcio, R.; Tucci, P.; Lauria, G.; Capello, A.R.; Dolce, V. Resistant starches and non-communicable diseases: A focus on Mediterranean diet. Foods 2021, 10, 2062. [Google Scholar] [CrossRef]
- Chen, M.; Guo, L.; Nsor-Atindana, J.; Goff, H.D.; Zhang, W.; Zhong, F. The effect of viscous soluble dietary fiber on nutrient digestion and metabolic responses II: In vivo digestion process. Food Hydrocoll. 2020, 107, 105908. [Google Scholar] [CrossRef]
- Larsen, N.; de Souza, C.B.; Krych, L.; Barbosa Cahú, T.; Wiese, M.; Kot, W.; Meyer Hansen, K.; Blennow, A.; Venema, K.; Jespersen, L. Potential of pectins to beneficially modulate the gut microbiota depends on their structural properties. Front. Microbiol. 2019, 10, 223. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, A.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; Young, V.B.; Henrissat, B.; Wilmes, P.; Stappenbeck, T.S.; Núñez, G.; Martens, E.C. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell, 2016, 167, 1339–1353. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J. ; Immune boosting functional foods and their mechanism: A critical evaluation of probiotics. Biomed. Pharmacother. 2020, 130, 110625. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanism mediated by probiotics and prebiotics and their impact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef] [PubMed]
- Ishisono, K.; Mano, T.; Yabe, T.; Kitaguchi, K. Dietary fiber pectin ameliorates experimental colitis in a neutral sugar side chain-dependent manner. Front. Immunol. 2019, 10, 2979. [Google Scholar] [CrossRef]
- Kimura, I.; Inoue, D.; Hirano, K.; Tsujimoto, G. The SCFA receptor GPR43 and energy metabolism. Front. Endocrinol. 2014, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Lo Conte, M.; Cosovich, I.; Ferrarense, R.; Nobili, A.; Palmieri, V.; Massimino, L.; Lamparelli, L.A.; Liang, W.; Riba, M.; Devecchi, E.; Bolla, A.M.; Pedone; E. ; Scavini, M.; Bosi, E.; Fasano, A.; Ungaro, F.; Diana, J.; Mancini, N.; Falcone, M. Alternations of the intestinal mucus layer correlate with dysbiosis and immune dysregulation in human Type 1 Diabetes. eBioMedicine 2023, 91, 104567. [Google Scholar] [CrossRef] [PubMed]
- Murga-Garrido, S.M.; Hong, Q.; Cross, T.-W. L.; Hutchinson, E.R.; Han, J.; Thomas, S.P.; Vivas, E.I.; Denu, J.; Geschin, D.G.; Tang, Z.-Z.; Rey, F.E. Gut microbiota variation modulates the effects of dietary fiber on host metabolism. Microbiome 2021, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska, P.; Hikawczuk, T.; Wiliczkiewicz, A. Dried sugar beet pulp as a source of soluble dietary fibre in equine nutrition: A review. Anim. Nutr. Feed Technol. 2021, 21, 405–420. [Google Scholar] [CrossRef]
- Long, J.; Li, X.; Xue, L.; Xie, Z.; Jiao, A.; Bai, Y.; Zhou, X.; Chen, L.; Qiu, C.; Xu, X.; Jin, Z. Continuous hydrolysis of mango peel pectin for the production of antibacterial pectic oligosaccharides in packed-bed reactor using immobilized polygalactouronase. Food Biosci. 2022, 50, 102117. [Google Scholar] [CrossRef]
- Vaz Rezende, E.S.V.; Lima, G.C.; Naves, M.M.V. Dietary fibers as beneficial microbiota modulators: A proposed classification by prebiotic categories. Nutrition, 2021, 89, 111217. [Google Scholar] [CrossRef] [PubMed]
- Cano, M.E.; García-Martin, A.; Comendador Morales, P.; Wojtusik, M.; Santos, V.E.; Kovensky, J.; Ladero, M. Production of oligosaccharides from agrofood wastes. Fermentation 2020, 6, 31. [Google Scholar] [CrossRef]
- de Moura, F.A.; Macagnan, F.T.; de Oliveira Petkowicz, C.L. da Silva, L.P. Partially hydrolyzed pectin extracted from passion fruit peel: Molar mass and physicochemical properties. Bioact. Carbohydr. Diet. Fibre 2020, 21, 100206. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, C.; Zhang, L.; Wang, Y.; Chen, G.; Fan, J.; Jia, Y.; Yan, F.; Ning, C. Pectin oligosaccharides from fruit of Actinidia arguta: Structure-activity relationship of prebiotic and antiglycation potentials. Carbohydr. Polym., 2019, 217, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Bennett, R.N.; Kirby, A.R.; Lo Curto, R.B.; Bisignano, G.; Waldron, K.W.; Faulds, G.B. Enzymatic hydrolysis of flavonoids and pectic oligosaccharides from bergamot (Citrus bergamia Risso) peel. J. Agric. Food Chem. 2006, 54, 8307–8313. [Google Scholar] [CrossRef] [PubMed]
- Hutnan, M.; Drtil, M.; Marfkova, L. Anaerobic biodegradation of sugar beet pulp. Biodegradation 2000, 11, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Tariq, A.; Sahar, A.; Usman, M.; Sameen, A.; Azhar, M.; Tahir, R.; Younas, R.; Khan, M.I. Extraction of dietary fiber and polyphenols from mango peel and its therapeutic potential to improve gut health. Food. Biosci. 2023, 53, 102669. [Google Scholar] [CrossRef]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-related gelling of pectins and linking with other natural components: A review. Polymers, 2018, 10, 762. [Google Scholar] [CrossRef] [PubMed]
- Usmani, Z.; Sharma, M.; Diwan, D.; Tripathi, M.; Whale, E.; Jayakody, L.N.; Moreau, B.; Thankur, V.K.; Tuohy, M.; Gupta, V.K. Valorization of sugar beet pulp to value-added products: A review. Bioresour. Technol. 2022, 346, 126580. [Google Scholar] [CrossRef]
- Wongkaew, M.; Tangjaidee, P.; Leksawasdi, N.; Jantansakulwong, K.; Rachtanapun, P.; Seesuriyachan, P.; Phimolsiripol, Y.; Chaiyaso, T.; Ruksiriwanich, W.; Jantrawut, P.; Sommano, S.R. Mango pectic oligosaccharides: A novel prebiotic for functional food. Front. Nutr. 2022, 9, 798543. [Google Scholar] [CrossRef] [PubMed]
- Verspreet, J.; Damen, B.; Broekaert, W.F.; Verbeke, K.; Delcour, J.A.; Courtin, C.M. A critical look at prebiotics within the dietary fiber concept. Annu. Rev. Food Sci. Technol. 2016, 7, 167–190. [Google Scholar] [CrossRef]
- Pasarin, D.; Ghizdareanu, A.-I.; Teodorescu, F.; Rovinaru, C.; Banu, A. Characterization of pectin oligosaccharides obtained from citrus peel pectin. Fermentation, 2023, 9, 312. [Google Scholar] [CrossRef]
- Kaur, B.; Panesar, P.S.; Anal, A.K. Standardization of ultrasound assisted extraction for the recovery of phenolic compounds from mango peels. J. Food Sci. Tech. 2022, 59, 2813–2820. [Google Scholar] [CrossRef]
- Wandee, Y.; Uttapap, D.; Mischnik, P.; Rungsardthong, V. Production of pectic-oligosaccharides from pomelo peel pectin by oxidative degradation with hydrogen peroxide. Food Chem. 2021, 348, 129078. [Google Scholar] [CrossRef]
- Gamonpilas, C.; Buathongjan, C.; Kirdsawasd, T.; Rattanaprasert, M.; Klomtun, M.; Phonsatta, N.; Methacanon, P. Pomelo pectin and fiber: Some perspectives and applications in food industry. Food Hydrocoll. 2021, 120, 106981. [Google Scholar] [CrossRef]
- Sivamani, S.; Binnal, P.; Roy, C.; Al Khaldi, A.; Al Hamar, F.; Maran, J.P.; Sivarajasekar, N.; Rajeshkumar, G.; Al Dhabi, N.A.; Karuppiah, P. Optimization and characterization of pectin recovered from (Persea americana) peel using statistical and nonstatistical techniques. Biomass Conv. Bioref. 2023, 13, 6501–6514. [Google Scholar] [CrossRef]
- Cosgove, D.J. Diffuse growth of plant cell walls. Plant Physiol. 2018, 176, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Lampugnani, E.R.; Khan, G.A.; Somssich, M.; Persson, S. Building a plant cell wall at a glance. J. Cell. Sci. 2018, 131. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, I.; Hotchkiss, A.T.; Fishman, M.L.; Rode, D. The effect of modified citrus pectin on urinary excretion of toxic elements. Phytother. Res. 2006, 20, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, S.; Dong, Y.; Zhu, R.; Lin, Y. Antioxidant activity of penta-oligogalacturonide isolated from haw pectin, supressed triglyceride synthesis in mice feed with a high-fat diet. Food Chem. 2014, 145, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Humerez-Flores, J.N.; Kyomugasho, C.; Gutiérrez-Ortiz, A.A.; De Bie, M.; Panozzo, A.; Van Loey, A.M.; Moldenaers, P.; Hendrickx, M.E. Production and molecular characterization of tailored citrus pectin-derived compounds. Food Chem. 2022, 367, 130635. [Google Scholar] [CrossRef] [PubMed]
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Zdunek, A.; Pieczywek, P.M.; Cybulska, J. The primary, secondary and structures of higher levels of pectin polysaccharides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1101–1117. [Google Scholar] [CrossRef] [PubMed]
- Babbar, N. Dejonghe, W.; Gatti, M.; Sforza, S.; Elst, K. Pectic oligosaccharides from agricultural by-products: Production, characterization and health benefits. Crit. Rev. Biotechnol. 2016, 36, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; Concha, D.; Vasquez, P.; Rodriguez-Nuñez, K.; Martinez, R.; Bernal, C. Effect of immobilization of pectinase on the molecular weight distribution of pectin oligosaccharides obtained from citrus pectin. Biocatal. Agric. Biotechnol. 2022, 43, 102389. [Google Scholar] [CrossRef]
- Hamaker, B.R.; Tuncil, Y.E. A perspective on the complexity of dietary fiber structures and their potential effect on gut microbiota. J. Mol. Biol. 2014, 426, 3838–3850. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Mao, G.; Wu, D.; Yu, C.; Cheng, H.; Xiao, H.; Ye, X.; Linhardt, R.J.; Orfila, C.; Chen, S. Highly branched RG-I domain enrichment is indispensable for pectin mitigating against high-fat diet-induced obesity. J. Agric. Food Chem. 2020, 68, 8688–8701. [Google Scholar] [CrossRef]
- Gullón, B.; Gómez, B.; Martínez-Sabajanes, M.; Yáñez, R.; Parajó, J.C.; Alonso, J.L. Pectic oligosaccharides: Manufacture and functional properties. Trends Food Sci. Technol. 2013, 30, 153–161. [Google Scholar] [CrossRef]
- Vogt, L.M.; Sahasrabudhe, N.M.; Ramasamy, U.; Meyer, D.; Pullens, G.; Faas, M.M.; Venema, K.; Schols, H.A.; de Vos, P. The impact of lemon pectin characteristics on TLR activation and T84 intestinal epithelial cell barrier function. J. Funct. Foods, 2016, 22, 398–407. [Google Scholar] [CrossRef]
- Sahasrabudhe, N.M.; Beukema, M.; Tian, L.; Troost, B.; Scholte, J.; Bruininx, E.; Bruggeman, G.; van den Berg, M.; Schurink, A.; Schols, H.A.; Faas, M.M.; de Vos, P. Dietary fiber pectin directly blocks tool-like receptor 2-1 and prevents doxorubicin-included ileitis. Front. Immunol. 2018, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Gao, X.; Wu, C.; Tian, F.; Lei, Q.; Bi, J.; Xie, B.; Wang, H.Y.; Chen, S.; Wang, X. Apple-derived pectin modulates gut microbiota, improves gut barrier function, and attenuates metabolic endotoxemia in rats with diet-induced obesity. Nutrients, 2016, 8, 126. [Google Scholar] [CrossRef]
- Naqash, F.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gani, A. Emerging concepts in the nutraceutical and functional properties of pectin – A Review. Carbohydr. Polym. 2017, 168, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Annapure, U.S. Trends in “green” and novel methods of pectin modification – A review. Carbohydr. Polym. 2022, 278, 118967. [Google Scholar] [CrossRef]
- Sila, D.N.; Van Buggenhout, S.; Duvetter, T.; Fraeye, I.; De Roeck, A.; Van Loey, A.; Hendrickx, M. Pectins in processed fruits and vegetables: Part II-structure-function relationships. Compr. Rev. 2009, 8, 86–104. [Google Scholar] [CrossRef]
- Pascale, N.; Gu, F.; Larsen, N.; Jespersen, L.; Respondek, F. The potential of pectins to modulate the human gut microbiota evaluated by in vitro fermentation. Nutrients, 2022, 14, 3629. [Google Scholar] [CrossRef] [PubMed]
- Zykwinska, A.; Boiffard, M.-H.; Kontkanen, H.; Buchert, J.; Thibault, J.-F.; Bonnin, E. Extraction of green labeled pectins and pectic oligosaccharides from plant byproducts. J. Agric. Food Chem. 2008, 56, 8926–8935. [Google Scholar] [CrossRef]
- Arrutia, F.; Adam, M; Calvo-Carrascal, M. Á.; Mao, Y.; Binner, E. Development of a continuous-flow system for microwave-assisted extraction of pectin-derived oligosaccharides from food waste. Chem. Eng. J. 2020, 395, 125056. [Google Scholar] [CrossRef]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current advancements in pectin: Extraction, properties and multifunctional applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef]
- Mao, G.; Li, S.; Orfila, C.; Shen, X; Zhou, S. ; Linhardt, R.J.; Ye, X.; Chen, S. Depolymerized RG-I-enriched pectin from citrus segment membranes modulates gut microbiota, increases SCFA production, and promotes the growth of Bifidobacterium spp., Lactobacillus spp. and Faecalibacterium spp. Food Funct. 2019, 10, 7828–7843. [Google Scholar] [CrossRef]
- Gómez, B.; Yáñez, R.; Parajó, J.C.; Alonso, J.L. Production of pectin-derived oligosaccharides from lemon peels by extraction, enzymatic hydrolysis and membrane filtration. J. Chem. Technol. Biotechnol. 2016, 91, 234–247. [Google Scholar] [CrossRef]
- Gómez, B.; Gullón, B.; Yáñez, R.; Schols, H.; Alonso, J.L. Prebiotic potential of pectins and pectic oligosaccharides derived from lemon peel wastes and sugar beet pulp: A comparative evaluation. J. Funct. Foods 2016, 20, 108–121. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, B.; Luo, H.; Meng, K.; Wang, Y.; Liu, M.; Bai, Y.; Yao, B.; Tu, T. Production pectin oligosaccharides using Humicola insolens Y1-derived unsual pectate lyase. J. Biosci. Bioeng. 2020, 129, 16–22. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, H.; Wang, L.; Liu, F.; Pan, S. Preparation and prebiotic potential of pectin oligosaccharides obtained from citrus peel pectin. Food Chem. 2018, 232–237. [Google Scholar] [CrossRef]
- Pham, V.T.; Catayud, M.; Rotsaert, C.; Seifert, N.; Richard, N.; van der Abbeele, P.; Marzorati, M.; Steinert, R.E. T.; Catayud, M.; Rotsaert, C.; Seifert, N.; Richard, N.; van der Abbeele, P.; Marzorati, M.; Steinert, R.E. Antioxidant vitamins and prebiotic FOS and XOS differentially shift microbiota composition and function and improve intestinal epithelial barrier in vitro. Nutrients 2021, 13, 1125. [Google Scholar] [CrossRef]
- Conçalves, D.A.; González, A.; Roupar, D.; Teixeira, J.A.; Nobre, C. How prebiotics have been produced from agro-industrial waste: An overview of the enzymatic technologies applied and the models used to validate their health claim. Trends. Food Sci. 2023, 135, 74–92. [Google Scholar] [CrossRef]
- Lunn, J.; Buttriss, J.L. Carbohydrates and dietary fibre. Nutr. Bull. 2007, 32, 21–64. [Google Scholar] [CrossRef]
- Dai, F.-J.; Chau, C.-F. Classification and regulatory perspectives of dietary fiber. J. Food Drug Anal. 2017, 25, 37–42. [Google Scholar] [CrossRef]
- Kang, H.J.; Kwon, J.H.; Ahn, D.U.; Lee, J.W.; Lee, W.K.; Jo, C. Effect of citrus pectin oligosaccharide prepared by irradiation on high cholesterol diet B6.KOR-ApoE mice. Food Sci. Biotechnol. 2009, 18, 884–888. [Google Scholar]
- Zhang, S.; Hu, H.; He, W.; Muhammad, Z.; Wang, L.; Liu, F.; Pan, S. Regulatory roles of pectin oligosaccharides on immunoglobulin production in healthy mice mediated by gut microbiota. Mol. Nutr. Food Res. 2019, 63, 1801363. [Google Scholar] [CrossRef]
- Prandi, B.; Baldassarre, S.; Babbar, N.; Bancalari, E.; Vandezande, P.; Hermans, D.; Bruggeman, G.; Gatti, M.; Elst, K.; Sforza, S. Pectin oligosaccharides from sugar beet pulp: Molecular characterization and potential prebiotic activity. Food Funct. 2018, 9, 1557. [Google Scholar] [CrossRef] [PubMed]
- Olano-Martin, E.; Gibson, G.R.; Rastall, R.A. Comparison of the in vitro bifidogenic properties of pectins and pectic-oligosaccharides. J. Appl. Microbiol. 2002, 93, 505–511. [Google Scholar] [CrossRef]
- Tan, H.; Chen, W.; Liu, Q.; Yang, G.; Li, K. Pectin oligosaccharides ameliorate colon cancer by regulating oxidative stress- and inflammation-activated signaling pathways. Front. Immunol. 2018, 9, 1504. [Google Scholar] [CrossRef]
- Darcy, J.L.; Washburne, A.D.; Robeson, M.S.; Prest, T.; Schmidt, S.K.; Lozupone, C.A. A phylogenic model for the recruitment of species into microbial communities and application to studies of the human microbiome. ISME J. 2020, 14, 1359–1368. [Google Scholar] [CrossRef]
- Scholz-Ahrens, K.E.; Ade, P.; Marten, B.; Weber, P.; Timm, W.; Açil, Y.; Glüer, C.-C.; Schrezenmeir, J. Prebiotics, probiotics, and synbiotics affect mineral absorption, bone mineral content, and bone structure. J. Nutr. 2007, 137, 838S–846S. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, X.; Liu, T.; Yu, Q.; Song, Z.; Wang, F.; Li, T. Pectin oligosaccharides improved lipid metabolism in white adipose tissue of high-fat diet fed mice. Food Sci. Biotechnol. 2022, 31, 1197–1205. [Google Scholar] [CrossRef]
- Li, S.; Li, T.; Jia, Y.; Zhu, R.; Wang, N.; Jin, S.; Guo, M. Fractination and structural characterization of haw pectin oligosaccharides. Eur. Food Res. Technol. 2011, 233, 731–734. [Google Scholar] [CrossRef]
- Zhu, R.; Sun, X.; Zhang, Y.; Yang, T.; Wang, C.; Zhang, J.; Duan, Z.; Shang, F.; Fan, J.; Liu, Y.; Peng, X.; Wang, N.; Chen, G. Effect of pectin oligosaccharides supplementation on infant formulas: The storage stability, formation and intestinal absorption of advanced glycation end products. Food Chem. 2022, 373 (Part B), 131571. [Google Scholar] [CrossRef]
- Kong, C.; Faas, M.M.; de Vos, P.; Akkerman, R. Impact of dietary fibers in infant formulas on gut microbiota on the intestinal immune barrier. Food Funct. 2022, 11, 9445. [Google Scholar] [CrossRef]
- Fanaro, S.; Jelinek, J.; Stahl, B.; Boehm, G.; Kock, R.; Vigi, V. Acidic oligosaccharides from pectin hydrolysate as new component for infant formulae: Effect of intestinal flora, stool characteristics, and pH. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Hong, M.; Zhuang, C.; Zhang, L.; Wang, C.; Liu, J.; Duan, Z.; Shang, F.; Hu, F.; Li, T.; Ning, C.; Chen, G. Pectin oligosaccharides from hawtorn (Crataefus pinnatifida Bunge. Var. major) inhibit the formation of advanced glycation end products in infant formula milk powder. Food Funct. 2019, 10, 8081. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R. Fibre and effects on probiotics (the probiotic concept). Clin. Nutr. Suppl. 2004, 1, 25–31. [Google Scholar] [CrossRef]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Tingirikari, J.M.R. Microbiota-accessible pectic poly- and oligosaccharides in gut health. Food Funct. 2018, 9, 5059. [Google Scholar] [CrossRef]
- Chung, W.S.F.; Walker, A.W.; Louis, P.; Parkhill, J.; Vermeiren, J.; Bosscher, D.; Duncan, S.H.; Flint, H.J. Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biology 2016, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liang, R.-H.; Lin, W.; Li, T.; Lin, C.M.; Wu, S.-S.; Wang, Z.-J. Pectic-oligosaccharides prepared by dynamic high pressure microfluidization and their in vitro fermentation properties. Carbohydr. Polym. 2013, 91, 175–182. [Google Scholar] [CrossRef]
- Duncan, S.H.; Holtop, G.; Lobley, G.E.; Colder, A.G.; Stewart, C.S.; Flint, H.J. Contribution of acetate to butyrate formation by human faecal bacteria. Brit. J. Nutr. 2004, 91, 915–923. [Google Scholar] [CrossRef]
- Manderson, K.; Pinart, M.; Tuohy, K.M.; Grace, W.E.; Hotchkiss, A.T.; Widmer, W.; Yadhav, M.P.; Gibons, G.R.; Rastall, R.A. In vitro determination of probiotic properties of oligosaccharides derived from an orange juice manufacturing by-product stream. Appl. Environ. Microbiol. 2005, 71. [Google Scholar] [CrossRef]
- Bang, S.-J.; Kim, G.; Lim, M.Y.; Song, E.-J.; Jung, D.-H.; Kum, J.-S.; Nam, Y.-D.; Park, C.-S.; Seo, D.-H. The influence of in vitro pectin fermentation on the human fecal microbiome. AMB Express 2018, 8, 98. [Google Scholar] [CrossRef]
- Gómez, B.; Gullón, B.; Remoroza, B.; Schols, H.A.; Parejó, J.C.; Alonso, J.L. Purification, characterization and prebiotic properties of pectic oligosaccharides from orange peel wastes. J. Agric. Food Chem. 2014, 62, 9769–9782. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Wilms, E.; Smolinska, A.; Hermes, G.D.A; Masclee, A.A.M; de Vos, P.; Schols, H.A; van Schooten, F.J.; Smidt, H.; Jonkers, D.M.A.E.; Zoetendal, E.G.; Troost, F.J. Sugar beet pectin supplementation did not alter profiles of fecal microbiota and exhaled breath in healthy young adults and healthy elderly. Nutrients 2019, 11, 2193. [Google Scholar] [CrossRef]
- Leijdekkers, A.G.M.; Aguirre, M.; Venema, K.; Bosch, G.; Gruppen, H.; Schols, H.A. In vitro fermentability of sugar beet pulp derived oligosaccharides using human and pig fecal inocula. J. Agric. Food Chem. 2014, 62, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Gulfi, M.; Arrigoni, E.; Amedó, R. Influence of structure on in vitro fermentability of commercial pectins and partially hydrolysed pectin preparations. Carbohydr. Polym. 2005, 59, 247–255. [Google Scholar] [CrossRef]
- Aguirre, M.; Jonkers, D.M.A.E.; Troost, F.J.; Roeselers, G.; Venema, K. In vitro characterization of the impact of different substrates on metabolite production, energy extraction and composition of gut microbiota from lean to obese subjects. PLoS ONE 2014, 9, e113864. [Google Scholar] [CrossRef] [PubMed]
- Holck, J.; Lorentzen, A.; Vigsnæs, L.K.; Licht, T.R.; Mikkelsen, J.D.; Meyer, A.S. Feruloylated and nonferuloylated arabino-oligosaccharides from sugar beet pectin selectively stimulate the growth of Bifidobacterium spp. in human fecal in vitro fermentations. J. Agric. Food Chem. 2011, 59, 6511–6519. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, N.; Yang, Z.; Zhao, K.; Pang, H.; Shao, K. Zhou, Z.; Li, S.; He, N. Preventive effect of pectic oligosaccharides on acute colitis model mice: Modulating epithelial barrier, gut microbiota and Treg/Th17 balance. Food Funct. 2022, 13, 9999–10012. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-Y.; Lin, C.-M.; Wu, M.-C. Evaluation of the prebiotic effects of citrus pectin hydrolysate. J. Food Drug Anal. 2017, 25, 550–558. [Google Scholar] [CrossRef]
- Gao, M.; Wang, X.; Lin, J.; Lin, X.Y.; Qi, D.; Luo, Y.; Aheyeli-kai, Y.; Ma, H. Separation, structural identification and antibacterial activity of pectin oligosaccharides derived from seed melon. Food Biosc. 2023, 53, 102616. [Google Scholar] [CrossRef]
- Amarasekera, M.; Prescott, S.L.; Palmer, D.J. Nutrition in early life, immune-programming and allergies: The role of epigenetics. Asian. Pac. J. Allergy Immunol. 2013, 31, 175–182. [Google Scholar]
- Despres, J.; Forano, E.; Lepercq, P.; Comtet-Marre, S.; Jubelin, G.; Chambon, C.; Yeoman, C.J.; Berg Miller, M.E.; Fields, C.J.; Martens, E.; Terrapon, N.; Henrissat, B.; White, B.A.; Mosoni, P. Xylan degradation by the human gut Bacteroides xylanisolvens XB1AT involves two distinct gene clusters that are linked at the transcriptional level. BMC Genom. 2016, 17, 326. [Google Scholar] [CrossRef]
- Chung, W.S.F.; Meijerink, M.; Zeuner, B.; Holck, J.; Louis, P.; Meyer, A.S.; Wells, J.M.; Flint, H.J. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol. Ecol. 2017, 93, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Holck, J.; Hjernø, K.; Lorentzen, A.; Visnæs, K.; Hemmingsen, L.; Licht, T.R.; Mikkelsen, J.D.; Meyer, A.S. Tailored enzymatic production of oligosaccharides from sugar beet pectin and evidence of differential effects of a single DP chain length difference on human faecal microbiota composition after in vitro fermentation. Process Biochem. 2011, 46, 1039–1049. [Google Scholar] [CrossRef]
- Fotschki, B.; Juśkiewicz, J.; Jurgoński, A.; Sójka, M. Fructo-oligosaccharides and pectins enhance beneficial effects of raspberry polyphenols in rats with nonalcoholic fatty liver. Nutrients 2021, 13, 833. [Google Scholar] [CrossRef]
- Gu, F.; Larsen, N.; Pascale, N.; Petersen, S.A.; Khakimov, B.; Respondek, F.; Jespersen, L. Age-related effects of the modulation of gut microbiota by pectins and their derivatives: An in vitro study. Front Microbiol. 2023, 14, 1207837. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Martínez, I.; Walter, J.; Keshavarzian, A.; Rose, D.J. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe 2013, 23, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Wilkowska, A.; Motyl, I.; Antczak-Chrobot, A.; Wojtczak, M.; Nowak, A.; Czyżowska, A.; Motyl, W. Influence of human age on the prebiotic effect of pectin-derived oligosaccharides obtained from apple pomace. Fermentation, 2021, 7, 224. [Google Scholar] [CrossRef]
- Luis, A.S.; Briggs, J.; Zhang, X.; Farnell, B.; Ndeh, D.; Labourel, A.; Baslé, A.; Cartmell, A.; Terrapon, N.; Stoott, K.; Lowe, E.C.; McLean, R.; Shearer, K.; Schükel, J.; Venditto, I.; Ralet, M.-C.; Henrissat, B.; Martens, E.C.; Mosimann, S.C.; Abbott, D.W.; Gilbert, H.J. Dietary pectic glycans are degraded by coordinated enzyme pathways in human colonic Bacteroides. Nat. Microbiol. 2018, 3, 210–219. [Google Scholar] [CrossRef]
- Shinohara, K.; Ohashi, Y.; Kawasumi, K.; Terada, A.; Fujisawa, T. Effect of apple intake on fecal microbiota and meatbolites in humans. Anaerobe 2010, 16, 510–515. [Google Scholar] [CrossRef]
- Paturi, G.; Butts, C.A.; Stoklosinski, H.; Herath, T.D.; Monro, J.A. Short-term feeding of fermentable dietary fibres influences the gut microbiota composition and metabolic activity in rats. Int. J. Food Sci. + Technol. 2017, 52, 2572–2581. [Google Scholar] [CrossRef]
- Koropatkin, N.M.; Cameron, E.A.; Martens, E.C. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 2012, 10, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N. Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; Marsland, B.J. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, S.; Lin, F. Zhang, P.; Muhammad, Z.; Pan, S. Role of the gut microbiota and their metabolites in modulating the cholesterol-lowering effects of citrus pectin oligosaccharides in C57BL/6 mice. J. Agric. Food Chem. 2019, 67, 11922–11930. [Google Scholar] [CrossRef] [PubMed]
- Reichart, N.; Duncan, S.H.; Young, P.; Brlenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phytogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef]
- Hirata, S.-I.; Kunisawa, J. Gut microbiome, metabolome and allergic diseases. Allergol. Int. 2017, 66, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Jakobsdottir, G.; Jädert, C.; Holm, L.; Nyman, M.E. Propionic and butyric acids, formed in the caecum of rats fed highly fermentable dietary fibre, are reflected in portal and aortic serum. Br. J. Nutr. 2013, 110, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, T.; Muto, O.; Yatsunami, K.; Echigo, T. Antibacterial activity of pectin hydrolyzates. Nippon Shokuhin Kogyo Gakkaishi, 1994, 41, 785–792. [Google Scholar] [CrossRef]
- Foti, P.; Ballistreri, G.; Timpanaro, N.; Rapisadra, P.; Romeo, F.V. Prebiotic effects of citrus pectic oligosaccharides. Nat. Prod. Res. 2022, 36, 3173–3176. [Google Scholar] [CrossRef]
- Sjögren, Y.M.; Jenmalmm, M.C.; Böttcher, M.F.; Björkstén, B.; Sverremark-Ekström, E. Altered early infant gut microbiota in children developing allergy up to 5 years of age. Clin. Exp. Allergy 2009, 39, 518–526. [Google Scholar] [CrossRef]
- Sung, J.; Kim, S.; Cabatbat, J.J.T; Jang, S.; Jin, Y.-S.; Jung, G.Y.; Chia, N.; Kim, P.-J. Global metabolic interaction network of the human gut microbiota for context-specific community-scale analysis. Nat. Commun. 2017, 8, 15393. [Google Scholar] [CrossRef]
- Goyal, A.; Wang, T.; Dubinkina, V.; Maslov, S. Ecology-guided prediction of cross-feeding interactions in the human gut microbiome. Nat. Commun. 2021, 12, 1335. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Yao, Z.; Tang, T. Intestinal microbiota-mediated dietary fiber bioavailability. Front. Nutr. 2022, 9, 1003571. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Tomé, S.; Ortega-Moreno, L.; Chaparro, M.; Gisbert, J.P. Gut microbiota and dietary factors as modulators of the mucus layer in inflammatory bowel disease. Int. J. Mol. Sci. 2021, 22, 10224. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Pérez, F.; Steióerwald, H.; Schülke, S.; Vieths, S.; Toda, M.; Scheurer, S. The dietary fiber pectin: Health benefits and potential for the treatment of allergies by modulation of gut microbiota. Curr. Allergy Asthma Rep. 2021, 21, 43. [Google Scholar] [CrossRef]
- Cai, J.; Sun, L.; Gonzalez, F.J. Gut microbiota-derived bile acids in intestinal immunity, inflammation and tumorigenesis. Cell Host Microbe 2022, 30, 289–300. [Google Scholar] [CrossRef]
- Cohen, Y.; Elinay, E. Dietary fibers & immunity – more than meets the eye. Cell Res. 2023, 33, 411–412. [Google Scholar] [CrossRef]
- Zuurveld, M.; van Witzenburg, N.P.; Garssen, J.; Folkerts, G.; Sthal, B.; van’t Land, B.; Willemsen, L.E.M. Immunomodulation by human milk oligosaccharides: The potential role in prevention of allergic diseases. Front. Immunol. 2020, 11, 801. [Google Scholar] [CrossRef]
- Bernard, H.; Desseyn, J.-L.; Gottrand, F.; Stahl, B.; Bartke, N.; Husson, M.-O. Pectic-derived acidic oligosaccharides improve the outcome of Pseudomonas aeruginosa lung infection in C57BL/6 Mice. PLoS ONE 2015, 10, e0139686. [Google Scholar] [CrossRef]
- Wikiera, A.; Irla, M.; Mika, M. Health promoting properties of pectin. Postepy Hig. Med. Dosw. 2014, 68, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, H.E.; Rodriguez-Piñeiro, A.M.; Schütte, A.; Ermund, A.; Boysen, P.; Bemark, M.; Sommer, F.; Bäckhed, F.; Hansson, G.C.; Johansson, M.E.V. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2015, 16, 164–177. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two Muc2 mucin dependent mucus layers in colon is devoid bacteria. PNAS 2008, 105, 15064–15069. [Google Scholar] [CrossRef]
- Leclere, L.; Van Cutsem, P.; Michiels, C. Anti-cancer activities of pH- or heat – modified pectin. Front. Pharmacal. 2013, 4, 128. [Google Scholar] [CrossRef] [PubMed]
- Hino, S.; Sonoyama, K.; Bito, H.; Kawagishi, H.; Aoe, S.; Morita, T. Low-methoyl pectin stimulates small intestinal mucin secretion irrespective of goblet cell proliferation and is characterized by jejunum Muc2 upregulation in rats. J. Nutr. 2013, 143, 34–40. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, J.; Li, J.; Kim, G.; Chen, E.S. , Xiao, S.; Snapper, S.B.; Bao, B.; An, D.; Blumberg, R.S.; Lin, C.-H.; Wang, S.; Zhong, J.; Lin, K.; Li, Q; Wu, C.; Kuchroo, V.K. Foxo1 controls gut homeostasis and commensalism by regulating mucus secretion. J. Exp. Med. 2021, 218, e20210324. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Minello, V.L.; Minello, A.; Ficele, L.; Skublewska-D’Elia, A.; Dargenio, V.N.; Cristofori, F.; Francavilla, R. Gut immunobiosis and biomodulators. Nutrients 2023, 15, 2114. [Google Scholar] [CrossRef]
- Bernard, H.; Desseyn, J.-L.; Bartke, N.; Kleijnjans, L.; Stahl, B.; Belzer, C.; Knol, J.; Gottrand, F.; Husson, M.-O. Dietary pectin-derived acidic oligosaccharides improve the pulmonary bacterial clearance of Pseudomonas aeruginosa lung infection in mice by modulating intestinal microbiota and immunity. J. Infect. Dis. 2015, 211, 156–165. [Google Scholar] [CrossRef]
- Montilla, A.; Muñoz-Alamagro, N.; Villamiel, M. Chapter 6 – A new approach of functional pectin and pectic oligosaccharides: Role as antioxidant and anti-inflammatory compounds. [In] Current Advances for Development of Functional Foods Modulating Inflammation and Oxidative Stress. 2022, 105-120. [CrossRef]
- Xie, J.; Yu, R.; Qi, J.; Zhang, G.; Peng, X.; Luo, J. Pectin and inulin stimulated the mucus formation at a similar level: An omics-based comparative analysis. Food Sci. 2020, 85, 1939–1947. [Google Scholar] [CrossRef]
- Singh, G.; Brass, A.; Knight, C.G; Cruickshank, S.M. Gut eosinophils and their impact on the mucus-resident microbiota. Immunology 2019, 158, 194–205. [Google Scholar] [CrossRef]
- Jeurink, P.V.; van Esch, B.C.; Rijnierse, A.; Garssen, J.; Knippels, L.M. Mechanism underlying immune effects of dietary oligosaccharides. Am. J. Clin. Nutr. 2013, 98, 572S–577S. [Google Scholar] [CrossRef]
- Lee, K.H. , Song, Y.; Wu, W.; Yu, W.; Zhang, G. The gut microbiota, environmental factors and links to the development of food allergy. Clin. Mol. Allergy 2020, 18, 5. [Google Scholar] [CrossRef]
- Nance, C.L.; Deniskin, R.; Diaz, V.C.; Paul, M.; Anvari, S.; Anagnostou, A. The role of the microbiome in food allergy: A Review. Children 2020, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Arauza, J.C.; Ornelas-Paz, J.J.; Pimentel-González, D.J.; Mendoza, S.R.; Guerra, R.E.S.; Paz Maldonado, L.M.T. Prebiotic effect of mucilage and pectic-derived oligosaccharides from Nopal (Opuntia ficus-indica). Food Sci. Biotechnol. 2012, 21, 997–1003. [Google Scholar] [CrossRef]
- Lee, J.H.; Shim, J.S.; Lee, J.S.; Kim, M.-K.; Chung, M.-S.; Kim, K.H. Pectin-like acidic polysaccharide from Panax ginseng with selective antiadhesive activity against pathogenic bacteria. Carbohydr Res. 2006, 341, 1154–1163. [Google Scholar] [CrossRef]
- Ouwerkerk, J.P.; de Vos, W.M.; Belzer, C. Glycobiome: Bacteria and mucus at the epithelial interface. Best. Pract. Res. Clin. Gastroenterol. 2013, 27, 25–38. [Google Scholar] [CrossRef]
| Item | Solubility | Fermentability | Viscosity |
|---|---|---|---|
| Cellulose | - | slow | - |
| β-glucan (cereals) | high | rapid | high |
| Inulin | variable | rapid | low |
| FOS | high | rapid | - |
| Pectin | high | rapid | high |
| POS | high | rapid | low |
| Item | DF (g /100 g edible portion) | Percentage of TDF (%) | For 30 g TDF, person must eat in kg (single component of diet) |
|||
|---|---|---|---|---|---|---|
| Total | IDF | SDF | IDF | SDF | ||
| Flaxseed | 22.3 | 10.1 | 12.2 | 45 | 55 | 0.13 |
| Soybean | 21.5 | 19.4 | 2.1 | 90 | 10 | 0.14 |
| Barley | 19.5 | 15.7 | 3.8 | 81 | 19 | 0.15 |
| Raw white beans | 17.7 | 13.4 | 4.3 | 76 | 24 | 0.17 |
| Wheat grain | 12.6 | 10.2 | 2.3 | 81 | 18 | 0.24 |
| Corn | 11.9 | 10.5 | 1.4 | 88 | 12 | 0.25 |
| Raw lentils | 11.4 | 10.3 | 1.1 | 90 | 10 | 0.26 |
| Almond | 11.2 | 10.1 | 1.1 | 90 | 10 | 0.27 |
| Oats | 10.3 | 6.5 | 3.8 | 63 | 37 | 0.29 |
| Raw coconut | 9.0 | 8.5 | 0.5 | 94 | 6 | 0.33 |
| Dry roasted peanut | 8.0 | 7.5 | 0.5 | 94 | 6 | 0.38 |
| Beetroot | 7.8 | 5.4 | 2.4 | 69 | 31 | 0.38 |
| Sesame seed | 7.8 | 5.9 | 1.9 | 76 | 24 | 0.38 |
| Frozen green peas | 3.5 | 3.2 | 0.3 | 91 | 9 | 0.86 |
| Kiwi | 3.4 | 2.6 | 0.8 | 76 | 24 | 0.88 |
| Pear | 3.0 | 2.0 | 1.0 | 67 | 33 | 1.00 |
| Raw spinach | 2.6 | 2.1 | 0.5 | 81 | 19 | 1.15 |
| Raw carrot | 2.5 | 2.3 | 0.2 | 92 | 8 | 1.20 |
| Strawberry | 2.2 | 1.3 | 0.9 | 59 | 41 | 1.36 |
| Unpeeled apple | 2.0 | 1.8 | 0.2 | 90 | 10 | 1.50 |
| Green beans | 1.9 | 1.4 | 0.5 | 74 | 26 | 1.58 |
| Peach | 1.9 | 1.0 | 0.9 | 53 | 47 | 1.58 |
| Raw cauliflower | 1.8 | 1.1 | 0.7 | 61 | 39 | 1.67 |
| Mango | 1.8 | 1.1 | 0.7 | 61 | 39 | 1.67 |
| Oranges | 1.8 | 0.7 | 1.1 | 39 | 61 | 1.67 |
| Bananas | 1.7 | 1.2 | 0.5 | 71 | 29 | 1.76 |
| Plums | 1.6 | 0.7 | 0.9 | 44 | 56 | 1.88 |
| Raw celery | 1.5 | 1.0 | 0.5 | 67 | 33 | 2.00 |
| Rice dry | 1.3 | 1.0 | 0.3 | 77 | 23 | 2.31 |
| Potato without skin | 1.3 | 1.0 | 0.3 | 77 | 23 | 2.31 |
| Raw tomato | 1.2 | 0.8 | 0.4 | 67 | 33 | 2.50 |
| Pineapple | 1.2 | 1.1 | 0.1 | 92 | 8 | 2.50 |
| Grapes | 1.2 | 0.7 | 0.5 | 58 | 42 | 2.50 |
| Rice cooked | 0.7 | 0.7 | 0.0 | 100 | 0 | 4.29 |
| Peeled cucumber | 0.6 | 0.5 | 0.1 | 83 | 17 | 5.00 |
| Watermelon | 0.5 | 0.3 | 0.2 | 60 | 40 | 6.00 |
| Research | |||||
|---|---|---|---|---|---|
| with pectin | Microbiota | SCFA | with POS | Microbiota | SCFA |
| Apple pectin [156] |
↑Bacteroidetes | *Acetate | Apple POS [157] |
↑Bifidobacteria | |
| ↑Firmicutes | *Propionate | ↑Lactobacilli | ↑Acetate | ||
| ↑Eubacterium eligens |
*Butyrate |
↓Bacteroides ↓Clostridia |
↑Propionate |
||
| Citrus pectin [111,150,151,152,153,154,155,156,157,158,159,160] |
↑Bifidobacteria | Orange POS [161] |
|||
| ↑Bacteroides | |||||
| ↑Eubacterium rectale | ↑Acetate |
↑Bifidobacteria |
*Acetate | ||
| ↑Lachnospira | ↑Propionate | *Propionate | |||
| ↑Dorea | *Bytyrate | ↑Lactobacilli | *Butyrate | ||
| ↑Clostridium | |||||
| ↑Sutterella | |||||
| Sugar beet Pectin [162] |
↑Prevotella | Sugar beet POS [163] |
↑Bacteroidetes | ||
| ↑Bacteroides | ↑Acetate | ||||
| ↑Bacteroidales | ↑Acetate |
↑Propionate | |||
| ↑Clostridium | ↑Butyrate | ||||
| ↓Lachnospiraceae | ↓Valerate | ||||
| ↓Ruminococcus | |||||
| Apple and Citrus pectin [164] |
↑Acetate | Apple POS and Citrus POS |
|||
| no available data | ↑Propionate | no available data | no available data | ||
| ↑Butyrate | |||||
| Apple and sugar beet pectin [165] | ↑Bifidobacteria |
↑Butyrate ↑Acetate | Apple POS and sugar beet POS | no available data | no available data |
| Citrus and sugar beet pectin [81] | ↑Bacteroidetes | ↑Propionate |
Citrus POS and sugar beet POS | no available data | no available data |
| ↑Enterobacteriaceae | |||||
| with pectin and POS | Microbiota | SCFA | |||
| Citrus pectin, sugar beet pectin and POS from lemon peel [Gomez 2016 JFF] |
↑Bifidobacteria ↑Lactobacilli ↑Faecalibacterium ↑Roseburia |
↑Acetate ↑Propionate ↑Butyrate |
|||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





