Submitted:
14 March 2024
Posted:
15 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
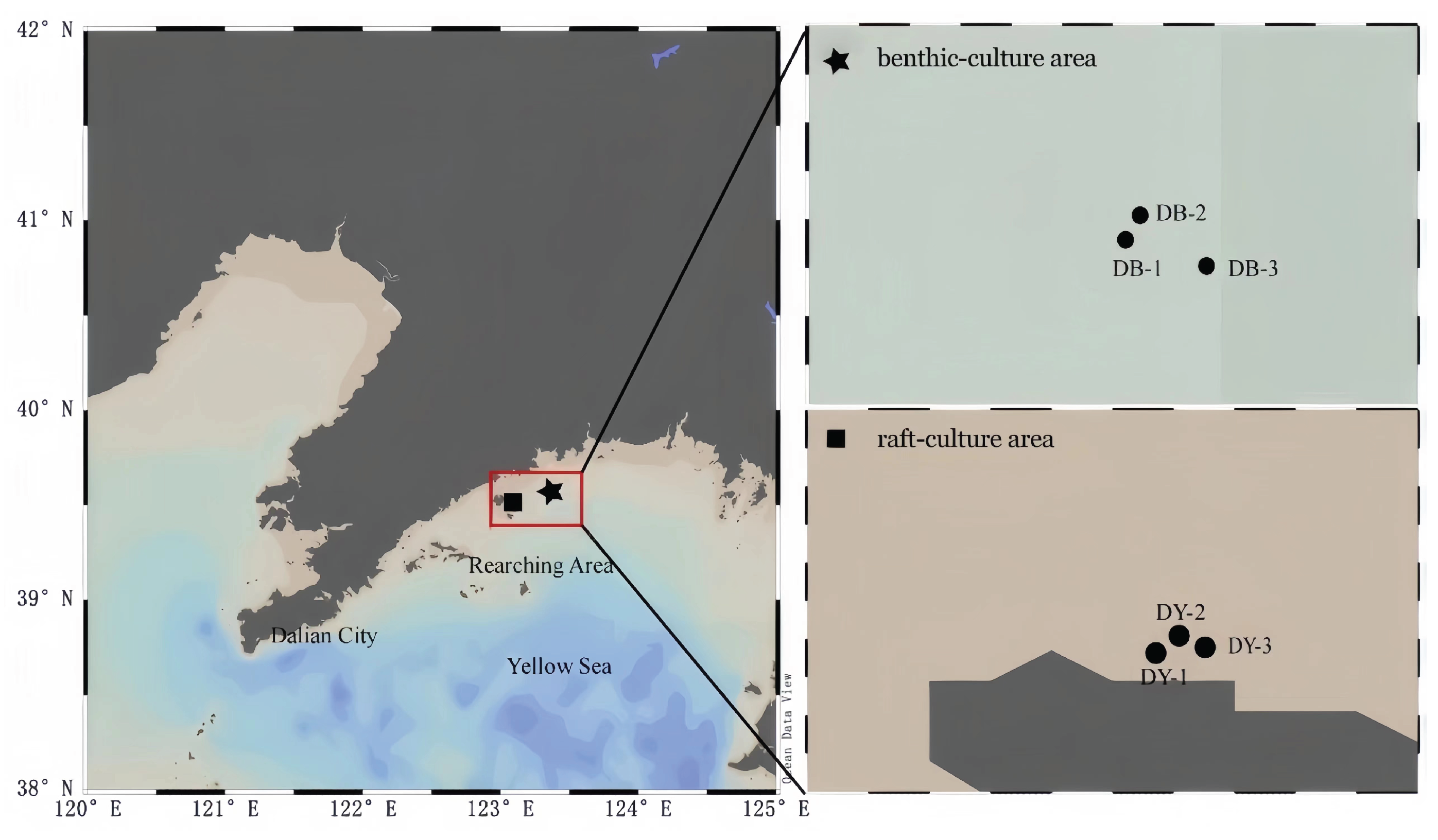
2.1. Sample Collection
2.2. Determination of Physicochemical Parameters of Seawater
2.3. Determination of Physical and Chemical Parameters of the Sediment
2.4. Intestinal Treatment of R. philippinarum
2.5. DNA Extraction and High-Throughput Sequencing
2.6. Data Analysis and Processing
3. Results
3.1. Physicochemical Properties of Seawater in R. philippinarum Culture Areas
3.2. Physicochemical Properties of Sediment in R. philippinarum Culture Areas
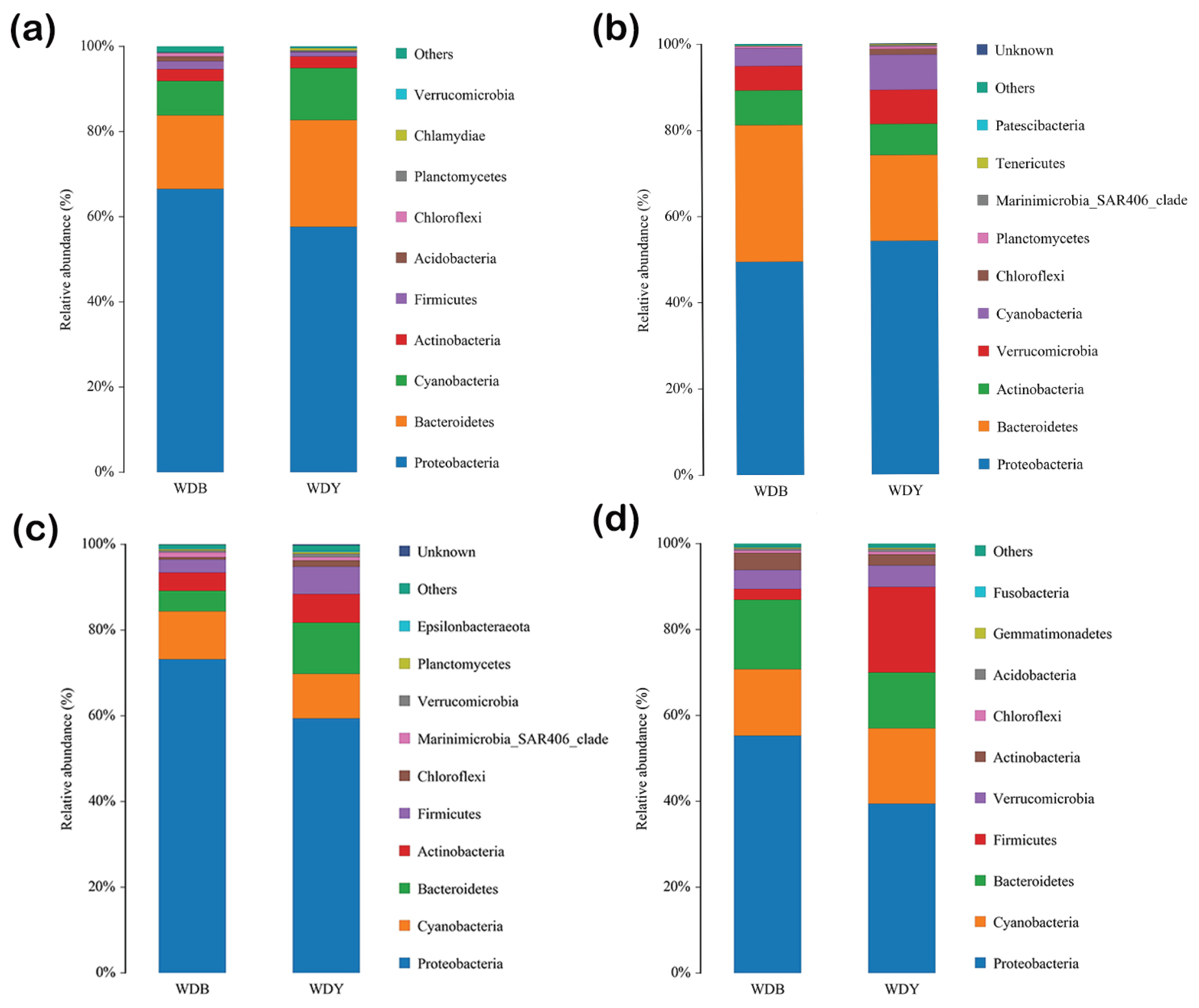
3.3. Seasonal Variation in Seawater Bacterial Diversity
3.4. Seasonal Variation in Sediment Bacterial
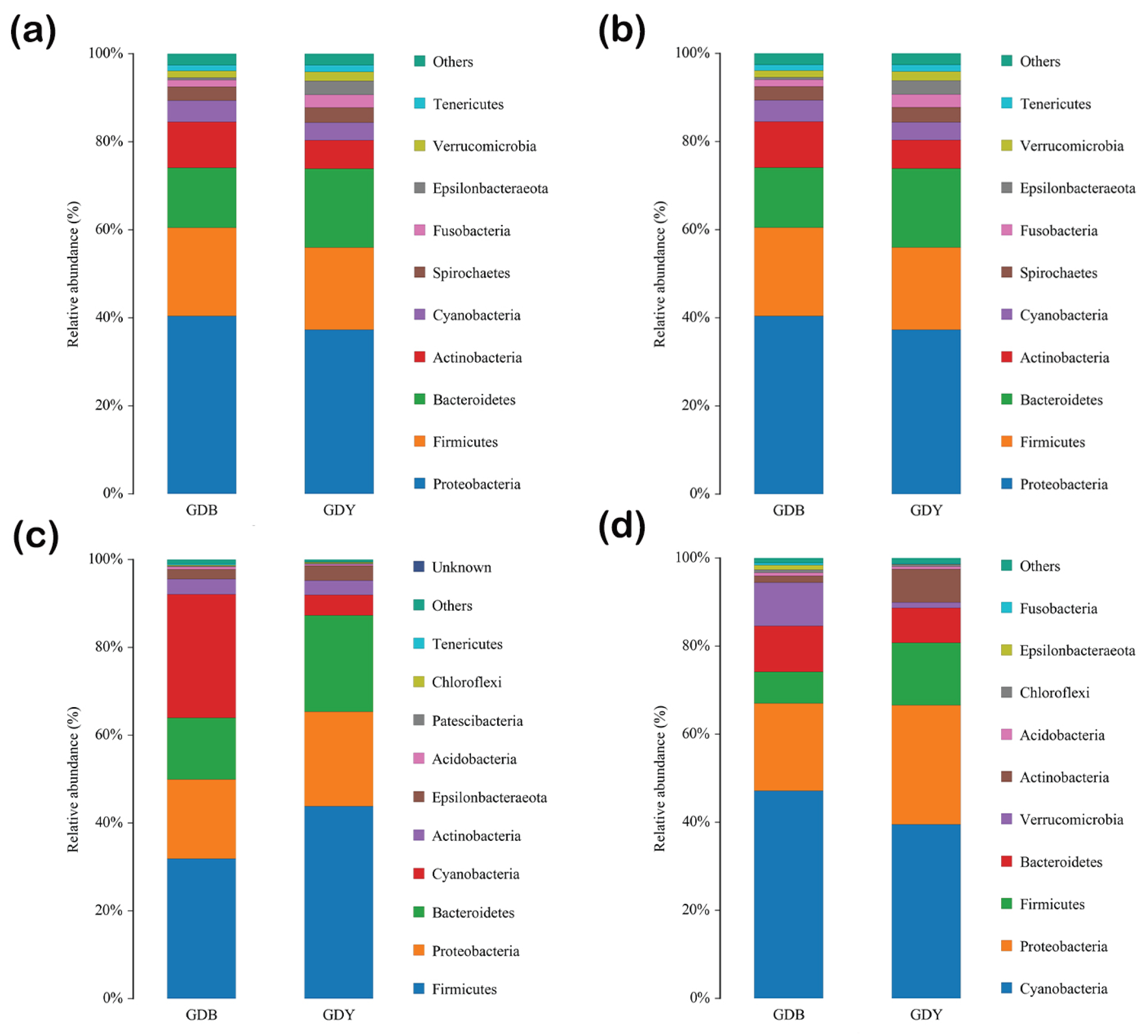
3.5. Seasonal Variation of Intestinal Bacteria Diversity in R. philippinarum
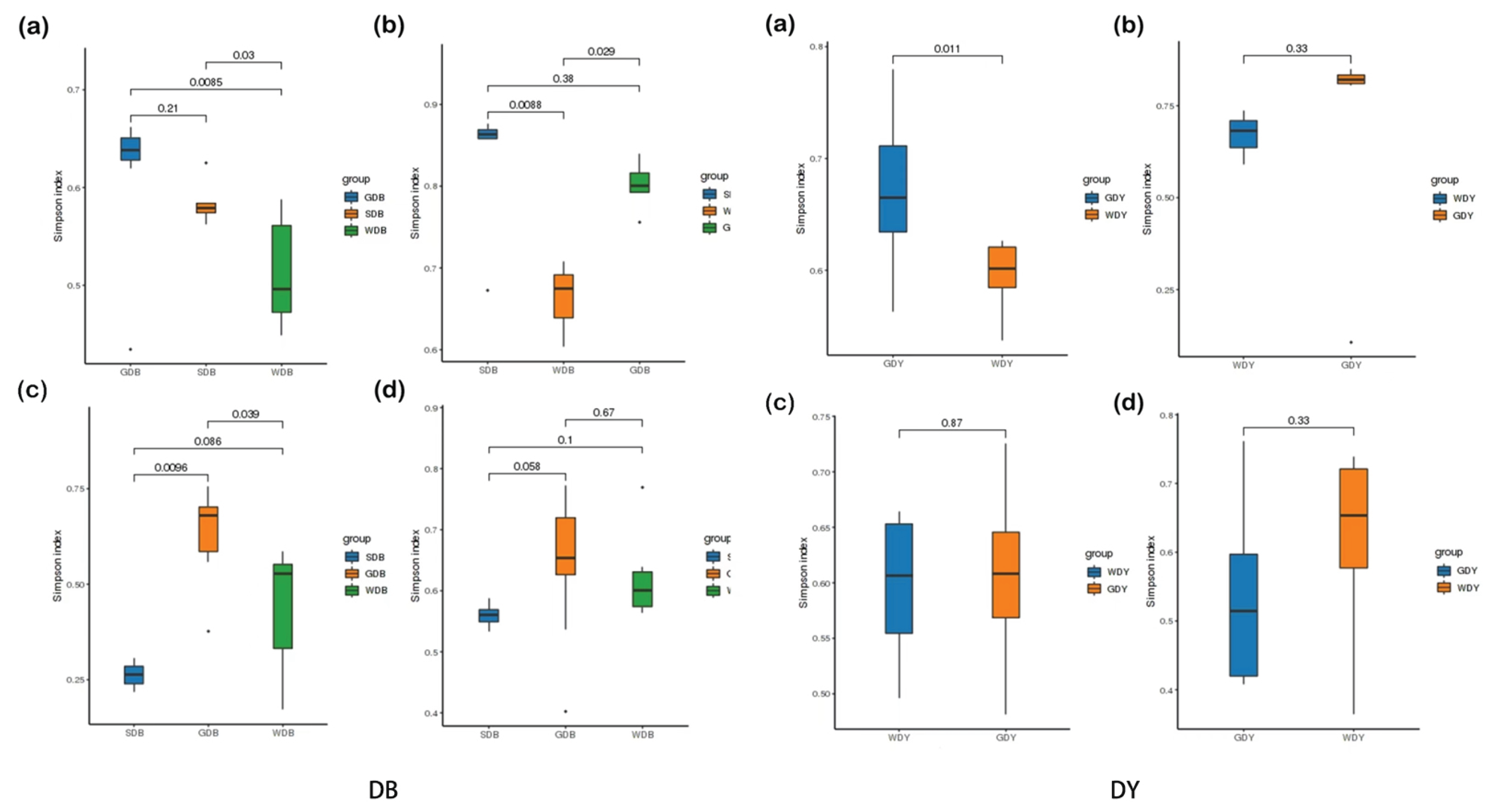
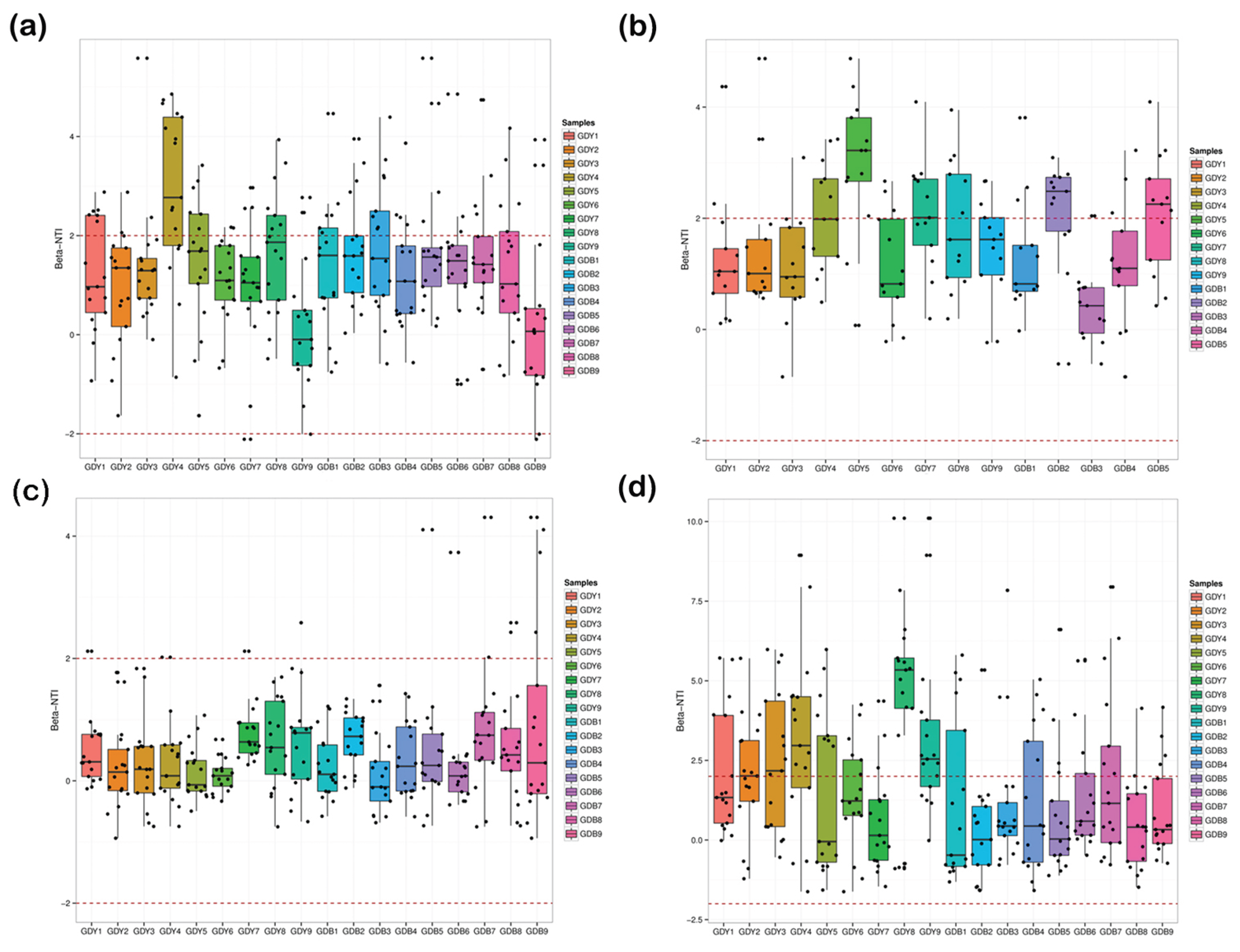
3.6. Alpha Diversity of the Intestinal Bacteria in R. philippinarum
3.7. Beta Diversity of the Intestinal Bacteria in R. philippinarum
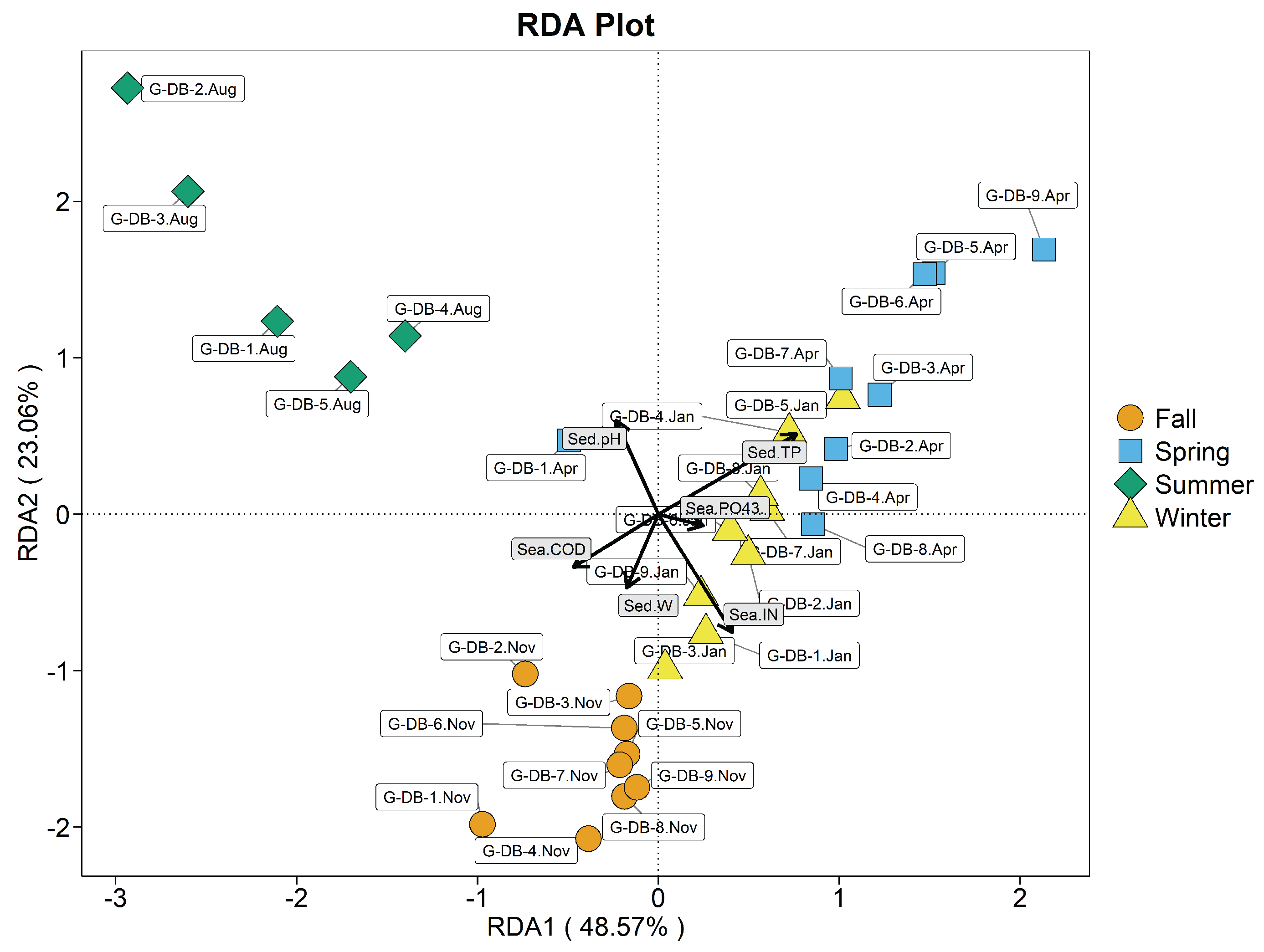
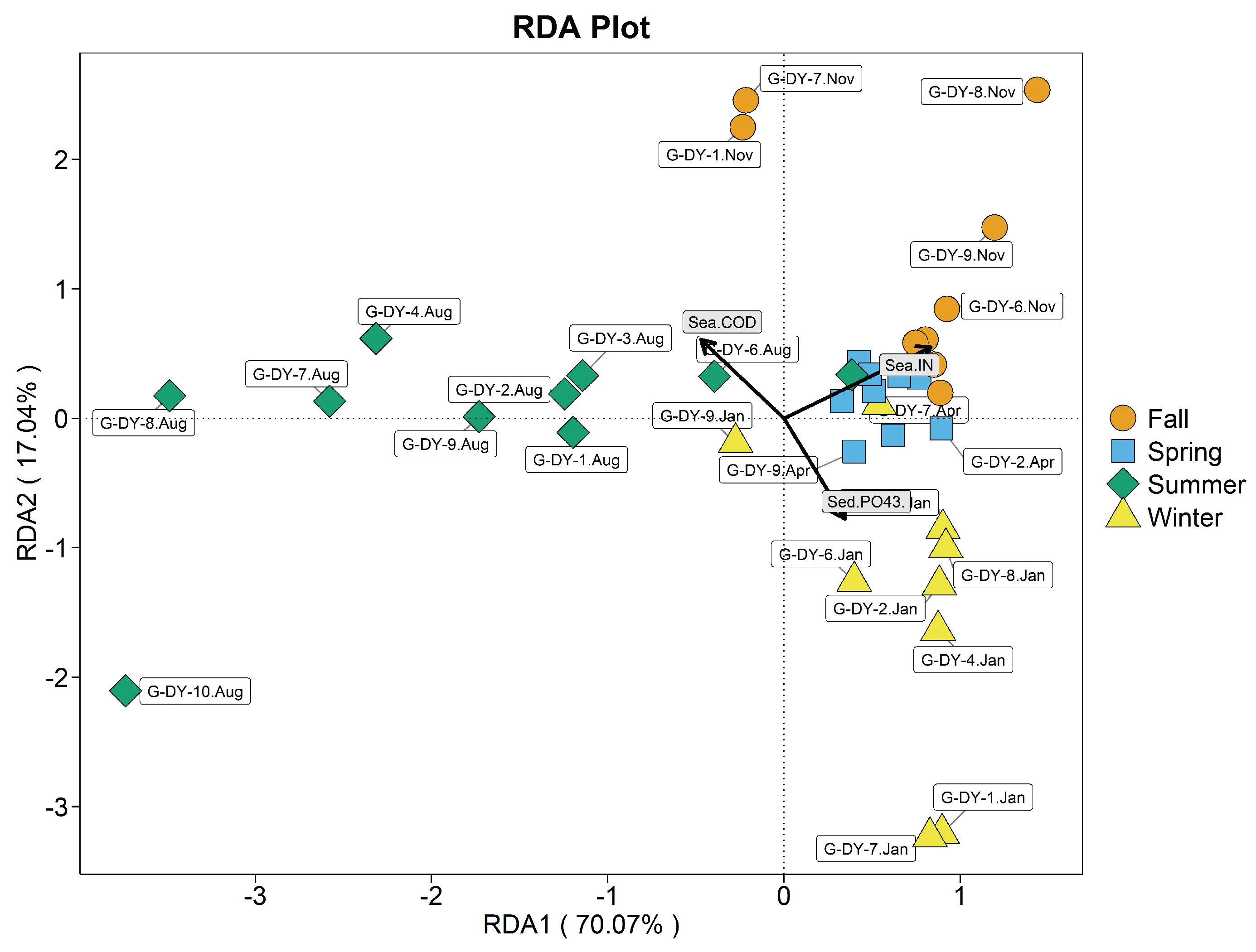
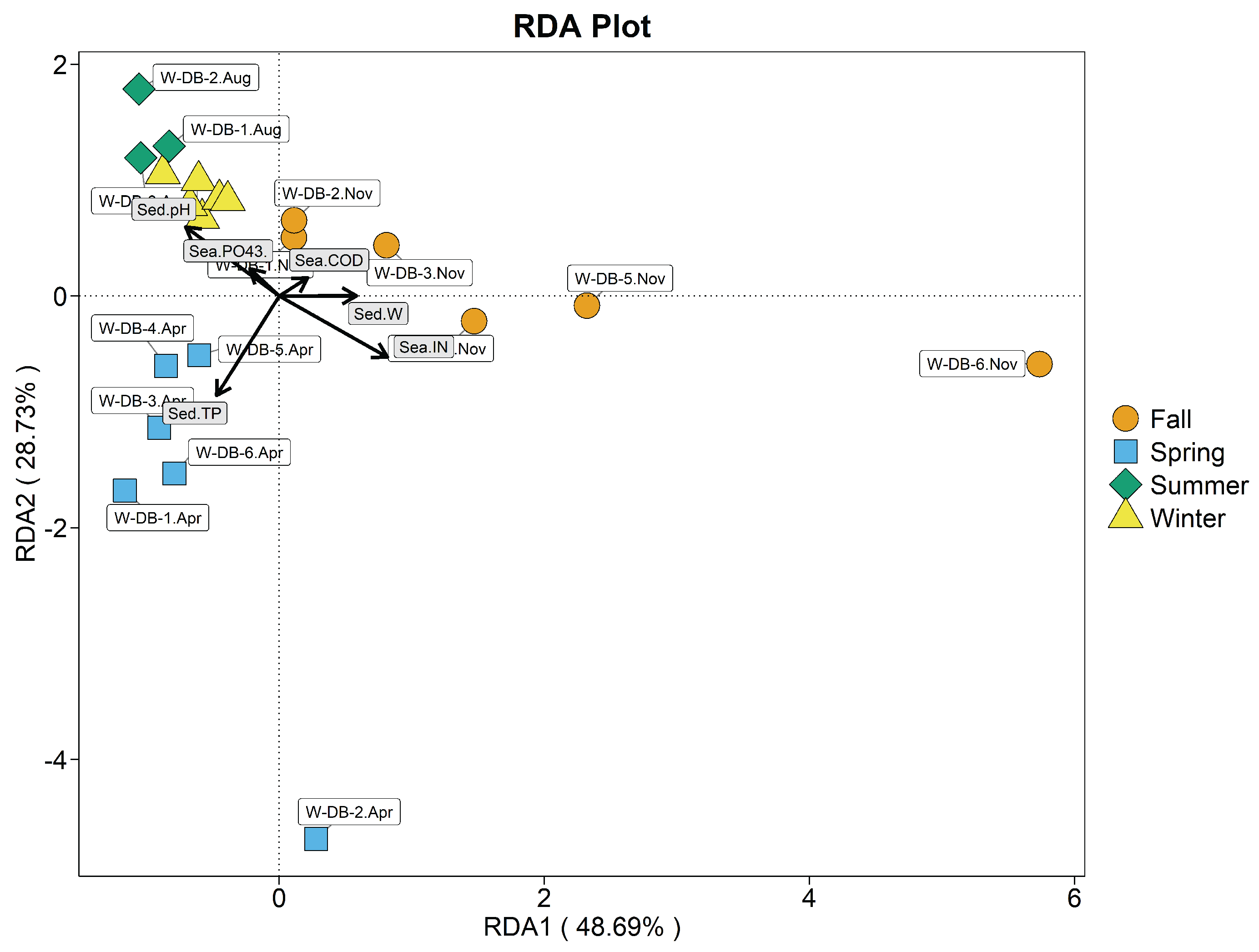
3.8. Correlation Analysis of the R. philippinarum Intestinal Bacterial Communities and Environmental variables
3.8.1. Correlation Analysis of the R. philippinarum Intestinal Bacterial Community and Environmental Factors
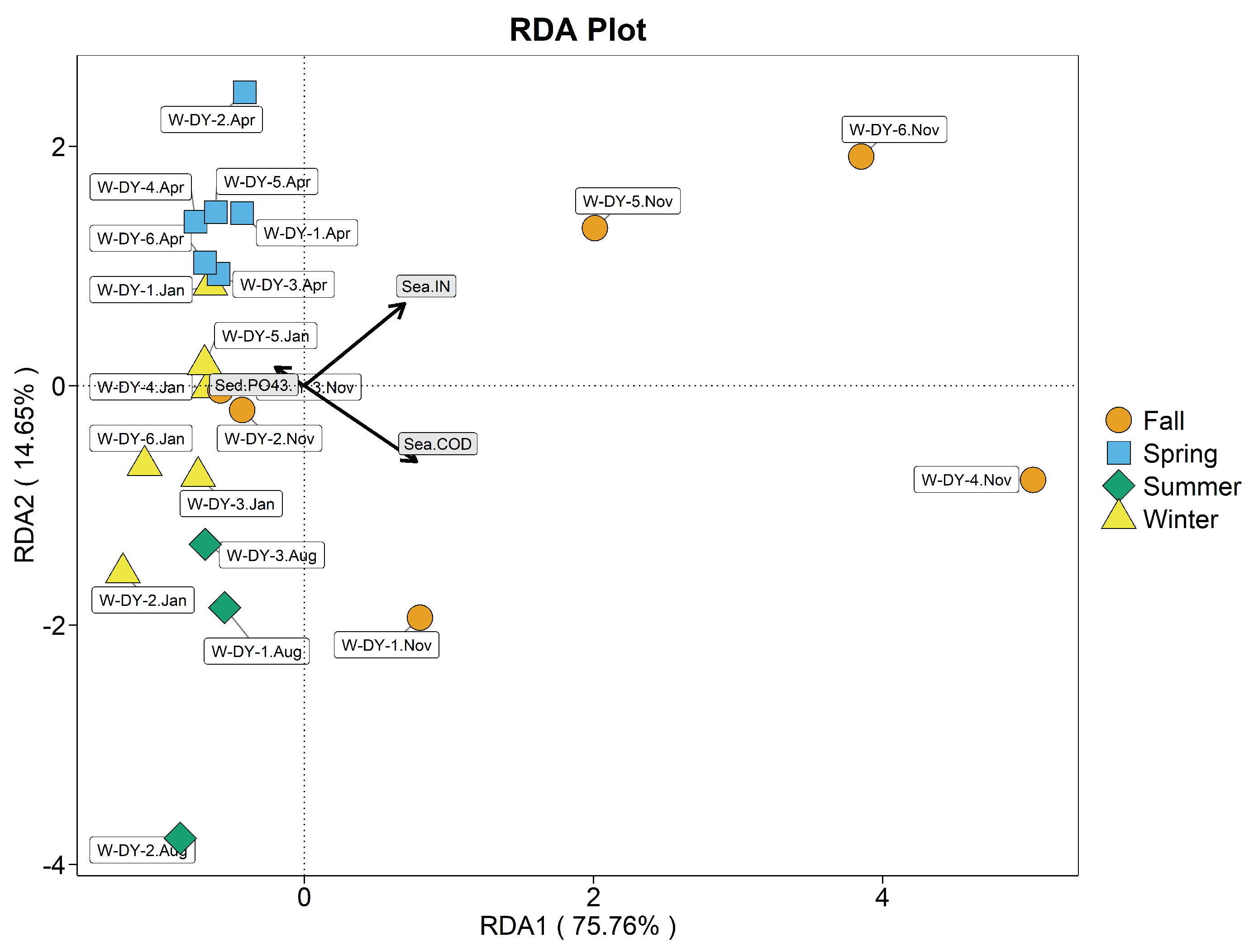
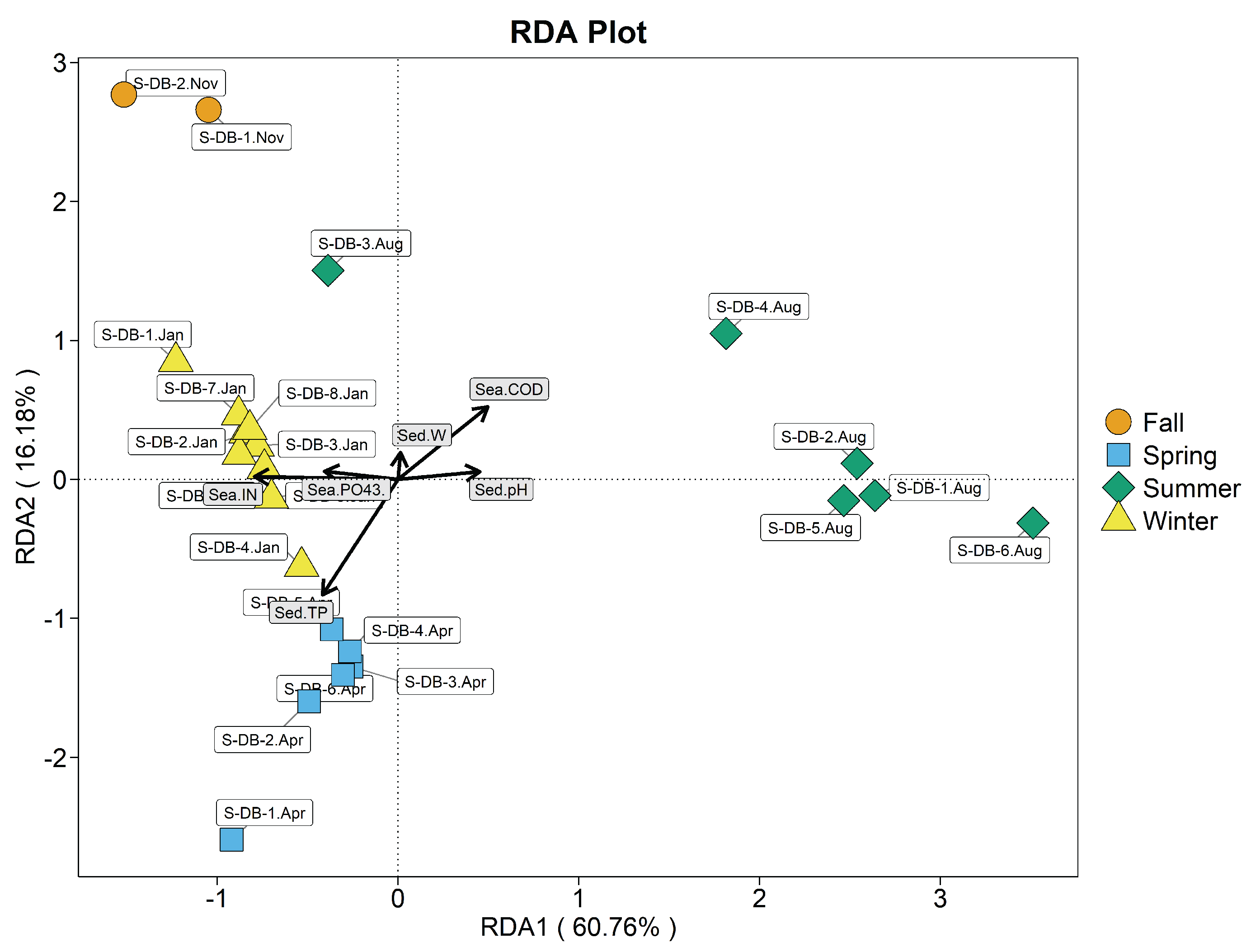
3.8.2. Correlation Analysis of the Seawater Bacteria Community Strucutre and Environmental Factors
3.8.3. Correlation Analysis of the Sediment Bacteria Community Structure and Environmental Factors
4. Discussion
4.1. Effects of Environmental Factors on Bacterial Community Structure in Seawater and Sediment
4.2. Effects of Environmental Factors on the Intestinal Bacteria Community Structure of Clams Collected from Benthic-Culture Area and Raft-Culture Area
4.3. Comparison of Alpha Diversity of Environmental Bacteria and Intestinal Bacteria of Benthic Cultured Clams and Raft Cultured Clams
4.4. Comparison of Intestinal Bacterial Community Structure between Benthic Cultured Clams and Raft Cultured Clams
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amalfitano, S.; Coci, M.; Corno, G.; Luna, G.M. A microbial perspective on biological invasions in aquatic ecosystems. Hydrobiologia 2015, 746, 13–22. [Google Scholar] [CrossRef]
- Nealson, K.H. Sediment bacteria: who’s there, what are they doing, and what’s new? Annual review of earth and planetary sciences 1997, 25, 403–34. [Google Scholar] [CrossRef]
- Lin, G.; Lin, X. Bait input altered microbial community structure and increased greenhouse gases production in coastal wetland sediment. Water Research 2022, 218, 118520. [Google Scholar] [CrossRef] [PubMed]
- Sehnal, L.; Brammer-Robbins, E.; Wormington, A.M.; Blaha, L.; Bisesi, J.; Larkin, I.; Martyniuk, C.J.; Simonin, M.; Adamovsky, O. Microbiome composition and function in aquatic vertebrates: small organisms making big impacts on aquatic animal health. Frontiers in Microbiology 2021, 12, 567408. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wang, Y.; Wang, C.; Zhang, L.; Tu, K.; Zheng, Z. Insights into the intestinal microbiota of several aquatic organisms and association with the surrounding environment. Aquaculture 2019, 507, 196–202. [Google Scholar] [CrossRef]
- Amalfitano, S.; Coci, M.; Corno, G.; Luna, G.M. A microbial perspective on biological invasions in aquatic ecosystems. Hydrobiologia 2015, 746, 13–22. [Google Scholar] [CrossRef]
- Tan, C.K.; Natrah, I.; Suyub, I.B.; Edward, M.J.; Kaman, N.; Samsudin, A.A. Comparative study of gut microbiota in wild and captive Malaysian Mahseer (Tor tambroides). Microbiologyopen 2019, 8, e00734. [Google Scholar] [CrossRef] [PubMed]
- Ringø, E.; Zhou, Z.; Vecino, J.G.; Wadsworth, S.; Romero, J.; Krogdahl, Å.; Olsen, R.E.; Dimitroglou, A.; Foey, A.; Davies, S.; others. Effect of dietary components on the gut microbiota of aquatic animals. A never-ending story? Aquaculture nutrition 2016, 22, 219–282. [Google Scholar] [CrossRef]
- Ruiz-González, C.; Niño-Garcia, J.; Berggren, M.; Del Giorgio, P. Contrasting dynamics and environmental controls of dispersed bacteria along a hydrologic gradient. Adv Oceanogr Limnol 2017, 8, 222–234. [Google Scholar] [CrossRef]
- Diwan, A.D.; Harke, S.N.; Gopalkrishna. ; Panche, A.N. Aquaculture industry prospective from gut microbiome of fish and shellfish: An overview. Journal of Animal Physiology and Animal Nutrition 2022, 106, 441–469. [Google Scholar] [CrossRef]
- Li, Y.F.; Yang, N.; Liang, X.; Yoshida, A.; Osatomi, K.; Power, D.; Batista, F.M.; Yang, J.L. Elevated seawater temperatures decrease microbial diversity in the gut of Mytilus coruscus. Frontiers in Physiology 2018, 9, 839. [Google Scholar] [CrossRef]
- Chen, C.Z.; Li, P.; Liu, L.; Li, Z.H. Exploring the interactions between the gut microbiome and the shifting surrounding aquatic environment in fisheries and aquaculture: A review. Environmental Research 2022, 214, 114202. [Google Scholar] [CrossRef]
- Wei, D.; Xing, C.; Hou, D.; Zeng, S.; Zhou, R.; Yu, L.; Wang, H.; Deng, Z.; Weng, S.; He, J.; others. Distinct bacterial communities in the environmental water, sediment and intestine between two crayfish-plant coculture ecosystems. Applied Microbiology and Biotechnology 2021, 105, 5087–5101. [Google Scholar] [CrossRef]
- Chakraborty, J.; Palit, K.; Das, S. Metagenomic approaches to study the culture-independent bacterial diversity of a polluted environment—a case study on north-eastern coast of Bay of Bengal, India. In Microbial Biodegradation and Bioremediation; Elsevier, 2022; pp. 81–107.
- Qin, Y.; Hou, J.; Deng, M.; Liu, Q.; Wu, C.; Ji, Y.; He, X. Bacterial abundance and diversity in pond water supplied with different feeds. Scientific reports 2016, 6, 35232. [Google Scholar] [CrossRef]
- Powell, S.M.; Chapman, C.; Bermudes, M.; Tamplin, M. Dynamics of seawater bacterial communities in a shellfish hatchery. Microbial ecology 2013, 66, 245–256. [Google Scholar] [CrossRef]
- Iwama, G.K. Interactions between aquaculture and the environment. Critical Reviews in Environmental Science and Technology 1991, 21, 177–216. [Google Scholar] [CrossRef]
- de Bruijn, I.; Liu, Y.; Wiegertjes, G.F.; Raaijmakers, J.M. Exploring fish microbial communities to mitigate emerging diseases in aquaculture. FEMS Microbiology Ecology 2018, 94, fix161. [Google Scholar] [CrossRef]
- Maji, U.J.; Mohanty, S.; Mahapatra, A.S.; Mondal, H.K.; Samanta, M.; Maiti, N.K. Exploring the gut microbiota composition of Indian major carp, rohu (Labeo rohita), under diverse culture conditions. Genomics 2022, 114, 110354. [Google Scholar] [CrossRef]
- Sandaa, R.A.; Magnesen, T.; Torkildsen, L.; Bergh, Ø. Characterisation of the bacterial community associated with early stages of great scallop (Pecten maximus), using denaturing gradient gel electrophoresis (DGGE). Systematic and Applied Microbiology 2003, 26, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, Y.; Lv, D.; Ge, Y.; Li, H.; You, Y. Change in the intestinal bacterial community structure associated with environmental microorganisms during the growth of Eriocheir sinensis. MicrobiologyOpen 2019, 8, e00727. [Google Scholar] [CrossRef]
- Hou, D.; Huang, Z.; Zeng, S.; Liu, J.; Weng, S.; He, J. Comparative analysis of the bacterial community compositions of the shrimp intestine, surrounding water and sediment. Journal of applied microbiology 2018, 125, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, F.; Yu, S.; Qin, S.; Wang, G. Impacts of mariculture on the diversity of bacterial communities within intertidal sediments in the Northeast of China. Microbial ecology 2013, 66, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Butt, R.L.; Volkoff, H. Gut microbiota and energy homeostasis in fish. Frontiers in endocrinology 2019, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, Z.; Chen, M.; Qu, Y.; Li, J.; Zhou, A.; Xie, S.; Zeng, F.; Zou, J. Microbiota comparison of Pacific white shrimp intestine and sediment at freshwater and marine cultured environment. Science of the Total Environment 2019, 657, 1194–1204. [Google Scholar] [CrossRef]
- Huang, F.; Pan, L.; Song, M.; Tian, C.; Gao, S. Microbiota assemblages of water, sediment, and intestine and their associations with environmental factors and shrimp physiological health. Applied microbiology and biotechnology 2018, 102, 8585–8598. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, G.; Ray, A.K. Bacterial symbiosis in the fish gut and its role in health and metabolism. Symbiosis 2017, 72, 1–11. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nature methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Che, L.; Hu, Q.; Wang, R.; Zhang, D.; Liu, C.; Zhang, Y.; Xin, G.; Fang, Z.; Lin, Y.; Xu, S.; others. Inter-correlated gut microbiota and SCFAs changes upon antibiotics exposure links with rapid body-mass gain in weaned piglet model. The Journal of nutritional biochemistry 2019, 74, 108246. [Google Scholar] [CrossRef]
- Fan, K.; Weisenhorn, P.; Gilbert, J.A.; Shi, Y.; Bai, Y.; Chu, H. Soil pH correlates with the co-occurrence and assemblage process of diazotrophic communities in rhizosphere and bulk soils of wheat fields. Soil Biology and Biochemistry 2018, 121, 185–192. [Google Scholar] [CrossRef]
- Feng, J.; Zhou, L.; Zhao, X.; Chen, J.; Li, Z.; Liu, Y.; Ou, L.; Xie, Z.; Wang, M.; Yin, X.; others. Evaluation of environmental factors and microbial community structure in an important drinking-water reservoir across seasons. Frontiers in Microbiology 2023, 14, 1091818. [Google Scholar] [CrossRef]
- Huang, Z.; Li, X.; Wang, L.; Shao, Z. Changes in the intestinal bacterial community during the growth of white shrimp, L itopenaeus vannamei. Aquaculture Research 2016, 47, 1737–1746. [Google Scholar] [CrossRef]
- Rungrassamee, W.; Klanchui, A.; Chaiyapechara, S.; Maibunkaew, S.; Tangphatsornruang, S.; Jiravanichpaisal, P.; Karoonuthaisiri, N. Bacterial population in intestines of the black tiger shrimp (Penaeus monodon) under different growth stages. PloS one 2013, 8, e60802. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.; Liu, B.; Xuan, Y.; Jiang, M.; Pan, Y.; Zhang, Y.; Gong, Y.; Lu, X.; Yu, D.; others. Dynamic changes of microbial communities in Litopenaeus vannamei cultures and the effects of environmental factors. Aquaculture 2016, 455, 97–108. [Google Scholar] [CrossRef]
- Schulze, A.D.; Alabi, A.O.; Tattersall-Sheldrake, A.R.; Miller, K.M. Bacterial diversity in a marine hatchery: balance between pathogenic and potentially probiotic bacterial strains. Aquaculture 2006, 256, 50–73. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Nobre, M.F.; Rainey, F.A.; Silva, M.T.; Wait, R.; Burghardt, J.; Chung, A.P.; Da Costa, M.S. Deinococcus geothermalis sp. nov. and Deinococcus murrayi sp. nov., two extremely radiation-resistant and slightly thermophilic species from hot springs. International Journal of Systematic and Evolutionary Microbiology 1997, 47, 939–947. [Google Scholar] [CrossRef]
- Kuang, T.; He, A.; Lin, Y.; Huang, X.; Liu, L.; Zhou, L. Comparative analysis of microbial communities associated with the gill, gut, and habitat of two filter-feeding fish. Aquaculture Reports 2020, 18, 100501. [Google Scholar] [CrossRef]
- Sylvain, F.É.; Holland, A.; Bouslama, S.; Audet-Gilbert, É.; Lavoie, C.; Val, A.L.; Derome, N. Fish skin and gut microbiomes show contrasting signatures of host species and habitat. Applied and environmental microbiology 2020, 86, e00789–20. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Han, W.; Liu, J.; Huang, X.; Zhou, W.; Zhang, J.; Cheng, Y. Bacterial community compositions of crab intestine, surrounding water, and sediment in two different feeding modes of Eriocheir sinensis. Aquaculture Reports 2020, 16, 100236. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, Q.; Wang, Y.; Zhang, J.; Xiong, D. Impairment of the intestine barrier function in Litopenaeus vannamei exposed to ammonia and nitrite stress. Fish & shellfish immunology 2018, 78, 279–288. [Google Scholar]
- Gao, S.; Pan, L.; Huang, F.; Song, M.; Tian, C.; Zhang, M. Metagenomic insights into the structure and function of intestinal microbiota of the farmed Pacific white shrimp (Litopenaeus vannamei). Aquaculture 2019, 499, 109–118. [Google Scholar] [CrossRef]
- Liu, H.; Guo, X.; Gooneratne, R.; Lai, R.; Zeng, C.; Zhan, F.; Wang, W. The gut microbiome and degradation enzyme activity of wild freshwater fishes influenced by their trophic levels. Scientific reports 2016, 6, 24340. [Google Scholar] [CrossRef]
- Wu, S.; Wang, G.; Angert, E.R.; Wang, W.; Li, W.; Zou, H. Composition, diversity, and origin of the bacterial community in grass carp intestine. PloS one 2012, 7, e30440. [Google Scholar] [CrossRef]
- Zhang, Z.; Xing, R.; Lv, Z.; Shao, Y.; Zhang, W.; Zhao, X.; Li, C. Analysis of gut microbiota revealed Lactococcus garviaeae could be an indicative of skin ulceration syndrome in farmed sea cucumber Apostichopus japonicus. Fish & shellfish immunology 2018, 80, 148–154. [Google Scholar]
- Shapleigh, J.P. Oxygen control of nitrogen oxide respiration, focusing on α-proteobacteria. Biochemical Society Transactions 2011, 39, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends in biotechnology 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Dai, L.; Liu, C.; Yu, L.; Song, C.; Peng, L.; Li, X.; Tao, L.; Li, G. Organic matter regulates ammonia-oxidizing bacterial and archaeal communities in the surface sediments of Ctenopharyngodon idellus aquaculture ponds. Frontiers in Microbiology 2018, 9, 2290. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, Q.; Cao, X.; Zhou, Y.; Song, C. Patterns of sediment fungal community dependent on farming practices in aquaculture ponds. Frontiers in Microbiology 2021, 12, 542064. [Google Scholar] [CrossRef]
- Zhao, K.; Yang, J.; Lv, J.; Liu, Q.; Liu, X.; Xie, S.; Feng, J. Structural Characteristics of Periphytic Algal Community and Its Relationship with Environmental Factors in the Taiyuan Region of the Fenhe River. Water 2022, 14, 2151. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.; Liu, B.; Xuan, Y.; Jiang, M.; Pan, Y.; Zhang, Y.; Gong, Y.; Lu, X.; Yu, D.; others. Dynamic changes of microbial communities in Litopenaeus vannamei cultures and the effects of environmental factors. Aquaculture 2016, 455, 97–108. [Google Scholar] [CrossRef]
- Del’Duca, A.; Cesar, D.E.; Abreu, P.C. Bacterial community of pond’s water, sediment and in the guts of tilapia (O reochromis niloticus) juveniles characterized by fluorescent in situ hybridization technique. Aquaculture Research 2015, 46, 707–715. [Google Scholar] [CrossRef]
- Green, T.; Barnes, A. Bacterial diversity of the digestive gland of Sydney rock oysters, Saccostrea glomerata infected with the paramyxean parasite, Marteilia sydneyi. Journal of Applied Microbiology 2010, 109, 613–622. [Google Scholar] [CrossRef]
- Lokmer, A.; Wegner, K.M. Hemolymph microbiome of Pacific oysters in response to temperature, temperature stress and infection. The ISME journal 2015, 9, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Milan, M.; Carraro, L.; Fariselli, P.; Martino, M.; Cavalieri, D.; Vitali, F.; Boffo, L.; Patarnello, T.; Bargelloni, L.; Cardazzo, B. Microbiota and environmental stress: how pollution affects microbial communities in Manila clams. Aquatic Toxicology 2018, 194, 195–207. [Google Scholar] [CrossRef]
- Pierce, M.L.; Ward, J.E. Microbial ecology of the Bivalvia, with an emphasis on the family Ostreidae. Journal of Shellfish Research 2018, 37, 793–806. [Google Scholar] [CrossRef]
- Brown, R.M.; Wiens, G.D.; Salinas, I. Analysis of the gut and gill microbiome of resistant and susceptible lines of rainbow trout (Oncorhynchus mykiss). Fish & shellfish immunology 2019, 86, 497–506. [Google Scholar]
- Egerton, S.; Culloty, S.; Whooley, J.; Stanton, C.; Ross, R.P. The gut microbiota of marine fish. Frontiers in microbiology 2018, 9, 873. [Google Scholar] [CrossRef] [PubMed]
- Nikapitiya, C. Marine bacteria as probiotics and their applications in aquaculture. Marine Microbiology 2013, pp. 97–126.
- Al-Harbi, A.H.; Uddin, N. Bacterial diversity of tilapia (Oreochromis niloticus) cultured in brackish water in Saudi Arabia. Aquaculture 2005, 250, 566–572. [Google Scholar] [CrossRef]
- Dabadé, D.S.; Wolkers-Rooijackers, J.C.; Azokpota, P.; Hounhouigan, D.J.; Zwietering, M.H.; Nout, M.R.; den Besten, H.M. Bacterial concentration and diversity in fresh tropical shrimps (Penaeus notialis) and the surrounding brackish waters and sediment. International journal of food microbiology 2016, 218, 96–104. [Google Scholar] [CrossRef]
- Vojvoda, J.; Lamy, D.; Sintes, E.; Garcia, J.A.; Turk, V.; Herndl, G.J. Seasonal variation in marine-snow-associated and ambient-water prokaryotic communities in the northern Adriatic Sea. Aquatic Microbial Ecology 2014, 73, 211–224. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Fu, B.; Mu, X. Improvement of aquaculture water quality by mixed Bacillus and its effects on microbial community structure. Environmental Science and Pollution Research 2022, 29, 69731–69742. [Google Scholar] [CrossRef]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Program, N.C.S.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; others. Topographical and temporal diversity of the human skin microbiome. science 2009, 324, 1190–1192. [Google Scholar] [CrossRef]
- Santisi, S.; Genovese, M.; Bonsignore, M.; Fiumara, E.; Maricchiolo, G.; Mancuso, M.; Genovese, L.; Giuliano, L.; Cappello, S. Study of bacterial communities in mussel Mytilus galloprovincialis by a combination of 16S crDNA and 16S rDNA sequencing. Int. J. Microbiol. Appl 2015, 2, 18–24. [Google Scholar]
- Li, Y.F.; Xu, J.K.; Chen, Y.W.; Ding, W.Y.; Shao, A.Q.; Liang, X.; Zhu, Y.T.; Yang, J.L. Characterization of gut microbiome in the mussel Mytilus galloprovincialis in response to thermal stress. Frontiers in Physiology 2019, 10, 1086. [Google Scholar] [CrossRef] [PubMed]
- Musella, M.; Wathsala, R.; Tavella, T.; Rampelli, S.; Barone, M.; Palladino, G.; Biagi, E.; Brigidi, P.; Turroni, S.; Franzellitti, S.; others. Tissue-scale microbiota of the Mediterranean mussel (Mytilus galloprovincialis) and its relationship with the environment. Science of the Total Environment 2020, 717, 137209. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Zhu, J.; Dai, W.; Dong, C.; Qiu, Q.; Li, C. Integrating gut microbiota immaturity and disease-discriminatory taxa to diagnose the initiation and severity of shrimp disease. Environmental microbiology 2017, 19, 1490–1501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zheng, X.; Ren, X.; Li, Y.; Wang, Y. Bacterial diversity in gut of large yellow croaker Larimichthys crocea and black sea bream Sparus macrocephalus reared in an inshore net pen. Fisheries science 2019, 85, 1027–1036. [Google Scholar] [CrossRef]
- Wei, N.; Wang, C.; Xiao, S.; Huang, W.; Lin, M.; Yan, Q.; Ma, Y. Intestinal microbiota in large yellow croaker, Larimichthys crocea, at different ages. Journal of the World Aquaculture Society 2018, 49, 256–267. [Google Scholar] [CrossRef]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nature Reviews Microbiology 2008, 6, 121–131. [Google Scholar] [CrossRef]
- Yukgehnaish, K.; Kumar, P.; Sivachandran, P.; Marimuthu, K.; Arshad, A.; Paray, B.A.; Arockiaraj, J. Gut microbiota metagenomics in aquaculture: factors influencing gut microbiome and its physiological role in fish. Reviews in Aquaculture 2020, 12, 1903–1927. [Google Scholar] [CrossRef]
- Bhatt, P.; Kumaresan, V.; Palanisamy, R.; Ravichandran, G.; Mala, K.; Amin, S.N.; Arshad, A.; Yusoff, F.M.; Arockiaraj, J. A mini review on immune role of chemokines and its receptors in snakehead murrel Channa striatus. Fish & shellfish immunology 2018, 72, 670–678. [Google Scholar]
- Shade, A.; Handelsman, J. Beyond the Venn diagram: the hunt for a core microbiome. Environmental microbiology 2012, 14, 4–12. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, Y.; Lin, X.; Zhang, H.; Zeng, J.; Hou, J.; Yang, Y.; Yao, T.; Knight, R.; Chu, H. Geographic distance and pH drive bacterial distribution in alkaline lake sediments across Tibetan Plateau. Environmental microbiology 2012, 14, 2457–2466. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Van Der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Zheng, Y.M.; Hu, H.W.; Li, J.; Zhang, L.M.; Chen, B.D.; Chen, W.P.; He, J.Z. Coupling of soil prokaryotic diversity and plant diversity across latitudinal forest ecosystems. Scientific Reports 2016, 6, 19561. [Google Scholar] [CrossRef] [PubMed]
- Dini-Andreote, F.; Stegen, J.C.; Van Elsas, J.D.; Salles, J.F. Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proceedings of the National Academy of Sciences 2015, 112, E1326–E1332. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Chu, H.; Zhang, B.; Wei, X.; Chen, W.; Wei, G. Linking soil fungi to bacterial community assembly in arid ecosystems. IMeta 2022, 1, e2. [Google Scholar] [CrossRef]

| Stations | Index (mg/L) | Spring | Summer | Autumn | Winter |
| IN | 0.159±0.021a | 0.050±0.004a | 0.217±0.025a | 0.102±0.008a | |
| DB | 0.025±0.009a | 0.005±0.003a | 0.217±0.006a | 0.126±0.128a | |
| COD IN |
1.182±0.284a 1.129±0.013a |
2.036±0.091a 0.052±0.003a |
2.214±1.219a 0.318±0.041b |
1.047±0.188a 0.104±0.013a |
|
| DY | 0.023±0.003a | 0.002±0.001a | 0.015±0.005a | 0.012±0.006a | |
| COD | 0.853±0.342a | 1.607±0.351a | 2.209±0.252a | 1.286±0.255a |
| Index | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|
| pH | 7.303±0.102 | 7.693±0.124 | 7.120±0.640 | 7.690±0.115 |
| TP (mg/L) | 0.089±0.006 | 0.011±0.003 | 0.012±0.0007 | 0.030±0.0009 |
| W(%) | 30.237±0.949 | 30.241±1.890 | 32.305±1.390 | 30.027±1.932 |
| Stations | Spring | Summer | Autumn | Winter | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Simpson | Shannon | Coverage | Simpson | Shannon | Coverage | Simpson | Shannon | Coverage | Simpson | Shannon | Coverage | |
| DB | 0.04±0.01a | 4.55±0.36a | 0.99 | 0.03±0.01a | 5.00±0.03a | 0.99 | 0.17±0.13a | 3.66±0.74a | 0.99 | 0.13±0.03a | 3.64±0.29a | 0.99 |
| DY | 0.06±0.02a | 4.02±0.23a | 0.99 | 0.02±0.00a | 4.78±0.70a | 0.99 | 0.11±0.03a | 3.80±0.33a | 0.99 | 0.27±0.02b | 3.08±0.16a | 0.99 |
| Stations | Spring | Summer | Autumn | Winter | ||||
|---|---|---|---|---|---|---|---|---|
| ACE | Chao 1 | ACE | Chao 1 | ACE | Chao 1 | ACE | Chao 1 | |
| DB | 496.70±123.01a | 523.19±119.35a | 1159.52±43.81a | 1042.6±11.01a | 641.90±25.3a | 660.78±14.57a | 686.14±19.63a | 700.22±21.61a |
| DY | 292.83±12.24a | 303.86±10.77a | 1097.87±55.31a | 1035.44±15.79a | 648.12±81.60a | 676.58±70.12a | 683.77±18.33a | 689.32±20.92a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





