1. Introduction
Trauma, systemic diseases, neoplastic fractures, infections, or a compromised blood supply are some of the causes that can result in large bone defects, which can delay or impair healing, leading to a loss of function in the affected individual. Consequently, the need for new treatments for patients with musculoskeletal diseases has increased during the last decades [
1,
2]. The regenerative capacity of bone has provided a paradigm for developing new bone regeneration strategies, making bone grafting the main treatment for bone injuries [
1]. Bone tissue engineering is the most developed research field in tissue engineering. It aims to induce the formation of new functional bone tissues based on the understanding of bone biology and its development [
2,
3,
4].
Bone is a highly vascularized and dynamic natural composite characterized by its mineralized extracellular matrix comprising collagen fibers, proteoglycans, and calcium and phosphate ions. This type of connective tissue develops different functions in human physiology, such as supplying reserved body minerals for homeostatic processes or structural functions, possessing excellent mechanical properties that provide load-bearing capacity for locomotion, and acting as a case protecting internal organs. Bone experiences a constantly dynamic growth, with continuous remodeling processes throughout an individual’s lifespan [
5,
6,
7]. Thus, being able to recover spontaneously after an injury under suitable physiological, environmental conditions is a phenomenon known as the bone healing process [
8,
9].
Large-sized bone defects cannot be healed by themselves when exceeding the so-called critical-sized defect. Furthermore, different factors may be involved in the failure of said process, such as the patient’s age and gender, unstable biomechanical properties, or an unfavorable wound environment [
5,
8,
9]. In those situations, surgical therapeutic interventions can be required due to the limited intrinsic regeneration potential, and in this area, tissue engineering has emerged as a promising approach for bone healing [
10]. Current clinical approaches mainly include autologous bone grafts, allogenic bone grafts, xenogenic bone grafts, or bone graft substitutes. Autologous grafts or autografts are still the gold standard due to their excellent osteoinductive, osteoconductive, osteointegrative, and non-immunogenic properties. However, this method requires a secondary surgical procedure, which is limited by several drawbacks such as dysesthesia, inflammation, infection, and limited donor bone availability. Likewise, allografts and xenografts are limited by the risk of high failure rates, immune rejection, blood disease transmission, and even poor osseointegration [
5,
11,
12].
Confronted with this situation, the search for new bone graft substitutes led researchers to explore new combinations of cells, biomaterials, and biological factors to achieve new therapeutic strategies for bone regeneration [
11,
12,
13]. However, none of the currently available bone graft substitutes possess all the desirable biological requirements for a biomaterial, such as bioactivity, biomimetism (including biocompatibility associated with osteoconductive and osteoinductive properties), angiogenic potential, biological safety, low patient morbidity, volumetric stability, biomechanical properties, non-immunogenic, biodegradability, easy market availability, long shelf life and reasonable production cost [
2,
12,
14].
Among the major bone tissue engineering approaches, novel scaffold-based treatments have recently been widely used [
10]. These methods are based on using three-dimensional porous structures that can support and actively guide tissue regeneration. [
12,
14]. Ideally, a scaffold suitable for BTE should aim to provide a microenvironment that mimics the properties of the native extracellular matrix (ECM) [
15], acting as space holders for cell infiltration, attachment, growth, and differentiation that also improves their viability. Besides, these three-dimensional structures facilitate the transmission of loads to surrounding tissues, instantly providing mechanical support to the defect site after implantation [
2,
10,
15]. Modifying types of fabrication process, structural features, biomaterial composition and biological requirements may modulate these characteristics. The latter are closely related to biomaterial selection and the incorporation of different substances such as growth factors, stem cells, etc. [
2].
Several manuscripts have previously analyzed the importance of different scaffold printing techniques, materials, and designs. Garot
et al. [
14] reviewed material and additive manufacturing techniques specifically used to repair bone defects. Similarly, Amini
et al. [
3] reviewed recent advances and challenges of bone tissue engineering, discussing widely investigated biomaterial scaffolds, structural properties, and the incorporation of biomimetic properties and/or growth factors. Likewise, scaffold design was studied by Ostrowska
et al. [
16], who analyzed in vitro the effect of different 3D-printed laydown patterns, Liang
et al. [
17], who designed 3 different 3D-printed scaffolds and evaluated their clinical applications in vitro and in vivo through subcutaneous implantation, or Gleadall
et al. [
18], who reviewed the relationship between geometry and performance of additive manufactured tissue engineering scaffold.
The design and fabrication of scaffolds were limited in the past since traditional technologies lacked the ability to incorporate internal architecture and control the porosity. However, currently, additive manufacturing (AM), also called rapid prototyping (RP) or solid freeform (SFF), has addressed these problems, allowing complete control over scaffold architecture at a very reasonable cost. AM offers the possibility to customize the scaffold's global shape and internal structure at high reproducibility and reliability. Besides, it may be used in personalized medicine since three-dimensional images acquired by Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) can be reproduced layer by layer based on CAD models [
14,
19,
20].
Extrusion-based, powder-based, and vat photopolymerization AM techniques allow the manufacture of polymeric, ceramic, and metallic scaffolds. Although AM techniques have been widely used in biomedical research, only a few products are available on the market to date [
14]. The desired traits of printable materials for tissue engineering are printability, biocompatibility, and good mechanical and structural properties. Polymers are particularly well-suited for additive manufacturing of scaffolds, and various printing techniques have been employed to fabricate polymeric scaffolds, such as fused deposition modeling, selective laser sintering, inkjet 3D printing, stereolithography, or 3D plotting [
19,
20].
Polylactic acid (PLA) is one of the most widely used synthetic polymers, commonly used as raw material in the Fused Deposition Modeling-Based 3D-printing process. Its versatility, mechanical properties, and biodegradability make it an exciting alternative for application in bone tissue engineering. Since its approval by the U.S. Food and Drug Administration (FDA), it has been used in human clinical applications such as surgical sutures, bone fixation or implantable scaffolds. However, PLA also presents several drawbacks, such as slow degradation rate, hydrophobic behavior, and release of acidic-by products during degradation processes [
12,
15,
20,
21,
22,
23]. To solve polymers’ drawbacks and obtain a more practical, functional, and valuable structure, composite materials are synthesized by combining two or more carefully integrated phases [
19,
24]. PLA composites can be fabricated by incorporating metal, ceramic, organic, inorganic, or nano-materials into the polymeric matrix [
21].
Calcium phosphate (CaP) ceramics, such as hydroxyapatite (HA) or beta-tricalcium phosphate (β-TCP), are some of the most commonly used synthetic bone substitutes due to their composition similarities to natural Bone [
12,
25]. CaP bioceramics are biomimetic materials standing out for their osteoconductivity, osteoinductivity, biocompatibility, and bioactivity. However, their brittleness represents a major disadvantage, mainly for load-bearing applications [
12,
14,
20]. Hence, the suitability of bioceramics for being printed through additive manufacturing techniques allows the manufacture of composite scaffolds [
21,
26]. Which optimizes the biocompatibility of the polymeric materials but maintains adequate mechanical properties and avoids the brittle behavior of the bioceramics [
14]. Furthermore, bioceramics also block the acidic environment originating from PLA’s degradation and increase its hydrophilicity and degradation rate [
27].
Despite the importance of bone graft composition, some specific requirements regarding the design of the scaffolds are also essential for bone tissue engineering. However, the design and optimization of the scaffolds for successful integration remain unclear, hypothesizing that results may depend on their fluid flow and nutrient/waste diffusion properties [
28,
29]. Geometrical characteristics of 3D scaffolds, such as pore architecture, pore size, porosity, and interconnectivity, may have a great influence on the bone regeneration capacity [
5,
12,
30]. These properties are essential for osteoblast and mesenchymal cell migration, proliferation, and differentiation. Besides, pore interconnection is essential for tissue ingrowth in porous material since it allows blood vessel invasion and nutrient supply [
31]. The scaffolds should also provide an effective support effect and maintain an appropriate mechanical environment at the defect site [
32]. Mechanical properties depend on porosity, pore size and shape, and material properties so that those characteristics will determine the amount of mechanical stimulus the scaffold carries [
29]. All these parameters may be affected by the scaffold’s design, which influences various factors in tissue engineering. Different geometries can be achieved by varying the position and orientation of the fibers, arising structures with aligned or staggered filaments, “repeated layers,” and orientations such as 0º/90º or 0º/60º/120º, depending on a print path [
18]. Different authors have already illustrated the effect of varying scaffold designs on bone healing, such as Berner et al. [
10], evaluating the effect of scaffold architecture on cranial bone healing; Lim et al. [
33], studying the effect of different pore architectures; or Entezari et al. [
34], demonstrating that manipulating pore size and permeability of 3D-printed scaffolds is a valuable strategy for enhancing bone regeneration outcomes. However, there is still a gap between the fabrication of different printing paths and their evaluation in vivo in a controlled study, being isolated from any other type of variable that could influence bone growth.
In the present manuscript, 3D-printed composite scaffolds were manufactured using fused deposition modeling to evaluate the impact of different laydown patterns. To synthesize the scaffolds, PLA was chosen as a polymeric matrix, and a previously proven shark teeth- marine derived bioapatites (bioCaP) [
36,
37,
38] were selected as a reinforcing agent. The primary objective of this study is to investigate and compare the effects of two distinct scaffold architectures — alternate and helical laydown patterns — on bone regeneration. The study aims to assess various scaffold properties, including pore size, porosity, mechanical strength, and overall biocompatibility, and their impact on the efficacy of bone healing in critical-sized defects. The null hypothesis was that scaffold architecture significantly influences the rate and quality of bone regeneration in critical-sized defects.
2. Material and Methods
2.1. Fabrication of 3D-Printed PLA-bioCaP Scaffold
Previously reported processes [
35] were utilized to obtain marine bioderived calcium phosphate (bioCaP) grains from
Prionace glauca shark teeth as a byproduct provided by IIM-CSIC (Vigo, Spain). The resulting bioCaP particles (20-63 µm diameter) showed a biphasic composition of ~70% apatitic (HA, apatite-CaF, fluorapatite) and ~30% non-apatitic phase (whitlockite, tricalcium bis(orthophosphate)), with contributions of F (1.0±0.5 %wt), Na (0.9±0.2 %wt) and Mg (0.65±0.04 %wt) [
35,
36].
PLA particles with a diameter of 80-250 µm were obtained from commercial polylactic acid pellets (SMARTFIL®, Smart Materials, Jaén, Spain) and then mixed with a contribution of 12.66 %wt of bioCaP using a Turbula® 3D mixer (WAB, Nidderau, Germany). A composite filament was produced using a filament extruder system (Filastruder, Snellville, Georgia), as published by Rojas-Lozano et al. [
37]. This 3D-FDM (Fused Deposition Modeling) printer works with two temperature control points (T1 and T2), whose temperature is adjusted according to the material of the pellet to be extruded. In our case T1 = 140ºC, T2 = 220ºC.
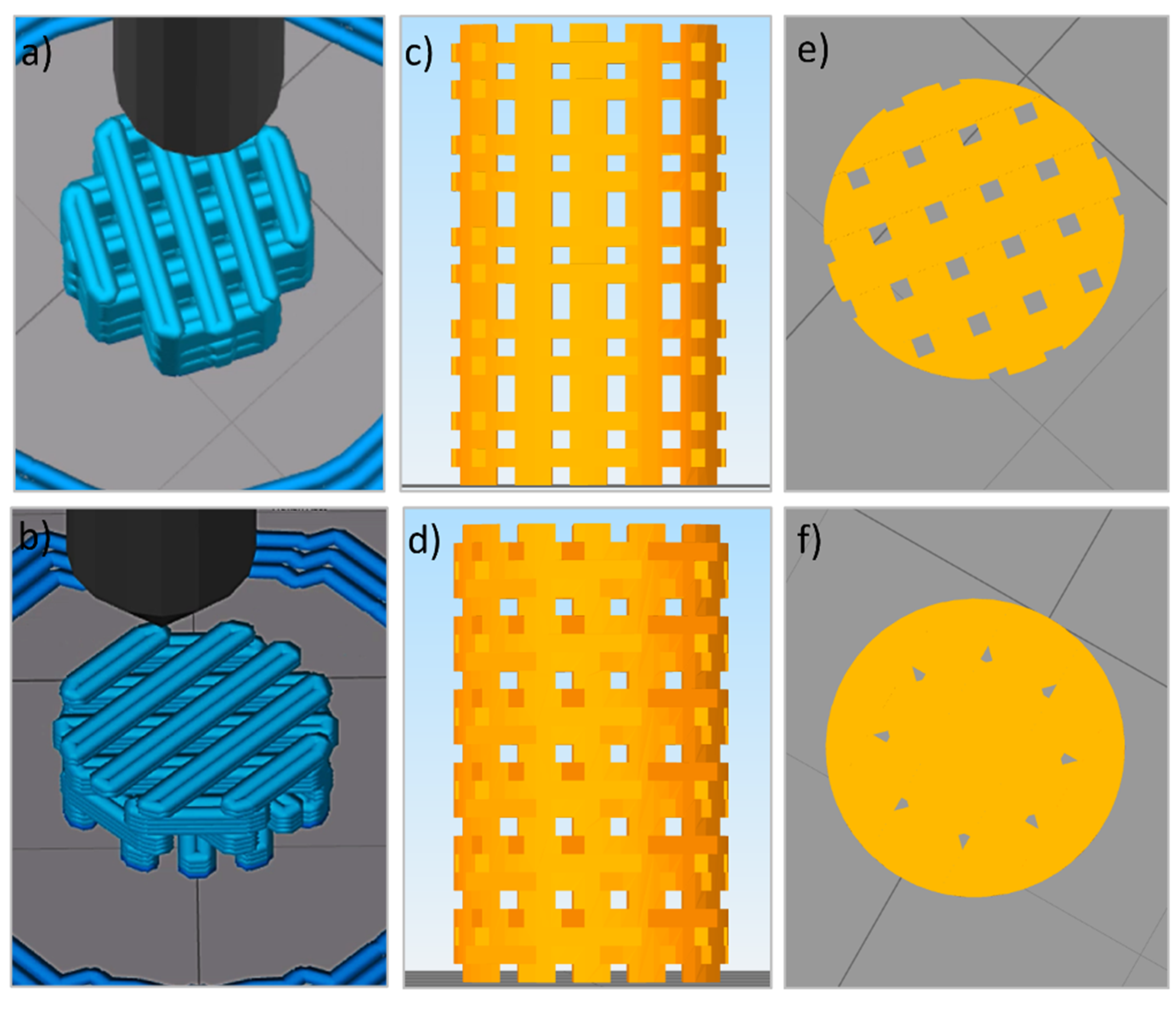
The filament was incorporated in a 3D printer (TUMAKER Voladora NX Pellet, Oiartzun, Spain) to manufacture cylindrical scaffolds with dimensions of 6 mm diameter and 10 mm height. A 0.8 mm diameter nozzle was utilized to synthesize scaffolds with two different laydown patterns namely O°/90°/180° (alternate structure (ALT)) and 0°/45°/90°/135°/180° (helical structure (HEL)). Simplify3D Professional Software was used to show simulations of the printing process and final scaffolds (
Figure 1). Before implanted, samples were packed in a laminar flow cabin and sterilized with a 15 kGy dose of gamma radiation.
2.2. Characterization of the 3D Printed Scaffolds
In a previous work, our group performed physicochemical characterization of the 3D printed PLA-12wt%bioCaP composite scaffolds obtained from the same filament after being subjected to the 3D printer (TUMAKER Voladora NX Pellet, Oiartzun, Spain), as mentioned above. It included the evaluation of the bonding configuration by Raman spectroscopy, with the quantitative analysis of the Raman spectra of different PLA-bioCaP scaffolds with increasing contributions in wt% of bioCaP, to obtain the bioCaP/PLA ratio at the printed scaffolds for each composite filament. Furthermore, the surface topography and the wettability were also respectively evaluated by SEM and contact angle measurements. Given that, the present work will be focus on the pore morphology, interconnectivity, and mechanical characterization of the mentioned scaffolds, as these data will be of great importance in evaluating the in vivo response of the implanted scaffolds.
2.2.1. Pore Morphology
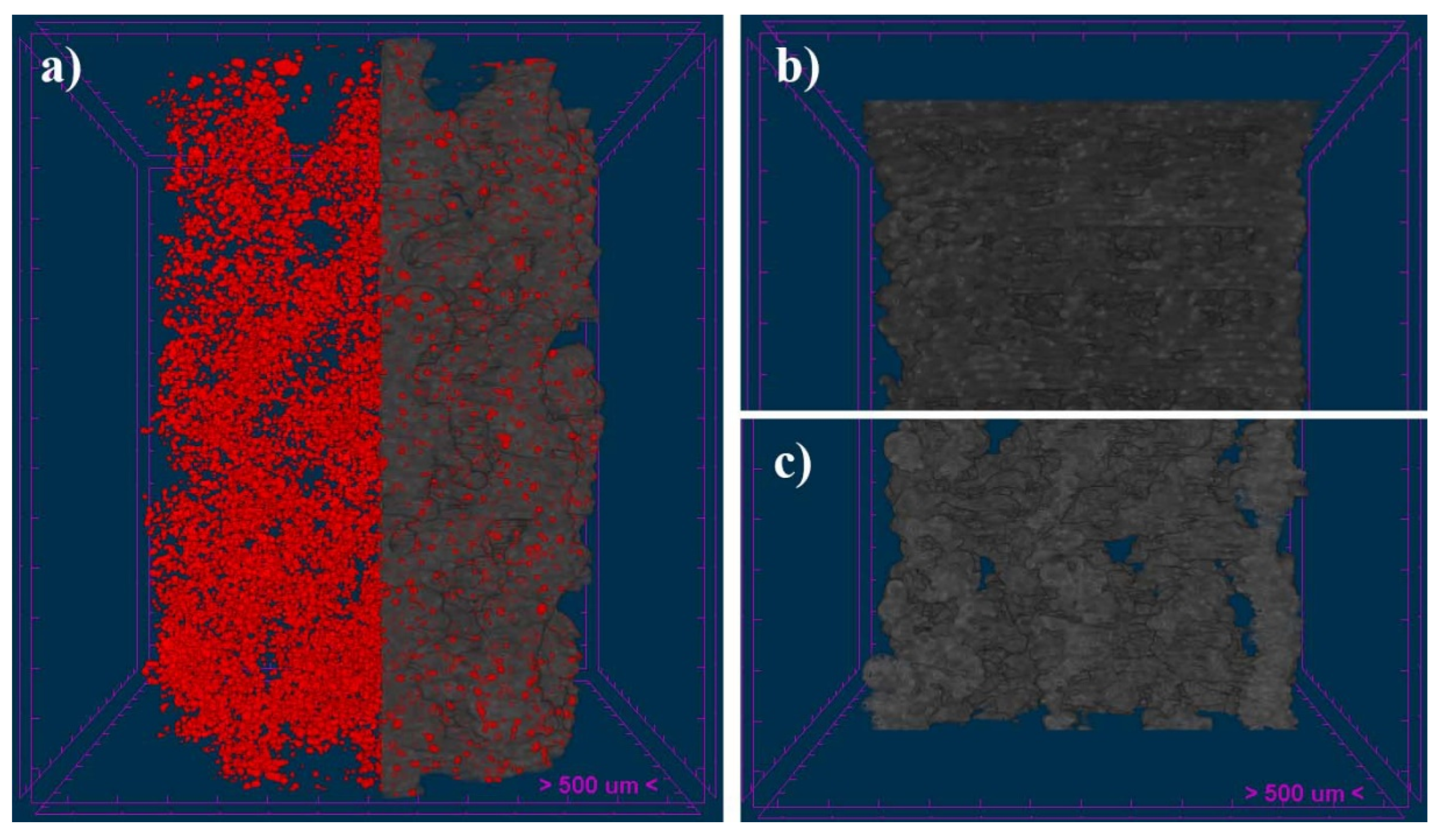
Three-dimensional images were obtained using Micro-CT (SkyScan 1172, Bruker-microCT, Kontich, Belgium). Scanning parameters were set to 13.54 µm pixels, X-ray source with 70 kV and 141 mA, and using a 0.5 mm Aluminum filter. The samples were rotated 360 degrees around their vertical axis with a rotational step of 0.4 degrees. The raw images of the scaffolds were reconstructed using the Skyscan standard software (NRecon, Bruker-microCT, Kontich, Belgium) to serial coronal-oriented tomograms using a modified back-projection algorithm [
38] and subsequently analyzed with another software (CTAn, Bruker-microCT, Kontich, Belgium). For 3D analysis, the volume of Interest (VOIs) was defined as a cylindrical region of 5.5 mm centered on the scaffold, with a total length of 8.0 mm (591 slides). Then, Anisotropic Diffusion filtering, histogram-based manual thresholding method [
39], and despeckle tool were applied to the images before quantification of specific parameters such as open porosity, object surface per volume ratio (ObjS/V ratio), Strut diameter (defined as Strut Thickness, St.Th) and pore size (defined as Strut Separation, St.Sp.). Likewise, interconnectivity was calculated with an algorithm considering only the open and accessible porosity volume within the scaffold, as previously described [
40,
41].
2.2.2. Mechanical Test
The mechanical behavior of the two different laydown patterns of 3D-printed scaffolds was assessed through compressive mechanical testing (Zwicki, Zwick/Roell, Ulm, Germany). Compression tests were performed, starting with a preloading force set at 0.5 N and then using a load cell of 1 kN. The cylindrical scaffolds were compressed at a 1 mm/min compression speed until failure. The force and displacement were recorded throughout the compression and converted to stress and strain based on the initial scaffold dimensions. The compressive strength was measured at the end of the elastic modulus.
2.3. Animal Model
The present manuscript was written following the Animals in Research Reporting In Vivo Experiments (ARRIVE) guidelines [
42].
Rabbits are the most used preclinical model for bone tissue’s testing. In addition to their easy housing and handling, they reach skeletal maturity at an early age after puberty, present similarities in bone mineral density and fracture toughness with humans, and their bone turnover is faster than other species like primates or rodents. Likewise, femoral condyles support defects more significant than 3 mm to test biocompatibility and osteoinduction in cancellous bone, so they allow the performance of critical defects to test biomaterials in load-bearing conditions, which should be 6 mm according to the species and location. [
8,
43,
44,
45,
46]. Critical size defect are defined as the smallest wound that does not heal spontaneously over a long period of time, so they are commonly performed to evaluate scaffold’s bone healing properties [
8,
27].
New Zealand White Rabbits (4-5 kg, male) were obtained from Granja San Bernardo, Navarra, Spain. All experiments were approved by the Ethics Committee of the University of Santiago de Compostela University (Spain) (Reference Number - 02/20/LU-002) and authorized by the Regional Government of Galicia. The animal housing and experimental procedures were conducted in the Animal Experimentation Facility of the University of Santiago de Compostela (Lugo, Spain).
To perform the surgical procedures, rabbits were premedicated by administering an intramuscular combination of medetomidine (50 ug/kg, Domtor, Esteve, Barcelona, Spain), ketamine (25 mg/kg, Imalgène 1000, Merial, Toulouse, France) and buprenorphine (0.03 mg/kg, Buprex, RB Pharmaceuticals, Berkshire, UK). Then, inhalatory anesthesia (Isoflurane, inspiratory Fraction ISO 2.5-4%, Isova-vet, Schering-Plow, Madrid, Spain) was utilized to induce and maintain general anesthesia. Furthermore, enrofloxacin (5 mg/kg SC, Ganadexil 5%, Invesa, Barcelona, Spain) and meloxicam (0.2 mg/kg SC, Metacam, Boehringer Ingelheim España, Barcelona, Spain) were administrated in order to obtain antibiotic prophylaxis and pain control, respectively. A circular bone defect of 6 mm in diameter was performed on the rabbit’s lateral femoral condyle bilaterally after cutting skin and muscle layer by layer, using a trephine burr (227B.204.060, Komet, Germany) connected to a surgical motor under irrigation. The fabricated scaffolds were implanted in the performed bone tunnels, and each one was allocated to one of the two treatment groups according to block randomization: PLA-12CaP-ALT (alternate structure) and PLA-12CaP-HEL (helical structure). After suturing, an intramuscular injection of atipamezole (25 µg/kg IM, Nosedorm, Karizoo, Barcelona, Spain) was administered to revert the sedation, and then rabbits were placed in the cages. Enrofloxacin (5 mg/kg, Ganadexil 10%, Invesa, Barcelona, Spain) and meloxicam (0.1 mg/kg, Metacam, Boehringer Ingelheim España, Barcelona, Spain) were utilized postoperatively for 5 days, with the same aim as reported above. In addition, veterinarians monitored weekly the rabbits for wound dehiscence, inflammation, infection, lameness, and general health.
Twelve weeks later, animals were sedated with medetomidine (50 ug/kg IM, Domtor, Esteve, Barcelona, Spain) and ketamine (25 mg/kg IM, Imalgène 1000, Merial, Toulouse, France) and then euthanized by sodium pentobarbital (100 mg/kg IV, Dolethal, Vétoquinol, Madrid, Spain) overdose injection in the lateral auricular vein. Samples were dissected free of skin and soft tissue, and femoral condyles were extracted, harvested, and fixed in a 10% buffered formalin solution for 2 weeks.
2.4. Micro-CT Analysis
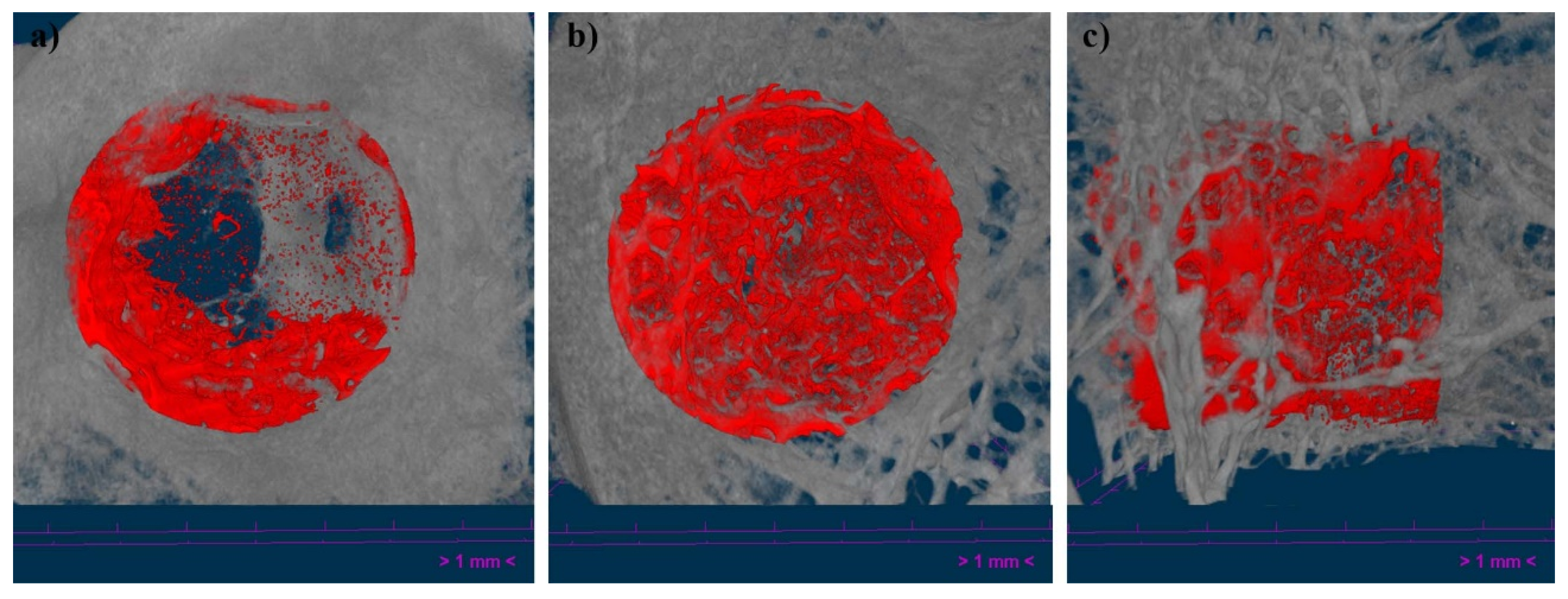
After the sample’s fixation, specimens were scanned using a high-resolution micro-computed tomography (uCT) machine (Skyscan 1172, Bruker microCT NV, Kontich, Belgium) equipped with an 11-Mpixel CCD camera. The acquisition parameters were set as described in a previous section. The reconstruction of the X-ray projections was performed using a modified back-projection algorithm [
38] (NRecon v.1.7.5, Bruker, Kontich, Belgium) with a final voxel size of 13.58 µm. Bone regeneration capacity of the implants was assessed using CT Analyser (CTAn 1.20.3.0+, Bruker, Kontich, Belgium), and parameters such as Bone Volume/Tissue Volume (BV/TV), Bone Surface/Bone Volume (BS/BV), Trabecular Thickness (Tb.Th.) and Trabecular Pattern Factor (Tb.Pf.), was evaluated inside a cylindrical region with a diameter of 5.987 mm and a total height of f 6 mm (442 slices), defined as the Volume of Interest (VOI).
2.5. Histologic and Histomorphometric Analysis
The specimens were later processed for undecalcified ground sections according to the method described by Donath [
47]. Briefly, the samples were dehydrated with EtOH and embedded within a methylmethacrylate resin
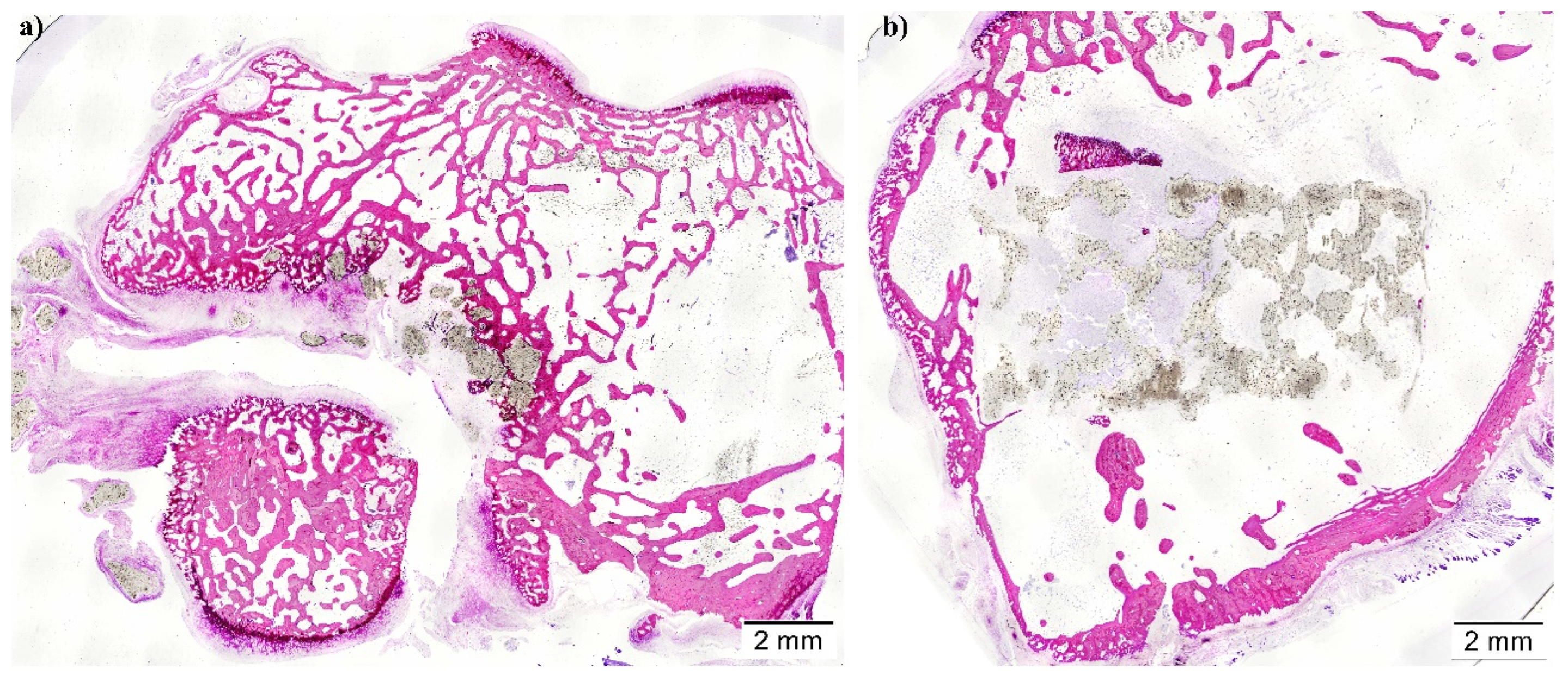
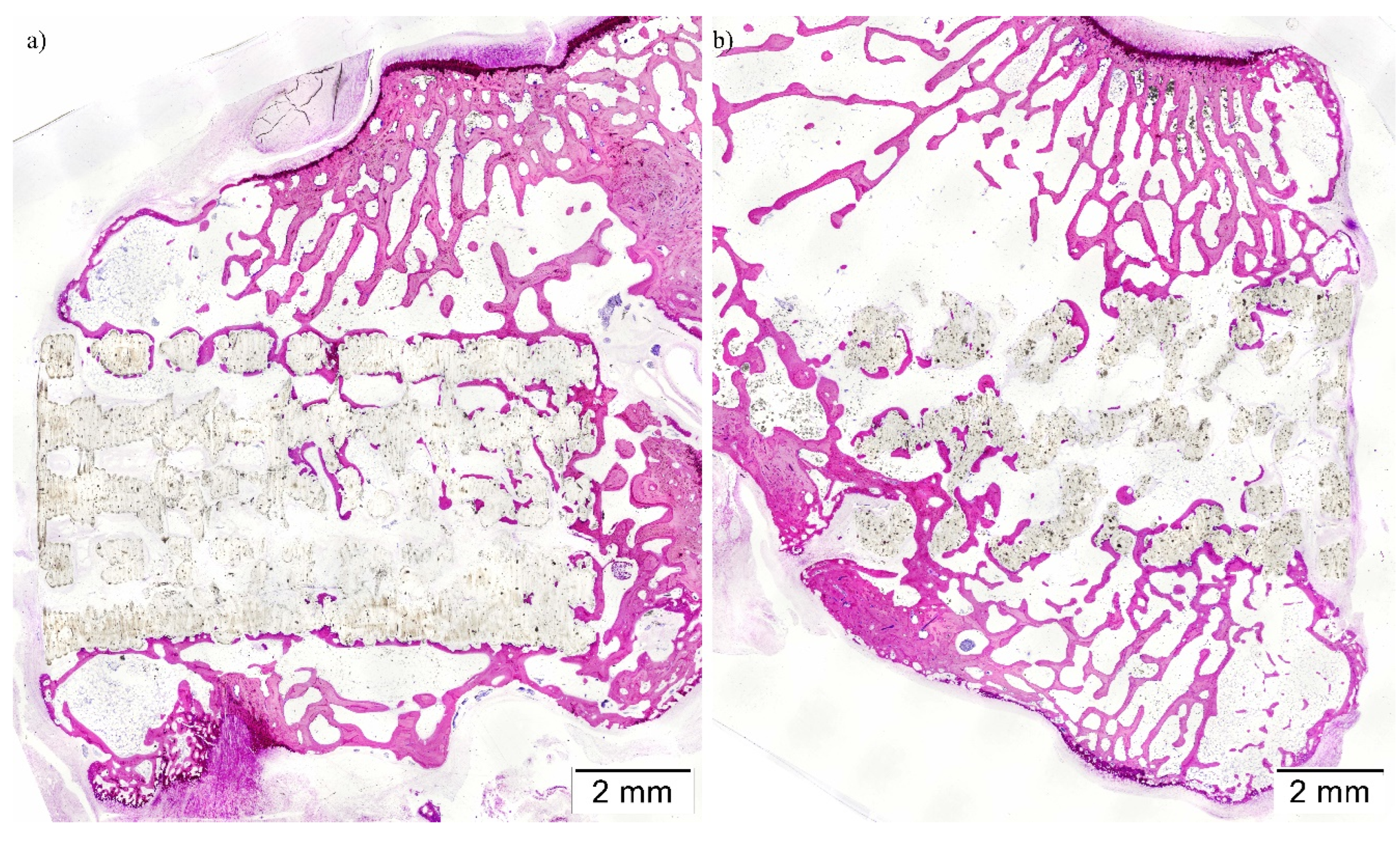
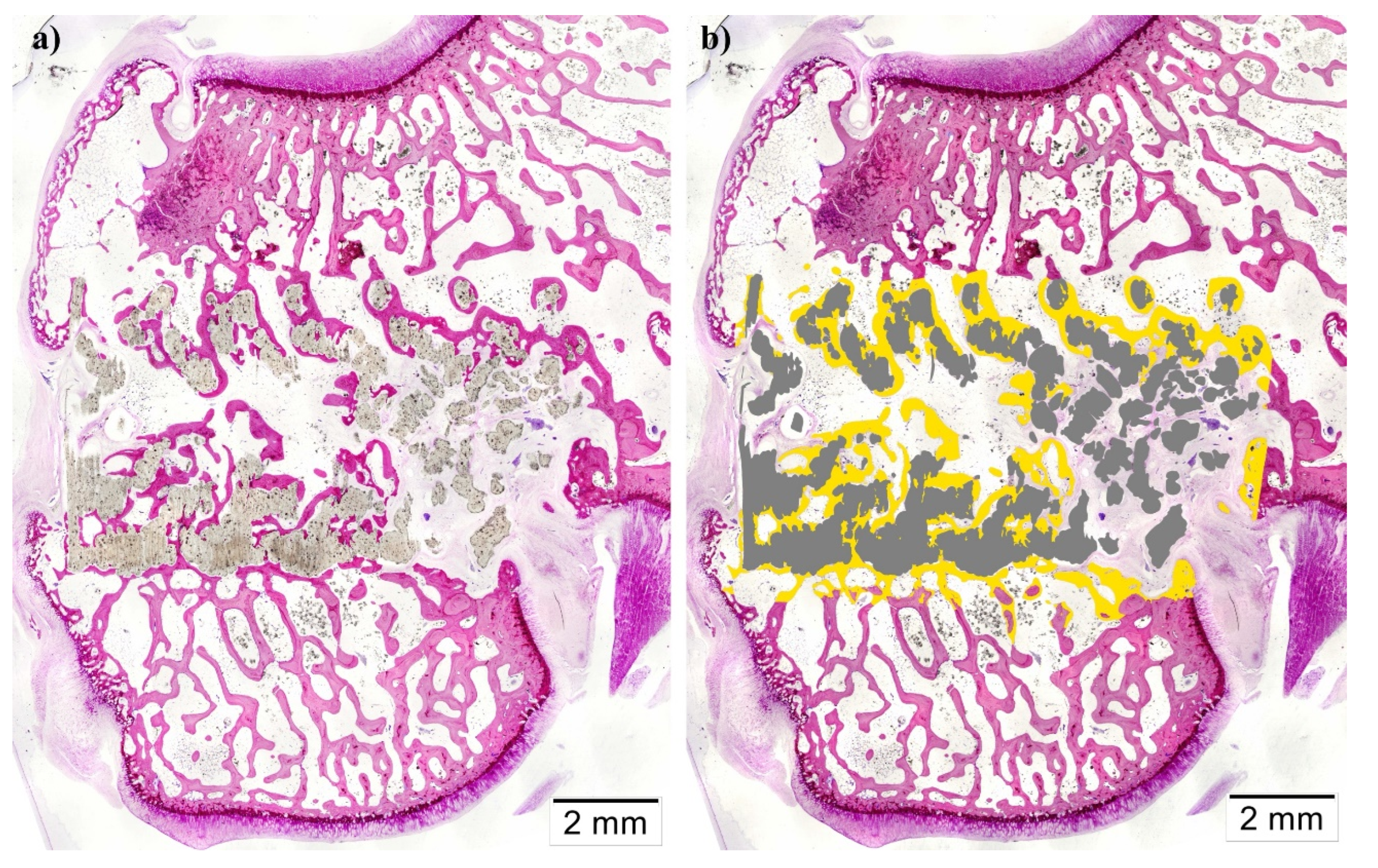
(Technovit 7200-VLC, Heraeus Kulzer GmbH, Wertheim, Germany). Resin blocks were cut using a band saw for the purpose of obtaining two central sections from each implant, which micro polished until they had a thickness of ∼40 μm. Furthermore, finally, tissue slides were stained with Lévai-Laczkó’s protocol.
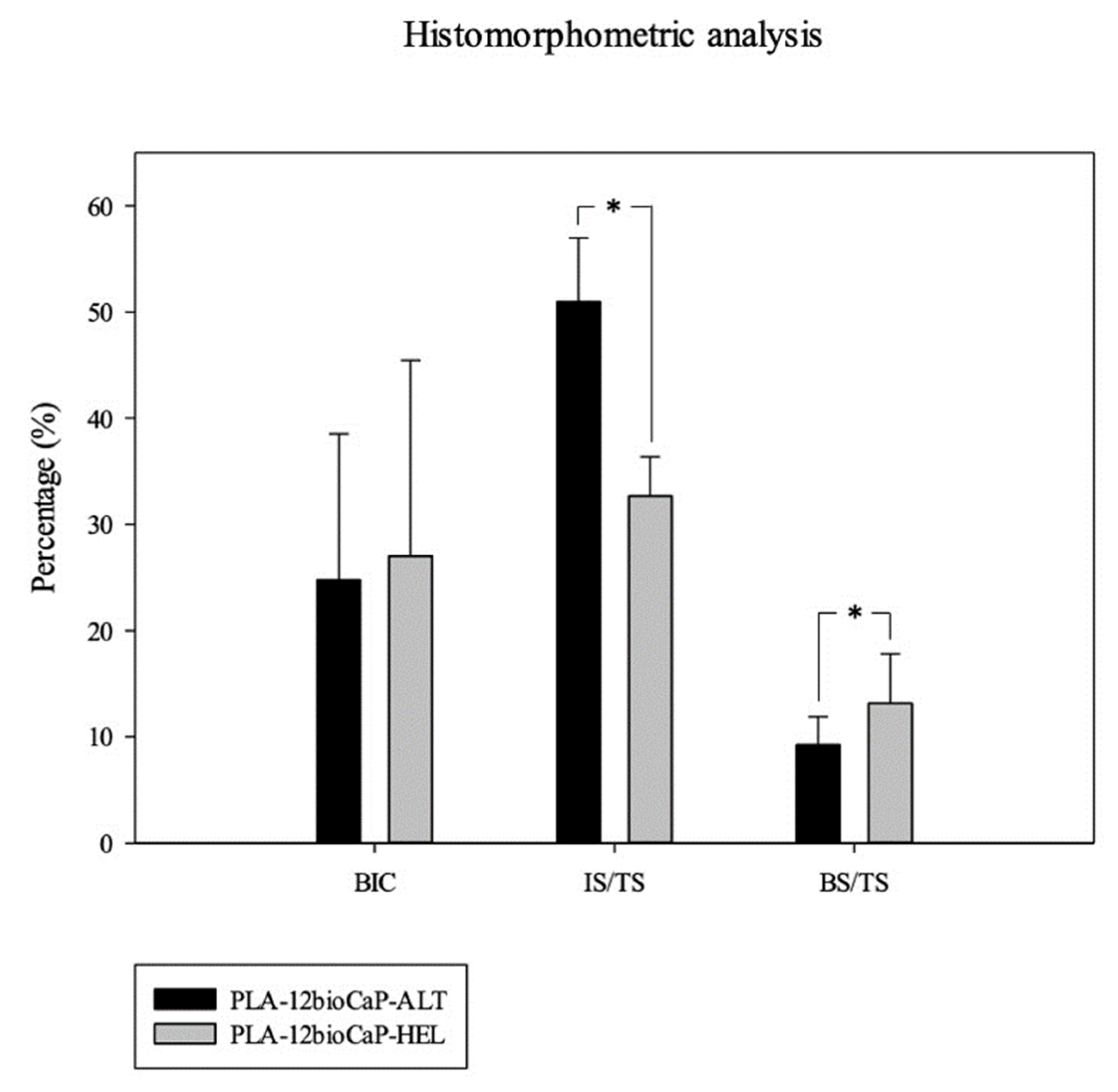
Once obtained, slides were imaged with an Olympus BX51 microscope (Olympus Corporation, Tokyo, Japan). Whole section images were captured at x4 augments and colored with Adobe Photoshop CS6 (Adobe Systems Incorporated, San Jose, USA), distinguishing new bone tissue, composite material, pristine Bone, and soft tissue. Colored images were analyzed using the Olympus CellSens 1.5 (Olympus Corporation) program in order to measure the following histomorphometric parameters: Bone-to-implant contact (BIC), Implant Surface/Tissue Surface (IS/TS), and Bone Surface/Tissue Surface (BS/TS); inside a defined Region of Interest (ROI). Scaffolds incorrectly placed proximally in the medullar cavity instead of the trabecular Bone were excluded from the analysis.
2.6. Statistical Analysis
Data were expressed as means ± standard deviations (SDs). The statistical analysis was performed with SigmaPlot 12.5 software for Windows (Systat Software Inc., Chicago, IL, USA). In the statistical analysis for pore morphology and mechanical test, the normality of the variables was assessed using the Kolmogoron-Smirnov test or Shapiro-Wilk test, and statistical comparison of different groups’ results was performed through a Paired t-test. Besides, correlation studies were performed by using Pearson’s correlation analysis. However, when analyzing micro-tomographic and histomorphometric results, the normality of the variables was evaluated using the Shapiro-Wilk test. Then the equality of variances was checked through an Equal Variance Test. If both tests were passed, the statistical comparison of the samples belonging to both groups was performed by a Student’s t-test. Nevertheless, a Mann-Whitney U test was selected for normal but non-equal variance variables to compare the results statistically. The statistical significance level was set at p<0.05 for all parameters.
4. Discussion
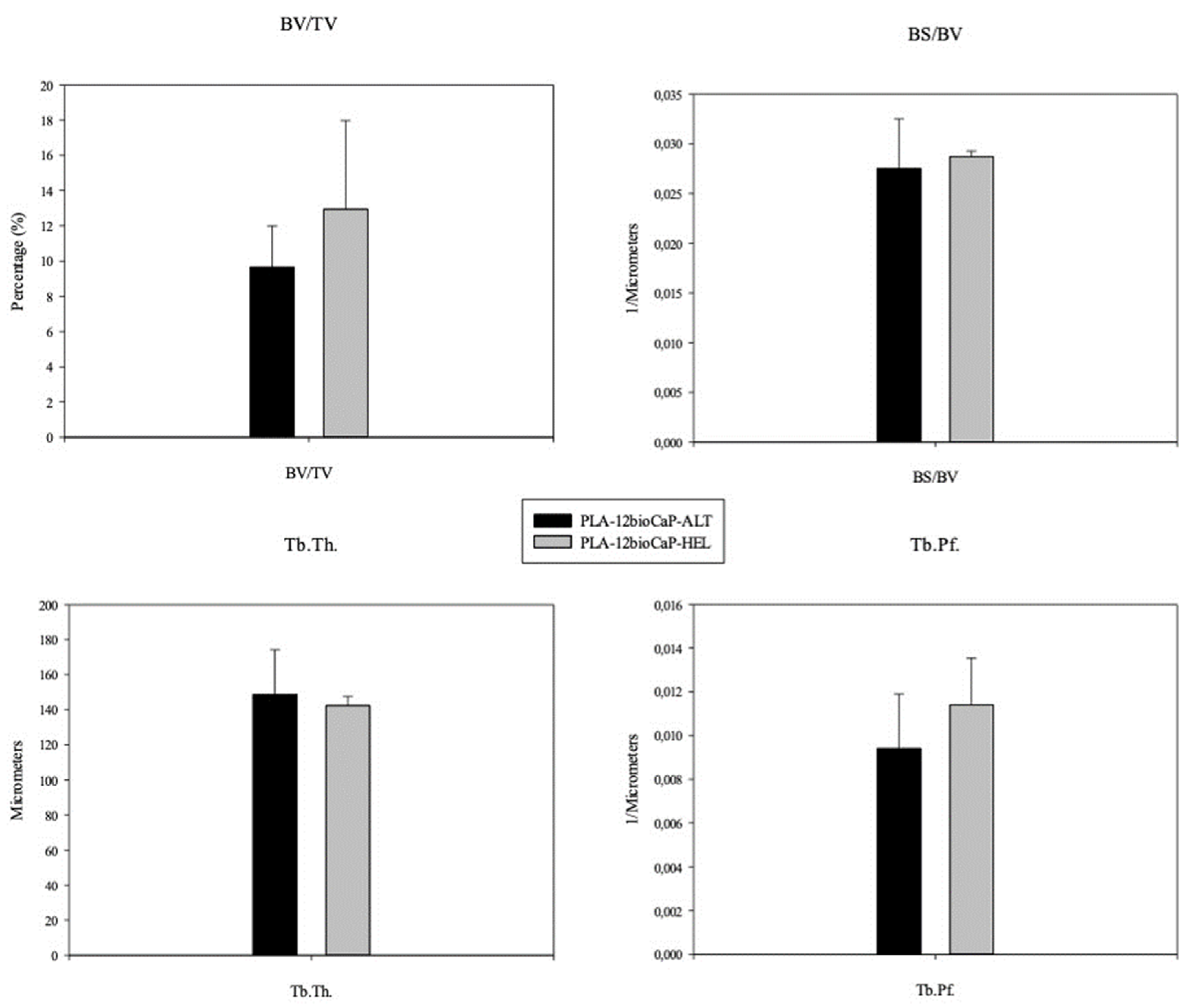
3D-printed scaffolds with different laydown patterns were designed and fabricated in the present manuscript to assess their morphological differences through the scaffold’s characterization and their capacity to promote bone regeneration when implanted in critical-sized bone defects. The results showed that helical structure presented higher values of pore size, porosity, and pore accessibility. However, the negative statistically significant correlation between porosity and compressive strength and how the struts were arranged in the helical scaffolds resulted in lower mechanical properties than the alternate group. Regarding bone regeneration, newly formed Bone obtained results were similar for micro-tomographic and histomorphometric analysis, respectively. The mean values for both groups were 9.65±2.34 % and 9.24±2,64 % when scaffolds with alternate structures were implanted, and 12.94±5.0 % and 13.13±4.70 %, in the case of the scaffolds with helical structure. Nevertheless, statistical significative differences between alternate and helical implanted structures were only found when analyzing the histomorphometric results.
The choice of appropriate materials to regenerate bone defects is crucial since they will determine some of the most important features of the implant, such as biomimetic, osteoconduction, osteoinduction, mechanical properties, biodegradability, hydrophilicity, etc. Hence, most published manuscripts are based on searching for the material or the combination of several that provide optimal conditions for bone regeneration, attending to the already mentioned characteristics [
7,
22,
23,
24,
49]. During the last decades, the tissue engineering field has evolved from particulate materials to more complex structures called scaffolds, which provide mechanical support and promote cell growth and vascularization. Furthermore, custom-designed 3D-printings have elevated these structures to another dimension, allowing complete control over the geometry of the implants [
33,
50,
51]. Thus, in addition to material selection, the scaffolds’ design has been postulated as another key aspect when facing bone tissue engineering, as demonstrated in the present manuscript. Understanding how the architectural properties work gives a better insight into the optimal structural design to improve bone regeneration could be provided [
34]. Currently, methods such as layer-by-layer deposition (Additive Manufacturing) are widely used to design complex porous scaffolds with well-defined architecture and optimized pore interconnectivity [
3].
Shark teeth-derived bioapatites (bioCaP) have been studied as an alternative bioceramic material for bone regeneration, which were obtained as fishing by-products of
Isurus oxyrinchus and
Prionace glauca [
35,
52]. Their biocompatibility has been proved in vitro [
38] and in vivo [
36], demonstrating the osteointegrative, osteoconductive, and osteoinductive properties. This bioceramic, already characterized [
35,
36], is based in a globular porous morphology with a biphasic composition of ~70% apatitic (HA, apatite-CaF, fluorapatite) and ~30% non-apatitic phase (whitlockite, tricalcium bis(orthophosphate)) together with contributions of F (1.0±0.5 wt%), Na (0.9±0.2 wt%) and Mg (0.65±0.04 wt%). The presence of these ions contributes to bone healing and regeneration since F enhances the synthesis of bone cell growth factor, and Mg is involved in synthesizing the parathyroid hormone that regulates bone homeostasis [
52]. This study confirmed the suitability of this biomaterial to be composited with a widely studied polymer, such as PLA [
22,
23], which has already been proven to be an adequate matrix to be reinforced with bioceramic materials to synthesize composites suitable for additive manufacturing techniques. Besides, mixed materials can negate some of each other’s disadvantages, improving their characteristics [
15,
21,
27].
The structural geometry or design of the scaffold is determined by the position and orientation of the fibers, affecting parameters such as pore size, porosity, mechanical properties, and biological performance [
18]. The pore features of the 3D-printed scaffolds play an essential role in cell adhesion, proliferation, and migration [
53]. As mentioned, the scaffold should be a 3D network with highly interconnected pores. Macroporosity promotes cell and ion transport and, consequently, osteogenesis, and microporosity (<10 μm) improves the surface area and roughness, providing attachment points for osteoblast [
54,
55]. Currently, no consensus on the optimal pore size has been achieved, and mainly approaches include regular and irregular pore structures [
32,
53]. Mean pore sizes ranging from 50 to 900 μm have been used in bone tissue engineering. The minimum recommended pore size is 100 μm; however, sizes under 300 µm still limit angiogenesis, resulting in small blood vessel diameter and bone formation due to reduced oxygen and nutrient diffusion. Larger ones provide more space for cell migration, tissue ingrowth, and vascularization, increasing osteoblast proliferation and differentiation throughout the scaffold [
3,
53,
54]. In addition, porosity and connectivity are critical parameters closely related to pore sizes. High porosity and large pores are supposed to enhance bone ingrowth and osseointegration [
3,
56,
57,
58]. Despite some reports showing differences in osteogenic income of implants with different porosities, no beneficial effects of low porosities were reported either. Other features, such as the materials' degradation rate and mechanical properties, should be considered when assessing porosity. Those materials with high degradation rates should not have high porosities (>90%) because higher surface areas interacting with host tissue will accelerate degradation due to macrophages via oxidation and/or hydrolysis. However, those with low degradation rates and robust mechanical properties can be highly porous [
55]. Trabecular Bone is characterized by a highly trabecular foam-like cellular microstructure, with porosity levels ranging from 30 to 95%. Likewise, trabecular Bone surrounded by cortical Bone creates a porous environment with pore sizes of 1 mm and 50-90% porosity [
53,
59].
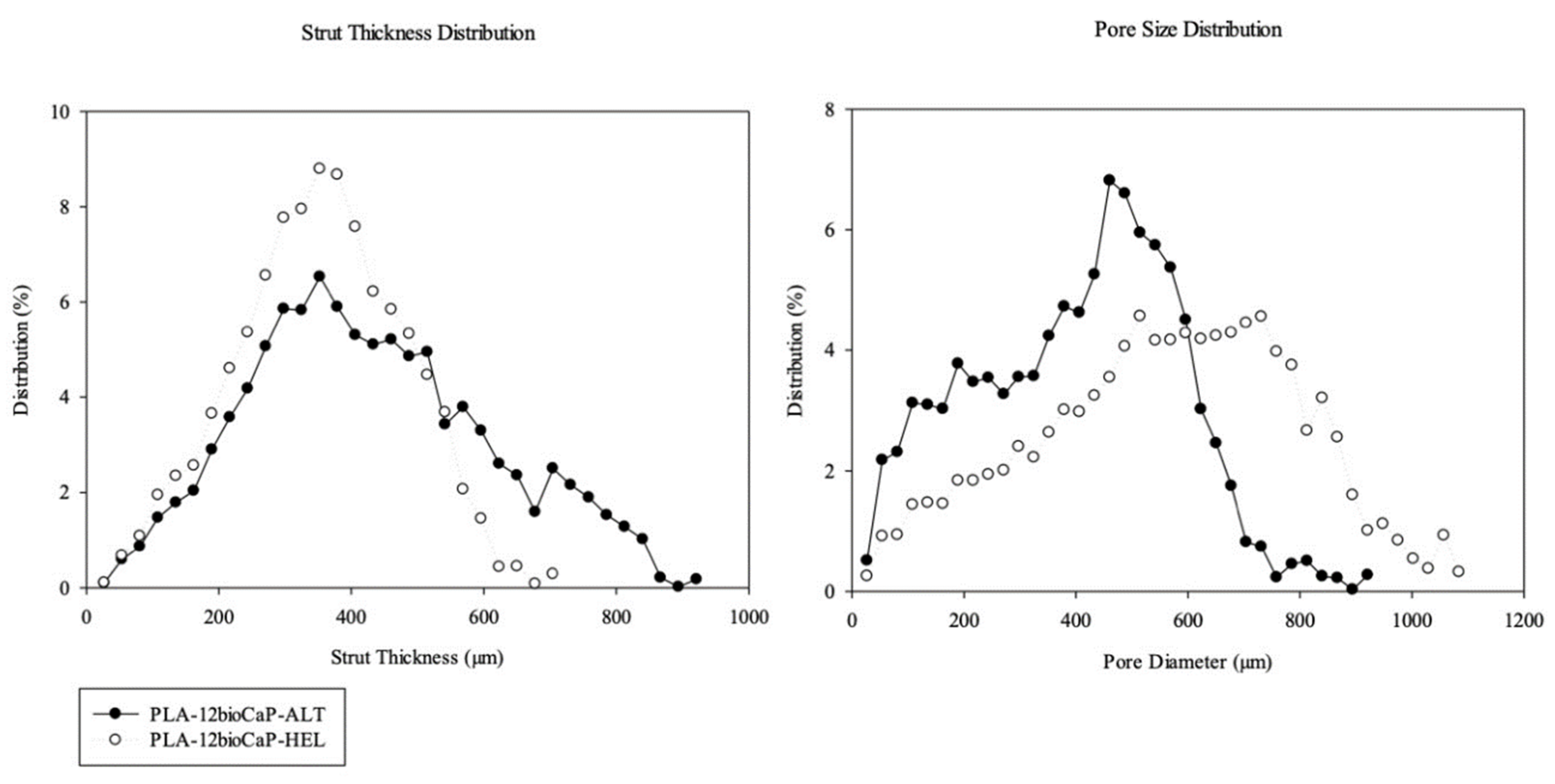
Alternate and helical structures resulted, respectively, in mean pore sizes of 400±20 μm and 560±6 μm, and porosity of 45±6 % and 63±1 %. After being implanted, helical PLA/bioCaP composite scaffolds achieved higher values of newly formed Bone than alternate ones. Greater pore size and large porosity of the helical structure, together with a greater available surface area, could explain the differences in bone regeneration between both groups, according to what was stated above. The effect on tissue formation of different 3D-printed scaffold designs has been extensively studied [
19,
31,
35]. However, no similar studies in vivo with PLA composites were found.
Larger pore sizes do not always mean a higher percentage of bone regeneration, mainly when working with sizes greater than 800 μm. Liu et al. [
53] manufactured macro-pore-sized (800, 1200, and 1600 μm) bioceramic scaffolds with identical porosity of 70%. For this, they used biphasic calcium phosphate (BCP), the combination of HA and β-TCP, and they could isolate the importance of pore sizes in a rabbit calvarial defect model by maintaining a constant porosity. The results showed higher BV values for BCP 800 and 1200 groups than for BCP1600. This was explained because the surface area of porous scaffolds is closely related to bone formation, and the specific surface area of the scaffolds decreases with the increasing pore size.
Nevertheless, in our study, the scaffolds with greater pore sizes also resulted in higher surface area values due to the differences between alternate and helical laydown patterns. To study the effect of pore size and permeability, Entezari et al. [
34] fabricated Strontium doped (Sr-HT-Gahnite) scaffolds with four different architectures, maintaining the same interconnectivity (100 %) and similar porosity (49.3±1.9 %). Architecture A was a conventional square mesh-like pattern; Architecture B was a double-lined pattern of bimodal pore sizes; Architecture C was a displaced double-layer pattern; and Architecture D was a quatrefoil pattern. Micro-tomographic results showed that architectures B and D presented greater volumes of regenerated Bone. Likewise, both presented higher permeability and larger pore sizes compared to the other two architectures. Authors concluded that higher tortuosity induced by displacing the layers (C) limited nutrient transportation and formed pillar-shaped bone constructs to carry bone loads. They also found that pore sizes to enhance bone formation should range between 390 and 590 µm since larger pores did not show any improvements. Those findings agreed with our results.
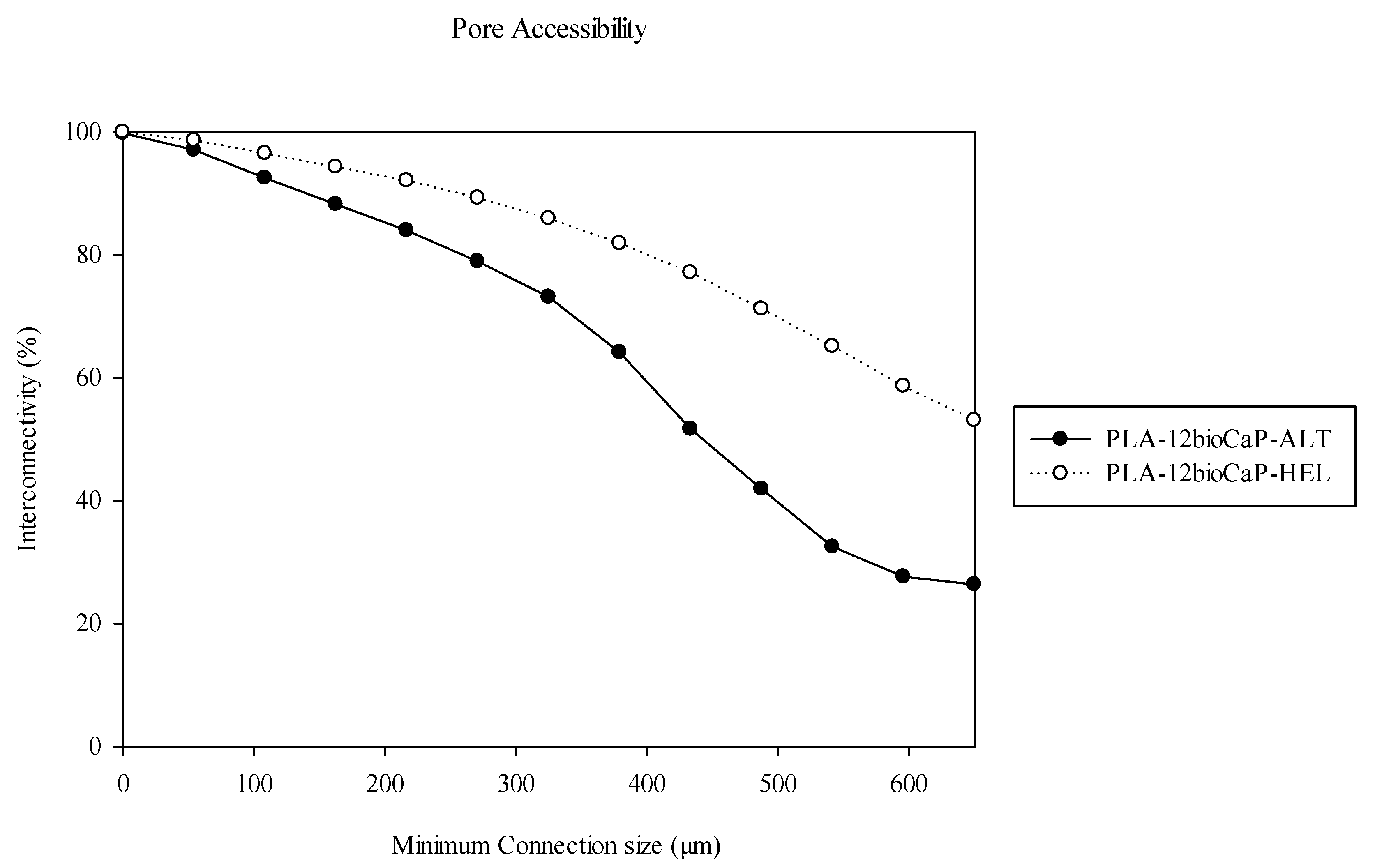
Even though our manuscript did not analyze the permeability, an equivalent parameter, such as the pore accessibility, was measured. It varies as a function of the scaffolds’ porosity and pore size [
34], and it reflects the interconnectivity of the network of pores, giving the smallest pore connection (pore throat) between the scaffold edge and any voxel within the scaffold pore space [
48]. Pore throat size determines whether cells can enter the pore structure smoothly and affects cell proliferation and differentiation functions since more pore accessibility provides the cells with a larger surface for attachment [
32]. In vivo trials confirmed these statements, showing higher values of bone formation and better access of cells to the inner parts of the scaffolds in the helical structures, the ones with superior pore accessibility. However, based on the results, both structures should ensure mass transfer and oxygen perfusion to allow bone regeneration [
18].
Regarding the effect of different laydown patterns, several authors studied their effects in vitro and in vivo. Domingos et al. [
60] designed polycaprolactone (PCL) scaffolds varying the pore size, with filament distances from 550 to 750 µm, and the laydown pattern, changing the deposition angle (0º/90º, 0º/60º/120º and 0º/45º/90º/135º), but maintaining a regular filament distance of 650 µm. Increasing the deposition angle achieves quadrangular, triangular, and complex polygonal internal pore geometries without modifying the scaffolds’ porosity. Then, biological experiments were carried out using hMSCs (human mesenchymal stem cell cultures), and a strong influence of pore size and geometry on cell viability was observed. Briefly, larger pore sizes could accommodate more viable cells, probably related to a large surface area and higher porosity. Regarding the influence of pore shape, a decreasing number of deposition angles resulted in incrementing cell viability, revealing that quadrangular pores (90º/90º) improved cell accessibility and colonization [
60]. Similarly, Kook et al. [
28] manufactured PCL scaffolds with combinations of different laydown patterns (0º/45º or 0º/90º) and pore sizes (150, 250, and 350 µm), and then the optimum scaffold architecture was determined by an MMT assay to measure the proliferation of MC3T3-E1 cells. Highest cell proliferation was observed in scaffolds with 0º/45º strut layout pattern and 150 µm pore size; thus, based on these results, authors selected scaffolds with 45º/150 µm and 45º/350 µm for in vivo trials. Their results were contrary to those obtained by Domingos et al. [
60], who found that the most suitable laydown pattern in vitro was 0º/90º but agreed with those obtained in the present manuscript. However, Kook et al. [
28] found that after in vivo implantation, the scaffolds with 150 µm pore size achieved higher amounts of newly formed bone than those with 350 µm, which is controversial considering the abovementioned results. The absence of a unanimous agreement on optimal pore size and shape can be attributed to the variability in materials employed and the specific sites of implantation [
5]. Additionally, the body of research examining scaffold geometry through in vivo studies is comparatively small. However, variations in pore shape for bone regeneration were studied by Berner et al. [
10] where they used different laydown patterns, and developed silanized polycaprolactone/tricalcium phosphate scaffolds with 0º/90º and 0º/60º/120º fiber orientations and implanted them in rat skull defects. The analysis showed that a higher degree of newly formed Bone was regenerated in 0º/90º scaffold, compared to 0º/60º/120º ones, where bone formation was closer to the host bone with less ingrowth to the center of the scaffold. The authors reported that differences could not only be explained by the variations in higher porosity, large pore size, and lower surface area of the 0º/90º groups but rather that struts’ architecture must also play a main role in bone formation.
In addition, one of the challenges when studying the effects of pore shape is the lack of isolation of architectural parameters. Mirkhalaf et al. [
30] synthesized Baghdadite 3D-printed scaffolds with 5 different structures, maintaining similar values of pore size (500 µm), porosity (50%) and surface-to-volume ratio (10 mm
-1). They were implanted in rabbit cranial defects to assess the effects of the surface convexity (cylindrical struts with convex surfaces vs. concave surfaces), the relative orientation of the scaffold concerning the defect site (scaffolds with cubic pores vs. rotated cubic pores), and interconnectivity (body-centered cubic scaffold). The results showed that only pore interconnectivity significantly affected the scaffold’s bone tissue regeneration capacity, which is in agreement with the data obtained for alternate and helical structures, where the one with greater interconnectivity got better results in bone regeneration trials.
Another important requirement for bone regeneration is the degradability of the implants, which, regardless of the material, is closely related to the porosity, the pore size, and the interconnectivity. In the present manuscript, the degradation of the scaffolds was subjectively more evident in the helical group, with lower values of IS/TS and a lack of connection between the struts in the histological sections. The degradation pattern of porous structures is linked to pore size, so smaller pores result in slower hydrolysis and, thus, low degradation rates. As expected, the same happens with the porosity since higher values result in further permeability and faster degradation. Likewise, it was observed that scaffolds with large pore sizes and lower porosity degraded faster than those with smaller pore sizes and higher porosity due to the effect of higher available surfaces in scaffolds with macropores [
53,
61]. Besides, scaffolds with square pores provided faster degradability and higher weight loss compared with other pore morphologies such as triangular or parallelogram [
62]. In addition to the hydrolytic process, osteoclasts and macrophages are responsible for resorbing or dissolving bone grafts [
12], which could explain the notable presence of macrophages in the PLA/bioCaP scaffolds’ periphery. Furthermore, macrophage colonization, giant cell engulfment, and cytokine secretion play a major role in the degradation of calcium phosphates. Thus, it relates to releasing calcium phosphate ions from biomaterials, which are very important to induce bone formation and maturation. As mentioned above, adequate porosity, pore size, and accessibility are also needed to allow cell adhesion, proliferation, and migration [
53]. By contrast, Domingos et al. [
63] reported after in vitro degradation studies that the degradation rate of PCL scaffolds was notably affected by porosity and pore size. At the same time, 0º/90º and 0º/45º/90º/135º filament orientations resulted in similar degradation rates when porosity and pore size values remained similar.
The scaffold design also has an essential effect on the mechanical properties of the scaffolds since as porosity and mean pore size increase, the mechanical strength is sacrificed. Thus, an adequate balance among these parameters is essential for synthesizing an ideal scaffold for bone tissue engineering [
3]. The present manuscript shows helical structures have demonstrated much lower mechanical properties than alternate structures. Furthermore, this lack of mechanical resistance in helical scaffolds could be the reason for the fracture of two femoral condyles after the scaffold’s implantation in rabbits, even though their compressive strength values were within the range for those stipulated for natural Bone (2-12 MPa) [
64]. Similarly, Domingos et al. [
60] reported that increases in pore size (filament’s distance from 550 µm to 750 µm) and reductions in laydown patterns’ deposition angle or increases in the number of deposition angles (from 0º/90º to 0º/45º/90º/135º) resulted in scaffolds with lower compressive modulus, and therefore in weaker structures. Regarding pore size, the compressive modulus decreases from 52.5±4.5 MPa to 23.1±2.8 MPa, and referring to deposition angle, the measurements showed reductions from 34.2±3.8 MPa to 19.1±2.8 MPa. The authors’ explanation for these results was the larger fused area between struts occasioned by a reduced deposition angle, leading to lower local stress experienced by scaffolds and a greater ease of sliding, increasing the scaffold’s deformability [
60,
65,
66]. Furthermore, high mechanical properties in scaffolds with 0º/90º and 0º/60º/120º laydown patterns are related to the aligned crossover points on every layer. By contrast, 0º/45º/90º/135º orientations produced misaligned crossover points among different layers, and thus the elastic modulus may be lower [
18]. Nevertheless, Liu et al. [
53], who synthesized scaffold using a brittle material such as BCP, observed that an increase in the size of the macropores resulted in similar compressive strength values.
Regarding differences between scaffolds with aligned or staggered fibers, many authors confirmed that staggered filaments had notably lower mechanical properties than aligned filaments, as reviewed by Gleadall et al. [
18]. Specifically, Serra et al. [
67] described that PLA-based composite scaffolds with staggered fibers showed 50-75 % lower elastic modulus than others with aligned fibers. Likewise, regarding the mechanism by which scaffolds collapse, it could be appreciated that a solid column from the top to the bottom of the scaffolds, as it happened with those with 0º/90ª laydown patterns, provides it with a pillar that strongly resists compression. When fibers are staggered, filaments bend slightly, and the structure easily collapses in a concertina manner [
18].
The present manuscript delved deeper into how specific scaffold designs resulted in significant changes in pore size, porosity, interconnectivity, available implant surface, and mechanical properties. But it also demonstrated, through the implantation of the scaffolds in an animal model, the effects of these modifications on bone regeneration, with their subsequent implications in the context of bone tissue engineering. In summary, higher pore sizes, porosity, and interconnectivity provided by the helical design give rise to more suitable structures for bone healing. However, they also present potential trade-offs due to their mechanical properties that could limit their applications in load-bearing sites in the absence of future improvements, which could be related to the redesign of new structures that provide more excellent mechanical resistance while maintaining the porosity and pore accessibility, or to the use of new biomaterials. In relation to this aspect, the 3D printing technique offers the possibility of easily modulating the scaffold properties, customizing it according to the specific final function in the body.
Thus, the study's results confirmed the initial hypothesis since they demonstrated that scaffold architecture could influence bone regeneration capabilities. Critical defects were performed in a rabbit femoral condyle model, which allowed us to confirm the advantages of using scaffolds with 0º/45º/90º/135º/180º (helical) orientations. As described above, laydown patterns have demonstrated great influences on the implant’s characteristics and suitability for bone graft use. Changes deriving from printing paths, which theoretically should trigger a series of biological changes in vivo, favored, or hindered bone ingrowth through different mechanisms. Mainly by facilitating or not facilitating the access of cells and new vessels to the inner part of the scaffolds, but also because of their differences in degradability rate. This study provides novel and interesting information to a field where significant advances are still necessary, mainly in the applications and evaluation “in vivo” of the different designs.
In addition, this report presents several constraints that should be pointed out. First, the limited osteogenic properties of the implants, although this was not one of the study’s main goals, due to already described PLA’s drawbacks and the impossibility in our case of manufacturing scaffolds with bioCaP concentrations higher than 12 %, so that could explain the low obtained values of newly formed Bone. Besides, the lack of degradation analysis prevents us from ensuring different degradability rates among both structures and their implication on scaffolds’ mechanical integrity. Regarding the animal model, the low number of animals used and the exclusion of several samples may limit the scope of the study. In the same way, long-term clinical trials could be interesting to assess the performance of the different scaffold designs over time. Furthermore, the variable distribution and limited amount of trabecular Bone in the rabbit’s femoral condyle, together with the use of long scaffolds (1 cm), failed in the task of maximizing the contact surface between host bone and implant, being in contact in some cases largely with bone marrow. So, the use of other animal models could be addressed for future research.
The publication of controversial results regarding scaffold architecture, as presented in this section, highlighted the importance of performing further investigations on this topic to validate and expand the knowledge. The synthesis and comparison of a wider variety of scaffold laydown patterns could provide more interesting information on physic-chemical characterization and degradation analysis. Besides, the analysis of their osteogenic capabilities in “in vivo” trials is also crucial since “in vitro” findings are not always correlated to “in vivo” ones [
14]. New alternative approaches may also be used to search highly porous and interconnected but, at the same time, resistant structures. An example is bimodal pore topologies, described by Entezari et al. [
35], which allowed the creation of larger pores without increasing porosity or sacrificing mechanical properties but enhancing the volume and functionality of newly formed Bone. Another alternative is the combination of different laydown patterns, such as 0º/90º/180º and 0º/45º/90º/135º/180º orientations, to achieve porous structures that facilitate bone ingrowth without compromising its mechanical resistance. Likewise, using different biomaterials, such as composites made with copolymers and 3D-printing techniques, to fabricate the scaffolds could be interesting since biomaterials’ characteristics influence printing patterns and techniques regarding ductility, printability, and mechanical resistance.
Challenges in the clinical adoption of 3D printed scaffolds include the need for medical-grade materials, high fabrication costs for patient-specific products, clinician training, and scaffold sterilization [
14]. Research and additive manufacturing efforts must focus on these areas to enhance clinical applicability. Adapting techniques for bone applications to weight-bearing sites, like long bone defects, is complex due to different bone environments and implant requirements. Therefore, extensive pre-clinical studies, particularly on larger animals at intended implantation sites, are crucial for clinical relevance.