Submitted:
12 March 2024
Posted:
14 March 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Result and Discussion
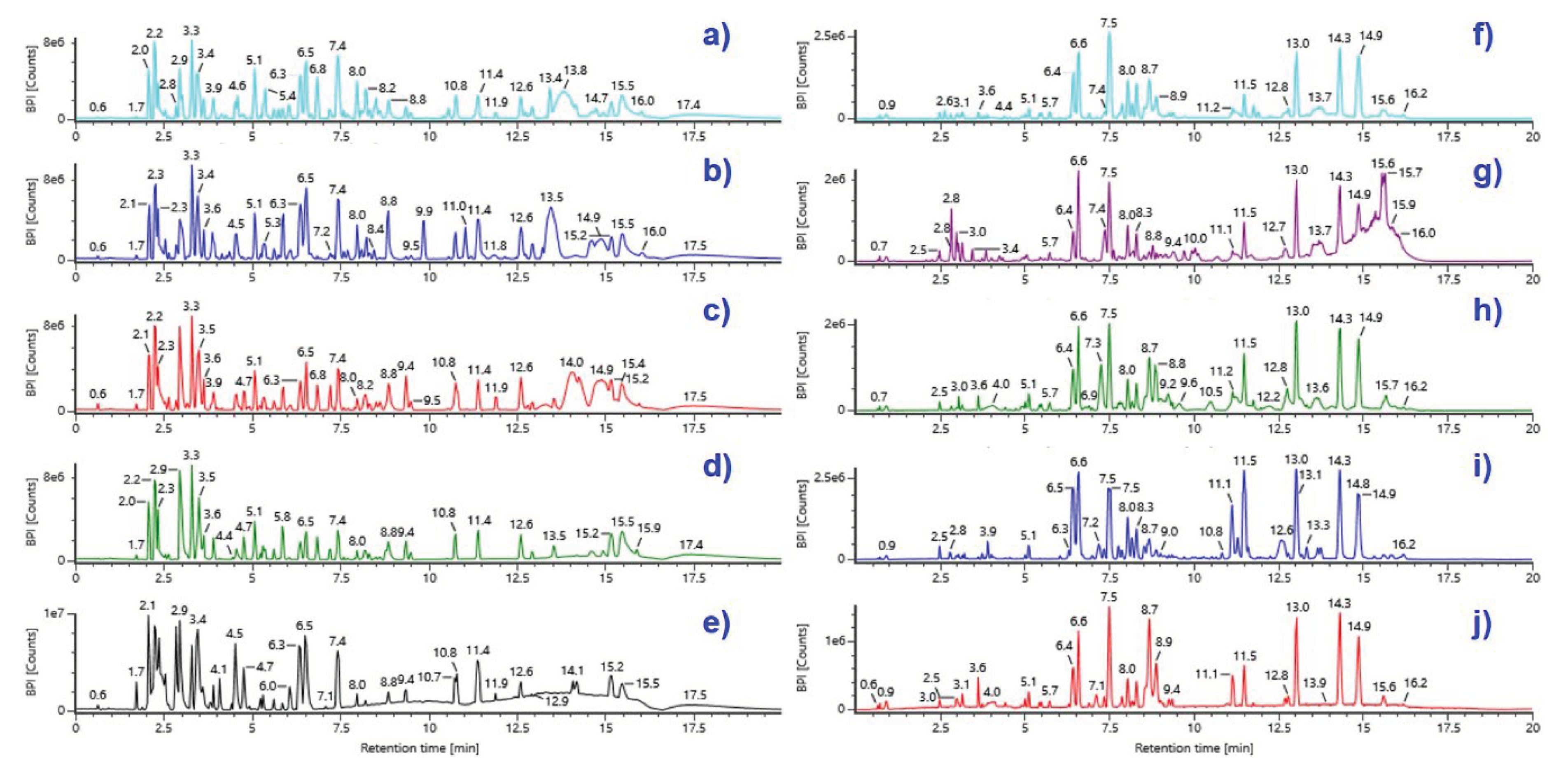
2.1. Optimization of Extraction Yield
2.1. UPLC-Q-TOF-MSE Analysis and Identification of Bioactive Compounds
2.1.1. Identification of Fatty Acid Amides
2.1.1. Identification of Cinnamic Acid Derivatives
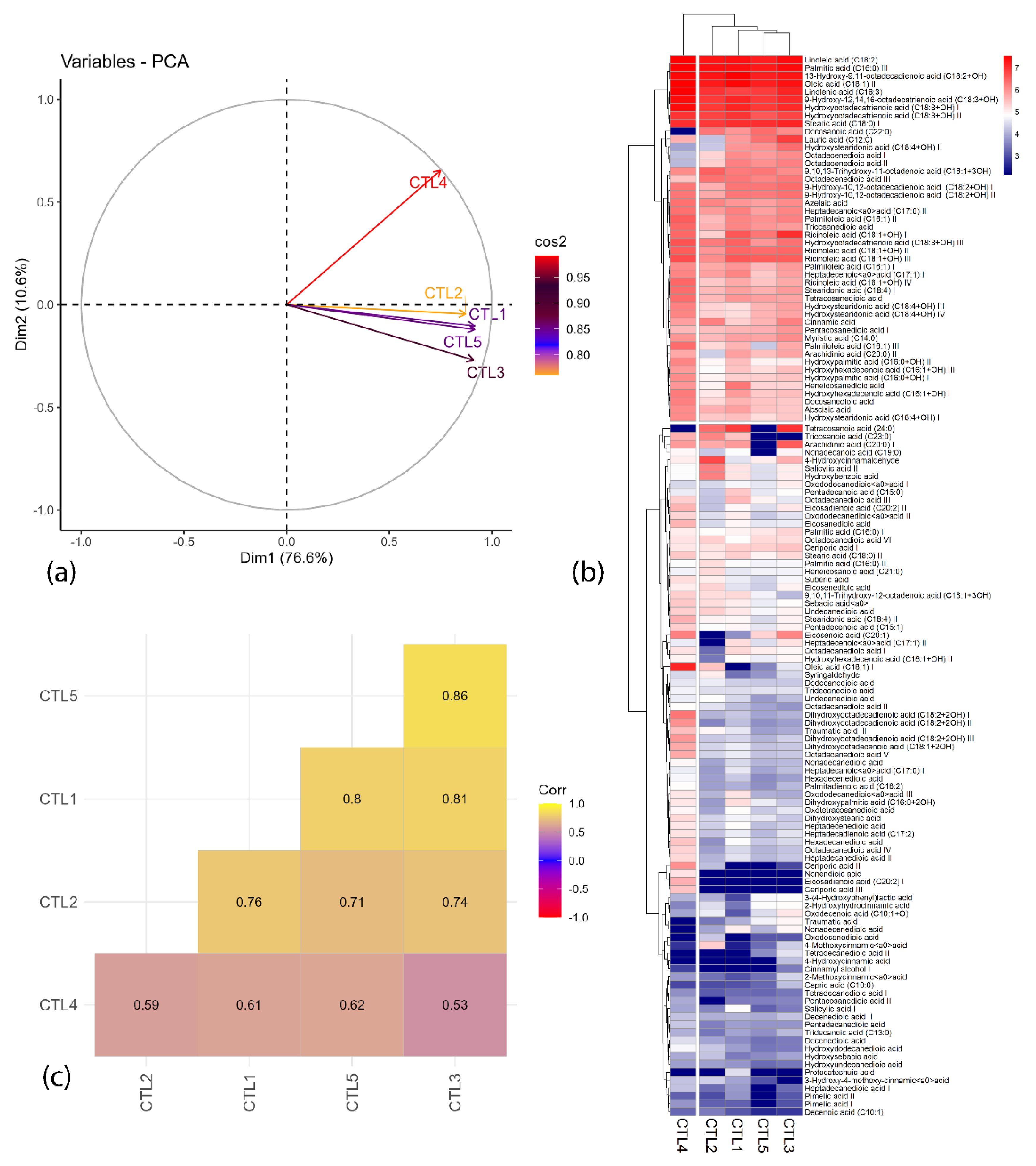
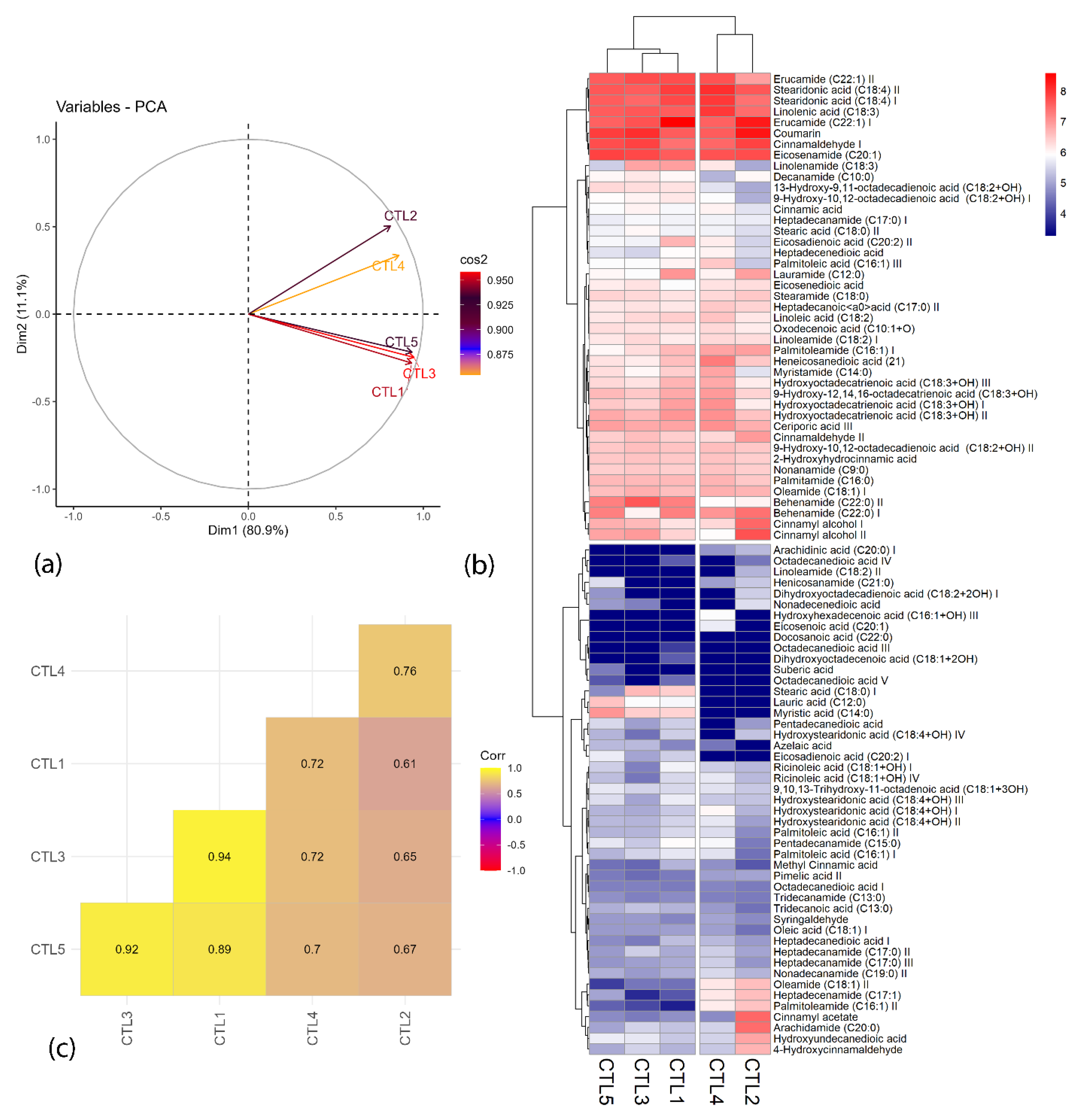
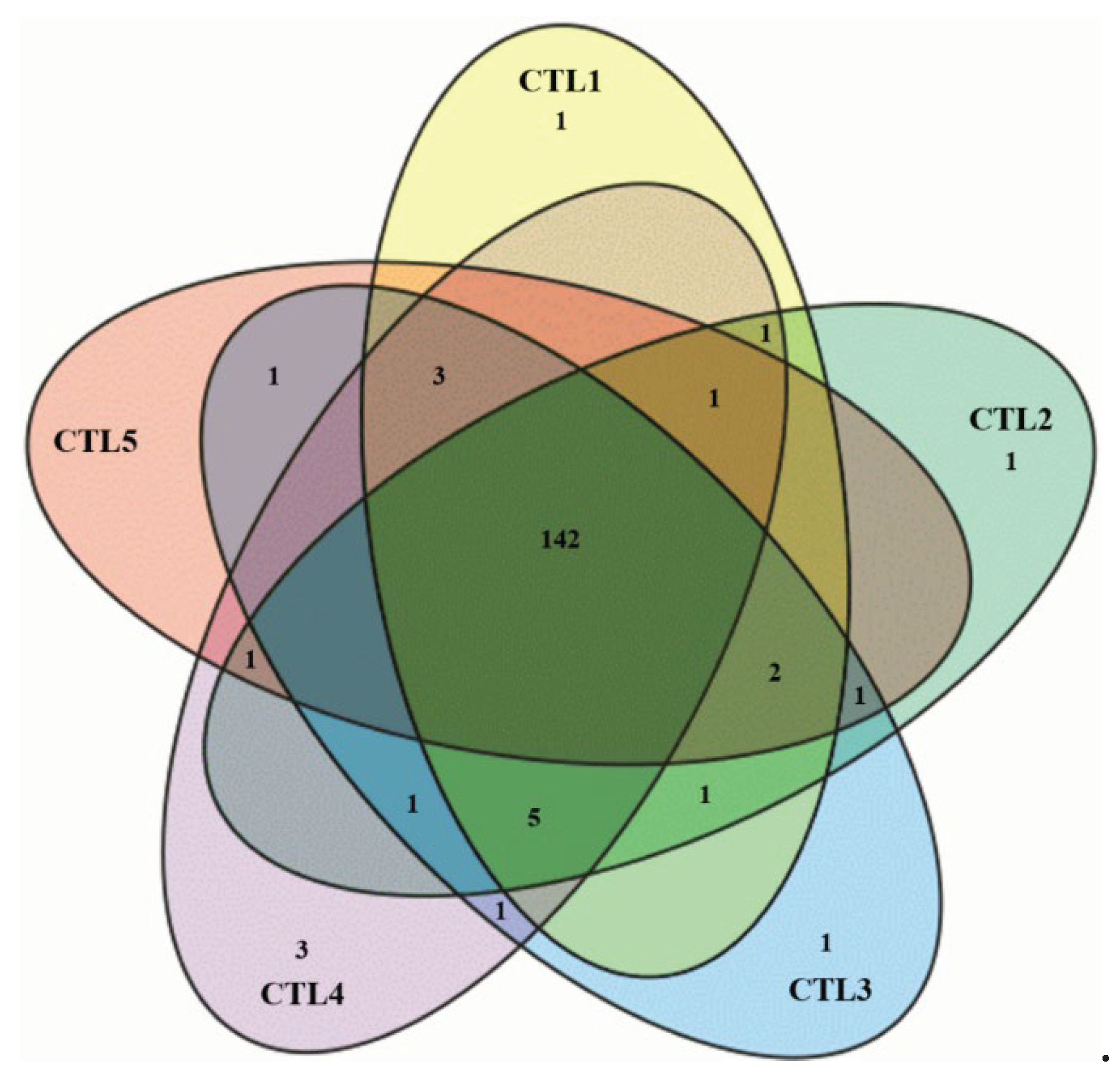
2.1. Chemometric Analysis
3. Experimental
3.1. Chemicals and Materials
3.2. Plant Materials
3.3. Supercritical Fluid (CO2) Extraction and Sample Preparation
3.4. UPLC-Q-TOF-MSE Analysis
3.5. Chemometric Analysis
Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants, Lauraceae, Vol. III, International Book Distributors, Dehradun, India 1935, 2146-2147.
- Sharma, V.; Lingamallu, J.M.R. An overview on chemical composition, bioactivity and processing of leaves of Cinnamomum tamala. Crit. Rev. Food Sci. Nutr. 2014, 54, 433–448. [Google Scholar] [CrossRef]
- Ahmed, A.; Choudhary, M.I.; Farooq, A.; Demirci, B.; Demirci, F.; Başer, K.H.C. ; Essential oil constituents of the spice Cinnamomum tamala (Ham. ) Nees & Eberm. Flavour Fragr. J. 2000, 15, 388–390. [Google Scholar]
- Jain, A.; Dubey, M.; Gupta, A.; Mahajan, S. Antimicrobial activity of Cinnamomum tamala (Tejpat) against some bacterial and fungal pathogens. J. Pharm. Res. 2011, 4, 3975–3977. [Google Scholar]
- Hassan, W.; Kazmi, S.N.Z.; Noreen, H.; Zaman, A.R.A.B. Antimicrobial Activity of Cinnamomum tamala Leaves. J. Nutr. Disord. Ther. 2015, 6. [Google Scholar] [CrossRef]
- Mir, S.R.; Ali, M.; Kapoor, R. Chemical composition of essential oil ofCinnamomum tamala Nees et Eberm. leaves. Flavour Fragr. J. 2004, 19, 112–114. [Google Scholar] [CrossRef]
- Farag, M.A.; Kabbash, E.M.; Mediani, A.; Döll, S.; Esatbeyoglu, T.; Afifi, S.M. Comparative Metabolite Fingerprinting of Four Different Cinnamon Species Analyzed via UPLC–MS and GC–MS and Chemometric Tools. Molecules 2022, 27, 2935. [Google Scholar] [CrossRef]
- Tiwari, S.; Talreja, S. An overview on coronil drug. J. Glob. Trends Pharm. Sci. 2020, 11, 8242–8247. [Google Scholar]
- Upadhyay, R.K. Therapeutic and pharmaceutical potential of Cinnamomum tamala. RRJoPS. 2017, 6, 18–28. [Google Scholar]
- Satyal, P.; Paudel, P.; Poudel, A. Dosoky; N. S.; Pokharel, K.K.; Setzer, W.N. Bioactivities and compositional analyses of Cinnamomum essential oils from Nepal: C. camphora, C. tamala, and C. glaucescens. Nat. Prod. Commun. 2013, 8, 1777–1784. [Google Scholar]
- Soni, R. , Mehta, N. M., Srivastava, D.N. Effect of ethanolic extract of Cinnamomum tamala leaves on wound healing in STZ induced diabetes in rats. Asian J. Pharm. Clin. Res. 2013, 6, 39–42. [Google Scholar]
- Sharma, G.; Nautiyal, A.R. Cinnamomum tamala: a valuable tree from Himalayas. Int. J. Med. Arom. Plants 2011, 1, 1–4. [Google Scholar]
- Mal, D.; Gharde, S.K.; Chatterjee, R. Chemical constituent of Cinnamomum tamala: An important tree spices. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 648–651. [Google Scholar] [CrossRef]
- Gambhire, N.M.; Juvekar, A.R.; Wankhede, S.S. Anti-inflammatory activity of aqueous extract of Cinnamomum tamala leaves by in vivo and in vitro methods. J. Pharm. Res. 2009, 2, 1521–1524. [Google Scholar]
- Sharma, G.; Nautiyal, A.R. Influence of explants type and plant growth regulators on in vitro multiple shoots regeneration of a Laurel from Himalaya. Nat. Sci. 2009, 7, 1–7. [Google Scholar]
- Samant, S.S.; Dhar, U.; Palni, L.M.S. Himalayan Medicinal Plants: Potential and Prospects, Gyanodaya Prakashan, Nainital, India 2001.
- Brown, T.J.; Brainard, J.; Song, F.; Wang, X.; Abdelhamid, A.; Hooper, L. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: systematic review and meta-analysis of randomised controlled trials. BMJ 2019, 366, l4697. [Google Scholar] [CrossRef] [PubMed]
- Boutros, C.; Somasundar, P.; Razzak, A.; Helton, S.; Espat, N.J. Omega-3 fatty acids: Investigations from cytokine regulation to pancreatic cancer gene suppression. Arch. Surg. 2010, 145, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Guil-Guerrero, J.; Isasa, M.T.; Delgado, A.D.; González, M.M. Fatty Acids and Carotenes in Some Ber (Ziziphus jujuba Mill) Varieties. Plant Foods Hum. Nutr. 2004, 59, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Sears, B.; Perry, M. The role of fatty acids in insulin resistance. Lipids Heal. Dis. 2015, 14, 1–9. [Google Scholar] [CrossRef]
- Mizunoya, W.; Haramizu, S.; Shibakusa, T.; Okabe, Y.; Fushiki, T. Dietary conjugated linoleic acid increases endurance capacity and fat oxidation in mice during exercise. Lipids 2005, 40, 265–71. [Google Scholar] [CrossRef]
- Savych, A.; Basaraba, R.; Muzyka, N.; Ilashchuk, P. Analysis of fatty acid composition content in the plant components of antidiabetic herbal mixture by GC-MS. Pharmacia 2021, 68, 433–439. [Google Scholar] [CrossRef]
- Murkar, A.; De Koninck, J.; Merali, Z. Cannabinoids: Revealing their complexity and role in central networks of fear and anxiety. Neurosci. Biobehav. Rev. 2021, 131, 30–46. [Google Scholar] [CrossRef]
- Hermes, D.J.; Xu, C.; Poklis, J.L.; Niphakis, M.J.; Cravatt, B.F.; Mackie, K.; Lichtman, A.H.; Ignatowska-Jankowska, B.M.; Fitting, S. Neuroprotective effects of fatty acid amide hydrolase catabolic enzyme inhibition in a HIV-1 Tat model of neuroAIDS. Neuropharmacology 2018, 141, 55–65. [Google Scholar] [CrossRef]
- Li, Z.; Dong, F.; Sun, Y.; Sun, Z.; Song, X.; Dong, Y.; Huang, X.; Zhong, J.; Zhang, R.; Wang, M.; et al. Qualitative and Quantitative Analysis of Six Fatty Acid Amides in 11 Edible Vegetable Oils Using Liquid Chromatography–Mass Spectrometry. Front. Nutr. 2022, 9, 857858. [Google Scholar] [CrossRef] [PubMed]
- Yerlikaya, S.; Baloglu, M.C.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Zengin, G. Investigation of chemical profile, biological properties of Lotus corniculatus L. extracts and their apoptotic-autophagic effects on breast cancer cells. J. Pharm. Biomed. Anal. 2019, 174, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Díaz, C.; Juárez-Oropeza, M.A.; Mascher, D.; Pavón, N.; Regla, I.; Paredes-Carbajal, M.C. Effects of Oleamide on the Vasomotor Responses in the Rat. Cannabis Cannabinoid Res. 2020, 5, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, H.B.; Leishman, E. Levels of bioactive lipids in cooking oils: olive oil is the richest source of oleoyl serine. J. Basic Clin. Physiol. Pharmacol. 2015, 27, 247–252. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; Ferracane, R.; Vitaglione, P. Food database of N-acyl-phosphatidylethanolamines, N-acylethanolamines and endocannabinoids and daily intake from a Western, a Mediterranean and a vegetarian diet. Food Chem. 2019, 300, 125218. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Wang, D. Chemical constituents of olive oil and from Camellia oleifera seed oil. J. Chin. Cereals Oils Assoc. 2008, 23, 121–123. [Google Scholar]
- Yang, J.Y.; Wu, C.F. Progress in the study of endogenous fatty acid amides. Chin. Pharmacol. Bull. 2000, 16, 1–3. [Google Scholar]
- Berg, B.; Lund, H.; Kringstad, A.; Kvernheim, A. Routine analysis of hydrocarbons, PCB and PAH in marine sediments using supercritical CO2 extraction. Chemosphere 1999, 38, 587–599. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Miller, D.J.; Krieger, M.S. Rapid and quantitative extraction and analysis of trace organics using directly coupled SFE-GC. J. High Resolut. Chromatogr. 1989, 12, 714–720. [Google Scholar] [CrossRef]
- Hartonen, K.; Bøwadt, S.; Dybdahl, H.P.; Nylund, K.; Sporring, S.; Lund, H.; Oreld, F. Nordic laboratory intercomparison of supercritical fluid extraction for the determination of total petroleum hydrocarbon, polychlorinated biphenyls and polycyclic aromatic hydrocarbons in soil. J. Chromatogr. A 2002, 958, 239–248. [Google Scholar] [CrossRef]
- Manilla, M.; Koistinen, J.; Vartiainen, T. Comparison of SFE with Soxhlet in the analysis of PCDD/PCDFs and PCBs in sediment. J. Environ. Monit. 2002, 4, 1047–1053. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef]
- Toishimanov, M.; Nurgaliyeva, M.; Serikbayeva, A.; Suleimenova, Z.; Myrzabek, K.; Shokan, A.; Myrzabayeva, N. Comparative Analysis and Determination of the Fatty Acid Composition of Kazakhstan’s Commercial Vegetable Oils by GC-FID. Appl. Sci. 2023, 13, 7910. [Google Scholar] [CrossRef]
- Bromke, M.A.; Hochmuth, A.; Tohge, T.; Fernie, A.R.; Giavalisco, P.; Burgos, A.; Willmitzer, L.; Brotman, Y. Liquid chromatography high-resolution mass spectrometry for fatty acid profiling. Plant J. 2015, 81, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Serafim, V.; Tiugan, D.-A.; Andreescu, N.; Mihailescu, A.; Paul, C.; Velea, I.; Puiu, M.; Niculescu, M.D. Development and Validation of a LC–MS/MS-Based Assay for Quantification of Free and Total Omega 3 and 6 Fatty Acids from Human Plasma. Molecules 2019, 24, 360. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bajpai, V.; Kumar, S.; Rawat, A.K.S. , Kumar, B. Analysis of isoquinoline alkaloids from Mahonia leschenaultia and Mahonia napaulensis roots using UHPLC-Orbitrap-MSn and UHPLC-QqQLIT-MS/MS. J. Pharm. Anal. 2017, 7, 77–86. [Google Scholar]
- Kumar, S.; Singh, A.; Kumar, B. Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal. 2017, 7, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, A.; Bajpai, V.; Srivastava, M.; Singh, B.P.; Kumar, B. Structural characterization of monoterpene indole alkaloids in ethanolic extracts of Rauwolfia species by liquid chromatography with quadrupole time-of-flight mass spectrometry. J. Pharm. Anal. 2016, 6, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Cui, Q.; Bai, G. Combining UPLC/Q-TOF-MS/MS with biological evaluation for NF-κB inhibitors in Uyghur medicine Althaea rosea flowers. Front. Plant Sci. 2019, 9, 19751984. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; An, Z.; Shi, C.; Li, P.; Liu, L. A sensitive and efficient method for simultaneous profiling of bile acids and fatty acids by UPLC-MS/MS. J. Pharm. Biomed. Anal. 2020, 178, 112815. [Google Scholar] [CrossRef] [PubMed]
- Divito, E.B.; Davic, A.P.; Johnson, M.E.; Cascio, M. Electrospray Ionization and Collision Induced Dissociation Mass Spectrometry of Primary Fatty Acid Amides. Anal. Chem. 2012, 84, 2388–2394. [Google Scholar] [CrossRef] [PubMed]
- Nichols, K.K.; Ham, B.M.; Nichols, J.J.; Ziegler, C.; Green-Church, K.B. Identification of Fatty Acids and Fatty Acid Amides in Human Meibomian Gland Secretions. Investig. Opthalmology Vis. Sci. 2007, 48, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef]
- Huang, J.-K.; Jan, C.-R. Linoleamide, a brain lipid that induces sleep, increases cytosolic Ca2+ levels in MDCK renal tubular cells. Life Sci. 2001, 68, 997–1004. [Google Scholar] [CrossRef]
- Sova, M.; Saso, L. Natural Sources, Pharmacokinetics, Biological Activities and Health Benefits of Hydroxycinnamic Acids and Their Metabolites. Nutrients 2020, 12, 2190. [Google Scholar] [CrossRef]
- Šojić, B.; Putnik, P.; Danilović, B.; Teslić, N.; Kovačević, D.B.; Pavlić, B. Lipid Extracts Obtained by Supercritical Fluid Extraction and Their Application in Meat Products. Antioxidants 2022, 11, 716. [Google Scholar] [CrossRef]
| No. | RT (min) |
Compound | Chemical Class |
Molecular Ion | Observed Mass (m/z) | Error (ppm) | MS/MS Fragments | SC-CO2 Extracts | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTL1 | CTL2 | CTL3 | CTL4 | CTL5 | ||||||||
| 1 | 1.62 | Protocatechuic acid | PC | [M-H]- | 153.0204 | -0.7 | 109.0297 | + | − | − | − | − |
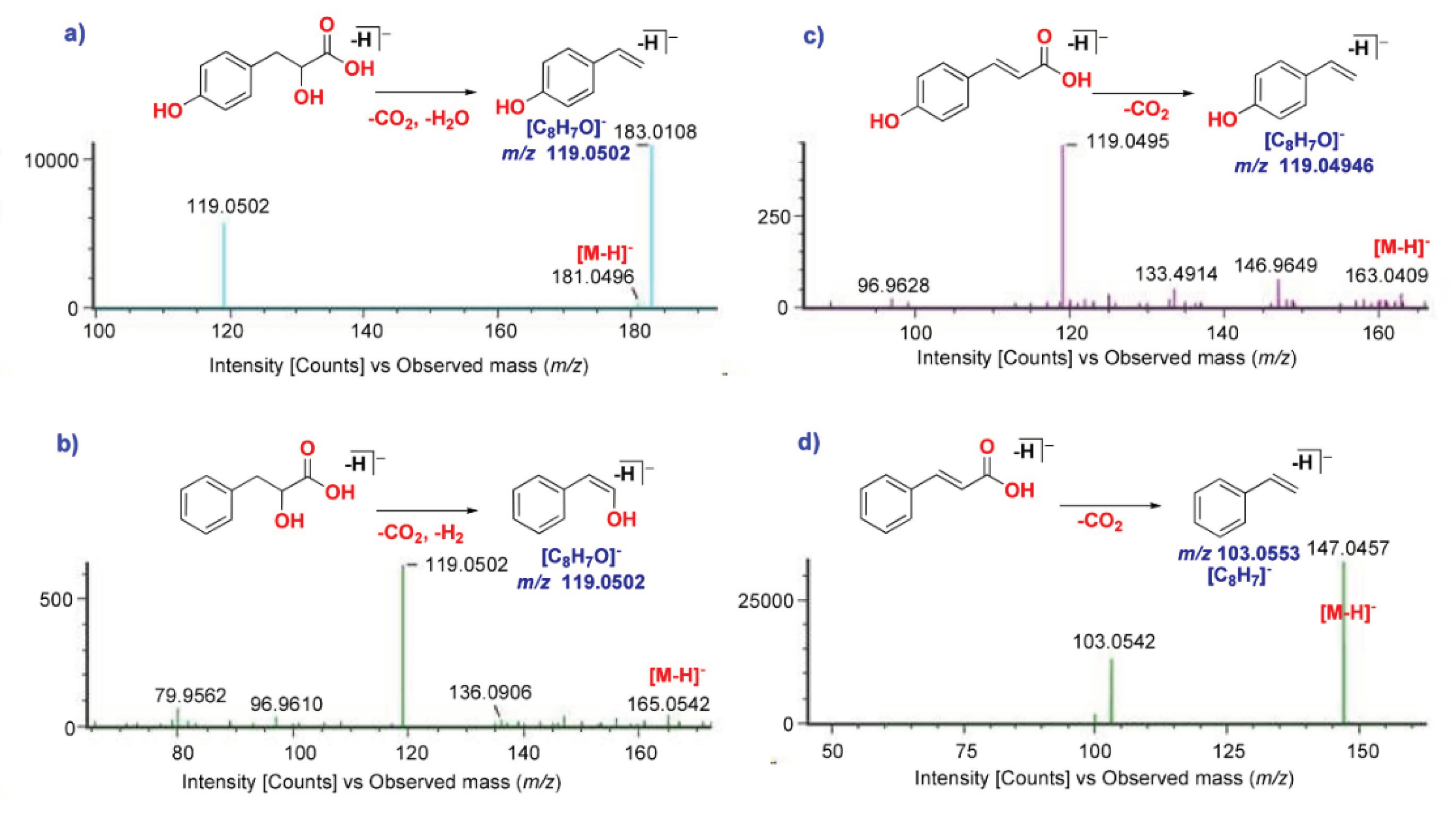
| 2 | 1.67 | 3-(4-Hydroxyphenyl)lactic acid | PC | [M-H]- | 181.0502 | 2.4 | 119.0502 | + | + | + | + | + |
| 3 | 1.86 | Oxodecanedioic acid | DFA | [M-H]- | 215.0928 | -1.4 | 197.0786, 171.1076, 155.0751, 153.0952 |
− | + | + | − | + |
| 4 | 1.88 | Heptanedioic acid (Pimelic acid I) |
DFA | [M-H]- | 159.0667 | -2.5 | 141.0542, 115.0772, 97.0673 |
+ | + | + | + | − |
| 5 | 1.90 | Salicylic acid | OC | [M-H]- | 137.0244 | 0.0 | 93.0348 | + | + | + | + | + |
| 6 | 2.16 | Heptanedioic acid (Pimelic acid II) |
DFA | [M-H]- | 159.0665 | -1.3 | 141.0542, 115.0772, 97.0673 |
+ | + | + | + | − |
| 7 | 2.18 | Octanedioic acid (Suberic acid) |
DFA | [M-H]- | 173.082 | -0.8 | 155.0687, 129.0986, 111.0816 |
+ | + | + | + | + |
| 8 | 2.21 | 2-Hydroxyhydrocinnamic acid | PC | [M-H]- | 165.0559 | -1.3 | 147.0906, 119.0502 | + | + | + | + | + |
| 9 | 2.22 | Hydroxysebacic acid | DFA | [M-H]- | 217.1095 | -6.5 | 199.0984, 173.1111, 171.1049, 155.1108 |
+ | + | + | + | + |
| 10 | 2.32 | 3-Hydroxy-4-methoxy-cinnamic acid | PC | [M-H]- | 193.0517 | -5.7 | 193.0517 | + | + | − | + | + |
| 11 | 2.33 | Hydroxyundecanedioic acid | DFA | [M-H]- | 231.1241 | -1.3 | 213.1229, 195.0973, 187.1238, 169.1233 |
+ | + | + | + | + |
| 12 | 2.40 | Syringaldehyde | PC | [M+H]+ | 183.0653 | -0.1 | 155.073, 140.050, 123.047, 105.0452, 95.053, 77.041 | + | + | + | + | + |
| 13 | 2.41 | Oxododecanedioic acid I | DFA | [M-H]- | 243.1215 | 5.4 | 225.1170, 207.1074, 199.1328, 181.1243 |
+ | + | + | + | + |
| 14 | 2.45 | Decenedioic acid I | DFA | [M-H]- | 199.0983 | -3.5 | 181.0865, 155.1055, 137.0939 |
+ | + | + | + | + |
| 15 | 2.47 | Nonanedioic acid (Azelaic acid) |
DFA | [M-H] | 187.0982 | -3.2 | 169.0861, 143.1065, 125.0966 |
+ | + | + | + | + |
| 16 | 2.50 | Oxododecanedioic acid II | DFA | [M-H]- | 243.1215 | 5.4 | 225.1170, 207.1074, 199.1328, 181.1243 |
+ | + | + | + | + |
| 17 | 2.60 | Oxododecanedioic acid III | DFA | [M-H]- | 243.1214 | 5.8 | 225.1170, 207.1074, 199.1328, 181.1243 |
+ | + | + | + | + |
| 18 | 2.65 | Dodecenedioic acid I | DFA | [M-H]- | 227.1301 | -5.3 | 209.1197, 183.1368, 165.1287 |
+ | + | + | + | + |
| 19 | 2.65 | Decenedioic acid II | DFA | [M-H]- | 199.0983 | -3.5 | 181.0865, 155.1055, 137.0939 |
+ | + | + | + | + |
| 20 | 2.65 | Hydroxydodecanedioic acid | DFA | [M-H]- | 245.1406 | -4.9 | 227.1334, 209.1108, 201.1317 |
+ | + | + | + | + |
| 21 | 2.75 | Sebacic acid | DFA | [M-H]- | 201.113 | 1.2 | 183.1021, 157.1214, 139.1119 |
+ | + | + | + | + |
| 22 | 2.77 | 4-Hydroxycinnamic acid | PC | [M-H]- | 163.0409 | -5.0 | 119.0495 | − | − | + | − | − |
| 23 | 2.78 | 4-Methoxycinnamic acid | OC | [M-H]- | 177.0556 | 0.6 | 133.0653, 117.0340, 103.0577, 92.0285 |
+ | + | + | + | + |
| 24 | 2.79 | Nonendioic acid | DFA | [M-H]- | 185.0815 | 2.2 | 167.0762, 141.0953, 123.0865 |
− | − | − | + | − |
| 25 | 2.79 | Salicylic acid | OC | [M-H]- | 137.0243 | 0.7 | 119.0515, 93.0348 | + | + | + | + | + |
| 26 | 2.82 | Abscisic acid | OC | [M-H]- | 263.1296 | -2.7 | 219.1398, 204.1162, 203.1083, 153.0899 |
+ | + | + | + | + |
| 27 | 2.82 | p-Hydroxybenzoic acid | PC | [M-H]- | 137.0249 | -3.1 | 93.0348 | + | + | + | + | + |
| 28 | 2.86 | 4-Hydroxycinnamaldehyde | PC | [M-H]- | 147.0457 | -3.9 | 119.0481, 117.0331 | + | + | + | + | + |
| 29 | 2.92 | Undecanedioic acid | DFA | [M-H]- | 213.1128 | 1.9 | 195.1116, 169.1233, 151.1254 |
+ | + | + | + | + |
| 30 | 2.93 | Decenoic acid | MFA | [M-H]- | 169.1233 | 0.6 | 169.1234, 151.1153, 125.1298 |
+ | + | + | + | + |
| 31 | 2.94 | Coumarin | OC | [M+H]+ | 147.0446 | 0.9 | 118.0454, 103.0603, 91.0597, 77.04313 |
+ | + | + | + | + |
| 32 | 2.95 | Oxodecenoic acid | MFA | [M-H]- | 183.1028 | -1.5 | 183.1027, 147.0874, 139.1129 |
+ | + | + | + | + |
| 33 | 3.04 | Decenedioic acid | DFA | [M-H]- | 215.1292 | -1.4 | 197.1188, 171.1410, 153.1279 |
+ | + | + | + | + |
| 34 | 3.06 | Cinnamic acid | OC | [M-H]- | 147.0457 | 0.5 | 103.0542, 77.0392 | + | + | + | + | + |
| 35 | 3.07 | Dodecanedioic acid II | DFA | [M-H]- | 227.1301 | -5.3 | 209.1197, 183.1368, 165.1287 |
+ | + | + | + | + |
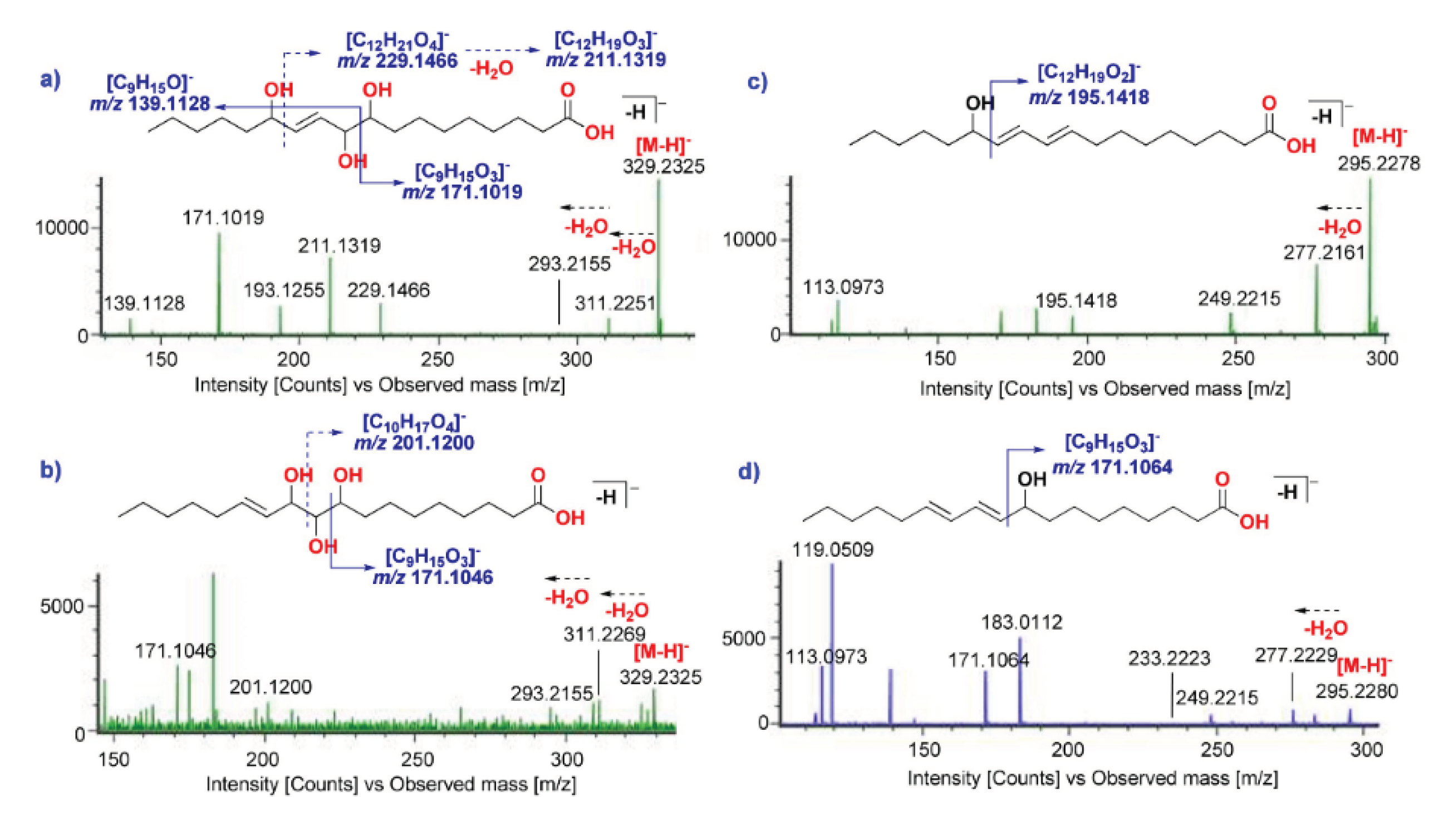
| 36 | 3.15 | 9,10,13-Trihydroxy-11-octadenoic acid | MFA | [M-H]- | 329.2325 | 2.4 | 311.2251, 293.2155, 229.1466, 211.1319, 209.1196, 193.1255, 171.1019, 139.1128 |
+ | + | + | + | + |
| 37 | 3.20 | 2-Methoxycinnamic acid | OC | [M-H]- | 177.056 | -1.5 | 133.0653, 117.0340, 103.0577, 92.0285 |
+ | + | + | + | + |
| 38 | 3.21 | Cinnamyl alcohol | OC | [M+H-H2O]+ | 117.0695 | 0.5 | 115.0555, 91.0559, 77.0384 |
− | − | + | + | − |
| 39 | 3.33 | Dihydroxyhexadecanoic acid | MFA | [M-H]- | 287.2232 | -1.4 | 287.2232, 269.2183, 241.2277 |
+ | + | + | + | + |
| 40 | 3.39 | Dodecanedioic acid | DFA | [M-H]- | 229.1439 | 2.9 | 211.1342, 167.1434 | + | + | + | + | + |
| 41 | 3.48 | Cinnamaldehyde I | OC | [M+H]+ | 133.0648 | 0.9 | 115.0601, 105.0752, 103.0603, 91.0597, 89.0436, 79.0593 |
+ | + | + | + | + |
| 42 | 3.60 | 9,10,11-Trihydroxy-12-octadenoic acid | MFA | [M-H]- | 329.2325 | 2.4 | 311.2269, 293.2155, 201.1200, 171.1046 |
+ | + | + | + | + |
| 43 | 3.72 | Octadecanedioic acid I | DFA | [M-H]- | 313.2375 | 3.2 | 295.2280, 269.2425, 251.2289 |
+ | + | + | + | + |
| 44 | 3.87 | Tridecanedioic acid | DFA | [M-H]- | 243.1601 | 0.4 | 225.1506, 199.1763, 181.1609 |
+ | + | + | + | + |
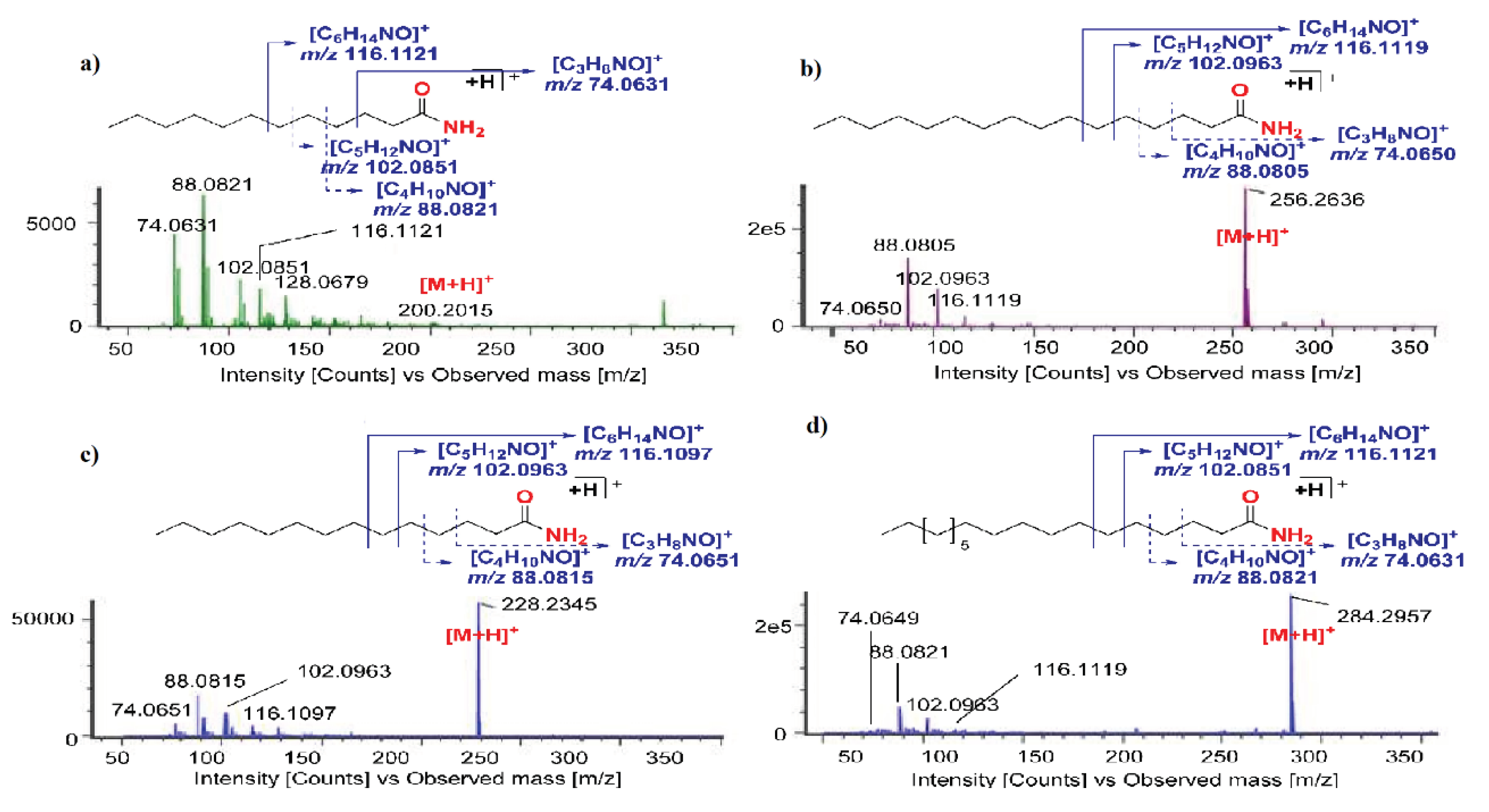
| 45 | 4.00 | Nonanamide | FAA | [M+H]+ | 158.1559 | 1.3 | 116.1119, 102.0963, 77.0431, 69.0753 |
+ | + | + | + | + |
| 46 | 4.00 | Methylcinnamic acid | OC | [M+H]+ | 163.0757 | -1.1 | 105.0356, 103.0569, 91.0519, 77.0379 |
+ | + | + | + | + |
| 47 | 4.23 | Cinnamyl acetate | OC | [M+H]+ | 177.0913 | -1.2 | 105.0356, 103.0569, 91.0519, 77.0379 |
+ | + | + | + | + |
| 48 | 4.41 | Decanamide | FAA | [M+H]+ | 172.1706 | -5.8 | 128.0678, 116.1181, 115.0579, 105.0731, 91.0597, 69.0751 |
+ | + | + | + | + |
| 49 | 4.49 | Tetradecanedioic acid I | DFA | [M-H]- | 257.1758 | 0.1 | 239.1580, 213.1841, 195.1700 |
+ | + | + | + | + |
| 50 | 4.75 | Cinnamyl alcohol II | OC | [M+H-H2O]+ | 117.0695 | 0.6 | 115.0555, 91.0559, 77.0384 |
+ | + | + | + | + |
| 51 | 4.84 | Hexadecanedioic acid | DFA | [M-H]- | 283.1912 | 1.1 | 265.1766, 221.1924 | + | + | + | + | + |
| 52 | 4.94 | Octadecanedioic acid II | DFA | [M-H]- | 313.2375 | 3.2 | 295.2280, 269.2425, 251.2289 |
+ | + | + | + | + |
| 53 | 5.08 | Cinnamaldehyde II | OC | [M+H]+ | 133.0649 | 0.7 | 115.0579, 105.0752, 103.0582, 77.0431 |
+ | + | + | + | + |
| 54 | 5.26 | Pentadecanedioic acid | DFA | [M-H]- | 271.1915 | 0.0 | 253.1779, 227.2038, 209.1932 |
+ | + | + | + | + |
| 55 | 5.40 | Octadecanedioic acid I | DFA | [M-H]- | 311.2224 | 1.3 | 293.2123, 267.2316, 249.2220 |
+ | + | + | + | + |
| 56 | 5.46 | Octadecanedioic acid III | DFA | [M-H]- | 313.2375 | 3.2 | 295.2280, 269.2425, 251.2289 |
+ | + | + | + | + |
| 57 | 5.50 | Octadecanedioic acid II | DFA | [M-H]- | 311.2224 | 1.3 | 293.2123, 267.2316, 249.2220 |
+ | + | + | + | + |
| 58 | 5.53 | Heptadecanedioic acid | DFA | [M-H]- | 297.2067 | 1.4 | 279.1973, 253.2210, 235.2145 |
+ | + | + | + | + |
| 59 | 5.70 | Octadecanedioic acid III | DFA | [M-H]- | 311.2224 | 1.3 | 293.2123, 267.2316, 249.2220 |
+ | + | + | + | + |
| 60 | 5.98 | Dihydroxystearic acid | MFA | [M-H]- | 315.2544 | -1.0 | 315.2544, 297.2490 | + | + | + | + | + |
| 61 | 6.03 | Hydroxystearidonic acid I | MFA | [M-H]- | 291.1964 | 0.7 | 273.1883, 255.2316, 245.1916 |
+ | + | + | + | + |
| 62 | 6.18 | Hexadecanedioic acid | DFA | [M-H]- | 285.2072 | -0.35 | 267.1978, 241.2069, 223.2130 |
+ | + | + | + | + |
| 63 | 6.32 | Decanoic acid (Capric acid) | MFA | [M-H]- | 171.1392 | -1.1 | 171.1396 | + | + | + | + | + |
| 64 | 6.40 | Stearidonic acid I | MFA | [M-H]- | 275.2027 | -3.6 | 275.2027, 257.1952, 231.2127, 229.1872 |
+ | + | + | + | + |
| 65 | 6.40 | Lauramide | FAA | [M+H]+ | 200.2015 | -3.0 | 116.1121, 115.0578, 105.0731, 102.0851, 91.0577, 77.0431 |
+ | + | + | + | + |
| 66 | 6.42 | 9-Hydroxy-12,14,16-octadecatrienoic acid | MFA | [M-H]- | 293.2125 | -1.0 | 275.2022, 235.1708, 183.1399, 171.1017 |
+ | + | + | + | + |
| 67 | 6.57 | Hydroxyoctadecatrienoic acid I | MFA | [M-H]- | 293.2125 | -1.0 | 275.2076, 257.1911, 185.1206, 171.1047 |
+ | + | + | + | + |
| 68 | 6.57 | Stearidonic acid II | MFA | [M-H]- | 275.2027 | -3.6 | 275.2027, 257.1952, 231.2127, 229.1872 |
+ | + | + | + | + |
| 69 | 6.80 | Hydroxystearidonic acid II | MFA | [M-H]- | 291.1964 | 0.7 | 273.1883, 255.2316, 245.1916 |
+ | + | + | + | + |
| 70 | 6.98 | Hydroxystearidonic acid III | MFA | [M-H]- | 291.1964 | 0.7 | 273.1883, 255.2316, 245.1916 |
+ | + | + | + | + |
| 71 | 7.16 | Hydroxystearidonic acid IV | MFA | [M-H]- | 291.1964 | 0.7 | 273.1883, 255.2316, 245.1916 |
+ | + | + | + | + |
| 72 | 7.17 | Tridecanamide | FAA | [M+H]+ | 214.2194 | 0.5 | 128.0678, 116.1123, 115.05788, 105.0761, 91.0597, 81.0739, 77.0431, 69.0781 |
|||||
| 73 | 7.22 | Heptadecanedioic acid I | DFA | [M-H]- | 299.2242 | -4.7 | 281.2143, 255.2352, 237.2166 |
+ | + | + | + | - |
| 74 | 7.49 | 13-Hydroxy-9,11-octadecadienoic acid | MFA | [M-H]- | 295.2278 | 0.3 | 295.2278, 277.2161, 249.2215, 195.1418, 171.1046, 113.0973 |
+ | + | + | + | + |
| 75 | 7.85 | Ricinoleic acid I | MFA | [M-H]- | 297.2438 | -1.0 | 297.2438, 279.2322, 253.2534, 183.1396, 111.0840, 93.0349 |
+ | + | + | + | + |
| 76 | 8.30 | Hydroxyoctadecatrienoic acid II | MFA | [M-H]- | 293.2125 | -1.0 | 275.2076, 257.1911, 185.1206, 171.1047 |
+ | + | + | + | + |
| 77 | 8.33 | Octadecanedioic acid IV | DFA | [M-H]- | 313.2375 | 3.2 | 295.2280, 269.2425, 251.2289 |
+ | + | + | + | + |
| 78 | 8.50 | Hydroxyoctadecatrienoic acid III | MFA | [M-H]- | 293.2125 | -1.0 | 275.2076, 257.1911, 185.1206, 171.1047 |
+ | + | + | + | + |
| 79 | 8.52 | Ricinoleic acid II | MFA | [M-H]- | 297.2438 | -1.0 | 297.2438, 279.2322, 253.2534, 183.1396, 111.0840, 93.0349 |
+ | + | + | + | + |
| 80 | 8.62 | Ricinoleic acid III | MFA | [M-H]- | 297.2438 | -1.0 | 297.2438, 279.2322, 253.2534, 183.1396, 111.0840, 93.0349 |
+ | + | + | + | + |
| 81 | 8.84 | Dodecanoic acid (Lauric acid) | MFA | [M-H]- | 199.1704 | -0.3 | 199.1704, 181.1572 | + | + | + | + | + |
| 82 | 9.01 | Hydroxyhexadecenoic acid I | MFA | [M-H]- | 269.213 | -3.0 | 269.2130, 251.2080, 225.2243, 223.2160 |
+ | + | + | + | + |
| 83 | 9.11 | Palmitoleamide I | FAA | [M+H]+ | 254.2483 | -1.8 | 237.2203, 219.2092, 165.0745, 146.6038, 135.1205, 121.1049, 116.0634, 111.0859, 109.1058, 107.0884, 105.0752, 95.0898, 93.0747, 91.0577, 83.0915, 81.0758, 79.0593, 77.0431, 69.0753, 67.0591 |
+ | + | + | + | + |
| 84 | 9.14 | Linolenamide | FAA | [M+H]+ | 278.2471 | 2.7 | 219.2102, 189.1640, 175.1480, 147.1168, 135.1170, 133.1010, 131.0860, 123.1170, 121.1010, 119.0860, 91.0578, 77.0449 |
+ | + | + | + | + |
| 85 | 9.17 | Tetradecanedioic acid II | DFA | [M-H]- | 257.1758 | 0.1 | 239.1580, 213.1841, 195.1700 |
- | - | + | - | + |
| 86 | 9.26 | 9-Hydroxy-10,12-octadecadienoic acid | MFA | [M-H]- | 295.2278 | 0.3 | 295.2280, 277.2229, 249.2215, 233.2223, 171.1064, 113.0973 |
+ | + | + | + | + |
| 87 | 9.29 | Myristamide | FAA | [M+H]+ | 228.2345 | -1.3 | 116.1097, 115.0578, 105.0731, 102.0963, 91.0597, 88.0805, 77.0431, 69.0753 |
+ | + | + | + | + |
| 88 | 9.36 | 9-Hydroxy-10,12-octadecadienoic acid | MFA | [M-H]- | 295.2278 | 0.3 | 295.2280, 277.2229, 249.2215, 233.2223, 171.1064, 113.0973 |
+ | + | + | + | + |
| 89 | 9.51 | Nonadecanedioic acid | DFA | [M-H]- | 327.2549 | -2.4 | 309.2492, 283.2639, 265.2502 |
+ | + | + | + | + |
| 90 | 9.81 | Hydroxyhexadecenoic acid II | MFA | [M-H]- | 269.213 | -3.0 | 269.2130, 251.2080, 225.2243, 223.2160 |
+ | + | + | + | + |
| 91 | 9.96 | Heptadecanedioic acid II | DFA | [M-H]- | 299.2242 | -4.7 | 281.2143, 255.2352, 237.2166 |
+ | + | + | + | + |
| 92 | 10.14 | Dihydroxyoctadecenoic acid | MFA | [M-H]- | 313.2378 | 1.9 | 295.2249, 277.2240, 269.2500, 171.1046, 155.1080, 125.0960 |
+ | + | + | + | + |
| 93 | 10.16 | Octadecanedioic acid V | DFA | [M-H]- | 313.2375 | 3.2 | 295.2280, 269.2425, 251.2289 |
+ | + | + | + | + |
| 94 | 10.22 | Tridecanoic acid | MFA | [M-H]- | 213.1856 | 1.9 | 213.1856, 195.1645 | + | + | + | + | + |
| 95 | 10.27 | Hydroxyhexadecenoic acid III | MFA | [M-H]- | 269.213 | -3.0 | 269.2130, 251.2080, 225.2243, 223.2160 |
+ | + | + | + | + |
| 96 | 10.29 | Hydroxyhexadecanoic acid I | MFA | [M-H]- | 271.2293 | -5.2 | 271.2293, 225.2244 | + | + | + | + | + |
| 97 | 10.35 | Pentadecanamide | FAA | [M+H]+ | 242.2466 | 116.0578, 115.0578, 102. 0954, 91.059 |
+ | + | + | + | + | |
| 98 | 10.50 | Dihydroxyoctadecadienoic acid I | MFA | [M-H]- | 311.2222 | 1.9 | 293.2160, 275.1958, 265.2173, 257.2183 |
+ | + | + | + | + |
| 99 | 10.60 | Palmitadienoic acid | MFA | [M-H]- | 251.2016 | 0.4 | 251.2016 | + | + | + | + | + |
| 100 | 10.66 | Linoleamide I | FAA | [M+H]+ | 280.2631 | 1.4 | 263.2333, 245.2219, 161.1178, 133.0839, 119.0683, 109.0826, 95.0667, 91.0353, 81.0513, 79.0352 |
+ | + | + | + | + |
| 101 | 10.70 | Dihydroxyoctadecadienoic acid II | MFA | [M-H]- | 311.2222 | 1.9 | 293.2160, 275.1958, 265.2173, 257.2183 |
+ | + | + | + | + |
| 102 | 10.74 | Eicosanedioic acid | DFA | [M-H]- | 341.2695 | 0.6 | 323.2603, 297.2877, 279.2632 |
+ | + | + | + | + |
| 103 | 10.77 | Nonadecanedioic acid | DFA | [M-H]- | 325.2368 | 4.9 | 307.2291, 281.2480, 263.2364, 237.2231 |
+ | + | + | - | + |
| 104 | 11.01 | Dihydroxyoctadecadienoic acid III | MFA | [M-H]- | 311.2222 | 1.9 | 293.2160, 275.1958, 265.2173, 257.2183 |
+ | + | + | + | + |
| 105 | 11.10 | Ceriporic acid I | DFA | [M-H]- | 351.2534 | 1.9 | 333.2467, 307.2613, 289.2500 |
+ | + | + | + | + |
| 106 | 11.14 | Oleic acid I | MFA | [M-H]- | 281.248 | 2.1 | 281.248, 263.2364, 237.2231 |
- | + | + | + | + |
| 107 | 11.17 | Pentacosanedioic acid I | DFA | [M-H]- | 411.3474 | 1.5 | 393.3307, 367.3678, 349.3567 |
+ | + | + | + | + |
| 108 | 11.24 | Stearic acid I | MFA | [M-H]- | 283.2642 | 0.2 | 283.2642, 265.2568 | + | + | + | + | + |
| 109 | 11.35 | Eicosenedioic acid | DFA | [M-H]- | 339.2542 | -0.3 | 321.2497, 295.2707, 277.2547 |
+ | + | + | + | + |
| 110 | 11.37 | Hydroxyhexadecanoic acid II | MFA | [M-H]- | 271.2293 | 1.5 | 271.2293, 225.2244 | + | + | + | + | + |
| 111 | 11.38 | Pentadecenoic acid | MFA | [M-H]- | 239.2015 | 0.8 | 239.2115, 221.1918 | + | + | + | + | + |
| 112 | 11.48 | Linolenic acid | MFA | [M-H]- | 277.2173 | 0.0 | 259.2143, 233.2348, 211.1382 |
+ | + | + | + | + |
| 113 | 11.61 | Myristic acid | MFA | [M-H]- | 227.2015 | 0.7 | 227.2015, 209.1939 | + | + | + | + | + |
| 114 | 11.80 | Oxotetracosanedioic acid | DFA | [M-H]- | 411.3118 | -0.5 | 393.3081, 375.2944, 367.3244, 349.3106 |
+ | + | + | + | + |
| 115 | 11.80 | Palmitamide | FAA | [M+H]+ | 256.2636 | -0.4 | 116.1119, 105.0730, 102.0963, 88.0805, 77.0431, 69.0752 |
+ | + | + | + | + |
| 116 | 11.83 | Heptadecadienoic acid | MFA | [M-H]- | 265.2167 | 2.3 | 265.2167, 247.2089 | + | + | + | + | + |
| 117 | 11.88 | Ceriporic acid II | DFA | [M-H]- | 351.2534 | 1.9 | 333.2467, 307.2613, 289.2500 |
- | + | + | + | - |
| 118 | 11.88 | Eicosadienoic acid I | MFA | [M-H]- | 307.2649 | -2.0 | 289.2500, 263.2529, 261.2602 |
- | - | - | + | - |
| 119 | 11.97 | Heneicosanedioic acid | DFA | [M-H]- | 355.285 | 1.1 | 337.2845, 311.2908, 293.2897 |
+ | + | + | + | + |
| 120 | 12.10 | Ceriporic acid III | DFA | [M-H]- | 351.2534 | 1.9 | 333.2467, 307.2613, 289.2500 |
- | - | - | + | - |
| 121 | 12.25 | Palmitoleic acid I | MFA | [M-H]- | 253.2177 | -1.6 | 253.2177, 235.2183 | + | + | + | + | + |
| 122 | 12.49 | Ricinoleic acid IV | MFA | [M-H]- | 297.2438 | -1.0 | 297.2438, 279.2322, 253.2534, 183.1396, 111.0840, 93.0349 |
+ | + | + | + | + |
| 123 | 12.51 | Oleamide I | FAA | [M+H]+ | 282.2787 | 1.4 | 265.2504, 247.2419, 177.1642, 165.0929, 149.1350, 135.1205, 121.1049, 111.0859, 107.0905, 97.1062, 91.0597, 83.0896, 81.0758, 79.0593, 69.0753, 55.059 |
+ | + | + | + | + |
| 124 | 12.52 | Arachidamide | FAA | [M+H]+ | 312.3257 | 1.3 | 207.0339, 165.0693, 159.1176, 145.1033, 116.0678, 115.0579, 105.0731, 102.0963, 91.0597, 77.0431, 69.0753, 67.05909 |
+ | + | + | + | + |
| 125 | 12.70 | Pentadecanoic acid | MFA | [M-H]- | 241.2173 | 0.0 | 241.2173, 223.2073 | + | + | + | + | + |
| 126 | 12.70 | Palmitic acid I | MFA | [M-H]- | 255.2328 | 0.6 | 255.2351, 237.2227 | + | + | + | + | + |
| 127 | 12.70 | Eicosenoic acid | MFA | [M-H]- | 309.2783 | 5.2 | 309.2799, 291.2735 | + | - | + | + | + |
| 128 | 12.85 | Heptadecanamide I | FAA | [M+H]+ | 270.2778 | 4.8 | 107.0884, 115.0579, 105.0752, 91.0597, 77.04313, 69.0753 |
+ | + | + | + | + |
| 129 | 13.04 | Linoleic acid | MFA | [M-H]- | 279.2329 | 0.4 | 279.2329, 261.2203, 243.2081 |
+ | + | + | + | + |
| 130 | 13.19 | Docosanedioic acid | DFA | [M-H]- | 369.301 | 0.0 | 335.3020, 325.3030, 307.2972 |
+ | + | + | + | + |
| 131 | 13.22 | Heptadecanamide II | FAA | [M+H]+ | 270.2778 | 4.8 | 107.0884, 115.0579, 105.0752, 91.0597, 77.04313, 69.0753 |
+ | + | + | + | + |
| 132 | 13.33 | Palmitoleic acid II | MFA | [M-H]- | 253.2177 | -1.6 | 253.2177, 235.2183 | + | + | + | + | + |
| 133 | 13.40 | Arachidinic acid I | MFA | [M-H]- | 311.295 | 1.9 | 311.2950, 293.2899, 267.2970 |
+ | + | + | + | - |
| 134 | 13.41 | Heptadecenamide | FAA | [M+H]+ | 268.2641 | -2.3 | 175.1539, 165.0745, 133.1033, 115.0573, 111.0876, 105.0731, 97.1032, 91.0578, 79.0598, 77.0431, 69.0763, 67.0591 |
+ | + | + | + | + |
| 135 | 13.41 | Behenamide I | FAA | [M+H]+ | 340.3575 | -0.3 | 144.0966, 130.0794, 116.1097, 117.0733, 102.0963, 88.0805 |
+ | + | + | + | + |
| 136 | 13.48 | Palmitoleamide II | FAA | [M+H]+ | 254.2481 | -1.0 | 237.2203, 219.2092, 165.0745, 146.6038, 135.1205, 121.1049, 116.0634, 111.0859, 109.1058, 107.0884, 105.0752, 95.0898, 93.0747, 91.0577, 83.0915, 81.0758, 79.0593, 77.0431, 69.0753, 67.0591 |
+ | + | + | + | + |
| 137 | 13.51 | Erucamide I | FAA | [M+H]+ | 338.3438 | -6.1 | 321.2128, 303.3040, 177.1675, 163.1533, 149.1386, 135.1241, 121.1086, 111.1242, 97.1100, 83.0933, 81.0795, 69.0789, 55.0626 |
+ | + | + | + | + |
| 138 | 13.57 | Heptadecenoic acid I | MFA | [M-H]- | 267.2331 | -0.4 | 267.2331, 249.2276 | + | + | + | + | + |
| 139 | 13.66 | Palmitoleic acid III | MFA | [M-H]- | 253.2177 | -1.6 | 253.2177, 235.2183 | + | + | + | + | + |
| 140 | 13.70 | Palmitic acid II | MFA | [M-H]- | 255.2328 | 0.6 | 255.2351, 237.2227 | + | + | + | + | + |
| 141 | 13.77 | Heneicosanoic acid | MFA | [M-H]- | 325.3113 | -0.3 | 325.3113, 307.3052, 281.3201 |
+ | + | + | + | + |
| 142 | 13.77 | Heptadecanamide III | FAA | [M+H]+ | 270.2778 | 4.8 | 107.0884, 115.0579, 105.0752, 91.0597, 88.0805, 77.0431, 69.0753 |
+ | + | + | + | + |
| 143 | 13.79 | Oleamide II | FAA | [M+H]+ | 282.2789 | 0.7 | 265.2504, 247.2419, 177.1642, 165.0929, 149.1350, 135.1205, 121.1049, 111.0859, 107.0905, 97.1062, 91.0597, 83.0896, 81.0758, 79.0593, 69.0753, 55.059 |
+ | + | + | + | + |
| 144 | 13.82 | Heptadecenoic acid II | MFA | [M-H]- | 267.2331 | -0.4 | 267.2331, 249.2276 | + | - | + | + | + |
| 145 | 14.12 | Arachidinic acid II | MFA | [M-H]- | 311.295 | 1.9 | 311.2950, 293.2899, 267.2970 |
+ | + | + | + | + |
| 146 | 14.30 | Palmitic acid III | MFA | [M-H]- | 255.2328 | 0.6 | 255.2351, 237.2227 | + | + | + | + | + |
| 147 | 14.37 | Heptadecanoic acid I | MFA | [M-H]- | 269.2482 | 1.5 | 269.2482, 251.2439, 225.2305 |
+ | + | + | + | + |
| 148 | 14.39 | Tricosanedioic acid | DFA | [M-H]- | 383.3176 | -2.4 | 365.3100, 339.3257, 321.3157 |
+ | + | + | + | + |
| 149 | 14.41 | Octadecanedioic acid VI | DFA | [M-H]- | 313.2375 | 3.19 | 295.2280, 269.2425, 251.2289 |
+ | + | + | + | + |
| 150 | 14.65 | Stearamide | FAA | [M+H]+ | 284.2957 | -3.2 | 207.0338, 116.1119, 102.0851, 88.0805, 81.0739, 74.0649, 69.0753 |
+ | + | + | + | + |
| 151 | 14.67 | Erucamide II | FAA | [M+H]+ | 338.3401 | 4.9 | 321.2128, 303.3040, 177.1675, 163.1533, 149.1386, 135.1241, 121.1086, 111.1242, 97.1100, 81.0795, 69.0789, 55.0626 |
+ | + | + | + | + |
| 152 | 14.87 | Stearic acid II | MFA | [M-H]- | 283.2642 | 0.2 | 283.2642, 265.2568 | + | + | + | + | + |
| 153 | 14.87 | Ocatdecanoic acid II | MFA | [M-H]- | 281.2478 | 2.8 | 281.2478, 263.2364 | + | + | + | + | + |
| 154 | 14.95 | Tetracosanoic acid | MFA | [M-H]- | 367.3573 | 2.4 | 367.3573 | + | + | + | - | - |
| 155 | 14.95 | Behenamide II | FAA | [M+H]+ | 340.3575 | -3.0 | 144.0966, 130.0794, 116.1097, 117.0733, 102.0963, 88.0805 |
+ | + | + | + | + |
| 156 | 15.04 | Nonadecanoic acid | MFA | [M-H]- | 297.2798 | 0.3 | 297.2798, 279.2667 | + | + | + | + | - |
| 157 | 15.16 | Eicosenamide | FAA | [M+H]+ | 310.3092 | 3.8 | 283.2647, 256.2669, 211.1508, 177.1669, 165.0719, 149.1350, 135.1205, 121.1049, 111.0859, 107.0884, 105.0752, 102.0942, 97.1063, 93.074, 91.0597, 81.0739, 79.0593, 77.0431, 69.0753, 55.0592 |
+ | + | + | + | + |
| 158 | 15.50 | Tricosanoic acid | MFA | [M-H]- | 353.3405 | 5.7 | 353.3405 | + | + | - | + | - |
| 159 | 15.56 | Eicosadienoic acid II | MFA | [M-H]- | 307.2649 | -2.0 | 289.2500, 263.2529, 261.2602 |
+ | + | + | + | + |
| 160 | 15.70 | Docosanoic acid (Behenic acid) |
MFA | [M-H]- | 339.3272 | -0.9 | 339.3272, 295.3106, 139.0407, 119.0496 |
+ | + | + | - | + |
| 161 | 15.70 | Nonadecanamide II | FAA | [M+H]+ | 298.3085 | 6.4 | 145.1033, 133.1057, 119.0907, 105.0752, 116.0642, 91.0597, 88.0845, 77.0431, 69.0745 |
+ | + | + | + | + |
| 162 | 15.70 | Linoleamide II | FAA | [M+H]+ | 280.2628 | 2.4 | 263.2333, 245.2219, 161.1178, 133.0839, 119.0683, 109.0826, 95.0667, 91.0353, 81.0513, 79.0352 |
- | + | - | - | - |
| 163 | 15.81 | Tetracosanedioic acid | DFA | [M-H]- | 397.3307 | 4.03 | 379.3195, 353.3482, 335.3321 |
+ | + | + | + | + |
| 164 | 15.87 | Heptadecanoic acid II (Margaric acid ) |
MFA | [M-H]- | 269.2482 | 1.5 | 269.2482, 251.2439, 225.2305 |
+ | + | + | + | + |
| 165 | 16.07 | Henicosanamide | FAA | [M+H]+ | 326.3426 | -2.6 | 165.0772, 159.1202, 121.1027, 109.1058, 105.0731, 102.0579, 91.0597, 69.0753, 67.0624 |
- | + | - | + | + |
| 166 | 16.23 | Pentacosanedioic acid II | DFA | [M-H]- | 411.3474 | 1.16 | 393.3307, 367.3678, 349.3567 |
+ | - | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





