Submitted:
12 March 2024
Posted:
13 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
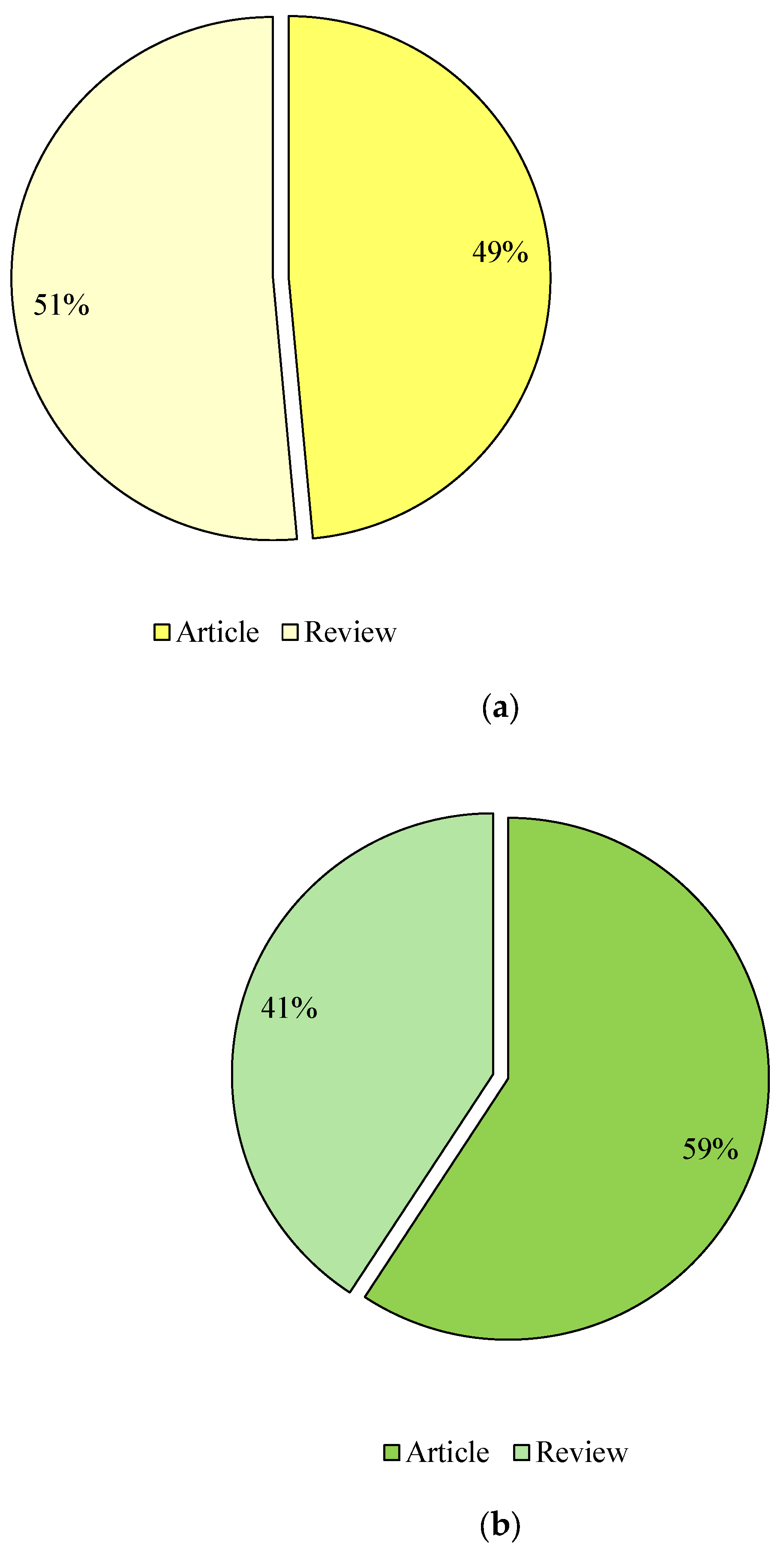
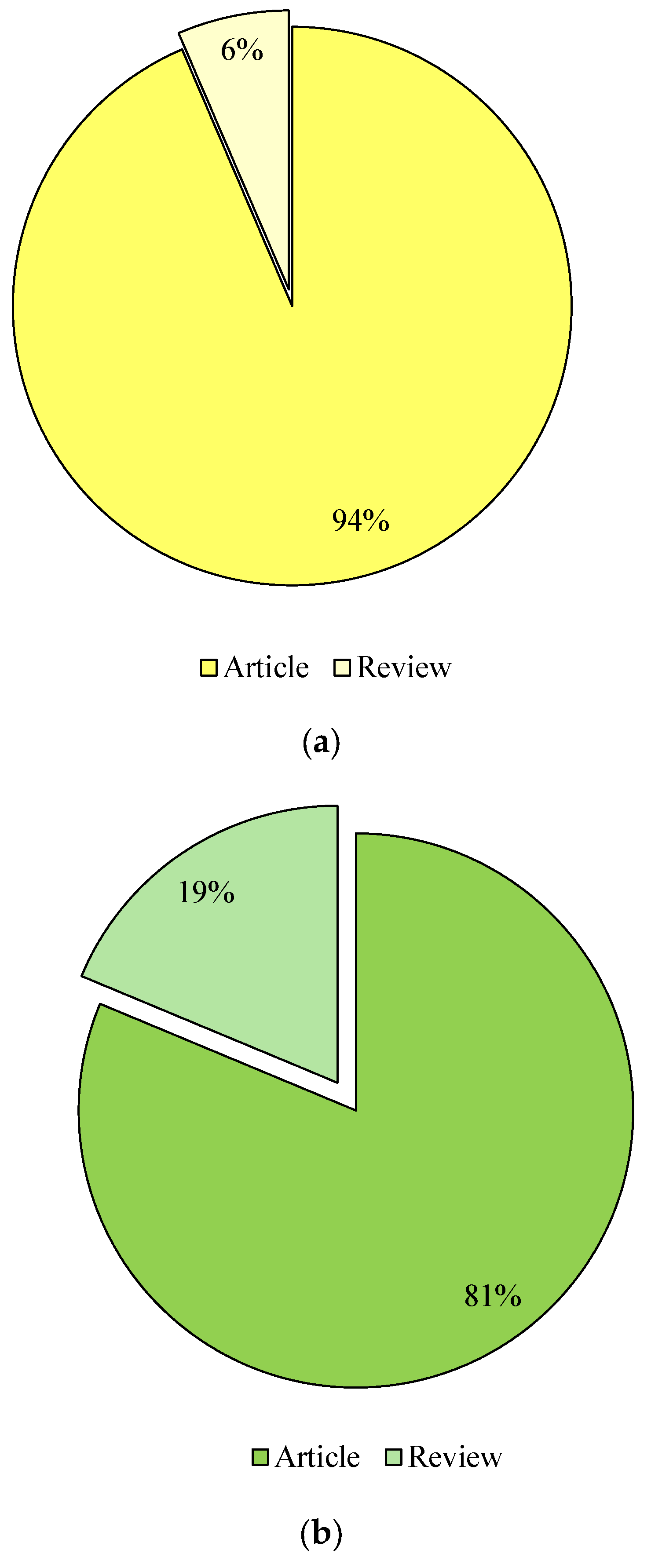
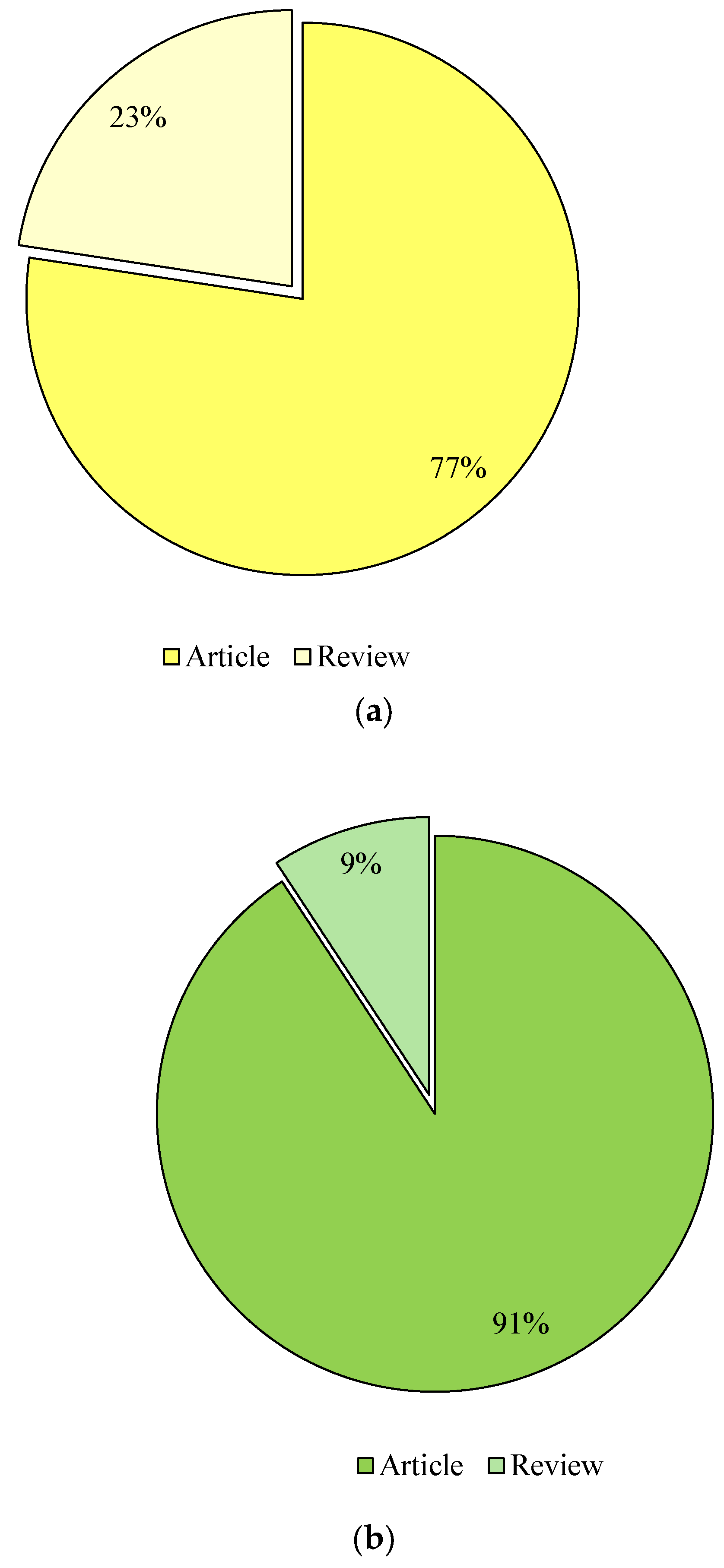
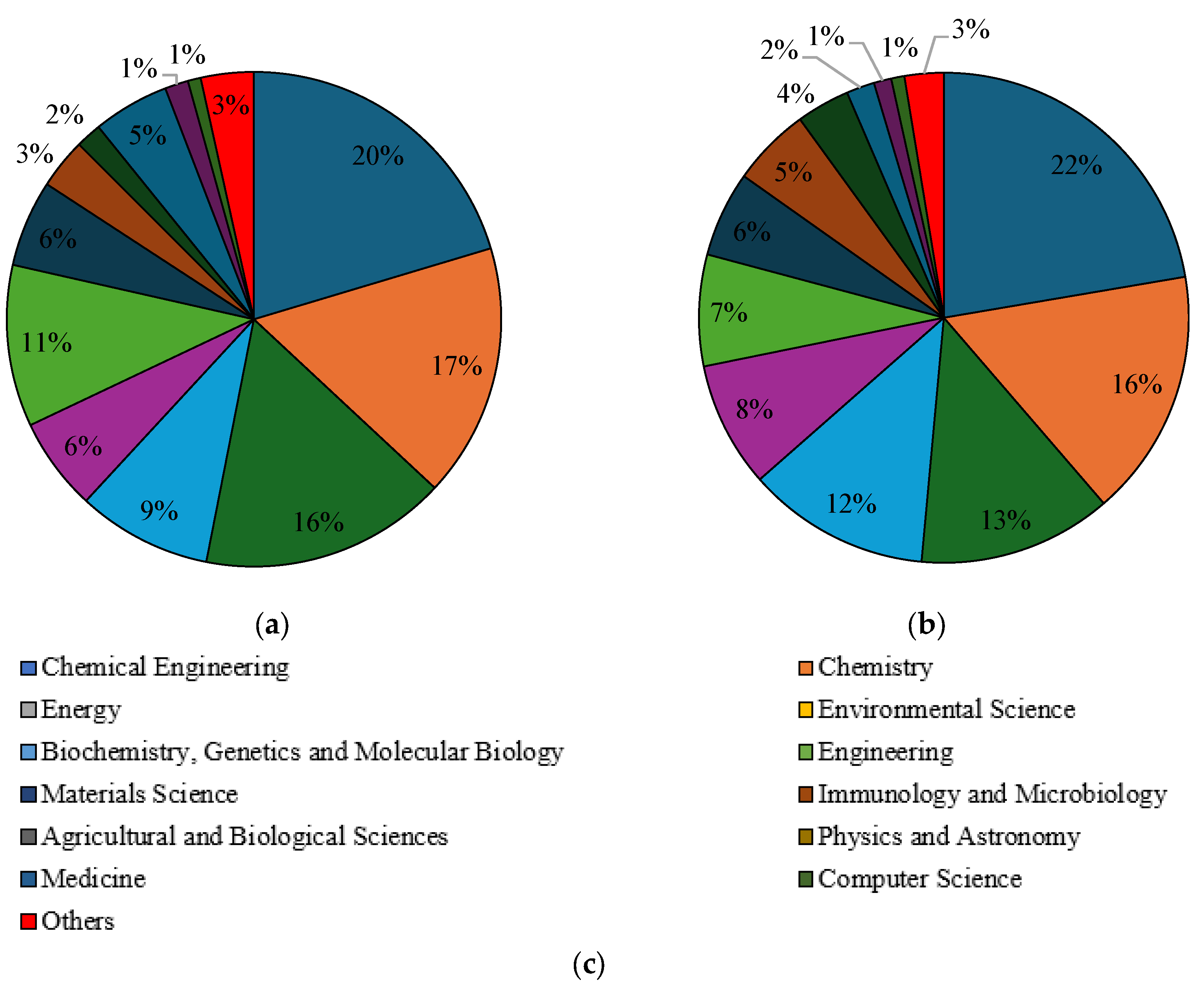
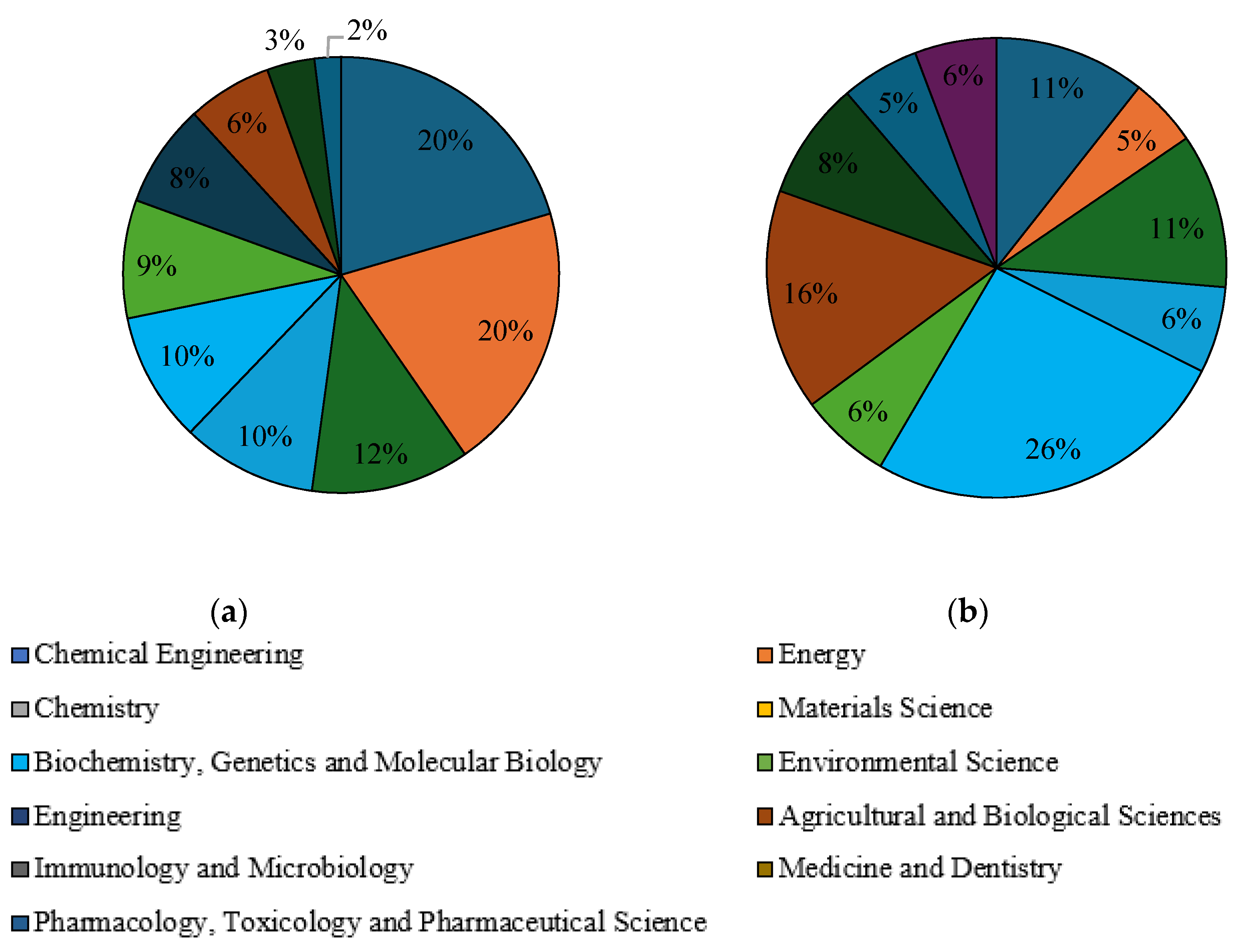
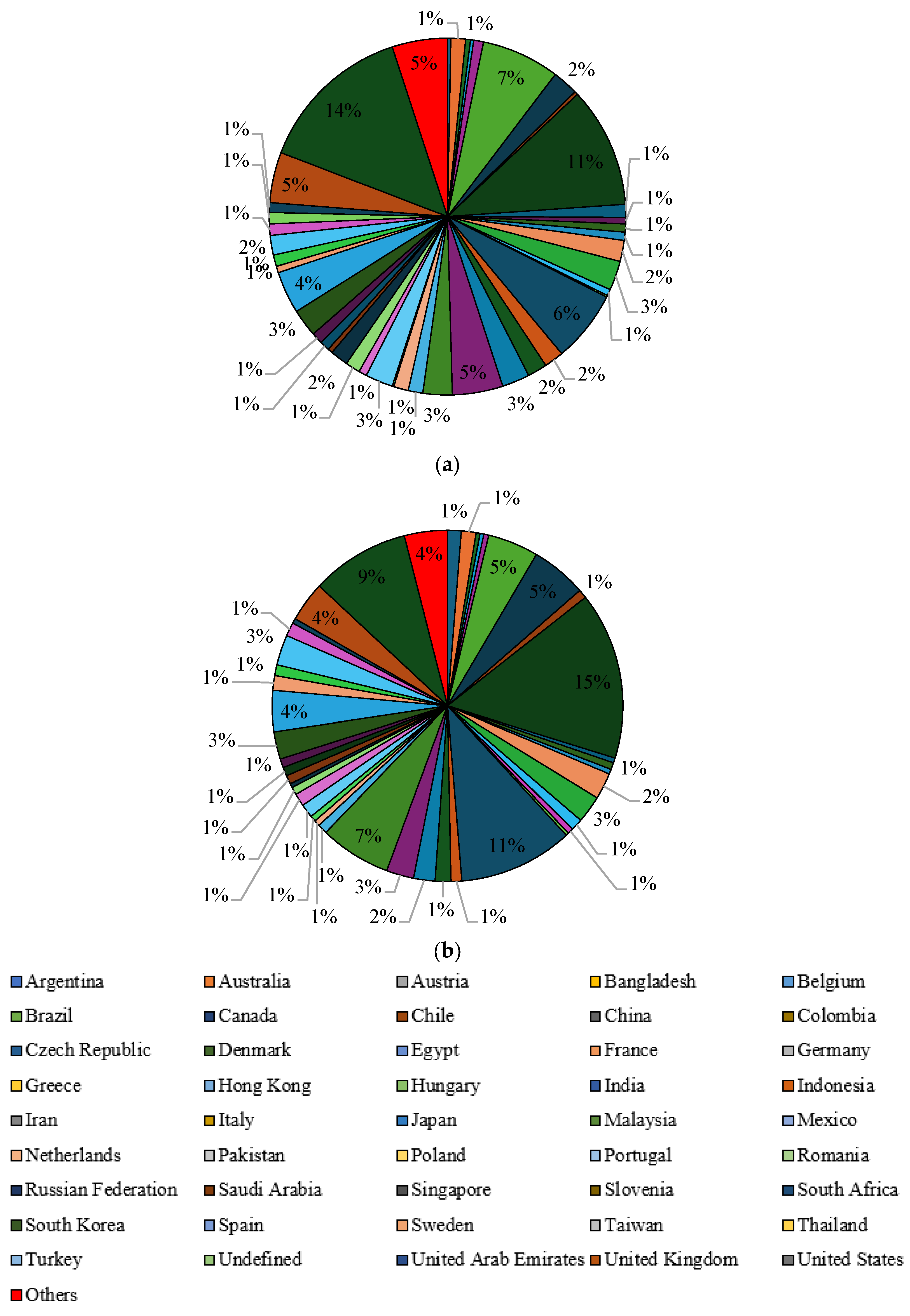
2. Bibliographical Analysis, Research Directions and Scientific Potential
3. Alcoholic Fermentation
3.1. Production of Bioethanol
3.2. Production of Biobutanol
4. Production of Bio-Oil
5. Production of Other Bio-Based Products
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhatia, L. Glycerol and Its Derivatives (Propanediol, Glycerolcarbonate, Epichlorohydrin): Implicit Role in Bioeconomy. Prod. Top 12 Biochem. Sel. by USDOE from Renew. Resour. Status Innov. 2022, 317–343. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Sakinah, A.M.M.; Zularisam, A.W.; Sirohi, R.; Khilji, I.A.; Reddy, V.J.; Pandey, A. Bioconversion of Glycerol into Biofuels—Opportunities and Challenges. BioEnergy Res. 2021 151 2021, 15, 46–61. [Google Scholar] [CrossRef]
- Hambali, E.; Fitria, R.; Sari, V.I. Glycerol and Derivatives. Biorefinery Oil Prod. Plants Value-Added Prod. Vol. 1 2022, 1, 469–491. [Google Scholar] [CrossRef]
- Miyuranga, K.A.V.; Arachchige, U.S.P.R.; Jayasinghe, R.A.; Samarakoon, G. Purification of Residual Glycerol from Biodiesel Production as a Value-Added Raw Material for Glycerolysis of Free Fatty Acids in Waste Cooking Oil. Energies 2022, Vol. 15, Page 8856 2022, 15, 8856. [Google Scholar] [CrossRef]
- Patel, H.K.; Patel, N.P.; Shah, M.P. Microbial Bioprospecting of Biodiesel Industry-Derived Crude Glycerol Waste Conversion into Value-Added Products. Green Approach to Altern. Fuel a Sustain. Futur. 2023, 71–87. [Google Scholar] [CrossRef]
- Tabassum, N.; Pothu, R.; Pattnaik, A.; Boddula, R.; Balla, P.; Gundeboyina, R.; Challa, P.; Rajesh, R.; Perugopu, V.; Mameda, N.; Radwan, A.B.; Abdullah, A.M.; Al-Qahtani, N. Heterogeneous Catalysts for Conversion of Biodiesel-Waste Glycerol into High-Added-Value Chemicals. Catal. 2022, Vol. 12, Page 767 2022, 12, 767. [Google Scholar] [CrossRef]
- Shamsudin, M.I.; Tan, L.S.; Tsuji, T.; Kiew, P.L. Production and Characterization of Biodiesel from Canola Oil through Enzymatic Transesterification. J. Phys. Conf. Ser. 2022, 2259, 012023. [Google Scholar] [CrossRef]
- Becerra-Ruiz, J.D.; Gonzalez-Huerta, R.G.; Gracida, J.; Amaro-Reyes, A.; Macias-Bobadilla, G. Using Green-Hydrogen and Bioethanol Fuels in Internal Combustion Engines to Reduce Emissions. Int. J. Hydrogen Energy 2019, 44, 12324–12332. [Google Scholar] [CrossRef]
- Bhan, C.; Verma, L.; Singh, J. Alternative Fuels for Sustainable Development. Environ. Concerns Sustain. Dev. 2020, 317–331. [Google Scholar] [CrossRef]
- Kaur, J.; Sarma, A.K.; Jha, M.K.; Gera, P. Valorisation of Crude Glycerol to Value-Added Products: Perspectives of Process Technology, Economics and Environmental Issues. Biotechnol. Reports 2020, 27, e00487. [Google Scholar] [CrossRef]
- Li, C.; Lesnik, K.L.; Liu, H. Microbial Conversion of Waste Glycerol from Biodiesel Production into Value-Added Products. Energies 2013, Vol. 6, Pages 4739-4768 2013, 6, 4739–4768. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Sakinah, A.M.M.; Zularisam, A.W.; Pandey, A. Glycerol Waste to Value Added Products and Its Potential Applications. Syst. Microbiol. Biomanufacturing 2021 14 2021, 1, 378–396. [Google Scholar] [CrossRef]
- He, Q. (Sophia); McNutt, J.; Yang, J. Utilization of the Residual Glycerol from Biodiesel Production for Renewable Energy Generation. Renew. Sustain. Energy Rev. 2017, 71, 63–76. [Google Scholar] [CrossRef]
- Babadi, A.A.; Rahmati, S.; Fakhlaei, R.; Barati, B.; Wang, S.; Doherty, W.; Ostrikov, K. Emerging Technologies for Biodiesel Production: Processes, Challenges, and Opportunities. Biomass and Bioenergy 2022, 163, 106521. [Google Scholar] [CrossRef]
- Suprun, V.Y.; Marukha, V.; Sylovaniuk, V.P. Recycling Technologies for Polyurethane Wastes (A Survey). Mater. Sci. 2022, 57, 755–764. [Google Scholar] [CrossRef]
- Md Radzi, M.R.; Manogaran, M.D.; Yusoff, M.H.M.; Zulqarnain; Anuar, M.R.; Shoparwe, N.F.; Rahman, M.F.A. Production of Propanediols through In Situ Glycerol Hydrogenolysis via Aqueous Phase Reforming: A Review. Catal. 2022, Vol. 12, Page 945 2022, 12, 945. [Google Scholar] [CrossRef]
- Carlucci, C. A Focus on the Transformation Processes for the Valorization of Glycerol Derived from the Production Cycle of Biofuels. Catal. 2021, Vol. 11, Page 280 2021, 11, 280. [Google Scholar] [CrossRef]
- Cichowska, J.; Figiel, A.; Stasiak-Różańska, L.; Witrowa-Rajchert, D. Modeling of Osmotic Dehydration of Apples in Sugar Alcohols and Dihydroxyacetone (DHA) Solutions. Foods 2019, Vol. 8, Page 20 2019, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Reshmy, R.; Paulose, T.A.P.; Philip, E.; Thomas, D.; Madhavan, A.; Sirohi, R.; Binod, P.; Kumar Awasthi, M.; Pandey, A.; Sindhu, R. Updates on High Value Products from Cellulosic Biorefinery. Fuel 2022, 308, 122056. [Google Scholar] [CrossRef]
- Rasrendra, C.B.; Culsum, N.T.U.; Rafiani, A.; Kadja, G.T.M. Glycerol Valorization for the Generation of Acrylic Acid via Oxidehydration over Nanoporous Catalyst: Current Status and the Way Forward. Bioresour. Technol. Reports 2023, 23, 101533. [Google Scholar] [CrossRef]
- Anto, L.; Warykas, S.W.; Torres-Gonzalez, M.; Blesso, C.N. Milk Polar Lipids: Underappreciated Lipids with Emerging Health Benefits. Nutr. 2020, Vol. 12, Page 1001 2020, 12, 1001. [Google Scholar] [CrossRef] [PubMed]
- Kalus, K.; Konkol, D.; Korczyński, M.; Koziel, J.A.; Opaliński, S. Effect of Biochar Diet Supplementation on Chicken Broilers Performance, NH3 and Odor Emissions and Meat Consumer Acceptance. Anim. 2020, Vol. 10, Page 1539 2020, 10, 1539. [Google Scholar] [CrossRef] [PubMed]
- Imbault, A.L.; Gong, J.; Farnood, R. Photocatalytic Production of Dihydroxyacetone from Glycerol on TiO 2 in Acetonitrile. RSC Adv. 2020, 10, 4956–4968. [Google Scholar] [CrossRef] [PubMed]
- Fehmberger, C.; Dos Santos, F.T.; Aloisio, C.M.; Hermes, E.; Zenatti, D.C.; Bautitz, I.R. Effectiveness of Incorporation of Crude Glycerin as a Source of Labile Carbon in the Composting of Poultry Production Residues. J. Clean. Prod. 2020, 251, 119739. [Google Scholar] [CrossRef]
- Villegas-Bolaños, P.A.; Gallego, J.A.; Dorkis, L.; Márquez, M.A. Colombian Olivine as a Potential Catalyst for Glycerol Valorization. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Luo, C.; Dou, B.; Zhang, H.; Liu, D.; Zhao, L.; Chen, H.; Xu, Y. Co-Production of Hydrogen and Syngas from Chemical Looping Water Splitting Coupled with Decomposition of Glycerol Using Fe-Ce-Ni Based Oxygen Carriers. Energy Convers. Manag. 2021, 238, 114166. [Google Scholar] [CrossRef]
- Kujawska, N.; Talbierz, S.; Dębowski, M.; Kazimierowicz, J.; Zieliński, M. Optimizing Docosahexaenoic Acid (DHA) Production by Schizochytrium Sp. Grown on Waste Glycerol. Energies 2021, 14, 1685. [Google Scholar] [CrossRef]
- Cannilla, C.; Giacoppo, G.; Frusteri, L.; Todaro, S.; Bonura, G.; Frusteri, F. Techno-Economic Feasibility of Industrial Production of Biofuels by Glycerol Etherification Reaction with Isobutene or Tert-Butyl Alcohol Assisted by Vapor-Permeation Membrane. J. Ind. Eng. Chem. 2021, 98, 413–424. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Muldoon, V.L.; Deng, S. Crude Glycerol and Glycerol as Fuels and Fuel Additives in Combustion Applications. Renew. Sustain. Energy Rev. 2022, 159, 112206. [Google Scholar] [CrossRef]
- Gonçalves, M.; Castro, C.S.; Boas, I.K.V.; Soler, F.C.; Pinto, E.D.C.; Lavall, R.L.; Carvalho, W.A. Glycerin Waste as Sustainable Precursor for Activated Carbon Production: Adsorption Properties and Application in Supercapacitors. J. Environ. Chem. Eng. 2019, 7, 103059. [Google Scholar] [CrossRef]
- Malaika, A.; Ptaszyńska, K.; Morawa Eblagon, K.; Pereira, M.F.R.; Figueiredo, J.L.; Kozłowski, M. Solid Acid Carbon Catalysts for Sustainable Production of Biofuel Enhancers via Transesterification of Glycerol with Ethyl Acetate. Fuel 2021, 304, 121381. [Google Scholar] [CrossRef]
- Bastos Lima, M.G. The Contested Sustainability of Biofuels in a North-South Context. Polit. Bioeconomy Sustain. 2021, 23–47. [Google Scholar] [CrossRef]
- Costa-Gutierrez, S.B.; Saez, J.M.; Aparicio, J.D.; Raimondo, E.E.; Benimeli, C.S.; Polti, M.A. Glycerol as a Substrate for Actinobacteria of Biotechnological Interest: Advantages and Perspectives in Circular Economy Systems. Chemosphere 2021, 279, 130505. [Google Scholar] [CrossRef] [PubMed]
- Seifert, C.; Bowien, S.; Gottschalk, G.; Daniel, R. Identification and Expression of the Genes and Purification and Characterization of the Gene Products Involved in Reactivation of Coenzyme B12-Dependent Glycerol Dehydratase of Citrobacter Freundii. Eur. J. Biochem. 2001, 268, 2369–2378. [Google Scholar] [CrossRef] [PubMed]
- Colin, T.; Bories, A.; Lavigne, C.; Moulin, G. Effects of Acetate and Butyrate during Glycerol Fermentation by Clostridium Butyricum. Curr. Microbiol. 2001, 43, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Nakashimada, Y.; Senba, K.; Matsui, T.; Nishio, N. Hydrogen and Ethanol Production from Glycerol-Containing Wastes Discharged after Biodiesel Manufacturing Process. J. Biosci. Bioeng. 2005, 100, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Barbirato, F.; Bories, A. Relationship between the Physiology of Enterobacter Agglomerans CNCM 1210 Grown Anaerobically on Glycerol and the Culture Conditions. Res. Microbiol. 1997, 148, 475–484. [Google Scholar] [CrossRef]
- Németh, Á.; Kupcsulik, B.; Sevella, B. 1,3-Propanediol Oxidoreductase Production with Klebsiella Pneumoniae DSM2026. World J. Microbiol. Biotechnol. 2003, 19, 659–663. [Google Scholar] [CrossRef]
- Talarico, T.L.; Axelsson, L.T.; Novotny, J.; Fiuzat, M.; Dobrogosz, W.J. Utilization of Glycerol as a Hydrogen Acceptor by Lactobacillus Reuteri: Purification of 1,3-Propanediol:NAD+ Oxidoreductase. Appl. Environ. Microbiol. 1990, 56, 943–948. [Google Scholar] [CrossRef]
- Gallardo, R.; Alves, M.; Rodrigues, L.R. Modulation of Crude Glycerol Fermentation by Clostridium Pasteurianum DSM 525 towards the Production of Butanol. Biomass and Bioenergy 2014, 71, 134–143. [Google Scholar] [CrossRef]
- Sarchami, T.; Munch, G.; Johnson, E.; Kießlich, S.; Rehmann, L. A Review of Process-Design Challenges for Industrial Fermentation of Butanol from Crude Glycerol by Non-Biphasic Clostridium Pasteurianum. Ferment. 2016, Vol. 2, Page 13 2016, 2, 13. [Google Scholar] [CrossRef]
- Johnson, E.E.; Rehmann, L. The Role of 1,3-Propanediol Production in Fermentation of Glycerol by Clostridium Pasteurianum. Bioresour. Technol. 2016, 209, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Petitdemange, H.; Cherrier, C.; Raval, G.; Gay, R. Regulation of the NADH and NADPH-Ferredoxin Oxidoreductases in Clostridia of the Butyric Group. Biochim. Biophys. Acta - Gen. Subj. 1976, 421, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.; Holtmann, D.; Ulber, R.; Tippkötter, N. Increased Biobutanol Production by Mediator-Less Electro-Fermentation. Biotechnol. J. 2019, 14, 1800514. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.R.; Seo, J.W.; Heo, S.Y.; Hong, W.K.; Luo, L.H.; Joe, M. ho; Park, D.H.; Kim, C.H. Efficient Production of Ethanol from Crude Glycerol by a Klebsiella Pneumoniae Mutant Strain. Bioresour. Technol. 2011, 102, 3918–3922. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; Hartono, M.R.; Chan, W.H.; Yeo, S.S. Ethanol Production from Biodiesel-Derived Crude Glycerol by Newly Isolated Kluyvera Cryocrescens. Appl. Microbiol. Biotechnol. 2011, 89, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jensen, P.R.; Workman, M. Bioconversion of Crude Glycerol Feedstocks into Ethanol by Pachysolen Tannophilus. Bioresour. Technol. 2012, 104, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.; Pankaew, S.; Boonsawang, P.; Tongurai, C. Anaerobic Fermentation of Crude Glycerol to Produce Value-Added Products. Appl. Eng. Agric. 2011, 27, 655–662. [Google Scholar] [CrossRef]
- Loaces, I.; Rodríguez, C.; Amarelle, V.; Fabiano, E.; Noya, F. Improved Glycerol to Ethanol Conversion by E. Coli Using a Metagenomic Fragment Isolated from an Anaerobic Reactor. J. Ind. Microbiol. Biotechnol. 2016, 43, 1405–1416. [Google Scholar] [CrossRef]
- Kata, I.; Semkiv, M.V.; Ruchala, J.; Dmytruk, K.V.; Sibirny, A.A. Overexpression of the Genes PDC1 and ADH1 Activates Glycerol Conversion to Ethanol in the Thermotolerant Yeast Ogataea (Hansenula) Polymorpha. Yeast 2016, 33, 471–478. [Google Scholar] [CrossRef]
- Thapa, L.P.; Lee, S.J.; Yang, X.; Lee, J.H.; Choi, H.S.; Park, C.; Kim, S.W. Improved Bioethanol Production from Metabolic Engineering of Enterobacter Aerogenes ATCC 29007. Process Biochem. 2015, 50, 2051–2060. [Google Scholar] [CrossRef]
- Maru, B.T.; López, F.; Kengen, S.W.M.; Constantí, M.; Medina, F. Dark Fermentative Hydrogen and Ethanol Production from Biodiesel Waste Glycerol Using a Co-Culture of Escherichia Coli and Enterobacter Sp. Fuel 2016, 186, 375–384. [Google Scholar] [CrossRef]
- Yazdani, S.S.; Gonzalez, R. Engineering Escherichia Coli for the Efficient Conversion of Glycerol to Ethanol and Co-Products. Metab. Eng. 2008, 10, 340–351. [Google Scholar] [CrossRef]
- Valle, A.; Cabrera, G.; Cantero, D.; Bolivar, J. Identification of Enhanced Hydrogen and Ethanol Escherichia Coli Producer Strains in a Glycerol-Based Medium by Screening in Single-Knock out Mutant Collections. Microb. Cell Fact. 2015, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cofré, O.; Ramírez, M.; Gómez, J.M.; Cantero, D. Pilot Scale Fed-Batch Fermentation in a Closed Loop Mixed Reactor for the Biotransformation of Crude Glycerol into Ethanol and Hydrogen by Escherichia Coli MG1655. Biomass and Bioenergy 2016, 91, 37–47. [Google Scholar] [CrossRef]
- Varrone, C.; Liberatore, R.; Crescenzi, T.; Izzo, G.; Wang, A. The Valorization of Glycerol: Economic Assessment of an Innovative Process for the Bioconversion of Crude Glycerol into Ethanol and Hydrogen. Appl. Energy 2013, 105, 349–357. [Google Scholar] [CrossRef]
- Nwachukwu, R.E.S.; Shahbazi, A.; Wang, L.; Ibrahim, S.; Worku, M.; Schimmel, K. Bioconversion of Glycerol to Ethanol by a Mutant Enterobacter Aerogenes. AMB Express 2012, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Durnin, G.; Clomburg, J.; Yeates, Z.; Alvarez, P.J.J.; Zygourakis, K.; Campbell, P.; Gonzalez, R. Understanding and Harnessing the Microaerobic Metabolism of Glycerol in Escherichia Coli. Biotechnol. Bioeng. 2009, 103, 148–161. [Google Scholar] [CrossRef]
- Hong, W.K.; Kim, C.H.; Heo, S.Y.; Luo, L.H.; Oh, B.R.; Seo, J.W. Enhanced Production of Ethanol from Glycerol by Engineered Hansenula Polymorpha Expressing Pyruvate Decarboxylase and Aldehyde Dehydrogenase Genes from Zymomonas Mobilis. Biotechnol. Lett. 2010, 32, 1077–1082. [Google Scholar] [CrossRef]
- Yang, G.; Tian, J.; Li, J. Fermentation of 1,3-Propanediol by a Lactate Deficient Mutant of Klebsiella Oxytoca under Microaerobic Conditions. Appl. Microbiol. Biotechnol. 2007, 73, 1017–1024. [Google Scholar] [CrossRef]
- Yu, K.O.; Kim, S.W.; Han, S.O. Engineering of Glycerol Utilization Pathway for Ethanol Production by Saccharomyces Cerevisiae. Bioresour. Technol. 2010, 101, 4157–4161. [Google Scholar] [CrossRef]
- Khanna, S.; Shukla, A.K.; Goyal, A.; Moholkar, V.S. Alcoholic Biofuels Production from Biodiesel Derived Glycerol by Clostridium Pasteurianum Whole Cells Immobilized on Silica. Waste and Biomass Valorization 2014, 5, 789–798. [Google Scholar] [CrossRef]
- Lin, D.S.; Yen, H.W.; Kao, W.C.; Cheng, C.L.; Chen, W.M.; Huang, C.C.; Chang, J.S. Bio-Butanol Production from Glycerol with Clostridium Pasteurianum CH4: The Effects of Butyrate Addition and in Situ Butanol Removal via Membrane Distillation. Biotechnol. Biofuels 2015, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Taconi, K.A.; Venkataramanan, K.P.; Johnson, D.T. Growth and Solvent Production by Clostridium Pasteurianum ATCC® 6013TM Utilizing Biodiesel-Derived Crude Glycerol as the Sole Carbon Source. Environ. Prog. Sustain. Energy 2009, 28, 100–110. [Google Scholar] [CrossRef]
- Biebl, H. Fermentation of Glycerol by Clostridium Pasteurianum — Batch and Continuous Culture Studies. J. Ind. Microbiol. Biotechnol. 2001, 27, 18–26. [Google Scholar] [CrossRef]
- Hong, W.K.; Yu, A.; Heo, S.Y.; Oh, B.R.; Kim, C.H.; Sohn, J.H.; Yang, J.W.; Kondo, A.; Seo, J.W. Production of Lipids Containing High Levels of Docosahexaenoic Acid from Empty Palm Fruit Bunches by Aurantiochytrium Sp. KRS101. Bioprocess Biosyst. Eng. 2013, 36, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Thyagarajan, T.; Puri, M.; Vongsvivut, J.; Barrow, C.J. Evaluation of Bread Crumbs as a Potential Carbon Source for the Growth of Thraustochytrid Species for Oil and Omega-3 Production. Nutr. 2014, Vol. 6, Pages 2104-2114 2014, 6, 2104–2114. [Google Scholar] [CrossRef]
- Ryu, B.G.; Kim, K.; Kim, J.; Han, J.I.; Yang, J.W. Use of Organic Waste from the Brewery Industry for High-Density Cultivation of the Docosahexaenoic Acid-Rich Microalga, Aurantiochytrium Sp. KRS101. Bioresour. Technol. 2013, 129, 351–359. [Google Scholar] [CrossRef]
- Fan, K.W.; Chen, F.; Jones, E.B.G.; Vrijmoed, L.L.P. Eicosapentaenoic and Docosahexaenoic Acids Production by and Okara-Utilizing Potential of Thraustochytrids. J. Ind. Microbiol. Biotechnol. 2001, 27, 199–202. [Google Scholar] [CrossRef]
- Unagul, P.; Assantachai, C.; Phadungruengluij, S.; Suphantharika, M.; Tanticharoen, M.; Verduyn, C. Coconut Water as a Medium Additive for the Production of Docosahexaenoic Acid (C22:6 N3) by Schizochytrium Mangrovei Sk-02. Bioresour. Technol. 2007, 98, 281–287. [Google Scholar] [CrossRef]
- Liang, Y.; Sarkany, N.; Cui, Y.; Yesuf, J.; Trushenski, J.; Blackburn, J.W. Use of Sweet Sorghum Juice for Lipid Production by Schizochytrium Limacinum SR21. Bioresour. Technol. 2010, 101, 3623–3627. [Google Scholar] [CrossRef]
- Quilodrán, B.; Hinzpeter, I.; Quiroz, A.; Shene, C. Evaluation of Liquid Residues from Beer and Potato Processing for the Production of Docosahexaenoic Acid (C22:6n-3, DHA) by Native Thraustochytrid Strains. World J. Microbiol. Biotechnol. 2009, 25, 2121–2128. [Google Scholar] [CrossRef]
- Sharma, K.K. Rapid Induction of Lipids and Harvesting of Microalgae for Biofuel and High Value Products, The University of Queensland, 2014.
- Abad, S.; Turon, X. Valorization of Biodiesel Derived Glycerol as a Carbon Source to Obtain Added-Value Metabolites: Focus on Polyunsaturated Fatty Acids. Biotechnol. Adv. 2012, 30, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, N.; Talbierz, S.; Dębowski, M.; Kazimierowicz, J.; Zieliński, M. Cultivation Method Effect on Schizochytrium Sp. Biomass Growth and Docosahexaenoic Acid (DHA) Production with the Use of Waste Glycerol as a Source of Organic Carbon. Energies 2021, Vol. 14, Page 2952 2021, 14, 2952. [Google Scholar] [CrossRef]
- Sun, X.M.; Ren, L.J.; Zhao, Q.Y.; Ji, X.J.; Huang, H. Enhancement of Lipid Accumulation in Microalgae by Metabolic Engineering. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 2019, 1864, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.Y.; Chen, F. Application of Statistically-Based Experimental Designs for the Optimization of Eicosapentaenoic Acid Production by the Diatom Nitzschia Laevis. Biotechnol. Bioeng. 2001, 75, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, F. Effects of Temperature and Temperature Shift on Docosahexaenoic Acid Production by the Marine Microalga Crypthecodinium Cohnii. JAOCS, J. Am. Oil Chem. Soc. 2000, 77, 613–617. [Google Scholar] [CrossRef]
- Richmond, A.E.; Soeder, C.J. Microalgaculture. Crit. Rev. Biotechnol. 1986, 4, 369–438. [Google Scholar] [CrossRef]
- Okoro, V.; Azimov, U.; Munoz, J.; Hernandez, H.H.; Phan, A.N. Microalgae Cultivation and Harvesting: Growth Performance and Use of Flocculants - A Review. Renew. Sustain. Energy Rev. 2019, 115, 109364. [Google Scholar] [CrossRef]
- Rawoof, S.A.A.; Kumar, P.S.; Vo, D.V.N.; Devaraj, K.; Mani, Y.; Devaraj, T.; Subramanian, S. Production of Optically Pure Lactic Acid by Microbial Fermentation: A Review. Environ. Chem. Lett. 2020 191 2020, 19, 539–556. [Google Scholar] [CrossRef]
- Li, S.; Hu, Z.; Yang, X.; Li, Y. Effect of Nitrogen Sources on Omega-3 Polyunsaturated Fatty Acid Biosynthesis and Gene Expression in Thraustochytriidae Sp. Mar. Drugs 2020, Vol. 18, Page 612 2020, 18, 612. [Google Scholar] [CrossRef]
- Orak, T.; Caglar, O.; Ortucu, S.; Ozkan, H.; Taskin, M. Chicken Feather Peptone: A New Alternative Nitrogen Source for Pigment Production by Monascus Purpureus. J. Biotechnol. 2018, 271, 56–62. [Google Scholar] [CrossRef]
- Pawar, P.R.; Lali, A.M.; Prakash, G. Integration of Continuous-High Cell Density-Fed-Batch Fermentation for Aurantiochytrium Limacinum for Simultaneous High Biomass, Lipids and Docosahexaenoic Acid Production. Bioresour. Technol. 2021, 325, 124636. [Google Scholar] [CrossRef]
- De Swaaf, M.E.; Sijtsma, L.; Pronk, J.T. High-Cell-Density Fed-Batch Cultivation of the Docosahexaenoic Acid Producing Marine Alga Crypthecodinium Cohnii. Biotechnol. Bioeng. 2003, 81, 666–672. [Google Scholar] [CrossRef]
- Hong, W.K.; Rairakhwada, D.; Seo, P.S.; Park, S.Y.; Hur, B.K.; Kim, C.H.; Seo, J.W. Production of Lipids Containing High Levels of Docosahexaenoic Acid by a Newly Isolated Microalga, Aurantiochytrium Sp. KRS101. Appl. Biochem. Biotechnol. 2011, 164, 1468–1480. [Google Scholar] [CrossRef]
- Chen, Y.H.; Walker, T.H. Biomass and Lipid Production of Heterotrophic Microalgae Chlorella Protothecoides by Using Biodiesel-Derived Crude Glycerol. Biotechnol. Lett. 2011, 33, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
- Talbierz, S.; Dębowski, M.; Kujawska, N.; Kazimierowicz, J.; Zieliński, M. Optimization of Lipid Production by Schizochytrium Limacinum Biomass Modified with Ethyl Methane Sulfonate and Grown on Waste Glycerol. Int. J. Environ. Res. Public Heal. 2022, Vol. 19, Page 3108 2022, 19, 3108. [Google Scholar] [CrossRef] [PubMed]
- Pyle, D.J.; Garcia, R.A.; Wen, Z. Producing Docosahexaenoic Acid (DHA)-Rich Algae from Biodiesel-Derived Crude Glycerol: Effects of Impurities on DHA Production and Algal Biomass Composition. J. Agric. Food Chem. 2008, 56, 3933–3939. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Sarkany, N.; Cui, Y.; Blackburn, J.W. Batch Stage Study of Lipid Production from Crude Glycerol Derived from Yellow Grease or Animal Fats through Microalgal Fermentation. Bioresour. Technol. 2010, 101, 6745–6750. [Google Scholar] [CrossRef] [PubMed]
- Rattanapoltee, P.; Dujjanutat, P.; Muanruksa, P.; Kaewkannetra, P. Biocircular Platform for Third Generation Biodiesel Production: Batch/Fed Batch Mixotrophic Cultivations of Microalgae Using Glycerol Waste as a Carbon Source. Biochem. Eng. J. 2021, 175, 108128. [Google Scholar] [CrossRef]
- Yokochi, T.; Honda, D.; Higashihara, T.; Nakahara, T. Optimization of Docosahexaenoic Acid Production by Schizochytrium Limacinum SR21. Appl. Microbiol. Biotechnol. 1998, 49, 72–76. [Google Scholar] [CrossRef]
- Kujawska, N.; Talbierz, S.; Dębowski, M.; Kazimierowicz, J.; Zieliński, M. Effect of the Concentration of Extracellular Polymeric Substances (EPS) and Aeration Intensity on Waste Glycerol Valorization by Docosahexaenoic Acid (DHA) Produced in Heterotrophic Culture of Schizochytrium Sp. Appl. Sci. 2021, Vol. 11, Page 9573 2021, 11, 9573. [Google Scholar] [CrossRef]
- Liu, L. ping; Hu, Y.; Lou, W. yong; Li, N.; Wu, H.; Zong, M. hua. Use of Crude Glycerol as Sole Carbon Source for Microbial Lipid Production by Oleaginous Yeasts. Appl. Biochem. Biotechnol. 2017, 182, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Polburee, P.; Limtong, S. Economical Lipid Production from Crude Glycerol Using Rhodosporidiobolus Fluvialis DMKU-RK253 in a Two-Stage Cultivation under Non-Sterile Conditions. Biomass and Bioenergy 2020, 138, 105597. [Google Scholar] [CrossRef]
- Souza, K.S.T.; Ramos, C.L.; Schwan, R.F.; Dias, D.R. Lipid Production by Yeasts Grown on Crude Glycerol from Biodiesel Industry. Prep. Biochem. Biotechnol. 2017, 47, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, J.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y.; Li, J. Lipid Production for Biodiesel from Sludge and Crude Glycerol. Water Environ. Res. 2017, 89, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Yan, S.; Tyagi, R.D.; Drogui, P. Lipid Production from Fed-Batch Fermentation of Crude Glycerol Directed by the Kinetic Study of Batch Fermentations. Fuel 2017, 209, 1–9. [Google Scholar] [CrossRef]
- Gupta, P.; Sahoo, P.C.; Sandipam, S.; Gupta, R.P.; Kumar, M. Fermentation of Biodiesel-Derived Crude Glycerol to 1,3-Propanediol with Bio-Wastes as Support Matrices: Polynomial Prediction Model. Enzyme Microb. Technol. 2023, 170, 110292. [Google Scholar] [CrossRef]
- Susmozas, A.; Martín-Sampedro, R.; Ibarra, D.; Eugenio, M.E.; Iglesias, R.; Manzanares, P.; Moreno, A.D. Process Strategies for the Transition of 1G to Advanced Bioethanol Production. Process 2020, 8, 1310. [Google Scholar] [CrossRef]
- Himmi, E.H.; Bories, A.; Barbirato, F. Nutrient Requirements for Glycerol Conversion to 1,3-Propanediol by Clostridium Butyricum. Bioresour. Technol. 1999, 67, 123–128. [Google Scholar] [CrossRef]
- Cardona, C.; Posada, J.; Montoya, M. Use of Glycerol from Biodiesel Production: Conversion to Added Value Products. Proceedings of European Congress of Chemical Engineering (ECCE-6), Copenhagen, 16-20 September 2007; 2007. [Google Scholar]
- Braun, M.; Santana, C.S.; Garcia, A.C.; Andronescu, C. From Waste to Value—Glycerol Electrooxidation for Energy Conversion and Chemical Production. Curr. Opin. Green Sustain. Chem. 2023, 41, 100829. [Google Scholar] [CrossRef]
- Ripoll, M.; Betancor, L. Opportunities for the Valorization of Industrial Glycerol via Biotransformations. Curr. Opin. Green Sustain. Chem. 2021, 28, 100430. [Google Scholar] [CrossRef]
- Ripoll, M.; Jackson, E.; Trelles, J.A.; Betancor, L. Dihydroxyacetone Production via Heterogeneous Biotransformations of Crude Glycerol. J. Biotechnol. 2021, 340, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Bumyut, A.; Champreda, V.; Singhakant, C.; Kanchanasuta, S. Effects of Immobilization of Actinobacillus Succinogenes on Efficiency of Bio-Succinic Acid Production from Glycerol. Biomass Convers. Biorefinery 2022, 12, 643–654. [Google Scholar] [CrossRef]
- D’ambrosio, S.; Alfano, A.; Cimini, D. Production of Succinic Acid From Basfia Succiniciproducens. Front. Chem. Eng. 2021, 3, 785691. [Google Scholar] [CrossRef]
- Bretz, K. Succinic Acid Production in Fed-Batch Fermentation of Anaerobiospirillum Succiniciproducens Using Glycerol as Carbon Source. Chem. Eng. Technol. 2015, 38, 1659–1664. [Google Scholar] [CrossRef]
- Leyton, A.; Shene, C.; Chisti, Y.; Asenjo, J.A. Production of Carotenoids and Phospholipids by Thraustochytrium Sp. in Batch and Repeated-Batch Culture. Mar. Drugs 2022, 20, 416. [Google Scholar] [CrossRef]
- Nabi, F.; Arain, M.A.; Rajput, N.; Alagawany, M.; Soomro, J.; Umer, M.; Soomro, F.; Wang, Z.; Ye, R.; Liu, J. Health Benefits of Carotenoids and Potential Application in Poultry Industry: A Review. J. Anim. Physiol. Anim. Nutr. (Berl). 2020, 104, 1809–1818. [Google Scholar] [CrossRef]
- Stachowiak, B.; Szulc, P. Astaxanthin for the Food Industry. Mol. 2021, Vol. 26, Page 2666 2021, 26, 2666. [Google Scholar] [CrossRef]
- Luna-Flores, C.H.; Wang, A.; Cui, Z.; von Hellens, J.; Speight, R.E. An Enhanced Electron Transport Chain Improved Astaxanthin Production in Phaffia Rhodozyma. Biotechnol. Bioeng. 2023, 120, 1382–1398. [Google Scholar] [CrossRef]
- Cardoso, L.A.C.; Jäckel, S.; Karp, S.G.; Framboisier, X.; Chevalot, I.; Marc, I. Improvement of Sporobolomyces Ruberrimus Carotenoids Production by the Use of Raw Glycerol. Bioresour. Technol. 2016, 200, 374–379. [Google Scholar] [CrossRef]
- Papadaki, E.; Mantzouridou, F.T. Natural β-Carotene Production by Blakeslea Trispora Cultivated in Spanish-Style Green Olive Processing Wastewaters. Foods 2021, Vol. 10, Page 327 2021, 10, 327. [Google Scholar] [CrossRef]
- Cutzu, R.; Coi, A.; Rosso, F.; Bardi, L.; Ciani, M.; Budroni, M.; Zara, G.; Zara, S.; Mannazzu, I. From Crude Glycerol to Carotenoids by Using a Rhodotorula Glutinis Mutant. World J. Microbiol. Biotechnol. 2013, 29, 1009–1017. [Google Scholar] [CrossRef]
- Cuaresma, M.; Casal, C.; Forján, E.; Vílchez, C. Productivity and Selective Accumulation of Carotenoids of the Novel Extremophile Microalga Chlamydomonas Acidophila Grown with Different Carbon Sources in Batch Systems. J. Ind. Microbiol. Biotechnol. 2011, 38, 167–177. [Google Scholar] [CrossRef]
- Morgunov, I.G.; Kamzolova, S.V.; Lunina, J.N. Citric Acid Production by Yarrowia Lipolytica Yeast on Different Renewable Raw Materials. Ferment. 2018, Vol. 4, Page 36 2018, 4, 36. [Google Scholar] [CrossRef]
- Volova, T.; Demidenko, A.; Kiselev, E.; Baranovskiy, S.; Shishatskaya, E.; Zhila, N. Polyhydroxyalkanoate Synthesis Based on Glycerol and Implementation of the Process under Conditions of Pilot Production. Appl. Microbiol. Biotechnol. 2019, 103, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Mothes, G.; Schnorpfeil, C.; Ackermann, J.U. Production of PHB from Crude Glycerol. Eng. Life Sci. 2007, 7, 475–479. [Google Scholar] [CrossRef]
- Tang, S.; Boehme, L.; Lam, H.; Zhang, Z. Pichia Pastoris Fermentation for Phytase Production Using Crude Glycerol from Biodiesel Production as the Sole Carbon Source. Biochem. Eng. J. 2009, 43, 157–162. [Google Scholar] [CrossRef]
- Habe, H.; Sato, Y.; Tani, H.; Matsutani, M.; Tanioka, K.; Theeragool, G.; Matsushita, K.; Yakushi, T. Heterologous Expression of Membrane-Bound Alcohol Dehydrogenase–Encoding Genes for Glyceric Acid Production Using Gluconobacter Sp. CHM43 and Its Derivatives. Appl. Microbiol. Biotechnol. 2021, 105, 6749–6758. [Google Scholar] [CrossRef] [PubMed]
- Volpato, G.; Rodrigues, R.C.; Heck, J.X.; Ayub, M.A.Z. Production of Organic Solvent Tolerant Lipase by Staphylococcus Caseolyticus EX17 Using Raw Glycerol as Substrate. J. Chem. Technol. Biotechnol. 2008, 83, 821–828. [Google Scholar] [CrossRef]
- Liu, Y.; Koh, C.M.J.; Ji, L. Bioconversion of Crude Glycerol to Glycolipids in Ustilago Maydis. Bioresour. Technol. 2011, 102, 3927–3933. [Google Scholar] [CrossRef]
- Nitayavardhana, S.; Khanal, S.K. Biodiesel-Derived Crude Glycerol Bioconversion to Animal Feed: A Sustainable Option for a Biodiesel Refinery. Bioresour. Technol. 2011, 102, 5808–5814. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.A.; Nemashkalov, V.A.; Stepanova, N.N.; Kamzolova, S.V.; Rymowicz, W.; Morgunov, I.G. The Effect of PH and Temperature on Arachidonic Acid Production by Glycerol-Grown Mortierella Alpina NRRL-A-10995. Ferment. 2018, Vol. 4, Page 17 2018, 4, 17. [Google Scholar] [CrossRef]
- Ji, Y. Recent Development of Heterogeneous Catalysis in the Transesterification of Glycerol to Glycerol Carbonate. Catal. 2019, Vol. 9, Page 581 2019, 9, 581. [Google Scholar] [CrossRef]
- Oliveira, M.; Ramos, A.; Monteiro, E.; Rouboa, A. Improvement of the Crude Glycerol Purification Process Derived from Biodiesel Production Waste Sources through Computational Modeling. Sustain. 2022, Vol. 14, Page 1747 2022, 14, 1747. [Google Scholar] [CrossRef]
- Shakeri, S.; Khoshbasirat, F.; Maleki, M. Rhodosporidium Sp. DR37: A Novel Strain for Production of Squalene in Optimized Cultivation Conditions. Biotechnol. Biofuels 2021, 14, 1–14. [Google Scholar] [CrossRef]
- Yang, Q.; Xie, Z.; Zheng, X.; Li, K.; Lu, T.; Lu, Y.; Chen, C.; Ling, X. Genetic Regulation and Fermentation Strategy for Squalene Production in Schizochytrium Sp. Appl. Microbiol. Biotechnol. 2022, 106, 2415–2431. [Google Scholar] [CrossRef]
- Kośmider, A.; Białas, W.; Kubiak, P.; Drozdzyńska, A.; Czaczyk, K. Vitamin B12 Production from Crude Glycerol by Propionibacterium Freudenreichii Ssp. Shermanii: Optimization of Medium Composition through Statistical Experimental Designs. Bioresour. Technol. 2012, 105, 128–133. [Google Scholar] [CrossRef]
- Kanagavalli, B.; Manoj, G.; Devaraj, S.; Hannah, S.; Sai Saran, K. Optimization of Vitamin B 12 Synthesis Using Propionibacterium Sp. Utilizing Dairy Wastes. Int. J. Adv. Eng. Manag. 2022, 4, 518. [Google Scholar] [CrossRef]
- Pawlicka-Kaczorowska, J.; Czaczyk, K. Effect of Crude and Pure Glycerol on Biomass Production and Trehalose Accumulation by Propionibacterium Freudenreichii Ssp. Shermanii 1. Acta Biochim. Pol. 2017, 64, 621–629. [Google Scholar] [CrossRef]
- Piwowarek, K.; Lipińska, E.; Hać-Szymańczuk, E.; Kot, A.M.; Kieliszek, M.; Bonin, S. Use of Propionibacterium Freudenreichii T82 Strain for Effective Biosynthesis of Propionic Acid and Trehalose in a Medium with Apple Pomace Extract and Potato Wastewater. Mol. 2021, Vol. 26, Page 3965 2021, 26, 3965. [Google Scholar] [CrossRef] [PubMed]
- Luna-Flores, C.H.; Wang, A.; von Hellens, J.; Speight, R.E. Towards Commercial Levels of Astaxanthin Production in Phaffia Rhodozyma. J. Biotechnol. 2022, 350, 42–54. [Google Scholar] [CrossRef] [PubMed]
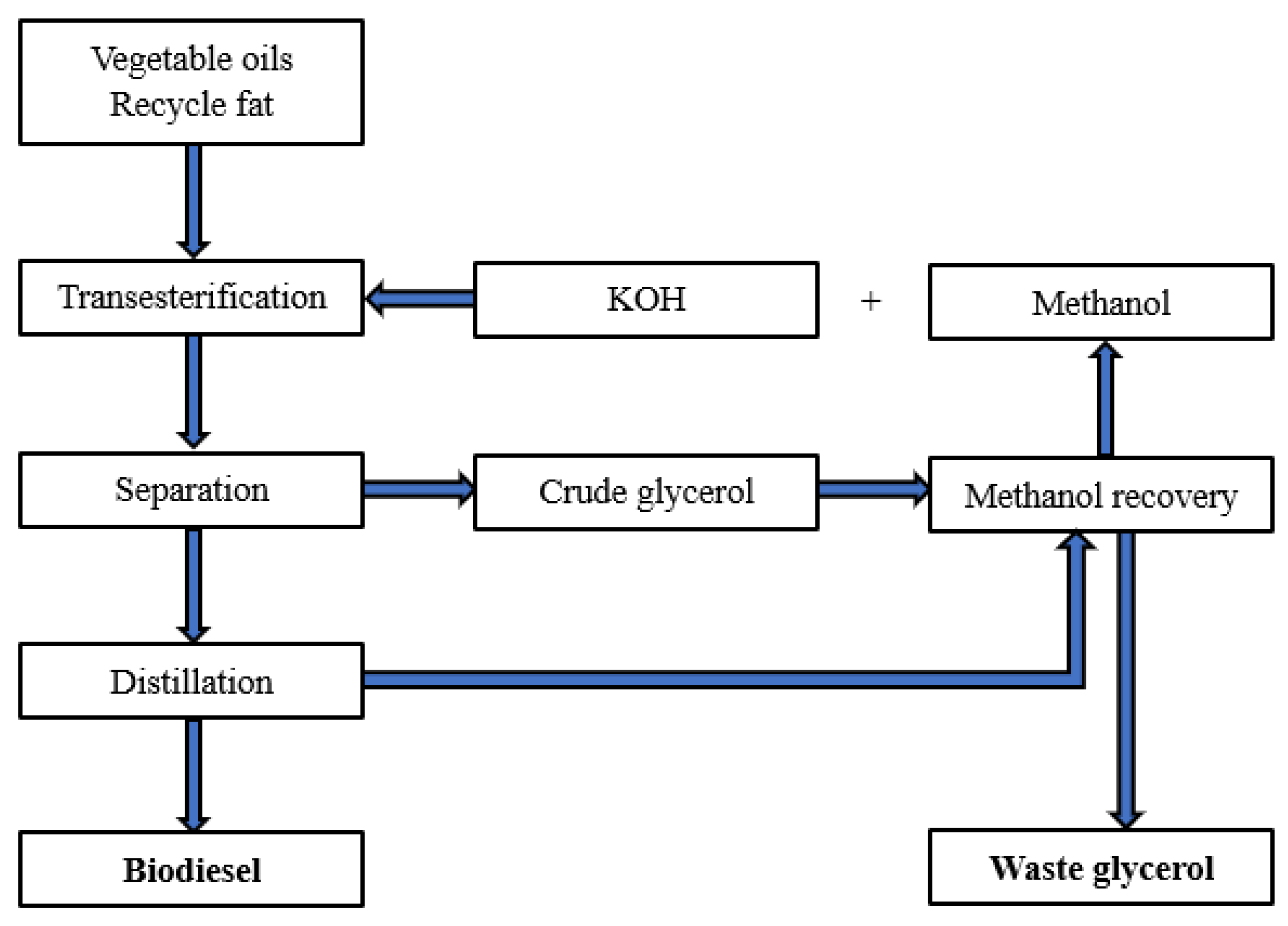
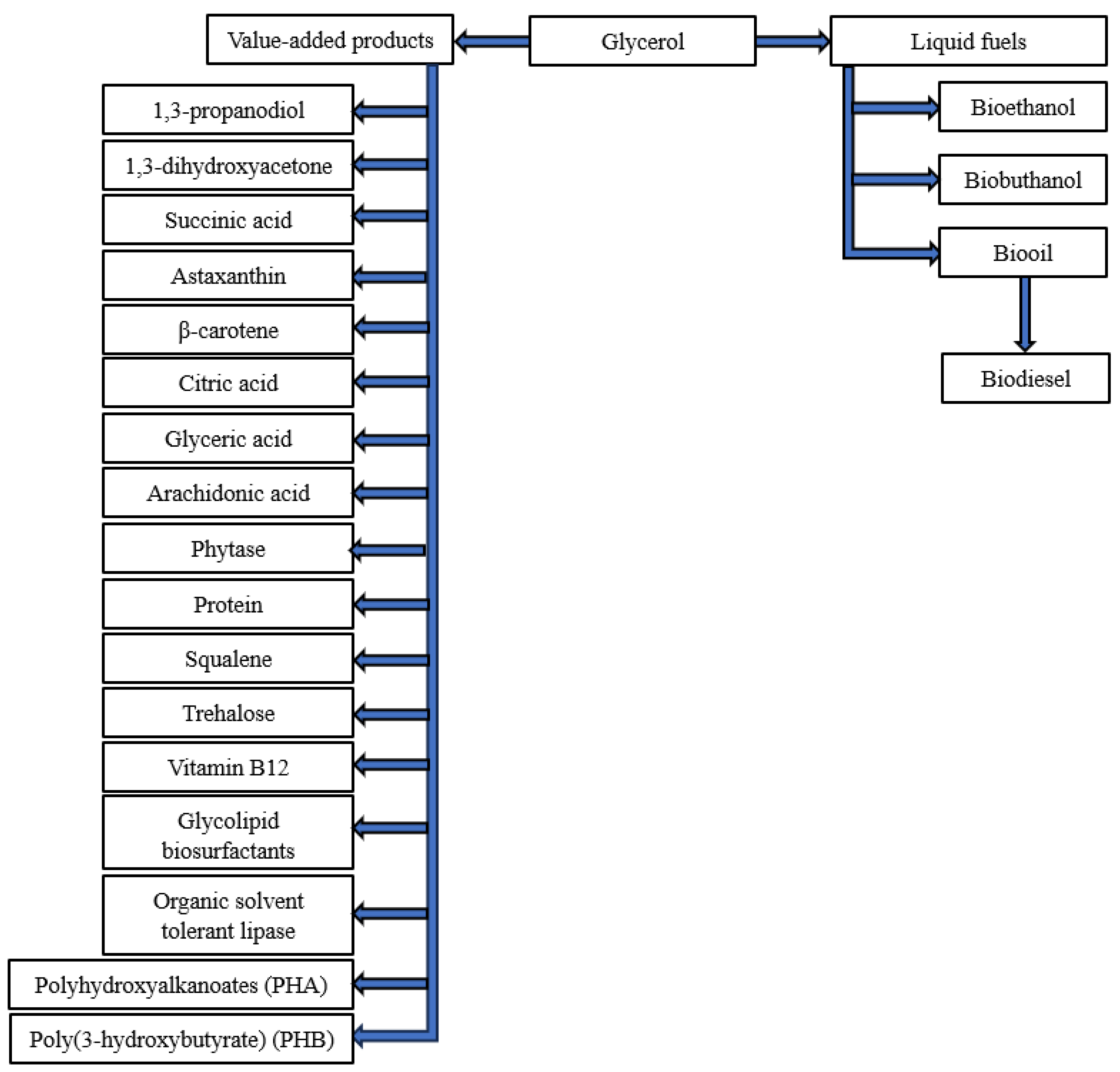


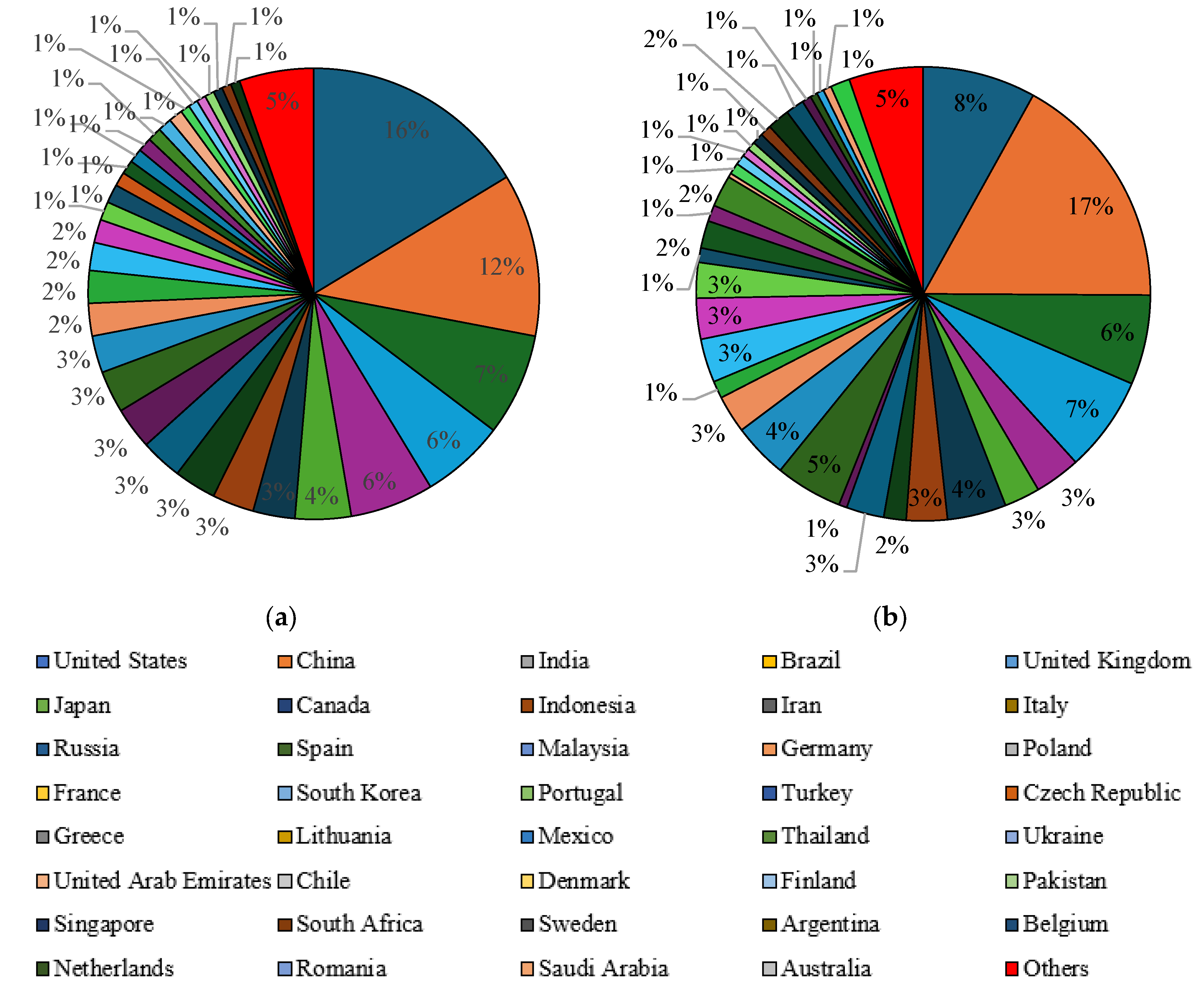
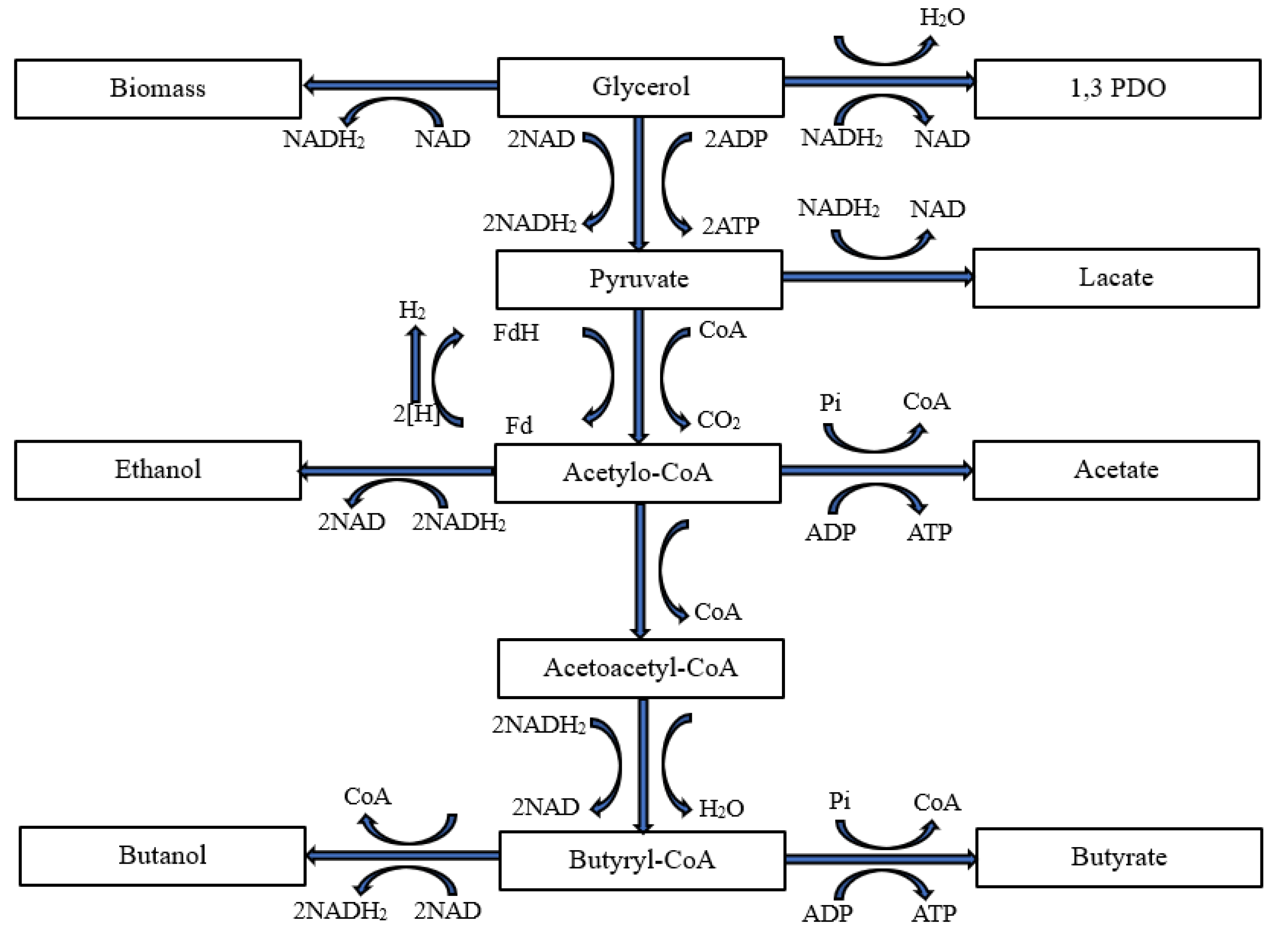
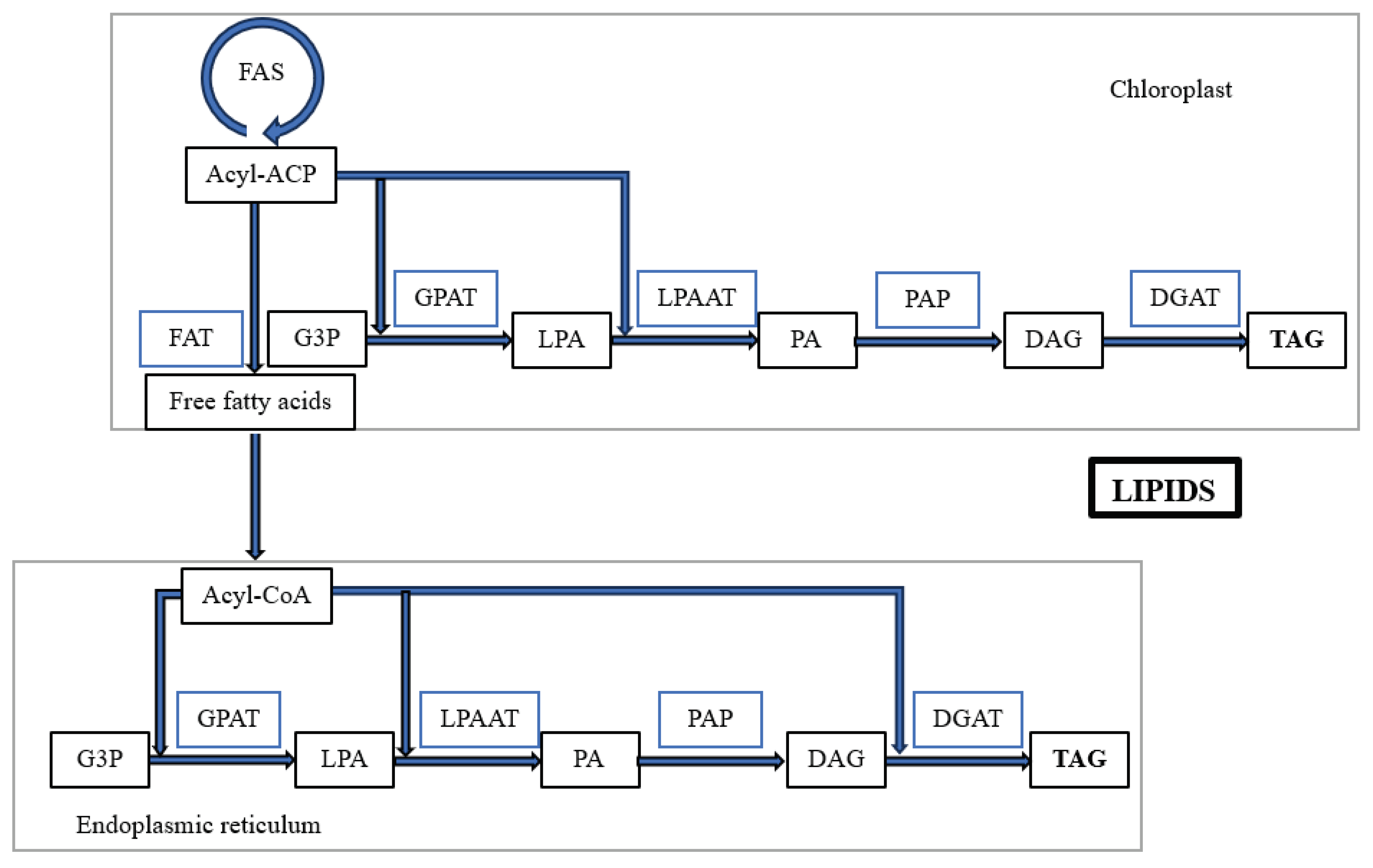
| Substrate | Experimental details | Glicerol concentration | Production/yield | Ref. |
|---|---|---|---|---|
| E. aerogenes SUMI014 | 100 mL serum bottle, 34°C, pH 7,5, 78 h, 200 rpm | 60 g/L | 34.54 g/L | [51] |
| E. aerogenes SUMI2008 | 60 g/L | 38.32 g/L | [51] | |
| E. aerogenes ATCC 29007 | 60 g/L | 13.09 g/L | [51] | |
| Enterobacter aerogenes HU-101 | Cylindrical glass column reactor, 37°C, pH 6.8 | 1.7 g/L | 0.96 g/L (ethanol), 1.12 g/L (hydrogen), 0.2 g/L (acetate), 0.2 g/L (1,3-PDO), 0.14 g/L (formate) |
[36] |
| 3.3 g/L | 0.83 g/L (ethanol), 0.9 g/L (hydrogen), 0.1 g/L (acetate), 0.22 g/L (1,3-PDO), 0.05 g/L (lactate), 0.2 g/L (formate) |
[36] | ||
| 10 g/L | 0.67 g/L (ethanol), 0.71 g/L (hydrogen), 0.09 g/L (acetate), 0.12 g/L (1,3-PDO), 0.11 g/L (lactate), 0.19 g/L (formate) |
[36] | ||
| 25 g/L | 0.56 g/L (ethanol), 0.71 g/L (hydrogen), 0.06 g/L (acetate), 0.17 g/L (1,3-PDO), 0.17 g/L (lactate) |
[36] | ||
| E. aerogenes ATCC 13048 | 125 mL serum bottles, 37°C, 120 rpm | 20 g/L | 12.8 g/L | [57] |
| E-Coli LY180 | 1.2 L fermenters, 37°C, pH 7.0, 24 h, 150 rpm | 50% v/v | 75 g/L | [49] |
| E-Coli EH05 | Multi-fermentation system with six 300 mL working volume vessels, 37.5°C, pH 6.3—7.5, 300 rpm | 20 g/L | 20.7 g/L | [58] |
| Hansenula polymorpha DL1-L | 250 mL flasks | 2% v/v | 2.74 g/L | [59] |
| K. cryocrescens | 2.5 L stirring bioreactor, 30°C, pH 7.0, 500 rpm | 25 g/L | 27 g/L | [46] |
| Klebsiella oxytoca M5al | 7.5 L stirring bioreactor, 37°C, pH 7, 300 rpm | 60 g/L | 12.26 g/L (ethanol), 13.31 g/L (acetate), 39.14 g/L (1,3-PDO), 16.73 g/L (lactate), 5.27 g/L (butanediol), 3.77 g/L (succinic acid) |
[60] |
| K. pneumoniae GEM167 | 5 L stirred-vessel system, 37°C, 200 rpm | 20 g/L | 20.5 g/L | [45] |
|
O. polymorpha with genes of PDC1 and ADH1 |
300 mL erlenmeyer flasks, 45°C, 140 rpm | 150 g/L | 5.0 g/L | [50] |
|
Enterobacter spH1 and E. coli CECT432 |
1.2 L jacketed bioreactor, 37°C, 72 h, 200 rpm | 289,7 mmol/L | 220,77 mmol/L (ethanol), 278,7 mmol/L (hydrogen) |
[52] |
| Saccharomyces cerevisiae YPH499 | batch | 20 g/L | 0.14 g/g glycerol | [61] |
| Microbial mixed culture | 3 L bioreactor, 37°C, pH 8, 120 rpm | 20 g/L | 26 g/L (ethanol), 9 L/L fermenter (hydrogen) |
[56] |
| Substrate | Experimental details | Glicerol concentration | Production/yield | Ref. |
|---|---|---|---|---|
| C. pasteurianum CH4 | 2 L bioreactor, 37°C, pH 5,5, 100 rpm | 100 g/L | 0.24 mol/mol crude glycerol (butanol) |
[63] |
| 2 L bioreactor, 37°C, pH 5,5, 100 rpm, 6 g/L butyrate as precursor | 100 g/L | 0.34 mol/mol crude glycerol (butanol) |
[63] | |
| 2 L bioreactor, 37°C, pH 5,5, 100 rpm, 6 g/L butyrate as precursor, vacuum membrane distillation (VMD) during the cultivation | 100 g/L | 0.39 mol/mol crude glycerol (butanol) |
[63] | |
| C. pasteurianum MTCC 116 (free cells) | 250 mL custom fabricated anaerobic flasks, 30°C, 24 h, 150 rpm | 5 g/L | 0.08 g/L (butanol), 0.25 g/L (1,3-PDO), 0,04 g/L (etanol) |
[62] |
| 10 g/L | 0.01 g/L (butanol), 0.28 g/L (1,3-PDO), 0,02 g/L (etanol) |
[62] | ||
| 15 g/L | 0.08 g/L (butanol), 0.11 g/L (1,3-PDO), 0,01 g/L (etanol) |
[62] | ||
|
C. pasteurianum MTCC 116 cells (immobilized cells) |
250 mL custom fabricated anaerobic flasks, 30°C, 24 h, 150 rpm | 5 g/L | 0.07 g/L (butanol), 0.31 g/L (1,3-PDO), 0,06 g/L (etanol) |
[62] |
| 10 g/L | 0.01 g/L (butanol), 0.26 g/L (1,3-PDO), 0,13 g/L (etanol) |
[62] | ||
| 15 g/L | 0.14 g/L (butanol), 0.17 g/L (1,3-PDO), 0,04 g/L (etanol) |
[62] | ||
| C. pasteurianum DSM 525 | 160 mL serum bottles, 37°C, pH 6,8±0,2 | 5 g/L | 0.17 g/L (butanol), 1.8 g/L (1,3-PDO), 0,06 g/L (ethanol), 0,74 g/L (acetic acid), 0,87 g/L (butyric acid), 0,33 g/L (lactic acid) |
[40] |
| 10 g/L | 0.77±0.19 g/L (butanol), 2.71±0.21 g/L (1,3-PDO), 0.18±0.01 g/L (ethanol), 0.99±0.05 g/L (acetic acid), 0.94±0.05 g/L (butyric acid), 0.51±0.07 g/L (lactic acid) |
[40] | ||
| 15 g/L | 2.21±0.11 g/L (butanol), 4.8±0.16 g/L (1,3-PDO), 0.34 g/L (ethanol), 1.52±0.12 g/L (acetic acid), 1.63±0.05 g/L (butyric acid), 1.61±0.05 g/L (lactic acid) |
[40] | ||
| 20 g/L | 2.42±0.1 g/L (butanol), 5.89±0.23 g/L (1,3-PDO), 0.26 g/L (ethanol), 1.66±0.1 g/L (acetic acid), 1.83±0.17 g/L (butyric acid), 0.84±0.1 g/L (lactic acid) |
[40] | ||
| 35 g/L | 6.71±0.43 g/L (butanol), 6.86±0.51 g/L (1,3-PDO), 0.59±0.05 g/L (ethanol), 0.83±0.08 g/L (acetic acid), 0.43±0.08 g/L (butyric acid), 0.73±0.18 g/L (lactic acid) |
[40] | ||
| 50 g/L | 6.73±0.39 g/L (butanol), 6.26±0.27 g/L (1,3-PDO), 0.68±0.04 g/L (ethanol), 0.69±0.03 g/L (acetic acid), 0.21±0.03 g/L (butyric acid), 1.24±0.04 g/L (lactic acid) |
[40] | ||
| C. pasteurianum ATCC 6013 | 35°C, pH 7 | 5 g/L | 0.04 mol/mol glycerol consumed (butanol), 0.02 mol/mol glycerol consumed (1,3-PDO), 0.46 mol/mol glycerol consumed (ethanol), 0.04 mol/mol glycerol consumed (acetate), 0.07 mol/mol glycerol consumed (butyrate) |
[64] |
| 25 g/L | 0.37 mol/mol glycerol consumed (butanol), 0.078 mol/mol glycerol consumed (1,3-PDO), 0.13 mol/mol glycerol consumed (ethanol), 0.057 mol/mol glycerol consumed (acetate), 0.056 mol/mol glycerol consumed (butyrate) |
[64] | ||
| C. pasteurianum DSM 525 | 1 L bioreactor, 35°C, pH 6 | 29.5 g/L | 17.1 mol/100 mol glycerol (butanol), 26.4 mol/100 mol glycerol (1,3-PDO), 1.5 mol/100 mol glycerol (ethanol), 3.2 mol/100 mol glycerol (acetate), 13.1 mol/100 mol glycerol (butyrate), 1.3 mol/100 mol glycerol (lactate) |
[65] |
| 54.2 g/L | 27 mol/100 mol glycerol (butanol), 10.5 mol/100 mol glycerol (1,3-PDO), 2.5 mol/100 mol glycerol (ethanol), 1.2 mol/100 mol glycerol (acetate), 3.9 mol/100 mol glycerol (butyrate) |
[65] | ||
| 83.7 g/L | 18 mol/100 mol glycerol (butanol), 23.4 mol/100 mol glycerol (1,3-PDO), 3.6 mol/100 mol glycerol (ethanol), 3.6 mol/100 mol glycerol (acetate), 3.9 mol/100 mol glycerol (butyrate), 3.2 mol/100 mol glycerol (lactate) |
[65] | ||
| 114.6 g/L | 28.1 mol/100 mol glycerol (butanol), 10.5 mol/100 mol glycerol (1,3-PDO), 4,2 mol/100 mol glycerol (ethanol), 4.2 mol/100 mol glycerol (acetate), 3.3 mol/100 mol glycerol (butyrate), 1.2 mol/100 mol glycerol (lactate) |
[65] |
| Substrate | Experimental details | Glicerol concentration | Production/yield | Ref. |
|---|---|---|---|---|
| Schizochytrium sp. | Biostat B Twin (Sartorius Stedim) bioreactor with a working capacity of 2 L, 27 °C, oxygen concentration 50%, initial peptone concentration 10 g/L, pH 6.5, volumetric airflow rate 0.3 Lair/min·Lreact., salinity 17.5 PSU, initial yeast extract concentration 0.4 g/L, 175 rpm | 150 g/L | 48.85±0.81 g/L | [75] |
| Schizochytrium limacinum SR-21 (ATCC MYA-1381) | 250 mL erlenmeyer flasks, 20 °C, 170 rpm, glycerol derived from soybean oil by Virginia Biodiesel Refinery (West Point, VA) | 75 g/L | 43.24±1.28% | [89] |
| 250 mL erlenmeyer flasks, 20 °C, 170 rpm, glycerol from a 50:50 (w/w) chicken fat and soybean oil mixture by Virginia Biodiesel | 75 g/L | 50.57±1.32% | ||
| 250 mL erlenmeyer flasks, 20 °C, 170 rpm, glycerol from canola oil by Seattle Biodiesel LLC (Seattle, WA) | 75 g/L | 46.71±1.01% | ||
| Schizochytrium sp. ATCC | 27 °C, peptone concentration 10 g/L, oxygen mass transfer rate kLa. 150 1/h, salinity 17.5 psu, pH 6.5, yeast extract concentration 0.4 g/L, 185 rpm, and inoculum DCW 5.0 g/L | 150 g/L | 69.44±0.76 g/L | [93] |
| Schizochytrium limacinum E20 | Biostat B Twin (Sartorius Stedim) bioreactor with a working capacity of 2 L, 26 °C, oxygen concentration 30%, pH 6.5±0,1 | 223,0 g/L | 48±1.2% | [88] |
| Schizochytrium limacinum C | 42±0.9% | |||
| Schizochytrium limacinum SR21 | 250-mL Erlenmeyer flasks, pH 7, 170 rpm | 23 g/L | 65.8±1.3% | [90] |
| Scenedesmus incrassulatus PPAY1 | 3-L tubular bioreactors, 28–32 ºC | 5 g/L | 31.50±0.71%; 0.51±0.01 g/dm3 |
[91] |
| 10 g/L | 38.49±0.26%; 0.74±0.02 g/dm3 |
|||
| 20 g/L | 44.64±0.19%; 1.26±0.01 g/dm3 |
|||
| 30 g/L | 50.25±0.35%; 1.22±0.02 g/dm3 |
|||
| 5 g/L | 38.03±0.35%; 1.08±0.02 g/dm3 |
|||
| 10 g/L | 47.15±0.49%; 1.46±0.03 g/dm3 |
|||
| 20 g/L | 58.27±0.05%; 2.45±0.01 g/dm3 |
|||
| 30 g/L | 52.01±0.01%; 2.94±0.03 g/dm3 |
|||
| Trichosporon fermentans | 250-mL conical flask, 28°C, pH 6, C/N 60, inoculum concentration 10% | 50 g/L | 5.2 g/L | [94] |
| Trichosporon cutaneum | 250-mL conical flask, 30°C, pH 6, C/N 60, inoculum concentration 10% | 70 g/L | 5.6 g/L | |
| Rhodosporidiobolus fluvialis DMKU-RK253 | 500-mL Erlenmeyer flask, 30°C pH 7, two-stage cultivation | 57 g/L | 27.81±1.86 g/L | [95] |
| Yarrowia lipolytica CCMA 0357 | 500-mL flasks, 28°C, 120 h, 150 rpm | 100 g/L | 70% (wt/wt) | [96] |
| Trichosporon oleaginosus ATCC 20905 | 15-L fermenter, 28°C, pH 6.5, 300-400 rpm | 25 g/L | 32.0% w/w (9.35 g/ L) | [97] |
| 50 g/L | 33.6% (10.13 g/L) | |||
| 100 g/L | 33.3% (9.13 g/L) | |||
| 150 g/L | 33.1% (9.03 g/L) | |||
| Trichosporon oleaginosus ATCC 20905 | 15-L fermenter, batch, 30°C, pH 5±0.1, 500 rpm, C/N 20 w/w | 45 mL | 22.98% w/w (3.08 g/L) | [98] |
| 15-L fermenter, batch, 30°C, pH 5±0.1, 500 rpm, C/N 30 w/w | 67 mL | 47.5% w/w (11.26 g/L) | ||
| 15-L fermenter, batch, 30°C, pH 5±0.1, 500 rpm, C/N 45 w/w | 101 mL | 48.95% w/w (12.14 g/L) | ||
| 15-L fermenter, batch, 30°C, pH 5±0.1, 500 rpm, C/N 60 w/w | 133 mL | 52.02% w/w (10.04 g/L) | ||
| 15-L fermenter, fed-batch, 30°C, pH 5±0.1, 500 rpm, C/N 45 w/w | 101 mL | 49.89% w/w (21.87 g/L) |
| Microorganism | Product | Ref. |
|---|---|---|
| Clostridium sp. |
1,3-propanediol |
[99,100] |
| Clostridium butyricum CNCM 1211 | [101] | |
| Klebsiella pneumoniae DSM-2026 | [102] | |
| Acetobacter xylinum | 1,3-dihydroxyacetone | [104] |
| Gluconobacter oxydans | [105] | |
| Actinobacillus succinogenes ATCC 55618™ | Succinic acid | [106] |
| Basfia succinoproducens | [107] | |
| Anaerobiospirillum succiniciproducens DSMZ 6400 | [108] | |
| Phaffia rhodozyma BPAX-A1 | Astaxanthin | [112] |
| Phaffia rhodozyma CBS 6938 | [134] | |
| Sporobolomyces ruberrimus H110 | [113] | |
| Blakeslea trispora ATCC 14271 | β-carotene | [114] |
| Rhodotorula glutinis | [115] | |
| Chlamydomonas acidophila | [116] | |
| Yarrowia lipolytica | Citric acid | [117] |
| Cupriavidus eutrophus B-10646 | Polyhydroxyalkanoates (PHA) | [118] |
| Paracoccus denitrificans i Cupriavidus necator JMP 134 | Poly(3-hydroxybutyrate) (PHB) | [119] |
| Pichia pastoris | Phytase | [120] |
| Gluconobacter sp. CHM43 | Glyceric acid | [121] |
| Staphylococcus caseolyticus EX17 | Organic solvent tolerant lipase | [122] |
| Ustilago maydis | Glycolipid biosurfactants | [123] |
| Rhizopus microsporus var. oligosporus | Protein | [124] |
| Mortierella alpina NRRL-A-10995 | Arachidonic acid | [125] |
| Rhodosporidium sp. DR37 | Squalene | [128] |
| Propionibacterium freudenreichii ssp. shermanii | Vitamin B12 | [130] |
| Propionibacterium freudenreichii ssp. shermanii 1 | Trehalose | [132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





