Submitted:
09 March 2024
Posted:
11 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Transthoracic Echocardiography and Formulas
Statistics
3. Results
3.1. Conventional Echocardiography Findings
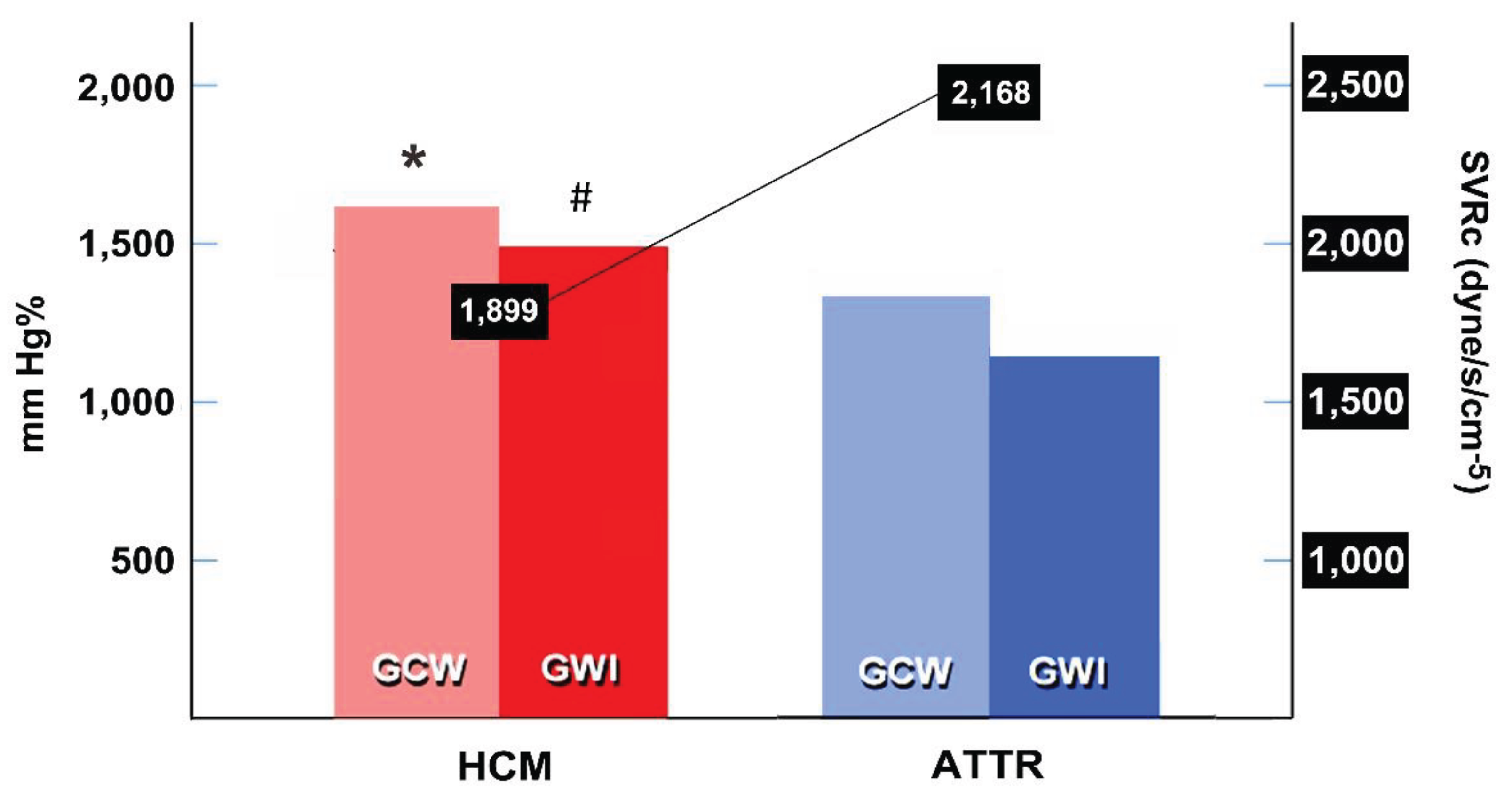
3.2. Systemic Vascular Resistance and Myocardial Work Indices
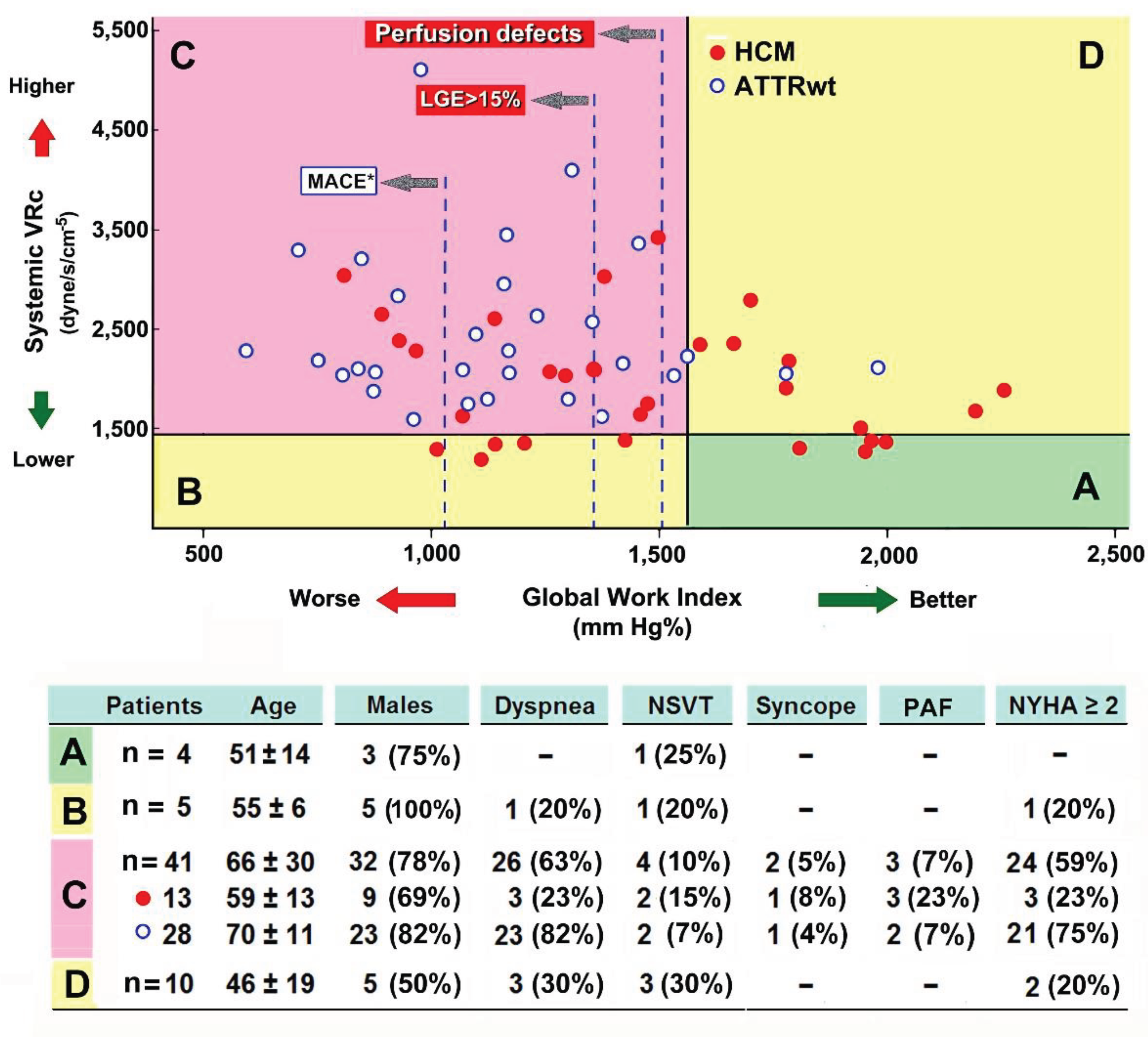
3.3. Intra-Observer Variability in MW and SVR Measurements
4. Discussion
4.1. Study Limitations
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiggers, C.J. Determinants of Cardiac Performance. Circulation 1951, 4, 485–495. [Google Scholar] [CrossRef]
- Ilebekk, A. Determinants of Cardiac Performance. J Oslo City Hosp 1979, 29, 91–102. [Google Scholar]
- Stefadouros, M.A.; Dougherty, M.J.; Grossman, W.; Craige, E. Determination of Systemic Vascular Resistance by a Noninvasive Technic. Circulation 1973, 47, 101–107. [Google Scholar] [CrossRef]
- Schiffrin, E.L. Remodeling of Resistance Arteries in Essential Hypertension and Effects of Antihypertensive Treatment. Am J Hypertens 2004, 17, 1192–1200. [Google Scholar] [CrossRef]
- Trammel, J.E.; Sapra, A. Physiology, Systemic Vascular Resistance. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2023. [Google Scholar]
- Sunagawa, K.; Maughan, W.L.; Burkhoff, D.; Sagawa, K. Left Ventricular Interaction with Arterial Load Studied in Isolated Canine Ventricle. Am J Physiol 1983, 245, H773–780. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol Rev 2018, 98, 1627–1738. [Google Scholar] [CrossRef] [PubMed]
- Naderi, N. Chapter 11 - Hemodynamic Study. In Practical Cardiology (Second Edition); Maleki, M., Alizadehasl, A., Haghjoo, M., Eds.; Elsevier, 2022; pp. 201–216 ISBN 978-0-323-80915-3.
- Cotter, G.; Moshkovitz, Y.; Kaluski, E.; Milo, O.; Nobikov, Y.; Schneeweiss, A.; Krakover, R.; Vered, Z. The Role of Cardiac Power and Systemic Vascular Resistance in the Pathophysiology and Diagnosis of Patients with Acute Congestive Heart Failure. Eur J Heart Fail 2003, 5, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Vriz, O.; Fadl Elmula, F.M.; Antonini-Canterin, F. Noninvasive Assessment of Ventricular-Arterial Coupling in Heart Failure. Heart Fail Clin 2021, 17(2), 245–254. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Aboyans, V.; Blacher, J.; Brodmann, M.; Brutsaert, D.L.; Chirinos, J.A.; De Carlo, M.; Delgado, V.; Lancellotti, P.; Lekakis, J.; et al. The Role of Ventricular–Arterial Coupling in Cardiac Disease and Heart Failure: Assessment, Clinical Implications and Therapeutic Interventions. A Consensus Document of the European Society of Cardiology Working Group on Aorta & Peripheral Vascular Diseases, European Association of Cardiovascular Imaging, and Heart Failure Association. European Journal of Heart Failure 2019, 21, 402–424. [Google Scholar] [CrossRef]
- Monte, I.P.; Faro, D.C.; Trimarchi, G.; de Gaetano, F.; Campisi, M.; Losi, V.; Teresi, L.; Di Bella, G.; Tamburino, C.; de Gregorio, C. Left Atrial Strain Imaging by Speckle Tracking Echocardiography: The Supportive Diagnostic Value in Cardiac Amyloidosis and Hypertrophic Cardiomyopathy. J Cardiovasc Dev Dis 2023, 10, 261. [Google Scholar] [CrossRef]
- de Gregorio, C.; Trimarchi, G.; Faro, D.C.; De Gaetano, F.; Campisi, M.; Losi, V.; Zito, C.; Tamburino, C.; Di Bella, G.; Monte, I.P. Myocardial Work Appraisal in Transthyretin Cardiac Amyloidosis and Nonobstructive Hypertrophic Cardiomyopathy. Am J Cardiol 2023, 208, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2020, 142, e558–e631. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and Treatment of Cardiac Amyloidosis. A Position Statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur J Heart Fail 2021, 23, 512–526. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; De Boer, R.A.; et al. 2023 ESC Guidelines for the Management of Cardiomyopathies. European Heart Journal 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015, 28, 1–39. [Google Scholar] [CrossRef]
- Olsen, F.J.; Skaarup, K.G.; Lassen, M.C.H.; Johansen, N.D.; Sengeløv, M.; Jensen, G.B.; Schnohr, P.; Marott, J.L.; Søgaard, P.; Gislason, G.; et al. Normal Values for Myocardial Work Indices Derived From Pressure-Strain Loop Analyses: From the CCHS. Circ Cardiovasc Imaging 2022, 15, e013712. [Google Scholar] [CrossRef]
- Authors/Task Force Members; McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). With the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2022, 24, 4–131. [Google Scholar] [CrossRef]
- Hiemstra, Y.L.; Bijl, P. van der; Mahdiui, M. el; Bax, J.J.; Delgado, V.; Marsan, N.A. Myocardial Work in Nonobstructive Hypertrophic Cardiomyopathy: Implications for Outcome. J Am Soc Echocardiogr 2020, 33, 1201–1208. [Google Scholar] [CrossRef]
- Garcia Brás, P.; Rosa, S.A.; Cardoso, I.; Branco, L.M.; Galrinho, A.; Gonçalves, A.V.; Thomas, B.; Viegas, J.M.; Fiarresga, A.; Branco, G.; et al. Microvascular Dysfunction Is Associated With Impaired Myocardial Work in Obstructive and Nonobstructive Hypertrophic Cardiomyopathy: A Multimodality Study. J Am Heart Assoc 2023, 12, e028857. [Google Scholar] [CrossRef]
- Clemmensen, T.S.; Eiskjær, H.; Ladefoged, B.; Mikkelsen, F.; Sørensen, J.; Granstam, S.-O.; Rosengren, S.; Flachskampf, F.A.; Poulsen, S.H. Prognostic Implications of Left Ventricular Myocardial Work Indices in Cardiac Amyloidosis. European Heart Journal - Cardiovascular Imaging 2021, 22, 695–704. [Google Scholar] [CrossRef]
- Licordari, R.; Trimarchi, G.; Teresi, L.; Restelli, D.; Lofrumento, F.; Perna, A.; Campisi, M.; de Gregorio, C.; Grimaldi, P.; Calabrò, D.; et al. Cardiac Magnetic Resonance in HCM Phenocopies: From Diagnosis to Risk Stratification and Therapeutic Management. J Clin Med 2023, 12, 3481. [Google Scholar] [CrossRef] [PubMed]
- Moura, B.; Aimo, A.; Al-Mohammad, A.; Keramida, K.; Ben Gal, T.; Dorbala, S.; Todiere, G.; Cameli, M.; Barison, A.; Bayes-Genis, A.; et al. Diagnosis and Management of Patients with Left Ventricular Hypertrophy: Role of Multimodality Cardiac Imaging. A Scientific Statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2023, 25, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Sagawa, K. Instantaneous Pressure-Volume Relationships and Their Ratio in the Excised, Supported Canine Left Ventricle. Circ Res 1974, 35, 117–126. [Google Scholar] [CrossRef]
- Duan, Q.; Tao, H.; Dong, Q.; Liao, K.; Yang, Y.; Cheng, X.; Ge, P. Non-Invasive Global Myocardial Work Index as a New Surrogate of Ventricular-Arterial Coupling in Hypertensive Patients with Preserved Left Ventricular Ejection Fraction. Front Cardiovasc Med 2022, 9, 958426. [Google Scholar] [CrossRef] [PubMed]
- Mihalcea, D.; Memis, H.; Balinisteanu, A.; Vladareanu, A.-M.; Mihaila, S.; Vinereanu, D. Myocardial Work-A New Tool for Early Detection of Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, Prednisone Chemotherapy Induced-Cardiotoxicity in Hematological Patients. J Clin Ultrasound 2023, 51, 377–384. [Google Scholar] [CrossRef]
- Palmiero, G.; Vetrano, E.; Rubino, M.; Monda, E.; Dongiglio, F.; Lioncino, M.; Di Fraia, F.; Caiazza, M.; Verrillo, F.; Capodicasa, L.; et al. The Role of New Imaging Technologies in the Diagnosis of Cardiac Amyloidosis. Heart Fail Clin 2022, 18, 61–72. [Google Scholar] [CrossRef]
- Henein, M.Y.; Lindqvist, P. Myocardial Work Does Not Have Additional Diagnostic Value in the Assessment of ATTR Cardiac Amyloidosis. J Clin Med 2021, 10, 4555. [Google Scholar] [CrossRef]
- Roger-Rollé, A.; Cariou, E.; Rguez, K.; Fournier, P.; Lavie-Badie, Y.; Blanchard, V.; Roncalli, J.; Galinier, M.; Carrié, D.; Lairez, O.; et al. Can Myocardial Work Indices Contribute to the Exploration of Patients with Cardiac Amyloidosis? Open Heart 2020, 7, e001346. [Google Scholar] [CrossRef]
- de Gregorio, C. , Micari, A., Di Bella, G., Carerj, S., Coglitore, S. Systolic wall stress may affect the intramural coronary blood flow velocity in myocardial hypertrophy, independently on the left ventricular mass. Echocardiography. [CrossRef]
- Briasoulis, A.; Bampatsias, D.; Petropoulos, I.; Rempakos, A.; Patras, R.; Theodorakakou, F.; Makris, N.; Dimopoulos, M.A.; Stamatelopoulos, K.; Kastritis, E. Left Ventricular Myocardial Work Improves in Response to Treatment and Is Associated with Survival Among Patients with Light-Chain Cardiac Amyloidosis. Eur Heart J Cardiovasc Imaging 2023, jead351. [Google Scholar] [CrossRef]
- Barroso, F.A.; Coelho, T.; Dispenzieri, A.; Conceição, I.; Waddington-Cruz, M.; Wixner, J.; Maurer, M.S.; Rapezzi, C.; Planté-Bordeneuve, V.; Kristen, A.V.; et al. Characteristics of Patients with Autonomic Dysfunction in the Transthyretin Amyloidosis Outcomes Survey (THAOS). Amyloid 2022, 29, 175–183. [Google Scholar] [CrossRef]
- Di Bella, G.; Cappelli, F.; Licordari, R.; Piaggi, P.; Campisi, M.; Bellavia, D.; Minutoli, F.; Gentile, L.; Russo, M.; de Gregorio, C.; et al. Prevalence and Diagnostic Value of Extra-Left Ventricle Echocardiographic Findings in Transthyretin-Related Cardiac Amyloidosis. Amyloid 2022, 29, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Holm, H.; Magnusson, M.; Jujić, A.; Bozec, E.; Girerd, N. How to Calculate Ventricular-Arterial Coupling? Eur J Heart Fail 2022, 24(4), 600–602. [Google Scholar] [CrossRef] [PubMed]
| HCM (n=30) | ATTR (n=30) | P value | |
|---|---|---|---|
| Age, years | 58 [54 – 73] | 69 [62 – 78] | <0.001 |
| Males | 24 (80%) | 23 (77%) | 0.754 |
| Body surface area, m2.7 | 1.9 [1.7 – 2.0] | 1.7 [1.6 – 1.9] | <0.001 |
| Systolic BP, mm Hg | 128.8 ± 11.9 | 128.5 ± 12.7 | 0.925 |
| Diastolic BP, mm Hg | 77.4 ± 12.3 | 80.7 ± 7.9 | 0.226 |
| Mean BP, mm Hg | 94.5 ± 10.3 | 96.6 ± 7.4 | 0.375 |
| Pulse pressure, mm Hg | 51.4 ± 13.5 | 47.9 ±13.5 | 0.311 |
| Heart rate, bpm | 67.2 ± 11.3 | 68.4 ± 11.2 | 0.690 |
| NYHA class I-III | 1.4 ± 0.56 | 1.9 ± 0.46 | <0.001 |
| Dyspnea | 7 (23%) | 23 (77%) | <0.001 |
| Family history of SD | 7 (23%) | 3 (10%) | 0.166 |
| Implantable cardioverter defibrillator | 3 (10%) | 1 (3%) | NA |
| Therapy | |||
| Beta-blockers | 25 (83%) | 10 (33%) | <0.001 |
| Calcium antagonists | 11 (37%) | 5 (17%) | 0.080 |
| ACE-inhibitors | 8 (27%) | 2 (7%) | 0.07 |
| ARB | 1 (3%) | 7 (23%) | 0.023 |
| Statins | 6 (20%) | 15 (50%) | 0.015 |
| Anti-coagulants | 6 (20%) | 6 (20%) | 1.000 |
| Anti-platelet drugs | 5 (17%) | 9 (30%) | 0.222 |
| Diuretics | 4 (13%) | 17 (57%) | <0.001 |
| Tafamidis 65 mg | - | 30 (100%) | NA |
| Values are numbers (%), mean ± SD, or median values [IQR], as appropriate. ACE, angiotensin-converting enzyme; ARB, angiotensin receptor antagonists; ATTR, wild-type transthyretin cardiac amyloidosis; BP, blood pressure; HCM, nonobstructive hypertrophic cardiomyopathy; NA, not available/performed; SCD, sudden cardiac death. | |||
| HCM (n=30) | ATTR (n=30) | P value | |
|---|---|---|---|
| Left ventricular end-diastolic volume index, mL/m2.7 | 51.4 [37.9 – 59.8] | 49.7 [38.7 – 60.0] | 0.831 |
| Left ventricular end-systolic volume index, mL/m2.7 | 18.8 [15.0 – 25.9] | 23.2 [17.2 – 27.1] | 0.020 |
| Left ventricular ejection fraction, % | 63.4 [54.4 – 63.8] | 55.0 [52.3 – 57.2] | <0.001 |
| Stroke volume index, mL/m2.7 | 32.5 [23.2 – 36.3] | 27.8 [21.5 – 31.8] | 0.444 |
| Cardiac output, L/min | 3.9 [2.8 – 4.4] | 3.4 [2.4 – 3.7] | 0.004 |
| Ventricular septum thickness, mm | 15.2 [14.6 – 18.0] | 15.5 [14.0 – 21.0] | 0.688 |
| Mitral E / tissue E' velocity ratio | 11.7 ± 5.0 | 16.8 ± 5.9 | 0.001 |
| Global longitudinal strain, -% | 15.0 [16.0 – 10.3] | 12.5 [14.5 – 10.0] | 0.022 |
| Global myocardial work index, mm Hg% | 1468.5 ± 403.5 | 1149.7 ± 319.3 | 0.001 |
| Global constructive work, mm Hg% | 1442 [989 – 1554] | 1141 [874 - 1356] | 0.008 |
| Left atrial volume index, ml/m2.7 | 43.7 ± 16.2 | 44.6 ± 15.5 | 0.991 |
| Systemic vascular resistance (corrected), dyne/s/cm-5 | 1899 [1693 – 2600] | 2168 [2051 – 2860] | 0.008 |
| Measurements are expressed as mean values ± SD or median values [IQR], as appropriate. | |||
| HCM (n=30) | ATTR (n=30) | Total (n=60) | |
|---|---|---|---|
| Class A (best class) | 4 (13%) | - | 4 (7%) |
| Class B (impaired GWI) | 5 (17%) | - | 5 (8%) |
| Class C (poorest class) | 13 (43%) | 28 (93%) | 41 (68%) |
| Class D (higher SVRc) | 8 (27%) | 2 (7%) | 10 (17%) |
| Differential characteristics in subclass patients with HCM | |||
| Class C (n=13) | Other Classes (n=17) | p-value | |
| Stroke volume index, ml/ m2.7 | 26.7 ± 8.7 | 37.5 ± 10.8 | 0.005 |
| LV end-diastolic volume index, ml/ m2.7 | 43.7 ± 12.8 | 57.5 ± 16.1 | 0.015 |
| GLS, -% | 13.0 ± 3.4 | 16.0 ± 4.3 | 0.043 |
| IVS thickness, mm | 18.2 ± 3.4 | 15.6 ± 2.8 | 0.029 |
| LV ejection fraction, % | 60.6 ± 6.1 | 64.8 ± 5.1 | 0.055 |
| Age, years | 58.7 ± 13.0 | 48.9 ± 16.2 | 0.076 |
| Left atrial volume index, ml/ m2.7 | 47.1 ± 20.8 | 41.14 ± 11.6 | 0.368 |
| Measurements are expressed as mean values ± SD. HCM, hypertrophic cardiomyopathy; GLS, global longitudinal strain; IVS, interventricular septum; LV, left ventricle/ventricular. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





