1. Introduction
Antimalarial drug development remains strongly linked to plant-based pharmaceuticals, as some of the most important therapeutics are based on their chemical scaffolds, such as aminoquinolines (e.g., chloroquine) and endoperoxides, i.e., artemisinin-based drugs [
1,
2]. The main causative agent of malaria,
Plasmodium falciparum (
P. falciparum), has developed resistance to all antimalarial drugs in clinical use, including aminoquinolines and endoperoxides [
3,
4,
5,
6]. Furthermore, the development of these antimalarials involves unaffordable environmental and economic costs for most malaria-endemic countries, hence the WHO’s encouragement of applying natural extracts or plant-based pharmaceuticals based on traditional medicines [
7,
8]. Therefore, we propose a strategy that aims to repurpose traditional medicinal plants for antimalarial applications.
Tropaeolum majus L. (
T. majus), an herbaceous plant commonly known as garden nasturtium, belongs to the family
Tropaeolaceae and it is native to Peru [
9,
10] It was first introduced in Europe in the sixteenth century and then spread to other parts of the world, including malaria-endemic countries, such as Angola, Rwanda, and Vietnam [
9,
10,
11].
T. majus was selected as a potential candidate for antimalarial drug development not only because of its widespread distribution, but also because of its traditional usages against bacterial infections, such as bronchitis, sinusitis, and urinary tract infections, as well as for its antifungal and antiviral activities [
12,
13,
14].
The broad therapeutic spectrum of
T. majus can be linked to a group of compounds known as glucosinolates [
15,
16,
17,
18]. Benzyl glucosinolate is a metabolite found in every part of the
T. majus plant, especially in the seeds [
15,
16,
17,
18,
19]. When hydrolyzed by the endogenous enzyme myrosinase (Thioglucoside hydrolase, EC 3.2.3.1), it generates a variety of breakdown products, including benzyl isothiocyanate (BITC) [
16,
17,
19], which has been reported to have antimicrobial [
20,
21,
22,
23], larvicidal [
24], anthelmintic [
25], and anticancer [
26] activities.
To our knowledge, there is no information on
T. majus and BITC usages as antimalarials. However, a recent study by Hashimoto et al. (2023) demonstrated the
in vivo antimalarial activity of another isothiocyanate, allyl isothiocyanate (AITC), and its metabolite, N-acetyl-S-(N-allyl thiocarbamoyl)-l-cysteine (NAC-AITC), by
in vitro and
in vivo assays, both extracted from
Wasabia japonica [
27]. Arianie et al. (2021) designed novel isothiocyanates based on eugenol and cinnamaldehyde derivatives and rhamnosyloxy benzyl isothiocyanate from
Moringa oleifera leaves, used in traditional medicine to treat malaria, by molecular docking and demonstrated their potential as antimalarials through
in silico approaches [
28,
29].
Hence, this study aimed to evaluate the antimalarial activity by flow cytometry of T. majus seeds, leaves, and stems aqueous extracts, traditionally applied as antimicrobials, and BITC, the major biologically active compound derived from the T. majus parts. This assessment prompted a subsequent investigation of BITC’s cross-resistance with aminoquinolines, also using flow cytometry, and BITC did not demonstrate the same resistance mechanism as this antimalarial drug class.
2. Results
2.1. Antimalarial Screening Assessment
Flow cytometry was first used to evaluate the aqueous extracts and BITC for antimalarial activity against the asexual blood stage GFP-expressing
P. falciparum (3D7-GFP), a chloroquine-sensitive strain. Using the 3D7-GFP strain obviates any staining procedure with a fluorescent dye since the parasites are auto-fluorescent, simplifying culture procedures [
30,
31]. The number of fluorescent events after drug exposure detected by flow cytometry, i.e., the percentage of surviving GFP parasites, allows the determination of the growth inhibition percentage, as described in Teixeira de Morais Gomes et al. (2020). The results were obtained from at least two experiments, each in triplicate, and are presented in
Table 1.
T. majus seed extract displayed a similar growth inhibition percentage for the tested concentrations (38.62 ± 22.89% at 132 µg/ml and 30.18 ± 13.47% at 13.2 µg/ml) and a higher growth inhibition percentage than the remaining extracts in all concentrations. The extract solvent had no antiplasmodial action at any concentration. Despite the better performance of the T. majus seed extract, none of the extracts presented more than 70% growth inhibition.
Benzyl isothiocyanate (BITC) at 0.50 µg/ml demonstrated a growth inhibition percentage above 70% (97.13 ± 0.62%) and was considered for antiplasmodial activity refinement. BITC solvent (DMSO) did not show a meaningful growth inhibition percentage in both concentrations (0.4% and 0.04%) and did not influence the inhibitory effect of BITC.
The reference antimalarial drug chloroquine (CQ) at 5.17 µg/ml and at 0.517 µg/ml exhibited growth inhibition percentages of 96.57 ± 0.57% and 94.71 ± 2.78%, respectively. BITC at the highest concentration (0.50 µg/ml) and CQ at a similar concentration (0.517 µg/ml) and ten times more concentrated (5.17 µg/ml) had comparable growth inhibitions against P. falciparum.
2.2. Dose-Response Evaluation
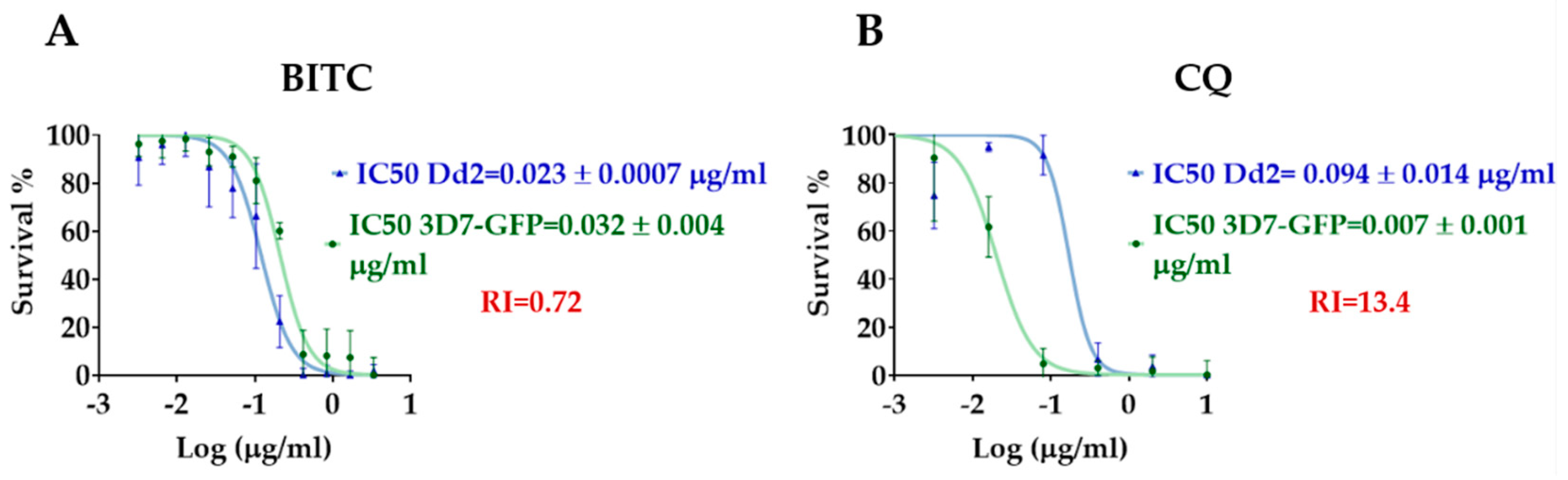
Since BITC was considered for further analysis, dose-response curves to determine the half-maximal inhibitory concentrations (IC50) against
P. falciparum strains 3D7-GFP and Dd2, a chloroquine-resistant strain, were also made based on the assessment of the parasite growth by flow cytometry. The results are demonstrated in
Figure 1 and were obtained from at least two experiments, each in triplicate.
Both BITC and the reference drug CQ displayed sigmoidal dose-response curves compatible with biological activity (
Figure 1). The IC50 values and the resistance index calculated for CQ were consistent with previous results in similar laboratory conditions [
32,
33,
34], hence validating the assay conditions. The resistance index provides a quantitative measure of activity against a resistant strain in comparison to a susceptible strain (RI=IC50 Dd2/IC50 3D7-GFP) [
35,
36].
The biological activity of BITC was similar against both susceptible and resistant-strains (p>0.05; Unpaired t-test), hence the absence of a right shift of the
P. falciparum Dd2 dose-response curve (
Figure 1) and a low resistance index (RI=0.72). As expected, the reference drug CQ, displayed lower biological activity against the resistant-strain, which was significantly different (p<0.05; Unpaired t-test), thus the right shift of the
P. falciparum Dd2 dose-response curve (
Figure 1), i.e., an increase of the IC50 value, and a higher resistance index (RI=13.4).
3. Discussion
When it comes to
T. majus aqueous extracts, the differences in the growth inhibition percentages between the seed extract and the others can be attributable to a variety of factors.
T. majus seeds contain a higher BITC content than the leaves or stems, and while benzyl glucosinolate is soluble in water, BITC, the active metabolite, is poorly soluble, volatile, and non-stable [
37], which can account for the differences in
P. falciparum growth inhibition.
The dose-response evaluation and the RI of BITC revealed that its phenotypic response was different than the reference drug CQ, i.e., if a compound exhibits a resistance mechanism similar to that of other antimalarials [
35,
36]. Since CQ is an aminoquinoline, our results suggest that BITC does not share the same resistance mechanism as aminoquinolines, which can be considered an advantage for antimalarial drug development.
The mechanism of action of CQ is linked to its diffusion through biological membranes and concentration inside the parasite's the food vacuole, which has an acidic pH (in contrast with the neutral pH of the cytosol) [
38,
39]. Resistance to aminoquinolines is related to mutations in various proteins, including the transporters
P. falciparum chloroquine resistance transporter (
PfCRT) and the
P. falciparum multidrug resistance 1 protein (
PfMDR1) [
40,
41]. Since
P. falciparum 3D7-GFP is a CQ-sensitive strain and
P. falciparum Dd2 a CQ-resistant strain and considering that the IC50 of BITC for both strains was identical, this strongly suggests that BITC does not have the same mechanism of action and resistance as CQ.
Isothiocyanates have been shown to interact mainly with thiol groups, forming labile dithiocarbamate derivatives, and with amine groups, which may result in increased oxidation, i.e., production of reactive oxygen species (ROS), and inhibition of key enzymes and/or proteins in microorganisms [
42,
43,
44].
The electrophilic properties of BITC can render a high affinity for cellular sulfhydryl groups, such as enzymes and/or proteins with functional or structural cysteine residues [
22,
42]. One of
P. falciparum most important enzymes involved in the redox equilibrium is glutathione reductase (GR), which has cysteine residues [
45,
46,
47].GR is an antioxidant enzyme that catalyzes the regeneration of reduced glutathione (GSH), the active form of glutathione, from oxidized glutathione using NADPH as the source of reducing equivalents [
46,
47]. This reaction helps to avoid the synthesis of hydroxyl radical •OH from H
2O
2 produced due to hemoglobin digestion (inside the parasite’s food vacuole) and mitochondrial electron chain reactions [
45,
47]. A study conducted by Li et al. (2020) characterized BITC as a potential GR inhibitor in human cancer cells and demonstrated that BITC was evaluated as a competitive and irreversible GR inhibitor in a time- and concentration-dependent mode and this reaction depended on the presence of NADPH [
48].
Also, BITC might interfere with the GSH
de novo synthesis
in P. falciparum. It is known that in
P. falciparum, GSH can be
de novo biosynthesized by two enzymes, glutamylcysteine synthetase and glutathione synthetase, respectively, that require a source of amino acid precursors from the inactive form of glutathione (glutamate, cysteine, and glycine) [
45,
46]. In biological systems, a reaction between the inactive form of glutathione and BITC, catalyzed by glutathione-S-transferases, allows the formation of a dithiocarbamate derivative, which is then effluxed from the cell [
42]. This reaction may increase the interference of BITC with
P. falciparum redox equilibrium.
T. majus is characterized as a low toxicity plant, with a LD50 above 5000 mg/kg in a
in vivo mice acute toxicity study of oral administration by Zanetti et al. (2003). The absence of toxicity signs in oral administration, up to 2 weeks, can be due to the low concentrations of glucosinolates presented in the extracts or due to their metabolization in the organism [
49]. For the compound BITC the results of
in vivo mice toxicology experiments [
26] showed that animals had no evidence of major drug induced toxicity at doses up to 100 mg/kg, being the LD50 of 140 mg/kg.
The complexation of BITC from cyclodextrins performed by Li et al. (2015) improved the stability and the aqueous solubility of this compound [
37]. Hence, the antimalarial activity of the aqueous crude extracts could be improved by the enhancement of the hydrolysis of benzyl glucosinolate and solubility of BITC in water, in particular the seed extracts.
4. Materials and Methods
4.1. Plant Material
Tropaeolum majus L. plant material was collected in November of 2022 in a cultivated field at Parque Bensaúde, Lisbon. Stems, leaves, and seeds were cleansed of residues, and the stems and leaves were also cut into small pieces. Stems, leaves, and seeds were weighted separately and kept at -20◦C.
4.2. Tropaeolum majus L. Extraction
T. majus seeds (6.37 g dw), leaves (18.74g dw), and stems (20 g dw), previously lyophilized for 72h, were powdered with a mill, and macerated in 50 ml phosphate buffer (pH 7.4) for 12h with occasional stirring, to allow endogenous myrosinase to promote glucosinolate degradation and filtered obtaining aqueous extracts.
Afterward, the aqueous extracts were filtrated and lyophilized for 96h, in previously tared volumetric flasks, obtaining the following 52.8 mg seed extract, 1.002 mg of leaves extract, and 527.2 mg of stem extract. Dry extracts were then dissolved in 4 ml of distilled water, obtaining the following final concentrations for the antimalarial assays: 13.2 mg/ml seed extract; 250.6 mg/ml leaves extract; 131.8 mg/ml stem extract.
4.3. Antimalarial Assays
4.3.1. P. falciparum In Vitro Culture
Laboratory-adapted
P. falciparum lines 3D7-GFP (MRA-1029, MR4, ATCC® Manassas Virginia), a chloroquine-sensitive strain, and Dd2 (cryopreserved collection from IHMT), a chloroquine-resistant strain, were continuously cultured using a modified method of Trager and Jensen [
50,
51]. Parasites were cultivated in 5% hematocrit, 37°C, and an atmosphere with 5% of CO
2 and supplemented with complete culture medium (cRPMI), as previously described [
51].
4.3.2. Sample Preparation
The stock solutions were made in compliance with the maximum solvent limits that can be used in antimalarial assays [
52]. Keeping this in mind, a stock solution of BITC (Sigma-Aldrich®) with 112.5 µg/ml (754 µM) containing 90% dimethyl sulfoxide (DMSO; Sigma-Aldrich®) was diluted in sterile PBS (VWR
TM) to achieve a DMSO percentage in the assays ≤ 0.4%. The aqueous extracts were diluted in cRPMI to attain a water percentage ≤ 1% in the assays and previously filtrated with a 0.45-micron filter. The stock solution of the reference drug chloroquine (Sigma-Aldrich®) with 2584,3 µg/ml (5 mM) containing 100% of DMSO was diluted in cRPMI to also achieve a DMSO percentage in the assays ≤0.4%. The extract solvent (water) previously filtrated with a 0.45-micron filter, and BITC solvent (DMSO) were also diluted in cRPMI or sterile PBS, respectively, following the respective percentages used in the assays.
4.3.3. Extracts and Compounds Screening Assessment
All extracts and compounds were screened for their
in vitro antimalarial activity against
P. falciparum 3D7-GFP in at least two independent experiments in triplicate, as previously described with modifications [
53]. In brief, unsynchronized culture with 2% hematocrit and 1% parasitemia was incubated in a 96-well flat-bottom plate with the following concentrations for 72h (37 °C and 5% CO2):
T. majus seed extract: 132 µg/ml (1% water) and 13.2 µg/ml (0.1% water);
T. majus leaf extract: 2510 µg/ml (1% water) and 251 µg/ml (0.1% water);
T. majus stem extract: 1320 µg/ml (1% water) and 132 µg/ml (0.1% water);
BITC: 0.50 µg/ml (3.32 µM; 0.4% DMSO) and 0.050 µg/ml (0.332 µM; 0.04% DMSO).
Each plate also included growth control wells: untreated culture, 1%, and 0.1% water, 0.4% and 0.04% DMSO, 5.17 µg/ml (10 µM), and 0.517 µg/ml (1 µM) of chloroquine (reference drug). After the incubation period, cells were diluted to achieve a 0,7% hematocrit and the parasite growth was assessed by flow cytometry (Beckman Coulter, Cytoflex) with a 96-well plate reader, using Fl-1 (green fluorescent protein [GFP]; excitation wavelength, 488 nm). Typically, 100.000 RBCs were counted for each well. Samples were analyzed using FlowJo software (Tree Star Inc.). The growth inhibition percentage was then determined by the following formula:
The extracts and/or compounds that displayed at least 70% of growth inhibition were selected as potential candidates and confirmed by IC50 estimation and resistance index determination [
53].
4.3.4. Dose-Response Evaluation
The antimalarial activity was estimated by previously described protocols with adjustments in at least two experiments, each in triplicate [
53,
54]. In short, unsynchronized cultures with 2% hematocrit and 1% parasitemia of
P. falciparum 3D7-GFP and Dd2 strains were incubated for 72h (37 °C and 5% CO
2) in a 96-well flat-bottom plate with BITC in 2-fold serial dilutions ranging from 0.50 µg/ml to 0.0005 µg/ml (3.32 µM to 0.0032 µM). Additionally, each plate included growth control wells with no drug added and chloroquine as a reference drug in a 5-fold serial dilution with concentrations ranging from 5.17 µg/ml to 0.0003 µg/ml (10 µM to 0.00064 µM). After 72 hours, cells were diluted to achieve a 0,7% hematocrit and the parasite growth was assessed by flow cytometry (Beckman Coulter, Cytoflex) in a 96-well plate reader, using FI-1 (green; excitation wavelength, 488 nm). Before the flow cytometry reading,
P. falciparum Dd2 strain was stained with a mixture of SYBR
TM Green I (Invitrogen, Thermo Fisher Scientific) 0.5X in PBS 30 minutes in the dark at standard culture conditions. Typically, 100.000 RBCs were counted for each well. Samples were analyzed using FlowJo software (Tree Star Inc.). The IC50 was estimated through a nonlinear regression by using the GraphPad Prism 9 software (trial version) and the resistance index calculated by the following formula:
A resistance index above 10 predicts a high level of resistance, whereas a resistance index below 10 might indicate an intermediate resistance level and a resistance index close to or below 1 could reveal an absence of resistance [
35].
4.3.5. Statistical Analysis
GraphPad Prism 9 software (trial version) was used for the non-parametric Mann-Whitney test and parametric Unpaired t-test. A significant difference was assumed when p<0.05.
5. Conclusions
BITC similar activity against both chloroquine -susceptible and resistant Plasmodium falciparum strains suggests a different mechanism of action. Hence, BITC and Tropaeolum majus L. extracts have a good potential to develop new antimalarial medicines.
Author Contributions
Conceptualization, A.P.; T.S..; F.N methodology, A.P.; T.S..; software, T.S.; F.N.; validation, A.P.; T.S..; F.N.; formal analysis, TS.; FN.; investigation, A.P.; T.S.; F.N., resources, A.P.; T.S..; F.N.; data curation, T.S.; writing—original draft preparation, A.P.; T.S.; writing—review and editing, AP.; TS..; FN.; visualization, A.P.; TS..; supervision, A.P; F.N.; project administration, F.N..; funding acquisition, F.N. All authors have read and agreed to the published version of the manuscript.” Please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
This research was funded by Fundação para a Ciência e Tecnologia (
https://www.fct.pt/) GHTMUID/04413/2020 and LA-REAL - LA/P/0117/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
Authors wish to acknowledge Denise Duarte for advice on cell culture.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Senerovic, L.; Opsenica, D.; Moric, I.; Aleksic, I.; Spasić, M.; Vasiljevic, B. Quinolines and Quinolones as Antibacterial, Antifungal, Anti-Virulence, Antiviral and Anti-Parasitic Agents. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2020; Volume 1282, pp. 37–69. [Google Scholar]
- Ma, N.; Zhang, Z.; Liao, F.; Jiang, T.; Tu, Y. The Birth of Artemisinin. Pharmacol Ther 2020, 216. [Google Scholar] [CrossRef]
- World Health Organization. Report on Antimalarial Drug Efficacy, Resistance and Response: 10 Years of Surveillance (2010–2019); WHO Global Malaria, Programme, Ed.; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-92-4-001281-3. [Google Scholar]
- Ippolito, M.M.; Moser, K.A.; Jean-Bertin; Kabuya, B.; Cunningham, C.; Juliano, J.J. Antimalarial Drug Resistance and Implications for the WHO Global Technical Strategy. Curr Epidemiol Rep 2021, 8, 46–62. [Google Scholar] [CrossRef]
- Rasmussen, C.; Alonso, P.; Ringwald, P. Current and Emerging Strategies to Combat Antimalarial Resistance. Expert Rev Anti Infect Ther 2021, 1–20. [Google Scholar] [CrossRef]
- Ward, K.E.; Fidock, D.A.; Bridgford, J.L. Plasmodium falciparum Resistance to Artemisinin-Based Combination Therapies. Curr Opin Microbiol 2022, 69. [Google Scholar] [CrossRef] [PubMed]
- De Joarder, D.; Mukhopadhyay, C.; Sarkar, R. Sustainable Green Technologies for Synthesis of Potential Drugs Targeted toward Tropical Diseases. In Green Approaches in Medicinal Chemistry for Sustainable Drug Design; Krishna Banik, B., Ed.; Elsevier, 2020; pp. 75–93. ISBN 9780128175927. [Google Scholar]
- Burton, A.; Falkenberg, T.; Smith, M.; Zhang, Q.; Zhang, X.; Boerma, T.; Lerberghe, W. WHO Traditional Medicine Strategy 2014-2023; Zhang, Q., Ed.; World Health Organization: Geneve, 2013; ISBN 9789241506090. [Google Scholar]
- Kew Royal Botanical Gardens Tropaeolum majus, L. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:310974-2 (accessed on 22 November 2023).
- Duenas-Lopez, M.A. Tropaeolum majus L. (Nasturtium). Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.54181 (accessed on 22 November 2023).
- Jakubczyk, K.P.; Janda-Milczarek, K.; Watychowicz, K.; Łukasiak, J.; Wolska, J. Garden Nasturtium (Tropaeolum majus L.)-a Source of Mineral Elements and Bioactive Compounds. Rocz Panstw Zakl Hig 2018, 69, 119–126. [Google Scholar] [PubMed]
- Goos, K.-H.; Albrecht, U.; Schneider, B. Efficacy and Safety Profile of a Herbal Drug Containing Nasturtium Herb and Horseradish Root in Acute Sinusitis, Acute Bronchitis and Acute Urinary Tract Infection in Comparison with Other Treatments in the Daily Practice/Results of a Prospective Cohort Study. Arzneim.-Forsch./Drug Res 2006, 56, 249–257. [Google Scholar] [CrossRef]
- Vrca, I.; Jug, B.; Fredotović, Ž.; Vuko, E.; Brkan, V.; Šestić, L.; Juretić, L.; Dunkić, V.; Nazlić, M.; Ramić, D.; et al. Significant Benefits of Environmentally Friendly Hydrosols from Tropaeolum majus L. Seeds with Multiple Biological Activities. Plants 2023, 12, 3897. [Google Scholar] [CrossRef] [PubMed]
- Valsalam, S.; Agastian, P.; Arasu, M.V.; Al-Dhabi, N.A.; Ghilan, A.K.M.; Kaviyarasu, K.; Ravindran, B.; Chang, S.W.; Arokiyaraj, S. Rapid Biosynthesis and Characterization of Silver Nanoparticles from the Leaf Extract of Tropaeolum majus L. and Its Enhanced in-Vitro Antibacterial, Antifungal, Antioxidant and Anticancer Properties. J Photochem Photobiol B 2019, 191, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Česlová, L.; Klikarová, J.; Šalomounová, T. The Content and Profile of Biologically Active Compounds Present in Individual Parts of Nasturtium (Tropaeolum majus L.): Comprehensive Study. European Food Research and Technology 2023, 249, 413–428. [Google Scholar] [CrossRef]
- Kleinwächter, M.; Schnug, E.; Selmar, D. The Glucosinolate-Myrosinase System in Nasturtium (Tropaeolum majus L.): Variability of Biochemical Parameters and Screening for Clones Feasible for Pharmaceutical Utilization. J Agric Food Chem 2008, 56, 11165–11170. [Google Scholar] [CrossRef]
- Bartnik, M.; Facey, P.C. Glycosides. In Pharmacognosy: Fundamentals, Applications and Strategy; Elsevier Inc., 2017; pp. 101–161. ISBN 9780128020999. [Google Scholar]
- Vrca, I.; Ramić, D.; Fredotović, Ž.; Možina, S.S.; Blažević, I.; Bilušić, T. Chemical Composition and Biological Activity of Essential Oil and Extract from the Seeds of Tropaeolum Majus L. Var. Altum. Food Technol Biotechnol 2022, 60, 533–542. [Google Scholar] [CrossRef]
- Traka, M.; Mithen, R. Glucosinolates, Isothiocyanates and Human Health. Phytochemistry Reviews 2009, 8, 269–282. [Google Scholar] [CrossRef]
- Pereira, C.; Calado, A.M.; Sampaio, A.C. The Effect of Benzyl Isothiocyanate on Candida albicans Growth, Cell Size, Morphogenesis, and Ultrastructure. World J Microbiol Biotechnol 2020, 36. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, K.; Song, J.; Wu, H.; Hao, H.; Bi, J.; Hou, H.; Zhang, G. Bacteriostatic Effects of Benzyl Isothiocyanate on Vibrio Parahaemolyticus: Transcriptomic Analysis and Morphological Verification. BMC Biotechnol 2021, 21. [Google Scholar] [CrossRef] [PubMed]
- Dufour, V.; Stahl, M.; Rosenfeld, E.; Stintzi, A.; Baysse, C. Insights into the Mode of Action of Benzyl Isothiocyanate on Campylobacter Jejuni. Appl Environ Microbiol 2013, 79, 6958–6968. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhao, Y. meng; Wang, C.; Zhu, H. ping Antibacterial Activity and Main Action Pathway of Benzyl Isothiocyanate Extracted from Papaya Seeds. J Food Sci 2021, 86, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Flor-Weiler, L.B.; Behle, R.W.; Berhow, M.A.; McCormick, S.P.; Vaughn, S.F.; Muturi, E.J.; Hay, W.T. Bioactivity of Brassica Seed Meals and Its Compounds as Ecofriendly Larvicides against Mosquitoes. Sci Rep 2023, 13. [Google Scholar] [CrossRef]
- Kermanshai, R.; Mccarry, B.E.; Rosenfeld, J.; Summers, P.S.; Weretilnyk, E.A.; Sorger, G.J. Benzyl Isothiocyanate Is the Chief or Sole Anthelmintic in Papaya Seed Extracts. Phytochemistry 2001, 57, 427–435. [Google Scholar] [CrossRef]
- Pintão, A.M.; Pais, M.S.; Coley, H.; Kelland, L.R.; Judson, I.R. In Vitro and In Vivo Antitumor Activity of Benzyl Isothiocyanate: A Natural Product from Tropaeolum majus L. Planta Med. 1995, 61, 233–236. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yoshioka, S.; Iwanaga, S.; Kanazawa, K. Anti-Malarial Activity of Allyl Isothiocyanate and N-Acetyl-S-(N-Allylthiocarbamoyl)-l-Cysteine. Mol Nutr Food Res 2023. [Google Scholar] [CrossRef]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, Genetic, Ethnopharmacology, Phytochemistry and Pharmacology of Moringa oleifera Leaves: An Overview. Int J Mol Sci 2015, 16, 12791–12835. [Google Scholar] [CrossRef] [PubMed]
- Arianie, L.; Widodo; Iftitah, E.D. Warsito Natural Isothiocyanate Anti-Malaria: Molecular Docking, Physicochemical, Adme, Toxicity and Synthetic Accessibility Study of Eugenol and Cinnamaldehyde. International Journal of Applied Pharmaceutics 2021, 13, 82–88. [Google Scholar] [CrossRef]
- BEI Reagent Search MRA-1029 Plasmodium falciparum, 3D7HT-GFP (Parasitic Protozoa). Available online: https://www.beiresources.org/Catalog/BEIParasiticProtozoa/MRA-1029.aspx (accessed on 15 October 2021).
- Kulkeaw, K. Progress and Challenges in the Use of Fluorescence-based Flow Cytometric Assays for Anti-malarial Drug Susceptibility Tests. Malar J 2021, 20. [Google Scholar] [CrossRef]
- Abdou, A.M.; Seddek, A. latif S.; Abdelmageed, N.; Badry, M.O.; Nishikawa, Y. Wild Egyptian Medicinal Plants Show in Vitro and in Vivo Cytotoxicity and Antimalarial Activities. BMC Complement Med Ther 2022, 22. [Google Scholar] [CrossRef] [PubMed]
- Camara, A.; Haddad, M.; Traore, M.S.; Chapeland-Leclerc, F.; Ruprich-Robert, G.; Fourasté, I.; Balde, M.A.; Royo, J.; Parny, M.; Batigne, P.; et al. Variation in Chemical Composition and Antimalarial Activities of Two Samples of Terminalia Albida Collected from Separate Sites in Guinea. BMC Complement Med Ther 2021, 21. [Google Scholar] [CrossRef]
- Silva, A.T.; Lobo, L.; Oliveira, I.S.; Gomes, J.; Teixeira, C.; Nogueira, F.; Marques, E.F.; Ferraz, R.; Gomes, P. Building on Surface-Active Ionic Liquids for the Rescuing of the Antimalarial Drug Chloroquine. Int J Mol Sci 2020, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nzila, A.; Mwai, L. In Vitro Selection of Plasmodium falciparum Drug-Resistant Parasite Lines. Journal of Antimicrobial Chemotherapy 2009, 65, 390–398. [Google Scholar] [CrossRef]
- Shamsuddin, M.A.; Ali, A.H.; Zakaria, N.H.; Mohammat, M.F.; Hamzah, A.S.; Shaameri, Z.; Lam, K.W.; Mark-Lee, W.F.; Agustar, H.K.; Abd Razak, M.R.M.; et al. Synthesis, Molecular Docking, and Antimalarial Activity of Hybrid 4-Aminoquinoline-Pyrano[2,3-c]Pyrazole Derivatives. Pharmaceuticals 2021, 14. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Yang, Q.; Zhang, N.; Du, Y.; Zhu, H. Preparation and Characterization of Inclusion Complex of Benzyl Isothiocyanate Extracted from Papaya Seed with β-Cyclodextrin. Food Chem 2015, 184, 99–104. [Google Scholar] [CrossRef]
- Geary, T.G.; Jensen, J.B.; Ginsburg, H. Uptake of [3H]Chloroquine by Drug-Sensitive and -Resistant Strains of the Human Malaria Parasite Plasmodium falciparum. Biochem Pharmacol 1986, 35, 3805–3812. [Google Scholar] [CrossRef]
- Wicht, K.J.; Mok, S.; Fidock, D.A. Molecular Mechanisms of Drug Resistance in Plasmodium falciparum Malaria. Annu Rev Microbiol 2020, 74, 431–454. [Google Scholar] [CrossRef]
- Shafik, S.H.; Cobbold, S.A.; Barkat, K.; Richards, S.N.; Lancaster, N.S.; Llinás, M.; Hogg, S.J.; Summers, R.L.; McConville, M.J.; Martin, R.E. The Natural Function of the Malaria Parasite’s Chloroquine Resistance Transporter. Nat Commun 2020, 11. [Google Scholar] [CrossRef]
- Sanchez, C.P.; Manson, E.D.T.; Moliner Cubel, S.; Mandel, L.; Weidt, S.K.; Barrett, M.P.; Lanzer, M. The Knock-Down of the Chloroquine Resistance Transporter PfCRT Is Linked to Oligopeptide Handling in Plasmodium falciparum. Microbiol Spectr 2022, 10. [Google Scholar] [CrossRef]
- Brown, K.K.; Hampton, M.B. Biological Targets of Isothiocyanates. Biochim Biophys Acta Gen Subj 2011, 1810, 888–894. [Google Scholar] [CrossRef]
- Holst, B.; Williamson, G. A Critical Review of the Bioavailability of Glucosinolates and Related Compounds. Nat Prod Rep 2004, 21, 425–447. [Google Scholar] [CrossRef]
- Juge, N.; Mithen, R.F.; Traka, M. Molecular Basis for Chemoprevention by Sulforaphane: A Comprehensive Review. Cellular and Molecular Life Sciences 2007, 64, 1105–1127. [Google Scholar] [CrossRef]
- Tiwari, S.; Sharma, N.; Guru; Sharma, P.; Mishra, N. Redox Interactome in Malaria Parasite Plasmodium falciparum. 2021. [Google Scholar] [CrossRef]
- Müller, S. Role and Regulation of Glutathione Metabolism in Plasmodium falciparum. Molecules 2015, 20, 10511–10534. [Google Scholar] [CrossRef]
- Egwu, C.O.; Augereau, J.M.; Reybier, K.; Benoit-Vical, F. Reactive Oxygen Species as the Brainbox in Malaria Treatment. Antioxidants 2021, 10, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ni, M.; Xu, X.; Chen, W. Characterisation of Naturally Occurring Isothiocyanates as Glutathione Reductase Inhibitors. J Enzyme Inhib Med Chem 2020, 35, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Dolejal Zanetti, G.; Palermo Manfron, M.; Cristina Da Silva Martins Hoelzel, S.; Pereira Pagliarin, V.; Farias Morel, A. Toxicidade Aguda e Atividade Antibacteriana Dos Extratos de Tropaeolum majus L. Acta Farm. Bonaerense 2003, 22, 159–162. [Google Scholar]
- Trager, W.; Jensen, J.B. Human Malaria Parasites in Continuous Culture. Science (1979) 1976, 193, 673–675. [Google Scholar] [CrossRef]
- Nogueira, F.; Diez, A.; Radfar, A.; Pérez-Benavente, S.; Rosario, V.E. do; Puyet, A.; Bautista, J.M. Early Transcriptional Response to Chloroquine of the Plasmodium falciparum Antioxidant Defence in Sensitive and Resistant Clones. Acta Trop 2010, 114, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.; Subramanian, G.; Lim, Y.B.; Lim, C.T.; Chandramohanadas, R. A Reference Document on Permissible Limits for Solvents and Buffers during in Vitro Antimalarial Screening. Sci Rep 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Teixeira de Moraes Gomes, P.A.; Veríssimo de Oliveira Cardoso, M.; dos Santos, I.R.; Amaro de Sousa, F.; da Conceição, J.M.; Gouveia de Melo Silva, V.; Duarte, D.; Pereira, R.; Oliveira, R.; Nogueira, F.; et al. Dual Parasiticidal Activities of Phthalimides: Synthesis and Biological Profile against Trypanosoma Cruzi and Plasmodium falciparum. ChemMedChem 2020, 15, 2164–2175. [Google Scholar] [CrossRef]
- Araújo, D.M.F.; da Cruz Filho, I.J.; Santos, T.; Pereira, D.T.M.; Marques, D.S.C.; da Conceição Alves de Lima, A.; de Aquino, T.M.; de Moraes Rocha, G.J.; do Carmo Alves de Lima, M.; Nogueira, F. Biological Activities and Physicochemical Characterization of Alkaline Lignins Obtained from Branches and Leaves of Buchenavia viridiflora with Potential Pharmaceutical and Biomedical Applications. Int J Biol Macromol 2022, 219, 224–245. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).