Submitted:
05 March 2024
Posted:
06 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Topical Formulations of Melatonin
2.1. Formulations with Liposomes
2.2. Lipid Nanoparticles
2.3. Polymeric Nanoparticles
2.4. Nanomicelles
2.5. Self-Nanoemulsifying Drug Delivery Systems
2.6. Biomechanical Delivery Systems
3. Melatonin as a Potential Therapeutic Approach for Dry Eye Disease
4. Melatonin and Cataract
5. Melatonin, a Versatile Molecule in the Retina
5.1. Melatonin’s Effects on Retinal Function:
6. Melatonin and Age-Related Macular Degeneration (AMD)
7. Melatonin Effects on Metabolic Syndrome and Diabetic Retinopathy
8. Melatonin and Retinal Ischemia
9. Melatonin and Glaucoma
10. Melatonin and Uveitis
11. Melatonin and Retinitis Pigmentosa
12. Melatonin and Myopia
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lerner, A.B.; Case, J.D.; Takahashi, Y. Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands. J Biol Chem 1960, 235, 1992–7. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.; Sengupta, A.; Maitra, S.K. Melatonin: fifty years of scientific journey from the discovery in bovine pineal gland to delineation of functions in human. Indian J Biochem Biophys 2008, 45, 289–304. [Google Scholar] [PubMed]
- Taverne, Y.J.; Merkus, D.; Bogers, A.J.; Halliwell, B.; Duncker, D.J.; Lyons, T.W. Reactive Oxygen Species: Radical Factors in the Evolution of Animal Life: A molecular timescale from Earth’s earliest history to the rise of complex life. Bioessays 2018, 40(3).
- Davies, K.J.A.; Ursini, F. The Oxygen Paradox. Cleup University Press 1995, p. 1-811.
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front Endocrinol 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Zheng, X.; Kong, J.; Manchester, L.C.; Hardeland, R.; Kim, S.J.; Xu, X.; Reiter, R.J. Fundamental issues related to the origin of melatonin and melatonin isomers during evolution: relation to their biological functions. Int J Mol Sci 2014, 15, 15858–90. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Liu, X.; Rosales-Corral, S.A.; Acuna-Castroviejo, D.; Reiter, R.J. Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary function and evolution in eukaryotes. J Pineal Res 2013, 54, 127–38. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Qin, L.; Reiter, R.J. Melatonin: A Mitochondrial Targeting Molecule Involving Mitochondrial Protection and Dynamics. Int J Mol Sci 2016, 17, 2124. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J Pineal Res 2013, 54, 245–57. [Google Scholar] [CrossRef]
- Singh, S.S.; Deb, A.; Sutradhar, S. Dexamethasone Modulates Melatonin MT2 Receptor Expression in Splenic Tissue and Humoral Immune Response in Mice Biol. Rhythm Res 2017, 48, 425–435. [Google Scholar] [CrossRef]
- Legros, C.; Dupré, C.; Brasseur, C.; Bonnaud, A.; Bruno, O.; Valour, D.; Shabajee, P.; Giganti, A.; Nosjean, O.; Kenakin, T.P.; Boutin, J.A. Characterization of the various functional pathways elicited by synthetic agonists or antagonists at the melatonin MT1 and MT2 receptors. Pharmacol Res Perspect 2019, 8, e00539. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin: signaling mechanisms of a pleiotropic agent. Biofactors 2009, 35, 183–92. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol 2008, 85, 335–53. [Google Scholar] [CrossRef]
- Scuderi, L.; Davinelli, S.; Iodice, C.M.; Bartollino, S.; Scapagnini, G.; Costagliola, C.; Scuderi, G. Melatonin: Implications for Ocular Disease and Therapeutic Potential. Curr Pharm Des 2019, 25, 4185–4191. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Q.; Wu, W.; Zeng, W.; Feng, Y. Therapeutic Effects of Melatonin on Ocular Diseases: Knowledge Map and Perspective. Front Pharmacol 2021, 12, 721869. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, A.; Hassan, M. Stability of melatonin in aqueous solution. J Pineal Res 1995, 18, 90–2. [Google Scholar] [CrossRef] [PubMed]
- Daya, S.; Walker, R.B.; Glass, B.D.; Anoopkumar-Dukie, S. The effect of variations in pH and temperature on stability of melatonin in aqueous solution. J Pineal Res 2001, 31, 155–8. [Google Scholar] [CrossRef] [PubMed]
- Vlachou, M.; Siamidi, A. Melatonin Modified Release Formulations Designed for Sleep Disorders [Internet]. Melatonin - Molecular Biology, Clinical and Pharmaceutical Approaches. IntechOpen 2018.
- Souto, E.B.; Dias-Ferreira, J.; López-Machado, A.; Ettcheto, M.; Cano, A.; Camins Espuny, A.; Espina, M.; Garcia, M.L.; Sánchez-López, E. Advanced Formulation Approaches for Ocular Drug Delivery: State-Of-The-Art and Recent Patents. Pharmaceutics 2019, 11, 460. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Rui, M. Liposomal Delivery System for Small Molecular Therapeutic Drugs. In The World Scientific Encyclopedia of Nanomedicine and Bioengineering II: Bioimplants, Regenerative Medicine, and Nano-Cancer Diagnosis and Phototherapy Volume 2, Advanced Nanomaterials for Bioimaging and Cancer Therapy 2017 235-253.
- Quinteros, D.; Vicario-de-la-Torre, M.; Andrés-Guerrero, V.; Palma, S.; Allemandi, D.; Herrero-Vanrell, R.; Molina-Martínez, I.T. Hybrid formulations of liposomes and bioadhesive polymers improve the hypotensive effect of the melatonin analogue 5-MCA-NAT in rabbit eyes. PLoS One 2014, 9, e110344. [Google Scholar] [CrossRef]
- Crooke, A.; Huete-Toral, F.; Martínez-Águila, A.; Martín-Gil, A.; Pintor, J. Melatonin and its analog 5-methoxycarbonylamino-N-acetyltryptamine potentiate adrenergic receptor-mediated ocular hypotensive effects in rabbits: significance for combination therapy in glaucoma. J Pharmacol Exp Ther 2013, 346, 138–45. [Google Scholar] [CrossRef]
- Alkozi, H.A.; Navarro, G.; Franco, R.; Pintor, J. Melatonin and the control of intraocular pressure. Prog Retin Eye Res 2020, 75, 100798. [Google Scholar] [CrossRef]
- Lou, Q.; Pan, L.; Xiang, S.; Li, Y.; Jin, J.; Tan, J.; Huang, B.; Nan, K.; Lin, S. Suppression of NLRP3/Caspase-1/GSDMD Mediated Corneal Epithelium Pyroptosis Using Melatonin-Loaded Liposomes to Inhibit Benzalkonium Chloride-Induced Dry Eye Disease. Int J Nanomedicine 2023, 18, 2447–2463. [Google Scholar] [CrossRef]
- Mrudula, B.; Sanjay, K.; Husain, M.; Kailas, M.; Vaibhav, I.; Kiran, S.; Vishal, S. Nanostructured Lipid Carriers Based Drug Delivery System: A Review. Indo American Journal of Pharmaceutical Research 2017, 7. [Google Scholar]
- Omerović, N.; Vranić, E. Application of nanoparticles in ocular drug delivery systems. Health and Technology 2020, 1. [Google Scholar] [CrossRef]
- Leonardi, A.; Bucolo, C.; Drago, F.; Salomone, S.; Pignatello, R. Cationic solid lipid nanoparticles enhance ocular hypotensive effect of melatonin in rabbit. Int J Pharm 2015, 478, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, L.; Wang, J.; Zhang, H.; Zhang, Z.; Xing, G.; Wang, X.; Liu, M. Drug-loaded PEG-PLGA nanoparticles for cancer treatment. Front Pharmacol 2022, 13, 990505. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, T.; Bucolo, C.; Carbone, C.; Pignatello, R.; Drago, F.; Puglisi, G. Polymeric nanoparticles augment the ocular hypotensive effect of melatonin in rabbits. Int J Pharm 2013, 440, 135–40. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; Santini, A.; Souto, E.B. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. , Oliveira, A.M., Neves, A., Pires, B., Venkatesh, D.N., Durazzo, A., Lucarini, M., Eder, P., Silva, A.M., Santini, A., Souto, E.B. Polymeric Nanoparticles: Production, Characterization, Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.V.R.; Silveira, P.L.; Spingolon, G.; Alves, G.A.L.; Peña, F.P.; Aguirre, T.A.S. Polymeric nanoparticles containing babassu oil: A proposed drug delivery system for controlled release of hydrophilic compounds. Chem Phys Lipids 2023, 253, 105304. [Google Scholar] [CrossRef]
- Bessone, C.D.V.; Martinez, S.M.; Luna, J.D.; Marquez, M.A.; Ramírez, M.L.; Allemandi, D.A.; Carpentieri, Á.R.; Quinteros, D.A. Neuroprotective effect of melatonin loaded in ethylcellulose nanoparticles applied topically in a retinal degeneration model in rabbits. Exp Eye Res 2020, 200, 108222. [Google Scholar] [CrossRef]
- Ye, D.; Xu, Y.; Shi, Y.; Fan, M.; Lu, P.; Bai, X.; Feng, Y.; Hu, C.; Cui, K.; Tang, X.; Liao, J.; Huang, W.; Xu, F.; Liang, X.; Huang, J. Anti-PANoptosis is involved in neuroprotective effects of melatonin in acute ocular hypertension model. J Pineal Res 2022, 73, e12828. [Google Scholar] [CrossRef]
- Carbone, C.; Manno, D.; Serra, A.; Musumeci, T.; Pepe, V.; Tisserand, C.; Puglisi, G. Innovative hybrid vs polymeric nanocapsules: The influence of the cationic lipid coating on the “4S”. Colloids Surf B Biointerfaces 2016, 141, 450–457. [Google Scholar] [CrossRef]
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J Control Release 2017, 248, 96–116. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, R.; Corsaro, R.; Bonaccorso, A.; Zingale, E.; Carbone, C.; Musumeci, T. Soluplus® polymeric nanomicelles improve solubility of BCS-class II drugs. Drug Deliv Transl Res 2022, 12, 1991–2006. [Google Scholar] [CrossRef] [PubMed]
- Dal Monte, M.; Cammalleri, M.; Amato, R.; Pezzino, S.; Corsaro, R.; Bagnoli, P.; Rusciano, D. A Topical Formulation of Melatoninergic Compounds Exerts Strong Hypotensive and Neuroprotective Effects in a Rat Model of Hypertensive Glaucoma. Int J Mol Sci 2020, 21, 9267. [Google Scholar] [CrossRef] [PubMed]
- Dal Monte, M.; Cammalleri, M.; Pezzino, S.; Corsaro, R.; Pescosolido, N.; Bagnoli, P.; Rusciano, D. Hypotensive Effect of Nanomicellar Formulation of Melatonin and Agomelatine in a Rat Model: Significance for Glaucoma Therapy. Diagnostics (Basel) 2020, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Hafner, A.; Lovrić, J.; Romić, M.D.; Juretić, M.; Pepić, I.; Cetina-Čižmek, B.; Filipović-Grčić, J. Evaluation of cationic nanosystems with melatonin using an eye-related bioavailability prediction model. Eur J Pharm Sci 2015, 75, 142–50. [Google Scholar] [CrossRef] [PubMed]
- Zingale, E.; Bonaccorso, A.; D’Amico, A.G.; Lombardo, R.; D’Agata, V.; Rautio, J.; Pignatello, R. Formulating Resveratrol and Melatonin Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) for Ocular Administration Using Design of Experiments. Pharmaceutics 2024, 16, 125. [Google Scholar] [CrossRef] [PubMed]
- Romeo, A.; Kazsoki, A.; Omer, S.; Pinke, B.; Mészáros, L.; Musumeci, T.; Zelkó, R. Formulation and Characterization of Electrospun Nanofibers for Melatonin Ocular Delivery. Pharmaceutics 2023, 15, 1296. [Google Scholar] [CrossRef]
- Serramito, M.; Pereira-da-Mota, A.F.; Carpena-Torres, C.; Huete-Toral, F.; Alvarez-Lorenzo, C.; Carracedo, G. Melatonin-Eluting Contact Lenses Effect on Tear Volume: In Vitro and In Vivo Experiments. Pharmaceutics 2022, 14, 1019. [Google Scholar] [CrossRef]
- Navarro-Gil, F.J.; Huete-Toral, F.; Domínguez-Godínez, C.O.; Carracedo, G.; Crooke, A. Contact Lenses Loaded with Melatonin Analogs: A Promising Therapeutic Tool against Dry Eye Disease. J Clin Med 2022, 11, 3483. [Google Scholar] [CrossRef]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv Transl Res. 2016, 6, 735–754. [Google Scholar] [CrossRef]
- Lemp, M.A.; Baudouin, C.; Baum, J.; Dogru, M.; Foulks, G.N.; Kinoshita, S.; Laibson, P.; McCulley, J.; Murube, J.; Pflugfelder, S.C.; et al. The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul. Surf 2007, 5, 75–92. [Google Scholar]
- Tsubota, K., Yokoi, N., Shimazaki, J., Watanabe, H., Dogru, M., Yamada, M., Kinoshita, S., Kim, H. M., Tchah, H. W., Hyon, J. Y., Yoon, K. C., Seo, K. Y., Sun, X., Chen, W., Liang, L., Li, M., Liu, Z., ùAsia Dry Eye Society. New Perspectives on Dry Eye Definition and Diagnosis: A Consensus Report by the Asia Dry Eye Society. The ocular surface. 2017, 15, 65–76.
- Ciuffi, M.; Pisanello, M.; Pagliai, G.; Raimondi, L.; Franchi-Micheli, S.; Cantore, M.; Mazzetti, L.; Failli, P. Antioxidant protection in cultured corneal cells and whole corneas submitted to UV-B exposure. Journal of photochemistry and photobiology. J Photochem Photobiol B 2003, 71, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zuo, X.; Peng, L.; Wang, X.; Zeng, H.; Zhong, J.; Li, S.; Xiao, Y.; Wang, L.; Ouyang, H.; Yuan, J. Melatonin ameliorates oxidative stress-mediated injuries through induction of HO-1 and restores autophagic flux in dry eye. Exp Eye Res 2021, 205, 108491. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Peng, R.; Shen, H.; Zhong, L.; Song, S.; Wang, T.; Ling, S. Treatment With Melatonin After Corneal Graft Attenuates Rejection. Front Pharmacol 2021, 12, 778892. [Google Scholar] [CrossRef]
- Carracedo, G., Carpena, C., Concepción, P., Díaz, V., García-García, M., Jemni, N., Lledó, V. E., Martín, M., Pastrana, C., Pelissier, R., Veselinova, A., Wang, X., Pintor, J. Presence of melatonin in human tears. J Optom. 2017, 10, 3–4.
- Crooke, A.; Guzman-Aranguez, A.; Mediero, A.; Alarma-Estrany, P.; Carracedo, G.; Pelaez, T.; Peral, A.; Pintor, J. Effect of melatonin and analogues on corneal wound healing: involvement of Mt2 melatonin receptor. Curr Eye Res 2015, 40, 56–65. [Google Scholar] [CrossRef]
- Navarro Gil, F.J.; Huete-Toral, F.; Crooke, A.; Dominguez Godinez, C.O.; Carracedo, G.; Pintor, J. Effect of Melatonin and Its Analogs on Tear Secretion. J Pharmacol Exp Ther 2019, 371, 186–190. [Google Scholar] [CrossRef]
- Alkozi, H.A.; Sánchez Montero, J.M.; Doadrio, A.L.; Pintor, J. Docking studies for melatonin receptors. Expert Opin Drug Discov 2018, 13, 241–248. [Google Scholar] [CrossRef]
- Hoyle, C.H.; Peral, A.; Pintor, J. Melatonin potentiates tear secretion induced by diadenosine tetraphosphate in the rabbit. Eur J Pharmacol 2006, 552, 159–61. [Google Scholar] [CrossRef]
- Xu, J.; Chen, P.; Zhao, G.; Wei, S.; Li, Q.; Guo, C.; Cao, Q.; Wu, X.; Di, G. Copolymer Micelle-administered Melatonin Ameliorates Hyperosmolarity-induced Ocular Surface Damage through Regulating PINK1-mediated Mitophagy. Curr Eye Res 2022, 47, 688–703. [Google Scholar] [CrossRef] [PubMed]
- Wahl, C.; Li, T.; Takagi, Y.; Howland, H. The effects of light regimes and hormones on corneal growth in vivo and in organ culture. J Anat 2011, 219, 766–75. [Google Scholar] [CrossRef]
- Dubbelman, M.; Van der Heijde, G.L.; Weeber, H.A.; Vrensen, G.F. Changes in the internal structure of the human crystalline lens with age and accommodation. Vision Res 2003, 43, 2363–75. [Google Scholar] [CrossRef]
- Petrash, J.M. Aging and age-related diseases of the ocular lens and vitreous body. Invest Ophthalmol Vis Sci 2013, 54, ORSF54–9. [Google Scholar] [CrossRef] [PubMed]
- Bassnett, S. Lens organelle degradation. Exp Eye Res 2002, 74, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Moreau, K.L.; King, J.A. Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends Mol Med 2012, 18, 273–82. [Google Scholar] [CrossRef] [PubMed]
- Benedek, G.B. Cataract as a protein condensation disease: the Proctor Lecture. Invest Ophthalmol Vis Sci 1997, 38, 1911–21. [Google Scholar] [PubMed]
- Bassnett, S. Mitochondrial dynamics in differentiating fiber cells of the mammalian lens. Curr Eye Res 1992, 11, 1227–32. [Google Scholar] [CrossRef]
- Bassnett, S. The fate of the Golgi apparatus and the endoplasmic reticulum during lens fiber cell differentiation. Invest Ophthalmol Vis Sci 1995, 36, 1793–803. [Google Scholar]
- Pendergrass, W.; Penn, P.; Possin, D.; Wolf, N. Accumulation of DNA, nuclear and mitochondrial debris, and ROS at sites of age-related cortical cataract in mice. Invest Ophthalmol Vis Sci 2005, 46, 4661–70. [Google Scholar] [CrossRef]
- Bubenik, G.A.; Brown, G.M.; Uhlir, I.; Grota, L.J. Immunohistological localization of N-acetylindolealkylamines in pineal gland, retina and cerebellum. Brain Res 1974, 81, 233–42. [Google Scholar] [CrossRef] [PubMed]
- Aimoto, T., Rohde, B.H., Chiou, G.C., Lauber, J.K. N-acetyltransferase activity and melatonin level in the eyes of glaucomatous chickens. J Ocul Pharmacol. 1985, 1, 149–60.
- Quay, W.B. Increases in volume, fluid content, and lens weight of eyes following systemic administration of melatonin. J Pineal Res 1984, 1, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Itoh, M.T.; Miyata, M.; Shimizu, K.; Sumi, Y. Circadian rhythm of serotonin N -acetyltransferase activity in rat lens. Exp Eye Res 2000, 70, 805–8. [Google Scholar] [CrossRef] [PubMed]
- Mhatre, M.C.; van Jaarsveld, A.S.; Reiter, R.J. Melatonin in the lacrimal gland: first demonstration and experimental manipulation. Biochem Biophys Res Commun 1988, 153, 1186–92. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Reiter, R.J.; Orhii, P.B.; Hara, M.; Poeggeler, B. Inhibitory effect of melatonin on cataract formation in newborn rats: evidence for an antioxidative role for melatonin. J Pineal Res 1994, 17, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Yağci, R., Aydin, B., Erdurmuş, M., Karadağ, R., Gürel, A., Durmuş, M., Yiğitoğlu, R. Use of melatonin to prevent selenite-induced cataract formation in rat eyes. Curr Eye Res 2006, 31, 845-50.
- Bai, J.; Dong, L.; Song, Z.; Ge, H.; Cai, X.; Wang, G.; Liu, P. The role of melatonin as an antioxidant in human lens epithelial cells. Free Radic Res 2013, 47, 635–42. [Google Scholar] [CrossRef]
- Costello, M.J.; Brennan, L.A.; Basu, S.; Chauss, D.; Mohamed, A.; Gilliland, K.O.; Johnsen, S.; Menko, S.; Kantorow, M. Autophagy and mitophagy participate in ocular lens organelle degradation. Exp Eye Res 2013, 116, 141–50. [Google Scholar] [CrossRef]
- Jenwitheesuk, A.; Nopparat, C.; Mukda, S.; Wongchitrat, P.; Govitrapong, P. Melatonin regulates aging and neurodegeneration through energy metabolism, epigenetics, autophagy and circadian rhythm pathways. Int J Mol Sci 2014, 15, 16848–84. [Google Scholar] [CrossRef]
- Mi, Y., Wei, C., Sun, L., Liu, H., Zhang, J., Luo, J., Yu, X., He, J., Ge, H., Liu, P. (2023). Melatonin inhibits ferroptosis and delays age-related cataract by regulating SIRT6/p-Nrf2/GPX4 and SIRT6/NCOA4/FTH1 pathways. Biomed Pharmacother 2023, 157, 114048. 114048.
- Lledó, V. E., Alkozi, H. A., Sánchez-Naves, J., Fernandez-Torres, M. A., Guzman-Aranguez, A. Melatonin counteracts oxidative damage in lens by regulation of Nrf2 and NLRP3 inflammasome activity. Exp Eye Res 2022, 215, 108912.
- Ohannes, A.K.; Hussain, S.A.R.; Rasool, A.A.H.; Mohammed, H.M.; Al Essa, A.; Abdulrazzaq, M.H. Protective effects of melatonin as an eye drops against selenite-induced cataract in rat pups. Saudi Pharmaceutical Journal 2009, 17, 2. [Google Scholar]
- Tosini, G.; Baba, K.; Hwang, C.K.; Iuvone, P.M. Melatonin: an underappreciated player in retinal physiology and pathophysiology. Exp Eye Res 2012, 103, 82–9. [Google Scholar] [CrossRef] [PubMed]
- Tosini, G.; Pozdeyev, N.; Sakamoto, K.; Iuvone, P.M. The circadian clock system in the mammalian retina. Bioessays 2008, 30, 624–33. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.; Wankiewicz, E.; Brown, G.M.; Fujieda, H. MT(1) melatonin receptor in the human retina: expression and localization. Invest Ophthalmol Vis Sc. 2002, 43, 889–97. [Google Scholar]
- Jockers, R.; Maurice, P.; Boutin, J.A.; Delagrange, P. Melatonin receptors, heterodimerization, signal transduction and binding sites: what’s new? Br J Pharmacol 2008, 154, 1182–95. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Pozdeyev, N.; Mazzoni, F.; Contreras-Alcantara, S.; Liu, C.; Kasamatsu, M.; Martinez-Merlos, T.; Strettoi, E.; Iuvone, P.M.; Tosini, G. Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc Natl Acad Sci U S A 2009, 106, 15043–8. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L.; Delagrange, P.; Krause, D.N.; Sugden, D.; Cardinali, D.P.; Olcese, J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev 2010, 62, 343–80. [Google Scholar]
- Marchiafava, P.L.; Longoni, B. Melatonin as an antioxidant in retinal photoreceptors. J Pineal Res 1999, 26, 184–9. [Google Scholar] [CrossRef]
- Piano, I., Baba, K., C Gargini, Tosini, G. Heteromeric MT1/MT2 melatonin receptors modulate the scotopic electroretinogram via PKCζ in mice. Exp Eye Res 2018, 177, 50-54.
- Emser, W.; Dechoux, R.; Weiland, M.; Wirz-Justice, A. Melatonin decreases the amplitude of the b-wave of the human electroretinogram. Experientia 1993, 49, 686–7. [Google Scholar] [CrossRef]
- Peters, J.L.; Cassone, V.M. Melatonin regulates circadian electroretinogram rhythms in a dose- and time-dependent fashion. J Pineal Res 2005, 38, 209–15. [Google Scholar] [CrossRef]
- Beatty, S.; Koh, H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol 2000, 45, 115–34. [Google Scholar] [CrossRef]
- Bellezza, I. Oxidative Stress in Age-Related Macular Degeneration: Nrf2 as Therapeutic Target. Front Pharmacol 2018, 9, 1280. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.; Hu, D.N.; Perez, V.; Tai, K.; Yu, G.P.; Chen, M.; Tone, P.; McCormick, S.A.; Walsh, J. Urinary 6-sulfatoxymelatonin level in age-related macular degeneration patients. Mol Vis 2009, 15, 1673–9. [Google Scholar]
- Yi, C.; Pan, X.; Yan, H.; Guo, M.; Pierpaoli, W. Effects of melatonin in age-related macular degeneration. Ann N Y Acad Sci 2005, 1057, 384–92. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.B.; Hu, D.N.; Chen, M.; McCormick, S.A.; Walsh, J.; Roberts, J.E. Effects of melatonin and its receptor antagonist on retinal pigment epithelial cells against hydrogen peroxide damage. Mol Vis 2012, 18, 1640–8. [Google Scholar] [PubMed]
- Lin, J.; Epel, E. Stress and telomere shortening: Insights from cellular mechanisms. Ageing Res Rev 2022, 73, 101507. [Google Scholar] [CrossRef] [PubMed]
- Rowe-Rendleman, C.; Glickman, R.D. Possible therapy for age-related macular degeneration using human telomerase. Brain Res Bull 2004, 62, 549–53. [Google Scholar] [CrossRef]
- Rastmanesh, R. Potential of melatonin to treat or prevent age-related macular degeneration through stimulation of telomerase activity. Med Hypotheses 2011, 76, 79–85. [Google Scholar] [CrossRef]
- Tisi, A.; Feligioni, M.; Passacantando, M.; Ciancaglini, M.; Maccarone, R. The Impact of Oxidative Stress on Blood-Retinal Barrier Physiology in Age-Related Macular Degeneration. Cells 2021, 10, 64. [Google Scholar] [CrossRef]
- Diéguez, H.H.; González Fleitas, M.F.; Aranda, M.L.; Calanni, J.S.; Keller Sarmiento, M.I.; Chianelli, M.S.; Alaimo, A.; Sande, P.H.; Romeo, H.E.; Rosenstein, R.E.; Dorfman, D. Melatonin protects the retina from experimental nonexudative age-related macular degeneration in mice. J Pineal Res 2020, 68, e12643. [Google Scholar] [CrossRef]
- Mehrzadi, S.; Hemati, K.; Reiter, R.J.; Hosseinzadeh, A. Mitochondrial dysfunction in age-related macular degeneration: melatonin as a potential treatment. Expert Opin Ther Targets 2020, 24, 359–378. [Google Scholar] [CrossRef]
- Ku, L.C.; Sheu, M.L.; Cheng, H.H.; Lee, C.Y.; Tsai, Y.C.; Tsai, C.Y.; Lin, K.H.; Lai, L.C.; Lai, D.W. Melatonin protects retinal integrity through mediated immune homeostasis in the sodium iodate-induced mouse model of age-related macular degeneration. Biomed Pharmacother 2023, 161, 114476. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Li, Y.; Che, M.; Li, C.; Wu, Q.; Sun, C. Melatonin relieves hepatic lipid dysmetabolism caused by aging via modifying the secondary bile acid pattern of gut microbes. Cell Mol Life Sci 2022, 79, 527. [Google Scholar] [CrossRef] [PubMed]
- Rong, B.; Wu, Q.; Reiter, R.J.; Sun, C. The Mechanism of Oral Melatonin Ameliorates Intestinal and Adipose Lipid Dysmetabolism Through Reducing Escherichia Coli-Derived Lipopolysaccharide. Cell Mol Gastroenterol Hepatol 2021, 12, 1643–1667. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, Y.; Han, H.; Ma, J.; Liu, G.; Wu, X.; Huang, X.; Fang, R.; Baba, K.; Bin, P.; Zhu, G.; Ren, W.; Tan, B.; Tosini, G.; He, X.; Li, T.; Yin, Y. Administration of Exogenous Melatonin Improves the Diurnal Rhythms of the Gut Microbiota in Mice Fed a High-Fat Diet. mSystems 2020, 5, e00002–20. [Google Scholar] [CrossRef] [PubMed]
- Onaolapo, A.Y.; Adebisi, E.O.; Adeleye, A.E.; Olofinnade, A.T.; Onaolapo, O.J. Dietary Melatonin Protects Against Behavioural, Metabolic, Oxidative, and Organ Morphological Changes in Mice that are Fed High-Fat, High- Sugar Diet. Endocr Metab Immune Disord Drug Targets 2020, 20, 570–583. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, Y.; Han, H.; Chen, S.; Gao, J.; Liu, G.; Wu, X.; Deng, J.; Yu, Q.; Huang, X.; Fang, R.; Li, T.; Reiter, R.J.; Zhang, D.; Zhu, C.; Zhu, G.; Ren, W.; Yin, Y. Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. J Pineal Res 2018, 6, e12524. [Google Scholar] [CrossRef] [PubMed]
- Onaolapo, A.Y.; Onaolapo, O.J. Circadian dysrhythmia-linked diabetes mellitus: Examining melatonin’s roles in prophylaxis and management. World J Diabetes 2018, 9, 99–114. [Google Scholar] [CrossRef]
- Ozdemir, G.; Ergün, Y.; Bakariş, S.; Kılınç, M.; Durdu, H.; Ganiyusufoğlu, E. Melatonin prevents retinal oxidative stress and vascular changes in diabetic rats. Eye (Lond) 2014, 28, 1020–7. [Google Scholar] [CrossRef]
- Salido, E.M.; Bordone, M.; De Laurentiis, A.; Chianelli, M.; Keller Sarmiento, M.I.; Dorfman, D.; Rosenstein, R.E. Therapeutic efficacy of melatonin in reducing retinal damage in an experimental model of early type 2 diabetes in rats. J Pineal Res 2013, 54, 179–89. [Google Scholar] [CrossRef]
- Jiang, T.; Chang, Q.; Cai, J.; Fan, J.; Zhang, X.; Xu, G. Protective Effects of Melatonin on Retinal Inflammation and Oxidative Stress in Experimental Diabetic Retinopathy. Oxid Med Cell Longev 2016, 2016, 3528274. [Google Scholar] [CrossRef]
- Mehrzadi, S.; Motevalian, M.; Rezaei Kanavi, M.; Fatemi, I.; Ghaznavi, H.; Shahriari, M. Protective effect of melatonin in the diabetic rat retina. Fundam Clin Pharmacol 2018, 32, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Yu, F.; Shi, L.; Ko, M.L.; Ko, G.Y. Melatonin Affects Mitochondrial Fission/Fusion Dynamics in the Diabetic Retina. J Diabetes Res 2019, 2019, 8463125. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, B., Cvetkovic, T., Stoimenov, T. J., Despotovic, M., Zivanovic, S., Basic, J., Veljkovic, A., Velickov, A., Kocic, G., Pavlovic, D., Sokolovic, D.Oral supplementation with melatonin reduces oxidative damage and concentrations of inducible nitric oxide synthase, VEGF and matrix metalloproteinase 9 in the retina of rats with streptozotocin/nicotinamide induced pre-diabetes. Eur J Pharmacol 2018, 833, 290-297.
- Tu, Y.; Zhu, M.; Wang, Z.; Wang, K.; Chen, L.; Liu, W.; Shi, Q.; Zhao, Q.; Sun, Y.; Wang, X.; Song, E.; Liu, X. Melatonin inhibits Müller cell activation and pro-inflammatory cytokine production via upregulating the MEG3/miR-204/Sirt1 axis in experimental diabetic retinopathy. J Cell Physiol 2020, 235, 8724–8735. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Song, E.; Wang, Z.; Ji, N.; Zhu, L.; Wang, K.; Sun, H.; Zhang, Y.; Zhu, Q.; Liu, X.; Zhu, M. Melatonin attenuates oxidative stress and inflammation of Müller cells in diabetic retinopathy via activating the Sirt1 pathway. Biomed Pharmacother 2021, 137, 111274. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhang, C.; Yang, Q.; Xie, H.; Liu, D.; Tian, H.; Lu, L.; Xu, J.Y.; Li, W.; Xu, G.; Qiu, Q.; Liu, K.; Luo, D.; Xu, G.T.; Zhang, J. Melatonin maintains inner blood-retinal barrier via inhibition of p38/TXNIP/NF-κB pathway in diabetic retinopathy. J Cell Physiol 2021, 236, 5848–5864. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Wang, H.; Gu, Y.; Li, X.; Tao, L.; Lu, P. Melatonin exerts protective effects on diabetic retinopathy via inhibition of Wnt/β-catenin pathway as revealed by quantitative proteomics. Exp Eye Res 2021, 205, 108521. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Rathnasamy, G.; Foulds, W.S.; Ling, E.A. Cellular and Molecular Mechanisms of Retinal Ganglion Cell Death in Hypoxic-Ischemic Injuries. J Neurol Exp Neurosci 2015, 1, 10–19. [Google Scholar] [CrossRef]
- Park, S.W.; Lee, H.S.; Sung, M.S.; Kim, S.J. The effect of melatonin on retinal ganglion cell survival in ischemic retina. Chonnam Med J 2012, 48, 116–22. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, X.; Hu, Y.; Yang, B.; Tsui, C.K.; Yu, S.; Lu, L.; Liang, X. Melatonin attenuated retinal neovascularization and neuroglial dysfunction by inhibition of HIF-1α-VEGF pathway in oxygen-induced retinopathy mice. J Pineal Res 2018, 64, e12473. [Google Scholar] [CrossRef]
- Miranda-Riestra, A.; Estrada-Reyes, R.; Torres-Sanchez, E.D.; Carreño-García, S.; Ortiz, G.G.; Benítez-King, G. Melatonin: A Neurotrophic Factor? Molecules 2022, 27, 7742. [Google Scholar] [CrossRef]
- Huang, R.; Xu, Y.; Lu, X.; Tang, X.; Lin, J.; Cui, K.; Yu, S.; Shi, Y.; Ye, D.; Liu, Y.; Liang, X. Melatonin protects inner retinal neurons of newborn mice after hypoxia-ischemia. J Pineal Res 2021, 71, e12716. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Sivakumar, V.; Yong, Z.; Lu, J.; Foulds, W.S.; Ling, E.A. Blood-retinal barrier disruption and ultrastructural changes in the hypoxic retina in adult rats: the beneficial effect of melatonin administration. J Pathol 2007, 212, 429–39. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A. Glaucoma. The Lancet 2011, 377, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Pascolini, D.; Mariotti, S.P. Global estimates of visual impairment: 2010. Br J Ophthalmol 2012, 96, 614–618. [Google Scholar] [CrossRef]
- Nucci, C.; Tartaglione, R.; Rombolà, L.; Morrone, L. A.; Fazzi, E.; Bagetta, G. Neurochemical Evidence to Implicate Elevated Glutamate in the Mechanisms of High Intraocular Pressure (IOP)-induced Retinal Ganglion Cell Death in Rat. NeuroToxicology 2005, 26, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: a review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Investigators, T.A. The advanced glaucoma intervention study (AGIS) : 7. the relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol 2000, 130, 429–440. [Google Scholar]
- Jin, S.W.; Noh, S.Y. Long-Term Clinical Course of Normal-Tension Glaucoma: 20 Years of Experience. Journal of Ophthalmology 2017, e2651645. [Google Scholar] [CrossRef]
- Marquis, R.E.; Whitson, J.T. Management of Glaucoma: Focus on Pharmacological Therapy. Drugs Aging 2005, 22, 1–21. [Google Scholar] [CrossRef]
- Agorastos, A.; Huber, C.G. The role of melatonin in glaucoma: implications concerning pathophysiological relevance and therapeutic potential. J Pineal Res 2011, 50, 1–7. [Google Scholar] [CrossRef]
- Alkozi, H.; Sánchez-Naves, J.; de Lara, M.J.; Carracedo, G.; Fonseca, B.; Martinez-Aguila, A.; Pintor, J. Elevated intraocular pressure increases melatonin levels in the aqueous humour. Acta Ophthalmologica 2017, 95, e185–e189. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Águila, A.; Martín-Gil, A.; Carpena-Torres, C.; Pastrana, C.; Carracedo, G. Influence of Circadian Rhythm in the Eye: Significance of Melatonin in Glaucoma. Biomolecules 2021, 11, 340. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Molecular and Cellular Endocrinology 2012, 351, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Crooke, A.; Huete-Toral, F.; Colligris, B.; Pintor, J. The role and therapeutic potential of melatonin in age-related ocular diseases. Journal of Pineal Research 2017, 63, e12430. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Águila, A.; Fonseca, B.; Pérez de Lara, M.J.; Miras-Portugal, M.T.; Gómez-Villafuertes, R.; Carracedo, G.; Pintor, J. Changes in melatonin receptor expression in a murine model of glaucoma. Mol Vis 2020 26, 530–539.
- Alcantara-Contreras, S.; Baba, K.; Tosini, G. Removal of Melatonin Receptor Type 1 Increases Intraocular Pressure and Retinal Ganglion Cells Death in the Mouse. Neurosci Lett 2011, 494, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Tosini, G.; Iuvone, M.; Boatright, J.H. Is the melatonin receptor type 1 involved in the pathogenesis of glaucoma? J Glaucoma 2013, 22 Suppl 5, S49–50. [Google Scholar] [CrossRef]
- Alkozi, H.; Sánchez-Naves, J.; de Lara, M.J.; Carracedo, G.; Fonseca, B.; Martinez-Aguila, A.; Pintor, J. Elevated intraocular pressure increases melatonin levels in the aqueous humour. Acta ophthalmologica 2017, 95, e185–e189. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-P.; Shen, M.-Y.; Shen, G.-L.; Qi, Q.-R.; Sun, X.-H. Melatonin concentrations in serum of primary glaucoma patients. International Journal of Ophthalmology 2018, 11, 1337–1341. [Google Scholar]
- Kim, J.Y.; Jeong, A.R.; Chin, H.S.; Kim, N.R. Melatonin Levels in Patients With Primary Open-angle Glaucoma With High or Low Intraocular Pressure. Journal of Glaucoma 2019, 28, 154–160. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Obayashi, K.; Miyata, K.; Saeki, K.; Ogata, N. Decreased melatonin secretion in patients with glaucoma: Quantitative association with glaucoma severity in the LIGHT study. J Pineal Res 2020, 69, e12662. [Google Scholar] [CrossRef] [PubMed]
- Gubin, D.G.; Malishevskaya Т, N.; Astakhov, Y.S.; Astakhov, S.Y.; Cornelissen, G.; Kuznetsov, V.A.; Weinert, D. Progressive retinal ganglion cell loss in primary open-angle glaucoma is associated with temperature circadian rhythm phase delay and compromised sleep. Chronobiol Int 2019, 36, 564–577. [Google Scholar] [CrossRef]
- Alarma-Estrany, P., Guzman-Aranguez, A., Huete, F., Peral, A., Plourde, R., Jr, Pelaez, T., Yerxa, B., Pintor, J. (2011). Design of Novel Melatonin Analogs for the Reduction of Intraocular Pressure in Normotensive Rabbits. J Pharmacol Exp Ther 2011, 337, 703–709.
- Crooke, A.; Huete-Toral, F.; Martínez-Águila, A.; Martín-Gil, A.; Pintor, J. Melatonin and Its Analog 5-Methoxycarbonylamino-N-Acetyltryptamine Potentiate Adrenergic Receptor-Mediated Ocular Hypotensive Effects in Rabbits: Significance for Combination Therapy in Glaucoma. J Pharmacol Exp Ther 2013, 346, 138–145. [Google Scholar] [CrossRef]
- Martinez-Aguila, A.; Fonseca, B.; Perez de Lara, M.J.; Pintor, J. Effect of Melatonin and 5-Methoxycarbonylamino-N-Acetyltryptamine on the Intraocular Pressure of Normal and Glaucomatous Mice. Journal of Pharmacology and Experimental Therapeutics 2016, 357, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, D.S.; Liebmann, J.M.; Ritch, R. Brimonidine: a new alpha2-adrenoreceptor agonist for glaucoma treatment. J Glaucoma 1997, 6, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Arranz-Romera, A.; Davis, B.M.; Bravo-Osuna, I.; Esteban-Pérez, S.; Molina-Martínez, I.T.; Shamsher, E.; Ravindran, N.; Guo, L.; Cordeiro, M.F.; Herrero-Vanrell, R. Simultaneous co-delivery of neuroprotective drugs from multi-loaded PLGA microspheres for the treatment of glaucoma. Journal of controlled release: official journal of the Controlled Release Society 2019, 297, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.A.; Mowafi, H.A. Melatonin Provides Anxiolysis, Enhances Analgesia, Decreases Intraocular Pressure, and Promotes Better Operating Conditions During Cataract Surgery Under Topical Anesthesia. Anesthesia & Analgesia 209, 108, 1146–1151.
- Pescosolido, N.; Gatto, V.; Stefanucci, A.; Rusciano, D. Oral treatment with the melatonin agonist agomelatine lowers the intraocular pressure of glaucoma patients. Ophthalmic and Physiological Optics 2015, 35, 201–205. [Google Scholar] [CrossRef]
- Martínez-Águila, A.; Fonseca, B.; Bergua, A.; Pintor, J. Melatonin analogue agomelatine reduces rabbit’s intraocular pressure in normotensive and hypertensive conditions. European Journal of Pharmacology 2013, 701, 213–217. [Google Scholar] [CrossRef]
- Southgate, G.; Schubert, M.; Daya, S. Melatonin Offers Protection against Glutamate Receptor Agonists in Neuronal Cultures. Annals of Neurosciences 2010, 15, 1–5. [Google Scholar] [CrossRef]
- Espinar, A.; García-Oliva, A.; Isorna, E.M.; Quesada, A.; Prada, F.A.; Guerrero, J.M. Neuroprotection by melatonin from glutamate-induced excitotoxicity during development of the cerebellum in the chick embryo. J Pineal Res 2000, 28, 81–88. [Google Scholar] [CrossRef]
- del Valle Bessone, C., Fajreldines, H. D., de Barboza, G. E. D., Tolosa de Talamoni, N. G., Allemandi, D. A., Carpentieri, A. R., et al. Protective role of melatonin on retinal ganglionar cell: In vitro an in vivo evidences. Life Sciences 2019, 218, 233–240.
- Gupta, N.; Yücel, Y.H. Glaucoma as a neurodegenerative disease. Curr Opin Ophthalmol 2007, 18, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Rusciano, D.; Pezzino, S.; Mutolo, M.G.; Giannotti, R.; Librando, A.; Pescosolido, N. Neuroprotection in Glaucoma: Old and New Promising Treatments. Adv Pharmacol Sci 2017, 4320408–4320408. [Google Scholar] [CrossRef] [PubMed]
- Egwuagu, C.E.; Alhakeem, S.A.; Mbanefo, E.C. Uveitis: Molecular Pathogenesis and Emerging Therapies. Front Immunol 2021, 12, 623725. [Google Scholar] [CrossRef]
- Aranda, M.L.; Fleitas, M.F.G.; Dieguez, H.; Iaquinandi, A.; Sande, P.H.; Dorfman, D.; Rosenstein, R.E. Melatonin as a Therapeutic Resource for Inflammatory Visual Diseases. Curr Neuropharmacol 2017, 15, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Sande, P.H.; Dorfman, D.; Fernandez, D.C.; Chianelli, M.; Domínguez Rubio, A.P.; Franchi, A.M.; Silberman, D.M.; Rosenstein, R.E.; Sáenz, D.A. Treatment with melatonin after onset of experimental uveitis attenuates ocular inflammation. Br J Pharmacol 2014, 171, 5696–707. [Google Scholar] [CrossRef] [PubMed]
- Chesnokova, N.B.; Beznos, O.V.; Lozinskaya, N.A.; Beyshenova, G.A.; Nesterova, T.V. Effect of melatonin instillations on the clinical course of experimental uveitis and biochemical processes in tears and aqueous humor. Biomed Khim 2016, 62, 164–8. [Google Scholar] [CrossRef] [PubMed]
- Banaś, I., Buntner, B., Niebrój, T., Ostrowska, Z. Stezenie melatoniny w surowicy chorych ze zwyrodnieniem barwnikowym siatkówki [Levels of melatonin in serum of patients with retinitis pigmentosa]. Klin Oczna 1995, 97, 321-3.
- Xu, X.J.; Wang, S.M.; Jin, Y.; Hu, Y.T.; Feng, K.; Ma, Z.Z. Melatonin delays photoreceptor degeneration in a mouse model of autosomal recessive retinitis pigmentosa. J Pineal Res 2017, 63. [Google Scholar] [CrossRef] [PubMed]
- Liang, F. Q., Aleman, T. S., ZaixinYang, Cideciyan, A. V., Jacobson, S. G., Bennett, J. Melatonin delays photoreceptor degeneration in the rds/rds mouse. Neuroreport 2001, 12, 1011-4.
- Pastor-Idoate, S.; Mateos-Olivares, M.; Sobas, E.M.; Marcos, M.; Toribio, A.; Pastor, J.C.; Usategui Martín, R. Short-Wavelength Light-Blocking Filters and Oral Melatonin Administration in Patients With Retinitis Pigmentosa: Protocol for a Randomized Controlled Trial. JMIR Res Protoc 2023, 12, e49196. [Google Scholar] [CrossRef]
- Morgan, I.G. The biological basis of myopic refractive error. Clin Exp Optom 2003, 86, 276–88. [Google Scholar] [CrossRef]
- Hoffmann, M.; Schaeffel, F. Melatonin and deprivation myopia in chickens. Neurochem Int 1996, 28, 95–107. [Google Scholar] [CrossRef]
- Morgan, I.G.; Rose, K.A.; Ashby, R.S. Animal Models of Experimental Myopia: Limitations and Synergies with Studies on Human Myopia. In: Spaide, R., Ohno-Matsui, K., Yannuzzi, L. (eds) Pathologic Myopia. Springer, New York, NY 2014.
- Kearney, S.; O’Donoghue, L.; Pourshahidi, L.K.; Cobice, D.; Saunders, K.J. Myopes have significantly higher serum melatonin concentrations than non-myopes. Ophthalmic Physiol Opt 2017, 37, 557–567. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, N.; Velpandian, T.; Gupta, V.; Vanathi, M.; Vashist, P.; Gowtham, L.; Saxena, R.; Tandon, R. Myopia, Melatonin and Conjunctival Ultraviolet Autofluorescence: A Comparative Cross-sectional Study in Indian Myopes. Curr Eye Res 2021, 46, 1474–1481. [Google Scholar] [CrossRef]
- Fulton, J.M.; Flanagan, S.C.; Sittlington, J.J.; Cobice, D.; Dobbin, S.; McCullough, S.J.; Orr, G.; Richardson, P.; Saunders, K.J. A Cross-Sectional Study of Myopia and Morning Melatonin Status in Northern Irish Adolescent Children. J Ophthalmol 2023, 2023, 7961623. [Google Scholar] [CrossRef]
- Chakraborty, R.; Seby, C.; Scott, H.; Tang, V.; Kemps, E.; Anstice, N.; Juers, E.; Lovato, N.; Taranath, D.A.; Mills, R.A.; Lack, L.C. Delayed melatonin circadian timing, lower melatonin output, and sleep disruptions in myopic, or short-sighted, children. Sleep 2024, 47, zsad265. [Google Scholar] [CrossRef]
- Hussain, A.; Gopalakrishnan, A.; Scott, H.; Seby, C.; Tang, V.; Ostrin, L.; Chakraborty, R. Associations between systemic melatonin and human myopia: A systematic review. Ophthalmic Physiol Opt 2023, 43, 1478–1490. [Google Scholar] [CrossRef]
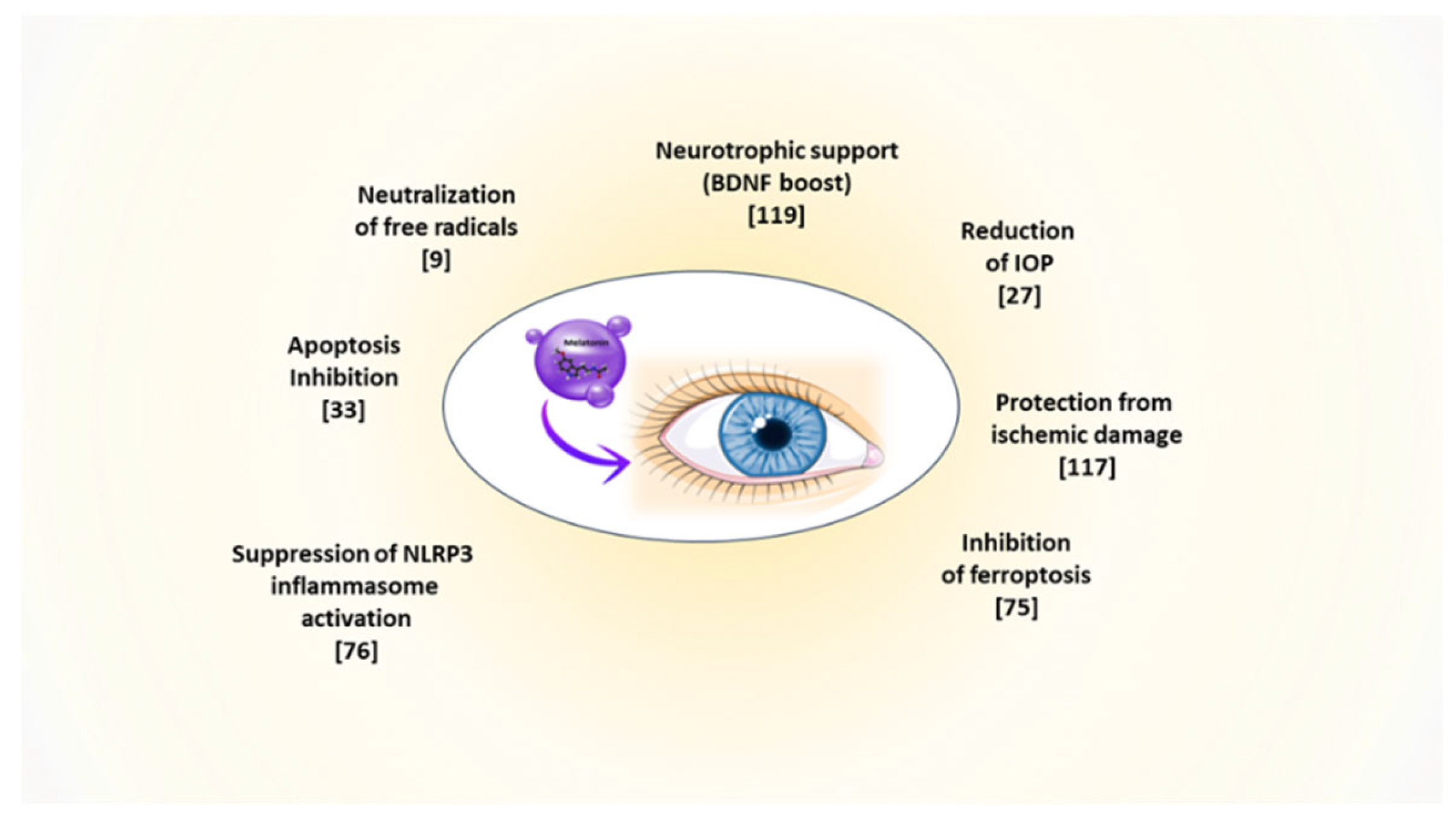
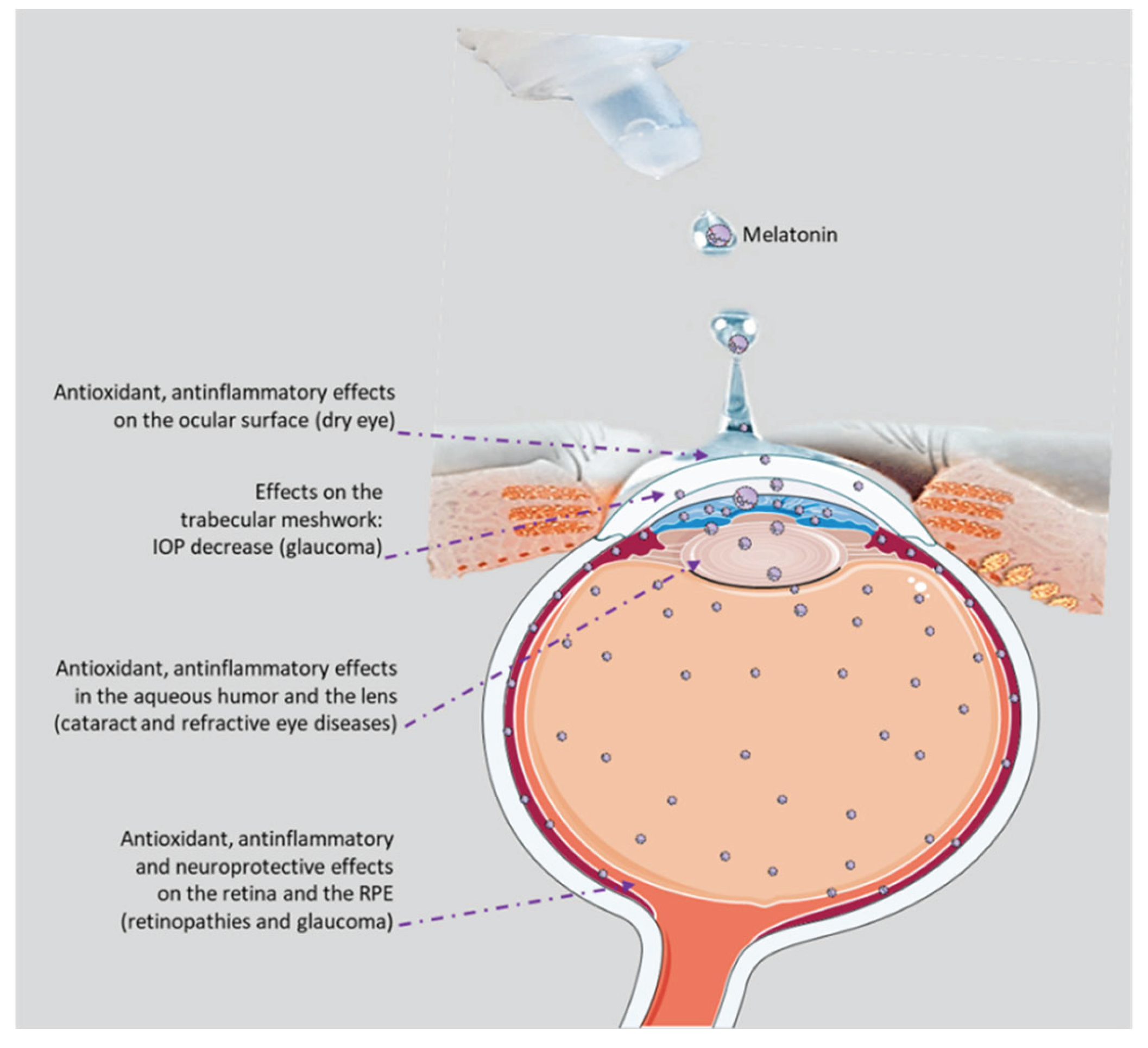
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




