1. Introduction
Post-partum hemorrhage (PPH) is a significant cause of maternal morbidity worldwide, with potential complications including loss of fertility and death [
1]. PPH is estimated to occur in 6–11% of all deliveries, with severe PPH (sPPH) affecting 1–3% [
2,
3]. Although treatment guidelines vary, uterotonics are recommended prophylactically as well as for PPH treatment [
1,
4,
5]. If bleeding persists, options may include minimally invasive obstetrical interventions (e.g., manual removal of placenta/manual exploration of the uterus and/or episiotomy/genital tract sutures), fluid replacement, hemostatic agents, and blood product transfusions [
1,
2,
4]; and non-invasive/minimally invasive mechanical methods (e.g. manual uterine compression/intra-uterine balloon tamponade) [
1,
2]. If bleeding remains uncontrolled, conservative invasive procedures may be considered, including selective arterial embolization, laparotomy, uterine/iliac artery ligation or uterine compression sutures [
1]. Life-threatening situations may require emergency hysterectomy [
4]. Risks associated with these invasive procedures include wound infection, vessel damage, sepsis, thromboembolic events (TEs), organ failure, loss of reproductive potential, and adverse psychological/social impact [
6,
7,
8,
9,
10].
Despite the multitude of options described above, there remains an unmet clinical need in sPPH management. Effective and preferably non-invasive treatments are desirable to minimize the need for surgical interventions, avoid future infertility, and reduce maternal mortality. Off-label use of recombinant activated human factor VII (rFVIIa) is recommended in some sPPH management guidelines, typically at a dosage of 60–90 µg/kg, although recommended timing varies (usually as a last resort before hysterectomy or in life-threatening situations) [
11,
12,
13,
14]. Data regarding rFVIIa treatment of sPPH have been reported; however, it is unclear whether the benefits outweigh the risks [
15,
16,
17,
18,
19]. Concerns regarding TEs remain, as the majority of studies involving rFVIIa were carried out in non-obstetric populations [
20]. Therefore, it has not yet been possible to establish a benefit-risk profile for this treatment in sPPH.
Here, we describe a collaborative project that aimed to evaluate efficacy and safety of rFVIIa for sPPH management. Data from a previously-published open-label randomized controlled trial (RCT) conducted across eight centers (seven in France and one in Switzerland; NCT00370877) [
17] were re-analyzed alongside four observational studies (PPH consortium [Denmark, The Netherlands, and the UK; NCT04723979; OS-1], Bern University Hospital [OS-2], the UniSeven registry [Czech Republic; OS-3], and the Australian and New Zealand Haemostasis Registry [ANZHR; OS-4]). Although some results from these studies have previously been published [
17,
18,
21,
22,
23,
24], in order to provide information on a more clinically relevant endpoint and an updated safety analysis, datasets were re-analyzed at the individual patient level using a common primary endpoint and propensity score-matching strategies where appropriate (OS-1 and OS-2).
2. Materials and Methods
2.1. Data Sets
In the RCT, women with sPPH were randomized 1:1 to rFVIIa treatment or standard-of-care following failure of sulprostone to control bleeding. Of the four observational studies, OS-1 and OS-2 provided data for rFVIIa-exposed and non-exposed women, and OS-3 and OS-4 provided data on rFVIIa-exposed women only. Inclusion criteria, sPPH definition and rFVIIa use varied amongst the studies (
Table 1;
Supplementary Materials Section S1.1; Table S1). Relevant ethical approvals and consent were obtained as required (
Supplementary Materials Section S1.1).
2.2. Patient Populations
Three populations were defined for this combined analysis. The full analysis set included all randomized women for the RCT and all women who met the inclusion criteria of the observational studies (
Table 1). The full analysis set was used for efficacy analyses for the RCT and for assessing safety in all studies. Secondly, a sub-population of women “at risk of further invasive procedures” was defined in the observational studies (i.e., women without a hysterectomy before or concurrent with first administration of rFVIIa, meaning that additional invasive procedures were still possible).
The third patient population was the propensity score analysis set (PSAS). Due to differences between women who were/were not exposed to rFVIIa in OS-1 [
23] and OS-2, propensity score matching was used to construct two comparable subgroups. As the number of comparable controls (non-exposed women) available per rFVIIa-exposed woman varied, a maximum of 4 controls were selected. To counteract numerical imbalance, controls were weighted according to the number of controls for each exposed woman.
2.3. Endpoints
The primary endpoint was occurrence of invasive procedures, including uterine compression sutures, uterine or iliac artery ligation, radiological arterial embolization or hysterectomy (timeframes provided in
Supplementary Materials Section S1.2, Figure S1). Secondary endpoints included amount of blood products transfused (including red blood cells [RBCs] and fresh frozen plasma [FFP]) and occurrence of hysterectomy. Safety endpoints included occurrence of venous TEs (VTEs), arterial TEs (ATEs), and maternal deaths (timeframes provided in
Supplementary Materials Section S1.3).
2.4. Statistical Analysis
For the RCT, the primary endpoint was compared between rFVIIa and reference (non-exposed) groups in the full analysis set according to the odds ratio (OR) and relative risk reduction (with 95% confidence intervals [CI] and p-value calculated from a two-sided chi-square test [5% significance level]).
For the comparative analysis of OS-1 and OS-2, the balance of confounding variables was checked to ensure the propensity score model had been specified correctly (
Supplementary Materials Section S1.4). An exact conditional logistic regression was used for the comparison between rFVIIa-exposed and non-exposed women for the primary and secondary endpoints. The test assessing the OR was two-sided (5% significance level) and no multiplicity adjustment was required as the analysis was based on only one primary endpoint. In OS-3 and OS-4, descriptive analysis of the primary endpoint was conducted in the population at risk.
Safety endpoints were analyzed descriptively. Meta-analyses were conducted on the occurrence of TEs in rFVIIa-exposed (all studies) and non-exposed women (RCT, OS-1, OS-2), using a generalized linear mixed model to estimate proportions, based on the binomial distribution using a logit link function with study included as a random effect.
3. Results
3.1. Patient Populations and Baseline Characteristics
Across all studies, 446 women were exposed to rFVIIa, with 1,717 women not exposed. The RCT full analysis set included 42 women randomized to early treatment with rFVIIa (≤60 minutes of sulprostone administration) and 42 to the reference group. Nine women in the reference group also received rFVIIa (8 on a compassionate use basis, 1 in error). The full analysis set of the 4 observational studies included 395 rFVIIa-exposed and 1,684 non-exposed women; and the overall full analysis set for all 5 studies comprised 437 rFVIIa-exposed and 1,726 non-exposed women (
Table 2). Two-hundred and eighteen rFVIIa-exposed women were included in the population at risk of invasive procedures across all observational studies. The PSAS of OS-1 included 40 rFVIIa-exposed women and 115 matched controls. Two of the 40 rFVIIa-exposed women and 4/115 matched controls were excluded from the primary analyses due to hysterectomy during the 20 min lag period, and 3/115 matched controls were excluded due to having been matched to the rFVIIa-exposed women who had hysterectomy in this time period. The PSAS of OS-2 included 18 rFVIIa-exposed women and 43 matched controls (one of whom was excluded from the primary analyses due to hysterectomy during the lag period). Full details of the outcomes of the propensity score matching process are presented in the
Supplementary Materials Section S2.1, Table S2 and Figure S2.
There were some variations in baseline characteristics in the full analysis set between studies, including mode of delivery and primary cause of PPH (
Table 2), and clinical characteristics of rFVIIa-exposed and matched controls in the PSAS were well-balanced (
Table S2). In the RCT, one woman had an invasive procedure prior to rFVIIa exposure, whereas in the full analysis set of the observational studies, the proportion of women with a prior invasive procedure ranged from 23% (UK cohort of OS-1) to 62% (OS-2). In the OS-1 and OS-2 PSAS, none of the women previously had a hysterectomy, as per the study design.
3.2. Efficacy of rFVIIa for the Treatment of sPPH (Comparative Studies)
3.2.1. Randomized Controlled Trial
For the primary endpoint of occurrence of invasive procedures, 21/42 (50%) rFVIIa-exposed women had a subsequent invasive procedure compared with 38/42 (91%) in the reference group, corresponding to a 45% relative reduction in risk (95% CI: 0.24–0.60; p<0.0001) of an invasive procedure in the rFVIIa group. The OR between the two groups was 0.11 (95% CI: 0.03–0.35;
Table 3 and
Figure 1).
A post-hoc subgroup analysis of this endpoint showed that amongst women with a baseline fibrinogen plasma level ≥2 g/L, invasive procedures occurred in 33% (9/27) of rFVIIa-exposed women versus 94% (31/33) of those in the reference group. For women with a baseline fibrinogen plasma level <2 g/L, invasive procedures occurred in 88% (7/8) of rFVIIa-exposed women versus 100% (5/5) in the reference group (
Supplementary Materials Section S2.2; Figure S3).
In the rFVIIa-exposed group, 3/42 (7%) women underwent a hysterectomy versus 8/42 (19%) in the reference group, corresponding to a 62.5% relative reduction in risk (95% CI: −0.32–0.89; p=0.19) of a hysterectomy in the rFVIIa group (
Table 3; odds ratio not prespecified for this endpoint). Median duration of bleeding in the rFVIIa group was 115.0 min (interquartile range [IQR] 60.0–195.0) versus 177.5 min (IQR 130.0–250.0) in the reference group (no statistical testing applied). Units of RBCs and FFP transfused were similar between groups (
Table S3).
3.2.2. Observational Studies (OS-1 and OS-2)
For the primary endpoint of occurrence of invasive procedures in OS-1, 22/38 (58%) rFVIIa-exposed women had a subsequent invasive procedure compared with 13.3/38.0 (35%) in the weighted matched control group (conditional OR: 2.46; 95% CI: 1.06–5.99, p=0.04;
Table 3 and
Figure 1). In OS-2, invasive procedures occurred in 3/18 (17%) of rFVIIa-exposed women and 5.6/17.8 (32%) of the weighted matched control group (conditional OR: 0.33; 95% CI: 0.03–1.75, p=0.27;
Table 3 and
Figure 1). Of note, the denominator of the control percentage is based on data from 108 women for OS-1 and 42 women for OS-2, and weighted according to the number of controls within pairs (
Supplementary Materials Sections S1.4 and S2.1). A sensitivity analysis performed for OS-1 and OS-2 to account for the fact that some matched control patients received rFVIIa at a later time point yielded similar results (
Supplementary Materials Section S2.2; Table S4).
In the rFVIIa-exposed group of OS-1, 13/38 (34%) women had a hysterectomy versus 7.8/38 (20%) in the reference group (OR: 2.23; 95% CI: 0.83–6.06; p=0.12) (
Table 3 and
Figure 1). In OS-2, 2/18 (11%) rFVIIa-exposed women had a hysterectomy compared with 3.1/17.8 (17%) in the weighted reference group (OR: 0.52; 95% CI: 0.05–3.03; p=0.68).
In OS-2, median duration of bleeding (from onset to stop of sPPH) for women in the PSAS was 167.5 min (IQR: 101.0–235.0 min) for matched-exposed women and 250.0 min (IQR: 138.0–673.5 min) for matched controls. Details of duration of bleeding were unavailable for OS-1. In the PSAS of both OS-1 and OS-2, mean volumes of RBCs and FFP before and after matching time were comparable between rFVIIa-exposed women and matched controls (
Table S3).
3.3. Clinical Outcomes Following rFVIIa Treatment of sPPH (Non-Comparative Studies)
In the observational studies without a comparator arm (OS-3 and OS-4), 23% (10/43) and 30% (22/74) of women in the population at risk had an invasive procedure following rFVIIa exposure, respectively. For the secondary endpoint of hysterectomy following rFVIIa administration, 21% (9/43) of rFVIIa-exposed women in OS-3 and 20% (15/76) of exposed women in OS-4 went on to have a hysterectomy.
3.4. Safety of rFVIIa in the Management of sPPH
A total of 446 women across all studies were exposed to rFVIIa, including 9 women in the RCT reference group. VTEs were reported in 2/51 rFVIIa-exposed and in none of the 33 non-exposed women in the RCT, with no ATEs reported in either group (
Table 4).
In the observational studies, VTEs were reported in 3/358 rFVIIa-exposed women (none were fatal). Additionally, 1 VTE occurred in the Dutch cohort of OS-1; however, TEs in this cohort were only reported if the patient underwent an embolization procedure (which was the case for 23 exposed women and 144 non-exposed), and these results are therefore presented separately. Data were missing for 9 non-exposed women in the Danish cohort of OS-1. A VTE was reported in 7/452 non-exposed women (from OS-2 and the Danish and UK cohorts of OS-1), and 2 VTEs were reported in the Dutch cohort of OS-1. An ATE was reported in 1/358 rFVIIa-exposed women across the observational studies (myocardial infarction, OS-4 [fatal]) and in 1/452 non-exposed women. One woman experienced an ATE in the non-exposed group of the Dutch cohort of OS-1 (
Table 4). Further details on all TEs are provided in the
Supplementary Materials section S2.3.
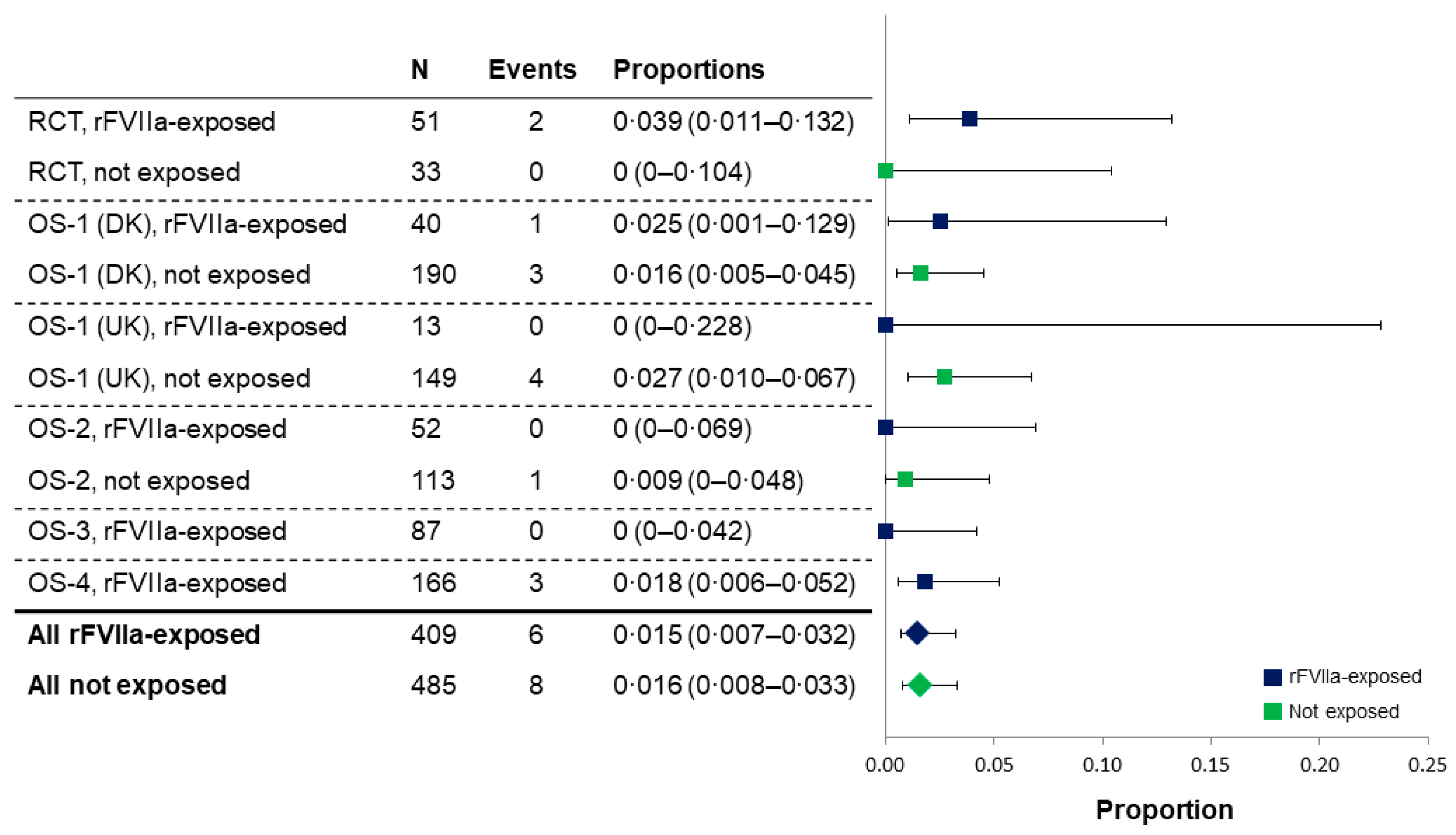
The results of a meta-analysis showed the proportion of women with a TE for all studies was 1.5% in rFVIIa-exposed women versus 1.6% in non-exposed (
Figure 2). The overall proportions of women with an ATE or VTE were comparable between groups (0.2% for ATEs in both groups, and 1.2% versus 1.4% for VTEs in exposed and non-exposed women, respectively).
Fifteen deaths were reported in 446 women exposed to rFVIIa across the studies, with 9 deaths reported among the 1,717 women in the reference groups (
Table S5). The cause of death for 9/15 exposed women was not related to a TE and unknown or not available for 4 women; the remaining 2 exposed women had experienced a TE, but the death was assessed as unlikely to be related to rFVIIa by a study clinician (please see the
Supplementary Materials Section S2.3 for further details).
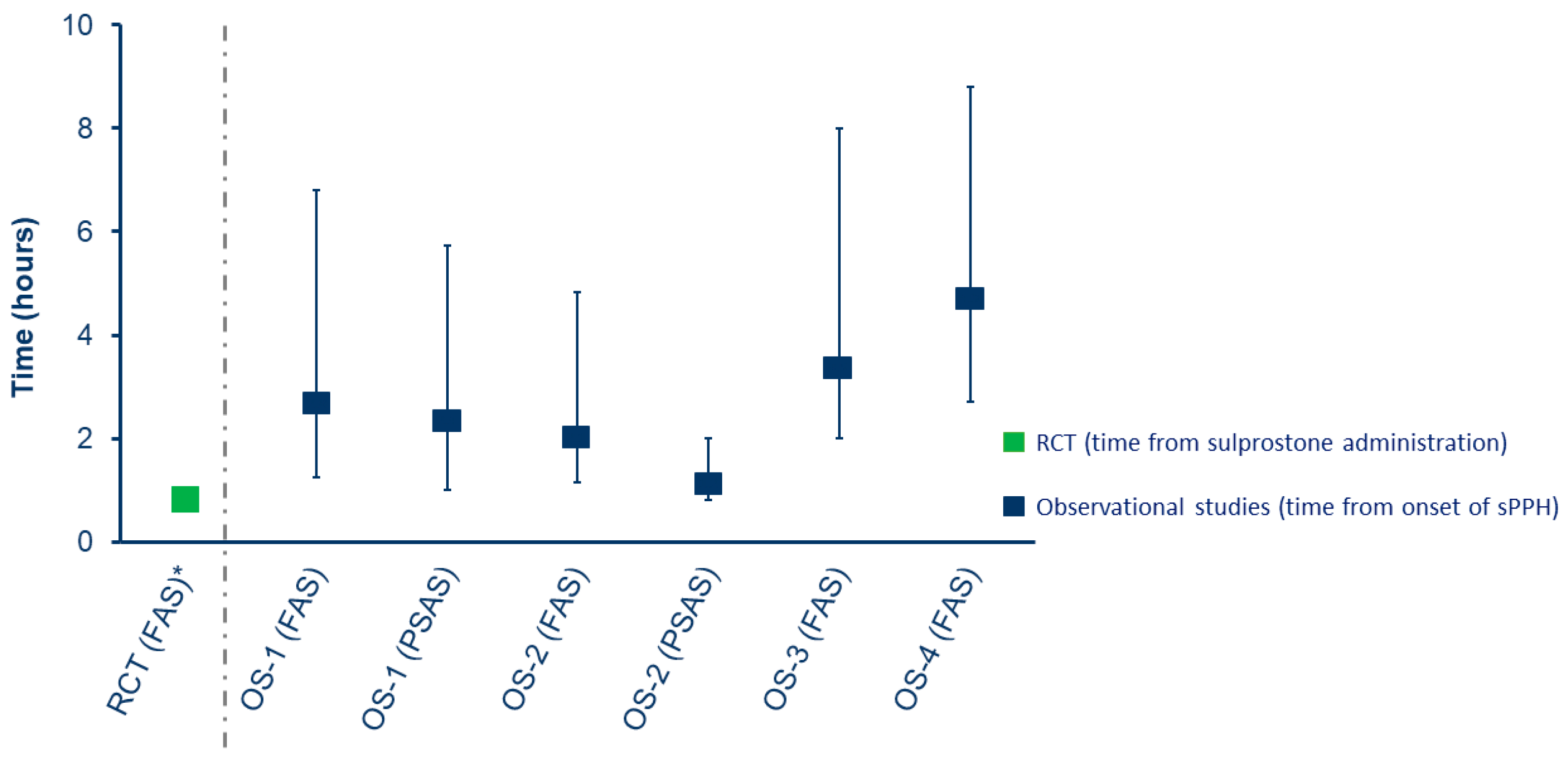
3.5. rFVIIa Dosing and Timing of Administration
In the RCT, women received rFVIIa within 60 minutes of sulprostone administration. In the full analysis set of the observational studies, median time (IQR) from onset of sPPH to first dose of rFVIIa varied, ranging from 127.5 min (71.0−290.5) in OS-2 to 291.0 min (160.0–525.0) in OS-4 (
Figure 3). Details of doses/dosage received in the studies are provided in the
Supplementary Materials Section S2.4, Figures S4 and S5.
4. Discussion
In this collaborative project, data regarding the use of rFVIIa in over 400 women with sPPH across one RCT and four observational studies were analyzed in parallel, allowing for the collation of efficacy and safety data from multiple international sources. Data analysis revealed contrasting results for the primary endpoint of occurrence of invasive procedures across the studies with a comparator arm. In the RCT, there was a reduction in invasive procedures in rFVIIa-exposed women compared with the reference group. In contrast, results from OS-1 showed that more rFVIIa-exposed women underwent an invasive procedure compared with non-exposed PS-matched controls; and in OS-2, there was no statistically significant difference between groups. Nevertheless, the safety analysis did not show any increased incidence of TEs with rFVIIa treatment.
There is a relevant safety concern of development of TEs following the use of rFVIIa in sPPH, due to a potential overstimulation of the coagulation system [
25]. Previously, a Cochrane review found a significant increase in ATEs with rFVIIa treatment of bleeding in patients without hemophilia [
25]; and women with sPPH may have an increased risk of developing TEs [
26]. The current analysis showed that proportions of TEs were similar in women with sPPH exposed to rFVIIa and non-exposed women, with the majority of events being VTEs. Of note, 13/15 deaths in rFVIIa-exposed women were recorded during OS-4, in which the median time from onset of sPPH until first administration of rFVIIa was just under 5 hours, with some of these women having been transferred from a local center (often in a remote location) before treatment. Real-world data presents challenges for analysis and the contrasting efficacy results found in the RCT, OS-1 and OS-2 may have been due to residual confounding effects, such as the severity of bleeding when rFVIIa was administered.
Although it is not possible to definitively conclude why the efficacy outcomes from the comparative studies varied, there are some potential hypotheses that can be considered. The studies were diverse in terms of design, patient populations and setting, with data collected through a variety of sources. Clinical experience from previously approved indications of rFVIIa suggested timing of administration may be critical, with earlier use potentially being more beneficial [
27]. The European Medicines Agency approval of rFVIIa authorizes its use as a treatment for sPPH after failure of uterotonics [
28], and it is likely that optimal timing of administration may depend on clinical circumstances, such as necessity for surgical repair of trauma or transfer to a larger treatment center.
As coagulopathy was not evaluated in this project, no conclusions could be drawn regarding its impact on clinical efficacy of rFVIIa treatment in this setting. Further research is necessary to investigate in which type of patient and within which timeframe rFVIIa would be relevant to treat sPPH.
There are some limitations of this project that should be considered when interpreting the results. Since a limited number of variables could be included in the PS-matching model for OS-1 and OS-2, it is possible that the PS-matching did not remove all confounding, leading to under or over estimation of a possible effect of rFVIIa. As previously discussed [
17], the RCT was a multi-center, open-label trial with a relatively small sample size, therefore confounding, observer bias or random (false-positive) error cannot be excluded. Women were treated at referral centers with facilities available for active management of PPH, which may have resulted in a higher likelihood of performing an invasive procedure within the comparator arm (no concurrent treatment), as some intervention was required to stop the hemorrhage. However, median times from randomization to invasive procedure initiation were similar between the two groups, and the level of reduction of invasive procedures with rFVIIa was fairly substantial [
17]. Another consideration is that the treatment landscape has changed since these sPPH events occurred, with some treatments (intrauterine balloon, fibrinogen replacement, and tranexamic acid), and rapid bedside coagulation assessment more widely used today [
29,
30]. Therefore, these results may not fully correspond with the current treatment landscape.
5. Conclusions
In order to establish the efficacy and safety for a drug, ideally the highest possible evidence is required from an adequately powered double-blinded, randomized, placebo-controlled trial. Although such data are currently unavailable regarding the use of rFVIIa in sPPH, in our collaborative project we have collected globally available information, and analyzed and presented it systematically. Our main finding from the safety meta-analysis indicated there was unlikely to be an increased incidence of either arterial or venous TEs associated with rFVIIa in women with sPPH. The multi-center, open-label RCT found a marked reduction in invasive procedures after rFVIIa treatment, whereas results from the comparative observational studies did not confirm this. Going forward, more data regarding the clinical efficacy and safety of rFVIIa in different circumstances and causes of sPPH are desirable to optimize treatment.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Supplementary methods; Supplementary Results; Table S1: Summary of additional study information for the randomized controlled trial and observational studies; Table S2: Patient characteristics in OS-1 and OS-2 (propensity score analysis set); Table S3: Blood transfusions administered before and after rFVIIa administration in the randomized controlled trial and observational studies (full analysis or propensity score analysis sets); Table S4: Sensitivity analysis of primary endpoint (occurrence of invasive procedures) to account for rFVIIa administration after matching time in OS-1 and OS-2 (propensity score analysis set); Table S5: Maternal deaths in women with available data from the randomized controlled trial and observational studies (rFVIIa exposed, n=446; unexposed, n=1,717); Figure S1: Definition of matching time for matched patients in relation to sPPH onset (A) and use of matching time in relation to the time window for invasive procedures (panel B); Figure S2: Standardized bias plot for propensity score matching in (A) OS-1 and (B) OS-2; Figure S3: Proportion of women with any invasive procedure after randomization in the randomized controlled trial by fibrinogen plasma level at baseline (full analysis set); Figure S4: Number of doses of rFVIIa received by women with sPPH in the randomized controlled trial and observational studies (full analysis set); Figure S5: Median dosage of rFVIIa administered in the randomized controlled trial, OS-2, OS-3 and OS-4 (full analysis set).
Author Contributions
All authors were either investigators in the randomized controlled trial (G.L.L. and F.J.M.), directly involved in one of the observational studies, or participated in the collection and analysis of the data. T.v.d.A., J.v.d.B., C.C.D., D.v.D., H.M.E., N.v.G., D.H., M.K. were responsible for data collection and analysis from the PPH Consortium (OS-1); G.C., D.S. and J.Z. were responsible for data from Bern University Hospital (OS-2); J.B. was responsible for data from the UniSeven registry (OS-3); and Z.K.M., C.W. and E.M.W. were responsible for data from the ANZHR (OS-4). Population-level data were analyzed by C.B., L.K. and A.L. for reporting purposes. C.C.D. (PPH Consortium): Conceptualization, Data collection, Methodology, Supervision. F.J.M. (randomized controlled trial): Conceptualization, Methodology, Supervision. H.M.E. (PPH Consortium): Conceptualization, Data collection, Methodology, Supervision. J.v.d.B. (PPH Consortium): Conceptualization, Methodology, Supervision. J.Z. (Bern University Hospital): Conceptualization, Data collection, Methodology, Supervision. C.C.D, H.M.E and J.Z. contributed equally to this to this work. All authors contributed to subsequent drafting and review of the manuscript, and approved the final version for submission.
Funding
This project was initiated and funded by Novo Nordisk A/S (Bagsværd, Denmark) and supported a regulatory submission for rFVIIa to the European Medicines Agency. The sponsor had access to anonymized/pseudonymized patient-level data and performed descriptive and statistical analyses for the RCT, OS-2 and OS-3. Patient-level data were analyzed by the Department of Clinical Epidemiology, Leiden University Medical Center (OS-1); and by the Department of Epidemiology and Preventive Medicine, Monash University (OS-4). These institutions then provided population-level data to the sponsor for reporting purposes.
Institutional Review Board Statement
Ethics committee approvals were obtained from country-specific institutional review boards/institutional ethics committees as appropriate for all studies. The RCT was conducted in accordance with the principles of the Declaration of Helsinki and ICH Good Clinical Practice; and for the observational studies, data were collected according to appropriate privacy guidelines.
Informed Consent Statement
In the RCT, written informed consent was obtained from each patient or the legally acceptable surrogate when the patient was unable to give consent.
Data Availability Statement
Nimes University Hospital is currently implementing a data sharing policy, and any requests for access to data that support the findings of the RCT included in this project will be managed by the Delegation for Clinical Research and Innovation (DRCI). Anonymized patient-level and study-level data from the PPH consortium will be shared upon reasonable request, provided that the requestor has appropriate ethics approval (any requests should be directed to
j.g.van_der_bom@lumc.nl in the first instance; data sharing agreements with individual countries may be required for onward data sharing of the consortium data). Study-level data and study protocol underlying the results from Bern University Hospital reported in this article will be shared upon reasonable request, in agreement with the study center’s data sharing policy. Anonymized patient-level, study-level data and study protocol/case report form underlying the results reported from UniSeven will be shared upon reasonable request. Anonymized patient-level and study-level data from the ANZHR will be shared upon reasonable request, provided that the requestor has appropriate ethics approval. Data will be shared via a Secure eResearch Platform hosted by Monash University (requests should be directed to
cameron.wellard@monash.edu).
Acknowledgments
The authors would like to thank the patients, their families and all study investigators for their participation and support in this collaborative project. This project was initiated and funded by Novo Nordisk A/S (Bagsværd, Denmark) and supported a regulatory submission for rFVIIa to the European Medicines Agency. The authors wish to thank the Australian and New Zealand Hemostasis Registry, Steering Committee, investigators and sites; and Dr Stephen McCall, Faculty of Health Sciences, American University of Beirut, for his valuable contributions to the PPH Consortium and for his assistance in developing this manuscript. Medical writing support was provided by Carol McNair, Ashfield MedComms GmbH (Mannheim, Germany, an Inizio company) and was funded by Novo Nordisk A/S.
Conflicts of Interest
Some of the authors received financial reimbursements to their institutions from Novo Nordisk for the presented work regarding efficacy and safety of recombinant factor VIIa, and the Australian and New Zealand Hemostasis Registry was funded by Novo Nordisk, paid to Monash University. C.C.D., C.W., D.H., D.v.D., E.W., G.C., G.L.L., H.E., J.Z., N.v.G., T.v.d.A. and Z.M. report no other conflicts of interest relevant to this study. M.K. did not receive any financial reimbursement from Novo Nordisk for the presented work and has no conflict of interest to report. C.B., L.K. and A.L. are employees of Novo Nordisk; and A.L. owns shares in Novo Nordisk. D.S. has received advisory board and lecture fees from Novo Nordisk and Ferring in favor of the departmental research fund. F.J.M. has received honoraria from Novo Nordisk as a speaker (symposia, webinars) and as a consultant; and has acted as an academic co-investigator in studies sponsored by Novo Nordisk. J.B. has received speaker and/or consultancy fees from Sobi, Pfizer, Octapharma, Roche, Takeda and Novo Nordisk. J.v.d.B. has received unrestricted research grants from Sanquin for the design and data collection of the TeMpOH1 study, and reimbursement for educational activities from Bayer, all paid to their institution.
References
- ACOG Committee on Practice Bulletins. Practice Bulletin No. 183: Postpartum Hemorrhage. Obstetrics & Gynecology 2017, 130, e168–e186. [Google Scholar] [CrossRef]
- Muñoz, M.; Stensballe, J.; Ducloy-Bouthors, A.S.; Bonnet, M.P.; De Robertis, E.; Fornet, I.; Goffinet, F.; Hofer, S.; Holzgreve, W.; Manrique, S.; et al. Patient blood management in obstetrics: prevention and treatment of postpartum haemorrhage. A NATA consensus statement. Blood transfusion = Trasfusione del sangue 2019, 17, 112–136. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.; Callaghan, W.M.; Berg, C.; Alexander, S.; Bouvier-Colle, M.-H.; Ford, J.B.; Joseph, K.S.; Lewis, G.; Liston, R.M.; Roberts, C.L.; et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy and Childbirth 2009, 9, 55. [Google Scholar] [CrossRef]
- Mavrides, E.; Allard, S.; Chandraharan, E.; Collins, P.; Green, L.; Hunt, B.J.; Riris, S.; Thomson, A.J.; on behalf of the Royal College of Obstetricians and Gynaecologists. Prevention and management of postpartum haemorrhage. BJOG 2016, 24, e106–e149. [Google Scholar]
- World Health Organization. WHO Recommendations: Uterotonics for the prevention of postpartum haemorrhage. Available online: https://iris.who.int/bitstream/handle/10665/277276/9789241550420-eng.pdf?ua=1&ua=1 (accessed on 12/02/2024).
- Gizzo, S.; Saccardi, C.; Patrelli, T.S.; Di Gangi, S.; Breda, E.; Fagherazzi, S.; Noventa, M.; D'Antona, D.; Nardelli, G.B. Fertility rate and subsequent pregnancy outcomes after conservative surgical techniques in postpartum hemorrhage: 15 years of literature. Fertil Steril 2013, 99, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Sentilhes, L.; Gromez, A.; Clavier, E.; Resch, B.; Verspyck, E.; Marpeau, L. Predictors of failed pelvic arterial embolization for severe postpartum hemorrhage. Obstet Gynecol 2009, 113, 992–999. [Google Scholar] [CrossRef]
- Sanad, A.S.; Mahran, A.E.; Aboulfotouh, M.E.; Kamel, H.H.; Mohammed, H.F.; Bahaa, H.A.; Elkateeb, R.R.; Abdelazim, A.G.; El-Din, M.A.Z.; Shawki, H.E. The effect of uterine artery ligation in patients with central placenta pevia: a randomized controlled trial. BMC Pregnancy Childbirth 2018, 18, 351. [Google Scholar] [CrossRef]
- Michelet, D.; Ricbourg, A.; Gosme, C.; Rossignol, M.; Schurando, P.; Barranger, E.; Mebazaa, A.; Gayat, E. Emergency hysterectomy for life-threatening postpartum haemorrhage: Risk factors and psychological impact. Gynecol Obstet Fertil 2015, 43, 773–779. [Google Scholar] [CrossRef]
- Thurn, L.; Wikman, A.; Lindqvist, P.G. Postpartum blood transfusion and hemorrhage as independent risk factors for venous thromboembolism. Thromb Res 2018, 165, 54–60. [Google Scholar] [CrossRef]
- Schlembach, D.; Helmer, H.; Henrich, W.; von Heymann, C.; Kainer, F.; Korte, W.; Kuhnert, M.; Lier, H.; Maul, H.; Rath, W.; et al. Peripartum Haemorrhage, Diagnosis and Therapy. Guideline of the DGGG, OEGGG and SGGG (S2k Level, AWMF Registry No. 015/063, March 2016). Geburtshilfe Frauenheilkd 2018, 78, 382–399. [Google Scholar] [CrossRef]
- Affronti, G.; Agostini, V.; Brizzi, A.; Bucci, L.; De Blasio, E.; Frigo, M.G.; Giorgini, C.; Messina, M.; Ragusa, A.; Sirimarco, F.; et al. The daily-practiced post-partum hemorrhage management: an Italian multidisciplinary attended protocol. Clin Ter 2017, 168, e307–e316. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.; Rodrigues, A.; Gomes, M.; Carrilho, A.; Nunes, A.R.; Orfao, R.; Alves, A.; Aguiar, J.; Campos, M. Interventional Algorithms for the Control of Coagulopathic Bleeding in Surgical, Trauma, and Postpartum Settings: Recommendations From the Share Network Group. Clinical and applied thrombosis/hemostasis: official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis 2016, 22, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Kadir, R.; McLintock, C.; Ducloy, A.S.; El-Refaey, H.; England, A.; Federici, A.B.; Grotegut, C.A.; Halimeh, S.; Herman, J.H.; Hofer, S.; et al. Evaluation and management of postpartum hemorrhage: consensus from an international expert panel. Transfusion 2014, 54, 1756–1768. [Google Scholar] [CrossRef]
- Park, S.C.; Yeom, S.R.; Han, S.K.; Jo, Y.M.; Kim, H.B. Recombinant Activated Factor VII as a Second Line Treatment for Postpartum Hemorrhage. Korean J Crit Care Med 2017, 32, 333–339. [Google Scholar] [CrossRef]
- Franchini, M.; Franchi, M.; Bergamini, V.; Montagnana, M.; Salvagno, G.L.; Targher, G.; Lippi, G. The use of recombinant activated FVII in postpartum hemorrhage. Clin Obstet Gynecol 2010, 53, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Lavigne-Lissalde, G.; Aya, A.G.; Mercier, F.J.; Roger-Christoph, S.; Chauleur, C.; Morau, E.; Ducloy-Bouthors, A.S.; Mignon, A.; Raucoules, M.; Bongain, A.; et al. Recombinant human FVIIa for reducing the need for invasive second-line therapies in severe refractory postpartum hemorrhage: a multicenter, randomized, open controlled trial. J Thromb Haemost 2015, 13, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Blatny, J.; Seidlova, D.; Penka, M.; Ovesna, P.; Brabec, P.; Sevcik, P.; Ventruba, P.; Cerny, V. Severe postpartum haemorrhage treated with recombinant activated factor VII in 80 Czech patients: analysis of the UniSeven registry. Int J Obstet Anesth 2011, 20, 367–368. [Google Scholar] [CrossRef]
- Alfirevic, Z.; Elbourne, D.; Pavord, S.; Bolte, A.; Van Geijn, H.; Mercier, F.; Ahonen, J.; Bremme, K.; Bødker, B.; Magnúsdóttir, E.M.; et al. Use of recombinant activated factor VII in primary postpartum hemorrhage: the Northern European registry 2000-2004. Obstet Gynecol 2007, 110, 1270–1278. [Google Scholar] [CrossRef]
- Lin, Y.; Stanworth, S.; Birchall, J.; Doree, C.; Hyde, C. Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia. The Cochrane database of systematic reviews 2011, Cd005011. [Google Scholar] [CrossRef]
- Colucci, G.; Helsing, K.; Biasiutti, F.D.; Raio, L.; Schmid, P.; Tsakiris, D.A.; Eberle, B.; Surbek, D.; Lämmle, B.; Alberio, L. Standardized management protocol in severe postpartum hemorrhage: A single-center study. Clinical and applied thrombosis/hemostasis: official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis 2018, 24, 884–893. [Google Scholar] [CrossRef]
- Phillips, L.E.; McLintock, C.; Pollock, W.; Gatt, S.; Popham, P.; Jankelowitz, G.; Ogle, R.; Cameron, P.A.; Australian; New Zealand Haemostasis, R. Recombinant activated factor VII in obstetric hemorrhage: experiences from the Australian and New Zealand Haemostasis Registry. Anesth Analg 2009, 109, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Henriquez, D.; Caram-Deelder, C.; le Cessie, S.; Zwart, J.J.; van Roosmalen, J.J.M.; Eikenboom, J.C.J.; So-Osman, C.; van de Watering, L.M.G.; Zwaginga, J.J.; Koopman-van Gemert, A.; et al. Association of timing of plasma transfusion with adverse maternal outcomes in women with persistent postpartum hemorrhage. JAMA Netw Open 2019, 2, e1915628. [Google Scholar] [CrossRef] [PubMed]
- McCall, S.J.; Henriquez, D.; Edwards, H.M.; van den Akker, T.; Bloemenkamp, K.W.M.; van der Bom, J.; Bonnet, M.P.; Deneux-Tharaux, C.; Donati, S.; Gillissen, A.; et al. A total blood volume or more transfused during pregnancy or after childbirth: Individual patient data from six international population-based observational studies. PLoS One 2021, 16, e0244933. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.; Lin, Y.; Stanworth, S.; Birchall, J.; Doree, C.; Hyde, C. Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia. The Cochrane database of systematic reviews 2012, CD005011. [Google Scholar] [CrossRef]
- Chauleur, C.; Cochery-Nouvellon, E.; Mercier, E.; Aya, G.; Marès, P.; Mismetti, P.; Lissalde-Lavigne, G.; Gris, J.C. Analysis of the venous thromboembolic risk associated with severe postpartum haemorrhage in the NOHA First cohort. Thrombosis and haemostasis 2008, 100, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Salaj, P.; Brabec, P.; Penka, M.; Pohlreichova, V.; Smejkal, P.; Cetkovsky, P.; Dusek, L.; Hedner, U. Effect of rFVIIa dose and time to treatment on patients with haemophilia and inhibitors: analysis of HemoRec registry data from the Czech Republic. Haemophilia: the official journal of the World Federation of Hemophilia 2009, 15, 752–759. [Google Scholar] [CrossRef]
- Novo Nordisk A/S. NovoSeven: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/novoseven-epar-product-information_en.pdf (accessed on 12/02/2024).
- Bienstock, J.L.; Eke, A.C.; Hueppchen, N.A. Postpartum hemorrhage. N Engl J Med 2021, 384, 1635–1645. [Google Scholar] [CrossRef]
- Rigouzzo, A.; Louvet, N.; Favier, R.; Ore, M.V.; Piana, F.; Girault, L.; Farrugia, M.; Sabourdin, N.; Constant, I. Assessment of coagulation by thromboelastography during ongoing postpartum hemorrhage: A retrospective cohort analysis. Anesth Analg 2020, 130, 416–425. [Google Scholar] [CrossRef]
Figure 1.
Odds of (A) any invasive procedure or (B) hysterectomy among rFVIIa exposed women compared with non-exposed women in the randomized controlled trial, OS-1 and OS-2. The occurrence of (A) any invasive procedures or (B) hysterectomy after randomization in the RCT, after rFVIIa administration in rFVIIa-exposed patients in the OS, and after time of propensity score matching in matched control patients from the OS. Odds ratio was not pre-specified for the hysterectomy endpoint for the RCT, and so is not available for inclusion in Panel B. The x-axes in the figures use a log scale. *Includes patients from the reference group of the RCT and matched controls from OS-1 and OS-2. **The propensity score analysis set of OS-1 included patients from Denmark and The Netherlands. †The number of matched control patients in these groups was not a whole number due to the weighting that was used for the matching process. Data for the non-exposed groups of OS-1 and OS-2 were based on 108 and 42 women, respectively (women from the PSAS with hysterectomy prior to matching time were excluded from this analysis). OS-1, PPH Consortium; OS-2, Bern University Hospital Study. FAS, full analysis set; OS, observational study; PPH, post-partum hemorrhage; PSAS, propensity score matched analysis set; RCT, randomized controlled trial; rFVIIa, recombinant activated factor VII.
Figure 1.
Odds of (A) any invasive procedure or (B) hysterectomy among rFVIIa exposed women compared with non-exposed women in the randomized controlled trial, OS-1 and OS-2. The occurrence of (A) any invasive procedures or (B) hysterectomy after randomization in the RCT, after rFVIIa administration in rFVIIa-exposed patients in the OS, and after time of propensity score matching in matched control patients from the OS. Odds ratio was not pre-specified for the hysterectomy endpoint for the RCT, and so is not available for inclusion in Panel B. The x-axes in the figures use a log scale. *Includes patients from the reference group of the RCT and matched controls from OS-1 and OS-2. **The propensity score analysis set of OS-1 included patients from Denmark and The Netherlands. †The number of matched control patients in these groups was not a whole number due to the weighting that was used for the matching process. Data for the non-exposed groups of OS-1 and OS-2 were based on 108 and 42 women, respectively (women from the PSAS with hysterectomy prior to matching time were excluded from this analysis). OS-1, PPH Consortium; OS-2, Bern University Hospital Study. FAS, full analysis set; OS, observational study; PPH, post-partum hemorrhage; PSAS, propensity score matched analysis set; RCT, randomized controlled trial; rFVIIa, recombinant activated factor VII.
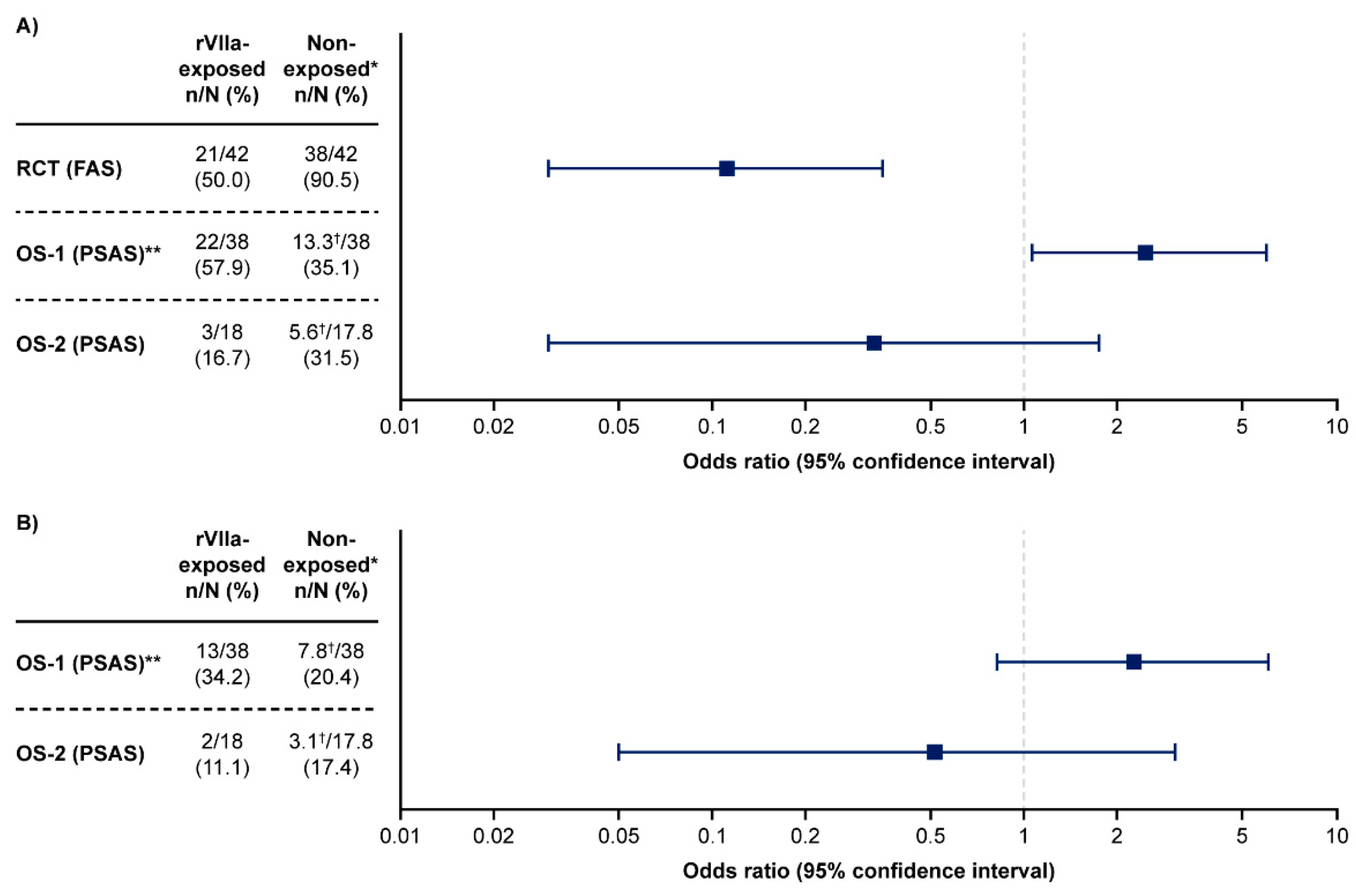
Figure 2.
Meta-analysis of thromboembolic events in rFVIIa-exposed and non-exposed women in the randomized controlled trial and observational studies (full analysis set, excluding patients with unavailable data). In the Dutch cohort of OS-1, a TE was only recorded if it was a complication of an embolization, therefore, TE data from this cohort were excluded from the meta-analysis. OS-1, PPH Consortium; OS-2, Bern University Hospital Study; OS-3, UniSeven; OS-4, Australian and New Zealand Hemostasis Registry. DK, Denmark; OS, observational studies; PPH, post-partum hemorrhage; RCT, randomized controlled trial; rFVIIa, recombinant activated factor VII; TE, thromboembolic event; UK, United Kingdom.
Figure 2.
Meta-analysis of thromboembolic events in rFVIIa-exposed and non-exposed women in the randomized controlled trial and observational studies (full analysis set, excluding patients with unavailable data). In the Dutch cohort of OS-1, a TE was only recorded if it was a complication of an embolization, therefore, TE data from this cohort were excluded from the meta-analysis. OS-1, PPH Consortium; OS-2, Bern University Hospital Study; OS-3, UniSeven; OS-4, Australian and New Zealand Hemostasis Registry. DK, Denmark; OS, observational studies; PPH, post-partum hemorrhage; RCT, randomized controlled trial; rFVIIa, recombinant activated factor VII; TE, thromboembolic event; UK, United Kingdom.
Figure 3.
Median time from onset of sPPH to administration of first dose of rFVIIa in the randomized controlled trial and observational studies (full analysis or propensity score analysis sets). Bars show interquartile range. Data shown for the OS-1 FAS are from Denmark and The Netherlands only. In the RCT, women were randomized if sulprostone had failed to control bleeding within 1 hour of administration; exact timing between sPPH onset and sulprostone administration was not available. OS-1, PPH Consortium; OS-2, Bern University Hospital Study; OS-3, UniSeven registry; OS-4, ANZHR. ANZHR, Australian and New Zealand Hemostasis Registry; FAS, full analysis set; OS, observational study; PPH, post-partum hemorrhage; PSAS, propensity score analysis set; RCT, randomized clinical trial; rFVIIa, recombinant activated factor VII; sPPH, severe post-partum hemorrhage.
Figure 3.
Median time from onset of sPPH to administration of first dose of rFVIIa in the randomized controlled trial and observational studies (full analysis or propensity score analysis sets). Bars show interquartile range. Data shown for the OS-1 FAS are from Denmark and The Netherlands only. In the RCT, women were randomized if sulprostone had failed to control bleeding within 1 hour of administration; exact timing between sPPH onset and sulprostone administration was not available. OS-1, PPH Consortium; OS-2, Bern University Hospital Study; OS-3, UniSeven registry; OS-4, ANZHR. ANZHR, Australian and New Zealand Hemostasis Registry; FAS, full analysis set; OS, observational study; PPH, post-partum hemorrhage; PSAS, propensity score analysis set; RCT, randomized clinical trial; rFVIIa, recombinant activated factor VII; sPPH, severe post-partum hemorrhage.
Table 1.
Study design overview for the randomized controlled trial and observational studies.
Table 1.
Study design overview for the randomized controlled trial and observational studies.
| Study name |
Randomized
controlled trial |
Observational studies |
| OS-1 |
OS-2 |
OS-3 |
OS-4 |
| Denmark |
Netherlands |
UK |
| Key inclusion criteria |
≥18 years
>27 weeks gestation
>1,500 mL blood loss Sulprostone failure |
≥10 U RBCs within 24 h |
Obstetric hemorrhage;
≥4 U RBCs, or
multicomponent blood
transfusion*, or plasma in
addition to RBCs |
≥8 U RBC within 24 h
≥20 weeks of
gestation |
≥1,500 mL blood loss within 24 h |
≥1,500 mL blood loss within 24 h |
Obstetric hemorrhage with
registered birth |
| Definition of PPH |
Severe PPH: Blood loss >1,500 mL measured in graduated bag and/or hemodynamically
unstable and/or need for packed cells
transfusion |
Massive PPH: ≥10 U RBCs within 24 h |
Persistent PPH:
>1,000 mL blood loss refractory to first-line interventions to control bleeding AND ≥1 of: ≥4 units RBCs, multicomponent blood transfusion (RBC and FFP and or/platelet concentrates), or plasma in addition to RBCs |
Major PPH:
≥8 U RBCs within
24 h |
Severe PPH:
Continuous bleeding ≥1,500 mL within 24 h |
Severe PPH: Blood loss ≥1,500 mL within 24 h |
Obstetric case of
hemorrhage with a
registered
delivery |
Protocol for rFVIIa
administration
|
60 ug/kg rFVIIa after sulprostone failure |
.. |
.. |
.. |
rFVIIa at a dose of
60–90 μg/kg was
administered |
.. |
.. |
Table 2.
Patient characteristics in the randomized controlled trial and observational studies.
Table 2.
Patient characteristics in the randomized controlled trial and observational studies.
| Study name |
Randomized
controlled trial |
Observational studies |
| OS-1 |
OS-2 |
OS-3 |
OS-4 |
| Denmark |
Netherlands |
UK |
| Baseline characteristics |
rFVIIa
N=42
|
Ref
N=42
|
rFVIIa
N=40
|
No rFVIIa
N=199
|
rFVIIa
N=37
|
No rFVIIa
N=1223
|
rFVIIa
N=13
|
No rFVIIa
N=149
|
rFVIIa
N=52
|
No rFVIIa
N=113
|
rFVIIa
N=87
|
rFVIIa
N=166
|
| Age at delivery, years |
| N |
·· |
·· |
40 |
199 |
37 |
1223 |
13 |
149 |
·· |
·· |
84 |
166 |
| Median |
·· |
·· |
33.0 |
33.0 |
31.0 |
32.0 |
34.0 |
33.0 |
·· |
·· |
31.5 |
33.0 |
| IQR |
·· |
·· |
29.0–38.0 |
30.0–36.0 |
28.0–34.0 |
28.0–35.0 |
28.0–36.0 |
29.0–36.0 |
·· |
·· |
28.0–36.0 |
29.0–37.0 |
| Maternal body weight, kg* |
| N |
42 |
40 |
·· |
·· |
·· |
·· |
·· |
·· |
52 |
108 |
87 |
135 |
| Median |
68.0 |
70.0 |
·· |
·· |
·· |
·· |
·· |
·· |
70.0 |
72.5 |
72.0 |
65.0 |
| IQR |
62.0–76.0 |
60.0–79.0 |
·· |
·· |
·· |
·· |
·· |
·· |
61.0–83.0 |
67.0–82.0 |
66.0–80.0 |
55.0–75.0 |
| Cause of PPH, n (%)** |
| AIP†
|
6 (14.3) |
8 (19.0) |
11 (27.5) |
51 (25.6) |
6 (16.2) |
119 (9.7) |
1 (7.7) |
28 (25.5) |
9 (17.3) |
17 (15.0) |
16 (18.4) |
28 (16.9) |
| Placental abruption |
·· |
·· |
4 (10.0) |
13 (6.5) |
0 |
12 (1.0) |
1 (7.7) |
14 (9.4) |
5 (9.6) |
8 (7.1) |
9 (10.3) |
15 (9.0) |
| Placental retention |
4 (9.5) |
1 (2.4) |
4 (10.0) |
21 (10.6) |
2 (5.4) |
217 (17.7) |
·· |
·· |
1 (1.9) |
31 (27.4) |
1 (1.1) |
·· |
| Trauma‡
|
7 (16.6) |
2 (4.8) |
7 (17.5) |
50 (25.1) |
3 (8.1) |
89 (7.3) |
1 (7.7) |
26 (17.5) |
1 (1.9) |
9 (8.0) |
6 (6.9) |
5 (3.0) |
| Uterine atony |
39 (92.9) |
36 (85.7) |
11 (27.5) |
37 (18.6) |
25 (67.6) |
780 (63.8) |
8 (61.5) |
56 (37.6) |
34 (65.4) |
48 (42.5) |
24 (27.6) |
39 (23.5) |
| Other |
·· |
·· |
3 (7.5) |
27 (13.6) |
1 (2.7) |
6 (0.5) |
2 (15.4) |
14 (9.4) |
2 (3.8) |
0 |
32 (36.8) |
87 (52.4) |
| Missing |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 (0.7) |
0 |
0 |
14 (16.1) |
24 (14.5) |
| Delivery type, n (%) |
| Caesarean section |
23 (54.8) |
20 (47.6) |
25 (62.5) |
133 (66.8) |
14 (37.8) |
279 (22.8) |
7 (53.9) |
101 (67.8) |
40 (76.9) |
57 (50.4) |
40 (46.0) |
117 (70.5) |
| Multiple birth (≥2), n (%) |
| Yes |
7 (16.7) |
7 (16.7) |
1 (2.5) |
12 (6.0) |
3 (8.1) |
71 (5.8) |
3 (23.1) |
4 (2.7) |
8 (15.4) |
15 (13.3) |
6 (6.9) |
8 (4.8) |
| Invasive procedure(s) prior to rFVIIa, n (%) |
| Any |
1 (2.4) |
NA |
23 (57.5) |
NA |
15 (40.5) |
NA |
3 (23.1) |
NA |
32 (61.5) |
NA |
21 (24.1) |
63 (38.0) |
| Hysterectomy |
0 |
NA |
15 (37.5) |
NA |
6 (16.2) |
NA |
1 (7.7) |
NA |
3 (5.8) |
NA |
16 (18.4) |
45 (27.1) |
Table 3.
Women with subsequent invasive procedure or hysterectomy in the randomized controlled trial and comparative observational studies.
Table 3.
Women with subsequent invasive procedure or hysterectomy in the randomized controlled trial and comparative observational studies.
| Study name |
Randomized
controlled trial (FAS)
|
Observational studies |
| OS-1 (PSAS)* |
OS-2 (PSAS) |
| Number of women |
rFVIIa
N=42
|
Ref
N=42
|
rFVIIa
N=38
|
Weighted matched controls
N=38** |
rFVIIa
N=18 |
Weighted matched controls
N=17.8** |
|
At least one invasive procedure after rFVIIa administration† (primary endpoint), n (%) |
21 (50.0) |
38 (90.5) |
22 (57.9) |
13.3‡ (35.1) |
3 (16.7) |
5.6‡ (31.5) |
| Odds ratio (95% CI) |
0.11 (0.03–0.35) |
2.46 (1.06–5.99) |
0.33 (0.03–1.75) |
| p-value |
·· |
0.04 |
0.27 |
Relative risk reduction,
% (95% CI)
|
44.7 (24–60) |
NP |
NP |
| p-value |
<0.0001 |
NP |
NP |
Women with hysterectomy,
n (%)
|
3 (7.1) |
8 (19.1) |
13 (34.2) |
7.8‡ (20.4) |
2 (11.1) |
3.1‡ (17.4) |
| Odds ratio (95% CI) |
NP |
2.23 (0.83–6.06) |
0.52 (0.05–3.03) |
| p value |
NP |
0.12 |
0.68 |
Relative risk reduction,
% (95% CI)
|
62.5 (−32–89) |
NP |
NP |
| p-value |
0.1944 |
NP |
NP |
Table 4.
Thromboembolic events in women with available data from the randomized controlled trial and observational studies.
Table 4.
Thromboembolic events in women with available data from the randomized controlled trial and observational studies.
| Study name |
Randomized
controlled trial (FAS)* |
Observational studies |
| OS-1 |
OS-2
(FAS)
|
OS-3‡
(FAS)
|
OS-4
(FAS)‡‡
|
Denmark
(FAS)**
|
Netherlands
(FAS)††
|
UK
(FAS)
|
| Number of women |
rFVIIa
N=51 |
Ref
N=33 |
rFVIIa
N=40 |
No rFVIIa
N=190†
|
rFVIIa
N=23 |
No rFVIIa
N=144 |
rFVIIa
N=13 |
No rFVIIa
N=149 |
rFVIIa
N=52 |
No rFVIIa
N=113 |
rFVIIa
N=87 |
rFVIIa
N=166 |
|
| Arterial TEs, n(%) |
0 |
0 |
0 |
1 (0.5) |
0 |
1 (0.7) |
0 |
0 |
0 |
0 |
0 |
1 (0.6)§
|
|
| Venous TEs, n(%) |
2 (3.9) |
0 |
1 (2.5) |
2 (1.1) |
1 (4.3) |
2 (1.4) |
0 |
4 (2.9) |
0 |
1 (0.9) |
0 |
2 (1.2) |
|
| All TEs, n(%) |
2 (3.9) |
0 |
1 (2.5) |
3 (1.6) |
1 (4.3) |
3 (2.1) |
0 |
4 (2.9) |
0 |
1 (0.9) |
0 |
3 (1.8) |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).