Submitted:
29 February 2024
Posted:
01 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
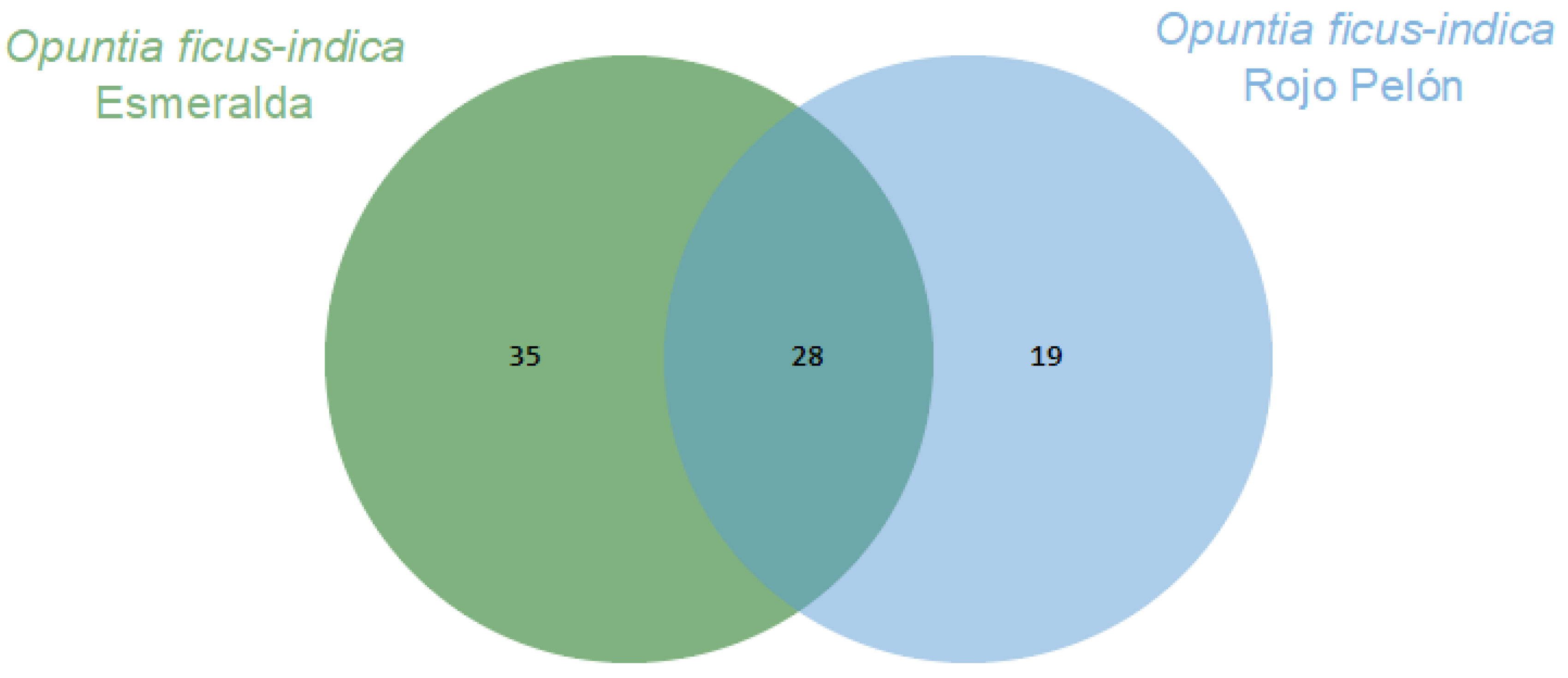
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Insects and Uninfested and Infested O. ficus-indica Cultivars
4.3. Essential oil of Dactylopius Species and Hosts
4.4. Derivatization for Alcohol Detection
4.5. Derivatization for Aldehydes and Carboxylic Acid Detection
4.6. Essential Oil GS-MS Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Morales, M.; Denno, B.D.; Miller, D.R.; Miller, G.L.; Ben-Dov, Y.; Hardy, N.B. ScaleNet: a literature-based model of scale insect biology and systematics. Database (Oxford) 2016, 1–5. [Google Scholar] [CrossRef]
- Van-Dam, A.R., Martinez L.P., Chavez A.J., May B.P. Range wide phylogeography of Dactylopius coccus (Hemiptera: Dactylopiidae). Ann Entomol Soc Am. 2015, 108, 299–310. [CrossRef]
- Campana, M.G. , Robles N.M., Tuross N. America’s red gold: Multiple lineages of cultivated cochineal in Mexico. Ecol Evol. 2015, 5, 607–617. [Google Scholar] [CrossRef]
- Barreto-García, O.A., Rodríguez-Leyva, E., Lomeli-Flores J.R., Vanegas-Rico J.M., Vigueras A.L., Portillo L. Laetilia coccidivora feeding on two cochineal insect species, Does the prey affect the fitness of the predator? BioControl 2020, 65, 727–736. [CrossRef]
- Eisner, T. , Nowicki S., Goetz M., Meinwald J. Red Cochineal Dye (Caminic Acid): Its Role in Nature. Science 1980, 208, 1039–1042. [Google Scholar] [CrossRef]
- Martínez-Martínez, S. , Rodríguez-Leyva E., Aranda-Ocampo S., Santillán-Galicia M.T., Hernández-López A., Guzmán-Franco A.W. Bacteria associated with carminic acid metabolism in the intestinal tract of three predators of Dactylopius opuntiae. Entomol Exp Appl. 2023, 172, 183–192. [Google Scholar] [CrossRef]
- Chávez-Moreno, C.K. , Tecante A., Casas A. The Opuntia (Cactaceae) and Dactylopius (Hemiptera: Dactylopiidae) in Mexico: A historical perspective of use, interaction and distribution. Biodiversity and Conservation 2009, 18, 3337–3355. [Google Scholar] [CrossRef]
- Borges, M.E. , Tejera R.L., Díaz L., Esparza P., Ibáñez E. Natural dyes extraction from cochineal (Dactylopius coccus). New extraction methods. Food Chemistry 2012, 132, 1855–1860. [Google Scholar] [CrossRef]
- Müller-Maatsch, J. , Gras C. The “Carmine Problem” and Potential Alternatives. Handbook on Natural Pigments in Food and Beverages 2016, 385–428. [Google Scholar] [CrossRef]
- Sánchez-García, M.A. , Bokhimi X., Velázquez S., Jiménez-González A.E. Dye-Sensitized solar cells prepared with Mexican pre-hispanic dyes. J. Nanotechnol 2018, 2018. [Google Scholar] [CrossRef]
- Vanegas-Rico, J.M. , Rodríguez-Leyva E., Lomeli-Flores J.R., González-Hernández H., Pérez-Panduro A., Mora-Aguilera G. Biology and life history of Hyperaspis trifurcata feeding on Dactylopius opuntiae. BioControl 2016, 61, 691–701. [Google Scholar] [CrossRef]
- Vanegas-Rico, J.M.; Lomeli-Flores, J.R.; Rodríguez-Leyva, E.; Mora-Aguilera, G.; Valdez, J.M. Natural enemies of Dactylopius opuntiae (Cockerell) on Opuntia ficus-indica (L.) Miller in Central Mexico. Acta Zool. Mexicana (n.s) 2010, 26, 415–433. [Google Scholar] [CrossRef]
- Griffith, M.P. The origins of an important cactus crop, Opuntia ficus-indica (Cactaceae): New molecular evidence. Am. J. Bot. 2004, 91, 1915–1921. [Google Scholar] [CrossRef]
- Mazzeo, G., Nucifora S., Russo A., Suma P. Dactylopius opuntiae, a new prickly pear cactus pest in the Mediterranean: an overview. Entomologia Experimentalis et Applicata 2019, 167, 59–72. [CrossRef]
- Torres, J.B. , Giorgi J.A. Management of the false carmine cochineal Dactylopius opuntiae (Cockerell): perspective from Pernambuco state, Brazil. Phytoparasitica 2018, 46, 331–340. [Google Scholar] [CrossRef]
- Mendel Z., Protasov A., Vanegas-Rico J.M., Lomeli-Flores J.R., Suma P., Rodríguez-Leyva E. Classical and fortuitous biological control of the prickly pear cochineal, Dactylopius opuntiae. Biological Control 2020, 142, 104157. [CrossRef]
- Silva M.A., Albuquerque T.G., Pereira P., Ramalho R., Vicente F., Oliveira M.B., Costa H. S. Opuntia ficus-indica (L.) mill.: A multi-benefit potential to be exploited. Molecules 2021, 26, 951. [CrossRef] [PubMed]
- Kiesling, R. Origen, domesticación y distribución de Opuntia ficus-indica. J. Prof. Assoc. Cactus Dev. 1998, 3, 50–60. [Google Scholar]
- Hernandez-Hernandez, F.C. , García-Gil F., Rojas-Martínez A., Hernández-Martínez S., Lanz-Mendoza H. Carminic acid dye from the homopteran Dactylopius coccus hemolymph is consumed during treatment with different microbial elicitors. Arch Insect Biochem Physiol 2003, 54, 37–45. [Google Scholar] [CrossRef]
- Bravo-Vinaja, A. , Méndez-Gallegos J. Emerging trends in scientific research on Dactylopius coccus Costa (Hemiptera: Dactylopiidae), carminic acid and its derivatives: a bibliometric analysis. Agricultura, Sociedad y Desarrollo 2023, 20, 139–165. [Google Scholar] [CrossRef]
- Sabbahi, R. , Hock V. Control of the prickly pear cochineal, Dactylopius opuntiae (Cockerell), in Morocco: an overview. Journal of Plant Diseases and Protection 2022, 129, 1323–1330. [Google Scholar] [CrossRef]
- García-Pascual E., González-Chávez M.M., Franco A., Rodríguez-Leyva E., Méndez-Gallegos S. J., Morales Rueda J.A., Bravo A. Dactylopius opuntiae (Hemiptera: Dactylopiidae) control tactics: a bibliometric analysis. Investigación Bibliotecológica 2023, 38, 13–29. [CrossRef]
- Paré, P.W. , Tumlinson J.H. Update on plant-insect interactions plant volatiles as a defense against insect herbivores. Plant Physiology 1999, 121, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Turlings, T.C.J. , Erb M. Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annual Review of Entomology 2017, 63, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Pophof, B. , Stange G., Abrell L. Volatile organic compounds as signals in a plant-herbivore system: Electrophysiological responses in olfactory sensilla of the moth Cactoblastis cactorum. Chem Senses 2005, 30, 51–68. [Google Scholar] [CrossRef]
- Beck, J.J. , Baig N., Cook D., Mahoney N.E., Marsico T.D. Semiochemicals from ex situ abiotically stressed cactus tissue: A contributing role of fungal spores? J. Agric. Food Chem. 2014, 62, 12273–12276. [Google Scholar] [CrossRef]
- Rasmann, S.; Köllner, T.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef]
- Maffei, M.E. Sites of synthesis, biochemistry and functional role of plant volatiles. South African Journal of Botany 2010, 76, 612–631. [Google Scholar] [CrossRef]
- Kasote, D. , Lee J., Sreenivasulu N. Editorial: Volatilomics in plant and agricultural research: recent trends. Frontiers in Plant Science 2023, 14. [Google Scholar] [CrossRef]
- Flath, R.A. , Takahashi J.M. Volatile Constituents of Prickly Pear (Opuntia ficus indica Mill., de Castilla Variety). J. Agric. Food Chem. 1971, 26, 835–837. [Google Scholar] [CrossRef]
- Chahdoura, H. , Barreira J.C.M., Fernández-Ruiz V., Morales P., Calhelha R.C., Flamini G. Soković M., Ferreira I. C. F. R., Achour L. Bioactivity, proximate, mineral and volatile profiles along the flowering stages of Opuntia microdasys (Lehm.): Defining potential applications. Food Funct 2016, 7, 1458–1467. [Google Scholar] [CrossRef]
- Nounah I., Chbani M., Matthäus B., Charrouf Z., Hajib A., Willenberg I. Profile of volatile aroma-active compounds of cactus seed oil (opuntia ficus-indica) from different locations in morocco and their fate during seed roasting. Foods 2020, 9. [CrossRef]
- Wright, C.R.; Setzer, W.N. Volatile compositions of two cactus species growing in the sonoran desert of Southern Arizona. Am. J. Essential Oils Nat. Prod. 2013, 1, 41–47. [Google Scholar]
- Apablaza, E. , Sáenz C., Prat L., Ubeda C. Comprehensive characterization and volatile profile of cactus pear fruits from six different species and cultivars. ACS Food Science and Technology 2021, 1, 928–936. [Google Scholar] [CrossRef]
- El-Hawary, S.S. , El-tantawy M.E., Rabeh M.A., Badr W.K. Chemical composition and antimicrobial activity of volatile constituents of cladodes, fruits peel and fruits pulp from Opuntia ficus indica (L.) Mill. (Prickly pear) growing in Egypt. Egypt J. Chem. 2020, 64, 437–444. [Google Scholar] [CrossRef]
- Zhang, W.W.; Zheng, H.; Zhang, H.; Chen, X.M.; Guo, Y.H. Volatile changes of Opuntia ficus-indica Mill before and after parasitizing by Dactylopius coccus Costa. Chin. J. Appl. Entomol. 2012, 49, 986–991. [Google Scholar]
- Stierlin, É. , Nicolè F., Costes T., Fernandez X., Michel T. Metabolomic study of volatile compounds emitted by lavender grown under open-field conditions: a potential approach to investigate the yellow decline disease. Metabolomics 2020, 16. [Google Scholar] [CrossRef]
- Cui, S. , Ling P., Zhu H., Keener H.M. Plant pest detection using an artificial nose system: A review. Sensors 2018, 18, 378. [Google Scholar] [CrossRef]
- Cázares-Samaniego, P.J. , Castillo C.G., Ramos-López M.A., González-Chávez M.M. Volatilome and essential oil of Ulomoides dermestoides: A broad-spectrum medical insect. Molecules 2021, 26, 6311. [Google Scholar] [CrossRef]
- De Lotto, G. On the status and identity of the cochineal insects (Homoptera: Coccoidea: Dactylopiidae). J. Entomol. Soc. South. Afr. 1974, 37, 167–193. [Google Scholar]
- Waku Y., Foldi I. The fine structure of insect glands secreting waxy substances. In Insect Ultrastructure; King, R.C., Akai, H., Eds.; Springer: Boston, MA, 1984; pp. 303–322. [Google Scholar] [CrossRef]
- Rodríguez, L.C.; Faúndez, E.H.; Niemeyer, H.M. Mate searching in the scale insect, Dactylopius coccus (Hemiptera: Coccoidea: Dactylopiidae). Eur. J. Entomol. 2005, 102, 305–306. [Google Scholar] [CrossRef]
- Palafox-Luna, J.A.; Rodríguez-Leyva, E.; Lomeli-Flores, J.R.; Vigueras-Guzmán, A.L.; Vanegas-Rico, J.M. Ciclo de vida y fecundidad de Dactylopius opuntiae (Hemiptera: Dactylopiidae) en Opuntia ficus-indica (Caryophyllales: Cactaceae). Agrociencia 2018, 52, 103–114. [Google Scholar]
- Martinez, T. , Fabriás G., Camps F. Sex pheromone biosynthetic pathway in Spodoptera littoralis and its activation by a neurohormone. The Journal of Biological Chemistry 1990, 265, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Tsfadia, O. , Azrielli A., Falach L., Zada A., Roelofs W., Rafaeli A. Pheromone biosynthetic pathways: PBAN-regulated rate-limiting steps and differential expression of desaturase genes in moth species. Insect Biochem Mol. Biol. 2008, 38, 552–567. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y. , Millar J.G. Chemistry of the pheromones of mealybug and scale insects. Nat Prod Rep. 2015, 32, 1067–1113. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues C., Paula C.D. de, Lahbouki S., Meddich A., Outzourhit A., Rashad M., Pari L., Coelhoso I., Fernando A.L., Souza V.G.L. Opuntia spp.: An overview of the bioactive profile and food applications of this versatile crop adapted to arid lands. Foods 2023, 12, 1465. [CrossRef] [PubMed]
- Holopainen, J.K. , Blande J.D. Where do herbivore-induced plant volatiles go? Frontiers in Plant Science 2013, 4. [Google Scholar] [CrossRef]
- Pérez-Hedo, M. , Bouagga S., Zhang N.X., Moerkens R., Messelink G., Jaques J.A., Flors V., Broufas G., Urbaneja A. Pappas M.L. Induction of plant defenses: the added value of zoophytophagous predators. Journal of Pest Science 2022, 95, 1501–1517. [Google Scholar] [CrossRef]
- Terrazas T, Mauseth J.D. Shoot Anatomy and Morphology', in Park Nobel (ed.), Cacti: Biology and Uses (Oakland, CA, 2002; online edn, California Scholarship Online, 22 Mar. 2012). [CrossRef]
- Dicke, M. , Sabelis M.W., Takabayashi J., Bruin J., Posthumus M.A. Plant strategies of manipulating predator-prey interactions through allelochemicals: prospects for application in pest control. Journal of Chemical Ecology 1990, 16, 3091–3118. [Google Scholar] [CrossRef]
- Ozawa, R. , Arimura G.I., Takabayashi J., Shimoda T., Nishioka T. Involvement of jasmonate-and salicylate-related signaling pathways for the production of specific herbivore-induced volatiles in plants. Plant CellPhysiol 2000, 41, 381–398. [Google Scholar] [CrossRef]
- Drukker, B. , Bruin J., Sabelis M.W. Anthocorid predators learn to associate herbivore-induced plant volatiles with presence or absence of prey. Physiological Entomology 2001, 25, 260–265. [Google Scholar] [CrossRef]
- Clavijo-McCormick, A. , Unsicker S.B., Gershenzon J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends in Plant Science 2012, 17, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A. , Baldwin I.T. Plant responses to insect herbivory: The emerging molecular analysis. Annual Review of Plant Biology 2002, 53, 299–328. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Q.; Zhang, Z.N. The influence of volatiles from the hindgut of the pine sawyer, Monochamus alternatus (Coleoptera: Cerambycidae), on its oviposition behavior. Zool. Stud. 2007, 46, 726–733. [Google Scholar]
- Smith, B.C. , Williams R.R. Temperature relations of adult Coleomegilla maculata lengi and C. M. medialzs (Coleoptera: Coccinellidae) and responses to ovipositional stimulants. The Canadian Entomologist 1976, 108, 925–930. [Google Scholar] [CrossRef]
- Berhe, Y.K. , Portillo L., Vigueras A.L. Resistance of Opuntia ficus-indica cv ‘Rojo Pelon’ to Dactylopius coccus (Hemiptera: Dactylopiidae) under greenhouse condition. Journal of the Professional Association for Cactus Development 2022, 24, 293–309. [Google Scholar] [CrossRef]
- Nakata, P.A. An assessment of engineered calcium oxalate crystal formation on plant growth and development as a step toward evaluating its use to enhance plant defense. Plos One 2015, 10. [Google Scholar] [CrossRef]
- War A.R., Buhroo A.A., Hussain B., Ahmad T., Nair R.M., Sharma H.C. Plant defense and insect adaptation with reference to secondary metabolites. Co-Evolution of Secondary Metabolites 2020, 795–822. [CrossRef]
- Ferris, G.F. Atlas of the Scale Insects of North America. Nature 1943, 151, 657. [Google Scholar] [CrossRef]
- Bardou, P. , Mariette J., Escudié F., Djemiel C., Klopp C. Jvenn: an interactive Venn diagram viewer. BMC Bioinformatics 2014, 15, 293. [Google Scholar] [CrossRef]



| No. | Compounds | D. coccus | D. opuntiae | |||
|---|---|---|---|---|---|---|
| RA (%) | KI Exp | RA (%) | KI Exp | KI ref | ||
| Carboxylic acids and derivatives | 59.28% | 78.29% | ||||
| 1 | Hexanoic acid | 1.09±0.08 | 903 | |||
| 2 | 2-methylhexanoic acid | 0.54±0.01 | 950 | |||
| 3 | Heptanoic acid | 0.20±0.05 | 1021 | 0.64±0.16 | 1021 | 1005 |
| 4 | 2,4-dimethylhexanoic acid | 0.37±0.02 | 1015 | |||
| 5 | 2-Ethylhexanoic acid | 10.90±0.21 | 1036 | 1031 | ||
| 6 | Lactic acid | 5.58±0.58 | 1061 | 0.86±0.03 | 1062 | 1057 |
| 7 | Glycolic acid | 0.11±0.03 | 1075 | 1072 | ||
| 8 | 2,6-dimethylheptanoic acid | 0.61±0.21 | 1087 | |||
| 9 | Octanoic acid | 1.90±0.74 | 1108 | 2.20±0.24 | 1108 | 1108 |
| 10 | 2,3,4-Trimethylpentanoic acid | 0.44±0.02 | 1127 | |||
| 11 | Ethyl benzoate | 0.12±0.01 | 1153 | 1141 | ||
| 12 | Ethyl octanoate | 0.05±1.77 | 1188 | 1175 | ||
| 13 | Nonanoic acid | 2.32±0.64 | 1214 | 3.57±0.37 | 1214 | 1205 |
| 14 | 2,4-dimethylnonanoic acid | 0.31±0.01 | 1234 | |||
| 15 | Benzoic acid | 2.04±0.00 | 1235 | 1.04±0.01 | 1235 | 1232 |
| 16 | Ethyl nonanoate | 0.26±0.03 | 1290 | 1282 | ||
| 17 | 2-Decenoic acid | 0.42±0.02 | 1310 | 1290 | ||
| 18 | Decanoic acid | 8.76±2.53 | 1316 | 2.38±0.12 | 1316 | 1309 |
| 19 | Butanedioic acid | 0.11±0.03 | 1318 | 1314 | ||
| 20 | (Z)-4-tert-butylcyclohexyl acetate | 0.80±0.02 | 1358 | 0.12±0.03 | 1356 | 1346 |
| 21 | Ethyl decanoate | 0.18±0.02 | 1388 | 1382 | ||
| 22 | Undecanoic acid | 0.63±0.02 | 1414 | 1410 | ||
| 23 | cis-5-Dodecenoic acid | 3.18±0.04 | 1504 | |||
| 24 | Dodecanoic acid | 4.93±0.90 | 1512 | 1509 | ||
| 25 | Nonanedioic acid | 0.40±0.03 | 1535 | 1511 | ||
| 26 | Ethyl dodecanoate | 1.06±0.07 | 1580 | 1566 | ||
| 27 | Tridecanoic acid | 0.49±0.04 | 1606 | 1606 | ||
| 28 | p-Hydroxybenzoic acid | 0.24±0.00 | 1615 | 0.05±0.01 | 1616 | 1621 |
| 29 | Hexyl salicylate | 1.05±0.01 | 1660 | 0.57±0.02 | 1658 | 1684 |
| 30 | (Z)-9-Tetradecenoic acid | 0.42±0.05 | 1702 | 0.22±0.09 | 1707 | 1691 |
| 31 | Tetradecanoic acid | 21.25±2.90 | 1717 | 30.15±1.35 | 1718 | 1713 |
| 32 | Ethyl tetradecanoate | 0.40±0.01 | 1793 | 1782 | ||
| 33 | (Z)-9-Hexadecenoic acid | 0.56±0.12 | 1909 | 1885 | ||
| 34 | Hexadecanoic acid | 2.30±1.83 | 1934 | 5.89±0.42 | 1934 | 1909 |
| 35 | Ethyl hexadecanoate | 0.23±0.01 | 1974 | 1968 | ||
| 36 | Heptadecanoic acid | 1.03±0.06 | 2039 | 2009 | ||
| 37 | (Z,Z) 9,12-Octadecadienoic acid | 0.83±0.73 | 2105 | 3.76±0.67 | 2105 | 2087 |
| 38 | (Z) -9-Octadecenoic acid | 0.82±1.11 | 2112 | 2.44±0.33 | 2112 | 2088 |
| 39 | Octadecanoic acid | 1.69±2.32 | 2139 | 2.58±0.22 | 2140 | 2133 |
| 40 | Ethyl octadecanoate | 0.33±0.03 | 2208 | 2181 | ||
| 41 | Dehydroabietic acid | 1.58±0.00 | 2375 | 0.12±0.02 | 2376 | 2385 |
| Aldehydes 5.80 7.68 | ||||||
| 42 | Hexanal | 1.79±0.36 | 964 | |||
| 43 | Heptanal | 1.24±0.11 | 1066 | 1069 | ||
| 44 | Octanal | 0.23±0.07 | 1165 | 1162 | ||
| 45 | Nonanal | 0.34±0.11 | 1235 | 2.57±0.17 | 1268 | 1267 |
| 46 | Decanal | 0.28±0.02 | 1367 | 1366 | ||
| 47 | Dodecanal | 0.28±0.10 | 1663 | |||
| 48 | α-Hexylcinnamaldehyde | 5.46±0.08 | 0.11±0.12 | 1719 | 1728 | |
| 49 | Heptadecanal | 1.18±0.33 | 2088 | |||
| Ether 0.09 | ||||||
| 50 | Benzyl methyl ether | 0.09±0.01 | 966 | |||
| Terpene 0.54 0.08 | ||||||
| 51 | p-Cymene | 0.08±0.04 | 1018 | 1025 | ||
| 52 | α-Ionone | 0.36±0.15 | 1415 | 1413 | ||
| 53 | β-Ionone | 0.18±0.02 | 1472 | 1486 | ||
| Ketones 0.54 | ||||||
| 54 | Benzophenone | 0.09±0.02 | 1600 | 1611 | ||
| 55 | 2-Nonadecanone | 0.45±0.08 | 2116 | 2087 | ||
| Alcohols 12.15 0.62 | ||||||
| 56 | Phenol | 0.31±0.09 | 1045 | 1043 | ||
| 57 | 2-Ethylhexanol | 0.22±0.04 | 1095 | |||
| 58 | 1-Dodecanol | 4.31±0.00 | 1559 | 0.09±0.01 | 1559 | 1575 |
| 59 | 1-Tridecanol | 0.42±0.00 | 1659 | 1656 | ||
| 60 | 1-Tetradecanol | 3.43±0.00 | 1765 | 1770 | ||
| 61 | 1-Hexadecanol | 3.47±0.00 | 1977 | 1965 | ||
| 62 | 1-Octadecanol | 0.52±0.00 | 2175 | 2159 | ||
| Alkene 0.42 | ||||||
| 63 | 1-Tridecene | 0.42±0.00 | 1284 | 1287 | ||
| Alkane 1.80 2.41 | ||||||
| 64 | Hexadecane | 1.80±0.00 | 1581 | 1.11±0.29 | 1581 | 1600 |
| 65 | Octadecane | 0.25±0.00 | 1797 | 1800 | ||
| 66 | Heneicosane | 1.05±0.21 | 2309 | |||
| Total | 79.57 | 90.13 | ||||
| No. | Compounds | OFI Esmeralda |
OFI Esmeralda infested by D. coccus |
KIExp | OFI Rojo Pelón |
OFI Rojo Pelón infested by D. opuntiae | KIExp | KIRef |
|---|---|---|---|---|---|---|---|---|
| RA (%) | RA (%) | RA (%) | RA (%) | |||||
| Carboxilic acid and derivatives 48.79 | 44.28 | 31.78 | 20.05 | |||||
| 1 | Hexanoic acid | 0.83±0.63 | 0.82±0.00 | 942 | 904 | |||
| 67 | 3-Methyl-2-pentenoic acid | 0.35±0.32 | 959 | 926 | ||||
| 68 | 2-Hexenoic acid | 0.66±0.01 | 0.36±0.00 | 972 | 939 | |||
| 69 | 4-Oxopentanoic acid | 0.22±0.14 | 991 | 956 | ||||
| 70 | Heptanoic acid | 0.59±0.50 | 0.39±0.05 | 1022 | 0.39±0.11 | 0.25±0.15 | 1030 | 1005 |
| 5 | 2-Ethylhexanoic acid | 0.11±0.07 | 1044 | 1031 | ||||
| 71 | 4-Methylvaleric acid | 0.15±0.10 | 1033 | 1039 | ||||
| 72 | 2-Methyl-4-pentenoic acid | 0.11±0.16 | 1062 | |||||
| 73 | Lactic acid | 3.22±1.91 | 1.63±0.16 | 1065 | 1057 | |||
| 15 | Benzoic acid | 1.49±1.77 | 0.29±0.03 | 1080 | 2.06±0.13 | 0.50±0.09 | 1080 | 1084 |
| 74 | Methyl benzoate | 0.41±0.14 | 1081 | 0.91±0.34 | 1084 | |||
| 9 | Octanoic acid | 1.23±1.52 | 1112 | 1.21±0.07 | 1.34±0.07 | 1112 | 1109 | |
| 11 | Ethyl Benzoate | 0.05±0.01 | 1156 | 0.08±0.04 | 0.24±0.11 | 1152 | 1141 | |
| 75 | Benzeneacetic acid | 0.17±0.05 | 1160 | 1150 | ||||
| 76 | Salicylic acid | 0.18±0.25 | 1171 | 1176 | ||||
| 77 | Methyl salicylate | 1.21±0.37 | 6.96±0.14 | 1181 | 0.32±0.07 | 1172 | 1176 | |
| 78 | 2-Nonenoic acid | 0.86±0.00 | 1179 | 0.33±0.08 | 1184 | |||
| 13 | Nonanoic acid | 1.09±0.24 | 0.76±0.07 | 1216 | 1.45±0.12 | 1.72±0.14 | 1212 | 1206 |
| 79 | Ethyl salycilate | 1.03±0.01 | 1244 | 1241 | ||||
| 18 | Decanoic acid | 0.77±0.75 | 0.63±0.00 | 1318 | 0.79±0.08 | 1311 | 1309 | |
| 19 | Butanedioic acid | 0.49±0.19 | 1320 | 1314 | ||||
| 80 | Gliceric acid | 0.73±0.02 | 1346 | 1342 | ||||
| 81 | 2-Methoxybenzoic acid | 0.10±0.01 | 1331 | 1362 | ||||
| 82 | Methyl 2-methoxy benzoate | 0.45±0.10 | 1319 | 1295 | ||||
| 83 | Glutaric acid | 0.16±0.03 | 1410 | 1400 | ||||
| 22 | Undecanoic acid | 0.16±0.02 | 0.14±0.07 | 1417 | 0.08±0.02 | 0.12±0.08 | 1408 | 1410 |
| 24 | Dodecanoic acid | 5.70±6.08 | 2.77±0.06 | 1516 | 7.19±0.50 | 2.35±0.16 | 1505 | 1509 |
| 84 | 2,5-Dimethoxy benzenemethanol acetate | 0.13±0.04 | 1523 | |||||
| 26 | Ethyl Dodecanoate | 0.26±1.27 | 0.17±0.09 | 1582 | 0.33±0.01 | 1571 | 1581 | |
| 28 | p-Hydroxybenzoic acid | 0.94±0.45 | 1620 | 1621 | ||||
| 29 | Hexylsalicylate | 0.25±3.04 | 0.46±0.07 | 1662 | 1652 | |||
| 85 | Methyl tetradecanoate | 0.84±0.16 | 1719 | 1714 | ||||
| 27 | Tridecanoic acid | 0.06±0.04 | 1611 | 1606 | ||||
| 86 | 12-Methyltridecanoic acid | 0.07±0.00 | 1678 | 1680 | ||||
| 31 | Tetradecanoic acid | 2.98±3.09 | 1.36±0.06 | 1720 | 1.56±0.10 | 0.19±0.07 | 1714 | 1714 |
| 87 | Methyl benzoate | 1.46±0.03 | 1752 | |||||
| 88 | Benzyl Benzoate | 2.65±0.51 | 1741 | 0.15±0.04 | 1754 | 1765 | ||
| 32 | Ethyl tetradecanoate | 0.10±0.13 | 1784 | 1782 | ||||
| 89 | Nonanedioic acid | 0.28±0.01 | 1808 | 1788 | ||||
| 90 | Pentadecanoic acid | 0.42±0.47 | 0.43±0.02 | 1826 | 1807 | |||
| 91 | Isopropyl tetradecanoate | 0.05±0.34 | 1820 | 1827 | ||||
| 92 | Benzyl salicylate | 0.31±0.12 | 1855 | 1860 | ||||
| 34 | Hexadecanoic acid | 7.19±2.16 | 4.88±0.16 | 1935 | 9.35±0.74 | 8.25±0.45 | 1916 | 1909 |
| 93 | 15-Methylhexadecanoic acid | 0.17±0.03 | 2040 | 1974 | ||||
| 37 | (Z,Z)-9,12-Octadecadienoic acid | 1.90± 0.44 | 1.03±0.12 | 2106 | 0.88±0.14 | 2.20±1.43 | 2087 | 2087 |
| 38 | (Z)-9-Octadecenoic acid | 2.35±1.66 | 1.94±0.10 | 2113 | 2.47±0.98 | 2090 | 2100 | |
| 39 | Octadecanoic acid | 2.53±0.59 | 1.76±0.01 | 2141 | 2.76±0.15 | 0.59±0.15 | 2119 | 2133 |
| 94 | Methyl octadecanoate | 0.33±0.04 | 1809 | |||||
| 40 | Ethyl octadecanoate | 0.11±0.00 | 2199 | 2202 | ||||
| 41 | Dehydroabietic acid | 9.32±2.24 | 9.99±0.04 | 2376 | 0.46±0.05 | 0.39±0.02 | 2344 | 2373 |
| Aldehides and derivatives 2.15 6.25 4.3 4.82 | ||||||||
| 42 | Hexanal | 0.44±0.44 | 0.47±0.00 | 984 | 964 | |||
| 43 | Heptanal | 0.18±0.02 | 1069 | 1068 | ||||
| 95 | Benzaldehyde | 0.15±0.07 | 1094 | 0.32±0.03 | 0.55±0.33 | 1094 | 1080 | |
| 96 | Diethyl acetal hexanal | 0.25±0.14 | 0.46±0.10 | 1086 | 1082 | |||
| 97 | 5,5-Dimethyl-3-oxo-1-cyclohexene-1-carboxaldehyde | 0.15±0.03 | 1104 | |||||
| 44 | Octanal | 0.17±0.01 | 1160 | 0.40±0.05 | 0.09±0.05 | 1165 | 1167 | |
| 98 | Phenylacetaldehyde | 0.62±0.61 | 0.56±0.06 | 1198 | 0.82±0.06 | 0.81±0.17 | 1201 | 1208 |
| 45 | Nonanal | 0.53±0.21 | 1.03±0.01 | 1271 | 1.73±0.12 | 1.32±0.09 | 1265 | 1267 |
| 46 | Decanal | 0.14±0.00 | 1370 | 0.14±0.08 | 1366 | 1366 | ||
| 99 | Nonanaldimethylacetal | 0.21±0.10 | 0.37±0.05 | 1374 | 1379 | |||
| 100 | 3-(4-(tert-butyl)phenyl-2-methylpropanal | 0.30±0.04 | 1497 | 1500 | ||||
| 101 | 4-Hydroxy-3-methoxybenzaldehyde | 0.10±0.04 | 0.62±0.02 | 1524 | 0.89±0.04 | 2.05±0.01 | 1511 | 1544 |
| 102 | 3-Ethoxy-4-hydroxybenzaldehyde | 0.11±0.02 | 1554 | 1560 | ||||
| 48 | α-Hexylcinnamaldehyde | 1.22±0.74 | 1725 | 1726 | ||||
| 103 | Octadecanal | 0.32±0.13 | 2187 | |||||
| Heterocycles | 0.67 | 8.91 | 1.38 | |||||
| 104 | 2-Isopropyl-3-metoxypirazina | 0.25±3.25 | 1070 | 1080 | ||||
| 105 | 2-Methoxy-3-isopropylpyrazine | 0.30± 7.22 | 1.05±0.33 | 1083 | 1089 | |||
| 106 | Ethyl 2-(5-methyl-5-vinyltetrahydrofuran-2-yl)propan-2-yl carbonate | 8.18±0.10 | 1064 | 1090 | ||||
| 107 | 3-Isobutyl-2-methoxypyrazine | 0.43±0.03 | 1164 | 1170 | ||||
| 108 | 3-Ethyl-4-methyl-1H-pyrrole-2,5-dione | 0.35±0.03 | 1209 | 1192 | ||||
| 109 | 3-Hydroxy-2-methylpyran-4-one | 0.07±0.00 | 1266 | 1293 | ||||
| 110 | 2,3-Dihydro-2,2,4,6-tetramethylbenzofuran | 0.33±0.01 | 1410 | |||||
| Ethers | 0.31 | 0.16 | ||||||
| 50 | Benzylmethylether | 0.31±0.20 | 966 | 966 | ||||
| 111 | 1,2-Dimethoxybenzene | 0.16±0.02 | 1111 | 1106 | ||||
| Ketones | 1.95 | 1.16 | 1.17 | 0.61 | ||||
| 112 | 5-Hexen-2-one | 0.29±0.01 | 1007 | |||||
| 113 | 2,2,6-Trimethylcyclohexanone | 0.14±0.09 | 1031 | |||||
| 114 | Acetophenone | 0.57±0.08 | 1047 | 0.45±0.05 | 1055 | 1049 | ||
| 115 | Isophorone | 0.24±0.06 | 0.01±0.22 | 1106 | 0.17±0.03 | 1038 | 1094 | |
| 116 | Phenylacetone | 0.48±0.05 | 0.44±0.00 | 1114 | 0.24±0.02 | 0.16±0.07 | 1110 | 1116 |
| 117 | 4-Oxoisophorone | 0.13±0.02 | 1131 | 0.07±0.04 | 1125 | 1105 | ||
| 118 | 2-(1-Hydroxybut-2-enylidene)cyclohexanone | 0.14±0.03 | 1145 | |||||
| 119 | 1-(1-cyclohexen-1-yl)(-1-1-Butenone) | 0.70±0.34 | 1214 | |||||
| 54 | Benzophenone | 0.11±0.07 | 0.14±0.01 | 1607 | 0.41±0.05 | 1584 | 1607 | |
| Terpenes | 17.89 | 13.92 | 0.8 | 15.52 | ||||
| 120 | Limonene | 0.85±0.23 | 1023 | 1020 | ||||
| 121 | Linalool oxide | 8.48±0.58 | 5.06±0.42 | 1063 | 1064 | |||
| 122 | trans-Linalool oxide | 5.70±0.03 | 1064 | 1068 | ||||
| 123 | 1,5,5-Trimethyl-3-methylene cyclohexene | 0.55±0.63 | 1071 | |||||
| 124 | β-Linalool | 5.00±0.58 | 0.26±0.35 | 1088 | 1082 | |||
| 125 | α-Terpineol | 2.00±0.36 | 1178 | 1172 | ||||
| 126 | Linalool | 0.19±0.22 | 1232 | 5.61±0.10 | 1227 | 1227 | ||
| 127 | Geraniol | 0.44±0.27 | 0.33±0.04 | 1250 | 1.84±0.06 | 1357 | 1238 | |
| 128 | Nerol | 0.33±0.06 | 1232 | 0.79±0.04 | 1328 | 1260 | ||
| 129 | β-Damascenone | 0.18±0.17 | 1362 | 0.55±0.30 | 1360 | 1361 | ||
| 52 | α-Ionona | 0.09±0.26 | 1404 | 1413 | ||||
| 130 | α-Isomethylionone | 0.88±0.15 | 1453 | 1478 | ||||
| 53 | β-Ionone | 0.40±0.06 | 1460 | 0.18±0.04 | 1458 | 1486 | ||
| 131 | Dihydroactinidiolide | 0.38±0.13 | 0.30±0.02 | 1537 | 1532 | |||
| 132 | Neophytadiene | 0.39±0.08 | 1832 | 1842 | ||||
| 133 | 28-Nor-17β(H)-hopane | 0.45±0.01 | 2942 | |||||
| 134 | β-Sitosterol | 0.35±0.03 | 6.55±0.04 | 3244 | 3284 | |||
| Alcohols | 12.72 | 9.91 | 29.37 | 30.78 | ||||
| 135 | 1,2-Dihydroxy-4-methylpentane | 0.27±0.02 | 990 | |||||
| 136 | Hexanol | 0.06±0.01 | 9994 | 992 | ||||
| 137 | (Z)-2-Hexen-1-ol | 0.36±0.19 | 1010 | 7.65±0.18 | 4.21±0.08 | 1025 | 1001 | |
| 56 | Phenol | 0.22±0.09 | 0.16±1.30 | 1045 | 1043 | |||
| 138 | Heptanol | 0.11±0.23 | 1067 | 1092 | ||||
| 57 | 2-Ethylhexanol | 0.58±0.23 | 0.22±0.24 | 1099 | 2.13±0.09 | 1.49±0.05 | 1103 | |
| 139 | Benzylalcohol | 0.27±0.11 | 0.56±0.22 | 1143 | 0.90±0.02 | 2.08±0.06 | 1132 | 1156 |
| 140 | 1-Octanol | 0.29±0.14 | 1158 | 1.27±0.04 | 1.51±0.18 | 1177 | 1177 | |
| 141 | Guaiacol | 0.35±0.03 | 1209 | 1192 | ||||
| 142 | Nonanol | 0.07±0.01 | ||||||
| 143 | Glycerol | 0.33±0.52 | 1290 | 1.46±0.09 | 1288 | 1292 | ||
| 144 | p-Vinylguaiacol | 10.62±7.24 | 1.76±0.34 | 1305 | 14.67±0.93 | 17.03±5.14 | 1294 | 1282 |
| 145 | 1-Methyl-1(4-methyl-3-cyclohexenyl)ethanol | 0.63±0.00 | 1318 | 1309 | ||||
| 146 | Isododecanol | 0.09±0.01 | 1479 | |||||
| 58 | 1-Dodecanol | 0.47±0.09 | 0.60±0.01 | 1563 | 0.76±0.04 | 1553 | 1575 | |
| 60 | 1-Tetradecanol | 0.66±0.00 | 1756 | 1768 | ||||
| 61 | 1-Hexadecanol | 0.23±0.07 | 0.98±0.08 | 1978 | 0.53±0.03 | 1960 | 1965 | |
| 62 | 1-Octadecanol | 0.92±0.04 | 2177 | 2159 | ||||
| 147 | 3,7,11,15-Tetramethyl-2-hexadecenol | 1.82±0.15 | 2198 | 3.52±0.17 | 2173 | 2179 | ||
| 148 | Octacosanol | 0.94±0.04 | 3125 | 3154 | ||||
| Aromatic derivatives | 0.77 | |||||||
| 149 | 1,2-Dihydro-1,1,6-trimethyl naphthalene | 0.18±0.07 | 1338 | 1332 | ||||
| 150 | 10,18,Bisnorabieta-8,11,13.triene | 0.59±0.03 | 2041 | |||||
| Alkanes | 0.69 | 0.55 | 0.78 | |||||
| 64 | Hexadecane | 0.69±0.19 | 0.07±0.01 | 1585 | 1600 | |||
| 151 | Heptadecane | 0.12±0.05 | 1692 | 1700 | ||||
| 152 | Nonadecane | 0.36±0.06 | 1906 | 1900 | ||||
| 153 | Eicosane | 0.19±2.08 | 1992 | 2000 | ||||
| 66 | Heneicosane | 0.23±0.42 | 2092 | 2100 | ||||
| 154 | Docosane | 0.36±0.07 | 2188 | 2200 | ||||
| Total | 85.27 | 76.9 | 77.11 | 73.16 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





