1. Introduction
The field of assisted reproductive technologies (ART) has undergone a significant revolution with the advent of in in-vitro fertilization (IVF), offering newfound hope to millions of individuals and couples worldwide struggling with infertility [
1,
2,
3,
4,
5,
6]. Although conventional IVF procedures have achieved success, they are limited by factors such as poor success rates, intrusive processes, and ethical considerations related to embryo manipulation [
7,
8,
9].
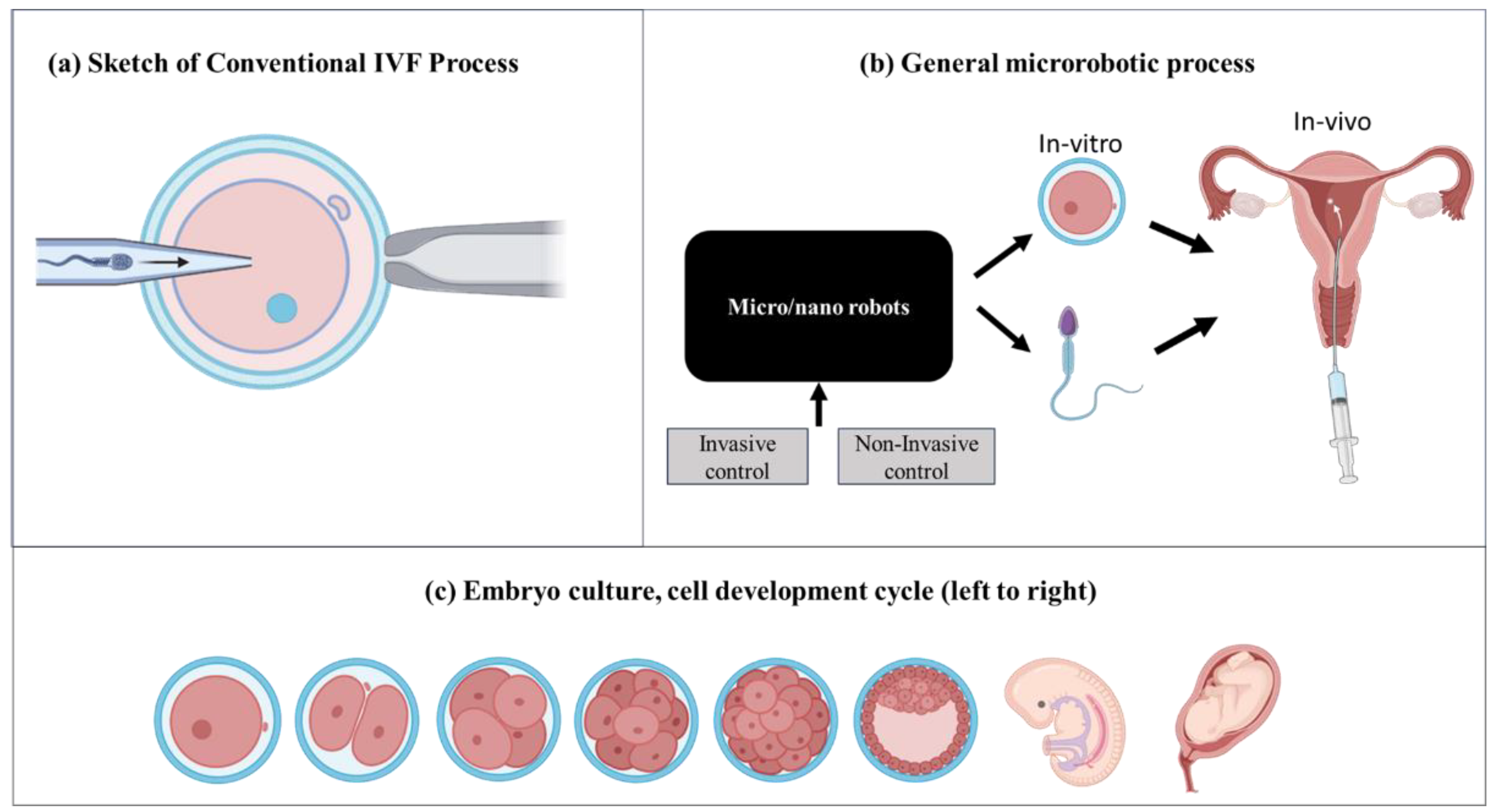
Figure 1a highlights a conventional IVF process wherein sperm is injected inside an egg cell called an oocyte. While manually manipulating oocyte or sperm, there will be mechanical damage. Integrating micro/nanorobotics into IVF operations offers a significant chance to overcome these restrictions and fundamentally change the area of assisted reproduction. These encompass refined accuracy in altering gametes and embryos, resulting in enhanced conception rates and less danger of harm. Furthermore, micro/nanorobotics provide precise administration of medication to reproductive tissues, enhancing the efficiency of fertility drugs while reducing the impact on the rest of the body. Microfluidic platforms facilitate the creation of controlled settings for the growth of gametes and embryos [
10,
11,
12,
13,
14,
15,
16,
17]. These platforms simulate the circumstances found in living organisms, hence improving the viability and development of the gametes and embryos. The utilization of micro/nanorobotic systems for real-time monitoring and imaging allows for precise evaluation of embryo quality, resulting in enhanced selection and increased rates of success. Micro/nanorobotics also facilitate the advancement of minimally invasive IVF techniques [
18,
19,
20,
21,
22], which effectively decrease patient discomfort and difficulties commonly connected with conventional surgeries (see
Figure 1b). Consequently, this enhances the overall results and accessibility of the treatment. On the whole, the integration of these technologies offers a promising chance to transform assisted reproduction by improving the accuracy, effectiveness, and success rates of fertility therapies.
Micro/nanorobotics, distinguished by their small dimensions and meticulous manipulation abilities, have arisen as favorable instruments for enhancing the effectiveness of IVF treatments. These robotic systems utilize the principles of precision engineering, miniaturization, and manipulation at the micro/nanoscale to navigate intricate biological environments, manipulate gametes and embryos with exceptional accuracy, and create controlled microenvironments to enhance fertilization and embryo development. Implementing various essential methods is crucial for the precise management of micro/nanorobots in order to improve the effectiveness and safety of IVF procedures. To begin with, external magnetic fields are employed to precisely guide micro/nanorobots throughout the female reproductive tract, directing them to specific destinations such as the fallopian tubes or uterus [
7,
23]. This enables them to assist in various IVF operations. Moreover, several micro/nanorobots are specifically engineered to react to chemical stimuli present in the reproductive environment [
24,
25]. This enables them to precisely navigate towards particular targets and carry out specialized tasks, such as delivering drugs or manipulating cells. Advanced micro/nanorobotic systems also include feedback control techniques, utilizing sensors to continuously monitor environmental variables such as temperature and pH [
26,
27,
28,
29]. The feedback data allows the control system to adapt the robot's behavior, guaranteeing both safety and accuracy in IVF treatments. Moreover, the presence of autonomous navigation capabilities enables certain micro/nanorobots to roam autonomously within the reproductive environment, adjusting to dynamic situations and executing intricate tasks without continuous external supervision. By utilizing these control methodologies, scientists can create micro/nanorobotic systems that efficiently aid in many phases of IVF, ultimately enhancing success rates and patient results.
Progress in micro/nanorobotics has facilitated the precise manipulation of sperm, less intrusive retrieval of oocytes, and controlled cultivation of embryos [
7,
11,
15,
19,
30,
31]. These advancements have the potential to raise the success rates of IVF, minimize invasiveness, and improve patient outcomes. Nevertheless, the clinical application of micro/nanorobotic technology in IVF requires addressing problems related to scalability, biocompatibility, and regulatory considerations. Various obstacles need to be overcome in order to permit the practical implementation of its clinical translation. An important obstacle is in scalability, as existing micro/nanorobotic systems may not possess the capacity to be readily expanded for extensive clinical use. Reducing the size of these systems while preserving their functionality and efficacy poses engineering obstacles that must be resolved. Moreover, it is important to guarantee biocompatibility in order to avert any unfavorable reactions or harm to the tissues when micro/nanorobots are introduced into the reproductive environment. It is necessary to meticulously choose materials and surface coatings in order to minimize immunological reactions and optimize compatibility with biological tissues. Further, it is imperative to tackle regulatory concerns in order to guarantee the safety and effectiveness of micro/nanorobotic technologies in clinical environments. Regulatory bodies mandate comprehensive proof of safety, efficacy, and quality assurance prior to granting approval for the clinical utilization of novel medical devices. Hence, it is imperative to conduct thorough testing and validation procedures in order to comply with regulatory requirements and secure permission for clinical implementation. To enable the clinical integration of micro/nanorobotic technologies in IVF and enhance outcomes for fertility patients, it is crucial to tackle obstacles pertaining to scalability, biocompatibility, and regulatory considerations.
This review seeks to offer a thorough examination of the utilization of micro/nanorobotics in IVF, delving into recent progress, advantages, obstacles, and forthcoming paths in this swiftly developing domain. Through an analysis of the fundamental concepts, methodologies, and prospective consequences of micro/nanorobotics in IVF, our objective is to elucidate the innovative capability of these technologies in enhancing the effectiveness and security of assisted reproductive treatments.
2. Discussion
Micro/nanorobotic devices have caused a significant change in the field of IVF sperm manipulation techniques. An innovative technology has been established, ushering in an unprecedented era marked by increased accuracy and regulation in critical procedures including sperm sorting, manipulation, and selection [
12,
18,
32]. By employing micro/nanorobots based on magnetism and acoustics, scientists have successfully facilitated targeted sperm delivery to the oocyte, which substantially increases fertilization rates and decreases the probability of multiple pregnancies. Moreover, the incorporation of actuators and sensors into these micro/nanorobots has brought about a paradigm shift in the discipline by permitting instantaneous surveillance and evaluation of sperm parameters. By enabling the optimization of sperm selection for fertilization, this capability ultimately improves the overall effectiveness of IVF procedures.
The utilization of micro/nanorobotic systems for sperm manipulation has revealed an abundance of favorable opportunities in the field of reproductive science [
18,
24,
27,
30,
33,
34]. These developments have presented new possibilities for the advancement of fertility treatments and ART, thus instilling renewed optimism among individuals and couples confronted with diverse fertility obstacles. Through the provision of cellular-level manipulation and control capabilities, micro/nanorobotics have successfully surmounted conventional constraints linked to sperm sorting, manipulation, and selection methodologies. This represents a substantial advancement in the discipline, as it tackles critical challenges that are commonly encountered in the pursuit of successful conception.
The ability of micro/nanorobotic systems to address common issues such as male infertility, limited sperm motility, and genetic abnormalities in sperm is one of their most significant contributions to IVF [
18,
35]. Micro/nanorobotics present an unprecedented opportunity for individuals attempting to conceive by offering a method to precisely identify and address these obstacles. By augmenting the ability of existing IVF procedures, this technology creates opportunities for additional investigations and advancements in the domain of reproductive science. Consequently, micro/nanorobotic systems are positioned to significantly influence the trajectory of fertility treatments and support the endeavors of individuals and couples in their pursuit of becoming parents.
The following sections provide an overview of the fundamental principles and developments that govern the manipulation of sperm and oocytes using micro/nano robotic technology. Additionally, they discuss the progress made in embryo culture techniques and the numerous advantages offered by micro/nano robotic technology. The purpose of these subsections is to clarify the groundbreaking approaches and substantial progress achieved in these pivotal domains of reproductive science. Furthermore, a concise summary of our current research pursuits in this area is provided, elucidating our contributions and commitment to furthering the field of assisted reproduction via the incorporation of state-of-the-art micro/nanorobotic technologies.
2.1. Principles of Micro/Nanorobotics in IVF
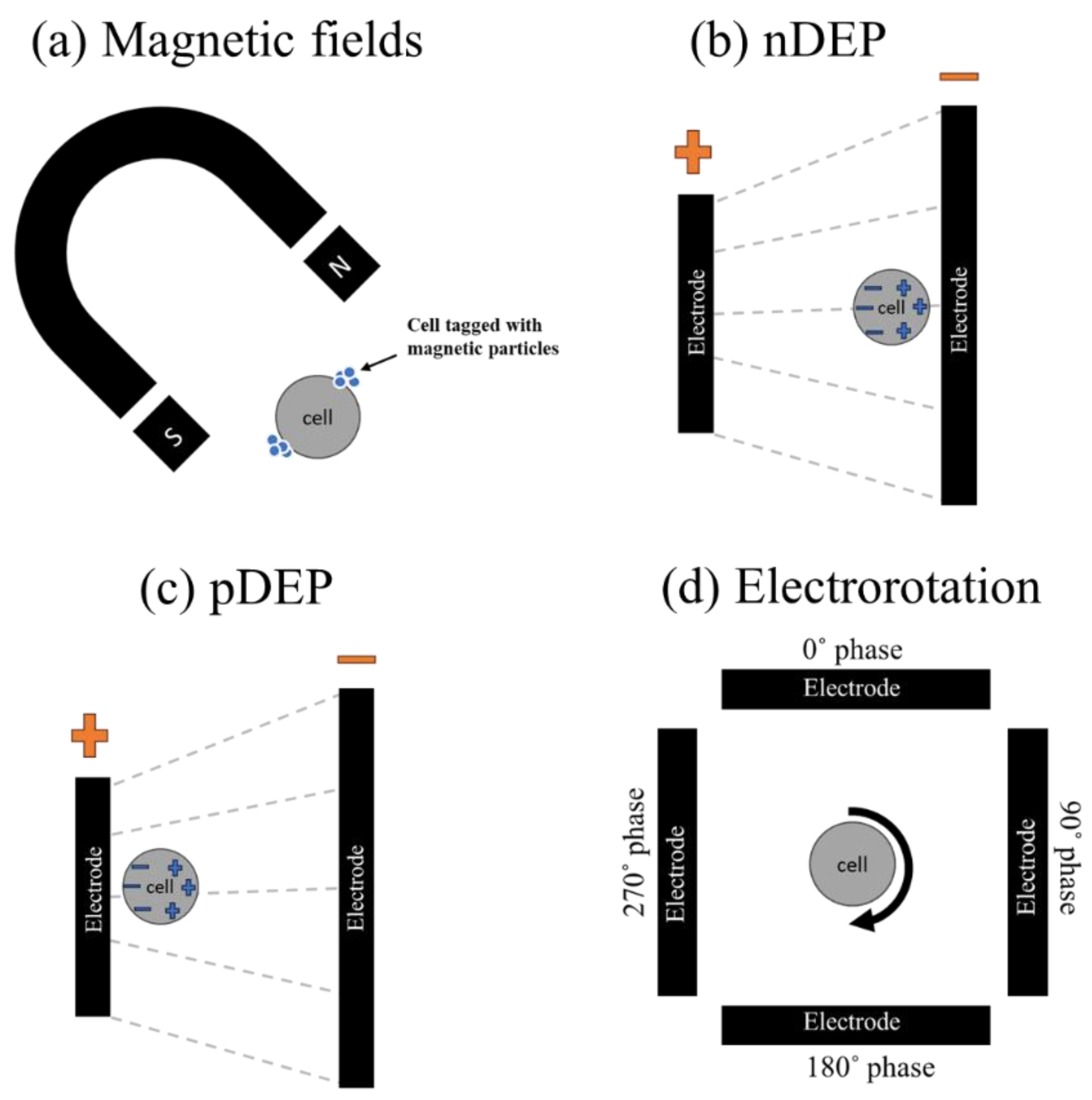
The principles of micro/nanorobotics in IVF involve several features, such as manipulating gametes, sperm and embryos, delivering drugs to specific targets, creating controlled environments, monitoring in real-time, and navigating within the reproductive environment. The research in this domain is around the advancement of robotic systems that can augment the accuracy, effectiveness, and security of IVF procedures. Micro/nanorobotic systems can be engineered to react to external stimuli (invasively and non-invasively), such as magnetic, electric fields (see
Figure 2a) chemical signals, optical and ultrasound signals [
19,
23,
25]. This enables accurate control over the manipulation of gametes and embryos during IVF operations [
16]. For example, Karcz et al., [
16] reported a review on electrically driven on-chip micro/nano robotic technologies to manipulate and handle gametes and embryos. The gametes and embryos are placed within a micro or nanodevice and can be precisely managed using electrical stimulation techniques such as dielectrophoresis (DEP), positive dielectrophoresis (pDEP) (see
Figure 1c), or negative dielectrophoresis (nDEP) (see
Figure 2b-d), and electrorotation (ER) (see
Figure 1d). As shown in
Figure 2, the principle of electrical phenomena includes combination of arrangement of micro patterned electrodes to induce non-uniform electric fields and field gradients. One can achieve precise manipulation and wireless control over the cells by optimizing the electric field amplitude, frequency and phase. In many cases, these magnetic and electric fields were used to activate micro/nano robots to harness the required manipulation tasks on embryos and gametes [
16] [
19,
23,
25].
In addition, micro/nanorobots can be designed to transport pharmaceuticals or therapeutic substances to reproductive organs with great accuracy, enhancing the efficiency of fertility therapies while reducing the occurrence of adverse effects across the body [
36,
37]. Furthermore, the integration of microfluidic devices with micro/nanorobotic systems allows for the creation of controlled habitats for the cultivation of gametes and embryos. This replication of in-vivo circumstances enhances the viability and development of these reproductive cells during in-vitro fertilization (IVF) operations [
38]. The incorporation of feedback control mechanisms into micro/nanorobotic systems allows for the continuous monitoring of environmental variables like temperature and pH [
26,
27,
28,
29]. This ensures accurate and secure operation throughout IVF procedures. In addition, several micro/nanorobots are specifically engineered to operate independently inside the reproductive environment. These robots employ built-in sensors and algorithms to assess their surroundings and determine their movements and behaviors [
39,
40,
41,
42]. Autonomous navigation enables micro/nanorobots to adjust to varying environments and execute intricate tasks without continuous external supervision, hence boosting the efficiency and safety of IVF treatments.
Ultimately, the study of micro/nanorobotics in IVF shows potential for enhancing the field of assisted reproduction through the enhancement of accuracy, effectiveness, and security in reproductive procedures. Micro/nanorobotic systems have the potential to revolutionize IVF procedures and improve outcomes for patients undergoing fertility treatments by utilizing principles such as external stimuli responsiveness, targeted drug delivery, controlled environment creation, real-time monitoring, and autonomous navigation.
2.2. Advancements in Sperm Manipulation
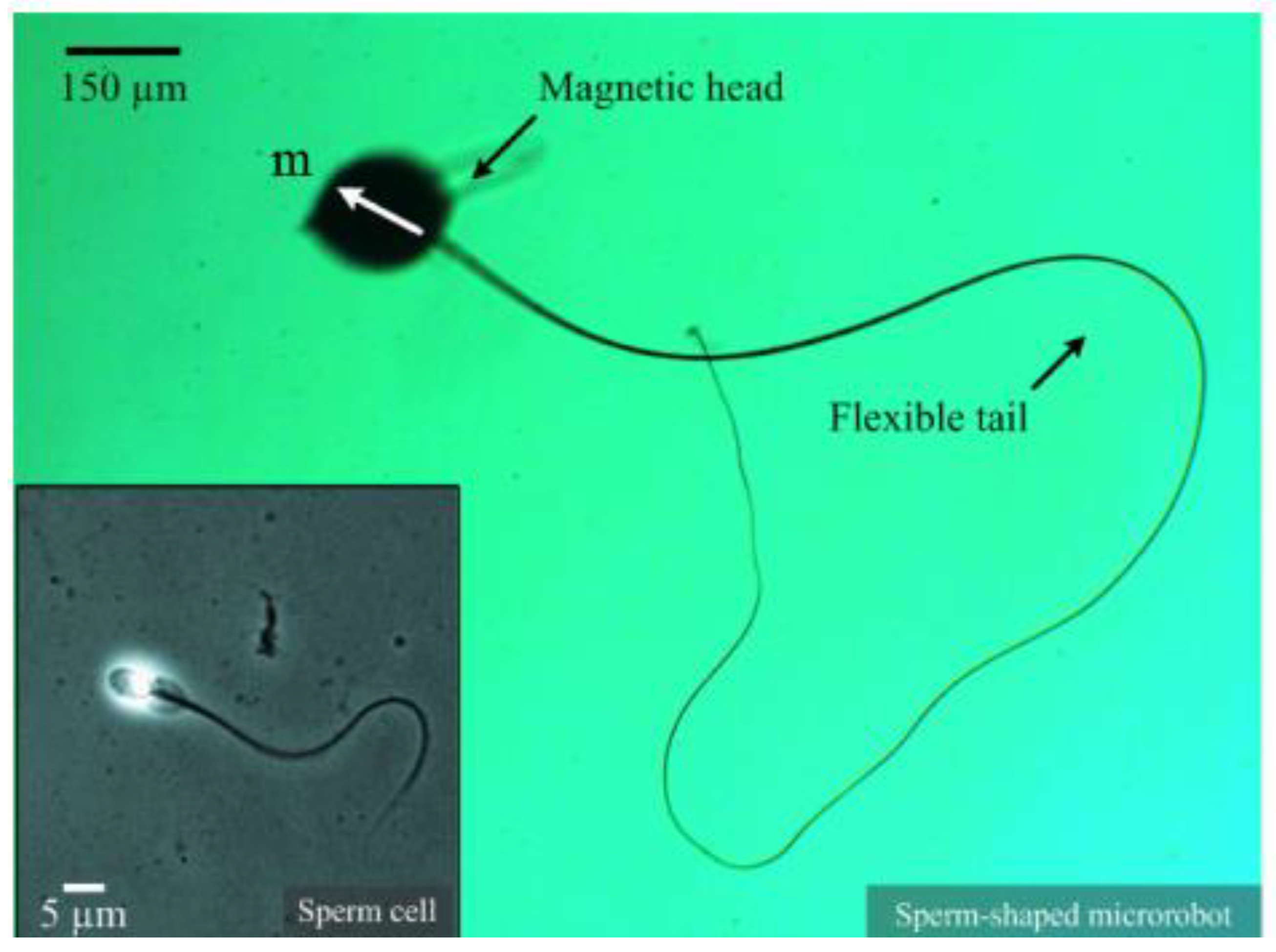
An aspect of reproductive science that has undergone significant progress is the creation of micro/nano robotic systems intended for the manipulation and selection of sperm. A group of researchers from the Department of Nano-Engineering at the University of California, San Diego and the Max Planck Institute for Intelligent Systems have pioneered the use of magnetic microbots to isolate and manipulate sperm cells according to their genetic and motility characteristics [
38,
43] (see
Figure 3). The microbots, which are operated by means of external magnetic fields, demonstrate the capacity to efficiently segregate viable, healthy sperm from immobile or aberrant sperm. As shown in
Figure 3, the researchers have created a microrobot that mimics the shape of a sperm using the advanced method of electro-spinning. This microrobot is intricately designed using a microbead and an ultra-fine fiber to accurately replicate the physical characteristics of a real sperm cell. The microbead, made from iron oxide nanoparticles, has a magnetic dipole moment, and the ultra-fine fiber is created to provide propulsive force when exposed to fluctuating magnetic fields. The microrobot's design utilizes the exceptional qualities of its components to perform precise movement and navigation in different situations. The microbead's magnetic dipole moment allows accurate control and direction of the microrobot in a fluid environment through the use of external magnetic fields. The ultra-fine fiber's reaction to alternating magnetic fields moves the microrobot ahead, enhancing its movement with exceptional agility and efficiency. This innovative advancement paves the way for creating small robotic systems designed for various purposes, such as delivering drugs precisely in the human body, performing detailed microsurgery, and exploring tight locations.
The manipulation of sperm cells using micro-robot represents a major development in microscale robotics by using ideas from nature and utilizing modern materials and fabrication techniques. The biomimetic design and functional characteristics of this technology enable the development of diverse and precisely controllable microscale platforms, offering innovative solutions in healthcare, industrial, and scientific fields. Advancements in downsizing and robotics are driving the study of microrobots, which have the potential to transform our interactions with the miniature world. As a result, IVF procedures are considerably improved in terms of efficiency. This innovation signifies a significant progression in reproductive technology, providing enhanced accuracy and specificity in the procedures used to select sperm.
Furthermore, substantial advancements in targeted drug delivery and gene editing methodologies have been enabled by micro/nano robotics, with the ultimate goal of augmenting sperm functionality and fertility potential. Through the integration of drug-loaded modules onto nano-scale carriers or robots, scientists are able to accurately deliver therapeutic agents or genetic modifiers to sperm cells [
18,
19,
22,
33]. This enables them to target specific molecular defects or enhance the motility and viability of sperm. As an illustration, a bio-hybrid magnetic microrobot was constructed by Mishra and Magdanz et al. (2020) [
33] via electrostatic self-assembly, wherein magnetic nanoparticles were integrated with non-motile sperm cells. The biocompatibility and drug-loading capability of these microrobots were validated by the researchers, underscoring their potential as biohybrid instruments capable of targeted therapy in living organisms. Moreover, these devices are programmable, detectable, and biocompatible. Personalized fertility treatments, which are customized to the unique characteristics of each sperm, have the potential to significantly transform the field of reproductive medicine.
The incorporation of micro/nano robotic systems into sperm manipulation methodologies not only improves the efficacy and accuracy of IVF procedures, but also presents novel opportunities for advancements in reproductive science research and innovation. These developments facilitate a more comprehensive exploration of the molecular mechanisms underlying sperm biology and function, thereby creating opportunities for the advancement of customized and efficacious fertility treatments. Moreover, the capacity to accurately regulate sperm properties via targeted drug delivery and gene editing methodologies signifies a substantial advancement in the resolution of diverse fertility obstacles encountered by both individuals and couples. This progressive technology possesses the capacity to fundamentally alter the reproductive medicine domain, providing individuals grappling with infertility challenges with renewed optimism and prospects.
In summary, the progress made in reproductive science with the creation of micro/nano robotic systems for sperm manipulation implies a significant turning point. By employing novel methodologies such as magnetic microbots and targeted drug delivery systems, scientists have attained an unparalleled level of accuracy and regulation in the manipulation and selection of sperm [
18,
19,
21,
22,
44]. These developments possess tremendous potential for improving the efficacy of IVF procedures and for creating individualized fertility treatments that cater to specific requirements. In the future, ongoing investigations and advancements in this domain hold the potential to significantly transform reproductive medicine, providing fresh prospects and optimism for individuals and couples attempting to surmount obstacles related to fertility.
2.3. Innovations in Oocyte Retrieval
The use of micro/nano robots has brought about a significant change in ART, especially in the field of oocyte retrieval methods. The miniature robots exhibit exceptional accuracy and manipulation abilities at the micro/nano scale, enabling less invasive and extremely precise retrieval procedures. By using micro/nano robots, physicians can maneuver through the complex components of the reproductive system with improved skill, reaching oocytes with minimal tissue damage and greatly decreasing patient discomfort. This novel method signifies a notable progression in ART, offering better results and improved patient experiences during oocyte retrieval procedures.
Advances in micro/nano robot-assisted oocyte retrieval have attracted scientific interest and have been published in scholarly journals [
31,
45,
46,
47]. A work by Zhau et al. (2021) [
46] in the Journal of Chemical Reviews demonstrates the effective use of magnetic nanorobots for precise oocyte retrieval. This study demonstrates how micro/nano robots have the potential to significantly improve current ART techniques by providing increased precision, efficiency, and patient comfort during oocyte retrieval procedures. This research highlights the significant impact of micro/nano robotic technology on enhancing ART, leading to better patient outcomes and experiences.
A review article was published by Sitti et al. [
48] in 2019 on biohybrid microrobots for oocyte retrieval. This study emphasizes the development of microrobots capable of navigating the fallopian tubes to retrieve oocytes with high precision and efficiency. The microrobots, propelled by biological motors, exhibit enhanced maneuverability within the intricate reproductive anatomy, ensuring targeted oocyte retrieval while minimizing tissue trauma. These findings underscore the potential of micro/nano robots to redefine the landscape of oocyte retrieval in ART, offering novel solutions for addressing challenges associated with conventional retrieval techniques [
45,
49]. Through such innovations, micro/nano robots hold promise for enhancing the efficacy and patient satisfaction in ART procedures, paving the way for future advancements in reproductive medicine.
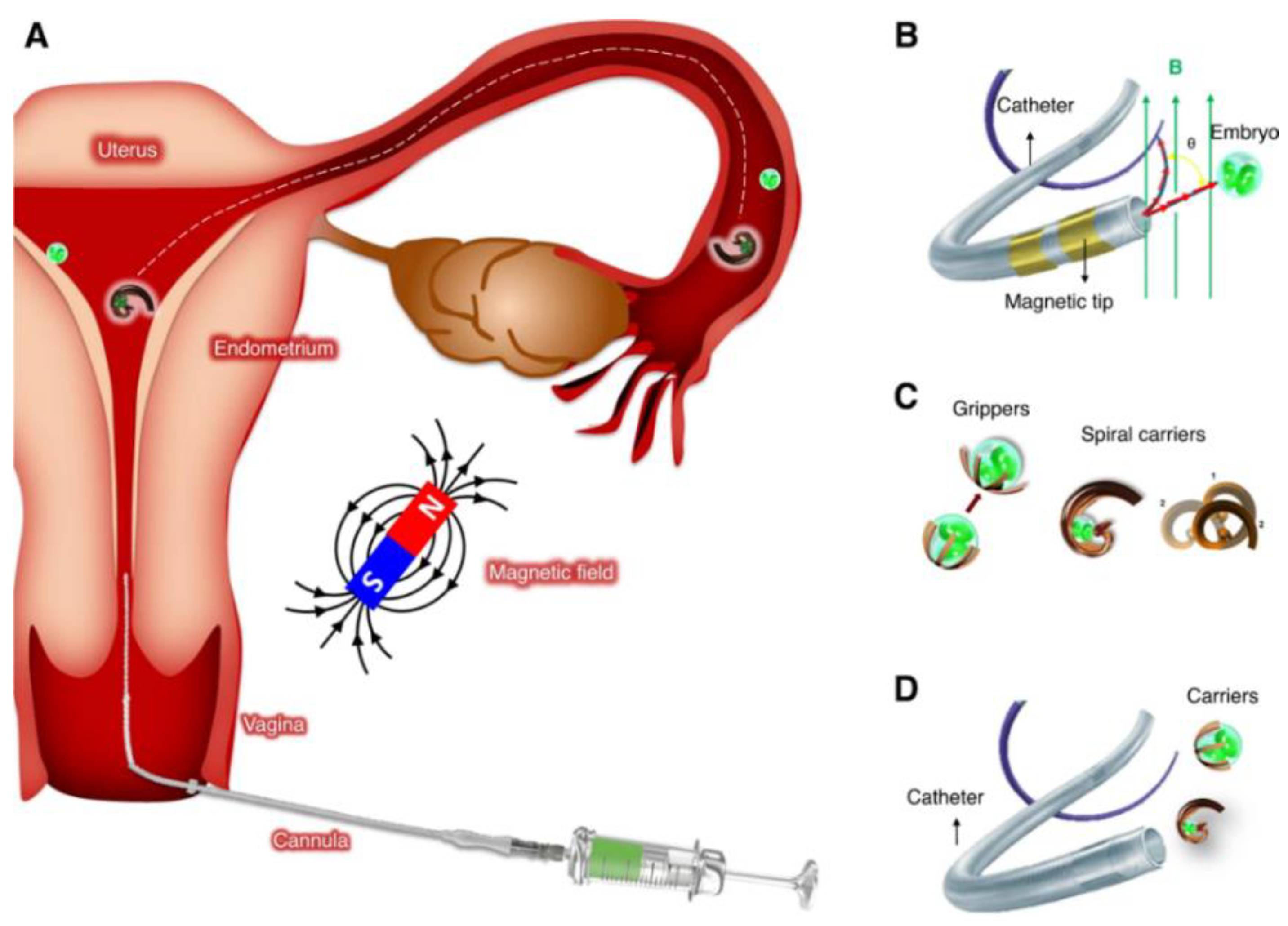
Furthermore, the integration of micro/nano robots in oocyte retrieval procedures has also been explored by Medina-Sánchez et al. (2023) [
7] published in Nature communication (see
Figure 4). The article delves into the developing topic of medical microrobotics, which aims to achieve non-invasive diagnosis and treatment inside the human body by utilizing small sensors and actuators. The article explored the possible uses of these technologies in assisted reproduction, namely in facilitating ART in in-vivo and improving embryo implantation. The article also discussed a case study demonstrating a possible intervention for repeated embryo implantation failure. It involves employing magnetically controlled microrobots to non-invasively bring an early embryo or an oocyte back to the fertilization site. Exposing the embryo to secretory oviduct fluid allows it to develop naturally and coordinate with the formation of the endometrium. The article describes different microrobot designs, focusing on material selection and fabrication techniques, with the goal of moving from laboratory testing to animal investigations and, ultimately, human treatment. The paper discussed the regulatory and ethical issues related to the clinical application of microrobotics in assisted reproduction, emphasizing the importance of thorough review and supervision as these technologies progress towards clinical adoption.
An important advancement in ART involves incorporating micro/nano robots into oocyte retrieval procedures. This offers a practical way to enhance both the precision of the process and the health of the patient. By integrating micro robots into the intricate procedure of oocyte extraction, researchers want to improve the efficacy of reproductive treatments and enhance the efficiency of current technology. This novel method in reproductive medicine focuses on the ongoing research and development of micro and nano robotic technologies. Recent studies in micro and nano robotic technology could greatly influence ART by improving the results and experiences of those receiving reproductive treatments. Scientists are working to improve the capabilities of these mini robots to better fulfill the needs of patients and medical professionals. Advancements in micro and nanorobotics in ART are enhancing procedure precision and patient happiness, resulting in more effective and personalized fertility treatments.
Overall, the incorporation of micro and nano robots into oocyte retrieval methods is a significant technological innovation in the field of ART. By incorporating innovative methods to enhance outcomes and improve the patient's journey in reproductive medicine, it is expected that advancements in this field would greatly influence the future of fertility treatments during their use.
2.4. Advancements in Embryo Culture
The integration of micro/nano robotics has brought about a new level of complexity in embryo culture techniques, leading to a significant change in the field of ART. Researchers can optimize embryo growing circumstances using advanced robotic equipment. These technologies offer researchers accurate control and manipulation abilities at the cellular level. Embryos can now get customized conditions to support their growth and development thanks to this breakthrough, which has led to the creation of microfluidic devices that can replicate the intricate microenvironments found in living organisms. Research, like the one conducted by Chan et al. in 2018 [
50], has demonstrated that microfluidic devices are successful in maintaining consistent culture conditions for embryos. This is achieved by meticulously controlling factors such as oxygen levels, nutritional gradients, and fluid flow dynamics.
The use of micro and nano robotics in embryo culture techniques has led to notable improvements in ART therapies, particularly in procedures like IVF and embryo transfer. Micro and nano scale robotics enhance embryo viability in assisted reproductive technology procedures by maintaining regular and stable culture conditions, resulting in increased success rates. Several research groups, such as those led by Li et al. and Wang et al., [
51,
52] have studied the use of microfluidic-based on-chip embryo culture. This research highlights the diverse capabilities and possibilities of micro/nano robots in improving embryo culture conditions. These findings represent a significant shift in ART, offering new possibilities for enhancing embryo development and ultimately improving the likelihood of successful conception for individuals and couples undergoing fertility treatments.
Micro/nano robotics could significantly impact reproductive medicine by providing precise and flexible control over the laboratory environment. For researchers to enhance their comprehension of the factors affecting embryo development and improve ART procedures, they need to create specialized environments for embryos that closely mimic natural physiological conditions. The integration of micro and nano robotics into embryo culture techniques has the potential to improve embryo viability and implantation, advancing ART and offering hope to individuals and couples struggling with infertility. Advancements in embryo culture techniques utilizing micro and nano robotics may be developed as research in this sector progresses. These advancements will greatly influence the future of assisted reproduction and reproductive treatments.
Furthermore, micro/nano robotics facilitate non-invasive monitoring and analysis of embryo development in real-time, offering insights into embryonic behavior and viability without disturbing the delicate developmental process. For instance, microfluidic devices integrated with sensors and imaging systems enable continuous monitoring of embryo morphology, metabolism, and gene expression patterns throughout the culture period Fang et al., 2023 [
32] and Camarillo et al., 2019 [
53]. Fang et al. [
32] conducted a comprehensive analysis on the most current breakthroughs in microfluidics technology that have the potential to be used at various phases of embryo development, such as sperm sorting and embryo vitrification. The authors noted how advances in alternative materials, organ-on-a-chip technology, functional units for integrated microfluidic systems, and 3D printing have accelerated the development of innovative microfluidic devices. The researchers emphasized the ability of a single microfluidic platform to integrate oocyte processing, sperm processing, and embryo culture, reducing multiple arduous laboratory procedures to a compact and effective system. The goal of researchers is to increase the effectiveness and accuracy of assisted reproductive procedures by integrating them into a single system. This integration would eliminate the requirement for human effort and smooth out disparities in the outcomes. The paper investigates the current applications of microfluidic technologies in IVF approaches, addressing the challenges and opportunities that accompany these advancements. Academics are using microfluidics to address challenges in ART therapies, such as uneven embryo quality and high costs, by creating a more regulated and customized embryonic environment. The review emphasizes microfluidics' potential to change ART procedures, allowing for more accessible and effective fertility treatments. Microfluidic technologies offer the potential to boost pregnancy rates while decreasing the chance of implantation failure or miscarriage by allowing embryologists to assess embryo quality and choose the most viable embryos for transfer.
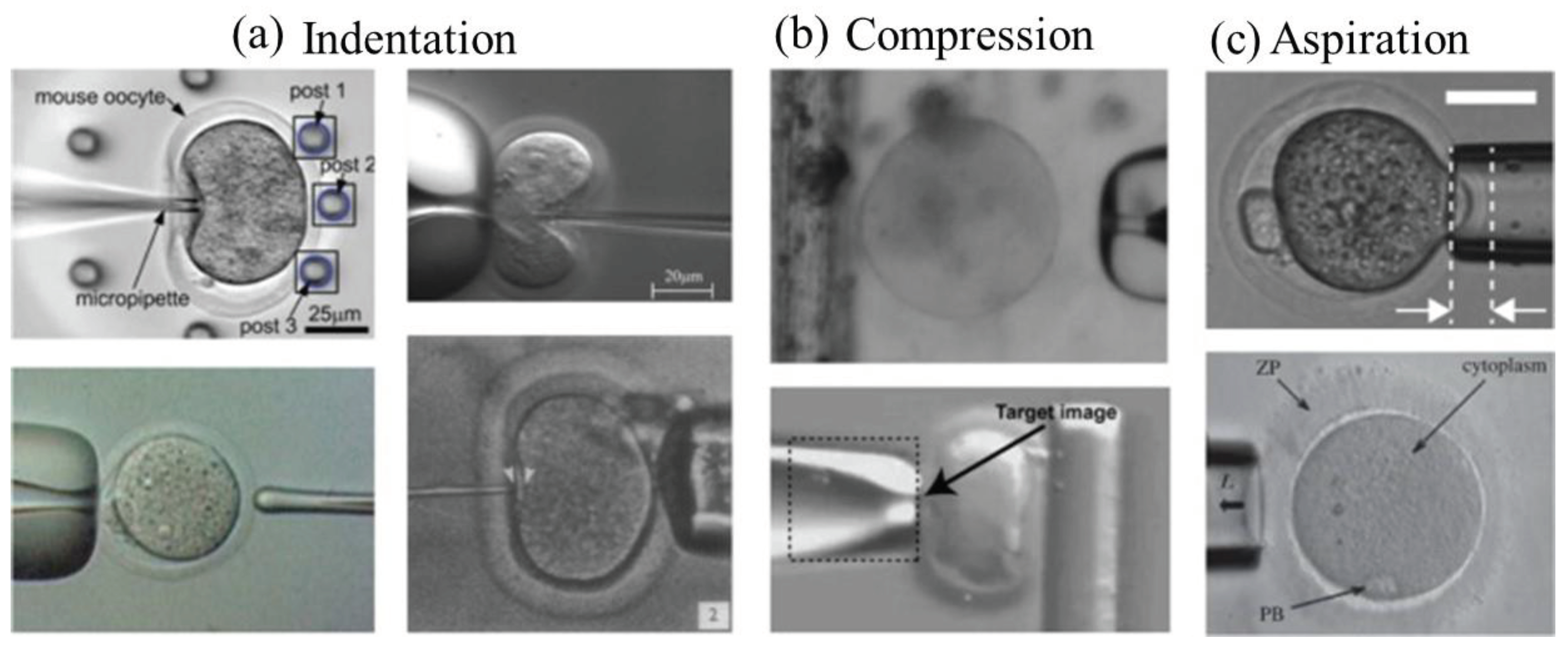
In early 2019, Camerillo et al. , [
53] investigated the application of microfluidic technologies to evaluate the biomechanical characteristics of oocytes and embryos with the goal of improving outcomes in ART. It explores how microfluidic platforms provide distinct capacities for examining the biomechanics of reproductive cells, such as oocytes and embryos, which are essential for their development and survival. The review emphasizes the significance of comprehending the biomechanical characteristics of these cells and their impact on several phases of the ART process, including fertilization, embryo growth, and implantation. The article explores how microfluidic-based biomechanical analysis can enhance the selection of high-quality oocytes and embryos for ART procedures, ultimately improving the success rates of reproductive treatments. The article by Camerillo et al., [
53] also discusses a range of microfluidic methods and tools created to assess biomechanical characteristics such cell stiffness, deformability, and adhesion strength, offering understanding into their structural and functional health (See
Figure 5). The review discusses obstacles and future paths in microfluidic-based biomechanical analysis of reproductive cells, emphasizing its ability to advance Assisted Reproductive Technology (ART) and enhance results for people and couples seeking fertility treatments.
Additionally, the integration of micro/nano robotics with artificial intelligence (AI) and machine learning algorithms represents a significant advancement in ART, particularly in the embryo assessment and selection process. combining micro/nano robots with AI and machine learning algorithms is a notable progression in ART, especially in the evaluation and selection of embryos [
35,
44,
55]. Embryologists can utilize a combination of robotics and AI systems to analyze extensive data obtained from microfluidic platforms and imaging technologies in order to enhance their understanding of embryo quality and developmental capacity. By doing advanced analysis, AI algorithms can detect intricate patterns and biomarkers linked to favorable pregnancy results, improving the precision of embryo evaluation.
AI-driven systems offer prediction models and decision-support tools to embryologists, enabling them to make more educated and tailored decisions about selecting embryos. Micro/nano robotics can enhance the embryo selection process in ART treatments by employing machine learning algorithms, hence increasing the likelihood of successful pregnancy outcomes. Integrating robots and AI improves embryo evaluation efficiency, advancing personalized medicine in reproductive healthcare by tailoring treatment decisions to specific patient features and needs.
2.5. Benefits, and Challenges
Micro/nano robotics have led to substantial breakthroughs in the field of IVF, bringing about a notable revolution in treatment procedures. These advanced devices provide accurate control of microenvironments, transforming the processes of choosing and handling embryos. Researchers may possibly now use micro/nano robotics to precisely manipulate the circumstances for embryo growth, controlling factors like temperature, pH, and nutrition availability at the cellular level. The precise regulation described in Nordhoff et al.'s (2019) [
14,
56] study enhances embryo growth, leading to greater embryo quality and higher success rates in IVF treatments.
Furthermore, the use of micro/nano robotics allows for real-time monitoring and manipulation of embryos, resulting in improved results in IVF. Advanced microfluidic devices with integrated sensors allow for ongoing monitoring of embryo development parameters, enabling immediate modifications when necessary [
57]. This real-time monitoring feature improves embryo selection, guaranteeing the transfer of the most viable embryos and ultimately resulting in improved pregnancy results. Although there has been significant development, the integration of micro/nano robots in IVF is faced with significant problems that need to be resolved.
Developing and integrating new technology in healthcare settings is challenging due to its complexity and cost, which hinder mainstream adoption. It is crucial to prioritize the safety and dependability of micro/nano robotic systems to avoid any possible harm to embryos and patients. Researchers and doctors encounter challenges that are worsened by regulatory factors and ethical issues associated with the use of these technologies [
9,
58,
59]. To address these problems, it is essential to utilize interdisciplinary teamwork and rigorous quality control measures to incorporate micro/nano robots safely and efficiently into IVF facilities. To maximize the potential of micro/nano robotics in enhancing IVF procedures and ensuring safety and efficacy for patients undergoing reproductive therapies, it is crucial to dedicate concentrated and meticulous efforts. IVF clinics can improve their operations and increase success rates for individuals undergoing reproductive treatments by overcoming hurdles and utilizing the advantages of micro/nano robotics.
3. Future Directions
Looking towards the future, micro/nano robotics stand poised to transform assisted reproductive technologies, promising to enhance the efficiency and success rates of these treatments. Continued advancements in micro/nano robotics technology, characterized by miniaturization, integration of sensors and actuators, and automation, hold the key to unlocking more precise and efficient embryo manipulation and culture processes. The ongoing evolution of these robotics systems will enable researchers and clinicians to exert greater control over microenvironments within which embryos develop, optimizing conditions for embryo growth and maturation. This heightened level of precision in embryo manipulation has the potential to significantly improve the overall success rates of assisted reproductive technologies, offering renewed hope to individuals and couples grappling with fertility challenges.
The integration of AI and machine learning algorithms represents a notable shift in the process of selecting embryos and forecasting pregnancy outcomes based on embryo features. AI systems can identify complex patterns and biomarkers associated with embryo quality and developmental potential by analyzing data from microfluidic devices, imaging technologies, and genetic studies [
44,
60,
61,
62,
63]. Prediction models and AI-driven decision-support systems let embryologists make more educated and personalized decisions when selecting embryos. The integration of micro/nano robotics with AI technology has the potential to significantly enhance the effectiveness of assisted reproductive technologies, leading to improved pregnancy outcomes for individuals undergoing fertility treatments.
Furthermore, current studies in tissue engineering and organ-on-a-chip technologies are set to enhance the capacities of microfluidic systems utilized in assisted reproductive technologies [
18,
34,
37,
51,
57]. Sophisticated microfluidic technologies have the potential to greatly enhance embryo culture settings in-vitro by closely replicating the intricate in vivo microenvironments where embryos normally develop. Advanced microfluidic devices can enhance reproductive results in IVF treatments by creating a more biologically accurate environment for embryo growth. The combination of micro/nano robotics with tissue engineering and organ-on-a-chip technologies shows great potential for enhancing assisted reproductive technologies. This integration could lead to improved success rates and overall experience for individuals and couples seeking fertility treatments.
3.1. Summary of our current research pursuits:
Our earlier research was primarily focused on automating the intricate 3D rotation process of individual oocytes through the innovative electrorotation method [
64]. By meticulously generating a precise 3D electro-rotational field within a specialized microdevice, we achieved the advancement in rotating single bovine oocytes with unparalleled precision. Moreover, our study introduced a significant approach for evaluating cell health by analyzing rotation spectra, thus contributing valuable insights to the field [
64].
Following our work on a single oocyte manipulation, our research trajectory shifted towards the manipulation and fabrication of magnetic bead swimmers that closely mimic the characteristics of sperm cells. This endeavor enabled us to achieve precise control over small particles and adeptly transport them to predefined target regions [
65,
66]. This phase of our research marked a significant advancement in the realm of microscale robotics and targeted delivery systems, with wide-ranging implications for various biomedical applications.
Presently, our research endeavors are directed towards evaluating the efficacy of microswimmers or micro/nano robotic technology in targeting rotatable cells within 3D environments. Leveraging cutting-edge image processing and AI technologies, we aim to streamline and enhance the efficiency of high-throughput automation in this domain. Ultimately, our ongoing efforts strive to make substantial contributions to the fields of IVF and ART, promising transformative advancements in reproductive healthcare.
4. Conclusions
Micro/nanorobotics are ready to revolutionize IVF by improving the levels of precision, safety, and effectiveness in assisted reproductive technologies. Advanced technology can transform different areas of IVF such as focused sperm manipulation, less invasive oocyte retrieval, and precision embryo culture. This has the potential to improve IVF success rates and enhance patient outcomes. Current research and development in micro/nanorobotics are crucial for overcoming hurdles and preparing for the widespread use of micro/nanorobotic-assisted IVF in clinical settings, leading to advancements in reproductive healthcare. Progress in micro/nanorobotics can improve current IVF methods and introduce new approaches to boost the efficiency and success of assisted reproductive technologies. Researchers are advancing micro/nanorobotic systems for use in IVF, aiming to enhance outcomes for individuals and couples undergoing fertility treatments.
Author Contributions
Conceptualization, P.B.; methodology, P.B.; software, P.B.; validation, P.B.; formal analysis, P.B.; investigation, P.B.; resources, P.B.; data curation, P.B.; writing—original draft preparation, P.B.; writing—review and editing, P.B.; visualization, P.B.; supervision, P.B.; project administration, P.B. All authors have read and agreed to the published version of the manuscript
Funding
This research received no external funding.
Acknowledgments
The author would like to acknowledge Prof. James Geoff Chase, and Georgina Lv for guidance and support in writing this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lazzari, E., et al., Projecting the Contribution of Assisted Reproductive Technology to Completed Cohort Fertility. Popul Res Policy Rev, 2023. 42(1): p. 6. [CrossRef]
- Chiware, T.M., et al., IVF and other ART in low- and middle-income countries: a systematic landscape analysis. Hum Reprod Update, 2021. 27(2): p. 213-228. [CrossRef]
- Chambers, G.M., et al., The economic impact of assisted reproductive technology: a review of selected developed countries. Fertil Steril, 2009. 91(6): p. 2281-94. [CrossRef]
- Eskew, A.M. and E.S. Jungheim, A History of Developments to Improve in vitro Fertilization. Mo Med, 2017. 114(3): p. 156-159.
- Silver, R.I., et al., In vitro fertilization is associated with an increased risk of hypospadias. J Urol, 1999. 161(6): p. 1954-7.
- Braude, P. and M. Johnson, Reflections on 40 years of IVF. BJOG: An International Journal of Obstetrics & Gynaecology, 2019. 126(2): p. 135-137. [CrossRef]
- Nauber, R., et al., Medical microrobots in reproductive medicine from the bench to the clinic. Nat Commun, 2023. 14(1): p. 728. [CrossRef]
- Wang, J. and M.V. Sauer, In vitro fertilization (IVF): a review of 3 decades of clinical innovation and technological advancement. Ther Clin Risk Manag, 2006. 2(4): p. 355-64. [CrossRef]
- Asplund, K., Use of in vitro fertilization-ethical issues. Ups J Med Sci, 2020. 125(2): p. 192-199. [CrossRef]
- Wheeler, M.B., E.M. Walters, and D.J. Beebe, Toward culture of single gametes: The development of microfluidic platforms for assisted reproduction. Theriogenology, 2007. 68: p. S178-S189. [CrossRef]
- Ferraz, M. and G.A. Ferronato, Opportunities involving microfluidics and 3D culture systems to the in vitro embryo production. Anim Reprod, 2023. 20(2): p. e20230058. [CrossRef]
- Alias, A.B., H.Y. Huang, and D.J. Yao, A Review on Microfluidics: An Aid to Assisted Reproductive Technology. Molecules, 2021. 26(14). [CrossRef]
- Yan, J., et al., Revolutionizing the female reproductive system research using microfluidic chip platform. Journal of Nanobiotechnology, 2023. 21(1): p. 490. [CrossRef]
- Le Gac, S. and V. Nordhoff, Microfluidics for mammalian embryo culture and selection: where do we stand now? Molecular Human Reproduction, 2016. 23(4): p. 213-226. [CrossRef]
- Karcz, A., et al., Development of a Microfluidic Chip Powered by EWOD for In Vitro Manipulation of Bovine Embryos. Biosensors, 2023. 13(4): p. 419. [CrossRef]
- Karcz, A., et al., Electrically-driven handling of gametes and embryos: taking a step towards the future of ARTs. Lab on a Chip, 2022. 22(10): p. 1852-1875. [CrossRef]
- Ferraz, M.A.M.M., et al., An oviduct-on-a-chip provides an enhanced in vitro environment for zygote genome reprogramming. Nature Communications, 2018. 9(1): p. 4934. [CrossRef]
- Kashaninejad, N., M.J.A. Shiddiky, and N.-T. Nguyen, Advances in Microfluidics-Based Assisted Reproductive Technology: From Sperm Sorter to Reproductive System-on-a-Chip. Advanced Biosystems, 2018. 2(3): p. 1700197. [CrossRef]
- Singh, A.V., et al., Sperm Cell Driven Microrobots-Emerging Opportunities and Challenges for Biologically Inspired Robotic Design. Micromachines (Basel), 2020. 11(4). [CrossRef]
- Ullrich, F., et al., Mobility Experiments With Microrobots for Minimally Invasive Intraocular Surgery. Investigative Ophthalmology & Visual Science, 2013. 54(4): p. 2853-2863. [CrossRef]
- Durfey, C.L., et al., Nanotechnology-based approach for safer enrichment of semen with best spermatozoa. Journal of Animal Science and Biotechnology, 2019. 10(1): p. 14. [CrossRef]
- Xu, H., et al., Sperm-Hybrid Micromotor for Targeted Drug Delivery. ACS Nano, 2018. 12(1): p. 327-337. [CrossRef]
- Lin, Z., et al., Magnetically Actuated Peanut Colloid Motors for Cell Manipulation and Patterning. ACS Nano, 2018. 12(3): p. 2539-2545. [CrossRef]
- Soto, F. and R. Chrostowski, Frontiers of Medical Micro/Nanorobotics: in vivo Applications and Commercialization Perspectives Toward Clinical Uses. Front Bioeng Biotechnol, 2018. 6: p. 170. [CrossRef]
- Beladi-Mousavi, S.M., et al., Recoverable Bismuth-Based Microrobots: Capture, Transport, and On-Demand Release of Heavy Metals and an Anticancer Drug in Confined Spaces. ACS Appl Mater Interfaces, 2019. 11(14): p. 13359-13369. [CrossRef]
- Li, Z., et al., Self-sensing intelligent microrobots for noninvasive and wireless monitoring systems. Microsystems & Nanoengineering, 2023. 9(1): p. 102. [CrossRef]
- Yesin, K.B., K. Vollmers, and B.J. Nelson. Analysis and design of wireless magnetically guided microrobots in body fluids. in IEEE International Conference on Robotics and Automation, 2004. Proceedings. ICRA '04. 2004. 2004. [CrossRef]
- Sitti, M., et al., Biomedical Applications of Untethered Mobile Milli/Microrobots. Proceedings of the IEEE, 2015. 103(2): p. 205-224. [CrossRef]
- Hou, Y., et al., Design and Control of a Surface-Dimple-Optimized Helical Microdrill for Motions in High-Viscosity Fluids. IEEE/ASME Transactions on Mechatronics, 2023. 28(1): p. 429-439. [CrossRef]
- Celi, N., D. Gong, and J. Cai, Artificial flexible sperm-like nanorobot based on self-assembly and its bidirectional propulsion in precessing magnetic fields. Scientific Reports, 2021. 11(1): p. 21728. [CrossRef]
- Feng, L., et al., On-chip microfluid induced by oscillation of microrobot for noncontact cell transportation. Applied Physics Letters, 2017. 111(20). [CrossRef]
- Fang, Y., et al., Microfluidic in-vitro fertilization technologies: Transforming the future of human reproduction. TrAC Trends in Analytical Chemistry, 2023. 160: p. 116959. [CrossRef]
- Magdanz, V., et al., IRONSperm: Sperm-templated soft magnetic microrobots. Science Advances, 2020. 6(28): p. eaba5855 . [CrossRef]
- Liu, D., et al., Magnetic Micro/Nanorobots: A New Age in Biomedicines. Advanced Intelligent Systems, 2022. 4(12): p. 2200208. [CrossRef]
- Fernandez, E.I., et al., Artificial intelligence in the IVF laboratory: overview through the application of different types of algorithms for the classification of reproductive data. J Assist Reprod Genet, 2020. 37(10): p. 2359-2376. [CrossRef]
- Xie, H., et al., Reconfigurable magnetic microrobot swarm: Multimode transformation, locomotion, and manipulation. Sci Robot, 2019. 4(28). [CrossRef]
- Cai, X., et al., Ultrasound-Responsive Materials for Drug/Gene Delivery. Front Pharmacol, 2019. 10: p. 1650. [CrossRef]
- Li, J., et al., Micro/Nanorobots for Biomedicine: Delivery, Surgery, Sensing, and Detoxification. Sci Robot, 2017. 2(4). [CrossRef]
- Jiang, J., et al., Control and Autonomy of Microrobots: Recent Progress and Perspective. Advanced Intelligent Systems, 2022. 4(5): p. 2100279. [CrossRef]
- Sun, H.C.M., et al., Magnetically Powered Biodegradable Microswimmers. Micromachines (Basel), 2020. 11(4). [CrossRef]
- Huang, L., et al., Driving modes and characteristics of biomedical micro-robots. Engineered Regeneration, 2023. 4(4): p. 411-426. [CrossRef]
- Wehner, M., et al., An integrated design and fabrication strategy for entirely soft, autonomous robots. Nature, 2016. 536(7617): p. 451-455. [CrossRef]
- Khalil, I.S.M., et al. Sperm-shaped magnetic microrobots: Fabrication using electrospinning, modeling, and characterization. in 2016 IEEE International Conference on Robotics and Automation (ICRA). 2016. [CrossRef]
- Jiang, V.S. and C.L. Bormann, Artificial intelligence in the in vitro fertilization laboratory: a review of advancements over the last decade. Fertil Steril, 2023. 120(1): p. 17-23. [CrossRef]
- Wang, Y., et al., Causes and Effects of Oocyte Retrieval Difficulties: A Retrospective Study of 10,624 Cycles. Front Endocrinol (Lausanne), 2021. 12: p. 564344. [CrossRef]
- Zhou, H., et al., Magnetically Driven Micro and Nanorobots. Chem Rev, 2021. 121(8): p. 4999-5041. [CrossRef]
- Yan, X., et al., Multifunctional biohybrid magnetite microrobots for imaging-guided therapy. Science Robotics, 2017. 2(12): p. eaaq1155 . [CrossRef]
- Alapan, Y., et al., Microrobotics and Microorganisms: Biohybrid Autonomous Cellular Robots. Annual Review of Control, Robotics, and Autonomous Systems, 2019. 2(1): p. 205-230. [CrossRef]
- Rose, B.I., Approaches to oocyte retrieval for advanced reproductive technology cycles planning to utilize in vitro maturation: a review of the many choices to be made. J Assist Reprod Genet, 2014. 31(11): p. 1409-19. [CrossRef]
- Chen, C., et al., Insert-based microfluidics for 3D cell culture with analysis. Anal Bioanal Chem, 2018. 410(12): p. 3025-3035. [CrossRef]
- Li, J., et al., Development of a magnetic microrobot for carrying and delivering targeted cells. Sci Robot, 2018. 3(19). [CrossRef]
- Wang, W., et al., Bubble-based microrobot: Recent progress and future perspective. Sensors and Actuators A: Physical, 2023. 360: p. 114567. [CrossRef]
- Yanez, L.Z. and D.B. Camarillo, Microfluidic analysis of oocyte and embryo biomechanical properties to improve outcomes in assisted reproductive technologies. Mol Hum Reprod, 2017. 23(4): p. 235-247. [CrossRef]
- Liu, X., et al., Elastic and viscoelastic characterization of mouse oocytes using micropipette indentation. Ann Biomed Eng, 2012. 40(10): p. 2122-30. [CrossRef]
- Abdullah, K.A.L., et al., Automation in ART: Paving the Way for the Future of Infertility Treatment. Reproductive Sciences, 2023. 30(4): p. 1006-1016. [CrossRef]
- Le Gac, S. and V. Nordhoff, Microfluidics for mammalian embryo culture and selection: where do we stand now? Mol Hum Reprod, 2017. 23(4): p. 213-226. [CrossRef]
- Sequeira, R.C., et al., Microfluidic Systems for Assisted Reproductive Technologies: Advantages and Potential Applications. Tissue Eng Regen Med, 2020. 17(6): p. 787-800. [CrossRef]
- von Schondorf-Gleicher, A., et al., Revisiting selected ethical aspects of current clinical in vitro fertilization (IVF) practice. J Assist Reprod Genet, 2022. 39(3): p. 591-604. [CrossRef]
- Beers, B.C.v., The Ethics of IVF. Bioethics in Faith and Practice, 2019. [CrossRef]
- Chow, D.J.X., et al., Does artificial intelligence have a role in the IVF clinic? Reprod Fertil, 2021. 2(3): p. C29-c34. [CrossRef]
- Sadeghi, M.R., Will Artificial Intelligence Change the Future of IVF? J Reprod Infertil, 2022. 23(3): p. 139-140. [CrossRef]
- Dimitriadis, I., et al., Artificial intelligence in the embryology laboratory: a review. Reprod Biomed Online, 2022. 44(3): p. 435-448. [CrossRef]
- Letterie, G., Artificial intelligence and assisted reproductive technologies: 2023. Ready for prime time? Or not. Fertility and Sterility, 2023. 120(1): p. 32-37. [CrossRef]
- Benhal, P., et al., AC electric field induced dipole-based on-chip 3D cell rotation. Lab on a Chip, 2014. 14(15): p. 2717-2727. [CrossRef]
- Benhal, P., et al., On-chip testing of the speed of magnetic nano- and micro-particles under a calibrated magnetic gradient. Journal of Magnetism and Magnetic Materials, 2019. 474: p. 187-198. [CrossRef]
- Benhal, P., et al., Propulsion kinematics of achiral microswimmers in viscous fluids. Applied Physics Letters, 2021. 118(20). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).