Submitted:
28 February 2024
Posted:
28 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patient History
2.2. Physical Examination
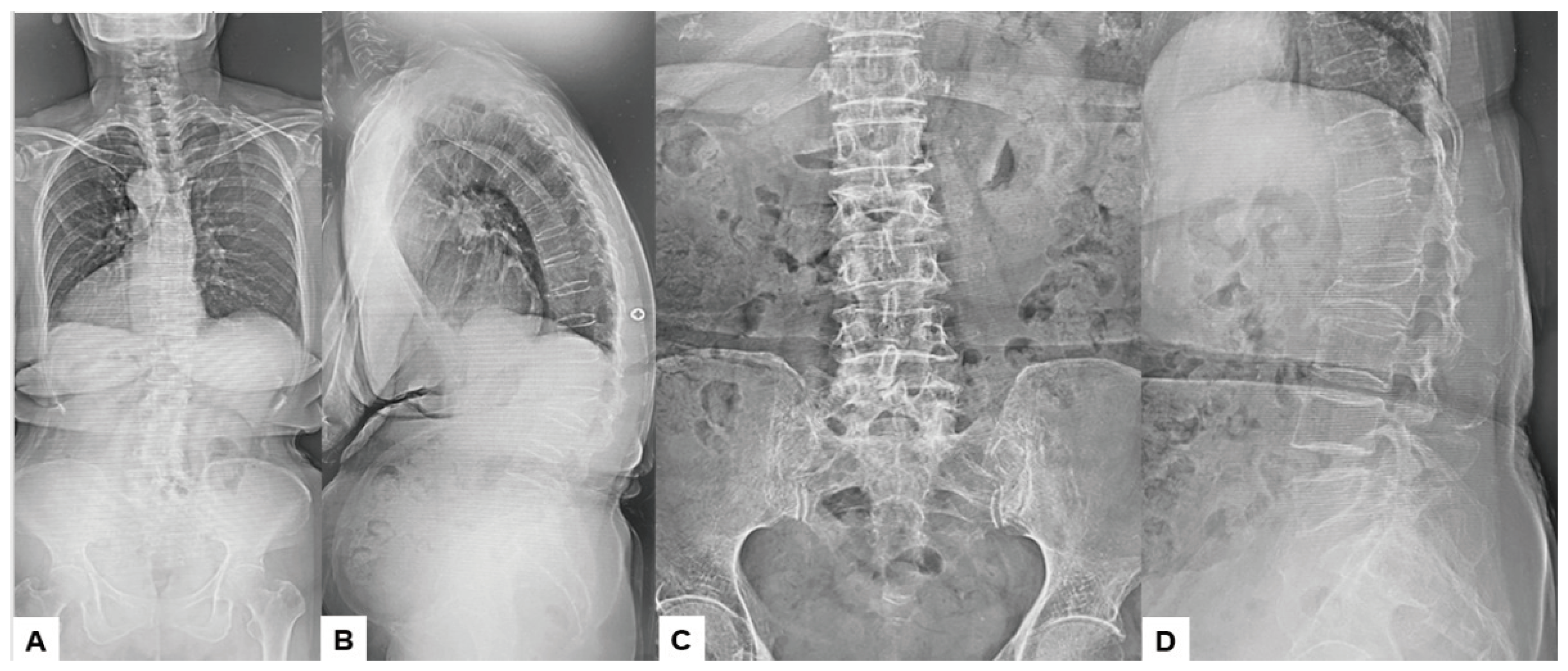
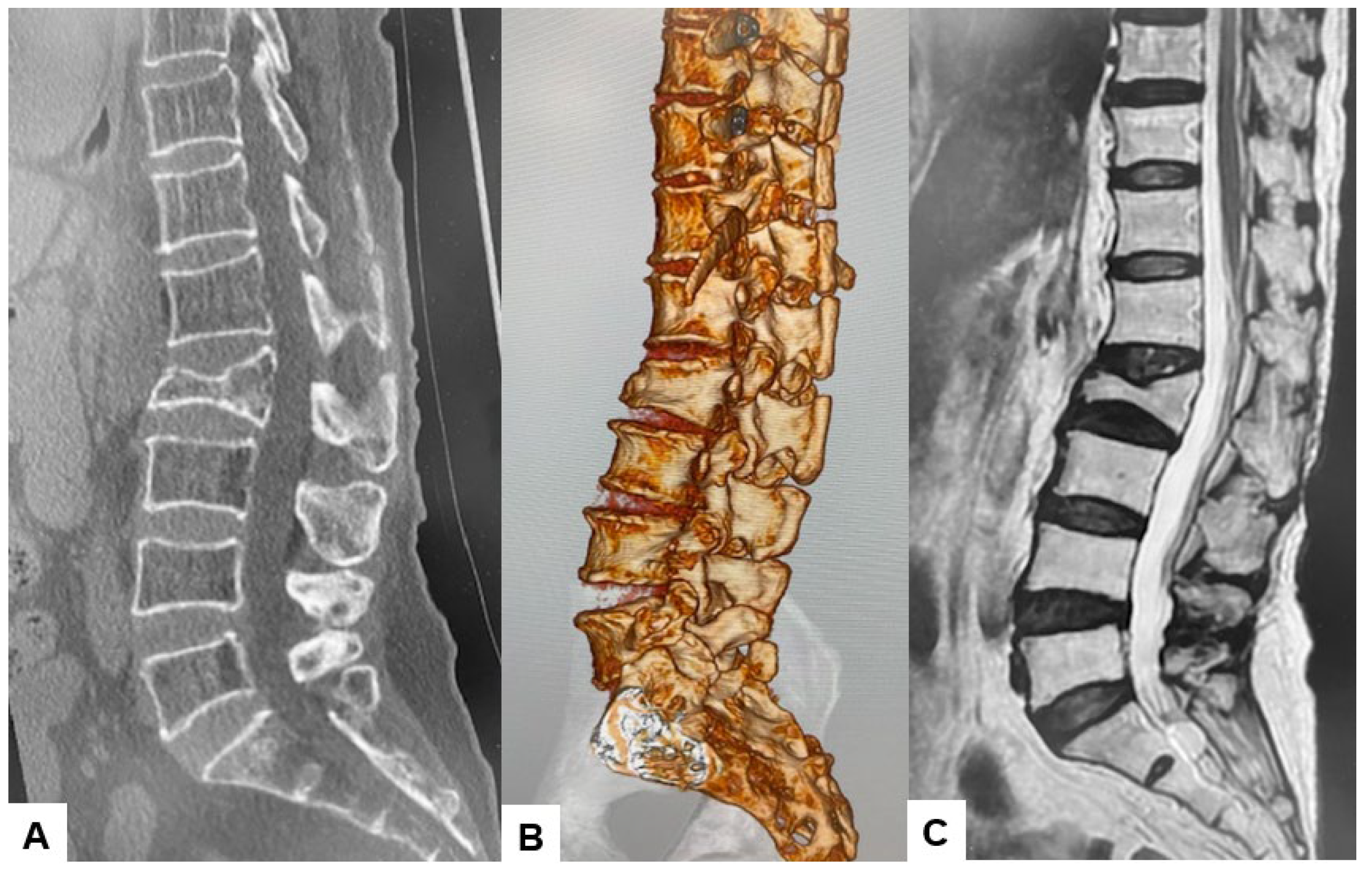
2.3. Preoperative Imaging
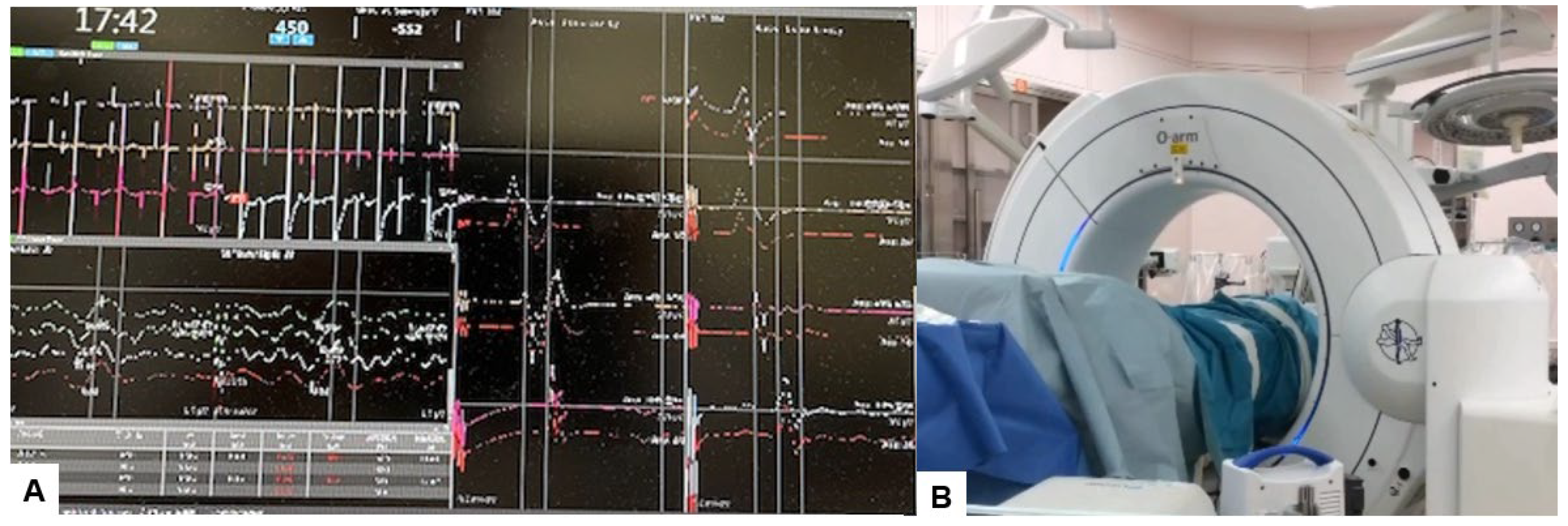
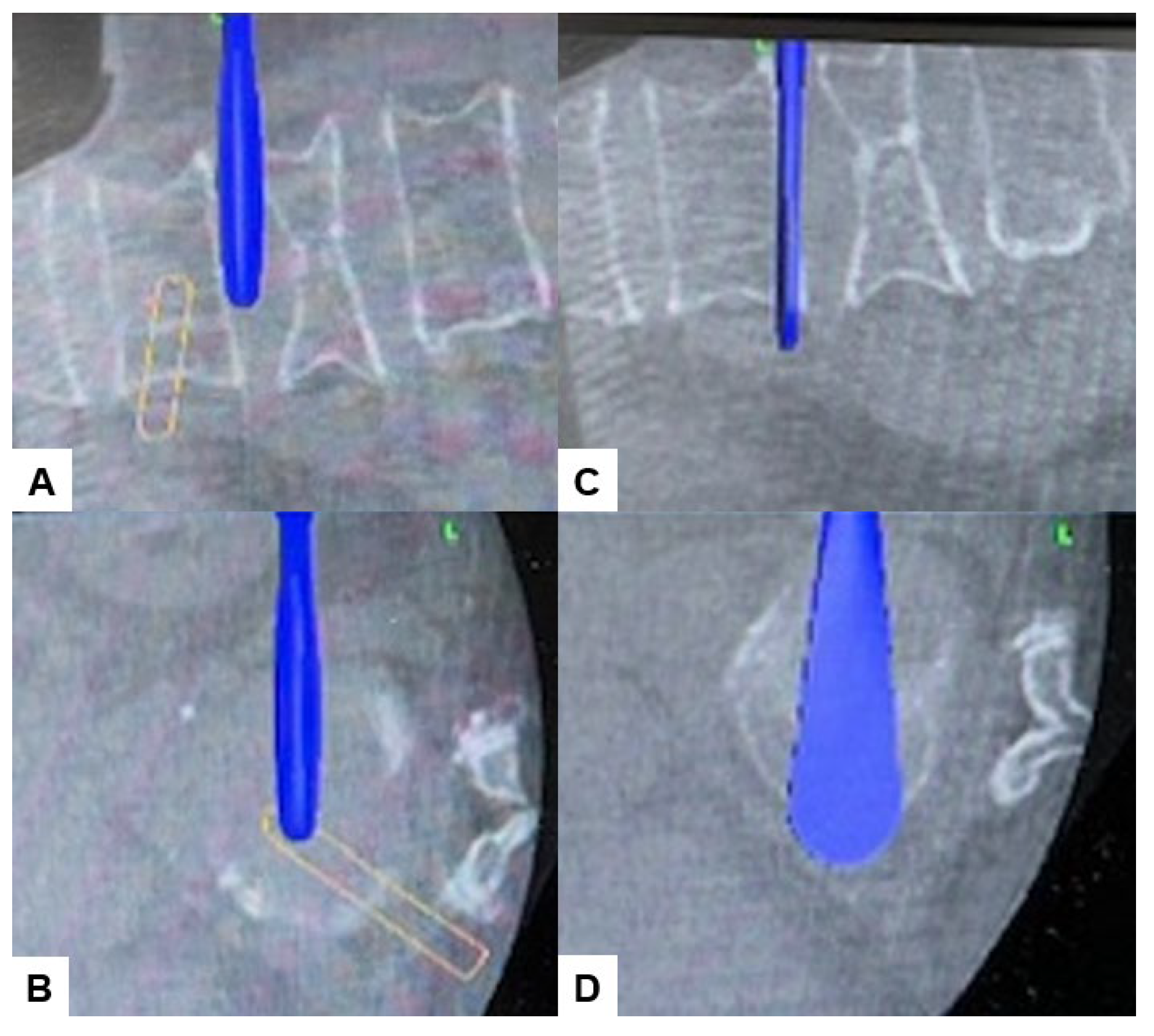
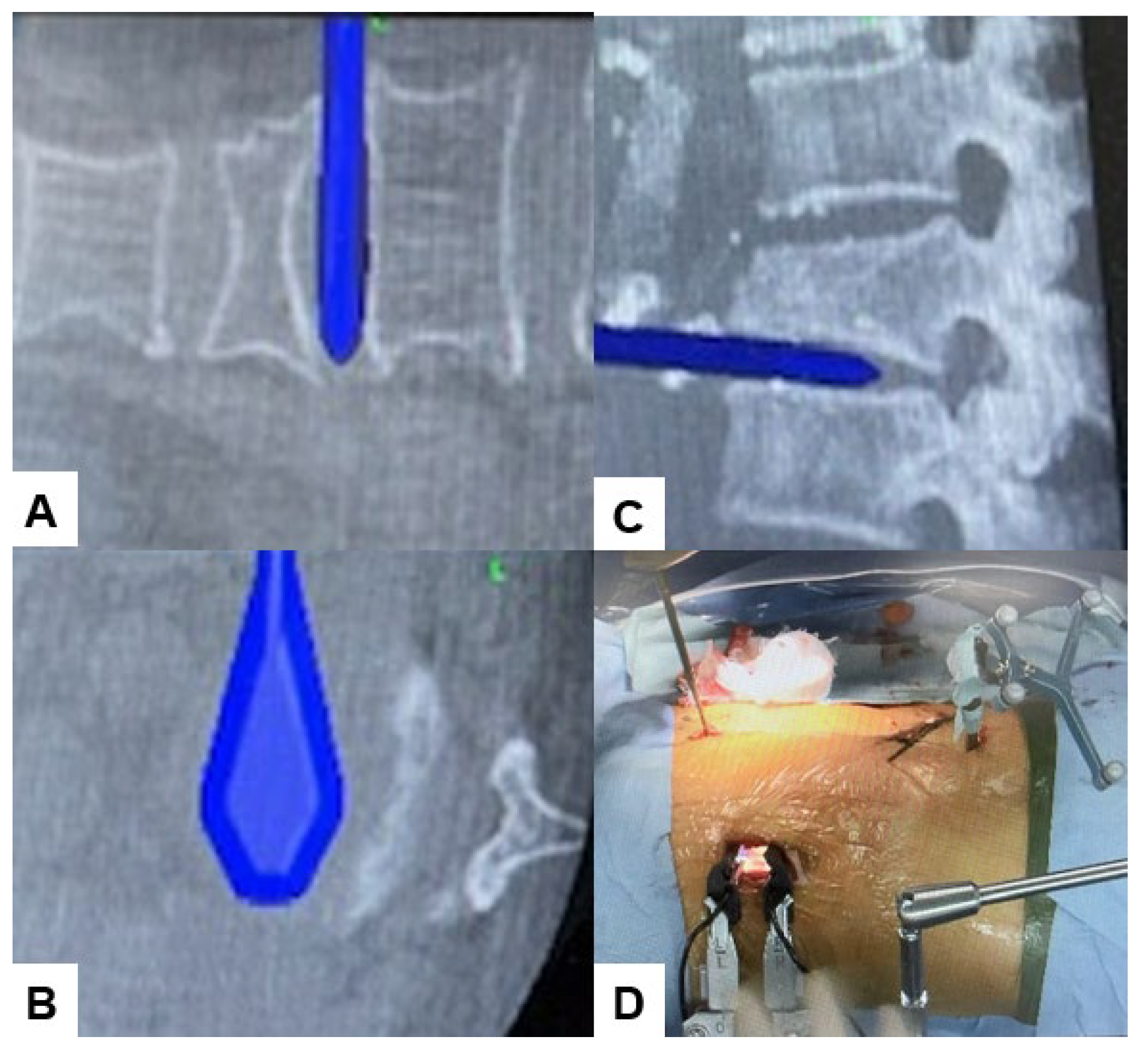
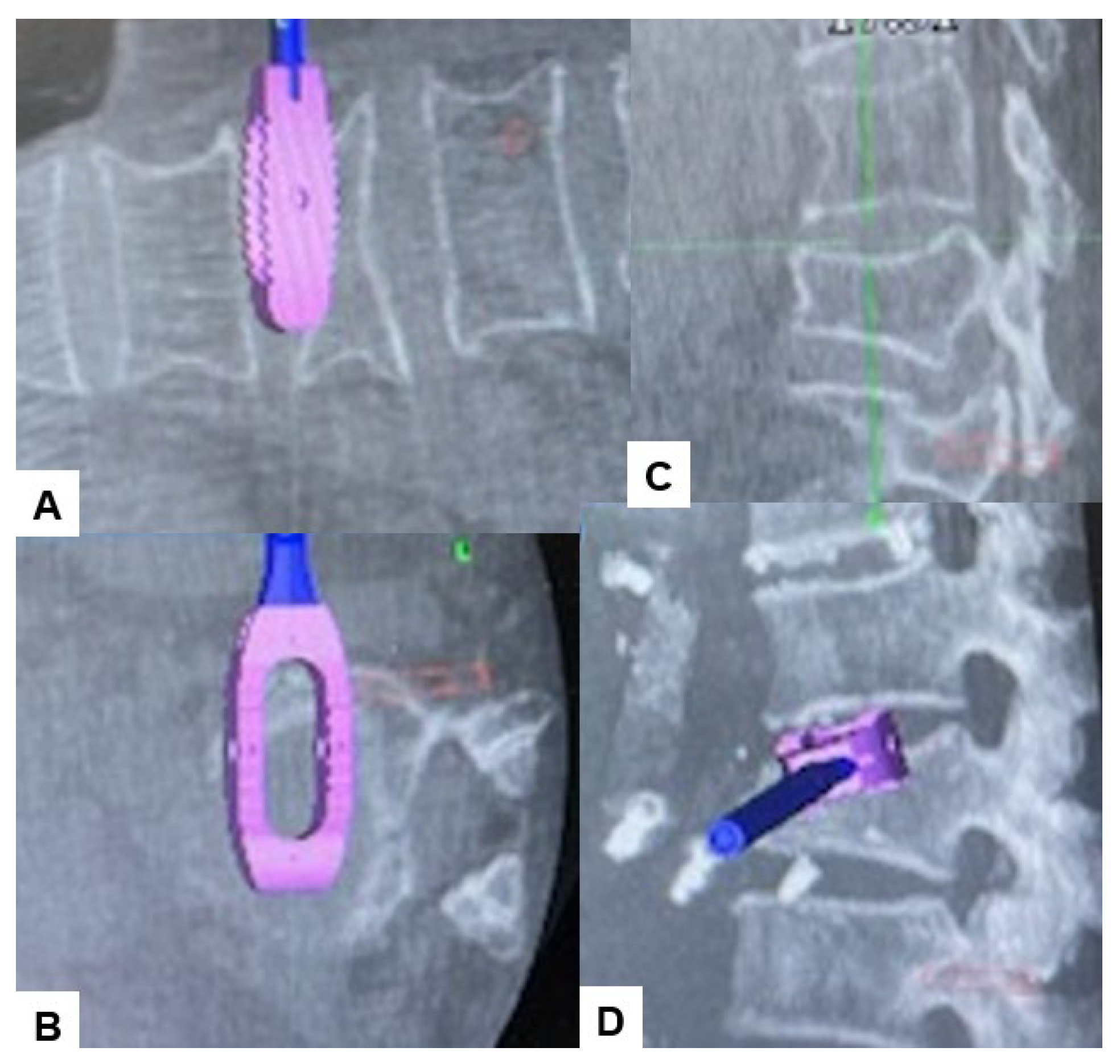
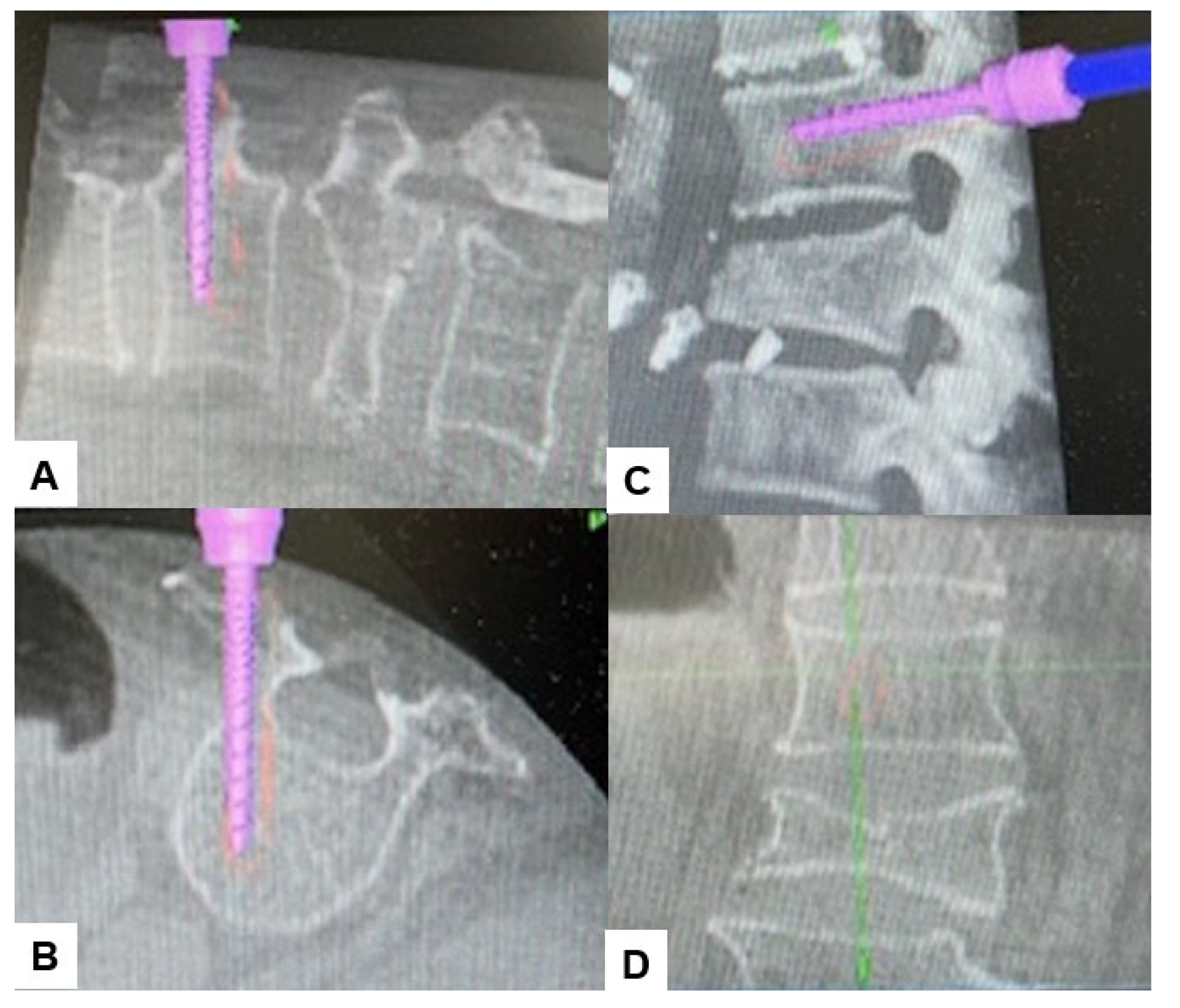
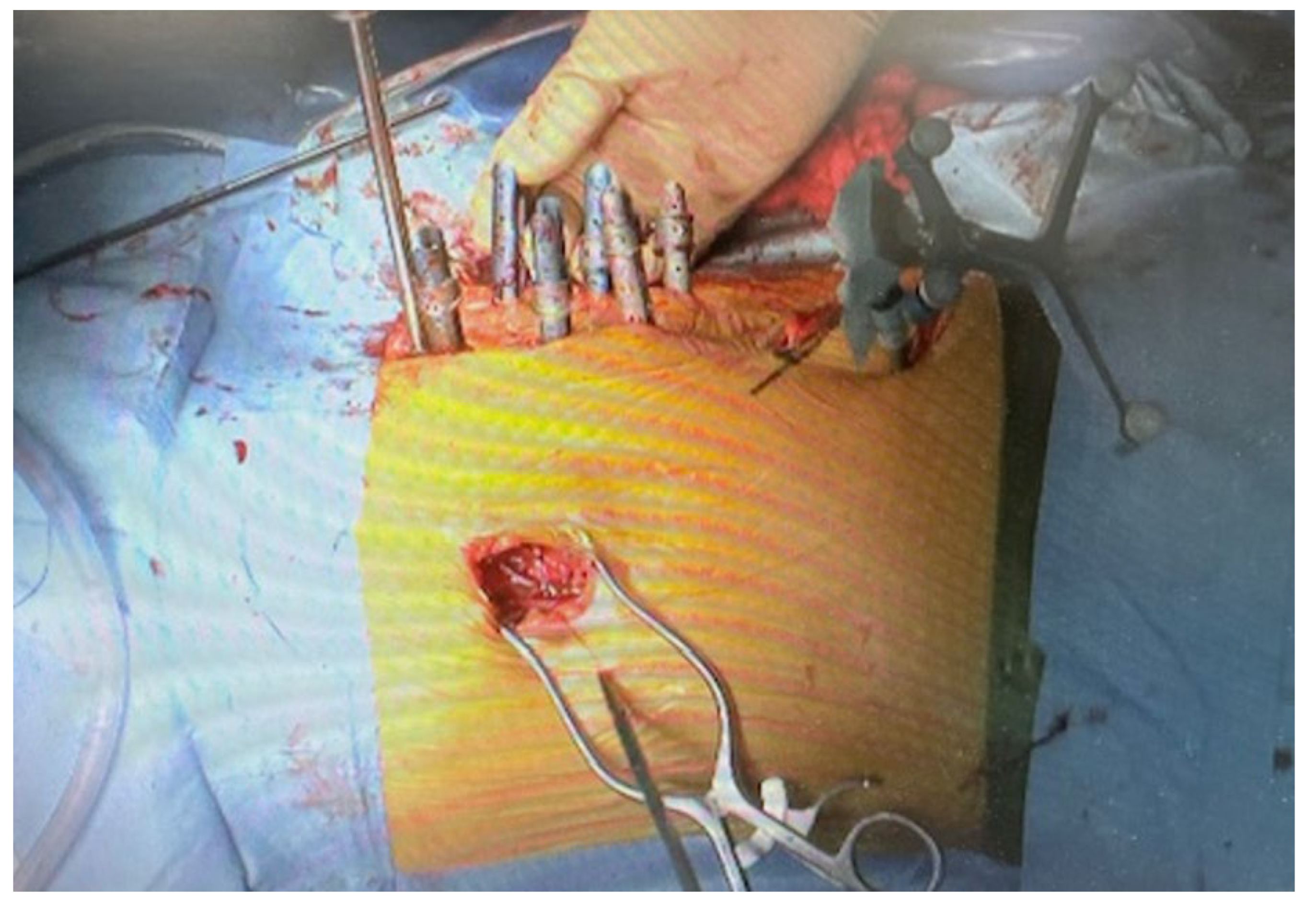
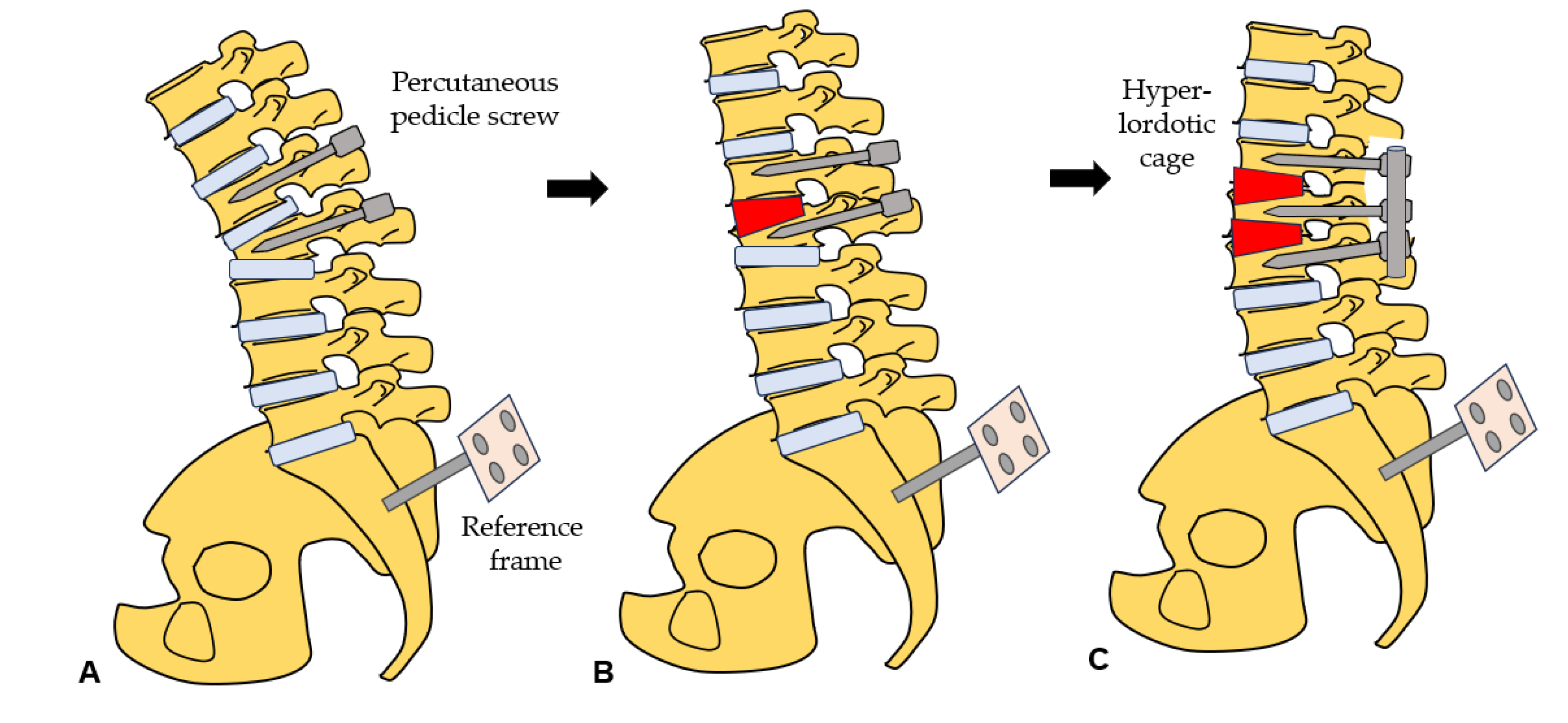
2.4. Surgery
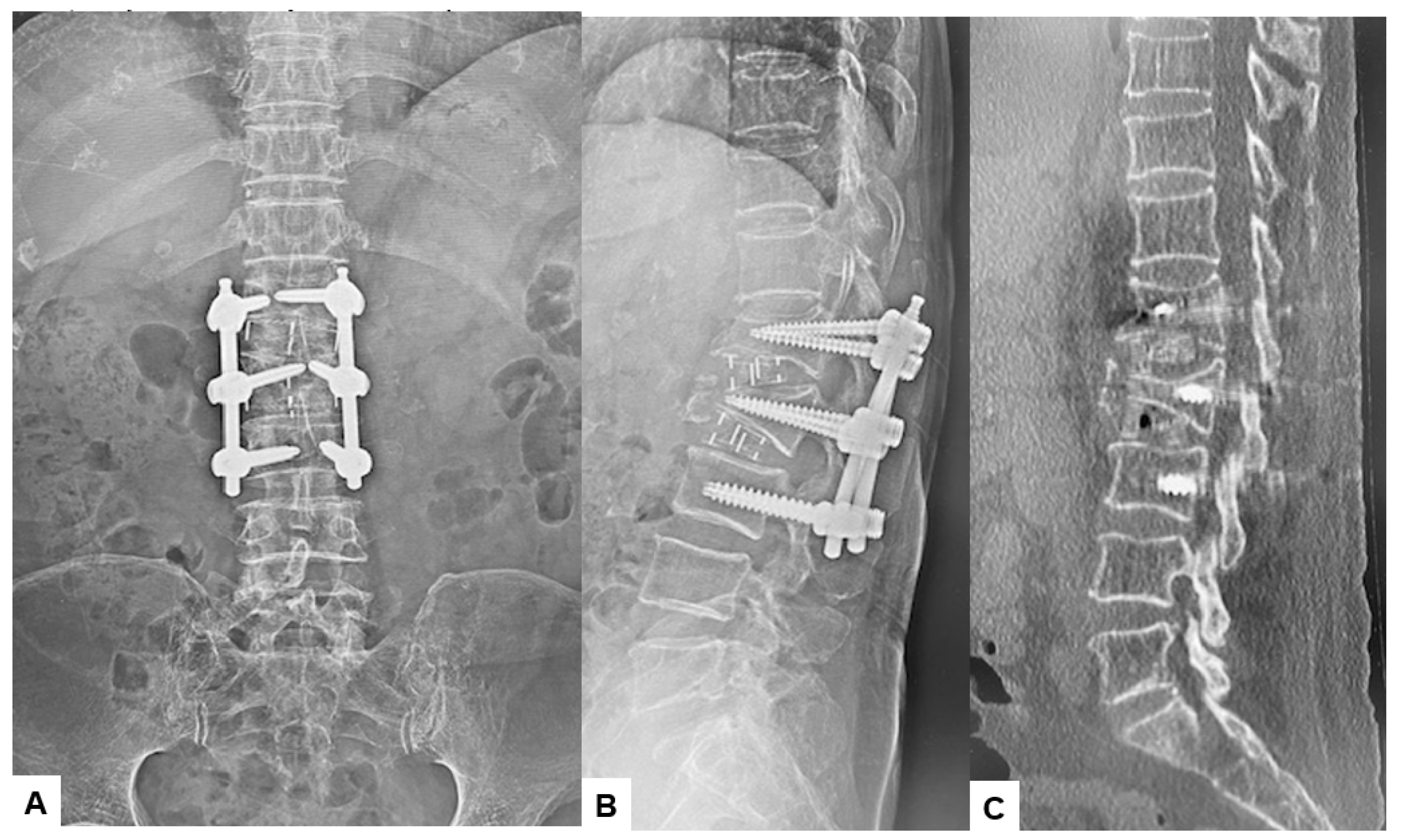
2.5. Postoperative Imagings
3. Results
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reginster JY, Burlet N. Osteoporosis: A still increasing prevalence. Bone 2006; 38:S4-S9. [CrossRef]
- Park JH, Lee SM, Shim SW, Baek SN, Choi YS. The Influence of Restrictive Pulmonary Dysfunction on Osteoporotic Thoracic Vertebral Fractures. Asian Spine J. 2021 Oct;15(5):659-663. [CrossRef]
- Jang HD, Kim EH, Lee JC, Choi SW, Kim HS, Cha JS, Shin BJ. Management of Osteoporotic Vertebral Fracture: Review Update 2022. Asian Spine J. 2022 Dec;16(6):934-946. PMC9827207. Ensrud KE, Schousboe JT. Clinical practice: vertebral fractures. N Engl J Med 2011;364:1634-42. [CrossRef]
- Rajasekaran S, Kanna RM, Schnake KJ, et al. Osteoporotic thoracolumbar fractures-how are they different?: classification and treatment algorithm. J Orthop Trauma 2017;31 Suppl 4:S49-56. [CrossRef]
- Sánchez-Pinto-Pinto B, Romero-Morales C, López-López D, de-Labra C, García-Pérez-de-Sevilla G. Efficacy of Bracing on Thoracic Kyphotic Angle and Functionality in Women with Osteoporosis: A Systematic Review. Medicina (Kaunas). 2022 May 24;58(6):693. [CrossRef]
- Kutsal FY, Ergin Ergani GO. Vertebral compression fractures: still an unpredictable aspect of osteoporosis. Turk J Med Sci. 2021; 51(2): 393–9. [CrossRef]
- Che H, Breuil V, Cortet B, et al. Vertebral fractures cascade: potential causes and risk factors. Osteoporosis Int. 2019; 30(3): 555–63. [CrossRef]
- Alpantaki K, Dohm M, Korovessis P, Hadjipavlou AG. Surgical options for osteoporotic vertebral compression fractures complicated with spinal deformity and neurologic deficit. Injury. 2018; 49(2): 261–71. [CrossRef]
- Cao Z, Wang G, Hui W, Liu B, Liu Z, Sun J. Percutaneous kyphoplasty for osteoporotic vertebral compression fractures improves spino-pelvic alignment and global sagittal balance maximally in the thoracolumbar region. PLoS One. 2020 Jan 30;15(1):e0228341. [CrossRef]
- Tanaka M, Singh M, Fujiwara Y, Uotani K, Oda Y, Arataki S, Yamauchi T, Takigawa T, Ito Y. Comparison of Navigated Expandable Vertebral Cage with Conventional Expandable Vertebral Cage for Minimally Invasive Lumbar/Thoracolumbar Corpectomy. Medicina (Kaunas). 2022 Mar 1;58(3):364. [CrossRef]
- Wen Z, Mo X, Zhao S, et al. Comparison of percutaneous kyphoplasty and pedicle screw fixation for treatment of thoracolumbar severe osteoporotic vertebral compression fracture with kyphosis. World Neurosurg. 2021; 152: e589–96. [CrossRef]
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006;17:1726–33. [CrossRef]
- Goldstein CL, Chutkan NB, Choma TJ, Orr RD. Management of the elderly with vertebral compression fractures. Neurosurgery. 2015;77(4):S33–S45. [CrossRef]
- Park HY, Ahn JH, Ha KY, et al. Clinical and radiologic features of osteoporotic spine fracture with delayed neurologic compromises. World Neurosurg. 2018;120:e1295–e1300. [CrossRef]
- Spiegl U, Jarvers JS, Heyde CE, Josten C. Osteoporotic vertebral body fractures of the thoracolumbar spine: indications and techniques of a 3608-stabilization. Eur J Trauma Emerg Surg. 2017;43(1):27–33. [CrossRef]
- Katsumi K, Hirano T, Watanabe K, et al.. Surgical treatment for osteoporotic thoracolumbar vertebral collapse using vertebroplasty with posterior spinal fusion: a prospective multicenter study. Int Orthop. 2016;40(11):2309–2315. [CrossRef]
- Goldstein CL, Chutkan NB, Choma TJ, et al. Management of the elderly with vertebral compression fractures. Neurosurgery. 2015;77(Suppl 4):S33-45. [CrossRef]
- Diebo BG, Henry J, Lafage V, Berjano P. Sagittal deformities of the spine:Factors influencing the outcomes and complications. Eur Spine J. 2015;24:S3–15. [CrossRef]
- Terai, H., Takahashi, S., Yasuda, H., Konishi, S., Maeno, T., Kono, H., Matsumura, A., Namikawa, T., Kato, M., Hoshino, M., Tamai, K., Toyoda, H., Suzuki, A., & Nakamura, H. (2021). Differences in surgical outcome after anterior corpectomy and reconstruction with an expandable cage with rectangular footplates between thoracolumbar and lumbar osteoporotic vertebral fracture. *North American Spine Society Journal (NASSJ)*, *6*, 100071. [CrossRef]
- Diebo B, Liu S, Lafage V, et al. Osteotomies in the treatment of spinal deformities: indications, classification, and surgical planning. Eur J Orthop Surg Traumatol 2014;24Suppl 1:S11–20. [CrossRef]
- Been HD, Poolman RW, Ubags LH. Clinical outcome and radiographic results after surgical treatment of post-traumatic thoracolumbar kyphosis following simple type A fractures. Eur Spine J. 2004;13:101–107. [CrossRef]
- Kamerlink JR, Errico T, Xavier S, et al. Major intraoperative neurologic monitoring deficits in consecutive pediatric and adult spinal deformity patients at one institution. Spine. 2010;35:240–245. [CrossRef]
- Zeng Y, Chen Z, Sun C, et al. Posterior surgical correction of posttraumatic kyphosis of the thoracolumbar segment. J Spinal Disord Tech. 2013;26:37–41. [CrossRef]
- McAfee PC. Complications of anterior approaches to the thoracolumbar spine. Emphasis on Kaneda instrumentation. Clin Orthop Relat Res. 1994;306:110–119.
- K Uchida, S Kobayashi, H Nakajima, Y Kokubo, T Yayama, RSato, G Timbihurira, H Baba. Anterior expandable strut cage replacement for osteoporotic thoracolumbar vertebral collapse.J Neurosurg Spine, 4 (2006), 454-462. [CrossRef]
- JP Grant, TR Oxland, MF Dvorak.Mapping the structural properties of the lumbosacral vertebral endplates. Spine (Phila Pa 1976), 26, (2001), 889-896. [CrossRef]
- M Pekmezci, E McDonald, A Kennedy, R Dedini, T McClellan, CAmes, V Deviren. Can a novel rectangular footplate provide higher resistance to subsidence than circular footplates. An ex vivo biomechanical study. Spine (Phila Pa 1976), 37 (2012), E1177-E1181,. [CrossRef]
- Chokshi JJ, Shah M. Outcomes of including fracture level in short-segment fixation for thoracolumbar fracture-dislocation. Asian Spine J. 2019;13:56–60. [CrossRef]
- Dobran M, Nasi D, Brunozzi D, et al. Treatment of unstable thoracolumbar junction fractures: short-segment pedicle fixation with inclusion of the fracture level versus long-segment instrumentation. Acta Neurochir (Wien) 2016;158:1883–9. [CrossRef]
- Hiyama A., Sakai D., Sato M., Watanabe M. The analysis of percutaneous pedicle screw technique with guide wire-less in lateral decubitus position following extreme lateral interbody fusion. J. Orthop. Surg. Res. 2019;14:304. [CrossRef]
- Yamauchi T, Jaiswal A, Tanaka M, Fujiwara Y, Oda Y, Arataki S, Misawa H. Minimally Invasive L5 Corpectomy with Navigated Expandable Vertebral Cage: A Technical Note. Brain Sci. 2021 Sep 19;11(9):1241. [CrossRef]
- Kwee M.M., Ho Y.H., Rozen W.M. The prone position during surgery and its complications: A systematic review and evidence-based guidelines. Int. Surg. 2015;100:292–303. [CrossRef]
- Robertson P.A., Rawlinson H.J., Hadlow A.T. Radiologic stability of titanium mesh cages for anterior spinal reconstruction following thoracolumbar corpectomy. J. Spinal Disord. Tech. 2004;17:44–52. [CrossRef]
- Suk, S.I.; Kim, J.H.; Lee, S.M.; Chung, E.R.; Lee, J.H. Anterior-posterior surgery versus posterior closing wedge osteotomy in posttraumatic kyphosis with neurologic compromised osteoporotic fracture. Spine 2003, 28, 2170–2175. [CrossRef]
- Tanaka M, Fujiwara Y, Uotani K, Ayhan S, Yamauchi T, Sonawane S, Nakanishi K. Minimally invasive thoracolumbar corpectomy with navigated expandable vertebral cage: A technical note, Interdiscip Neurosurg, (2021), 24. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




