Submitted:
23 February 2024
Posted:
28 February 2024
You are already at the latest version
Abstract
Keywords:
Introduction
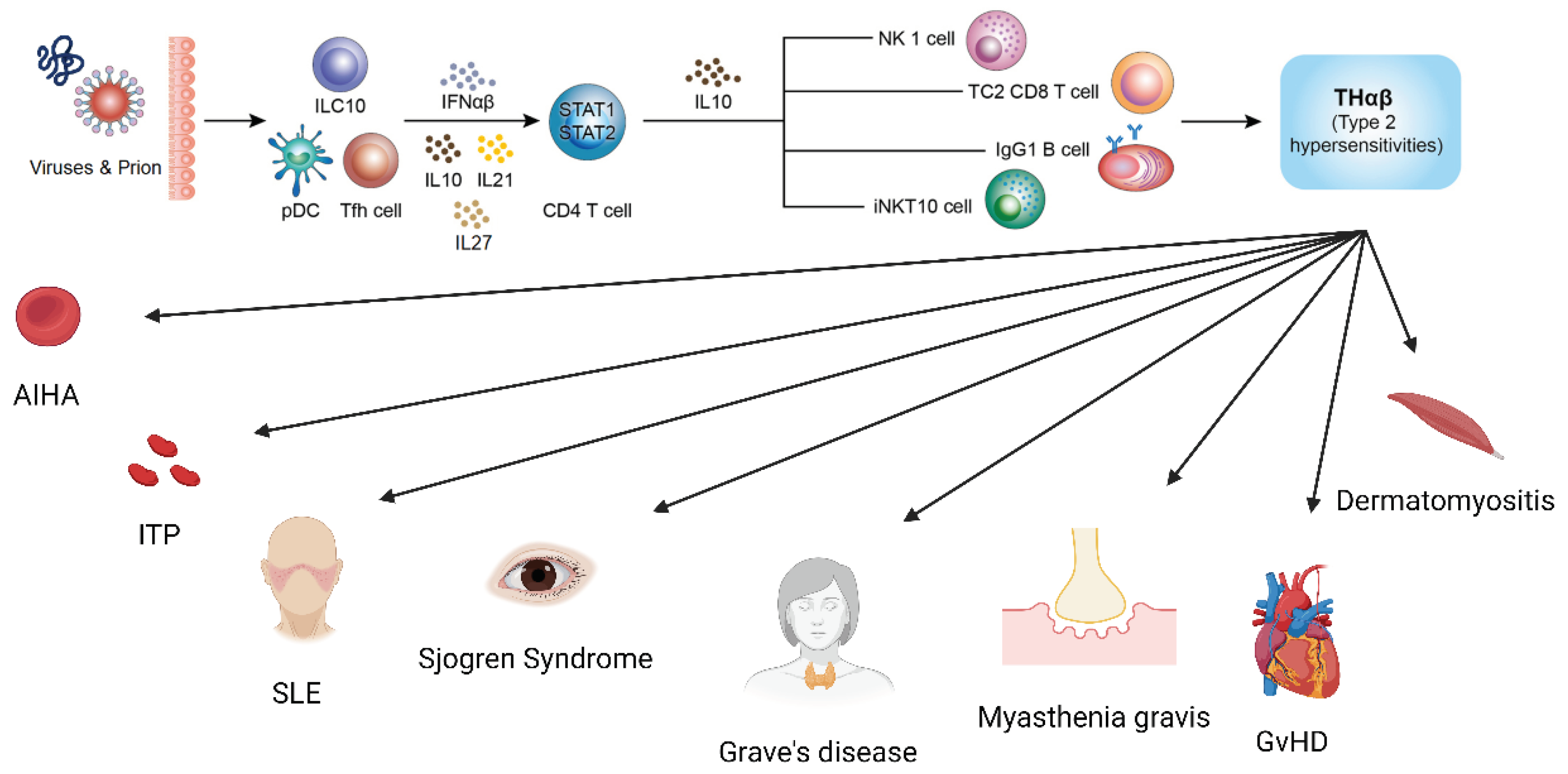
The Framework of Host Immunological Pathways
THαβ Immunological Pathway Related with SLE
THαβ Immunological Pathway Related with Sjogren’s Syndrome
THαβ Immunological Pathway Related with Myasthenia Gravis
THαβ Immunological Pathway Related with Grave’s Disease
THαβ Immunological Pathway Related with Graft Versus Host Disease
THαβ Immunological Pathway Related with Immune Thrombocytop Enia
THαβ Immunological Pathway Related with Autoimmune Hemolytic Anemia
THαβ Immunological Pathway Related with Dermatomyositis
Conclusions
Author Contributions
Funding
Ethical Statement and Consent
Data Availibility Statement
Acknowledgments
Conflicts of Interest
References
- Villalta, D.; Bizzaro, N.; Bassi, N.; Zen, M.; Gatto, M.; Ghirardello, A.; Iaccarino, L.; Punzi, L.; Doria, A. Anti-dsDNA antibody isotypes in systemic lupus erythematosus: IgA in addition to IgG anti-dsDNA help to identify glomerulonephritis and active disease. PLoS One 2013, 8, e71458. [Google Scholar] [CrossRef] [PubMed]
- Poole, B.D.; Scofield, R.H.; Harley, J.B.; James, J.A. Epstein-Barr virus and molecular mimicry in systemic lupus erythematosus. Autoimmunity 2006, 39, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zan, H.; Cerutti, A.; Dramitinos, P.; Schaffer, A.; Casali, P. CD40 engagement triggers switching to IgA1 and IgA2 in human B cells through induction of endogenous TGF-beta: evidence for TGF-beta but not IL-10-dependent direct S mu-->S alpha and sequential S mu-->S gamma, S gamma-->S alpha DNA recombination. J Immunol 1998, 161, 5217–5225. [Google Scholar] [CrossRef] [PubMed]
- Breitfeld, D.; Ohl, L.; Kremmer, E.; Ellwart, J.; Sallusto, F.; Lipp, M.; Forster, R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med 2000, 192, 1545–1552. [Google Scholar] [CrossRef]
- Hu, W.C. A Framework of All Discovered Immunological Pathways and Their Roles for Four Specific Types of Pathogens and Hypersensitivities. Front Immunol 2020, 11, 1992. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 1986, 136, 2348–2357. [Google Scholar] [CrossRef]
- Kobayashi, N.; Kondo, T.; Takata, H.; Yokota, S.; Takiguchi, M. Functional and phenotypic analysis of human memory CD8+ T cells expressing CXCR3. J Leukoc Biol 2006, 80, 320–329. [Google Scholar] [CrossRef]
- Tomiyama, H.; Takata, H.; Matsuda, T.; Takiguchi, M. Phenotypic classification of human CD8+ T cells reflecting their function: inverse correlation between quantitative expression of CD27 and cytotoxic effector function. Eur J Immunol 2004, 34, 999–1010. [Google Scholar] [CrossRef]
- Wen, T.H.; Tsai, K.W.; Wu, Y.J.; Liao, M.T.; Lu, K.C.; Hu, W.C. The Framework for Human Host Immune Responses to Four Types of Parasitic Infections and Relevant Key JAK/STAT Signaling. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Fort, M.M.; Cheung, J.; Yen, D.; Li, J.; Zurawski, S.M.; Lo, S.; Menon, S.; Clifford, T.; Hunte, B.; Lesley, R.; et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 2001, 15, 985–995. [Google Scholar] [CrossRef]
- Farne, H.A.; Wilson, A.; Powell, C.; Bax, L.; Milan, S.J. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev 2017, 9, Cd010834. [Google Scholar] [CrossRef]
- Masure, D.; Vlaminck, J.; Wang, T.; Chiers, K.; Van den Broeck, W.; Vercruysse, J.; Geldhof, P. A role for eosinophils in the intestinal immunity against infective Ascaris suum larvae. PLoS Negl Trop Dis 2013, 7, e2138. [Google Scholar] [CrossRef]
- Vliagoftis, H.; Lacy, P.; Luy, B.; Adamko, D.; Hollenberg, M.; Befus, D.; Moqbel, R. Mast cell tryptase activates peripheral blood eosinophils to release granule-associated enzymes. Int Arch Allergy Immunol 2004, 135, 196–204. [Google Scholar] [CrossRef]
- Komai-Koma, M.; Brombacher, F.; Pushparaj, P.N.; Arendse, B.; McSharry, C.; Alexander, J.; Chaudhuri, R.; Thomson, N.C.; McKenzie, A.N.; McInnes, I.; et al. Interleukin-33 amplifies IgE synthesis and triggers mast cell degranulation via interleukin-4 in naive mice. Allergy 2012, 67, 1118–1126. [Google Scholar] [CrossRef]
- Obata, K.; Mukai, K.; Tsujimura, Y.; Ishiwata, K.; Kawano, Y.; Minegishi, Y.; Watanabe, N.; Karasuyama, H. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood 2007, 110, 913–920. [Google Scholar] [CrossRef]
- Romagnani, P.; De Paulis, A.; Beltrame, C.; Annunziato, F.; Dente, V.; Maggi, E.; Romagnani, S.; Marone, G. Tryptase-Chymase Double-Positive Human Mast Cells Express the Eotaxin Receptor CCR3 and Are Attracted by CCR3-Binding Chemokines. The American Journal of Pathology 1999, 155, 1195–1204. [Google Scholar] [CrossRef]
- Basu, R.; O’Quinn, D.B.; Silberger, D.J.; Schoeb, T.R.; Fouser, L.; Ouyang, W.; Hatton, R.D.; Weaver, C.T. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity 2012, 37, 1061–1075. [Google Scholar] [CrossRef]
- Eyerich, S.; Eyerich, K.; Pennino, D.; Carbone, T.; Nasorri, F.; Pallotta, S.; Cianfarani, F.; Odorisio, T.; Traidl-Hoffmann, C.; Behrendt, H.; et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest 2009, 119, 3573–3585. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.C. Human immune responses to Plasmodium falciparum infection: molecular evidence for a suboptimal THalphabeta and TH17 bias over ideal and effective traditional TH1 immune response. Malaria journal 2013, 12, 392. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.C. The Central THalphabeta Immunity Associated Cytokine: IL-10 Has a Strong Anti-Tumor Ability Toward Established Cancer Models In Vivo and Toward Cancer Cells In Vitro. Front Oncol 2021, 11, 655554. [Google Scholar] [CrossRef] [PubMed]
- Tsou, A.; Chen, P.J.; Tsai, K.W.; Hu, W.C.; Lu, K.C. THαβ Immunological Pathway as Protective Immune Response against Prion Diseases: An Insight for Prion Infection Therapy. Viruses 2022, 14. [Google Scholar] [CrossRef]
- Krovi, S.H.; Gapin, L. Invariant Natural Killer T Cell Subsets-More Than Just Developmental Intermediates. Front Immunol 2018, 9, 1393. [Google Scholar] [CrossRef]
- Prochazkova, J.; Pokorna, K.; Holan, V. IL-12 inhibits the TGF-beta-dependent T cell developmental programs and skews the TGF-beta-induced differentiation into a Th1-like direction. Immunobiology 2012, 217, 74–82. [Google Scholar] [CrossRef]
- Anuradha, R.; George, P.J.; Hanna, L.E.; Chandrasekaran, V.; Kumaran, P.; Nutman, T.B.; Babu, S. IL-4-, TGF-beta-, and IL-1-dependent expansion of parasite antigen-specific Th9 cells is associated with clinical pathology in human lymphatic filariasis. J Immunol 2013, 191, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, K.; Hwang, Y.; Nikolaev, A.; Atreya, R.; Dornhoff, H.; Steiner, S.; Lehr, H.A.; Wirtz, S.; Vieth, M.; Waisman, A.; et al. TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nature immunology 2014, 15, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Ando, M.; Kamada, N.; Nagano, Y.; Narushima, S.; Suda, W.; Imaoka, A.; Setoyama, H.; Nagamori, T.; et al. Th17 Cell Induction by Adhesion of Microbes to Intestinal Epithelial Cells. Cell 2015, 163, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Backert, I.; Koralov, S.B.; Wirtz, S.; Kitowski, V.; Billmeier, U.; Martini, E.; Hofmann, K.; Hildner, K.; Wittkopf, N.; Brecht, K.; et al. STAT3 activation in Th17 and Th22 cells controls IL-22-mediated epithelial host defense during infectious colitis. J Immunol 2014, 193, 3779–3791. [Google Scholar] [CrossRef]
- Kumar, S.; Naqvi, R.A.; Khanna, N.; Pathak, P.; Rao, D.N. Th3 immune responses in the progression of leprosy via molecular cross-talks of TGF-beta, CTLA-4 and Cbl-b. Clin Immunol 2011, 141, 133–142. [Google Scholar] [CrossRef]
- Jiang, R.; Feng, X.; Guo, Y.; Lu, Q.; Hou, J.; Luo, K.; Fu, N.; Venuprasad, K.; Banchereau, J.; Ueno, H. T helper cells in patients with chronic hepatitis B virus infection. Chinese medical journal 2002, 115, 422–424. [Google Scholar] [CrossRef]
- Huang, X.; Dorta-Estremera, S.; Yao, Y.; Shen, N.; Cao, W. Predominant Role of Plasmacytoid Dendritic Cells in Stimulating Systemic Autoimmunity. Front Immunol 2015, 6, 526. [Google Scholar] [CrossRef]
- Elkon, K.B.; Stone, V.V. Type I interferon and systemic lupus erythematosus. J Interferon Cytokine Res 2011, 31, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Elkon, K.B.; Wiedeman, A. Type I IFN system in the development and manifestations of SLE. Curr Opin Rheumatol 2012, 24, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Eloranta, M.L.; Ronnblom, L. Cause and consequences of the activated type I interferon system in SLE. J Mol Med (Berl) 2016, 94, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Khamashta, M.; Merrill, J.T.; Werth, V.P.; Furie, R.; Kalunian, K.; Illei, G.G.; Drappa, J.; Wang, L.; Greth, W.; investigators, C.D.s. Sifalimumab, an anti-interferon-alpha monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann Rheum Dis 2016, 75, 1909–1916. [Google Scholar] [CrossRef]
- Morand, E.F.; Furie, R.; Tanaka, Y.; Bruce, I.N.; Askanase, A.D.; Richez, C.; Bae, S.C.; Brohawn, P.Z.; Pineda, L.; Berglind, A.; et al. Trial of Anifrolumab in Active Systemic Lupus Erythematosus. N Engl J Med 2020, 382, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Amezcua-Guerra, L.M.; Marquez-Velasco, R.; Chavez-Rueda, A.K.; Castillo-Martinez, D.; Masso, F.; Paez, A.; Colin-Fuentes, J.; Bojalil, R. Type III Interferons in Systemic Lupus Erythematosus: Association Between Interferon lambda3, Disease Activity, and Anti-Ro/SSA Antibodies. J Clin Rheumatol 2017, 23, 368–375. [Google Scholar] [CrossRef]
- Goel, R.R.; Wang, X.; O’Neil, L.J.; Nakabo, S.; Hasneen, K.; Gupta, S.; Wigerblad, G.; Blanco, L.P.; Kopp, J.B.; Morasso, M.I.; et al. Interferon lambda promotes immune dysregulation and tissue inflammation in TLR7-induced lupus. Proc Natl Acad Sci U S A 2020, 117, 5409–5419. [Google Scholar] [CrossRef]
- Celhar, T.; Fairhurst, A.M. Toll-like receptors in systemic lupus erythematosus: potential for personalized treatment. Front Pharmacol 2014, 5, 265. [Google Scholar] [CrossRef]
- Christensen, S.R.; Kashgarian, M.; Alexopoulou, L.; Flavell, R.A.; Akira, S.; Shlomchik, M.J. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med 2005, 202, 321–331. [Google Scholar] [CrossRef]
- Ehlers, M.; Fukuyama, H.; McGaha, T.L.; Aderem, A.; Ravetch, J.V. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J Exp Med 2006, 203, 553–561. [Google Scholar] [CrossRef]
- Elloumi, N.; Fakhfakh, R.; Abida, O.; Hachicha, H.; Marzouk, S.; Fourati, M.; Bahloul, Z.; Masmoudi, H. RNA receptors, TLR3 and TLR7, are potentially associated with SLE clinical features. Int J Immunogenet 2021, 48, 250–259. [Google Scholar] [CrossRef]
- Enevold, C.; Kjaer, L.; Nielsen, C.H.; Voss, A.; Jacobsen, R.S.; Hermansen, M.L.; Redder, L.; Oturai, A.B.; Jensen, P.E.; Bendtzen, K.; et al. Genetic polymorphisms of dsRNA ligating pattern recognition receptors TLR3, MDA5, and RIG-I. Association with systemic lupus erythematosus and clinical phenotypes. Rheumatol Int 2014, 34, 1401–1408. [Google Scholar] [CrossRef]
- Fillatreau, S.; Manfroi, B.; Dorner, T. Toll-like receptor signalling in B cells during systemic lupus erythematosus. Nat Rev Rheumatol 2021, 17, 98–108. [Google Scholar] [CrossRef]
- Shen, N.; Fu, Q.; Deng, Y.; Qian, X.; Zhao, J.; Kaufman, K.M.; Wu, Y.L.; Yu, C.Y.; Tang, Y.; Chen, J.Y.; et al. Sex-specific association of X-linked Toll-like receptor 7 (TLR7) with male systemic lupus erythematosus. Proc Natl Acad Sci U S A 2010, 107, 15838–15843. [Google Scholar] [CrossRef]
- Horton, C.G.; Pan, Z.J.; Farris, A.D. Targeting Toll-like receptors for treatment of SLE. Mediators Inflamm 2010, 2010. [Google Scholar] [CrossRef]
- Baglaenko, Y.; Manion, K.P.; Chang, N.H.; Gracey, E.; Loh, C.; Wither, J.E. IL-10 Production Is Critical for Sustaining the Expansion of CD5+ B and NKT Cells and Restraining Autoantibody Production in Congenic Lupus-Prone Mice. PLoS One 2016, 11, e0150515. [Google Scholar] [CrossRef]
- Beebe, A.M.; Cua, D.J.; de Waal Malefyt, R. The role of interleukin-10 in autoimmune disease: systemic lupus erythematosus (SLE) and multiple sclerosis (MS). Cytokine Growth Factor Rev 2002, 13, 403–412. [Google Scholar] [CrossRef]
- Sung, Y.K.; Park, B.L.; Shin, H.D.; Kim, L.H.; Kim, S.Y.; Bae, S.C. Interleukin-10 gene polymorphisms are associated with the SLICC/ACR Damage Index in systemic lupus erythematosus. Rheumatology (Oxford) 2006, 45, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Facciotti, F.; Larghi, P.; Bosotti, R.; Vasco, C.; Gagliani, N.; Cordiglieri, C.; Mazzara, S.; Ranzani, V.; Rottoli, E.; Curti, S.; et al. Evidence for a pathogenic role of extrafollicular, IL-10-producing CCR6(+)B helper T cells in systemic lupus erythematosus. Proc Natl Acad Sci U S A 2020, 117, 7305–7316. [Google Scholar] [CrossRef] [PubMed]
- Geginat, J.; Vasco, M.; Gerosa, M.; Tas, S.W.; Pagani, M.; Grassi, F.; Flavell, R.A.; Meroni, P.; Abrignani, S. IL-10 producing regulatory and helper T-cells in systemic lupus erythematosus. Semin Immunol 2019, 44, 101330. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Muchamuel, T.; Sakaguchi, S.; Andrade, S.; Menon, S.; Howard, M. Continuous administration of anti-interleukin 10 antibodies delays onset of autoimmunity in NZB/W F1 mice. J Exp Med 1994, 179, 305–310. [Google Scholar] [CrossRef]
- Conley, M.E.; Koopman, W.J. Serum IgA1 and IgA2 in normal adults and patients with systemic lupus erythematosus and hepatic disease. Clin Immunol Immunopathol 1983, 26, 390–397. [Google Scholar] [CrossRef]
- Otsuka, K.; Sato, M.; Tsunematsu, T.; Ishimaru, N. Virus Infections Play Crucial Roles in the Pathogenesis of Sjogren’s Syndrome. Viruses 2022, 14. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J. Preferentially immunoglobulin (IgG) subclasses production in primary Sjogren’s syndrome patients. Clin Chem Lab Med 2011, 50, 345–349. [Google Scholar] [CrossRef]
- Karlsen, M.; Hansen, T.; Nordal, H.H.; Brun, J.G.; Jonsson, R.; Appel, S. Expression of Toll-like receptor -7 and -9 in B cell subsets from patients with primary Sjogren’s syndrome. PLoS One 2015, 10, e0120383. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Horai, Y.; Suzuki, T.; Okada, A.; Ichinose, K.; Yamasaki, S.; Koji, T.; Kawakami, A. TLR3-mediated apoptosis and activation of phosphorylated Akt in the salivary gland epithelial cells of primary Sjogren’s syndrome patients. Rheumatol Int 2013, 33, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Bodewes, I.L.A.; Versnel, M.A. Interferon activation in primary Sjogren’s syndrome: recent insights and future perspective as novel treatment target. Expert Rev Clin Immunol 2018, 14, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Verstappen, G.M.; Kroese, F.G.M.; Bootsma, H. T cells in primary Sjogren’s syndrome: targets for early intervention. Rheumatology (Oxford) 2021, 60, 3088–3098. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Kubo, S.; Nakayamada, S.; Shimajiri, S.; Zhang, X.; Yamaoka, K.; Tanaka, Y. Association of plasmacytoid dendritic cells with B cell infiltration in minor salivary glands in patients with Sjogren’s syndrome. Mod Rheumatol 2016, 26, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Bertorello, R.; Cordone, M.P.; Contini, P.; Rossi, P.; Indiveri, F.; Puppo, F.; Cordone, G. Increased levels of interleukin-10 in saliva of Sjogren’s syndrome patients. Correlation with disease activity. Clin Exp Med 2004, 4, 148–151. [Google Scholar] [CrossRef]
- Ciecko, A.E.; Foda, B.; Barr, J.Y.; Ramanathan, S.; Atkinson, M.A.; Serreze, D.V.; Geurts, A.M.; Lieberman, S.M.; Chen, Y.G. Interleukin-27 Is Essential for Type 1 Diabetes Development and Sjogren Syndrome-like Inflammation. Cell Rep 2019, 29, 3073–3086 e3075. [Google Scholar] [CrossRef]
- Lu, C.; Pi, X.; Xu, W.; Qing, P.; Tang, H.; Li, Y.; Zhao, Y.; Liu, X.; Tang, H.; Liu, Y. Clinical significance of T cell receptor repertoire in primary Sjogren’s syndrome. EBioMedicine 2022, 84, 104252. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.Y.; Wang, X.; Meyerholz, D.K.; Lieberman, S.M. CD8 T cells contribute to lacrimal gland pathology in the nonobese diabetic mouse model of Sjogren syndrome. Immunol Cell Biol 2017, 95, 684–694. [Google Scholar] [CrossRef]
- Pertovaara, M.; Silvennoinen, O.; Isomaki, P. Cytokine-induced STAT1 activation is increased in patients with primary Sjogren’s syndrome. Clin Immunol 2016, 165, 60–67. [Google Scholar] [CrossRef]
- Ramos, H.L.; Valencia-Pacheco, G.; Alcocer-Varela, J. Constitutive STAT3 activation in peripheral CD3(+) cells from patients with primary Sjogren’s syndrome. Scand J Rheumatol 2008, 37, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.C.; Park, C.S.; You, I.C.; Choi, H.J.; Lee, K.H.; Im, S.K.; Park, H.Y.; Pflugfelder, S.C. Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Invest Ophthalmol Vis Sci 2010, 51, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Baber, A.; Nocturne, G.; Krzysiek, R.; Henry, J.; Belkhir, R.; Mariette, X.; Seror, R. Large granular lymphocyte expansions in primary Sjogren’s syndrome: characteristics and outcomes. RMD Open 2019, 5, e001044. [Google Scholar] [CrossRef]
- Leite, M.I.; Jacob, S.; Viegas, S.; Cossins, J.; Clover, L.; Morgan, B.P.; Beeson, D.; Willcox, N.; Vincent, A. IgG1 antibodies to acetylcholine receptors in ‘seronegative’ myasthenia gravis. Brain 2008, 131, 1940–1952. [Google Scholar] [CrossRef]
- Chien, P.J.; Yeh, J.H.; Chiu, H.C.; Hsueh, Y.M.; Chen, C.T.; Chen, M.C.; Shih, C.M. Inhibition of peripheral blood natural killer cell cytotoxicity in patients with myasthenia gravis treated with plasmapheresis. Eur J Neurol 2011, 18, 1350–1357. [Google Scholar] [CrossRef]
- Alseth, E.H.; Nakkestad, H.L.; Aarseth, J.; Gilhus, N.E.; Skeie, G.O. Interleukin-10 promoter polymorphisms in myasthenia gravis. J Neuroimmunol 2009, 210, 63–66. [Google Scholar] [CrossRef]
- Meng, H.; Zheng, S.; Zhou, Q.; Gao, Y.; Ni, Y.; Liang, H.; Chen, S. FoxP3(-) Tr1 Cell in Generalized Myasthenia Gravis and Its Relationship With the Anti-AChR Antibody and Immunomodulatory Cytokines. Front Neurol 2021, 12, 755356. [Google Scholar] [CrossRef]
- Cavalcante, P.; Barzago, C.; Baggi, F.; Antozzi, C.; Maggi, L.; Mantegazza, R.; Bernasconi, P. Toll-like receptors 7 and 9 in myasthenia gravis thymus: amplifiers of autoimmunity? Ann N Y Acad Sci 2018, 1413, 11–24. [Google Scholar] [CrossRef]
- Robinet, M.; Maillard, S.; Cron, M.A.; Berrih-Aknin, S.; Le Panse, R. Review on Toll-Like Receptor Activation in Myasthenia Gravis: Application to the Development of New Experimental Models. Clin Rev Allergy Immunol 2017, 52, 133–147. [Google Scholar] [CrossRef]
- Yapici, Z.; Tuzun, E.; Altunayoglu, V.; Erdogan, A.; Eraksoy, M. High interleukin-10 production is associated with anti-acetylcholine receptor antibody production and treatment response in juvenile myasthenia gravis. Int J Neurosci 2007, 117, 1505–1512. [Google Scholar] [CrossRef]
- Jeong, H.N.; Lee, J.H.; Suh, B.C.; Choi, Y.C. Serum interleukin-27 expression in patients with myasthenia gravis. J Neuroimmunol 2015, 288, 120–122. [Google Scholar] [CrossRef]
- Cufi, P.; Dragin, N.; Ruhlmann, N.; Weiss, J.M.; Fadel, E.; Serraf, A.; Berrih-Aknin, S.; Le Panse, R. Central role of interferon-beta in thymic events leading to myasthenia gravis. J Autoimmun 2014, 52, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Cufi, P.; Dragin, N.; Weiss, J.M.; Martinez-Martinez, P.; De Baets, M.H.; Roussin, R.; Fadel, E.; Berrih-Aknin, S.; Le Panse, R. Implication of double-stranded RNA signaling in the etiology of autoimmune myasthenia gravis. Ann Neurol 2013, 73, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, P.; Maggi, L.; Colleoni, L.; Caldara, R.; Motta, T.; Giardina, C.; Antozzi, C.; Berrih-Aknin, S.; Bernasconi, P.; Mantegazza, R. Inflammation and epstein-barr virus infection are common features of myasthenia gravis thymus: possible roles in pathogenesis. Autoimmune Dis 2011, 2011, 213092. [Google Scholar] [CrossRef]
- Leopardi, V.; Chang, Y.M.; Pham, A.; Luo, J.; Garden, O.A. A Systematic Review of the Potential Implication of Infectious Agents in Myasthenia Gravis. Front Neurol 2021, 12, 618021. [Google Scholar] [CrossRef] [PubMed]
- Feferman, T.; Maiti, P.K.; Berrih-Aknin, S.; Bismuth, J.; Bidault, J.; Fuchs, S.; Souroujon, M.C. Overexpression of IFN-induced protein 10 and its receptor CXCR3 in myasthenia gravis. J Immunol 2005, 174, 5324–5331. [Google Scholar] [CrossRef]
- Nisihara, R.; Pigosso, Y.G.; Prado, N.; Utiyama, S.R.R.; De Carvalho, G.A.; Skare, T.L. Rheumatic Disease Autoantibodies in Patients with Autoimmune Thyroid Diseases. Med Princ Pract 2018, 27, 332–336. [Google Scholar] [CrossRef]
- Ueki, I.; Abiru, N.; Kawagoe, K.; Nagayama, Y. Interleukin 10 deficiency attenuates induction of anti-TSH receptor antibodies and hyperthyroidism in a mouse Graves’ model. J Endocrinol 2011, 209, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Pedro, A.B.; Romaldini, J.H.; Americo, C.; Takei, K. Association of circulating antibodies against double-stranded and single-stranded DNA with thyroid autoantibodies in Graves’ disease and Hashimoto’s thyroiditis patients. Exp Clin Endocrinol Diabetes 2006, 114, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Ferrari, S.M.; Giuggioli, D.; Ferrannini, E.; Ferri, C.; Fallahi, P. Chemokine (C-X-C motif) ligand (CXCL)10 in autoimmune diseases. Autoimmun Rev 2014, 13, 272–280. [Google Scholar] [CrossRef]
- Kim, K.J.; Lee, K.W.; Choi, J.H.; An, J.H. Interferon-Alpha Induced Severe Hypothyroidism Followed by Graves’ Disease in a Patient Infected with Hepatitis C Virus. International Journal of Thyroidology 2015, 8. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, C.; Zhao, K.; Yu, N.; Li, Y.; Yu, Y.; Zhang, Y.; Song, Z.; Huang, Y.; Lu, G.; et al. Glycosylation of Anti-Thyroglobulin IgG1 and IgG4 Subclasses in Thyroid Diseases. Eur Thyroid J 2021, 10, 114–124. [Google Scholar] [CrossRef]
- Liu, N.; Lu, H.; Tao, F.; Guo, T.; Liu, C.; Cui, B.; Ning, G. An association of interleukin-10 gene polymorphisms with Graves’ disease in two Chinese populations. Endocrine 2011, 40, 90–94. [Google Scholar] [CrossRef]
- Peng, S.; Li, C.; Wang, X.; Liu, X.; Han, C.; Jin, T.; Liu, S.; Zhang, X.; Zhang, H.; He, X.; et al. Increased Toll-Like Receptors Activity and TLR Ligands in Patients with Autoimmune Thyroid Diseases. Front Immunol 2016, 7, 578. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, L.; Guo, C.; Kou, C.; Long, Y.; Li, J.; Zhang, H.-Q. Reduced proportion and activity of natural killer cells in patients with Graves’ disease. European Journal of Inflammation 2020, 18. [Google Scholar] [CrossRef]
- Villarroel, V.A.; Okiyama, N.; Tsuji, G.; Linton, J.T.; Katz, S.I. CXCR3-mediated skin homing of autoreactive CD8 T cells is a key determinant in murine graft-versus-host disease. J Invest Dermatol 2014, 134, 1552–1560. [Google Scholar] [CrossRef]
- Abraham, S.; Guo, H.; Choi, J.G.; Ye, C.; Thomas, M.B.; Ortega, N.; Dwivedi, A.; Manjunath, N.; Yi, G.; Shankar, P. Combination of IL-10 and IL-2 induces oligoclonal human CD4 T cell expansion during xenogeneic and allogeneic GVHD in humanized mice. Heliyon 2017, 3, e00276. [Google Scholar] [CrossRef] [PubMed]
- Duffner, U.; Lu, B.; Hildebrandt, G.C.; Teshima, T.; Williams, D.L.; Reddy, P.; Ordemann, R.; Clouthier, S.G.; Lowler, K.; Liu, C.; et al. Role of CXCR3-induced donor T-cell migration in acute GVHD. Exp Hematol 2003, 31, 897–902. [Google Scholar] [CrossRef]
- Roychowdhury, S.; Blaser, B.W.; Freud, A.G.; Katz, K.; Bhatt, D.; Ferketich, A.K.; Bergdall, V.; Kusewitt, D.; Baiocchi, R.A.; Caligiuri, M.A. IL-15 but not IL-2 rapidly induces lethal xenogeneic graft-versus-host disease. Blood 2005, 106, 2433–2435. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, Y.; Zheng, H. The Effects of Interferons on Allogeneic T Cell Response in GVHD: The Multifaced Biology and Epigenetic Regulations. Front Immunol 2021, 12, 717540. [Google Scholar] [CrossRef]
- Martin-Antonio, B.; Suarez-Lledo, M.; Arroyes, M.; Fernandez-Avilés, F.; Martinez, C.; Rovira, M.; Espigado, I.; Gallardo, D.; Bosch, A.; Buno, I.; et al. A Gene Variant in IRF3 Impacts On the Clinical Outcome of Acute Myeloid Leukemia (AML) Patients Submitted to Allogeneic Stem Cell Transplantation (allo-SCT). Blood 2012, 120, 468–468. [Google Scholar] [CrossRef]
- Hakim, F.T.; Memon, S.; Jin, P.; Imanguli, M.M.; Wang, H.; Rehman, N.; Yan, X.Y.; Rose, J.; Mays, J.W.; Dhamala, S.; et al. Upregulation of IFN-Inducible and Damage-Response Pathways in Chronic Graft-versus-Host Disease. J Immunol 2016, 197, 3490–3503. [Google Scholar] [CrossRef] [PubMed]
- Betts, B.C.; Sagatys, E.M.; Veerapathran, A.; Lloyd, M.C.; Beato, F.; Lawrence, H.R.; Yue, B.; Kim, J.; Sebti, S.M.; Anasetti, C.; et al. CD4+ T cell STAT3 phosphorylation precedes acute GVHD, and subsequent Th17 tissue invasion correlates with GVHD severity and therapeutic response. J Leukoc Biol 2015, 97, 807–819. [Google Scholar] [CrossRef]
- Ziegler, J.A.; Lokshin, A.; Sepulveda, A.R.; Bedeir, A.; Lentzsch, S.; Mapara, M.Y. Role of STAT1 Expression during Graft-Versus-Host Disease (GVHD) in the Gastrointestinal Tract: Association with Lamina Propria Cell Infiltration and Tissue Cytokine/Chemokine Expression. Blood 2006, 108, 3175–3175. [Google Scholar] [CrossRef]
- Belle, L.; Agle, K.; Zhou, V.; Yin-Yuan, C.; Komorowski, R.; Eastwood, D.; Logan, B.; Sun, J.; Ghilardi, N.; Cua, D.; et al. Blockade of interleukin-27 signaling reduces GVHD in mice by augmenting Treg reconstitution and stabilizing Foxp3 expression. Blood 2016, 128, 2068–2082. [Google Scholar] [CrossRef]
- Calcaterra, C.; Sfondrini, L.; Rossini, A.; Sommariva, M.; Rumio, C.; Menard, S.; Balsari, A. Critical role of TLR9 in acute graft-versus-host disease. J Immunol 2008, 181, 6132–6139. [Google Scholar] [CrossRef]
- Suthers, A.N.; Su, H.; Anand, S.M.; Poe, J.C.; Rose, J.J.; Hakim, F.T.; Pavletic, S.Z.; Rizzieri, D.A.; Horwitz, M.E.; Yang, Y.; et al. Increased TLR7 Signaling of BCR-Activated B Cells in Chronic Graft-Versus Host Disease (cGVHD). Blood 2017, 130, 75–75. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Nogai, A.; Bereswill, S.; Plickert, R.; Fischer, A.; Loddenkemper, C.; Steinhoff, U.; Tchaptchet, S.; Thiel, E.; Freudenberg, M.A.; et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut 2010, 59, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Koyama, M.; Hashimoto, D.; Aoyama, K.; Matsuoka, K.; Karube, K.; Niiro, H.; Harada, M.; Tanimoto, M.; Akashi, K.; Teshima, T. Plasmacytoid dendritic cells prime alloreactive T cells to mediate graft-versus-host disease as antigen-presenting cells. Blood 2009, 113, 2088–2095. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Sayed, A.A.; Han, P.; Tan, M.M.H.; Watt, E.; Constantinescu-Bercu, A.; Cocker, A.T.H.; Khoder, A.; Saputil, R.C.; Thorley, E.; et al. The role of CD8+ T-cell clones in immune thrombocytopenia. Blood 2023, 141, 2417–2429. [Google Scholar] [CrossRef] [PubMed]
- Tesse, R.; Del Vecchio, G.C.; De Mattia, D.; Sangerardi, M.; Valente, F.; Giordano, P. Association of interleukin-(IL)10 haplotypes and serum IL-10 levels in the progression of childhood immune thrombocytopenic purpura. Gene 2012, 505, 53–56. [Google Scholar] [CrossRef]
- Hua, F.; Ji, L.; Zhan, Y.; Li, F.; Zou, S.; Chen, L.; Gao, S.; Li, Y.; Chen, H.; Cheng, Y. Aberrant frequency of IL-10-producing B cells and its association with Treg/Th17 in adult primary immune thrombocytopenia patients. Biomed Res Int 2014, 2014, 571302. [Google Scholar] [CrossRef]
- Hassan, T.; Abdel Rahman, D.; Raafat, N.; Fathy, M.; Shehab, M.; Hosny, A.; Fawzy, R.; Zakaria, M. Contribution of interleukin 27 serum level to pathogenesis and prognosis in children with immune thrombocytopenia. Medicine (Baltimore) 2022, 101, e29504. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Y.; Xue, F.; Xu, J.; Fang, Z. Interleukin-27 rs153109 polymorphism and the risk for immune thrombocytopenia. Autoimmunity 2013, 46, 509–512. [Google Scholar] [CrossRef]
- Yamane, A.; Nakamura, T.; Suzuki, H.; Ito, M.; Ohnishi, Y.; Ikeda, Y.; Miyakawa, Y. Interferon-alpha 2b-induced thrombocytopenia is caused by inhibition of platelet production but not proliferation and endomitosis in human megakaryocytes. Blood 2008, 112, 542–550. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, S.; Li, X.; Wang, B.; Wang, X.; Ma, D.; Yang, L.; Peng, J.; Hou, M. Pathway of Toll-like receptor 7/B cell activating factor/B cell activating factor receptor plays a role in immune thrombocytopenia in vivo. PLoS One 2011, 6, e22708. [Google Scholar] [CrossRef]
- Chan, H.; Moore, J.C.; Finch, C.N.; Warkentin, T.E.; Kelton, J.G. The IgG subclasses of platelet-associated autoantibodies directed against platelet glycoproteins IIb/IIIa in patients with idiopathic thrombocytopenic purpura. Br J Haematol 2003, 122, 818–824. [Google Scholar] [CrossRef]
- Liu, Y.; Zuo, X.; Chen, P.; Hu, X.; Sheng, Z.; Liu, A.; Liu, Q.; Leng, S.; Zhang, X.; Li, X.; et al. Deciphering transcriptome alterations in bone marrow hematopoiesis at single-cell resolution in immune thrombocytopenia. Signal Transduct Target Ther 2022, 7, 347. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Guo, Z.; Ma, J.; Liu, F.; Gao, C.; Liu, S.; Wang, A.; Wu, R. STAT1 single nucleotide polymorphisms and susceptibility to immune thrombocytopenia. Autoimmunity 2015, 48, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ke, Y.; Cheng, Y.; Zhan, Y.; Wu, B. Aberrant Phosphorylation of STAT3 Protein of the CD4+ T Cells in Patients with Primary Immune Thrombocytopenia. Blood 2016, 128, 3740–3740. [Google Scholar] [CrossRef]
- Ward, F.J.; Hall, A.M.; Cairns, L.S.; Leggat, A.S.; Urbaniak, S.J.; Vickers, M.A.; Barker, R.N. Clonal regulatory T cells specific for a red blood cell autoantigen in human autoimmune hemolytic anemia. Blood 2008, 111, 680–687. [Google Scholar] [CrossRef]
- Sonneveld, M.E.; de Haas, M.; Koeleman, C.; de Haan, N.; Zeerleder, S.S.; Ligthart, P.C.; Wuhrer, M.; van der Schoot, C.E.; Vidarsson, G. Patients with IgG1-anti-red blood cell autoantibodies show aberrant Fc-glycosylation. Sci Rep 2017, 7, 8187. [Google Scholar] [CrossRef]
- Rangnekar, A.; Shenoy, M.S.; Mahabala, C.; Balanthimogru, P. Impact of baseline fluorescent antinuclear antibody positivity on the clinical outcome of patients with primary autoimmune hemolytic anemia. Hematol Transfus Cell Ther 2023, 45, 204–210. [Google Scholar] [CrossRef]
- Pattanakitsakul, P.; Sirachainan, N.; Tassaneetrithep, B.; Priengprom, T.; Kijporka, P.; Apiwattanakul, N. Enterovirus 71-Induced Autoimmune Hemolytic Anemia in a Boy. Clin Med Insights Case Rep 2022, 15, 11795476221132283. [Google Scholar] [CrossRef]
- Gao, Y.; Jin, H.; Nan, D.; Yu, W.; Zhang, J.; Yang, Y.; Hou, R.; Qin, R.; Hao, H.; Sun, Y.; et al. The Role of T Follicular Helper Cells and T Follicular Regulatory Cells in the Pathogenesis of Autoimmune Hemolytic Anemia. Sci Rep 2019, 9, 19767. [Google Scholar] [CrossRef] [PubMed]
- Toriani-Terenzi, C.; Fagiolo, E. IL-10 and the cytokine network in the pathogenesis of human autoimmune hemolytic anemia. Ann N Y Acad Sci 2005, 1051, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, E.; Elgohary, T.; Ibrahim, H. Naturally occurring regulatory T cells and interleukins 10 and 12 in the pathogenesis of idiopathic warm autoimmune hemolytic anemia. J Investig Allergol Clin Immunol 2011, 21, 297–304. [Google Scholar]
- Wang, S.; Qin, E.; Zhi, Y.; Hua, R. Severe autoimmune hemolytic anemia during pegylated interferon plus ribavirin treatment for chronic hepatitis C: a case report. Clin Case Rep 2017, 5, 1490–1492. [Google Scholar] [CrossRef]
- Xie, Y.; Shao, F.; Lei, J.; Huang, N.; Fan, Z.; Yu, H. Case report: A STAT1 gain-of-function mutation causes a syndrome of combined immunodeficiency, autoimmunity and pure red cell aplasia. Front Immunol 2022, 13, 928213. [Google Scholar] [CrossRef]
- Ciullini Mannurita, S.; Goda, R.; Schiavo, E.; Coniglio, M.L.; Azzali, A.; Fotzi, I.; Tondo, A.; Tintori, V.; Frenos, S.; Sanvito, M.C.; et al. Case Report: Signal Transducer and Activator of Transcription 3 Gain-of-Function and Spectrin Deficiency: A Life-Threatening Case of Severe Hemolytic Anemia. Front Immunol 2020, 11, 620046. [Google Scholar] [CrossRef]
- Poulet, F.M.; Penraat, K.; Collins, N.; Evans, E.; Thackaberry, E.; Manfra, D.; Engstrom, L.; Geissler, R.; Geraci-Erck, M.; Frugone, C.; et al. Drug-induced hemolytic anemia and thrombocytopenia associated with alterations of cell membrane lipids and acanthocyte formation. Toxicol Pathol 2010, 38, 907–922. [Google Scholar] [CrossRef]
- Greenberg, S.A. Dermatomyositis and type 1 interferons. Curr Rheumatol Rep 2010, 12, 198–203. [Google Scholar] [CrossRef]
- Zampieri, S.; Ghirardello, A.; Iaccarino, L.; Tarricone, E.; Gambari, P.F.; Doria, A. Anti-Jo-1 antibodies. Autoimmunity 2005, 38, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Fasth, A.E.; Dastmalchi, M.; Rahbar, A.; Salomonsson, S.; Pandya, J.M.; Lindroos, E.; Nennesmo, I.; Malmberg, K.J.; Soderberg-Naucler, C.; Trollmo, C.; et al. T cell infiltrates in the muscles of patients with dermatomyositis and polymyositis are dominated by CD28null T cells. J Immunol 2009, 183, 4792–4799. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, K.A.; Throm, A.A.; Pingel, J.T.; Saucier, N.; Zaher, H.S.; French, A.R. Expansion of a novel population of NK cells with low ribosome expression in juvenile dermatomyositis. Front Immunol 2022, 13, 1007022. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Tang, L.; Zhang, L.; Ren, Y.; Peng, H.; Xiao, Y.; Xu, J.; Mao, D.; Liu, L.; Liu, L. Identification of Biomarkers Associated With CD4(+) T-Cell Infiltration With Gene Coexpression Network in Dermatomyositis. Front Immunol 2022, 13, 854848. [Google Scholar] [CrossRef] [PubMed]
- Houtman, M.; Ekholm, L.; Hesselberg, E.; Chemin, K.; Malmstrom, V.; Reed, A.M.; Lundberg, I.E.; Padyukov, L. T-cell transcriptomics from peripheral blood highlights differences between polymyositis and dermatomyositis patients. Arthritis Res Ther 2018, 20, 188. [Google Scholar] [CrossRef]
- Piper, C.J.M.; Wilkinson, M.G.L.; Deakin, C.T.; Otto, G.W.; Dowle, S.; Duurland, C.L.; Adams, S.; Marasco, E.; Rosser, E.C.; Radziszewska, A.; et al. CD19(+)CD24(hi)CD38(hi) B Cells Are Expanded in Juvenile Dermatomyositis and Exhibit a Pro-Inflammatory Phenotype After Activation Through Toll-Like Receptor 7 and Interferon-alpha. Front Immunol 2018, 9, 1372. [Google Scholar] [CrossRef]
- Greenberg, S.A.; Higgs, B.W.; Morehouse, C.; Walsh, R.J.; Kong, S.W.; Brohawn, P.; Zhu, W.; Amato, A.; Salajegheh, M.; White, B.; et al. Relationship between disease activity and type 1 interferon- and other cytokine-inducible gene expression in blood in dermatomyositis and polymyositis. Genes Immun 2012, 13, 207–213. [Google Scholar] [CrossRef]
- Kishi, T.; Chipman, J.; Evereklian, M.; Nghiem, K.; Stetler-Stevenson, M.; Rick, M.E.; Centola, M.; Miller, F.W.; Rider, L.G. Endothelial Activation Markers as Disease Activity and Damage Measures in Juvenile Dermatomyositis. J Rheumatol 2020, 47, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Szodoray, P.; Alex, P.; Knowlton, N.; Centola, M.; Dozmorov, I.; Csipo, I.; Nagy, A.T.; Constantin, T.; Ponyi, A.; Nakken, B.; et al. Idiopathic inflammatory myopathies, signified by distinctive peripheral cytokines, chemokines and the TNF family members B-cell activating factor and a proliferation inducing ligand. Rheumatology (Oxford) 2010, 49, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Xia, L.; Lu, J. Pilot study of interleukin-27 in pathogenesis of dermatomyositis and polymyositis: associated with interstitial lung diseases. Cytokine 2012, 60, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Li, C.K.; Varsani, H.; Holton, J.L.; Gao, B.; Woo, P.; Wedderburn, L.R. MHC Class I overexpression on muscles in early juvenile dermatomyositis. J Rheumatol 2004, 31, 605–609. [Google Scholar] [PubMed]
- Nombel, A.; Fabien, N.; Coutant, F. Dermatomyositis With Anti-MDA5 Antibodies: Bioclinical Features, Pathogenesis and Emerging Therapies. Front Immunol 2021, 12, 773352. [Google Scholar] [CrossRef]
- Ladislau, L.; Suarez-Calvet, X.; Toquet, S.; Landon-Cardinal, O.; Amelin, D.; Depp, M.; Rodero, M.P.; Hathazi, D.; Duffy, D.; Bondet, V.; et al. JAK inhibitor improves type I interferon induced damage: proof of concept in dermatomyositis. Brain 2018, 141, 1609–1621. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.T.; Cho, M.L.; Park, Y.E.; Yoo, W.H.; Kim, J.H.; Oh, H.J.; Kim, D.S.; Baek, S.H.; Lee, S.H.; Lee, J.H.; et al. Expression of TLR2, TLR4, and TLR9 in dermatomyositis and polymyositis. Clin Rheumatol 2010, 29, 273–279. [Google Scholar] [CrossRef]
- Bellutti Enders, F.; van Wijk, F.; Scholman, R.; Hofer, M.; Prakken, B.J.; van Royen-Kerkhof, A.; de Jager, W. Correlation of CXCL10, tumor necrosis factor receptor type II, and galectin 9 with disease activity in juvenile dermatomyositis. Arthritis Rheumatol 2014, 66, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




