Submitted:
21 February 2024
Posted:
25 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
- a)
- HFE gene homozygosity – the greatest risk factor;
- b)
- positive family history for HH in the first-line relatives;
- c)
- Northern European ethnicity – the disease is less prevalent in populations of Afro-American, Hispanic, and Asian origin;
- d)
- male gender – men are susceptible to developing HH symptoms at an earlier age, however, females’ risk increases after menopause or a hysterectomy.
2. Gene mutations in hereditary hemochromatosis
3. The classification of hereditary hemochromatosis
4. Clinical presentation of hereditary hemochromatosis
4.1. Hereditary hemochromatosis and the skeletomuscular system
4.2. Hereditary hemochromatosis and the central nervous system
4.3. Hereditary hemochromatosis and the liver
4.4. Hereditary hemochromatosis and the cardiovascular system
4.5. Hereditary hemochromatosis and the endocrine system
4.6. Hereditary hemochromatosis and the skin
4.7. Hereditary hemochromatosis and the immune system
5. Diagnostic approach to the patient with suspected iron overload
5.1. Blood tests
- Serum transferrin saturation (TSAT) - the ratio between serum iron and total iron-binding capacity (TIBC), expressed as a percentage; increased transferrin saturation (>45%) is the earliest biochemical sign observed in all hemochromatosis subtypes [23]. However, other than iron overload disorders can cause increased transferrin saturation (eg. hemolysis, cytolysis) or decreased blood transferrin concentration (eg. hepatocellular failure, poor nutrition, proteinuria, genetic alterations) [76]. Normal or even low transferrin saturation can be observed in patients with ferroportin disease or hereditary aceruloplasminemia despite overt iron overload [77,78].
- Serum ferritin level (SF) - determining serum ferritin is the simplest and the most used diagnostic tool for body iron storage assessment, even if not a very specific method [79]. Since ferritin is an acute-phase protein, its concentration depends on many factors. Elevated SF levels (≥300 μg/L in men and ≥200 μg/L in women) require precise explanation before they are assigned to iron overload. Other conditions of hyperferritinemia such as metabolic syndrome, alcoholism, inflammation, and marked cytolysis should be ruled out [11]. Nevertheless, SF is an important prognostic factor in patients with HH. It is a predictor of advanced liver fibrosis and cirrhosis in patients with previously diagnosed congenital hemochromatosis.
5.2. Genetic testing
5.3. Additional diagnostic assessment for hereditary hemochromatosis
- Liver enzymes and function tests – the pattern of liver function alterations helps monitor liver damage in the course of HH.
- Liver biopsy – determining the hepatic iron concentration (HIC) is rarely required to establish a final HH diagnosis, therefore liver biopsy has been replaced by genetic testing and imaging findings. Seldom, it may be used to confirm or exclude other co-existing chronic liver diseases and to determine the degree of hepatic fibrosis, especially in C282Y homozygotes with SF >1000 ng/ml. The HIC may also be indicated in cases of suspected genetic iron overload with negative results towards common mutations including C282Y, H63D, and S65C. In remaining cases, liver biopsy is an option for individual consideration [84]. HIC determined in “good quality” biopsy specimen (i.e., sample gross weight ≥1 mg dry weight) is a reliable indicator of whole liver iron concentration [85].
- Magnetic resonance imaging (MRI) - the reference standard imaging modality for the detection and quantification of hepatic iron concentration (HIC); highly sensitive to the presence of tissue iron, the correlation between MRI findings and results of liver biopsy for the detection of moderate to high iron overload was excellent; the imagine technique increasingly adopted as a noninvasive alternative to biopsy for detection, severity grading, and treatment monitoring in patients with known or suspected iron overload [86,87].
6. Screening of healthy people for hereditary hemochromatosis
7. Treatment of hereditary hemochromatosis
7.1. Phlebotomy- the first-line treatment for iron depletion
7.2. Erythrocytapheresis as an alternative to phlebotomy
7.3. Iron chelators as a therapeutic alternative in hereditary hemochromatosis
7.4. Proton pump inhibitors - an adjunct therapy for hereditary hemochromatosis?
7.5. Liver transplantation for hereditary hemochromatosis
7.6. Recommended lifestyle and diet modifications in patients with hereditary hemochromatosis
- withdrawal of additional iron sources such as iron supplements, iron-containing multivitamins, and iron-fortified foods and drinks (eg. breakfast cereals, sports energy bars, etc.);
- recommendation of a varied vegetarian, semi-vegetarian or flexitarian diet;
- avoidance of vitamin C supplements which increase iron absorption, but there is no need to restrict natural vitamin C in their diet (fruit and vegetables); fruit juices should be consumed between meals;
- recommendation of complete alcohol abstinence as its hepatotoxic impact aggravates liver damage, there is no safe alcohol amount;
8. Hereditary hemochromatosis in women - pregnancy and fertility issues
9. Future directions in hereditary hemochromatosis
10. Conclusions
References
- Brissot, P.; Cavey, T.; Ropert, M.; Gaboriau, F.; Loréal, O. Hemochromatosis: A Model of Metal-Related Human Toxicosis. Environ. Sci. Pollut. Res. Int. 2018, 25, 2007–2013. [Google Scholar] [CrossRef] [PubMed]
- Bacon, B.R. Hemochromatosis: Discovery of the HFE Gene. Mo. Med. 2012, 109, 133–136. [Google Scholar] [PubMed]
- Von Recklinghausen: Uber Haemochromatose. Taggeblatt... - Google Scholar. Available online: https://scholar.google.com/scholar_lookup?journal=Tagebl+Versamml+Natur+%C3%84rzte+Heidelberg&title=%C3%9Cber+Hamochromatose&author=FD+von+Recklinghausen&volume=62&publication_year=1889&pages=324& (accessed on 18 February 2024).
- Feder, J.N.; Gnirke, A.; Thomas, W.; Tsuchihashi, Z.; Ruddy, D.A.; Basava, A. The Discovery of the New Haemochromatosis Gene. 1996. J Hepatol 2003, 38, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Fleming, R.E.; Ponka, P. Iron Overload in Human Disease. N. Engl. J. Med. 2012, 366, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Hepcidin and Iron Homeostasis. Biochim. Biophys. Acta 2012, 1823, 1434–1443. [Google Scholar] [CrossRef]
- Camaschella, C.; Nai, A.; Silvestri, L. Iron Metabolism and Iron Disorders Revisited in the Hepcidin Era. Haematologica 2020, 105, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A Red Carpet for Iron Metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef]
- Lal, A. Iron in Health and Disease: An Update. Indian. J. Pediatr. 2020, 87, 58–65. [Google Scholar] [CrossRef]
- Brissot, P.; Pietrangelo, A.; Adams, P.C.; de Graaff, B.; McLaren, C.E.; Loréal, O. Haemochromatosis. Nat. Rev. Dis. Primers 2018, 4, 18016. [Google Scholar] [CrossRef]
- Altés, A.; Sanz, C.; Bruguera, M. Hemocromatosis hereditaria. Probl. En. El Diagnóstico Y Tratamiento. Med. Clínica 2015, 144, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, T. Iron-Induced Oxidative Stress in Human Diseases. Cells 2022, 11, 2152. [Google Scholar] [CrossRef] [PubMed]
- Abe, C.; Miyazawa, T.; Miyazawa, T. Current Use of Fenton Reaction in Drugs and Food. Molecules 2022, 27, 5451. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, S.A.; Brown, K.E. Iron-Induced Liver Injury: A Critical Reappraisal. Int. J. Mol. Sci. 2019, 20, 2132. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox System in Health and Disease: The Latest Update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.J.; Bardou-Jacquet, E. Revisiting Hemochromatosis: Genetic vs. Phenotypic Manifestations. Ann. Transl. Med. 2021, 9, 731. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, J.; Mukhopadhya, A. European Association for Study of the Liver (EASL) Clinical Practice Guidelines on Haemochromatosis. Frontline Gastroenterol. 2023, 14, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.C.; Richard, L.; Weir, M.; Speechley, M. Survival and Development of Health Conditions after Iron Depletion Therapy in C282Y-Linked Hemochromatosis Patients. Can. Liver J. 2021, 4, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Brissot, P.; Troadec, M.-B.; Loréal, O. Intestinal Absorption of Iron in HFE-1 Hemochromatosis: Local or Systemic Process? J Hepatol 2004, 40, 702–709. [Google Scholar] [CrossRef]
- Barton, J.C.; McLaren, C.E.; Chen, W.; Ramm, G.A.; Anderson, G.J.; Powell, L.W.; Subramaniam, V.N.; Adams, P.C.; Phatak, P.D.; Gurrin, L.C.; et al. Cirrhosis in Hemochromatosis: Independent Risk Factors in 368 HFE p.C282Y Homozygotes. Ann. Hepatol. 2018, 17, 871–879. [Google Scholar] [CrossRef]
- Kowdley, K.V.; Brown, K.E.; Ahn, J.; Sundaram, V. ACG Clinical Guideline: Hereditary Hemochromatosis. Am. J. Gastroenterol. 2019, 114, 1202–1218. [Google Scholar] [CrossRef] [PubMed]
- Cancado, R.D.; Alvarenga, A.M.; Santos, P.C.J. HFE Hemochromatosis: An Overview about Therapeutic Recommendations. Hematol., Transfus. Cell Ther. 2022, 44, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Turshudzhyan, A.; Wu, D.C.; Wu, G.Y. Primary Non-HFE Hemochromatosis: A Review. J. Clin. Transl. Hepatol. 2023, 11, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Girelli, D.; Busti, F.; Brissot, P.; Cabantchik, I.; Muckenthaler, M.U.; Porto, G. Hemochromatosis Classification: Update and Recommendations by the BIOIRON Society. Blood 2022, 139, 3018–3029. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver EASL Clinical Practice Guidelines on Haemochromatosis. J. Hepatol. 2022, 77, 479–502. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.H.G.; Ramm, G.A.; Bridle, K.R.; Nicoll, A.J.; Delatycki, M.B.; Olynyk, J.K. Clinical Practice Guidelines on Hemochromatosis: Asian Pacific Association for the Study of the Liver. Hepatol. Int. 2023, 17, 522–541. [Google Scholar] [CrossRef] [PubMed]
- Delatycki, M.B.; Powell, L.W.; Allen, K.J. Hereditary Hemochromatosis Genetic Testing of At-Risk Children: What Is the Appropriate Age? Genet Test 2004, 8, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, H.R. HEMOCHROMATOSIS AND ARTHRITIS. Arthritis Rheum. 1964, 7, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.; Prideaux, A.; Kiely, P. Haemochromatosis: Unexplained Metacarpophalangeal or Ankle Arthropathy Should Prompt Diagnostic Tests: Findings from Two UK Observational Cohort Studies. Scand. J. Rheumatol. 2017, 46, 69–74. [Google Scholar] [CrossRef]
- Calori, S.; Comisi, C.; Mascio, A.; Fulchignoni, C.; Pataia, E.; Maccauro, G.; Greco, T.; Perisano, C. Overview of Ankle Arthropathy in Hereditary Hemochromatosis. Med Sci (Basel) 2023, 11, 51. [Google Scholar] [CrossRef]
- Adams, P.C.; Kertesz, A.E.; Valberg, L.S. Clinical Presentation of Hemochromatosis: A Changing Scene. Am. J. Med. 1991, 90, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Loughnan, R.; Ahern, J.; Tompkins, C.; Palmer, C.E.; Iversen, J.; Thompson, W.K.; Andreassen, O.; Jernigan, T.; Sugrue, L.; Dale, A.; et al. Association of Genetic Variant Linked to Hemochromatosis With Brain Magnetic Resonance Imaging Measures of Iron and Movement Disorders. JAMA Neurol. 2022, 79, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Rizek, P.; Sadikovic, B.; Adams, P.C.; Jog, M. Movement Disorders Associated With Hemochromatosis. Can. J. Neurol. Sci. 2016, 43, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Topiwala, A.; Wang, C.; Ebmeier, K.P.; Burgess, S.; Bell, S.; Levey, D.F.; Zhou, H.; McCracken, C.; Roca-Fernández, A.; Petersen, S.E.; et al. Associations between Moderate Alcohol Consumption, Brain Iron, and Cognition in UK Biobank Participants: Observational and Mendelian Randomization Analyses. PLoS Med. 2022, 19, e1004039. [Google Scholar] [CrossRef] [PubMed]
- Harrison-Findik, D.D.; Schafer, D.; Klein, E.; Timchenko, N.A.; Kulaksiz, H.; Clemens, D.; Fein, E.; Andriopoulos, B.; Pantopoulos, K.; Gollan, J. Alcohol Metabolism-Mediated Oxidative Stress down-Regulates Hepcidin Transcription and Leads to Increased Duodenal Iron Transporter Expression. J. Biol. Chem. 2006, 281, 22974–22982. [Google Scholar] [CrossRef] [PubMed]
- Harrison-Findik, D.D. Role of Alcohol in the Regulation of Iron Metabolism. World J. Gastroenterol. 2007, 13, 4925–4930. [Google Scholar] [CrossRef] [PubMed]
- Sadasivam, N.; Kim, Y.-J.; Radhakrishnan, K.; Kim, D.-K. Oxidative Stress, Genomic Integrity, and Liver Diseases. Molecules 2022, 27, 3159. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, L.M.; Dixon, J.L.; Purdie, D.M.; Powell, L.W.; Crawford, D.H.G. Excess Alcohol Greatly Increases the Prevalence of Cirrhosis in Hereditary Hemochromatosis. Gastroenterology 2002, 122, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.C.; Agnew, S. Alcoholism in Hereditary Hemochromatosis Revisited: Prevalence and Clinical Consequences among Homozygous Siblings. Hepatology 1996, 23, 724–727. [Google Scholar] [CrossRef]
- Alvi, A.T.; Santiago, L.E.; Nadeem, Z.; Chaudhry, A. Fulminant Hepatic Failure With Minimal Alcohol Consumption in a 25-Year-Old Female With Hereditary Hemochromatosis: A Rare Case. Cureus 2023, 15, e44544. [Google Scholar] [CrossRef]
- Kew, M.C. Hepatic Iron Overload and Hepatocellular Carcinoma. Liver Cancer 2014, 3, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, B.; Pammer, L.M.; Pfeifer, B.; Neururer, S.; Troppmair, M.R.; Panzer, M.; Wagner, S.; Pertler, E.; Gieger, C.; Kronenberg, F.; et al. Penetrance, Cancer Incidence and Survival in HFE Haemochromatosis-A Population-Based Cohort Study. Liver Int. 2024. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kaur, H.; Lerner, R.G.; Patel, R.; Rafiyath, S.M.; Singh Lamba, G. Hepatocellular Carcinoma in Non-Cirrhotic Liver Without Evidence of Iron Overload in a Patient with Primary Hemochromatosis. Review. J. Gastrointest. Canc 2012, 43, 36–39. [Google Scholar] [CrossRef]
- Bardou-Jacquet, E.; Morandeau, E.; Anderson, G.J.; Ramm, G.A.; Ramm, L.E.; Morcet, J.; Bouzille, G.; Dixon, J.; Clouston, A.D.; Lainé, F.; et al. Regression of Fibrosis Stage With Treatment Reduces Long-Term Risk of Liver Cancer in Patients With Hemochromatosis Caused by Mutation in HFE. Clin. Gastroenterol. Hepatol. 2020, 18, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Daniłowicz-Szymanowicz, L.; Świątczak, M.; Sikorska, K.; Starzyński, R.R.; Raczak, A.; Lipiński, P. Pathogenesis, Diagnosis, and Clinical Implications of Hereditary Hemochromatosis—The Cardiological Point of View. Diagnostics (Basel) 2021, 11, 1279. [Google Scholar] [CrossRef] [PubMed]
- Seferović, P.M.; Polovina, M.; Bauersachs, J.; Arad, M.; Ben Gal, T.; Lund, L.H.; Felix, S.B.; Arbustini, E.; Caforio, A.L.P.; Farmakis, D.; et al. Heart Failure in Cardiomyopathies: A Position Paper from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 553–576. [Google Scholar] [CrossRef] [PubMed]
- Rozpoznanie i leczenie hemochromatozy dziedzicznej. Podsumowanie wytycznych American College of Gastroenterology (AGA). Available online: http://www.mp.pl/social/article/260811 (accessed on 18 February 2024).
- Cortés, P.; Elsayed, A.A.; Stancampiano, F.F.; Barusco, F.M.; Shapiro, B.P.; Bi, Y.; Heckman, M.G.; Peng, Z.; Kempaiah, P.; Palmer, W.C. Clinical and Genetic Predictors of Cardiac Dysfunction Assessed by Echocardiography in Patients with Hereditary Hemochromatosis. Int. J. Cardiovasc. Imaging 2024, 40, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.-H.; Fefelova, N.; Pamarthi, S.H.; Gwathmey, J.K. Molecular Mechanisms of Ferroptosis and Relevance to Cardiovascular Disease. Cells 2022, 11, 2726. [Google Scholar] [CrossRef]
- Wood, M.J.; Powell, L.W.; Dixon, J.L.; Ramm, G.A. Clinical Cofactors and Hepatic Fibrosis in Hereditary Hemochromatosis: The Role of Diabetes Mellitus. Hepatology 2012, 56, 904–911. [Google Scholar] [CrossRef]
- Altamura, S.; Müdder, K.; Schlotterer, A.; Fleming, T.; Heidenreich, E.; Qiu, R.; Hammes, H.-P.; Nawroth, P.; Muckenthaler, M.U. Iron Aggravates Hepatic Insulin Resistance in the Absence of Inflammation in a Novel Db/Db Mouse Model with Iron Overload. Mol. Metab. 2021, 51, 101235. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.V.; Lorenzo, F.R.; McClain, D.A. Iron and the Pathophysiology of Diabetes. Annu. Rev. Physiol. 2023, 85, 339–362. [Google Scholar] [CrossRef] [PubMed]
- Asberg, A.; Hveem, K.; Thorstensen, K.; Ellekjter, E.; Kannelønning, K.; Fjøsne, U.; Halvorsen, T.B.; Smethurst, H.B.; Sagen, E.; Bjerve, K.S. Screening for Hemochromatosis: High Prevalence and Low Morbidity in an Unselected Population of 65,238 Persons. Scand. J. Gastroenterol. 2001, 36, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Felitti, V.J.; Koziol, J.A.; Ho, N.J.; Gelbart, T. Penetrance of 845G--> A (C282Y) HFE Hereditary Haemochromatosis Mutation in the USA. Lancet 2002, 359, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.J.; Gurrin, L.C.; Constantine, C.C.; Osborne, N.J.; Delatycki, M.B.; Nicoll, A.J.; McLaren, C.E.; Bahlo, M.; Nisselle, A.E.; Vulpe, C.D.; et al. Iron-Overload-Related Disease in HFE Hereditary Hemochromatosis. N. Engl. J. Med. 2008, 358, 221–230. [Google Scholar] [CrossRef] [PubMed]
- McLaren, G.D.; McLaren, C.E.; Adams, P.C.; Barton, J.C.; Reboussin, D.M.; Gordeuk, V.R.; Acton, R.T.; Harris, E.L.; Speechley, M.R.; Sholinsky, P.; et al. Clinical Manifestations of Hemochromatosis in HFE C282Y Homozygotes Identified by Screening. Can. J. Gastroenterol. 2008, 22, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.C.; Acton, R.T. Diabetes in HFE Hemochromatosis. J. Diabetes Res. 2017, 2017, 9826930. [Google Scholar] [CrossRef]
- Pelusi, C.; Gasparini, D.I.; Bianchi, N.; Pasquali, R. Endocrine Dysfunction in Hereditary Hemochromatosis. J. Endocrinol. Invest. 2016, 39, 837–847. [Google Scholar] [CrossRef]
- Parkash, O.; Akram, M. Hereditary Hemochromatosis. J. Coll. Physicians Surg. Pak. 2015, 25, 644–647. [Google Scholar]
- Chevrant-Breton, J.; Simon, M.; Bourel, M.; Ferrand, B. Cutaneous Manifestations of Idiopathic Hemochromatosis. Study of 100 Cases. Arch. Dermatol. 1977, 113, 161–165. [Google Scholar] [CrossRef]
- Varada, N.; Tun, K.M.; Chang, M.J.; Bomberger, S.; Calagari, R.; Varada, N.; Tun, K.M.; Chang, M.J.; Bomberger, S.; Calagari, R. A Rare Case of Hereditary Hemochromatosis Presenting With Porphyria Cutanea Tarda. Cureus 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.V.; Ray, J.M.; Bacon, B.R. Sporadic Porphyria Cutanea Tarda as the Initial Manifestation of Hereditary Hemochromatosis. ACG Case Rep. J. 2019, 6, e00247. [Google Scholar] [CrossRef] [PubMed]
- Sredoja Tišma, V.; Bulimbašić, S.; Jaganjac, M.; Stjepandić, M.; Larma, M. Progressive Pigmented Purpuric Dermatitis and Alopecia Areata as Unusual Skin Manifestations in Recognizing Hereditary Hemochromatosis. Acta Dermatovenerol. Croat. 2012, 20, 181–186. [Google Scholar] [PubMed]
- Leung, B.; Lindley, L.; Cruz, P.D.; Cole, S.; Ayoade, K.O. Iron Screening in Alopecia Areata Patients May Catch Hereditary Hemochromatosis Early. Cutis 2022, 110, E30–E32. [Google Scholar] [CrossRef] [PubMed]
- Reuben, A.; Chung, J.W.; Lapointe, R.; Santos, M.M. The Hemochromatosis Protein HFE 20 Years Later: An Emerging Role in Antigen Presentation and in the Immune System. Immun. Inflamm. Dis. 2017, 5, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Porto, G.; Sousa, M.D. Iron Overload and Immunity. World J. Gastroenterol. 2007, 13, 4707–4715. [Google Scholar] [CrossRef] [PubMed]
- Hubacek, J.A.; Philipp, T.; Adamkova, V.; Majek, O.; Dusek, L. A Haemochromatosis-Causing HFE Mutation Is Associated with SARS-CoV-2 Susceptibility in the Czech Population. Clin. Chim. Acta 2023, 538, 211–215. [Google Scholar] [CrossRef]
- Ristić, S.; Milić, S.; Tatalović, T.; Bilobrk, M.; Rončević, D.; Ćurko-Cofek, B.; Barac-Latas, V.; Čizmarević, N.S. The Influence of Hemochromatosis Gene (HFE) Mutations on SARS-CoV-2 Susceptibility and COVID-19 Severity. Balkan Med. J. 2023, 40, 229–231. [Google Scholar] [CrossRef]
- Sazawal, S.; Black, R.E.; Kabole, I.; Dutta, A.; Dhingra, U.; Ramsan, M. Effect of Iron/Folic Acid Supplementation on the Outcome of Malaria Episodes Treated with Sulfadoxine-Pyrimethamine. Malar. Res. Treat. 2014, 2014, 625905. [Google Scholar] [CrossRef]
- Carcillo, J.A.; Sward, K.; Halstead, E.S.; Telford, R.; Jimenez-Bacardi, A.; Shakoory, B.; Simon, D.; Hall, M. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network Investigators A Systemic Inflammation Mortality Risk Assessment Contingency Table for Severe Sepsis. Pediatr. Crit. Care Med. 2017, 18, 143–150. [Google Scholar] [CrossRef]
- Bennett, T.D.; Hayward, K.N.; Farris, R.W.D.; Ringold, S.; Wallace, C.A.; Brogan, T.V. Very High Serum Ferritin Levels Are Associated with Increased Mortality and Critical Care in Pediatric Patients. Pediatr. Crit. Care Med. 2011, 12, e233–e236. [Google Scholar] [CrossRef] [PubMed]
- Kernan, K.F.; Carcillo, J.A. Hyperferritinemia and Inflammation. Int. Immunol. 2017, 29, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Senussi, N.H.; Fertrin, K.Y.; Kowdley, K.V. Iron Overload Disorders. Hepatol. Commun. 2022, 6, 1842–1854. [Google Scholar] [CrossRef] [PubMed]
- Beaumont-Epinette, M.-P.; Delobel, J.-B.; Ropert, M.; Deugnier, Y.; Loréal, O.; Jouanolle, A.-M.; Brissot, P.; Bardou-Jacquet, E. Hereditary Hypotransferrinemia Can Lead to Elevated Transferrin Saturation and, When Associated to HFE or HAMP Mutations, to Iron Overload. Blood Cells Mol. Dis. 2015, 54, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Montosi, G.; Donovan, A.; Totaro, A.; Garuti, C.; Pignatti, E.; Cassanelli, S.; Trenor, C.C.; Gasparini, P.; Andrews, N.C.; Pietrangelo, A. Autosomal-Dominant Hemochromatosis Is Associated with a Mutation in the Ferroportin (SLC11A3) Gene. J. Clin. Investig. 2001, 108, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, H. Aceruloplasminemia. Neuropathology 2015, 35, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Bruzzese, A.; Martino, E.A.; Mendicino, F.; Lucia, E.; Olivito, V.; Bova, C.; Filippelli, G.; Capodanno, I.; Neri, A.; Morabito, F.; et al. Iron Chelation Therapy. Eur. J. Haematol. 2023, 110, 490–497. [Google Scholar] [CrossRef]
- Liu Yin, J.; Cussen, C.; Harrington, C.; Foskett, P.; Raja, K.; Ala, A. Guideline Review: European Association for the Study of Liver (EASL) Clinical Practice Guidelines on Haemochromatosis. J. Clin. Exp. Hepatol. 2023, 13, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Brissot, P.; Brissot, E. What’s Important and New in Hemochromatosis? Clin. Hematol. Int. 2020, 2, 143–148. [Google Scholar] [CrossRef]
- Milic, S.; Mikolasevic, I.; Orlic, L.; Devcic, E.; Starcevic-Cizmarevic, N.; Stimac, D.; Kapovic, M.; Ristic, S. The Role of Iron and Iron Overload in Chronic Liver Disease. Med. Sci. Monit. 2016, 22, 2144–2151. [Google Scholar] [CrossRef]
- Pinyopornpanish, K.; Tantiworawit, A.; Leerapun, A.; Soontornpun, A.; Thongsawat, S. Secondary Iron Overload and the Liver: A Comprehensive Review. J. Clin. Transl. Hepatol. 2023, 11, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Bassett, M.L.; Hickman, P.E.; Dahlstrom, J.E. The Changing Role of Liver Biopsy in Diagnosis and Management of Haemochromatosis. Pathology 2011, 43, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Urru, S.A.M.; Tandurella, I.; Capasso, M.; Usala, E.; Baronciani, D.; Giardini, C.; Visani, G.; Angelucci, E. Reproducibility of Liver Iron Concentration Measured on a Biopsy Sample: A Validation Study in Vivo. Am. J. Hematol. 2015, 90, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Golfeyz, S.; Lewis, S.; Weisberg, I.S. Hemochromatosis: Pathophysiology, Evaluation, and Management of Hepatic Iron Overload with a Focus on MRI. Expert. Rev. Gastroenterol. Hepatol. 2018, 12, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Reeder, S.B.; Yokoo, T.; França, M.; Hernando, D.; Alberich-Bayarri, Á.; Alústiza, J.M.; Gandon, Y.; Henninger, B.; Hillenbrand, C.; Jhaveri, K.; et al. Quantification of Liver Iron Overload with MRI: Review and Guidelines from the ESGAR and SAR. Radiology 2023, 307, e221856. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, N.; Herich, L. Measurement of Liver Iron Concentration by Superconducting Quantum Interference Device Biomagnetic Liver Susceptometry Validates Serum Ferritin as Prognostic Parameter for Allogeneic Stem Cell Transplantation. Eur. J. Haematol. 2016, 97, 336–341. [Google Scholar] [CrossRef] [PubMed]
- European Association For The Study Of The Liver EASL Clinical Practice Guidelines for HFE Hemochromatosis. J. Hepatol. 2010, 53, 3–22. [CrossRef] [PubMed]
- Savatt, J.M.; Johns, A.; Schwartz, M.L.B.; McDonald, W.S.; Salvati, Z.M.; Oritz, N.M.; Masnick, M.; Hatchell, K.; Hao, J.; Buchanan, A.H.; et al. Testing and Management of Iron Overload After Genetic Screening-Identified Hemochromatosis. JAMA Netw. Open 2023, 6, e2338995. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Kalafateli, M.; Thorburn, D.; Davidson, B.R.; Tsochatzis, E.; Gurusamy, K.S. Interventions for Hereditary Haemochromatosis: An Attempted Network Meta-Analysis. Cochrane Database Syst. Rev. 2017, 3, CD011647. [Google Scholar] [CrossRef]
- Adams, P.; Altes, A.; Brissot, P.; Butzeck, B.; Cabantchik, I.; Cançado, R.; Distante, S.; Evans, P.; Evans, R.; Ganz, T.; et al. Therapeutic Recommendations in HFE Hemochromatosis for p.Cys282Tyr (C282Y/C282Y) Homozygous Genotype. Hepatol. Int. 2018, 12, 83–86. [Google Scholar] [CrossRef]
- Stussi, G.; Buser, A.; Holbro, A. Red Blood Cells: Exchange, Transfuse, or Deplete. Transfus. Med. Hemother 2019, 46, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Rombout-Sestrienkova, E.; Brandts, L.; Koek, G.H.; van Deursen, C.Th.B.M. Patients with Hereditary Hemochromatosis Reach Safe Range of Transferrin Saturation Sooner with Erythrocytaphereses than with Phlebotomies. J. Clin. Apher. 2022, 37, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Sundic, T.; Hervig, T.; Hannisdal, S.; Assmus, J.; Ulvik, R.J.; Olaussen, R.W.; Berentsen, S. Erythrocytapheresis Compared with Whole Blood Phlebotomy for the Treatment of Hereditary Haemochromatosis. Blood Transfus. 2014, 12, s84–s89. [Google Scholar] [CrossRef] [PubMed]
- Cançado, R.; Melo, M.R.; de Moraes Bastos, R.; Santos, P.C.J.L.; Guerra-Shinohara, E.M.; Chiattone, C.; Ballas, S.K. Deferasirox in Patients with Iron Overload Secondary to Hereditary Hemochromatosis: Results of a 1-Yr Phase 2 Study. Eur. J. Haematol. 2015, 95, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Entezari, S.; Haghi, S.M.; Norouzkhani, N.; Sahebnazar, B.; Vosoughian, F.; Akbarzadeh, D.; Islampanah, M.; Naghsh, N.; Abbasalizadeh, M.; Deravi, N. Iron Chelators in Treatment of Iron Overload. J. Toxicol. 2022, 2022, 4911205. [Google Scholar] [CrossRef] [PubMed]
- Gomber, S.; Jain, P.; Sharma, S.; Narang, M. Comparative Efficacy and Safety of Oral Iron Chelators and Their Novel Combination in Children with Thalassemia. Indian. Pediatr. 2016, 53, 207–210. [Google Scholar] [CrossRef]
- Angelucci, E. Another Step Forward in Iron Chelation Therapy. Acta Haematol. 2015, 134, 231–232. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghe, C.N.; Kontoghiorghes, G.J. New Developments and Controversies in Iron Metabolism and Iron Chelation Therapy. World J. Methodol. 2016, 6, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bauchner, H.; Fontanarosa, P.B.; Golub, R.M. Evaluation of the Trial to Assess Chelation Therapy (TACT): The Scientific Process, Peer Review, and Editorial Scrutiny. JAMA 2013, 309, 1291–1292. [Google Scholar] [CrossRef]
- Mobarra, N.; Shanaki, M.; Ehteram, H.; Nasiri, H.; Sahmani, M.; Saeidi, M.; Goudarzi, M.; Pourkarim, H.; Azad, M. A Review on Iron Chelators in Treatment of Iron Overload Syndromes. Int. J. Hematol. Oncol. Stem Cell Res. 2016, 10, 239–247. [Google Scholar]
- Vanclooster, A.; van Deursen, C.; Jaspers, R.; Cassiman, D.; Koek, G. Proton Pump Inhibitors Decrease Phlebotomy Need in HFE Hemochromatosis: Double-Blind Randomized Placebo-Controlled Trial. Gastroenterology 2017, 153, 678–680. [Google Scholar] [CrossRef]
- Dirweesh, A.; Anugwom, C.M.; Li, Y.; Vaughn, B.P.; Lake, J. Proton Pump Inhibitors Reduce Phlebotomy Burden in Patients with HFE-Related Hemochromatosis: A Systematic Review and Meta-Analysis. Eur. J. Gastroenterol. Hepatol. 2021, 33, 1327–1331. [Google Scholar] [CrossRef]
- Lymberopoulos, P.; Prakash, S.; Shaikh, A.; Bhatnagar, A.; Allam, A.K.; Goli, K.; Goss, J.A.; Kanwal, F.; Rana, A.; Kowdley, K.V.; et al. Long-Term Outcomes and Trends in Liver Transplantation for Hereditary Hemochromatosis in the United States. Liver Transpl. 2023, 29, 15–25. [Google Scholar] [CrossRef]
- McPhail, M.J.W.; Khorsandi, S.E.; Abbott, L.; Al-Kadhimi, G.; Kane, P.; Karani, J.; O’Grady, J.; Heaton, N.; Bomford, A.; Suddle, A. Modern Outcomes Following Treatment of Hepatocellular Carcinoma in Hereditary Hemochromatosis: A Matched Cohort Study. Am. J. Clin. Oncol. 2019, 42, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Charlebois, E.; Pantopoulos, K. Nutritional Aspects of Iron in Health and Disease. Nutrients 2023, 15, 2441. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, A.J.; Gajewska, J.; Chajęcka-Wierzchowska, W.; Załuski, D.; Zadernowska, A. Prevalence of Listeria Monocytogenes and Other Listeria Species in Fish, Fish Products and Fish Processing Environment: A Systematic Review and Meta-Analysis. Sci. Total Environ. 2024, 907, 167912. [Google Scholar] [CrossRef] [PubMed]
- Lasagabaster, A.; Jiménez, E.; Lehnherr, T.; Miranda-Cadena, K.; Lehnherr, H. Bacteriophage Biocontrol to Fight Listeria Outbreaks in Seafood. Food Chem. Toxicol. 2020, 145, 111682. [Google Scholar] [CrossRef]
- Krahulcová, M.; Cverenkárová, K.; Koreneková, J.; Oravcová, A.; Koščová, J.; Bírošová, L. Occurrence of Antibiotic-Resistant Bacteria in Fish and Seafood from Slovak Market. Foods 2023, 12, 3912. [Google Scholar] [CrossRef]
- Milman, N.T. Managing Genetic Hemochromatosis: An Overview of Dietary Measures, Which May Reduce Intestinal Iron Absorption in Persons With Iron Overload. Gastroenterology Res. 2021, 14, 66–80. [Google Scholar] [CrossRef]
- Moirand, R.; Adams, P.C.; Bicheler, V.; Brissot, P.; Deugnier, Y. Clinical Features of Genetic Hemochromatosis in Women Compared with Men. Ann. Intern. Med. 1997, 127, 105–110. [Google Scholar] [CrossRef]
- Durazzo, M.; Belci, P.; Collo, A.; Prandi, V.; Pistone, E.; Martorana, M.; Gambino, R.; Bo, S. Gender Specific Medicine in Liver Diseases: A Point of View. World J. Gastroenterol. 2014, 20, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Zhang, J.; Goldenberg, I.; Gill, S.; Saeed, H.; Iyer, C.; Dunnigan, K. Maternal and Prenatal Outcomes of Hemochromatosis in Pregnancy: A Population-Based Study. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102221. [Google Scholar] [CrossRef] [PubMed]
- Scotet, V.; Saliou, P.; Uguen, M.; L’Hostis, C.; Merour, M.-C.; Triponey, C.; Chanu, B.; Nousbaum, J.-B.; Le Gac, G.; Ferec, C. Do Pregnancies Reduce Iron Overload in HFE Hemochromatosis Women? Results from an Observational Prospective Study. BMC Pregnancy Childbirth 2018, 18, 53. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.V.; Olsson, I.A.S.; Porto, G.; Rodrigues, P.N. Hemochromatosis and Pregnancy: Iron Stores in the Hfe-/- Mouse Are Not Reduced by Multiple Pregnancies. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G525–G529. [Google Scholar] [CrossRef]
- Sayaf, K.; Gabbia, D.; Russo, F.P.; De Martin, S. The Role of Sex in Acute and Chronic Liver Damage. Int. J. Mol. Sci. 2022, 23, 10654. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, S. Beyond the X Factor: Relevance of Sex Hormones in NAFLD Pathophysiology. Cells 2021, 10, 2502. [Google Scholar] [CrossRef] [PubMed]
- Bizzaro, D.; Crescenzi, M.; Di Liddo, R.; Arcidiacono, D.; Cappon, A.; Bertalot, T.; Amodio, V.; Tasso, A.; Stefani, A.; Bertazzo, V.; et al. Sex-Dependent Differences in Inflammatory Responses during Liver Regeneration in a Murine Model of Acute Liver Injury. Clin Sci (Lond) 2018, 132, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Shamas, A.G. Primary Hereditary Haemochromatosis and Pregnancy. GastroHep 2023, 2023, e2674203. [Google Scholar] [CrossRef]
- Phatak, P.D.; Bonkovsky, H.L.; Kowdley, K.V. Hereditary Hemochromatosis: Time for Targeted Screening. Ann. Intern. Med. 2008, 149, 270–272. [Google Scholar] [CrossRef]
| Novel HH classification | Molecular pattern | Comments |
|---|---|---|
| HFE-related | p.Cys282Tyr homozygosity or compound heterozygosity of p.Cys282Tyr with other rare HFE variants or HFE deletion |
Low penetrance; consider the presence of host-related or environmental cofactors for iron overload. In subjects with other HFE genotypes (eg, p.Cys282Tyr/His63Asp compound heterozygosity or p.His63Asp homozygosity) consider second-line genetic testing for rarer variants |
| Non-HFE-related | Rare variants in “non-HFE” genes: • HJV-related • HAMP-related • TFR2-related • SLC40A1 (GOF)-related |
Potentially, mutations in any hepcidin-regulatory gene may be causative (the effects of novel mutations should be confirmed through functional and epidemiological studies) Molecular subtypes characterization only in specialized centers, but the diagnosis of non-HFE related HH is sufficient to start phlebotomies at nonspecialized centers* |
| Digenic** | Double heterozygosity and/or double homozygosity/heterozygosity for mutations in 2 different genes involved in iron metabolism (HFE and/or non-HFE) |
More commonly, p.Cys282Tyr mutation in the HFE gene might coexist with mutations in other genes; rarely, both mutations involve non-HFE genes |
| Molecularly undefined | Molecular characterization (still) not available after sequencing of known genes (provisional diagnosis) | Patients should be referred (or DNA should be sent) to specialized centers |
| Organ/system | Symptoms |
|---|---|
| Skeletomuscular system | arthralgia, arthritis, chondrocalcinosis, reduced bone mineral density, fatigue, weakness |
| Central nervous system | lack of energy (lethargy), irritability, memory fog, mood swings, depression, anxiety, movement disorders, tremors |
| Liver | high liver enzymes, hepatosplenomegaly, liver fibrosis and cirrhosis, hepatocellular carcinoma |
| Cardiovascular system | cardiomyopathy, arrhythmia, heart failure |
| Endocrine system | hypogonadism, testicular atrophy, reproductive disorders with loss of libido, impotence, amenorrhea, hyperglycemia, diabetes mellitus, hypopituitarism |
| Skin | bronze or gray skin tone (hypermelanotic pigmentation), hair loss, porphyria cutanea tarda (?) |
| Immune system | immune defects, infections |
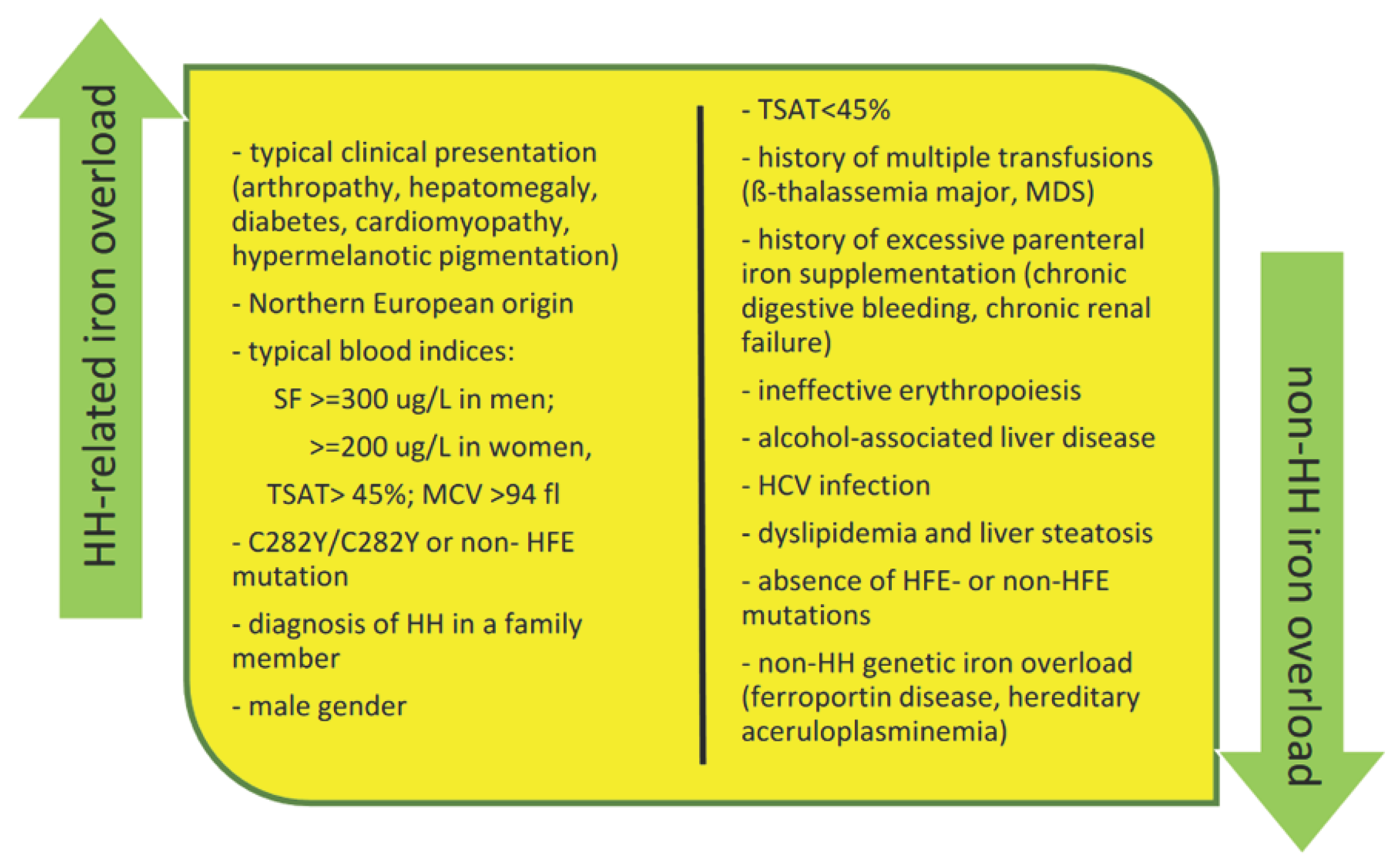
| Risk factors related to severe liver disease |
|---|
|
| Study Title (brief description) |
NCT Number | Conditions | Interventions | Study Type | Location |
|---|---|---|---|---|---|
| Impact of Transferrin Saturation Guided Maintenance Treatment on Quality of Life in HFE Haemochromatosis (biological test results guide the treatment modification: either time schedule or volume of bloodletting according to randomization group (patients treated with bloodletting according to current guidelines “ferritin alone” versus patients treated with bloodletting according to “transferrin saturation and serum ferritin”) |
NCT04779593 | Hemochromatosis | Clinical examination SF36 Quest AIMS2_SF Quest Bloodletting - experimental group 11 more |
Interventional | France |
| Treatment of Hemochromatosis (comparison of ferritin and MCV in guiding phlebotomy in pts with HH) |
NCT00007150 | Hemochromatosis | Phlebotomy | Interventional | United States |
| MRI QSM Imaging for Iron Overload (validation of a novel technique i.e., MRI-based QSM of the abdomen, for non-invasive assessment of liver iron deposition) |
NCT04631718 | Hemochromatosis Iron Overload |
Radiation: MRI-based QSM | Observational | United States |
| Iron Overload and Endocrinological Diseases (evaluation of the prevalence of endocrinological diseases in adult pts with iron overload due to β-thalassemia or hemochromatosis and their impact on quality of life) |
NCT06137079 | Thalassemia (major or intermedia) Hereditary Hemochromatosis |
Observational | Italy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




