1. Introduction
Fenretinide (
Figure 1), N-(4-hydroxyphenyl)retinamide (4-HPR) is a synthetic retinoid chemically related to all-trans-retinoic acid (ATRA), the acidic form of vitamin A [
1].
Although it shares with ATRA the property of specific binding to retinoic receptors, its spectrum of activity is not confined to promyelocytic leukemias, but is extended to solid neoplasms including ovarian, prostate, cervical, lung, renal, bladder, breast, glioma, skin, head and neck carcinoma, non-Hodgkin’s lymphoma, neuroblastoma, and Ewing’s sarcoma [
2,
3].
The main mechanism of action of 4-HPR is the induction of apoptosis rather than cellular differentiation, which in contrast is mainly induced by ATRA. 4-HPR induces tumor cell apoptosis through the generation of radical oxygen species, the imbalance of ceramides/dehydroceramides ratio and the induction of RARβ. 4-HPR has also shown antiangiogenic and anti-metastatic effects in different tumour models. For these peculiar characteristics, 4-HPR is currently the most studied retinoid both as chemopreventive and chemotherapeutic agent [
2,
3].
4-HPR has been evaluated in solid tumors and hematological malignancies by several clinical trials [
4] which demonstrated its excellent tolerability but a limited therapeutic efficacy due to its poor bioavailability. In fact, the scant aqueous solubility of 4-HPR restrains its absorption thus preventing the achievement of plasma concentrations suitable to elicit a therapeutic response. The clinical studies with 4-HPR have been mainly conducted by conventional formulations such as soft gelatin capsules, available at the National Cancer Institute, containing 100 mg 4-HPR in corn oil and polysorbate 80. Multiple and protracted administrations of 4-HPR by the capsules provided low plasma concentrations of the drug, always below the minimum threshold required for therapeutic activity [
4], demonstrating the unsuitability of this formulation to raise drug bioavailability at the levels within the therapeutic window. To improve 4-HPR bioavailability, nanofenretinide, a new formulation of fenretinide complexed with 2-hydroxypropyl-beta-cyclodextrin, was delivered intravenously and showed effectiveness against multiple solid tumors including lung and colorectal cancers [
5]. Then, an improved oral formulation was developed, named bionanofenretinide (BNF), based on drug complexation with a mixture of phospholipids and triglycerides providing nanocapsules. BNF was characterized by high drug loading, high aqueous solubility and increased oral absorption [
6].
To study the new formulation during pre-clinical investigation, it was necessary to set up and validate a method to measure 4-HPR concentrations in plasma and tumors. In the present paper we report the new methodology developed and applied during both pharmacokinetic and activity studies performed with BNF [
6]. Despite several methods based on mass spectrometry or traditional HPLC exists for the determination of retinoids in plasma [
7,
8,
9,
10,
11] only few methods for the specific quantitation of 4-HPR are available [
10,
12,
13,
14].In order to obtain a sensitive, specific and rapid procedure to quantify fenretinide in plasma and tumor, we developed and validated a high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) method that requires only 30 µL of plasma, a little amount of tumor homogenate, a simple solvent treatment and a short time of analysis. High selectivity and sensitivity are guaranteed by working in the Multiple Reaction Monitoring (MRM) mode. Differently from the existing methods, the presented method is able to quantify a wide range of 4-HPR concentrations (from 1 ng/mL to 2000 ng/mL). Moreover, 4-HPR metabolites were identified by high-resolution mass spectrometry (HRMS), using an Orbitrap instrument operating in ESI positive ion mode. Subsequently, the transitions identified throughout HRMS were used for the quantitation of metabolites.
The present method was successfully applied in a pharmacokinetic study in mice, also highlighting the tumor distribution of BNF and providing for the first-time data on its metabolism.
2. Materials and Methods
2.1. Reagents and Chemicals
Fenretinide (N-(4-hydroxyphenyl) retinamide), was purchased by Olon Spa (Milan, Italy). Deuterated fenretinide (N(-4-hydroxyphenyl-d4) retinamide), C26H29D4NO2 ([2H4]-4-HPR), used as internal standard (IS), was obtained from Tocris Bioscience (Bristol, UK).
HPLC-grade methanol and acetonitrile were purchased from Carlo Erba (Milan, Italy) and analytical grade formic acid (98%) from Sigma-Aldrich Co. (Milan, Italy). Deionized water was prepared using a Milli-Q water purifying system from Millipore Corp. (Bedford, MA, USA).
2.2. Animals
Female CD1 mice, 7 weeks old, were supplied by Envigo RMS SRL (Udine, Italy). Animals were housed in the Institute’s Animal Care Facilities, which meet international standards; they are regularly checked by a certified veterinarian who is responsible for health monitoring, animal welfare supervision, experimental protocols and procedures revision.
2.3. Preparation of Standard and Quality Control Solutions and Samples
Two separate ethanol stock solutions of 4-HPR for the preparation of standards and quality controls (QCs), necessary to the assay of plasma and tumor samples, were prepared at 1 mg/mL and further diluted in acetonitrile to obtain the appropriate working solutions from 10 to 20000 ng/mL. The stock solution for IS was prepared in ethanol at 1 mg/mL, and then diluted to working solutions at 300 ng/mL and 3200 ng/mL for plasma and tumor respectively. All stock and working solutions were stored in the dark at -20 °C until use.
To assay plasma, eight calibration standards and three levels of QCs samples were prepared adding 10 µL of different working solutions to 90 µL of control murine plasma to obtain final standard concentrations of 1, 5, 10, 25, 50, 100, 250 and 500 ng/mL, and 8, 80, 400 ng/mL for QCs (QL, QM, QH).
To validate the quantification method of 4-HPR in tumor, a six-point calibration curve was prepared for each analytical session by adding 15 µl of working solution of standards or QC to 135 µl of tumor control homogenate, specifically, A2780 ovarian cancer model was used. Final 4-HPR standards at 50, 100, 250, 500 1000 and 2000 ng/mL, corresponding to tissue concentrations of 350, 700, 1750, 3500, 7000 and 14000 ng/g, and QCs of 150, 750 and 900 ng/mL, corresponding to tissue concentrations of 1050, 5250 and 6300 ng/g were obtained.
2.4. Preparation of Plasma Samples
After thawing plasma samples at room temperature, an aliquot of 30 µL was transferred to a 1.5 mL Eppendorf polypropylene tube, spiked with 3 ng of IS and diluted with 90 µL of acetonitrile to deproteinize plasma. Each tube was thoroughly vortexed for 30 sec, shaken for 5 min at 1250 rpm and centrifuged for 5 min at 4000 g. The obtained supernatant was then transferred to an amber glass vial and 10 µL were injected into the HPLC-MS/MS system. Amber glass vials and aluminum foil were used as precautions to minimize exposure of the analytes to the light to avoid photodegradation.
2.5. Preparation of Tumor Homogenate Samples
Since 4-HPR is highly lipophilic, acetonitrile was used for tumor homogenization. Removed or control tumor was weighed, mixed with acetonitrile in a ratio of 1:6 (w/v) and homogenized (1 min) using an Ultra-Turrax (IK A, Staufen, Germany). An aliquot of 150 µL of tumor homogenate was spiked with 10 µL of IS working solution, mixed and centrifuged at 4000 g for 10 min at 4 °C. The supernatant was transferred to an amber glass vial and 10 µL were injected into the HPLC-MS/MS system.
2.6. Liquid Chromatographic Conditions
The HPLC system consisted of a Series 200 autosampler and micropump (Perkin Elmer, MA, USA) with an online vacuum degasser and a temperature (32 °C) controlled column compartment. Chromatographic separation was achieved on a Gemini-C18 column (50 mm x 2.0 mm, 5 µm particle size; Phenomenex Inc., Torrance, CA, USA) protected with a Security Guard™ ULTRA cartridges C18 (Phenomenex Inc., Torrance, CA, USA). The mobile phases consisted of 0.05% formic acid in water (MP-A) and 100% methanol (MP-B). The chromatographic separation was performed at a flow rate of 0.3 mL/min, applying the following gradient steps: from 32 to 2% MP-A in 3 min; 2% MP-A held for 2 min; from 2 to 32% MP-A in 30 sec and, as last step, re-equilibration at the initial condition for 4 min. The autosampler was maintained at 4 °C and the injected volume was 10 µL.
2.7. Mass Spectrometry Conditions
Mass spectrometric detection was carried out on a triple quadrupole API 4000 mass spectrometer (Sciex, MA, USA) equipped with atmospheric pressure chemical ionization source (APCI) operating in positive ion mode at 350 °C, with a needle current of 4 µA. The nebulizer gas (Gas 1), heater gas (Gas 2), curtain gas (CUR) and collision activated dissociation gas (CAD) were set to 40, 50, 30 and 5 instrument units, respectively. Declustering potentials (DP) was set at 60 V and the collision exit potential (CXP) at 15 V. All source parameters were optimized by direct infusion of 4-HPR under LC conditions. The quantification of 4-HPR and IS was carried out in MRM mode, using the pseudo-molecular (Q1) to fragment (Q3) ion transitions and the optimal collision energy (CE) reported in the following scheme.
| Analyte |
Q1
(m/z)
|
Q3
(m/z)
|
CE
(eV)
|
| 4-HPR |
392.3 |
283.3 |
16 |
| 4-oxo-4-HPR |
406.2 |
297.1 |
16 |
| 4-MPR |
406.3 |
283.2 |
16 |
| DH-4-HPR |
390.2 |
281.1 |
16 |
| [2H4]-4-HPR |
396.3 |
283.2 |
18 |
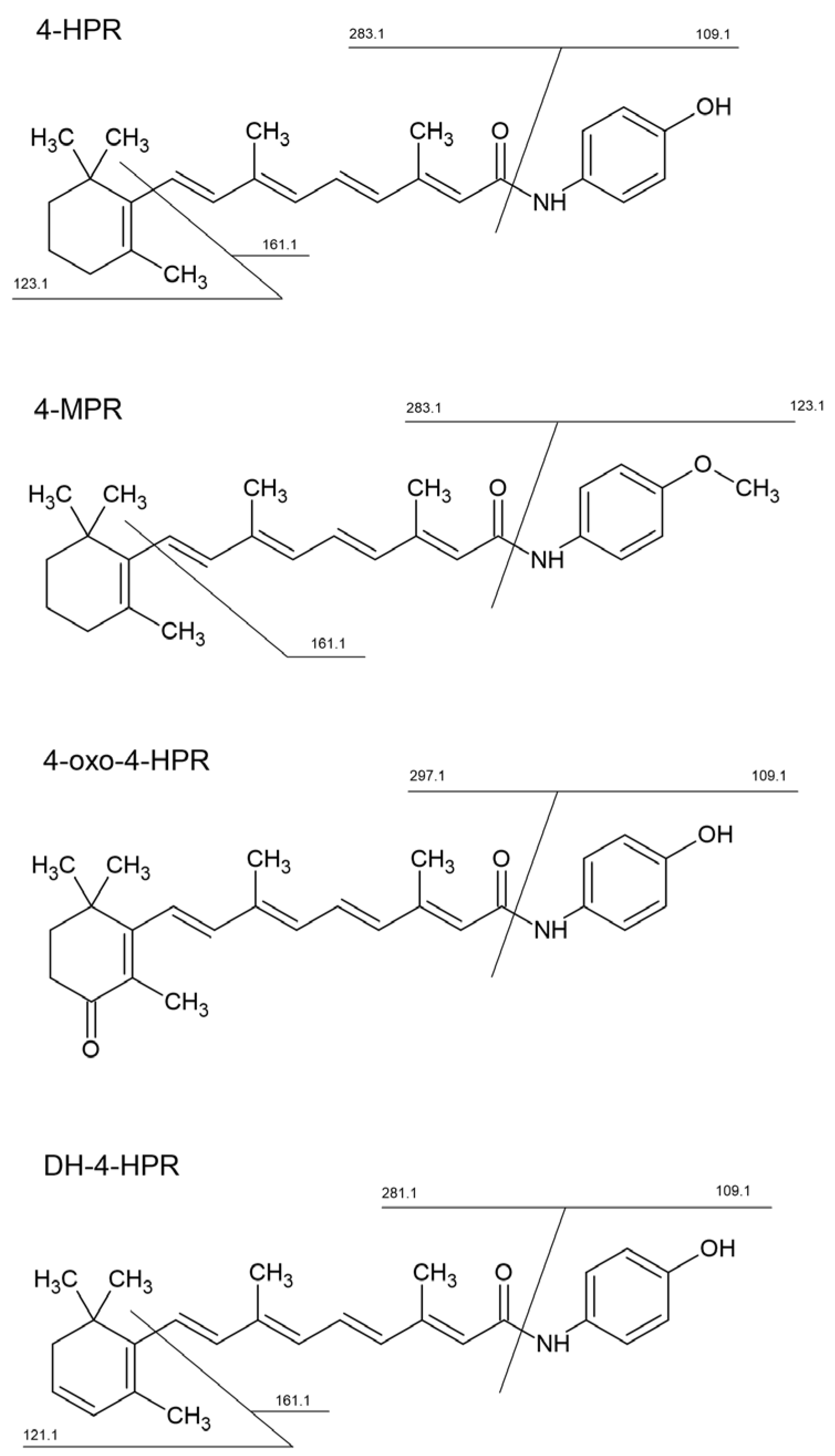
The scheme above also lists the specific transition used to quantify 4-HPR metabolites, which were previously identified using a high-resolution LTQ-Orbitrap XL mass spectrometer (Thermo Scientific Inc, Waltham, MA, USA), equipped with an electrospray source (ESI) operating in positive ion mode (
Figure 1). Chromatographic separation was performed using a 1200 series pump and auto sampler (Agilent Technologies, Santa Clara, CA, USA), with a Gemini-C18 column and a mobile phase composed of 0.1% formic acid in water (MP-A) and acetonitrile (MP-B). The injection volume was 8 ul and the flow rate was 200 ul/min. The elution gradient was from 2% to 99% of MP-B in 28 minutes; held to 99% of MP-B for 2 minutes and re-equilibration at 2% of MP-B for ten minutes. Full scan MS spectra were acquired in the m/z range 100-800 (60000 resolving power), while MS/MS spectra were acquired in the m/z range 50-500 with a collision energy of 28 eV (15000 resolving power).
2.8. Validation procedures
Method validation was performed according to the European Medicines Agency and the Food Drug and Administration guidelines on bio-analytical method validation [
15,
16]. These methods were validated in terms of linearity, carry-over, intra- and inter-day precision and accuracy, Lowest Limit of Quantification (LLOQ), selectivity, matrix effect and recovery. Moreover, stability tests were performed both in plasma and in tumor homogenate.
2.8.1. Limit of quantification, matrix effect and recovery
Six different batches of control plasma and different tumor type were spiked with 4-HPR at the LLOQ level of 1 ng/mL and 50 ng/mL (corresponding to 350 ng/g in tissue) respectively, to investigate the selectivity of the method. As defined by the guidelines of the main regulatory agencies, the LLOQ precision must be ≤ 20% and accuracy in the range 80–120% of the nominal value. During the preliminary phase of method development, we noticed that the actual concentrations found in samples from treated mice were quite superior to the instrumental detection limit (i.e., 0.5 ng/mL), so we decided to validate a LLOQ of 1 ng/mL for plasma and 50 ng/mL for tumor homogenate.
On the same independent sources of plasma and tumors, matrix effects and recovery were also investigated, analyzing the lowest 4-HPR QC concentrations.
Matrix effect was calculated evaluating the normalized Matrix Factor. Matrix Factor (MF) is the ratio between the peak area of analyte in spiked matrix and the peak area in the absence of matrix (pure solution of the analyte). A value of MF close to 1 defines absence of matrix effect.
The normalized matrix factor is the ratio of the analyte MF to the internal standard MF, a value close to 1 defines the absence of matrix effect. The normalized MF coefficient of variation (CV), calculated as percentage ratio of the standard deviation to the mean calculated concentration, had to be lower than 15%.
4-HPR extraction efficiency (recovery) was established comparing the peak area of the analyte extracted from plasma or tissue homogenate QC samples with the peak area of the extracted matrix samples with the same amount of analyte added following the extraction procedures. The IS recovery was determined in the same way at the concentration of 30 ng/mL and 200 ng/mL. The CV% had to be within 15%.
2.8.2. Linearity
The linearity of the standard calibration curve between 1 to 500 ng/mL for plasma and between 50 to 2000 ng/mL in tumor tissue was evaluated during different analytical runs using fresh preparations. Each calibration curve consisted of a double blank sample, a blank sample and eight calibrators for plasma or six for tumor homogenate. The double blank sample was re-injected also after the highest concentration standard in each run to monitor the 4-HPR and IS carry-over.
The ratio of the HPLC–MS/MS peak area for 4-HPR to the IS (y) was determined for each standard point and plotted versus the nominal concentration of 4-HPR in the sample (x) using a weighted (1/x2) least squares linear regression analysis. The acceptance criteria for accuracy of the back-calculated values of each standard point had to be in the range of 85–115% of the nominal concentration, and the LLOQ in the range 80–120%. At least 75% of the standard points of calibration curve must comply with the described criteria.
2.8.3. Accuracy and precision
Intra-day precision and accuracy over one day and inter-day precision and accuracy on different days were checked measuring the analytes in five and three replicates of QC samples, respectively. Three levels of QC were analyzed at the nominal concentrations of 8, 80 and 400 ng/mL for plasma and 150, 750 and 900 ng/mL for tumor. To analyze the QCs, we prepared and processed a fresh standard calibration curve for each analytical session. The precision of the method was determined using the CV% and the accuracy expressed as the percentage ratio of the mean calculated concentration to the nominal concentration.
For each QC, the measured concentration must be within 15% of the nominal value in at least 67% of QCs samples (2/3).
The ability to dilute plasma sample originally above the upper limit of quantification (ULOQ) was assayed by analyzing QC samples containing 4 and 10 times the concentration of the high QC sample, after a 4-fold and a 10-fold dilution in control murine plasma.
2.8.4. Stability
The stability of the 4-HPR in plasma and in tumor homogenate was tested by analyzing QC samples in triplicate during storage and handling. Bench-top stability was determined after 4 h at room temperature, stability in the autosampler by reanalyzing the processed QC samples 24 h after the first injection and long-term stability was assessed on QC samples stored 4 months for plasma and 3 weeks for tumor homogenate at -20 °C. The drug was considered stable at each QC concentration when the differences between the freshly prepared samples and the stability testing samples deviated no more than 15% from the nominal concentrations.
2.8.5. Application to the pharmacokinetic study
In this paper we report the results obtained in the first exploratory pharmacokinetics carried out to test the performance of the method, which allowed us to obtain preliminary information on the bioavailability of BNF. The oral and intravenous pharmacokinetics were investigated in CD1 female mice, 7 weeks of age, divided in groups, randomized to receive single dose of BNF at the 4-HPR equivalent dose of 5 and 10 mg/Kg in comparison with free 4-HPR. BNF was dissolved in sterile water while free 4-HPR, due to its water insolubility, was dissolved in the same mixture of corn oil and polysorbate 80 (0.92: 0.08, w:w) as contained in the soft gelatin capsules, for oral administration or in ethanol for iv administration. Both BNF and free 4-HPR were administered by gavage or bolus injection. After treatment, a series of blood samples were taken at 0.08 (iv) or 0.25 (po), 0.5, 1, 2, 4, 10, 24 and 48 h. Blood was collected in heparinized tubes from the retro-orbital plexus of the mice under isoflurane anesthesia. To obtain plasma, blood samples were centrifuged at 4000 rpm for 10 min at 4 °C. All the collected samples were stored at -20 °C until analysis.
In a different experiment of antitumor activity in mice growing melanoma (MEL 3) and lung cancer (LCSC-136) xenografts, the intratumor 4-HPR concentration was determined after repeated oral treatments with BNF or free 4-HPR at the 4-HPR equivalent dose of 100 mg/kg twice daily.
3. Results
3.1. Method Development and Validation
3.1.1. HPLC-MS/MS
Following the direct infusion of standard solution using both APCI and ESI source in positive ion mode, we decided to use the APCI source since we obtained a better and more stable signal of the analyte and IS. 4-HPR and [2H4]-4-HPR formed mainly a pseudo-molecular protonated molecule [M+H]+ at m/z 392.3 and 396.3, respectively. These precursor ions passed through the first quadrupole into the collision cell and the CE was optimized to obtain the product ions with the higher signal. The characteristic product ions were monitored in the third quadrupole at m/z 283.2 (16 eV), and 161.1 (25 eV), both for 4-HPR and [2H4]-4-HPR.
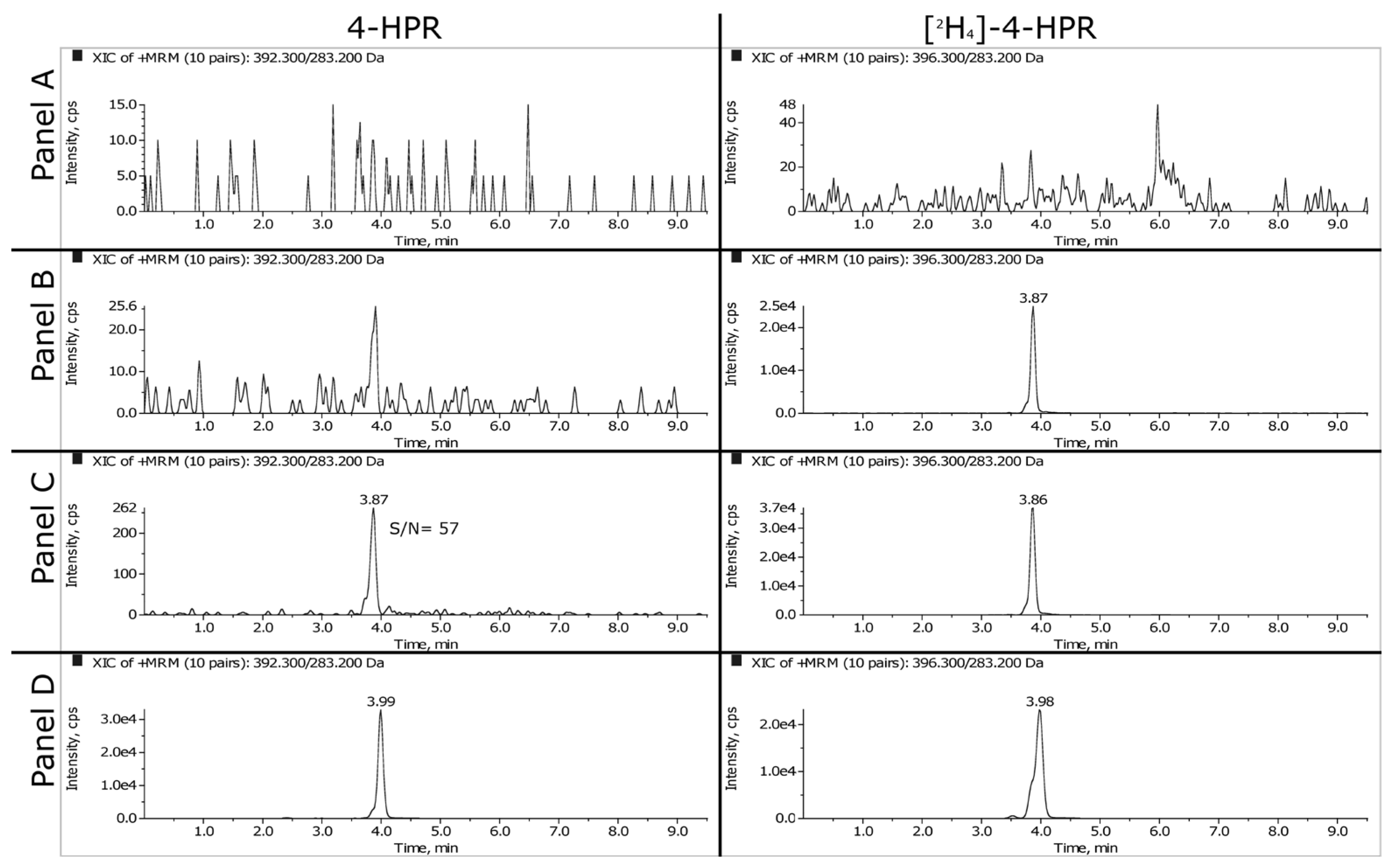
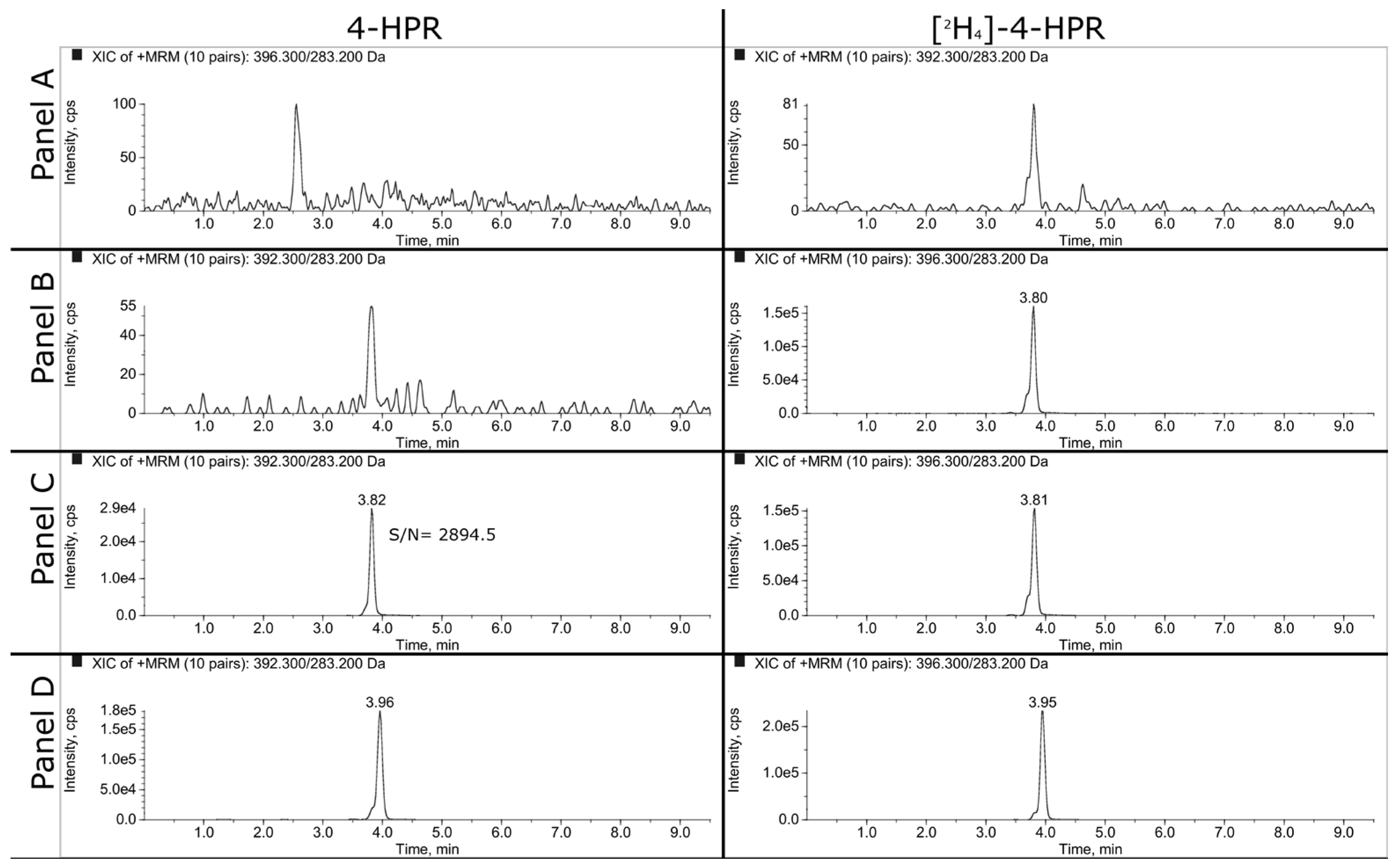
Representative SRM chromatograms of extracted plasma and tumor samples are shown in
Figure 2 and
Figure 3. The panels A, B and C refer respectively to double blank, blank and LLOQ samples at 1 ng/mL for plasma and 50 ng/mL (i.e., 350 ng/g) for tumor homogenate. Panel D shows the extracted plasma and tumor samples of a mouse treated daily for 3 weeks with 100 mg/kg of BNF and sacrificed 2 hours after the last dose. The measured concentrations of 4-HPR were 1669 ng/mL in plasma and 1774 ng/g in tumor.
3.1.2. LLOQ, Matrix Effect and Recovery
As explained in the materials and methods, we decided to validate the concentration of 1 ng/mL as LLOQ in plasma and 50 ng/mL in tumor homogenate. At this concentration, the precision and the accuracy were 7.6% and 107.6% respectively for plasma and 5.1% and 104.8% for tumor.
The matrix effect was calculated at the low QC concentration level. The normalized matrix factor ranging from 0.96 to 1.15 for the six sources of plasma samples and from 0.97 to 1.03 for tumor homogenate. The calculated normalized matrix factors, close to 1, indicate that no co-eluting substances of the matrices affect the analyte signal. In particular, considering the tumor matrix, the procedure of homogenization in a large volume of solvent, together with an appropriate chromatographic separation, allows the achievement of satisfactory results.
We use acetonitrile for both plasma deproteinization and tumor homogenization. The recoveries, evaluated processing five replicates at low and high QC concentrations, were: 85.5% (CV 3.0%) for QL and 98.7% (CV 11.8%) for QH in plasma and 93.8% (CV 5.8%) for QL and 94.9% (CV 7.6%) for QH in tumor. The IS recovery were 94.0% (CV 6.1%) and 103.2 (CV 8.6%) for tumor and plasma, respectively.
3.1.3. Linearity
In
Table 1 is reported the 4-HPR standard calibration curves in plasma and tumor, prepared on different days during the validation study. These calibration curves were generated plotting the peak area ratios of the analyte to the IS versus the 4-HPR nominal concentrations and applying weighted quadratic regression function (1/x
2). The plasma and tumor calibration curves were linear over the concentration ranges of 1-500 ng/mL and 50-2000 ng/mL, respectively. The back-calculated concentration accuracy was ≥ 96.8% in plasma and ≥ 96.6% in tumour with a precision, expressed as CV%, ≤ 4.5 and ≤ 3.4%. The Pearson’s coefficients of determination, R
2, were ≥ 0.9966 for both plasma and tumor.
Data for carry-over evaluation were obtained in analytical runs injecting blank samples following the injection of the ULOQ. No signal of 4-HPR and of IS was observed during this analysis.
3.1.4. Accuracy and Precision
Intra-day accuracy and precision of the method were evaluated by analyzing five replicates of QC samples at 8, 80 and 400 ng/mL for plasma and 150, 750, 900 ng/mL for tumor homogenate, within a single-run analysis. The method appears accurate and extremely precise, in fact, as shown in
Table 2 and
Table 3, the accuracy and precision were comprised in the ranges 89.7-90.1% and 2.1-5.5% in plasma and 103.3-107.0% and 1.0-2.3% in tumour.
The same satisfactory results were obtained when the inter-day variability was determined. As shown in
Table 2 and
Table 3, the precision and accuracy assessed in triplicate samples, over at least four days of analysis showed a range of 6.9-7.5% and 99.3-101.0% in plasma and 1.9-3.2% and 102.3-105.8% in tumor.
No dependence from dilution was observed in the analysis of 4-HPR in plasma, being mean accuracy of the found concentrations of 86.7% and 88.0%, respectively; the precision was 1.41% and 1.78% for the dilution ratio 1:4 and 1:10, respectively.
3.1.5. Stability
In order to evaluate 4-HPR stability in plasma and in tumor, QC samples were analyzed: three replicates at 8, 80 and 400 ng/mL for plasma samples and three replicates at 150, 750, 900 ng/mL for tumor samples.
As result, in plasma 4-HPR remained stable after 4 months at -20°C resulting in the ranges 98.0-101.9% of the nominal concentrations. In tumor homogenate, 4-HPR resulted stable at -20°C for three weeks being 98.1-112.6% of the nominal concentrations. In both cases, the coefficient of variation was ≤10.0%.
Bench top stability (4 h at room temperature) and autosampler stability (24 h at 4°C) for matrices was assessed to cover the preparation and injection period of analysis. We obtained an accuracy in the range of 106.1-108.4% (CV 1.2-3.5%) and 97.1-100.6% (CV 3.1-4.6%) for the bench-top stability in plasma and tumor, respectively. In the autosampler, samples resulted stable for 24 h with accuracy of 92.1-97.6% (CV 4.0-6.7%) and 104.1-110.0% (CV 0.8-3.0%) for plasma and tumor, respectively.
3.2. Identification of 4-HPR metabolites
Metabolites of 4-HPR were identified using a high-resolution mass spectrometer (LTQ-Orbitrap XL), which facilitates the identification of chemical structures. The analyses were carried out in plasma and tumor samples extracted as described above. The chemical structures of the identified metabolites and the corresponding MS/MS spectra used for the identification are shown in
supplementary Figure 1 and Figure 2. High resolutions MS/MS spectra allowed the identification of three main metabolites and their principal fragments, which were then used for the MRM quantitative analysis. They corresponded to: N-(4-methoxyphenyl) retinamide (4-MPR), 4-oxo-N-(4-hydroxyphenyl) retinamide (4-oxo-4-HPR) and dehydrogenated 4-HPR (DH-4-HPR).
3.3. Application to the pharmacokinetic study
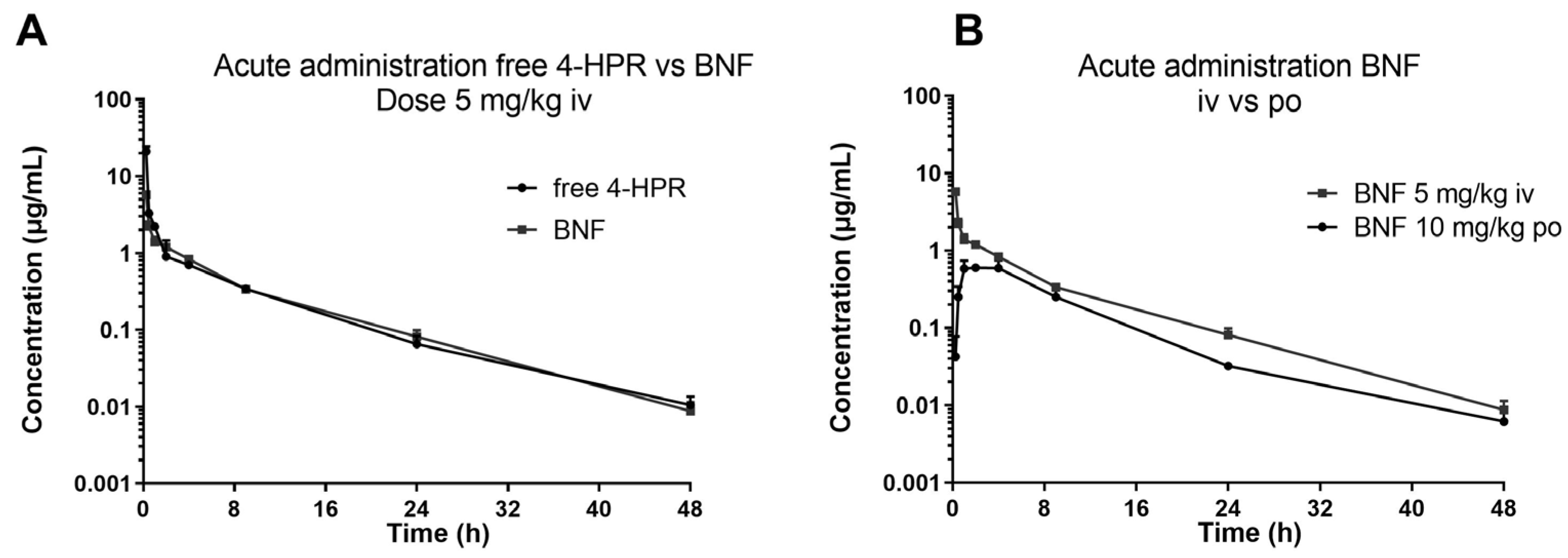
The described method was successfully applied in an explorative pharmacokinetic study with BNF, in mice treated by oral gavage and intravenous bolus with 10 and 5 mg/kg of 4-HPR dose equivalent, respectively.
Figure 4 shows the profiles of the measured plasma concentration-versus-time of 4-HPR obtained after BNF and for comparison the profile after free 4-HPR administered intravenously at 5 mg/Kg.
From a visual inspection of the curves, it can be seen that the two intravenous profiles are superimposable and by oral BNF, 4-HPR achieves plasma Cmax between 2 and 4 hours, it is distributed rapidly and eliminated with a HL of about 6-7 hr, warranting measurable drug plasma levels up to 48 hr. From the comparison of the calculated experimental AUC, the bioavailability of BNF resulted 25% (AUC0-last 4-HPR iv: 18.23 hr* µg/mL; AUC0-last BNF iv: 13.56 hr* µg/mL; AUC0-last BNF po: 6.745 hr* µg/mL).
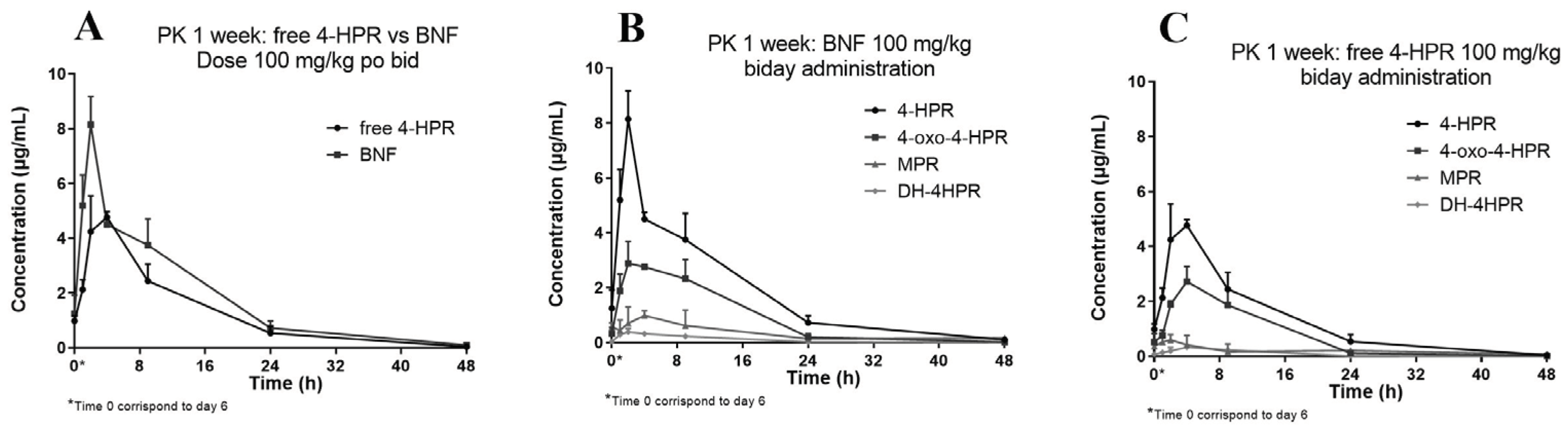
We subsequently studied the pharmacokinetics of BNF compared with free 4-HPR in one of the oral repeated treatment schedules employed during activity studies. The treatment consisted in doses of 100 mg/kg, given twice a day, for one week.
Figure 5 reports the obtained 4-HPR plasma concentration profile also showing the measured metabolites of 4-HPR.
The relative percent amounts of the active metabolite 4-oxo-4-HPR, and the inactive metabolite MPR, versus 4-HPR, corresponded to about 50% and 17%, respectively. A third metabolite, DH-4-HPR, previously described by Cooper et al. [
4] amounted to 5% 4-HPR (
Table 4).
3.4. Application to the activity study
The method was also successfully applied to measurements of 4-HPR and its metabolites in xenografts generated from subcutaneous injection of melanoma (#mel-3) and lung cancer (LCSC-136) stem cells [
6]. We measured mean 4-HPR concentrations of 2126 (5.4 µM) and 2228 ng/g (N=3), 2 hours after dosing. Interestingly, these concentrations are higher than the IC50 value necessary for in vitro inhibitory activity (i.e., 1-5 µM), therefore potentially effective. But even more interestingly, thanks to the developed method, we discovered that 4-oxo-4-HPR, the active metabolite, was present in the tumor tissues in quantities comparable to 4-HPR, probably contributing to the overall antitumor activity triggered by BNF administration.
4. Discussion
Limited availability of methods for determination of retinoids in plasma and tissues prevent to fully understand their effects, the correlations between concentration levels and activity in the different body compartments and the role of the pharmaceutical formulations in influencing their bioavailability and pharmacokinetics.
In this study, we describe the development of an accurate and reproducible bioanalytical method for the assessment of 4-HPR in plasma and tumour tissue. The method proved to be reproducible, precise, and highly accurate being inter-days precision and accuracy determined on quality controls in ranges of 6.9%-7.5% and 99.3-101.0% and 0.96-1.91% and 102.3-105.8% for plasma and tumour, respectively.
The APCI source and MRM mode together ensure the high selectivity and sensitivity of the HPLC-MS/MS method. Differently from the existing methods, the presented method is able to quantify a wide range of 4-HPR concentrations (from 1 ng/mL to 500 ng/mL).
Moreover, 4-HPR metabolites were identified by HRMS, using an Orbitrap instrument operating in ESI positive ion mode. Subsequently, the transitions identified were used for the quantitation of metabolites.
A description of the similarities and differences between this method and the other available methods [
10,
12,
13,
14] is provided in Supplementary (
Table S1). Unlike other methods, this one is able to measure the analytes in the dual plasma and tumor matrix. In addition to this finding, the main advantages of the present method are that a wider range of linearity reduces the need for dilutions and was introduced the possibility to the combined assessment of fenretinide and main metabolites allowing a more comprehensive pharmacokinetic analysis and limiting the required amounts of matrices, at only 30µL of plasma and 25 mg of tumor.
The new method was successfully applied to assess preliminarily the pharmacokinetics and bioavailability of BNF, a new oral nanoformulation of 4-HPR, in CD1 mice following acute and chronic oral treatment compared with the free drug. In addition, for the first time, it was determined the tumour distribution of 4-HPR and metabolites in a limited number of xenograft models (i.e., melanoma and lung cancer). The results of these assessments indicate that BNF is rapidly available into 4-HPR in vivo, and chronic oral treatment of daily administration allows the achievement of concentration potentially effective and higher than those obtained after treatment of free 4-HPR. Furthermore, there was no evidence of plasma accumulation of 4-HPR during chronic treatment, i.e., making the treatment well tolerated. The treatment allows 4-HPR to penetrate the tumor tissue at levels of the same order of magnitude found in plasma, and most interesting, at concentrations superior the IC50 value of in vitro inhibitory activity. It is also important to emphasize that we observed the presence of comparable tumor levels of the active metabolite 4-oxo-4-HPR, so raising the possibility that the reported in vivo effects of BNF might also depend on the conversion to this metabolite in vivo [
17]. All this leads to a significant exceeding of the aforementioned inhibition activity threshold.
The pharmacokinetic results and the preliminary tumour distribution data reported in this study allowed the optimal planning of activity studies, successfully conducted in different xenograft models [
6]. Specifically, the plasma and tumour exposure which we determined at active concentrations allowed to foresee daily treatments with BNF for long periods of time (2 weeks), also given the good tolerability observed in this study.
Considering the positive data originated by the combined exposure of the 4-HPR and metabolite, the newly developed HPLC-MS/MS method appears particularly useful for investigating extensively the in vivo pharmacokinetics of BNF, and after validation in human matrices, to be applied in future clinical pharmacokinetic studies.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Supplementary Figure S1. High resolution ion chromatogram obtained for the m/z value (± 10 ppm) of 4-HPR and its main metabolites in a tumor sample. Supplementary Figure S2. MS/MS spectra of 4-HPR and its identified metabolites, highlighting some of the observed ion fragments. Supplementary Table S1.
Author Contributions
“Conceptualization, MZ, CM and IO; methodology, validation, and formal analysis, CM, MZ, MF, EB, RF, AP and MP; data curation, MZ, CM, EB, RB, IO, AE, AZ; writing—original draft preparation, MZ, CM and RF; writing—review and editing, CM, IO, RF, AE, AZ and RB; supervision, MZ, RF and IO; funding acquisition IO and AZ. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by AIRC (IG 20744 to AZ).
Institutional Review Board Statement
Animal experimentation was conducted in conformance with the following laws, regulations, and policies governing the care and use of laboratory animals: Italian Governing Law (D. l. 26/2014; Authorization n.19/2008-A issued March 6, 2008 by the Ministry of Health). Mario Negri Institutional Regulations and Policies providing internal authorizations for persons conducting animal experiments (Quality Management System Certificate –UNI EN ISO 9001:2008 – Reg. N 86121). The experimental protocol was reviewed by the Mario Negri Institute Ethical Committee and approved by the Italian Ministry of Health (Aut. Min. No 489/2016-Pr).
Data Availability Statement
The data supporting the reported results are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Barua, A.B.; Olson, J.A. Preparation of Retinamides by Use of Retinoyl Fluoride. Journal of Lipid Research 1985, 26, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Companioni, O.; Mir, C.; Garcia-Mayea, Y.; LLeonart, M.E. Targeting Sphingolipids for Cancer Therapy. Front Oncol 2021, 11, 745092. [Google Scholar] [CrossRef] [PubMed]
- Potenza, R.L.; Lodeserto, P.; Orienti, I. Fenretinide in Cancer and Neurological Disease: A Two-Face Janus Molecule. Int J Mol Sci 2022, 23, 7426. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.P.; Reynolds, C.P.; Cho, H.; Kang, M.H. Clinical Development of Fenretinide as an Antineoplastic Drug: Pharmacology Perspectives. Exp Biol Med (Maywood) 2017, 242, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Orienti, I.; Francescangeli, F.; De Angelis, M.L.; Fecchi, K.; Bongiorno-Borbone, L.; Signore, M.; Peschiaroli, A.; Boe, A.; Bruselles, A.; Costantino, A.; et al. A New Bioavailable Fenretinide Formulation with Antiproliferative, Antimetabolic, and Cytotoxic Effects on Solid Tumors. Cell Death Dis 2019, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Orienti, I.; Salvati, V.; Sette, G.; Zucchetti, M.; Bongiorno-Borbone, L.; Peschiaroli, A.; Zolla, L.; Francescangeli, F.; Ferrari, M.; Matteo, C.; et al. A Novel Oral Micellar Fenretinide Formulation with Enhanced Bioavailability and Antitumour Activity against Multiple Tumours from Cancer Stem Cells. J Exp Clin Cancer Res 2019, 38, 373. [Google Scholar] [CrossRef] [PubMed]
- Rühl, R. Method to Determine 4-Oxo-Retinoic Acids, Retinoic Acids and Retinol in Serum and Cell Extracts by Liquid Chromatography/Diode-Array Detection Atmospheric Pressure Chemical Ionisation Tandem Mass Spectrometry. Rapid Communications in Mass Spectrometry 2006, 20, 2497–2504. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, T.E.; Bastani, N.E.; Blomhoff, R. Quantitative High-Throughput Determination of Endogenous Retinoids in Human Plasma Using Triple-Stage Liquid Chromatography/Tandem Mass Spectrometry. Rapid Communications in Mass Spectrometry 2007, 21, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Ni, K.-Y.; Shen, Y.; Ma, P.-C.; Cao, L.; Wang, W.-P.; Wang, Y. Development and Validation of a LC-MS/MS Method for the Determination of Viaminate in Human Plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2007, 856, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Nguyen, Vu.T.; Chen, M.-L.; Adamson, P.C. A Rapid, Sensitive and Selective Liquid Chromatography/Atmospheric Pressure Chemical Ionization Tandem Mass Spectrometry Method for Determination of Fenretinide (4-HPR) in Plasma. Journal of Chromatography B 2008, 862, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Karppi, J.; Nurmi, T.; Olmedilla-Alonso, B.; Granado-Lorencio, F.; Nyyssönen, K. Simultaneous Measurement of Retinol, α-Tocopherol and Six Carotenoids in Human Plasma by Using an Isocratic Reversed-Phase HPLC Method. Journal of Chromatography B 2008, 867, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Formelli, F.; Clerici, M.; Campa, T.; Di Mauro, M.G.; Magni, A.; Mascotti, G.; Moglia, D.; De Palo, G.; Costa, A.; Veronesi, U. Five-Year Administration of Fenretinide: Pharmacokinetics and Effects on Plasma Retinol Concentrations. JCO 1993, 11, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Vratilova, J.; Frgala, T.; Maurer, B.J.; Patrick Reynolds, C. Liquid Chromatography Method for Quantifying N-(4-Hydroxyphenyl)Retinamide and N-(4-Methoxyphenyl)Retinamide in Tissues. J Chromatogr B Analyt Technol Biomed Life Sci 2004, 808, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.E.; Min, H.K. Analysis of Fenretinide and Its Metabolites in Human Plasma by Liquid Chromatography-Tandem Mass Spectrometry and Its Application to Clinical Pharmacokinetics. J Pharm Biomed Anal 2017, 132, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration Bioanalytical Method Validation Guidance for Industry. Bioanalytical Method Validation Guidance for Industry 2018, 44.
- European Medicines Agency Bioanalytical Method Validation. Available online: https://www.ema.europa.eu/en/bioanalytical-method-validation (accessed on 14 December 2021).
- Villani, M.G.; Appierto, V.; Cavadini, E.; Bettiga, A.; Prinetti, A.; Clagett-Dame, M.; Curley, R.W.; Formelli, F. 4-Oxo-Fenretinide, a Recently Identified Fenretinide Metabolite, Induces Marked G2-M Cell Cycle Arrest and Apoptosis in Fenretinide-Sensitive and Fenretinide-Resistant Cell Lines. Cancer Res 2006, 66, 3238–3247. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).