Submitted:
20 February 2024
Posted:
20 February 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Case Report
2.2. Laboratory Investigations
2.3. Imagistic investigations
2.4. Molecular investigations
3. Results
3.1. Personal data
3.2. Clinical evaluation of the patient
3.3. Laboratory investigations
3.4. Interdisciplinary consultations and imagistic investigations
3.5. Molecular investigations
4. Discussion
4.1. NF1 and CRX genes
4.1.1. NF1 gene
4.1.2. CRX Gene
5. Neurofibromatosis
5.1. Clinical aspects
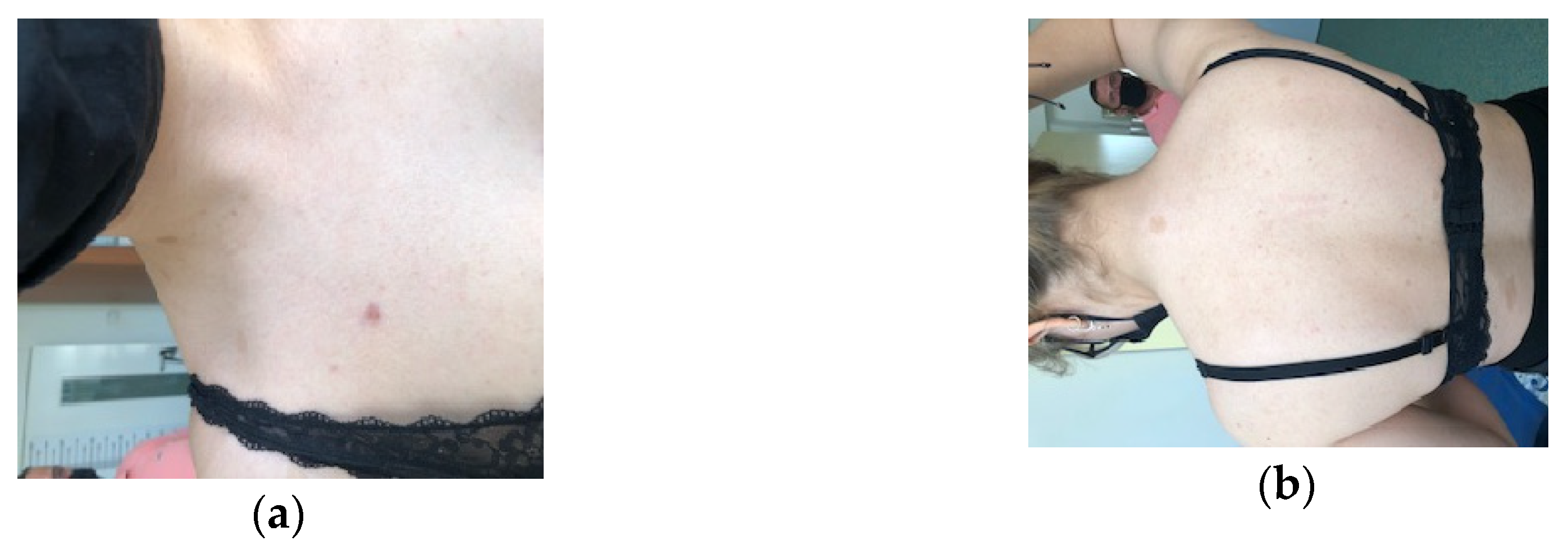
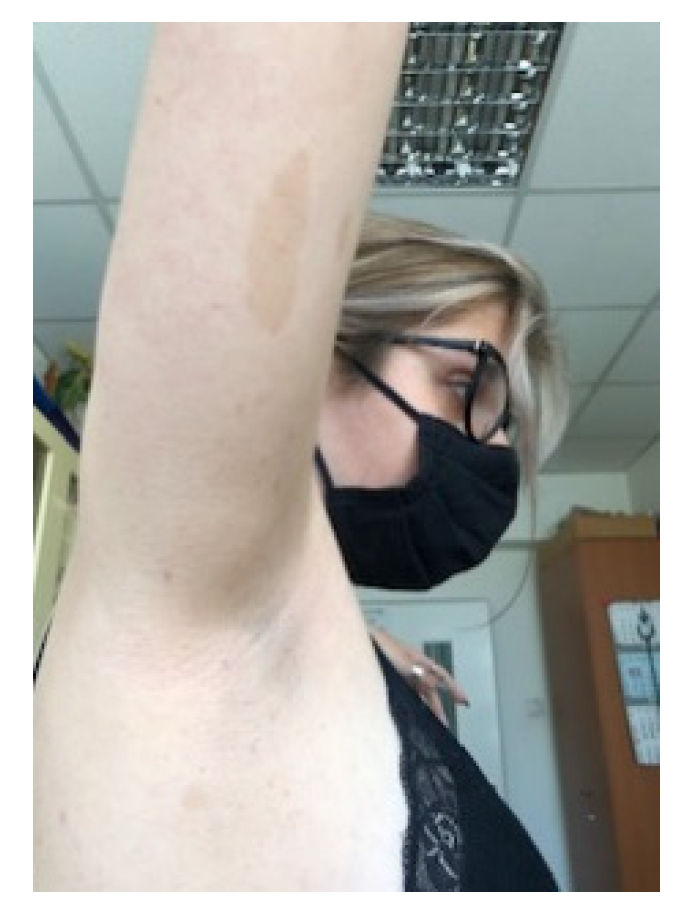
5.2. Cutaneous manifestations in NF1
5.2.1. Café au lait spots and frecklings.
5.2.2. Neurofibromas.
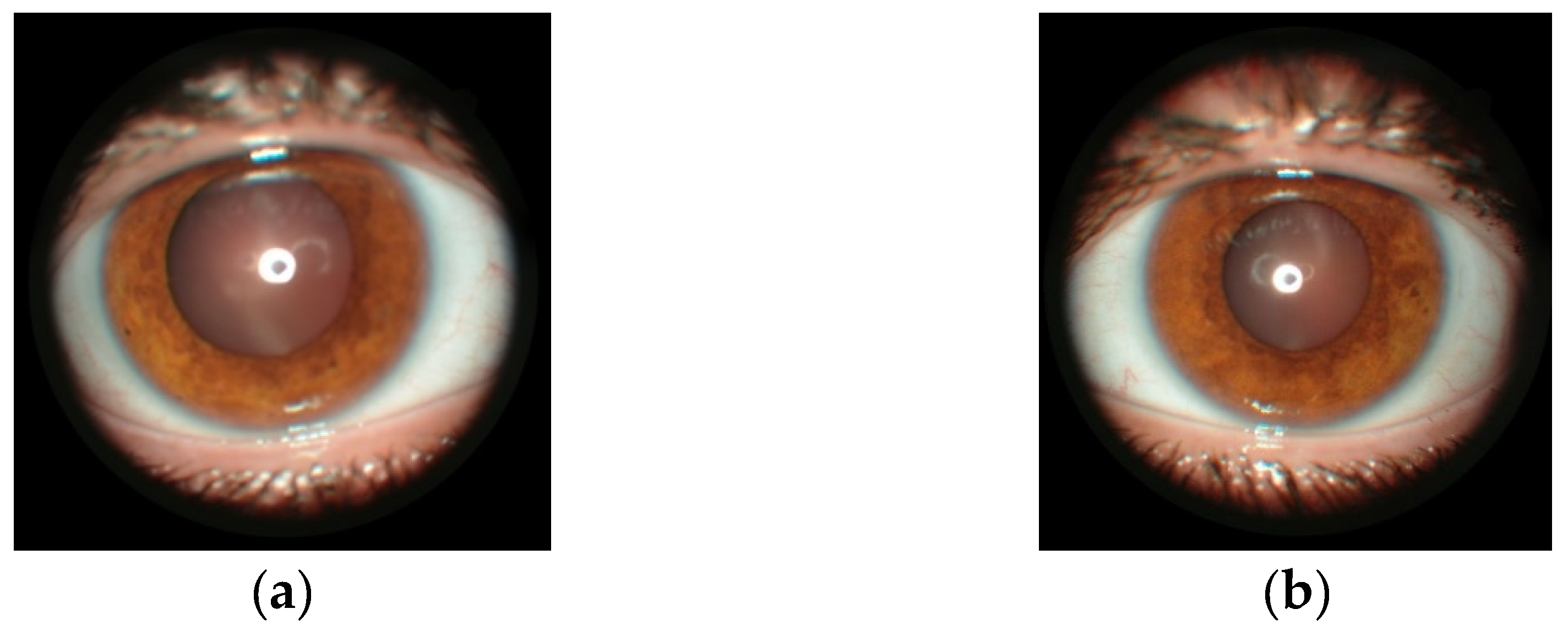
5.3. Ocular manifestations in NF1
5.4. Cerebral manifestations in NF1
5.5. Genotype-phenotype correlation in NF1
5.6. Treatment
5.6.1.
5.6.2. Drug Therapy
6. Cone rod dystrophies
6.1. Inheritance
6.2. Clinical aspects
6.3. Diagnosis of cone-rod dystrophy
6.4. Treatment
6.5. Genotype-phenotype correlation in CORD
7. The association of NF1 with cone-rod dystrophy
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedman, J. M. Neurofibromatosis 1; University of Washington, Seattle 1993 updated, 2022, April. Book editor. https://www.ncbi.nlm.nih.gov/books/NBK1109/.
- Jurca, C. M.; Kozma, K.; Petchesi, C. D.; Zaha, D. C.; Magyar, I.; Munteanu, M.; Faur, L.; Jurca, A.; Bembea, D.; Severin, E.; Jurca, A. D. Tuberous Sclerosis, Type II Diabetes Mellitus and the PI3K/AKT/MTOR Signaling Pathways—Case Report and Literature Review. Genes (Basel) 2023, 14 (2), 433. [CrossRef]
- Yoshida, Y. Neurofibromatosis 1 (von Recklinghausen Disease). Keio J. Med. 2023, No. 2023-0013-IR. [CrossRef]
- Adil, A.; Koritala, T.; Munakomi, S.; Singh, A. K. Neurofibromatosis Type 1; StatPearls Publishing, 2023.
- Farschtschi, S.; Mautner, V.-F.; Lawson McLean, A. C.; Schulz, A.; Friedrich, R. E.; Rosahl, S. K. The Neurofibromatoses. Dtsch. Arztebl. Int. 2020, 117 (20). [CrossRef]
- Jurca, C. M.; Frățilă, O.; Iliaș, T.; Jurca, A.; Cătana, A.; Moisa, C.; Jurca, A. D. A New Frameshift Mutation of PTEN Gene Associated with Cowden Syndrome—Case Report and Brief Review of the Literature. Genes (Basel) 2023, 14 (10), 1909. [CrossRef]
- Neurofibromatosis: Conference Statement. Arch. Neurol. 1988, 45 (5), 575. [CrossRef]
- Ferner, R. E.; Huson, S. M.; Thomas, N.; Moss, C.; Willshaw, H.; Evans, D. G.; Upadhyaya, M.; Towers, R.; Gleeson, M.; Steiger, C.; Kirby, A. Guidelines for the Diagnosis and Management of Individuals with Neurofibromatosis 1. J. Med. Genet. 2007, 44 (2), 81–88. [CrossRef]
- DeBella, K.; Szudek, J.; Friedman, J. M. Use of the National Institutes of Health Criteria for Diagnosis of Neurofibromatosis 1 in Children. Pediatrics 2000, 105 (3), 608–614. [CrossRef]
- Legius, E.; Messiaen, L.; Wolkenstein, P.; Pancza, P.; Avery, R. A.; Berman, Y.; Blakeley, J.; Babovic-Vuksanovic, D.; Cunha, K. S.; Ferner, R.; Fisher, M. J.; Friedman, J. M.; Gutmann, D. H.; Kehrer-Sawatzki, H.; Korf, B. R.; Mautner, V.-F.; Peltonen, S.; Rauen, K. A.; Riccardi, V.; Schorry, E.; Stemmer-Rachamimov, A.; Stevenson, D. A.; Tadini, G.; Ullrich, N. J.; Viskochil, D.; Wimmer, K.; Yohay, K.; Gomes, A.; Jordan, J. T.; Mautner, V.; Merker, V. L.; Smith, M. J.; Stevenson, D.; Anten, M.; Aylsworth, A.; Baralle, D.; Barbarot, S.; Barker, F., II; Ben-Shachar, S.; Bergner, A.; Bessis, D.; Blanco, I.; Cassiman, C.; Ciavarelli, P.; Clementi, M.; Frébourg, T.; Giovannini, M.; Halliday, D.; Hammond, C.; Hanemann, C. O.; Hanson, H.; Heiberg, A.; Joly, P.; Kalamarides, M.; Karajannis, M.; Kroshinsky, D.; Larralde, M.; Lázaro, C.; Le, L.; Link, M.; Listernick, R.; MacCollin, M.; Mallucci, C.; Moertel, C.; Mueller, A.; Ngeow, J.; Oostenbrink, R.; Packer, R.; Papi, L.; Parry, A.; Peltonen, J.; Pichard, D.; Poppe, B.; Rezende, N.; Rodrigues, L. O.; Rosser, T.; Ruggieri, M.; Serra, E.; Steinke-Lange, V.; Stivaros, S. M.; Taylor, A.; Toelen, J.; Tonsgard, J.; Trevisson, E.; Upadhyaya, M.; Varan, A.; Wilson, M.; Wu, H.; Zadeh, G.; Huson, S. M.; Evans, D. G.; Plotkin, S. R. Revised Diagnostic Criteria for Neurofibromatosis Type 1 and Legius Syndrome: An International Consensus Recommendation. Genet. Med. 2021, 23 (8), 1506–1513. [CrossRef]
- Hirbe, A. C.; Gutmann, D. H. Neurofibromatosis Type 1: A Multidisciplinary Approach to Care. Lancet Neurol. 2014, 13 (8), 834–843. [CrossRef]
- Gonzalez-Gonzalez, L. A.; Scanga, H.; Traboulsi, E.; Nischal, K. K. Novel Clinical Presentation of a CRX Rod-Cone Dystrophy. BMJ Case Rep. 2021, 14 (4), e233711. [CrossRef]
- Hamel, C. P. Cone Rod Dystrophies. Orphanet J. Rare Dis. 2007, 2 (1), 7. Sci. 2000, 41 (11), 3268–3277. [CrossRef]
- Furukawa, T.; Morrow, E. M.; Cepko, C. L. Crx, a Novel Otx-like Homeobox Gene, Shows Photoreceptor-Specific Expression and Regulates Photoreceptor Differentiation. Cell 1997, 91 (4), 531–541. [CrossRef]
- Wang, L.; Qi, A.; Pan, H.; Liu, B.; Feng, J.; Chen, W.; Wang, B. A Novel CRX Frameshift Mutation Causing Cone-Rod Dystrophy in a Chinese Family: A Case Report. Medicine (Baltimore) 2018, 97 (32), e11499. [CrossRef]
- Peng, G.H.; Ahmad, O.; Ahmad, F.; Liu, J.; Chen, S. The Photoreceptor-Specific Nuclear Receptor Nr2e3 Interacts with Crx and Exerts Opposing Effects on the Transcription of Rod versus Cone Genes. Hum. Mol. Genet. 2005, 14 (6), 747–764. [CrossRef]
- Kehrer-Sawatzki, H.; Cooper, D. N. Challenges in the Diagnosis of Neurofibromatosis Type 1 (NF1) in Young Children Facilitated by Means of Revised Diagnostic Criteria Including Genetic Testing for Pathogenic NF1 Gene Variants. Hum. Genet. 2022, 141 (2), 177–191. [CrossRef]
- Bergoug, M.; Doudeau, M.; Godin, F.; Mosrin, C.; Vallée, B.; Bénédetti, H. Neurofibromin Structure, Functions and Regulation. Cells 2020, 9 (11), 2365. [CrossRef]
- Mallone, F.; Alisi, L.; Lucchino, L.; Di Martino, V.; Nebbioso, M.; Armentano, M.; Lambiase, A.; Moramarco, A. Insights into Novel Choroidal and Retinal Clinical Signs in Neurofibromatosis Type 1. Int. J. Mol. Sci. 2023, 24 (17). [CrossRef]
- Stenson, P. D.; Mort, M.; Ball, E. V.; Evans, K.; Hayden, M.; Heywood, S.; Hussain, M.; Phillips, A. D.; Cooper, D. N. The Human Gene Mutation Database: Towards a Comprehensive Repository of Inherited Mutation Data for Medical Research, Genetic Diagnosis and next-Generation Sequencing Studies. Hum. Genet. 2017, 136 (6), 665–677. [CrossRef]
- Tadini, G.; Brems, H.; Legius, E. Proposal of New Diagnostic Criteria. In Multidisciplinary Approach to Neurofibromatosis Type 1; Springer International Publishing: Cham, 2020; pp 309–313. Book chapter https://link.springer.com/chapter/10.1007/978-3-319-92450-2_21.
- Koczkowska, M.; Chen, Y.; Callens, T.; Gomes, A.; Sharp, A.; Johnson, S.; Hsiao, M.-C.; Chen, Z.; Balasubramanian, M.; Barnett, C. P.; Becker, T. A.; Ben-Shachar, S.; Bertola, D. R.; Blakeley, J. O.; Burkitt-Wright, E. M. M.; Callaway, A.; Crenshaw, M.; Cunha, K. S.; Cunningham, M.; D’Agostino, M. D.; Dahan, K.; De Luca, A.; Destrée, A.; Dhamija, R.; Eoli, M.; Evans, D. G. R.; Galvin-Parton, P.; George-Abraham, J. K.; Gripp, K. W.; Guevara-Campos, J.; Hanchard, N. A.; Hernández-Chico, C.; Immken, L.; Janssens, S.; Jones, K. J.; Keena, B. A.; Kochhar, A.; Liebelt, J.; Martir-Negron, A.; Mahoney, M. J.; Maystadt, I.; McDougall, C.; McEntagart, M.; Mendelsohn, N.; Miller, D. T.; Mortier, G.; Morton, J.; Pappas, J.; Plotkin, S. R.; Pond, D.; Rosenbaum, K.; Rubin, K.; Russell, L.; Rutledge, L. S.; Saletti, V.; Schonberg, R.; Schreiber, A.; Seidel, M.; Siqveland, E.; Stockton, D. W.; Trevisson, E.; Ullrich, N. J.; Upadhyaya, M.; van Minkelen, R.; Verhelst, H.; Wallace, M. R.; Yap, Y.-S.; Zackai, E.; Zonana, J.; Zurcher, V.; Claes, K.; Martin, Y.; Korf, B. R.; Legius, E.; Messiaen, L. M. Genotype-Phenotype Correlation in NF1: Evidence for a More Severe Phenotype Associated with Missense Mutations Affecting NF1 Codons 844–848. Am. J. Hum. Genet. 2018, 102 (1), 69–87. [CrossRef]
- Koczkowska, M.; Callens, T.; Chen, Y.; Gomes, A.; Hicks, A. D.; Sharp, A.; Johns, E.; Uhas, K. A.; Armstrong, L.; Bosanko, K. A.; Babovic-Vuksanovic, D.; Baker, L.; Basel, D. G.; Bengala, M.; Bennett, J. T.; Chambers, C.; Clarkson, L. K.; Clementi, M.; Cortés, F. M.; Cunningham, M.; D’Agostino, M. D.; Delatycki, M. B.; Digilio, M. C.; Dosa, L.; Esposito, S.; Fox, S.; Freckmann, M.-L.; Fauth, C.; Giugliano, T.; Giustini, S.; Goetsch, A.; Goldberg, Y.; Greenwood, R. S.; Griffis, C.; Gripp, K. W.; Gupta, P.; Haan, E.; Hachen, R. K.; Haygarth, T. L.; Hernández-Chico, C.; Hodge, K.; Hopkin, R. J.; Hudgins, L.; Janssens, S.; Keller, K.; Kelly-Mancuso, G.; Kochhar, A.; Korf, B. R.; Lewis, A. M.; Liebelt, J.; Lichty, A.; Listernick, R. H.; Lyons, M. J.; Maystadt, I.; Martinez Ojeda, M.; McDougall, C.; McGregor, L. K.; Melis, D.; Mendelsohn, N.; Nowaczyk, M. J. M.; Ortenberg, J.; Panzer, K.; Pappas, J. G.; Pierpont, M. E.; Piluso, G.; Pinna, V.; Pivnick, E. K.; Pond, D. A.; Powell, C. M.; Rogers, C.; Ruhrman Shahar, N.; Rutledge, S. L.; Saletti, V.; Sandaradura, S. A.; Santoro, C.; Schatz, U. A.; Schreiber, A.; Scott, D. A.; Sellars, E. A.; Sheffer, R.; Siqveland, E.; Slopis, J. M.; Smith, R.; Spalice, A.; Stockton, D. W.; Streff, H.; Theos, A.; Tomlinson, G. E.; Tran, G.; Trapane, P. L.; Trevisson, E.; Ullrich, N. J.; Van den Ende, J.; Schrier Vergano, S. A.; Wallace, S. E.; Wangler, M. F.; Weaver, D. D.; Yohay, K. H.; Zackai, E.; Zonana, J.; Zurcher, V.; Claes, K. B. M.; Eoli, M.; Martin, Y.; Wimmer, K.; De Luca, A.; Legius, E.; Messiaen, L. M. Clinical Spectrum of Individuals with Pathogenic N F1 Missense Variants Affecting p.Met1149, p.Arg1276, and p.Lys1423: Genotype–Phenotype Study in Neurofibromatosis Type 1. Hum. Mutat. 2020, 41 (1), 299–315. [CrossRef]
- Peduto, C.; Zanobio, M.; Nigro, V.; Perrotta, S.; Piluso, G.; Santoro, C. Neurofibromatosis Type 1: Pediatric Aspects and Review of Genotype–Phenotype Correlations. Cancers (Basel) 2023, 15 (4), 1217. [CrossRef]
- Finkelstein, R.; Boncinelli, E. From Fly Head to Mammalian Forebrain: The Story of Otd and Otx. Trends Genet. 1994, 10 (9), 310–315. [CrossRef]
- Freund, C. L.; Gregory-Evans, C. Y.; Furukawa, T.; Papaioannou, M.; Looser, J.; Ploder, L.; Bellingham, J.; Ng, D.; Herbrick, J.-A. S.; Duncan, A.; Scherer, S. W.; Tsui, L.-C.; Loutradis-Anagnostou, A.; Jacobson, S. G.; Cepko, C. L.; Bhattacharya, S. S.; McInnes, R. R. Cone-Rod Dystrophy Due to Mutations in a Novel Photoreceptor-Specific Homeobox Gene () Essential for Maintenance of the Photoreceptor. Cell 1997, 91 (4), 543–553. [CrossRef]
- Cui, H.; Senior Department of Ophthalmology, the Third Medical Center of PLA General Hospital, Beijing 100853, China; Yang, Q.-H.; Qu, L.-H.; Hou, B.-K.; Li, Z.-H.; Huang, H.-B.; Senior Department of Ophthalmology, the Third Medical Center of PLA General Hospital, Beijing 100853, China; Department of Ophthalmology, the 74th Army Group Hospital, Guangzhou 510318, Guangdong Province, China; Senior Department of Ophthalmology, the Third Medical Center of PLA General Hospital, Beijing 100853, China; Senior Department of Ophthalmology, the Third Medical Center of PLA General Hospital, Beijing 100853, China; Senior Department of Ophthalmology, the Third Medical Center of PLA General Hospital, Beijing 100853, China; Department of Ophthalmology, Hainan Hospital of Chinese PLA General Hospital, Sanya 572000, Hainan Province, China. Various Phenotypes of Autosomal Dominant Cone-Rod Dystrophy with Cone-Rod Homeobox Mutation in Two Chinese Families. Int. J. Ophthalmol. 2022, 15 (12), 1915–1923. [CrossRef]
- Hodges, M. D.; Vieira, H.; Gregory-Evans, K.; Gregory-Evans, C. Y. Characterization of the Genomic and Transcriptional Structure of the CRX Gene: Substantial Differences between Human and Mouse. Genomics 2002, 80 (5), 531–542. [CrossRef]
- Hull, S.; Arno, G.; Plagnol, V.; Chamney, S.; Russell-Eggitt, I.; Thompson, D.; Ramsden, S. C.; Black, G. C. M.; Robson, A.; Holder, G. E.; Moore, A. T.; Webster, A. R. The Phenotypic Variability of Retinal Dystrophies Associated with Mutations in CRX, with Report of a Novel Macular Dystrophy Phenotype. Invest. Ophthalmol. Vis. Sci. 2014, 55 (10), 6934–6944. [CrossRef]
- Swain, P. K.; Chen, S.; Wang, Q.-L.; Affatigato, L. M.; Coats, C. L.; Brady, K. D.; Fishman, G. A.; Jacobson, S. G.; Swaroop, A.; Stone, E.; Sieving, P. A.; Zack, D. J. Mutations in the Cone-Rod Homeobox Gene Are Associated with the Cone-Rod Dystrophy Photoreceptor Degeneration. Neuron 1997, 19 (6), 1329–1336. [CrossRef]
- Doudeau, M.; Godin, F.; Mosrin, C.; Vallée, B.; Bénédetti, H. Neurofibromin Structure, Functions and Regulation. Cells 2020, 9 (11), 2365. [CrossRef]
- Clanor, P.-M. B.; Buchholz, C. N.; Hayes, J. E.; Friedman, M. A.; White, A. M.; Enke, R. A.; Berndsen, C. E. Structural and Functional Analysis of the Human Cone-rod Homeobox Transcription Factor. Proteins 2022, 90 (8), 1584–1593. [CrossRef]
- Perrault, I. Evidence of Autosomal Dominant Leber Congenital Amaurosis (LCA) Underlain by a CRX Heterozygous Null Allele. J. Med. Genet. 2003, 40 (7), 90e–990. [CrossRef]
- Tamura, R. Current Understanding of Neurofibromatosis Type 1, 2, and Schwannomatosis. Int. J. Mol. Sci. 2021, 22 (11), 5850. [CrossRef]
- Jurcă, M. C.; Iuhas, O. A.; Puiu, M.; Chiriţă-Emandi, A.; Andreescu, N. I.; Petcheşi, C. D.; Jurcă, A. D.; Magyar, I.; Jurcă, S. I.; Kozma, K.; Severin, E. M.; Bembea, M. Cardiofaciocutaneous Syndrome - a Longitudinal Study of a Case over 33 Years: Case Report and Review of the Literature. Rom. J. Morphol. Embryol. 2021, 62 (2), 563–568. [CrossRef]
- Rasmussen, S. A.; Yang, Q.; Friedman, J. M. Mortality in Neurofibromatosis 1: An Analysis Using U.s. Death Certificates. Am. J. Hum. Genet. 2001, 68 (5), 1110–1118. [CrossRef]
- Messersmith, L.; Krauland, K. Neurofibroma; StatPearls Publishing, 2023 book https://www.ncbi.nlm.nih.gov/books/NBK539707/.
- Ehara, Y.; Yamamoto, O.; Kosaki, K.; Yoshida, Y. Natural Course and Characteristics of Cutaneous Neurofibromas in Neurofibromatosis 1. J. Dermatol. 2018, 45 (1), 53–57. [CrossRef]
- Treglia, G.; Taralli, S.; Bertagna, F.; Salsano, M.; Muoio, B.; Novellis, P.; Vita, M. L.; Maggi, F.; Giordano, A. Usefulness of Whole-Body Fluorine-18-Fluorodeoxyglucose Positron Emission Tomography in Patients with Neurofibromatosis Type 1: A Systematic Review. Radiol. Res. Pract. 2012, 2012, 431029. [CrossRef]
- Viola, F.; Villani, E.; Natacci, F.; Selicorni, A.; Melloni, G.; Vezzola, D.; Barteselli, G.; Mapelli, C.; Pirondini, C.; Ratiglia, R. Choroidal Abnormalities Detected by Near-Infrared Reflectance Imaging as a New Diagnostic Criterion for Neurofibromatosis 1. Ophthalmology 2012, 119 (2), 369–375. [CrossRef]
- Pan, Y.; Hysinger, J. D.; Barron, T.; Schindler, N. F.; Cobb, O.; Guo, X.; Yalçın, B.; Anastasaki, C.; Mulinyawe, S. B.; Ponnuswami, A.; Scheaffer, S.; Ma, Y.; Chang, K.-C.; Xia, X.; Toonen, J. A.; Lennon, J. J.; Gibson, E. M.; Huguenard, J. R.; Liau, L. M.; Goldberg, J. L.; Monje, M.; Gutmann, D. H. NF1 Mutation Drives Neuronal Activity-Dependent Initiation of Optic Glioma. Nature 2021, 594 (7862), 277–282. [CrossRef]
- Vagge, A.; Camicione, P.; Capris, C.; Sburlati, C.; Panarello, S.; Calevo, M. G.; Traverso, C. E.; Capris, P. Choroidal Abnormalities in Neurofibromatosis Type 1 Detected by Near-Infrared Reflectance Imaging in Paediatric Population. Acta Ophthalmol. 2015, 93 (8), e667-71. [CrossRef]
- Sellmer, L.; Farschtschi, S.; Marangoni, M.; Heran, M. K. S.; Birch, P.; Wenzel, R.; Friedman, J. M.; Mautner, V.-F. Non-Optic Glioma in Adults and Children with Neurofibromatosis 1. Orphanet J. Rare Dis. 2017, 12 (1). [CrossRef]
- Well, L.; Döbel, K.; Kluwe, L.; Bannas, P.; Farschtschi, S.; Adam, G.; Mautner, V.-F.; Salamon, J. Genotype-Phenotype Correlation in Neurofibromatosis Type-1: NF1 Whole Gene Deletions Lead to High Tumor-Burden and Increased Tumor-Growth. PLoS Genet. 2021, 17 (5), e1009517. [CrossRef]
- Mukhopadhyay, S.; Maitra, A.; Choudhury, S. Selumetinib: The First Ever Approved Drug for Neurofibromatosis-1 Related Inoperable Plexiform Neurofibroma. Curr. Med. Res. Opin. 2021, 37 (5), 789–794. [CrossRef]
- Armstrong, A. E.; Belzberg, A. J.; Crawford, J. R.; Hirbe, A. C.; Wang, Z. J. Treatment Decisions and the Use of MEK Inhibitors for Children with Neurofibromatosis Type 1-Related Plexiform Neurofibromas. BMC Cancer 2023, 23 (1). [CrossRef]
- Sohocki, M. M.; Perrault, I.; Leroy, B. P.; Payne, A. M.; Dharmaraj, S.; Bhattacharya, S. S.; Kaplan, J.; Maumenee, I. H.; Koenekoop, R.; Meire, F. M.; Birch, D. G.; Heckenlively, J. R.; Daiger, S. P. Prevalence of AIPL1 Mutations in Inherited Retinal Degenerative Disease. Mol. Genet. Metab. 2000, 70 (2), 142–150. [CrossRef]
- 48. Payne AM, Downes SM, Bessant DA, Taylor R, Holder GE, Wa Pan, Y rren MJ, Bird AC, Bhattacharya SS. A mutation in guanylate cyclase activator 1A (GUCA1A) in an autosomal dominant cone dystrophy pedigree mapping to a new locus on chromosome 6p21.1. Hum. Mol. Genet. 1998, (2):273-7. [CrossRef]
- Kelsell, R. E.; Gregory-Evans, K.; Payne, A. M.; Perrault, I.; Kaplan, J.; Yang, R. B.; Garbers, D. L.; Bird, A. C.; Moore, A. T.; Hunt, D. M. Mutations in the Retinal Guanylate Cyclase (RETGC-1) Gene in Dominant Cone-Rod Dystrophy. Hum. Mol. Genet. 1998, 7 (7), 1179–1184. [CrossRef]
- Köhn, L.; Kadzhaev, K.; Burstedt, M. S. I.; Haraldsson, S.; Sandgren, O.; Golovleva, I. Mutation in the PYK2-Binding Domain of PITPNM3 Causes Autosomal Dominant Cone Dystrophy (CORD5) in Two Swedish Families. Adv. Exp. Med. Biol. 2008, 613, 229–234. [CrossRef]
- Yang, Z.; Chen, Y.; Lillo, C.; Chien, J.; Yu, Z.; Michaelides, M.; Klein, M.; Howes, K. A.; Li, Y.; Kaminoh, Y.; Chen, H.; Zhao, C.; Chen, Y.; Al-Sheikh, Y. T.; Karan, G.; Corbeil, D.; Escher, P.; Kamaya, S.; Li, C.; Johnson, S.; Frederick, J. M.; Zhao, Y.; Wang, C.; Cameron, D. J.; Huttner, W. B.; Schorderet, D. F.; Munier, F. L.; Moore, A. T.; Birch, D. G.; Baehr, W.; Hunt, D. M.; Williams, D. S.; Zhang, K. Mutant Prominin 1 Found in Patients with Macular Degeneration Disrupts Photoreceptor Disk Morphogenesis in Mice. J. Clin. Invest. 2008, 118 (8), 2908–2916. [CrossRef]
- Fishman, G. A.; Stone, E. M.; Alexander, K. R.; Gilbert, L. D.; Derlacki, D. J.; Butler, N. S. Serine-27-Phenylalanine Mutation within the Peripherin/RDS Gene in a Family with Cone Dystrophy. Ophthalmology 1997, 104 (2), 299–306. [CrossRef]
- Kelsell, R. E.; Gregory-Evans, K.; Gregory-Evans, C. Y.; Holder, G. E.; Jay, M. R.; Weber, B. H.; Moore, A. T.; Bird, A. C.; Hunt, D. M. Localization of a Gene (CORD7) for a Dominant Cone-Rod Dystrophy to Chromosome 6q. Am. J. Hum. Genet. 1998, 63 (1), 274–279. [CrossRef]
- Abid, A.; Ismail, M.; Mehdi, S. Q.; Khaliq, S. Identification of Novel Mutations in the SEMA4A Gene Associated with Retinal Degenerative Diseases. J. Med. Genet. 2006, 43 (4), 378–381. [CrossRef]
- Kobayashi, A.; Higashide, T.; Hamasaki, D.; Kubota, S.; Sakuma, H.; An, W.; Fujimaki, T.; McLaren, M. J.; Weleber, R. G.; Inana, G. HRG4 (UNC119) Mutation Found in Cone-Rod Dystrophy Causes Retinal Degeneration in a Transgenic Model. Invest. Ophthalmol. Vis. 2000, 41(11):3268-77. https://pubmed.ncbi.nlm.nih.gov/9425234/.
- Lu, Q.-K.; Zhao, N.; Lv, Y.-S.; Gong, W.-K.; Wang, H.-Y.; Tong, Q.-H.; Lai, X.-M.; Liu, R.-R.; Fang, M.-Y.; Zhang, J.-G.; Du, Z.-F.; Zhang, X.-N. A Novel CRX Mutation by Whole-Exome Sequencing in an Autosomal Dominant Cone-Rod Dystrophy Pedigree. International Journal of Ophthalmolog 2015,8 (6), 1112. [CrossRef]
- Fujinami-Yokokawa, Y.; Fujinami, K.; Kuniyoshi, K.; Hayashi, T.; Ueno, S.; Mizota, A.; Shinoda, K.; Arno, G.; Pontikos, N.; Yang, L.; Liu, X.; Sakuramoto, H.; Katagiri, S.; Mizobuchi, K.; Kominami, T.; Terasaki, H.; Nakamura, N.; Kameya, S.; Yoshitake, K.; Miyake, Y.; Kurihara, T.; Tsubota, K.; Miyata, H.; Iwata, T.; Tsunoda, K.; Nishimura, T.; Hayashizaki, Y.; Kondo, M.; Shimozawa, N.; Horiguchi, M.; Yamamoto, S.; Kuze, M.; Naoi, N.; Machida, S.; Shimada, Y.; Nakamura, M.; Fujikado, T.; Hotta, Y.; Takahashi, M.; Mochizuki, K.; Murakami, A.; Kondo, H.; Ishida, S.; Nakazawa, M.; Hatase, T.; Matsunaga, T.; Maeda, A.; Noda, K.; Tanikawa, A.; Yamamoto, S.; Yamamoto, H.; Araie, M.; Aihara, M.; Nakazawa, T.; Sekiryu, T.; Kashiwagi, K.; Kosaki, K.; Piero, C.; Fukuchi, T.; Hayashi, A.; Hosono, K.; Mori, K.; Tanaka, K.; Furuya, K.; Suzuki, K.; Kohata, R.; Yanagi, Y.; Minegishi, Y.; Iejima, D.; Suga, A.; Rossmiller, B. P.; Pan, Y.; Oshima, T.; Nakayama, M.; Teruyama, Y.; Yamamoto, M.; Minematsu, N.; Sanbe, H.; Mori, D.; Kijima, Y.; Mawatari, G.; Kurata, K.; Yamada, N.; Itoh, M.; Kawaji, H.; Murakawa, Y.; Japan Eye Genetics Consortium. Clinical and Genetic Characteristics of 18 Patients from 13 Japanese Families with CRX-Associated Retinal Disorder: Identification of Genotype-Phenotype Association. Sci. Rep. 2020, 10 (1). [CrossRef]
- Birtel, J.; Eisenberger, T.; Gliem, M.; Müller, P. L.; Herrmann, P.; Betz, C.; Zahnleiter, D.; Neuhaus, C.; Lenzner, S.; Holz, F. G.; Mangold, E.; Bolz, H. J.; Charbel Issa, P. Clinical and Genetic Characteristics of 251 Consecutive Patients with Macular and Cone/Cone-Rod Dystrophy. Sci. Rep. 2018, 8 (1). [CrossRef]
- Georgiou, M.; Fujinami, K.; Michaelides, M. Inherited Retinal Diseases: Therapeutics, Clinical Trials and End Points—A Review. Clin. Experiment. Ophthalmol. 2021, 49 (3), 270–288. [CrossRef]
- Boulanger-Scemama, E.; El Shamieh, S.; Démontant, V.; Condroyer, C.; Antonio, A.; Michiels, C.; Boyard, F.; Saraiva, J.-P.; Letexier, M.; Souied, E.; Mohand-Saïd, S.; Sahel, J.-A.; Zeitz, C.; Audo, I. Next-Generation Sequencing Applied to a Large French Cone and Cone-Rod Dystrophy Cohort: Mutation Spectrum and New Genotype-Phenotype Correlation. Orphanet J. Rare Dis. 2015, 10 (1). [CrossRef]
- Thiadens, A. A. H. J.; Phan, T. M. L.; Zekveld-Vroon, R. C.; Leroy, B. P.; van den Born, L. I.; Hoyng, C. B.; Klaver, C. C. W.; Roosing, S.; Pott, J.-W. R.; van Schooneveld, M. J.; van Moll-Ramirez, N.; van Genderen, M. M.; Boon, C. J. F.; den Hollander, A. I.; Bergen, A. A. B.; De Baere, E.; Cremers, F. P. M.; Lotery, A. J. Clinical Course, Genetic Etiology, and Visual Outcome in Cone and Cone–Rod Dystrophy. Ophthalmology 2012, 119 (4), 819–826. [CrossRef]
- Carss, K. J.; Arno, G.; Erwood, M.; Stephens, J.; Sanchis-Juan, A.; Hull, S.; Megy, K.; Grozeva, D.; Dewhurst, E.; Malka, S.; Plagnol, V.; Penkett, C.; Stirrups, K.; Rizzo, R.; Wright, G.; Josifova, D.; Bitner-Glindzicz, M.; Scott, R. H.; Clement, E.; Allen, L.; Armstrong, R.; Brady, A. F.; Carmichael, J.; Chitre, M.; Henderson, R. H. H.; Hurst, J.; MacLaren, R. E.; Murphy, E.; Paterson, J.; Rosser, E.; Thompson, D. A.; Wakeling, E.; Ouwehand, W. H.; Michaelides, M.; Moore, A. T.; Webster, A. R.; Raymond, F. L.; Aitman, T.; Alachkar, H.; Ali, S.; Allen, L.; Allsup, D.; Ambegaonkar, G.; Anderson, J.; Antrobus, R.; Armstrong, R.; Arno, G.; Arumugakani, G.; Ashford, S.; Astle, W.; Attwood, A.; Austin, S.; Bacchelli, C.; Bakchoul, T.; Bariana, T. K.; Baxendale, H.; Bennett, D.; Bethune, C.; Bibi, S.; Bitner-Glindzicz, M.; Bleda, M.; Boggard, H.; Bolton-Maggs, P.; Booth, C.; Bradley, J. R.; Brady, A.; Brown, M.; Browning, M.; Bryson, C.; Burns, S.; Calleja, P.; Canham, N.; Carmichael, J.; Carss, K.; Caulfield, M.; Chalmers, E.; Chandra, A.; Chinnery, P.; Chitre, M.; Church, C.; Clement, E.; Clements-Brod, N.; Clowes, V.; Coghlan, G.; Collins, P.; Cooper, N.; Creaser-Myers, A.; DaCosta, R.; Daugherty, L.; Davies, S.; Davis, J.; De Vries, M.; Deegan, P.; Deevi, S. V. V.; Deshpande, C.; Devlin, L.; Dewhurst, E.; Doffinger, R.; Dormand, N.; Drewe, E.; Edgar, D.; Egner, W.; Erber, W. N.; Erwood, M.; Everington, T.; Favier, R.; Firth, H.; Fletcher, D.; Flinter, F.; Fox, J. C.; Frary, A.; Freson, K.; Furie, B.; Furnell, A.; Gale, D.; Gardham, A.; Gattens, M.; Ghali, N.; Ghataorhe, P. K.; Ghurye, R.; Gibbs, S.; Gilmour, K.; Gissen, P.; Goddard, S.; Gomez, K.; Gordins, P.; Gräf, S.; Greene, D.; Greenhalgh, A.; Greinacher, A.; Grigoriadou, S.; Grozeva, D.; Hackett, S.; Hadinnapola, C.; Hague, R.; Haimel, M.; Halmagyi, C.; Hammerton, T.; Hart, D.; Hayman, G.; Heemskerk, J. W. M.; Henderson, R.; Hensiek, A.; Henskens, Y.; Herwadkar, A.; Holden, S.; Holder, M.; Holder, S.; Hu, F.; Huissoon, A.; Humbert, M.; Hurst, J.; James, R.; Jolles, S.; Josifova, D.; Kazmi, R.; Keeling, D.; Kelleher, P.; Kelly, A. M.; Kennedy, F.; Kiely, D.; Kingston, N.; Koziell, A.; Krishnakumar, D.; Kuijpers, T. W.; Kumararatne, D.; Kurian, M.; Laffan, M. A.; Lambert, M. P.; Allen, H. L.; Lawrie, A.; Lear, S.; Lees, M.; Lentaigne, C.; Liesner, R.; Linger, R.; Longhurst, H.; Lorenzo, L.; Machado, R.; Mackenzie, R.; MacLaren, R.; Maher, E.; Maimaris, J.; Mangles, S.; Manson, A.; Mapeta, R.; Markus, H. S.; Martin, J.; Masati, L.; Mathias, M.; Matser, V.; Maw, A.; McDermott, E.; McJannet, C.; Meacham, S.; Meehan, S.; Megy, K.; Mehta, S.; Michaelides, M.; Millar, C. M.; Moledina, S.; Moore, A.; Morrell, N.; Mumford, A.; Murng, S.; Murphy, E.; Nejentsev, S.; Noorani, S.; Nurden, P.; Oksenhendler, E.; Ouwehand, W. H.; Papadia, S.; Park, S.-M.; Parker, A.; Pasi, J.; Patch, C.; Paterson, J.; Payne, J.; Peacock, A.; Peerlinck, K.; Penkett, C. J.; Pepke-Zaba, J.; Perry, D. J.; Pollock, V.; Polwarth, G.; Ponsford, M.; Qasim, W.; Quinti, I.; Rankin, S.; Rankin, J.; Raymond, F. L.; Rehnstrom, K.; Reid, E.; Rhodes, C. J.; Richards, M.; Richardson, S.; Richter, A.; Roberts, I.; Rondina, M.; Rosser, E.; Roughley, C.; Rue-Albrecht, K.; Samarghitean, C.; Sanchis-Juan, A.; Sandford, R.; Santra, S.; Sargur, R.; Savic, S.; Schulman, S.; Schulze, H.; Scott, R.; Scully, M.; Seneviratne, S.; Sewell, C.; Shamardina, O.; Shipley, D.; Simeoni, I.; Sivapalaratnam, S.; Smith, K.; Sohal, A.; Southgate, L.; Staines, S.; Staples, E.; Stauss, H.; Stein, P.; Stephens, J.; Stirrups, K.; Stock, S.; Suntharalingam, J.; Tait, R. C.; Talks, K.; Tan, Y.; Thachil, J.; Thaventhiran, J.; Thomas, E.; Thomas, M.; Thompson, D.; Thrasher, A.; Tischkowitz, M.; Titterton, C.; Toh, C.-H.; Toshner, M.; Treacy, C.; Trembath, R.; Tuna, S.; Turek, W.; Turro, E.; Van Geet, C.; Veltman, M.; Vogt, J.; von Ziegenweldt, J.; Vonk Noordegraaf, A.; Wakeling, E.; Wanjiku, I.; Warner, T. Q.; Wassmer, E.; Watkins, H.; Webster, A.; Welch, S.; Westbury, S.; Wharton, J.; Whitehorn, D.; Wilkins, M.; Willcocks, L.; Williamson, C.; Woods, G.; Wort, J.; Yeatman, N.; Yong, P.; Young, T.; Yu, P. Comprehensive Rare Variant Analysis via Whole-Genome Sequencing to Determine the Molecular Pathology of Inherited Retinal Disease. Am. J. Hum. Genet. 2017, 100 (1), 75–90. [CrossRef]
- Oh, J. K.; Nuzbrokh, Y.; Lee, W.; Lima de Carvalho, J. R., Jr; Wang, N. K.; Sparrow, J. R.; Allikmets, R.; Tsang, S. H. A Mutation in CRX Causing Pigmented Paravenous Retinochoroidal Atrophy. Eur. J. Ophthalmol. 2022, 32 (1), NP235–NP239. [CrossRef]
- Zobor, D.; Kaufmann, D. H.; Weckerle, P.; Sauer, A.; Wissinger, B.; Wilhelm, H.; Kohl, S. Cone-Rod Dystrophy Associated with Amelogenesis Imperfecta in a Child with Neurofibromatosis Type 1. Ophthalmic Genet. 2012, 33 (1), 34–38. [CrossRef]
- Kylstra, J. A.; Aylsworth, A. S. Cone-Rod Retinal Dystrophy in a Patient with Neurofibromatosis Type 1. Can. J. Ophthalmol. 1993, 28 (2), 79–80.

| Neurofibromatosis type 1 |
| A: The diagnostic criteria for NF1 are met in an individual who does not have a parent diagnosed with NF1 if two or more of the following are present: |
| 2 or more of the criteria |
| ≥ 6 café-au-lait spots (> 5 mm before puberty, > 15 mm after puberty) |
| Freckling in the axillary or inguinal region |
| Two or more neurofibromas of any type or one plexiform neurofibroma |
| ≥ 2 Lisch nodules identified by slit lamp examination or two or more choroidal abnormalities (CAs)—defined as bright, patchy nodules imaged by optical coherence tomography (OCT) or near-infrared reflectance (NIR) imaging |
| optic pathway glioma |
| typical bony changes (dysplasia of sphenoid bone, thinning of cortex in long bones (with or without pseudarthrosis) |
| A heterozygous pathogenic NF1 variant with a variant allele fraction of 50% in apparently normal tissue, such as white blood cells. |
| B: A child of a parent who meets the diagnostic criteria specified in A merit a diagnosis of NF1 if one or more of the criteria in A are present. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





