1. Introduction
Cassava (manihoti esculenta, Crantz), a tuberous crop, providing a livelihood for over 500 million people is among the most important food staple worldwide(1). Infact, about 70 million people in Africa depend on it as a major source of carbohydrates(2). Its tubers can be processed into various products for use in pharmaceuticals, confectioneries, and breweries(1,3). In Uganda, Cassava is grown by about 29% of the agricultural households, producing 4.4 million tons under a land area of 941,000 Hectares(4). Despite the huge economic potential, cassava production in Africa is increasingly under threat by whiteflies; Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) and, millions of smallholder farms have been greatly ravaged by this pest (5). The pest is known to vector cassava mosaic begomoviruses (CMBs) and cassava brown streak geminiviruses (CBSVs), the causative agents of cassava mosaic disease (CMD) and cassava brown streak disease (CBSD)(6,7). According to (8), the transmission of these cassava viruses causes an estimated yield loss of over 1 billion USD annually in sub-Saharan Africa. In Uganda, during the early 1990s of the CMD epidemic, high whitefly populations were observed in the central and northern parts of the country which had been devastated by the epidemic (9). CBSD, which had previously been confined to the East African coastal region abruptly spread over into the mainland of the Great Lakes Region of East and Central Africa in 2004 (8,10). According to (7), the major factor responsible for the spread of this viral disease was the unprecedented increase in the abundance of the whitefly, Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae). To combat these diseases, CMD-resistant and CBSD tolerant varieties were developed and deployed by the National cassava breeding program in Uganda. However, these varieties have increasingly become susceptible to whitefly infestation and damage.
The whitefly also causes direct damage to cassava by feeding on the phloem of leaves, inducing leaf chlorosis and abscission which can result in considerable yield loss of up to about 50% on susceptible varieties (11). In addition, the honeydew excreted by Bemisia tabaci supports sooty mold formation which decreases the photosynthetic capacity of the cassava plant (12). In Uganda, previous research to identify possible sources of resistance to cassava whitefly among Latin America and Ugandan cassava landraces revealed good tolerance levels in Ecu 72, Ofumba Chai, Nabwire 1, and Njule red genotype (13). However, some of these varieties have become susceptible to Cassava Mosaic Disease (CMD) and whitefly infestation over the years. This situation is worsened by the limited research efforts focused on controlling the pest directly. More to that, the injudicious use of chemical pesticides for whitefly control increases the production costs, has adverse effects on the ecosystem, and is uneconomical for small-scale farmers (14). Therefore, stable host plant resistance offers a practical, low cost and long-term solution for sustainable whitefly management in Uganda. This, therefore, called for cassava genotypes with combined disease and whitefly resistance. As such it is imperative that the research taps into wider genetic cassava germplasm of which the best bet lines would be advanced into the cassava breeding scheme for introgression of genes of resistance to whitefly in Uganda. Therefore in 2016, a study was conducted to evaluate the reaction of 24 improved cassava varieties which were sourced from 5 different countries in the region (Uganda, Kenya, Tanzania, Malawi, and Mozambique) in a bid to identify cassava genotypes with putative resistance to whitefly in Uganda.
2. Materials and methods
2.1. Experimental sites
The trial was established in the second rains of 2016 in the North-Western Savannah Grassland (Lira - N 0229.812, E 032 91.879), Kyoga Plains (Kamuli - N 0081.056, E 033 12.402), and Lake Victoria Crescent (Wakiso - N 0051.917, E 032 63.679) agroecological zones. These sites were selected for the study based on their differential whitefly population pressure and their known history of cassava production in Uganda.
Lake Victoria Crescent is characterized by sandy clay alluvial soils with moist semi-deciduous forest, savanna, and swamps. The area receives bimodal rains ranging from 1750-2000mm in April to May and October to December for the first and second rains respectively. The average temperature is between 110C and 330C. Climate is warm and wet with high relative humidity and an altitude of 1134m above sea level. Northwestern Savanah Grassland is however made up of ferruginous sandy loam soils with intermediate savanna grassland and scattered trees. It also has a bimodal rainfall pattern ranging from 1340-1371mm. This is followed by a dry spell for about 5 months with temperature and altitude of 15-25oC and 951 – 1341m above sea level respectively.
Similar to the Lake Victoria Crescent, Kyoga plains agroecological zone is characterized by sandy clay alluvial soils with moist semi-deciduous forest, savannas, and swamps. The area, however, experiences bimodal rains of 1215-1328mm from March to May (first rains) and October to December (second rains). Temperature ranges from 15-32.5oC. It is generally warm and wet with relatively high humidity with an altitude of 1134m above sea level.
2.2. Source of planting materials
These materials were introduced in Uganda from Kenya, Tanzania, Malawi, and Mozambique as Tissue culture plantlets. These were hardened at the National Crops Resources Research Institute (NaCRRI) and then established at the cassava breeding multiplication center at RwebiZARDI in Kabarole district, western Uganda for multiplication. This site is on a high altitude and predominantly a tea-growing area with limited cassava cultivation and thus has a low disease and whitefly pressure. Materials from this site are routinely monitored and no incidence of both viral diseases has been recorded.
2.3. Experimental layout and design
The trial was laid out in RCBD with a plot size of 5m by 3m in 3 replications using 24 cassava genotypes. Stem cuttings of 20cm long with about 4 nodes each were planted at a spacing of 1m x 1m. In each replication, plots were separated by 2m from each other and 3m between replications. The trial was weeded manually using hand hoes to minimize the impact of weeds and alternate hosts of the pests. No plant protection measures, including pesticide application, were applied.
2.4. Data Collection
Monthly data were recorded from 2-9 Months After Planting (MAP) on the following parameters; adult and nymph count, whitefly feeding and sooty mold damage, CMD, and CBSD severity.
2.4.1. Whitefly adult and nymph abundance
Adult whitefly populations were assessed on the top 5 fully expanded apical leaves of 10 randomly selected plants in a plot. Each leaf was gently turned by the petiole to expose the adults on the underside (15). These were counted manually using a tally counter.
Nymphs were counted on the 14th leaf (16). This was with exception of 2 MAP where the lower mature leaves with the highest nymph numbers were assessed. The assessment was done on the same 10 plants sampled for the adult whiteflies with aid of a x10 magnifying lens.
2.4.2. Whitefly feeding damage
10 plants per plot were visually inspected using a severity scale of 1-5 where 1: No leaf damage, 2: > 25% of leaves damaged, mild chlorosis on few apical leaves, 3: >25-<50% leaves damaged with mild chlorosis, curled and twisted, 4: >50 - <75% of leaves damaged with moderate chlorosis and or wilting and 5: >75% leaves damaged with defoliation(17).
2.4.3. Sooty mold severity
This was assessed on 10 plants using a severity scale of 1-5 where 1: No leaf soot on leaves, 2: <25% of plant covered with soot, 3: >25 - <50% of plant covered with soot, 4: >50 - <75% of plant covered with soot, 5: >75% of plant covered with soot (17).
2.4.4. Cassava Mosaic and Cassava Brown Streak Disease severity
Cassava mosaic disease severity was scored on a scale of 1-5 where 1: Un-affected shoots or no symptoms observed, 2: Mild chlorotic pattern on most leaves, mild distortions at the bases of most leaves, while the remaining parts of the leaves and leaflets appear green and healthy, 3: Pronounced mosaic pattern on most leaves, narrowing and distortion of the lower one- third of the leaves, 4: Severe mosaic distortion of two-thirds of most leaves and general reduction of leaf size, and some stunting of shoots and 5: Very severe mosaic symptoms on all leaves, distortion, twisting and severe leaf reduction of most leaves accompanied by severe stunting of plants (18). The assessment was done on all 24 plants per plot.
Cassava Brown Streak Disease foliar severity was assessed in the entire plot using a severity scale of 1-5, where; 1: No apparent symptoms, 2: Foliar mosaic/ chlorosis, but no stem lesions, 3: Foliar mosaic, mild or moderate stem lesions, no die-back, 4: Foliar mosaic, severe stem lesions, or wilting but no die back, 5: Defoliation, severe stem lesions and dieback (19).
Both CMD and CBSD incidence was calculated as a percentage of the infected plants over the total number of sampled plants per plot.
2.5. Data Analysis
The data was analyzed using the R Version 3.5.1 statistical package. Whitefly adults and nymph counts from 2 to 5 MAP were subjected to the GLM (generalized linear model). The data were subjected to ANOVA followed by mean separations using the Turkeys student test (p≤0.05). The means followed by the same letter are not significantly different (p≤0.05). Mean and standard errors were calculated for damage and sooty mold, CMD, and CBSD incidence.
3. Results
3.1. Adult whitefly population on different cassava genotypes
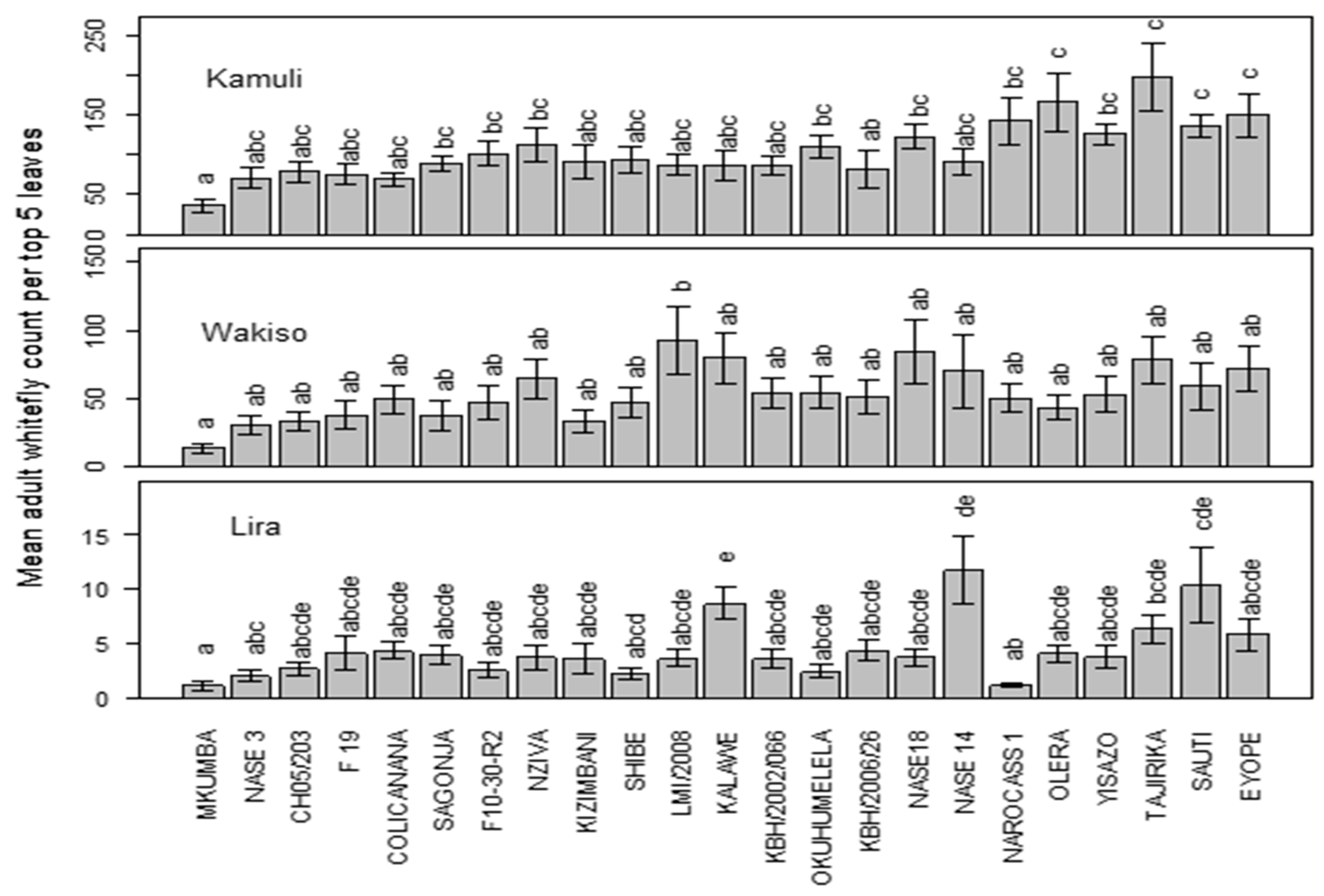
There was a significant variation in the population of the adult whiteflies between the three locations (p < 0.0001) and genotype (p <0.000). Mean adult whitefly populations were generally low in Lira compared to Wakiso and Kamuli. In Kamuli, genotypes Mkumba and KBH/2006/026 harbored the least adult whitefly numbers. The lowest populations were also observed on Mkumba and CH05/203 in Wakiso while in Lira NAROCASS1 and Mkumba were the least preferred genotypes by the adult whiteflies. Generally, Mkumba consistently registered the lowest mean adult whitefly population across the three locations (
Figure 1).
3.2. Nymph population on different cassava genotypes
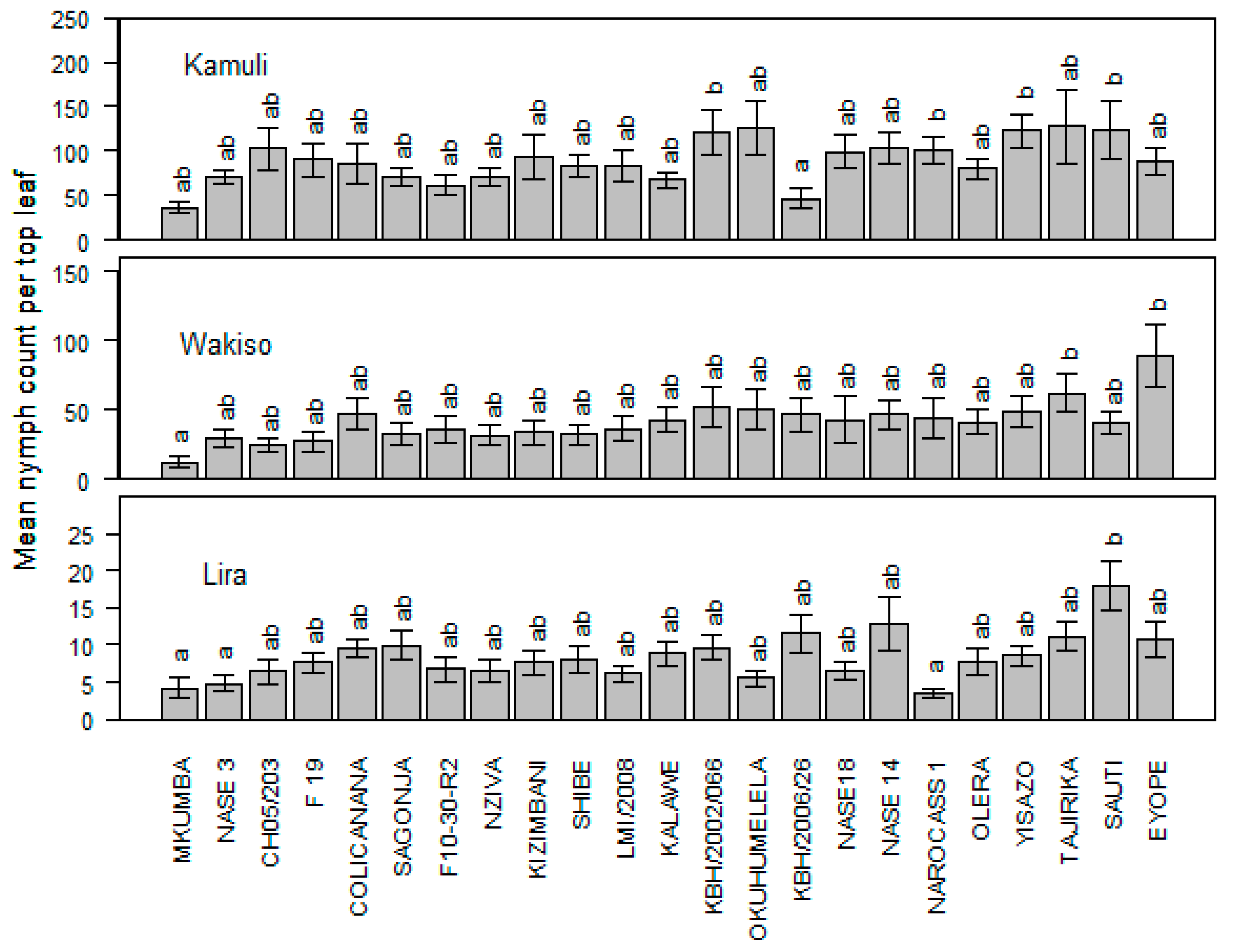
Mean nymph population varied significantly between locations (p < 0.0001) and genotypes (p = 0.001). The least nymph population was recorded on genotypes Mkumba and KBH/2006/26 in Kamuli while in Wakiso, CH05/203 and still Mkumba registered very low populations. Lira, on the other hand, had genotypes NAROCASS 1, Mkumba and NASE 3 with the least nymphal preference. Mkumba consistently maintained very low nymph numbers across the three locations. Of these locations, Lira recorded the lowest nymph populations (
Figure 2).
3.3. Whitefly damage and sooty mold severity on the different cassava genotypes
Across the three locations, average whitefly damage severity among the cassava genotypes ranged from score 1 in Lira to 2 in Wakiso. No whitefly and sooty mold damage were observed on any genotype in Lira and thus it is not included in the results in
Table 1. The lowest mean whitefly damage in Wakiso was observed on genotypes Kalawe, Kizimbani, Sauti, Shibe, and Tajirika. In Kamuli however, no damage symptoms were scored on CH05/203, Eyope, F19, F10-30-R2, Kalawe, KBH/2006/26, Kizimbani, LMI/2008, Mkumba, NAROCASS 1, NASE 3, NZIVA, Okuhumelela, Olera, Sagonja, Sauti and Yisazo (
Table 1).
Sooty mold damage was not evident on CH05/203 and NASE 14 in Wakiso. On the contrary, very low sooty mold damage was recorded on Kalawe, F10-30-R2, KBH/2006/26, Olera, Kizimbani and Mkumba in Kamuli. Sooty mold severities were generally higher in Kamuli than in Wakiso (
Table 1).
3.4. Cassava Mosaic Disease Incidence
Cassava mosaic disease symptoms were not observed on Mkumba, NASE 14, NASE 18, and KBH/2006/26 in Wakiso district. Except for Colicanana, Eyope, KBH/2002/066, Kizimbani, Nziva, and Sagonja, no disease symptoms were observed on any other genotype in Lira. Cassava genotypes; CH05/203, KBH/2006/26, LMI/2008, NAROCASS 1, NASE 14, NASE 18, Tajirika, and Yisazo did not exhibit any disease symptoms in Kamuli. Largely, the lowest CMD incidence was observed in Lira and the highest in Wakiso (
Table 2).
3.5. Cassava Brown Streak Disease Incidence
Foliar Cassava Brown Streak Disease symptoms were not observed on genotypes; Mkumba and Okhumumelela in all three locations. Generally, in Kamuli and Lira, most of the cassava genotypes did not exhibit any foliar symptoms of the disease. This was with exception of CH05/203, Eyope, F19, Nziva, Olera, Tajirika in Kamuli and Colicanana, Kalawe, Sauti, CH05/203 in Lira. The disease was least prevalent among genotypes in Lira as compared to Wakiso (
Table 2).
4. Discussion
The study revealed variation in the whitefly population in the three locations. This variation could be attributed to the different weather factors in these areas. High temperature and relative humidity are key factors that influence whitefly populations. These factors increase B. tabaci rate of development by reducing the time the nymphs take to complete the full life cycle. Kamuli and Wakiso which had high whitefly numbers are surrounded by water bodies and thus experience high relative humidity and temperature compared to Lira with a high temperature but low relative humidity. A similar study conducted by (20) revealed that weather parameters were crucial in the development of the whitefly (B.tabaci) population on tomato. Multiple regression analysis in this study showed that temperature, relative humidity, rainfall and sunshine hours combined were responsible for 89% of the variation in the whitefly population observed. More to that, (21) showed that the high adult B.tabaci populations were linked to high temperatures and low annual average rainfall around the cassava fields at the Tanzania coast. Land use pattern is another factor that could be responsible for the difference in the whitefly population structure observed in the three locations. Kamuli and Wakiso districts have had a lot of natural resources like forests, swamps reclaimed for human settlement, economic activity, and thus the habitats for the natural enemies of cassava whitely could have been destroyed and their population greatly reduced almost to extinction. The reverse is true for Lira district where most of the forest cover is still intact and thus harboring the predators and parasitoids that are proven to effectively control the whitefly population. This could explain the very low adult whitefly and nymph numbers observed in Lira as compared to Kamuli and Wakiso. The observation is supported by the study carried out by (22) in the southern part of France which showed that undisturbed vegetation cover harbored more predatory mirid bugs that are natural enemies of several pests of tomato. (23) further demonstrated that vegetation cover was among the factors responsible for colonization by Dicyphus tamaninii and Macrolophus caliginosus which are known predators of the greenhouse whitefly (Trialeurodes vaporariorum) on tomato in Spain.
Cassava genotypes reacted differently to the adult whitefly and nymph population infestation. This unearths the host plant resistance mechanisms possessed by these cassava genotypes. Host plant resistance can be categorized into antixenosis; which involves the use of inherent morphological traits like hairiness, plant architecture, leaf thickness to help repel heavy pest infestations (non-preference), antibiosis; which employs the biochemical attributes that alter the development and survival of the pest and, tolerance; where the plant can grow and substantially yield well even amidst heavy pest colonization. Plant morphological traits like the presence of glandular trichomes (hairs) have been reported as one of the main factors responsible for resistance towards small sucking pests conferred by hindering their egg-laying ability and feeding mechanisms in watermelon cultivars (24). These glandular trichomes especially type IV, have been reported to influence whitefly (B. tabaci) colonization depending on their angle on the leaf surface, type and length on tomato (25). This finding is in agreement with (26) who suggested that plant morphology and biochemistry play a key role in determining the suitability for growth and development of B. tabaci populations. According to (27), cassava defense compounds like flavonoids, cyanogenic glucosides, and hydroxycoumarins impact the population of phloem feeders like B. tabaci.
(17) indicated that antibiosis-linked resistance to whitefly in cassava was mainly influenced by biochemical and anti-nutrient compounds like free sugars, phenolics, and free proteins. These phenolic compounds have been reported to repel the feeding ability of B. tabaci on the plant as well as impacting the development, behavior, and growth of insects (28,29).
Adult whitefly and nymph population, cassava age, and leaf morphology are the main drivers of whitefly-associated feeding damage on Cassava. In this study, Kamuli and Wakiso which harbored high whitefly numbers exhibited the highest levels of damage compared to Lira where no signs of damage were observed at all. Studies carried out by (30) on sweet potato whitefly on Squash revealed that whitefly nymph and adult density was positively correlated to damage. Across the three locations, whitefly damage peaked at 3 months after planting. At this time, the plant has young tender leaves that are more preferred by the huge whitefly populations that had already colonized the plant. (31) also confirmed a similar observation on Lemon.
In addition, leaf morphology especially hairiness could have influenced the levels of feeding damage observed on the different genotypes. Earlier findings by (32) on cotton revealed that cultivars with a smooth leaf surface supported a high whitefly population that culminated into high feeding damage. The reverse was true for the hairy cultivars.
Different cassava genotypes expressed different levels of sooty mold damage. This is because different genotypes possess variations in the leaf morphological characteristics. This is further reechoed by (26) that, cassava leaf area affects sooty mold severity i.e. a genotype could have high sooty mold severity yet it harbors low whitefly population because of its broader leaf surface. However, this contradicts the findings of (13) where they observed no obvious correlation between the whitefly population and genotype traits like leaf width and color.
Sooty mold damage was greatest in Kamuli and least in Lira. This could be associated with the high whitefly populations observed in Kamuli compared to Lira. Previous studies on Cantaloupe have associated high whitefly nymph populations with heavy sooty mold establishment in Arizona, United States (33). Cassava leaf area is another probable factor driving sooty mold damage levels among the different genotypes. According to (26), some cassava genotypes with few whiteflies (B.tabaci) supported high sooty mold damage scores probably because of their large leaf area.
Unlike Lira and Kamuli, the highest CMD and CBSD incidence was recorded in Wakiso. Disease pressure and whitefly population could explain this observed trend. Studies by (34) found a positive relationship between the whitefly population and disease pressure. The study further attributed the two factors for the unprecedented spread of CBSD in Kamuli and Wakiso. Furthermore, the initial inoculum in the surrounding fields and high vector population influenced CMD incidence in susceptible cassava varieties (35,36). However, the variation in the reaction of the different genotypes to the viral diseases could be attributed to their inherent genetic make-up. Some genotypes were bred for tolerance or resistance to the two diseases.
5. Conclusions
The genotype Mkumba consistently exhibited high levels of resistance to field populations of whitefly (B. tabaci) across the three locations. The findings have demonstrated the potential of the improved cassava varieties as possible sources of combined disease and whitefly resistance for sustainable management of the whitefly. This study further recommends the inclusion of Mkumba for participatory variety selection. However, research to ascertain its mode of resistance as well as profiling its biochemical properties should be carried out.
Author Contributions
Conceptualization, O.A.C., and J.C.; methodology, O.A.C., J.C., W.S., OSM., OT., and OP.; validation, O.A.C., J.C., W.S., OSM., OT., and OP; formal analysis, OSM; investigation, W.S., OSM., OT., and OP; resources, O.A.C and J.C.; data curation, OSM.; and writing—original draft preparation, W.S and OSM.; writing—review and edit, O.A.C., J.C., W.S., OSM., OT., and OP; visualization, W.S and OSM.; supervision, O.A.C.; project administration, O.A.C and JC.; funding acquisition, O.A.C and JC. All authors have read and agreed to the published version of the manuscript.
Funding
This work received funding from the Bill and Melinda Gates Foundation through the University of Greenwich; grant number INV-010435 and previously OPP1200124. The authors are grateful to the Foundation for funding this research through the African Cassava Whitefly Project Phase one (ACWP1).
Data Availability Statement
Data for this study will be available on request.
Acknowledgments
Research teams both at National Crops Resources Research Institute and Ngetta Zonal Agricultural Research and Development Institute (Acipa Alexandrina and Tekkara Allan Obonyom) are hailed for the technical efforts rendered towards the study.
Conflicts of Interest
The authors declare no conflict of interestReferences.
References
- Ceballos H, Sánchez T, Chávez AL, Iglesias C, Debouck D, Mafla G, et al. Variation in crude protein content in cassava (Manihot esculenta Crantz) roots. J Food Compos Anal [Internet]. 2006 Sep;19(6–7):589–93. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0889157505001225. [CrossRef]
- Kawano, K. Thirty Years of Cassava Breeding for Productivity—Biological and Social Factors for Success. Crop Sci [Internet]. 2003 Jul;43(4):1325–35. Available from: https://acsess.onlinelibrary.wiley.com/doi/10.2135/cropsci2003.1325. [CrossRef]
- Gunorubon J, Kekpugile K. Journal of Engineering Modification of Cassava Starch for Industrial Uses. Int J Eng Technol. 2012;2(6).
- Oxford U of. Strategic Plan 2013–18. 2013.
- Otim-Nape GW, Bua A, Thresh JM, Baguma Y, Ogwal S, Ssemakula GN, et al. The current pandemic of cassava mosaic virus disease in East Africa and its control. Curr pandemic cassava mosaic virus Dis East Africa its Control. 2000.
- Maruthi MN, Hillocks RJ, Mtunda K, Raya MD, Muhanna M, Kiozia H, et al. Transmission of Cassava brown streak virus by Bemisia tabaci (Gennadius). J Phytopathol. 2005 May;153(5):307–12. [CrossRef]
- Legg JP, Jeremiah SC, Obiero HM, Maruthi MN, Ndyetabula I, Okao-Okuja G, et al. Comparing the regional epidemiology of the cassava mosaic and cassava brown streak virus pandemics in Africa. Virus Res. 2011 Aug;159(2):161–70. [CrossRef]
- Mbanzibwa DR, Tian YP, Tugume AK, Patil BL, Yadav JS, Bagewadi B, et al. Evolution of cassava brown streak disease- associated viruses. 2011;(2011):974–87. [CrossRef]
- Gibson RW, Legg JP, Otim-Nape GW. Unusually severe symptoms are a characteristic of the current epidemic of mosaic virus disease of cassava in Uganda. Ann Appl Biol. 1996;128(3):479–90. [CrossRef]
- Alicai T, Omongo CA, Maruthi MN, Hillocks RJ, Baguma Y, Kawuki R, et al. Re-emergence of Cassava Brown Streak Disease in Uganda. Plant Dis. 2007;91(1):24–9. [CrossRef]
- Legg JP, Thresh JM. Cassava virus diseases in Africa. Plant Virol sub-Saharan Africa. 2002;(June 2003):517–52.
- Nelson S. Sooty Mold. 2008.
- Omongo CA, Kawuki R, Bellotti AC, Alicai T, Baguma Y, Maruthi M, et al. African Cassava Whitefly, Bemisia tabaci, Resistance in African and South American Cassava Genotypes. J Integr Agric. 2012 Feb;11(2):327–36.
- Bellotti A, Herrera Campo BV, Hyman G. Cassava Production and Pest Management: Present and Potential Threats in a Changing Environment. Trop Plant Biol. 2012 Mar;5(1):39–72. [CrossRef]
- Fargette D, Fauquet C, Thouvenel J-C. With I plate. Vol. 106. 1985.
- Abisgold JD, Fishpool LDC. A method for estimating population sizes of whitefly nymphs (Bemisia tabaci genn.) on cassava. Trop Pest Manag. 1990 Jan;36(3):287–92. [CrossRef]
- Bellotti A., Arias B. Host plant resistance to whiteflies with emphasis on cassava as a case study. Crop Prot. 2001 Nov;20(9):813–23. [CrossRef]
- Hahn SK, Terry ER, Leuschner K. Breeding cassava for resistance to cassava mosaic disease. Euphytica. 1980 Nov;29(3):673–83. [CrossRef]
- Gondwe FMT, Mahungu NM, Hillocks RJ, Raya MD, Moyo CC, Soko MM, et al. Economic losses experienced by smallscale farmers in Malawi due to cassava brown streak virus disease. 2003.
- Sharma D, Maqbool A, Ahmad H, Srivastava K, Kumar M, Vir V. Effect of meteorological factors on the population dynamics of insect pests. 2013;(August 2015). 20 August.
- Jeremiah SC, Ndyetabula IL, Mkamilo GS, Haji S, Muhanna MM, Chuwa C, et al. The Dynamics and Environmental Influence on Interactions Between Cassava Brown Streak Disease and the Whitefly, Bemisia tabaci. Phytopathology. 2015 May;105(5):646–55. [CrossRef]
- Aviron S, Poggi S, Varennes Y-D, Lefèvre A. Local landscape heterogeneity affects crop colonization by natural enemies of pests in protected horticultural cropping systems. Agric Ecosyst Environ. 2016 Jul;227:1–10. [CrossRef]
- Alomar Ò, Goula M, Albajes R. Colonisation of tomato fields by predatory mirid bugs (Hemiptera: Heteroptera) in northern Spain. Agric Ecosyst Environ. 2002 Apr;89(1–2):105–15. [CrossRef]
- McAuslane HJ, Webb SE, Elmstrom GW. Resistance in Germplasm of Cucurbita pepo to Silverleaf, a Disorder Associated with Bemisia argentifolii (Homoptera: Aleyrodidae). Florida Entomol. 1996 Jun;79(2):206. [CrossRef]
- Toscano LC, Boiça Jr. AL, Maruyama WI. Nonpreference of whitefly for oviposition in tomato genotypes. Sci Agric [Internet]. 2002 Dec;59(4):677–81. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-90162002000400009&lng=en&tlng=en.
- Macfadyen S, Scientific TC, Paull CA, Scientific TC, Boykin LM, Gowda MMN. Cassava whitefly, Bemisia tabaci ( Gennadius ) ( Hemiptera : Aleyrodidae ) in East African farming landscapes : a review of the factors determining abundance. 2018;(February).
- Pinto-Zevallos DM, Pareja M, Ambrogi BG. Current knowledge and future research perspectives on cassava (Manihot esculenta Crantz) chemical defenses: An agroecological view. Phytochemistry. 2016 Oct;130:10–21. [CrossRef]
- SULISTYO A, INAYATI A. Mechanisms of antixenosis, antibiosis, and tolerance of fourteen soybean genotypes in response to whiteflies (Bemisia tabaci). Biodiversitas J Biol Divers. 2016 May;17(2). [CrossRef]
- Fürstenberg-Hägg J, Zagrobelny M, Bak S, Fürstenberg-Hägg J, Zagrobelny M, Bak S. Plant Defense against Insect Herbivores. Int J Mol Sci. 2013 May;14(5):10242–97. [CrossRef]
- Schuster DJ, Kring JB, Price JF. Association of the Sweetpotato Whitefly with a Silverleaf Disorder of Squash. HortScience. 2019;26(2):155–6. [CrossRef]
- Walker GP, Zareh N. Leaf age preference for opposition by three species of whitefly on lemon. Entomol Exp Appl. 1990 Jul;56(1):31–45. [CrossRef]
- Butler GD, Henneberry TJ, Wilson FD. Bemisia tabaci (Homoptera: Aleyrodidae) on Cotton: Adult Activity and Cultivar Oviposition Preference. J Econ Entomol. 1986 Apr;79(2):350–4. [CrossRef]
- Riley DG, Palumbo JC. Interaction of Silverleaf Whitefly (Homoptera: Aleyrodidae) with Cantaloupe Yield. J Econ Entomol. 1995 Dec;88(6):1726–32. [CrossRef]
- Katono K, Alicai T, Baguma Y, Edema R, Bua A, Omongo C. Influence of Host Plant Resistance and Disease Pressure on Spread of Cassava Brown Streak Disease in Uganda. Am J Exp Agric [Internet]. 2015 Jan 10;7(5):284–93. Available from: https://journaljeai.com/index.php/JEAI/article/view/675. [CrossRef]
- Colvin J, Omongo CA, Maruthi MN, Otim-Nape GW, Thresh JM. Dual begomovirus infections and high Bemisia tabaci populations: Two factors driving the spread of a cassava mosaic disease pandemic. Plant Pathol. 2004;53(5):577–84. [CrossRef]
- Legg JP, Sseruwagi P, Boniface S, Okao-Okuja G, Shirima R, Bigirimana S, et al. Spatio-temporal patterns of genetic change amongst populations of cassava Bemisia tabaci whiteflies driving virus pandemics in East and Central Africa. Virus Res. 2014 Jun;186:61–75. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).