Submitted:
08 February 2024
Posted:
08 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Three simple assumptions of 4G model of final unification
- 1)
- There exists a characteristic weak fermion of rest energy, . It can be considered as the zygote of all elementary particles [7].
- 2)
- There exists a nuclear elementary charge in such a way that, = Strong coupling constant [13] and .
- 3)
- Each atomic interaction is associated with a characteristic large gravitational coupling constant. Their fitted magnitudes are,
- a)
- Interaction range is inversely proportional to interaction strength.
- b)
- Effective magnitude of the operating gravitational constant is,
3. Atomic, nuclear and sub-nuclear applications
- a)
- Up, Charm and Top quark’s electromagnetic charge is
- b)
- Down, Strange and Bottom quark’s electromagnetic charge is
- c)
- Quarks having an electromagnetic charge of and can be called as integral charge quarks.
- d)
- There exists no repulsion between any two particles having same kind of nuclear charge.
4. Understanding the rest mass of electron neutrino
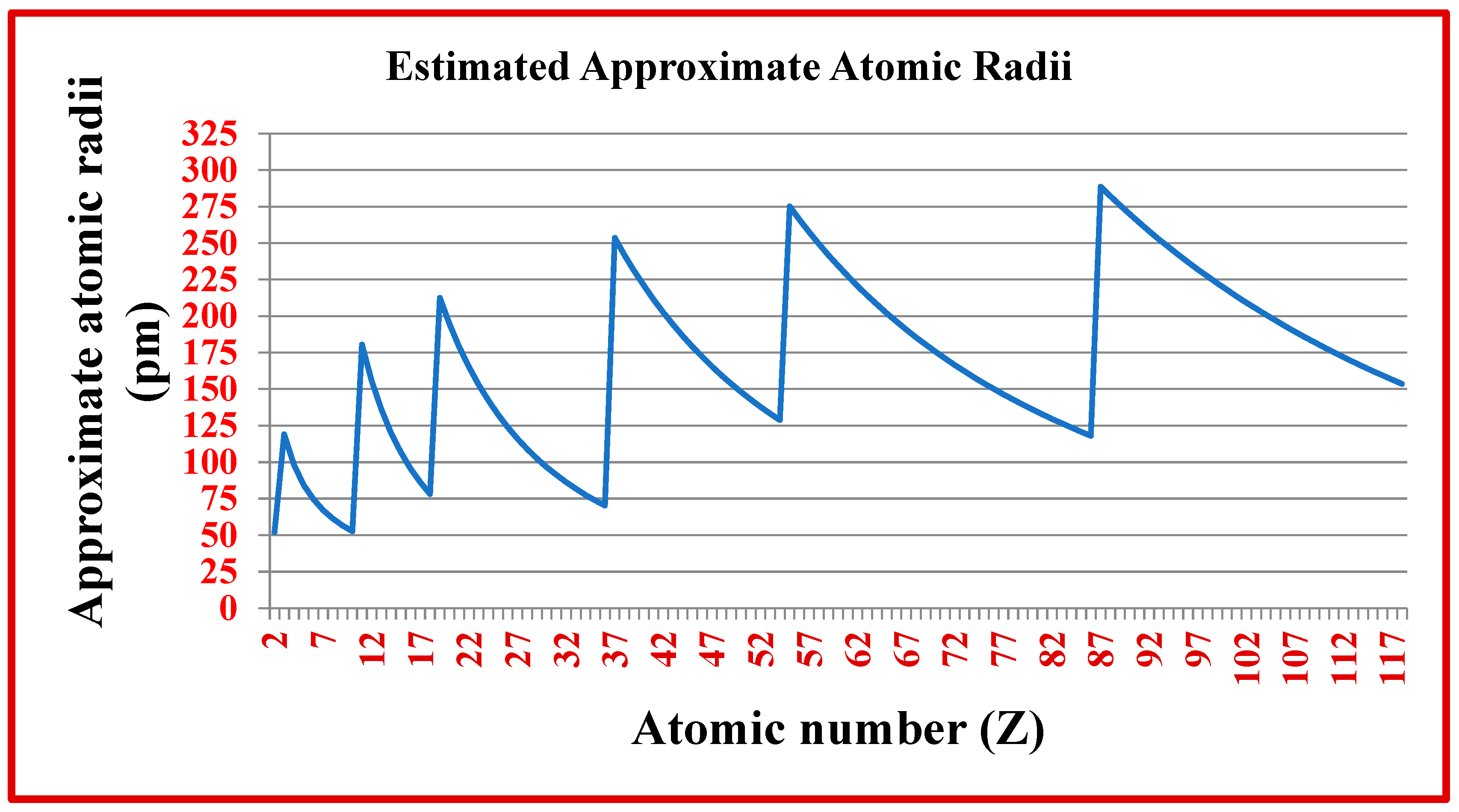
5. Understanding and estimating atomic radii
6. Understanding Unified atomic mass unit, Avogadro number and Electrochemical equivalents
7. Conclusion
References
- Salam A, Sivaram C. Strong gravity approach to QCD and confinement. Modern Physics Letters A.8,4,321,1993. [CrossRef]
- Onofrio, R. On weak interactions as short-distance manifestations of gravity. Modern Physics Letters A.28,7,1350022, 2013. [CrossRef]
- Seshavatharam U.V.S and Lakshminarayana S. Final unification with Schwarzschild’s Interaction. Journal of Applied Physical Science International 3(1): 12-22, 2015.
- Seshavatharam, U. V. S, Lakshminarayana S. Understanding the Basics of Final Unification With Three Gravitational Constants Associated With Nuclear, Electromagnetic and Gravitational Interactions. J. Nucl. Phy. Mat. Sci. Rad. A. 4, 355-373, 2017. [CrossRef]
- Seshavatharam, U.V.S, Lakshminarayana S. Role of Four Gravitational Constants in Nuclear Structure. Mapana-Journal of Sciences.18,1,21, 2019. [CrossRef]
- Seshavatharam, U.V.S, Lakshminarayana S. To Validate the Role of Electromagnetic and Strong Gravitational Constants via the Strong Elementary Charge. Universal Journal of Physics and Application. 9(5): 216-225, 2015. [CrossRef]
- Seshavatharam U. V. S, Gunavardhana Naidu T, Lakshminarayana S. To confirm the existence of heavy weak fermion of rest energy 585 GeV. AIP Conf. Proc. 2451, 020003-1–020003-6, 2022.
- Seshavatharam U.V.S, Lakshminarayana S. EPR argument and mystery of the reduced Planck’s constant. Algebras, Groups, and Geometries. 36(4), 801-822, 2020,.
- Seshavatharam U.V.S, Lakshminarayana S. 4G model of final unification – A brief report. Journal of Physics: Conference Series 2197, 012029, 2022.
- Seshavatharam U.V.S and Lakshminarayana S. Is reduced Planck’s constant - an outcome of electroweak gravity? Mapana Journal of Sciences. 19,1,1, 2020.
- Mukhi, S. String theory: a perspective over the last 25 years. Classical and Quantum Gravity. 28,15, 153001, 2011. [CrossRef]
- Seshavatharam U.V.S and Lakshminarayana S. On the Compactification and Reformation of String Theory with Three Large Atomic Gravitational Constants. International Journal of Physical Research, 9(1), 42-48, 2021.
- R.L. Workman et al. (Particle Data Group), Prog. Theor. Exp. Phys. 2022, 083C01 (2022) and 2023 update.
- Tiesinga, E., Mohr, P. J., Newell, D. B. & Taylor, B. N. CODATA recommended values of the fundamental physical constants: 2018. Rev. Mod. Phys. 93, 025010, 2021. [CrossRef]
- Seshavatharam U. V. S and Lakshminarayana S. Understanding the Origins of Quark charges, Quantum of Magnetic Flux, Planck’s radiation constant and celestial magnetic moments with 4G model of nuclear charge. (In review). [CrossRef]
- Seshavatharam U.V.S, Lakshminarayana S. H. K. Cherop and K.M. Khanna. Three Unified Nuclear Binding Energy Formulae. World Scientific News, 163, 30-77, 2022.
- Seshavatharam U.V.S, Lakshminarayana S. On the Role of Nuclear Binding Energy in Understanding Cold Nuclear Fusion. Mapana Journal of Sciences, 20(3), 29-42, 2021.
- Seshavatharam U.V.S, Lakshminarayana, S. IGEC Transactions. To Develop an Eco-friendly Cold Nuclear Thermal Power Plant by Considering Iron-56 as A Fuel. Proceedings of the 15th International Green Energy Conference (IGEC-XV). Springer Nature Switzerland, 2024.
- Seshavatharam U. V. S and Lakshminarayana S. Understanding condensed matter physics with refined strong and electroweak mass formula. (In review).
- E. S. Green. Nuclear Physics. McGraw Hill Book Co. 1955.
- Seshavatharam U.V.S and Lakshminarayana. Understanding nuclear stability range with 4G model of nuclear charge. World scientific news. 177, 118-136, 2023.
- Gao Z. P, Wang YJ, Lü HL et al., Machine learning the nuclear mass. NUCL. SCI. TECH. 32, 109, 2021.
- Tuncay Bayram, Serkan Akkoyun, S.Okan Kara, Alper Sinan. New Parameters for Nuclear Charge Radius Formulas. Acta Phys.Polon.B 44, 8, 1791-1799, 2013. [CrossRef]
- Angeli, K.P. Marinova, Table of experimental nuclear ground state charge radii: An update, Atomic Data and Nuclear Data Tables, 99(1), 69-95, 2013.
- Seshavatharam U.V.S, Lakshminarayana S. Super symmetry in strong and weak interactions. International Journal of Modern Physics E. 19,2,263, 2010.
- Seshavatharam U.V.S, Lakshminarayana S. Integral charge SUSY in Strong nuclear gravity. Proceedings of the DAE Symp. on Nucl. Phys. 56, 842, 2011.
- Seshavatharam U. V. S and Lakshminarayana S. 4G Model of Fractional Charge Strong-Weak Super Symmetry. International Astronomy and Astrophysics Research Journal, 2(1), 31-55, 2020.
- Seshavatharam U. V. S and Lakshminarayana S. Understanding the Origins of Quark charges, Quantum of Magnetic Flux, Planck’s radiation constant and celestial magnetic moments with 4G model of nuclear charge. Preprints 2023, 2023102015.
- The KATRIN Collaboration. Direct neutrino-mass measurement with sub-electron volt sensitivity. Nat. Phys. 18, 160–166, 2022.
- Jyotsna Singh 1 and M. Ibrahim Mirza - Theoretical and Experimental Challenges in the Measurement of Neutrino Mass. Advances in High Energy Physics. 2023, Article ID 8897375, 2023.
- https://pubchem.ncbi.nlm.nih.gov/ptable/atomic-radius/.
- https://en.wikipedia.org/wiki/Atomic_radii_of_the_elements_(data_page).
- Owolabi, Taoreed, Akande Kabiru and Olatunji Sunday. Estimation of the atomic radii of periodic elements using support vector machine. International Journal of Advanced Information Science and Technology. 28, 39-49. 2014.
- Martin Rahm, Roald Hoffmann, N. W. Ashcroft. Atomic and Ionic Radii of Elements 1–96. Chemistry (Weinheim an der Bergstrasse, Germany), 22(41):14625-14632,2016.
- Krishnamohan G. Prasanna, Sooraj Sunil, Ajith Kumar, James. T. Joseph. Theoretical atomic radii of elements (H-Cm): A non-relativistic study with Gaussian basis set using HF, post-HF and DFT methods. [CrossRef]
- Yadav, P., Tandon, H., Malik, B. et al. A quest for the universal atomic radii. Struct Chem 33, 389–394.2022. [CrossRef]
- Seshavatharam U.V.S and Lakshminarayana S. A very brief review on strong and electroweak mass formula pertaining to 4G model of final unification. 67th DAE Symposium on Nuclear Physics, Dec’2023, Electroweak Interaction in Nuclei, F11, p. 1173-1174.
- ChtMavrodiev S, Deliyergiyev MA. Modification of the nuclear landscape in the inverse problem framework using the generalized Bethe-Weizsäcker mass formula. Int. J. Mod. Phys. E 27: 1850015, 2018. [CrossRef]
- Y. Azuma et al. Improved measurement results for the Avogadro constant using a 28Si-enriched crystal. Metrologia, 52, 360-375, 2015. [CrossRef]
- Farschad Torabi, Pouria Ahmadi, Chapter 2 - Fundamentals of batteries, Editor(s): Farschad Torabi, Pouria Ahmadi, Simulation of Battery Systems, Academic Press, Pages 55-81, 2020.
| Particle | Integral Quark charges | Internal Decay mode | Decay Result |
|---|---|---|---|
| Neutron | +e, +e, (-2e) | -2e → -e, -e | [+e, +e, (-e)] = Proton and Electron (-e) |
| Anti neutron | -e, -e, (+2e) | +2e → +e,+e | [-e,-e,(+e)] = Anti proton and Positron (+e) |
| Proton | (+2e), (-2e), +e +e, +e, -e |
+2e → +e,+e -2e → -e, -e |
[(+e), -2e, +e] = Neutron and Positron (+e) [+2e, (-e),+e] = (Baryon+2e) and (electron or muon-) Lepton+ and Gamma or Pion0 Lepton- and meson+2e |
| Anti proton | (-2e), (+2e), -e -e,-e, +e |
-2e → -e, -e +2e → +e,+e |
[(-e), +2e, -e] = Anti neutron and Electron (-e) [-2e, (+e),-e] = (Baryon-2e) and (positron or muon+) Lepton- and Gamma or Pion0 Lepton+ and meson-2e |
| Pion+ | (+2e),-e | +2e → +e, +e | Positron or Muon+ and [(+e),-e] = Gamma or Pion0 |
| Pion- | (-2e),+e | -2e → -e,-e | Electron or Muon- and [(-e),+e] = Gamma or Pion0 |
| Pion0 | (+2e),(-2e); -e,+e |
(+e,+e),(-e,-e) -e,+e |
Two Gamma Electron, Positron and Gamma Two electrons and Two positrons Electron and positron One Gamma |
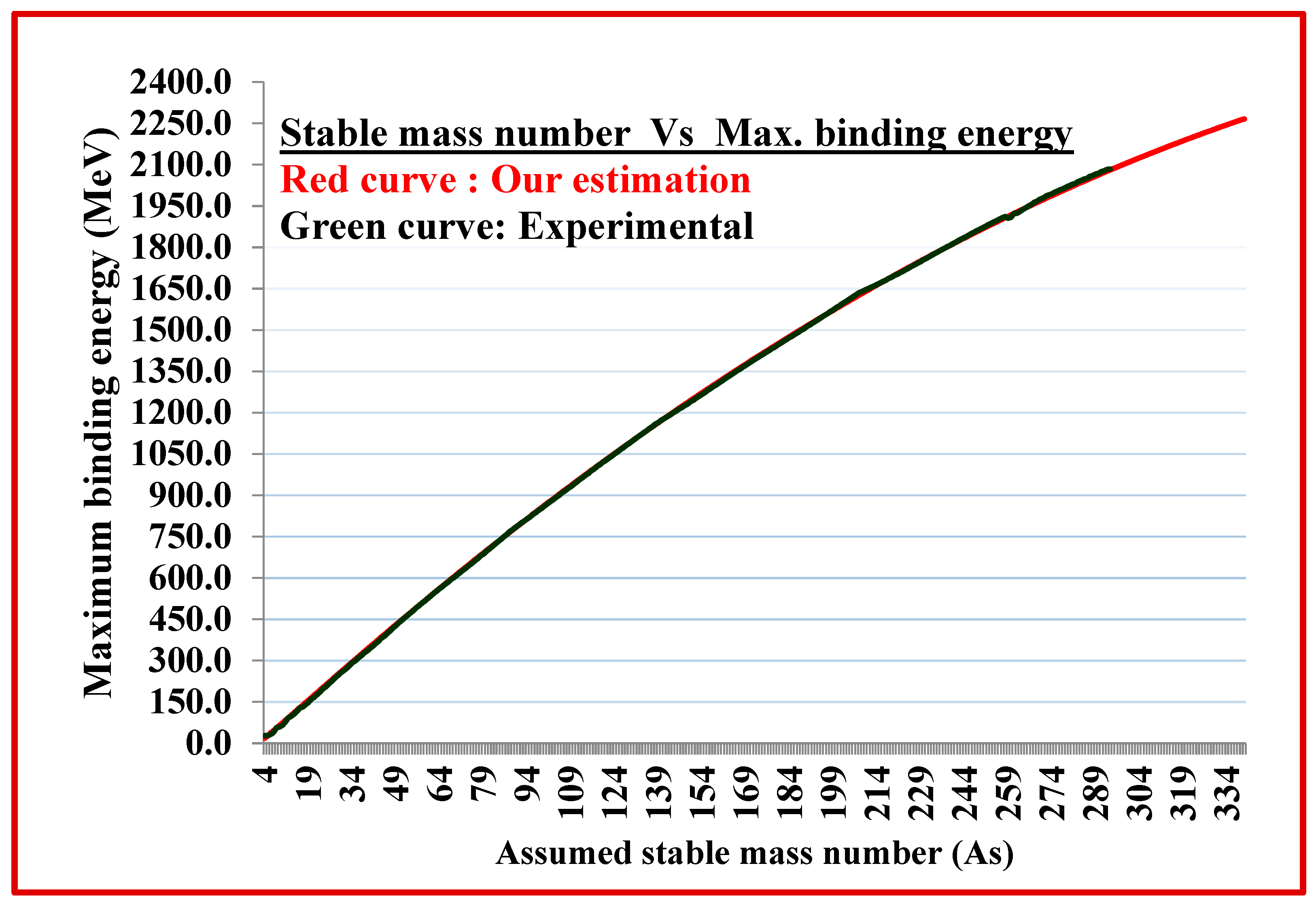
| Assumed stable mass number As | Estimated Max. Binding energy of As (MeV) | Experimental Max. Binding energy of As (MeV) |
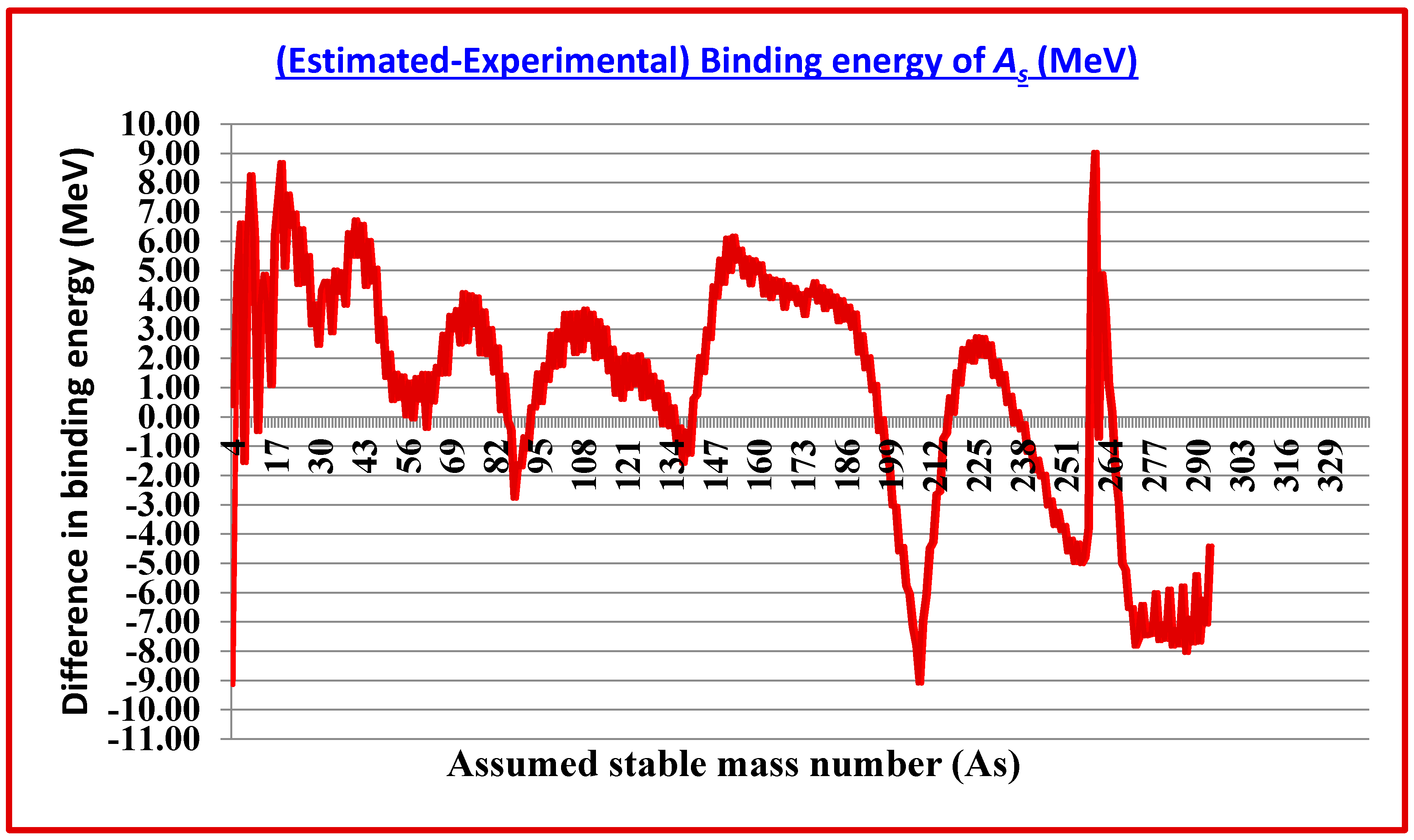
(Est.- Exp.) Binding energy (MeV) |
Estimated Binding energy per nucleon (MeV) | Experimental Binding energy per nucleon (MeV) |
|---|---|---|---|---|---|
| 4 | 19.2 | 28.30 | -9.13 | 4.79 | 7.07 |
| 5 | 27.94 | 27.56 | 0.38 | 5.59 | 5.51 |
| 6 | 36.86 | 31.99 | 4.86 | 6.14 | 5.33 |
| 7 | 45.87 | 39.25 | 6.62 | 6.55 | 5.61 |
| 8 | 54.95 | 56.50 | -1.55 | 6.87 | 7.06 |
| 9 | 64.08 | 58.16 | 5.91 | 7.12 | 6.46 |
| 10 | 73.25 | 64.98 | 8.27 | 7.32 | 6.50 |
| 11 | 82.45 | 76.20 | 6.24 | 7.50 | 6.93 |
| 12 | 91.67 | 92.16 | -0.49 | 7.64 | 7.68 |
| 13 | 100.91 | 97.11 | 3.80 | 7.76 | 7.47 |
| 14 | 110.16 | 105.28 | 4.87 | 7.87 | 7.52 |
| 15 | 119.42 | 115.49 | 3.92 | 7.96 | 7.70 |
| 16 | 128.68 | 127.62 | 1.06 | 8.04 | 7.98 |
| 17 | 137.95 | 131.76 | 6.19 | 8.11 | 7.75 |
| 18 | 147.22 | 139.81 | 7.41 | 8.18 | 7.77 |
| 19 | 156.49 | 147.80 | 8.69 | 8.24 | 7.78 |
| 20 | 165.76 | 160.64 | 5.11 | 8.29 | 8.03 |
| 21 | 175.02 | 167.41 | 7.61 | 8.33 | 7.97 |
| 22 | 184.28 | 177.77 | 6.51 | 8.38 | 8.08 |
| 23 | 193.53 | 186.56 | 6.97 | 8.41 | 8.11 |
| 24 | 202.78 | 198.26 | 4.52 | 8.45 | 8.26 |
| 25 | 212.02 | 205.59 | 6.43 | 8.48 | 8.22 |
| 26 | 221.24 | 216.68 | 4.56 | 8.51 | 8.33 |
| 27 | 230.47 | 224.95 | 5.51 | 8.54 | 8.33 |
| 28 | 239.68 | 236.54 | 3.14 | 8.56 | 8.45 |
| 29 | 248.88 | 245.01 | 3.87 | 8.58 | 8.45 |
| 30 | 258.07 | 255.62 | 2.45 | 8.60 | 8.52 |
| 31 | 267.25 | 262.92 | 4.33 | 8.62 | 8.48 |
| 32 | 276.41 | 271.78 | 4.63 | 8.64 | 8.49 |
| 33 | 285.57 | 280.96 | 4.61 | 8.65 | 8.51 |
| 34 | 294.71 | 291.84 | 2.87 | 8.67 | 8.58 |
| 35 | 303.84 | 298.82 | 5.02 | 8.68 | 8.54 |
| 36 | 312.96 | 308.71 | 4.25 | 8.69 | 8.58 |
| 37 | 322.07 | 317.10 | 4.97 | 8.70 | 8.57 |
| 38 | 331.16 | 327.34 | 3.81 | 8.71 | 8.61 |
| 39 | 340.24 | 333.94 | 6.29 | 8.72 | 8.56 |
| 40 | 349.30 | 343.81 | 5.49 | 8.73 | 8.60 |
| 41 | 358.35 | 351.62 | 6.73 | 8.74 | 8.58 |
| 42 | 367.38 | 361.90 | 5.49 | 8.75 | 8.62 |
| 43 | 376.40 | 369.83 | 6.58 | 8.75 | 8.60 |
| 44 | 385.41 | 380.96 | 4.45 | 8.76 | 8.66 |
| 45 | 394.40 | 388.37 | 6.03 | 8.76 | 8.63 |
| 46 | 403.38 | 398.77 | 4.61 | 8.77 | 8.67 |
| 47 | 412.34 | 407.26 | 5.08 | 8.77 | 8.67 |
| 48 | 421.29 | 418.70 | 2.58 | 8.78 | 8.72 |
| 49 | 430.22 | 426.85 | 3.37 | 8.78 | 8.71 |
| 50 | 439.13 | 437.78 | 1.35 | 8.78 | 8.76 |
| 51 | 448.03 | 445.85 | 2.19 | 8.78 | 8.74 |
| 52 | 456.92 | 456.35 | 0.57 | 8.79 | 8.78 |
| 53 | 465.78 | 464.29 | 1.50 | 8.79 | 8.76 |
| 54 | 474.64 | 474.01 | 0.63 | 8.79 | 8.78 |
| 55 | 483.47 | 482.08 | 1.40 | 8.79 | 8.77 |
| 56 | 492.29 | 492.26 | 0.03 | 8.79 | 8.79 |
| 57 | 501.10 | 499.91 | 1.19 | 8.79 | 8.77 |
| 58 | 509.89 | 509.95 | -0.06 | 8.79 | 8.79 |
| 59 | 518.66 | 517.31 | 1.34 | 8.79 | 8.77 |
| 60 | 527.41 | 526.85 | 0.57 | 8.79 | 8.78 |
| 61 | 536.15 | 534.67 | 1.49 | 8.79 | 8.77 |
| 62 | 544.87 | 545.26 | -0.39 | 8.79 | 8.79 |
| 63 | 553.58 | 552.10 | 1.48 | 8.79 | 8.76 |
| 64 | 562.27 | 561.76 | 0.51 | 8.79 | 8.78 |
| 65 | 570.94 | 569.21 | 1.73 | 8.78 | 8.76 |
| 66 | 579.60 | 578.14 | 1.46 | 8.78 | 8.76 |
| 67 | 588.24 | 585.41 | 2.83 | 8.78 | 8.74 |
| 68 | 596.86 | 595.39 | 1.47 | 8.78 | 8.76 |
| 69 | 605.46 | 602.00 | 3.47 | 8.77 | 8.72 |
| 70 | 614.05 | 611.09 | 2.97 | 8.77 | 8.73 |
| 71 | 622.62 | 618.95 | 3.67 | 8.77 | 8.72 |
| 72 | 631.18 | 628.69 | 2.49 | 8.77 | 8.73 |
| 73 | 639.71 | 635.47 | 4.25 | 8.76 | 8.71 |
| 74 | 648.23 | 645.66 | 2.57 | 8.76 | 8.73 |
| 75 | 656.74 | 652.57 | 4.17 | 8.76 | 8.70 |
| 76 | 665.22 | 662.07 | 3.15 | 8.75 | 8.71 |
| 77 | 673.69 | 669.59 | 4.10 | 8.75 | 8.70 |
| 78 | 682.14 | 679.99 | 2.15 | 8.75 | 8.72 |
| 79 | 690.58 | 686.95 | 3.62 | 8.74 | 8.70 |
| 80 | 698.99 | 696.87 | 2.13 | 8.74 | 8.71 |
| 81 | 707.39 | 704.37 | 3.02 | 8.73 | 8.70 |
| 82 | 715.77 | 714.27 | 1.50 | 8.73 | 8.71 |
| 83 | 724.14 | 721.74 | 2.39 | 8.72 | 8.70 |
| 84 | 732.48 | 732.27 | 0.22 | 8.72 | 8.72 |
| 85 | 740.81 | 739.38 | 1.43 | 8.72 | 8.70 |
| 86 | 749.12 | 749.23 | -0.11 | 8.71 | 8.71 |
| 87 | 757.42 | 757.86 | -0.44 | 8.71 | 8.71 |
| 88 | 765.70 | 768.47 | -2.77 | 8.70 | 8.73 |
| 89 | 773.95 | 775.54 | -1.59 | 8.70 | 8.71 |
| 90 | 782.20 | 783.90 | -1.70 | 8.69 | 8.71 |
| 91 | 790.42 | 791.09 | -0.67 | 8.69 | 8.69 |
| 92 | 798.63 | 799.73 | -1.10 | 8.68 | 8.69 |
| 93 | 806.81 | 806.46 | 0.35 | 8.68 | 8.67 |
| 94 | 814.98 | 814.68 | 0.30 | 8.67 | 8.67 |
| 95 | 823.14 | 821.63 | 1.51 | 8.66 | 8.65 |
| 96 | 831.27 | 830.78 | 0.49 | 8.66 | 8.65 |
| 97 | 839.39 | 837.60 | 1.79 | 8.65 | 8.64 |
| 98 | 847.49 | 846.25 | 1.24 | 8.65 | 8.64 |
| 99 | 855.57 | 852.75 | 2.82 | 8.64 | 8.61 |
| 100 | 863.63 | 861.93 | 1.70 | 8.64 | 8.62 |
| 101 | 871.68 | 868.73 | 2.95 | 8.63 | 8.60 |
| 102 | 879.71 | 877.95 | 1.76 | 8.62 | 8.61 |
| 103 | 887.72 | 884.19 | 3.53 | 8.62 | 8.58 |
| 104 | 895.71 | 893.09 | 2.62 | 8.61 | 8.59 |
| 105 | 903.69 | 900.13 | 3.55 | 8.61 | 8.57 |
| 106 | 911.64 | 909.48 | 2.16 | 8.60 | 8.58 |
| 107 | 919.58 | 916.02 | 3.57 | 8.59 | 8.56 |
| 108 | 927.50 | 925.24 | 2.26 | 8.59 | 8.57 |
| 109 | 935.41 | 931.72 | 3.68 | 8.58 | 8.55 |
| 110 | 943.29 | 940.64 | 2.65 | 8.58 | 8.55 |
| 111 | 951.16 | 947.62 | 3.54 | 8.57 | 8.54 |
| 112 | 959.01 | 957.01 | 2.00 | 8.56 | 8.54 |
| 113 | 966.84 | 963.55 | 3.29 | 8.56 | 8.53 |
| 114 | 974.65 | 972.59 | 2.06 | 8.55 | 8.53 |
| 115 | 982.44 | 979.40 | 3.04 | 8.54 | 8.52 |
| 116 | 990.22 | 988.68 | 1.54 | 8.54 | 8.52 |
| 117 | 997.98 | 995.62 | 2.35 | 8.53 | 8.51 |
| 118 | 1005.72 | 1004.95 | 0.77 | 8.52 | 8.52 |
| 119 | 1013.44 | 1011.43 | 2.01 | 8.52 | 8.50 |
| 120 | 1021.15 | 1020.54 | 0.61 | 8.51 | 8.50 |
| 121 | 1028.83 | 1026.71 | 2.12 | 8.50 | 8.49 |
| 122 | 1036.50 | 1035.52 | 0.98 | 8.50 | 8.49 |
| 123 | 1044.15 | 1042.10 | 2.05 | 8.49 | 8.47 |
| 124 | 1051.78 | 1050.69 | 1.10 | 8.48 | 8.47 |
| 125 | 1059.40 | 1057.27 | 2.12 | 8.48 | 8.46 |
| 126 | 1066.99 | 1066.37 | 0.62 | 8.47 | 8.46 |
| 127 | 1074.57 | 1072.66 | 1.91 | 8.46 | 8.45 |
| 128 | 1082.13 | 1081.44 | 0.69 | 8.45 | 8.45 |
| 129 | 1089.67 | 1088.24 | 1.42 | 8.45 | 8.44 |
| 130 | 1097.19 | 1096.91 | 0.29 | 8.44 | 8.44 |
| 131 | 1104.69 | 1103.51 | 1.19 | 8.43 | 8.42 |
| 132 | 1112.18 | 1112.45 | -0.27 | 8.43 | 8.43 |
| 133 | 1119.65 | 1118.88 | 0.77 | 8.42 | 8.41 |
| 134 | 1127.10 | 1127.43 | -0.34 | 8.41 | 8.41 |
| 135 | 1134.53 | 1134.18 | 0.35 | 8.40 | 8.40 |
| 136 | 1141.94 | 1142.77 | -0.83 | 8.40 | 8.40 |
| 137 | 1149.34 | 1149.68 | -0.34 | 8.39 | 8.39 |
| 138 | 1156.71 | 1158.29 | -1.58 | 8.38 | 8.39 |
| 139 | 1164.07 | 1164.55 | -0.47 | 8.37 | 8.38 |
| 140 | 1171.41 | 1172.69 | -1.27 | 8.37 | 8.38 |
| 141 | 1178.74 | 1178.12 | 0.62 | 8.36 | 8.36 |
| 142 | 1186.04 | 1185.28 | 0.75 | 8.35 | 8.35 |
| 143 | 1193.32 | 1191.26 | 2.06 | 8.34 | 8.33 |
| 144 | 1200.59 | 1199.08 | 1.51 | 8.34 | 8.33 |
| 145 | 1207.84 | 1204.83 | 3.01 | 8.33 | 8.31 |
| 146 | 1215.07 | 1212.40 | 2.67 | 8.32 | 8.30 |
| 147 | 1222.28 | 1217.80 | 4.48 | 8.31 | 8.28 |
| 148 | 1229.47 | 1225.39 | 4.09 | 8.31 | 8.28 |
| 149 | 1236.65 | 1231.26 | 5.39 | 8.30 | 8.26 |
| 150 | 1243.81 | 1239.24 | 4.56 | 8.29 | 8.26 |
| 151 | 1250.95 | 1244.84 | 6.11 | 8.28 | 8.24 |
| 152 | 1258.07 | 1253.10 | 4.97 | 8.28 | 8.24 |
| 153 | 1265.17 | 1258.99 | 6.18 | 8.27 | 8.23 |
| 154 | 1272.25 | 1266.93 | 5.32 | 8.26 | 8.23 |
| 155 | 1279.32 | 1273.58 | 5.73 | 8.25 | 8.22 |
| 156 | 1286.36 | 1281.59 | 4.77 | 8.25 | 8.22 |
| 157 | 1293.39 | 1287.95 | 5.44 | 8.24 | 8.20 |
| 158 | 1300.40 | 1295.89 | 4.51 | 8.23 | 8.20 |
| 159 | 1307.39 | 1302.02 | 5.37 | 8.22 | 8.19 |
| 160 | 1314.37 | 1309.45 | 4.92 | 8.21 | 8.18 |
| 161 | 1321.32 | 1316.09 | 5.23 | 8.21 | 8.17 |
| 162 | 1328.26 | 1324.10 | 4.16 | 8.20 | 8.17 |
| 163 | 1335.17 | 1330.37 | 4.80 | 8.19 | 8.16 |
| 164 | 1342.07 | 1338.03 | 4.05 | 8.18 | 8.16 |
| 165 | 1348.95 | 1344.25 | 4.71 | 8.18 | 8.15 |
| 166 | 1355.82 | 1351.56 | 4.25 | 8.17 | 8.14 |
| 167 | 1362.66 | 1358.00 | 4.66 | 8.16 | 8.13 |
| 168 | 1369.49 | 1365.77 | 3.71 | 8.15 | 8.13 |
| 169 | 1376.29 | 1371.78 | 4.52 | 8.14 | 8.12 |
| 170 | 1383.08 | 1379.03 | 4.05 | 8.14 | 8.11 |
| 171 | 1389.85 | 1385.42 | 4.43 | 8.13 | 8.10 |
| 172 | 1396.60 | 1392.76 | 3.85 | 8.12 | 8.10 |
| 173 | 1403.34 | 1399.13 | 4.21 | 8.11 | 8.09 |
| 174 | 1410.05 | 1406.59 | 3.46 | 8.10 | 8.08 |
| 175 | 1416.75 | 1412.41 | 4.34 | 8.10 | 8.07 |
| 176 | 1423.43 | 1419.28 | 4.15 | 8.09 | 8.06 |
| 177 | 1430.09 | 1425.46 | 4.62 | 8.08 | 8.05 |
| 178 | 1436.73 | 1432.80 | 3.92 | 8.07 | 8.05 |
| 179 | 1443.35 | 1438.90 | 4.45 | 8.06 | 8.04 |
| 180 | 1449.95 | 1446.29 | 3.66 | 8.06 | 8.03 |
| 181 | 1456.54 | 1452.24 | 4.30 | 8.05 | 8.02 |
| 182 | 1463.11 | 1459.33 | 3.77 | 8.04 | 8.02 |
| 183 | 1469.65 | 1465.52 | 4.13 | 8.03 | 8.01 |
| 184 | 1476.18 | 1472.94 | 3.25 | 8.02 | 8.01 |
| 185 | 1482.70 | 1478.69 | 4.01 | 8.01 | 7.99 |
| 186 | 1489.19 | 1485.88 | 3.31 | 8.01 | 7.99 |
| 187 | 1495.66 | 1491.88 | 3.78 | 8.00 | 7.98 |
| 188 | 1502.12 | 1499.09 | 3.03 | 7.99 | 7.97 |
| 189 | 1508.56 | 1505.01 | 3.55 | 7.98 | 7.96 |
| 190 | 1514.98 | 1512.80 | 2.17 | 7.97 | 7.96 |
| 191 | 1521.38 | 1518.56 | 2.82 | 7.97 | 7.95 |
| 192 | 1527.76 | 1526.12 | 1.64 | 7.96 | 7.95 |
| 193 | 1534.12 | 1532.06 | 2.06 | 7.95 | 7.94 |
| 194 | 1540.47 | 1539.58 | 0.89 | 7.94 | 7.94 |
| 195 | 1546.79 | 1545.68 | 1.11 | 7.93 | 7.93 |
| 196 | 1553.10 | 1553.60 | -0.50 | 7.92 | 7.93 |
| 197 | 1559.39 | 1559.45 | -0.06 | 7.92 | 7.92 |
| 198 | 1565.66 | 1567.00 | -1.34 | 7.91 | 7.91 |
| 199 | 1571.91 | 1573.48 | -1.57 | 7.90 | 7.91 |
| 200 | 1578.14 | 1581.18 | -3.03 | 7.89 | 7.91 |
| 201 | 1584.36 | 1587.41 | -3.05 | 7.88 | 7.90 |
| 202 | 1590.56 | 1595.16 | -4.61 | 7.87 | 7.90 |
| 203 | 1596.73 | 1601.16 | -4.42 | 7.87 | 7.89 |
| 204 | 1602.89 | 1608.65 | -5.76 | 7.86 | 7.89 |
| 205 | 1609.03 | 1615.07 | -6.04 | 7.85 | 7.88 |
| 206 | 1615.16 | 1622.32 | -7.17 | 7.84 | 7.88 |
| 207 | 1621.26 | 1629.06 | -7.80 | 7.83 | 7.87 |
| 208 | 1627.34 | 1636.43 | -9.09 | 7.82 | 7.87 |
| 209 | 1633.41 | 1640.37 | -6.96 | 7.82 | 7.85 |
| 210 | 1639.46 | 1645.55 | -6.09 | 7.81 | 7.84 |
| 211 | 1645.49 | 1649.97 | -4.48 | 7.80 | 7.82 |
| 212 | 1651.50 | 1655.77 | -4.27 | 7.79 | 7.81 |
| 213 | 1657.49 | 1660.13 | -2.64 | 7.78 | 7.79 |
| 214 | 1663.46 | 1666.01 | -2.55 | 7.77 | 7.79 |
| 215 | 1669.42 | 1670.16 | -0.74 | 7.76 | 7.77 |
| 216 | 1675.35 | 1675.90 | -0.55 | 7.76 | 7.76 |
| 217 | 1681.27 | 1680.58 | 0.69 | 7.75 | 7.74 |
| 218 | 1687.17 | 1687.05 | 0.12 | 7.74 | 7.74 |
| 219 | 1693.05 | 1691.51 | 1.55 | 7.73 | 7.72 |
| 220 | 1698.91 | 1697.79 | 1.12 | 7.72 | 7.72 |
| 221 | 1704.76 | 1702.42 | 2.34 | 7.71 | 7.70 |
| 222 | 1710.58 | 1708.66 | 1.92 | 7.71 | 7.70 |
| 223 | 1716.39 | 1713.82 | 2.56 | 7.70 | 7.69 |
| 224 | 1722.17 | 1720.30 | 1.87 | 7.69 | 7.68 |
| 225 | 1727.94 | 1725.21 | 2.74 | 7.68 | 7.67 |
| 226 | 1733.69 | 1731.60 | 2.09 | 7.67 | 7.66 |
| 227 | 1739.42 | 1736.71 | 2.72 | 7.66 | 7.65 |
| 228 | 1745.14 | 1743.08 | 2.06 | 7.65 | 7.65 |
| 229 | 1750.83 | 1748.33 | 2.50 | 7.65 | 7.63 |
| 230 | 1756.51 | 1755.13 | 1.38 | 7.64 | 7.63 |
| 231 | 1762.16 | 1760.25 | 1.92 | 7.63 | 7.62 |
| 232 | 1767.80 | 1766.69 | 1.12 | 7.62 | 7.62 |
| 233 | 1773.42 | 1771.93 | 1.49 | 7.61 | 7.60 |
| 234 | 1779.02 | 1778.57 | 0.46 | 7.60 | 7.60 |
| 235 | 1784.61 | 1783.86 | 0.74 | 7.59 | 7.59 |
| 236 | 1790.17 | 1790.41 | -0.24 | 7.59 | 7.59 |
| 237 | 1795.71 | 1795.53 | 0.18 | 7.58 | 7.58 |
| 238 | 1801.24 | 1801.69 | -0.45 | 7.57 | 7.57 |
| 239 | 1806.75 | 1806.97 | -0.23 | 7.56 | 7.56 |
| 240 | 1812.24 | 1813.45 | -1.21 | 7.55 | 7.56 |
| 241 | 1817.71 | 1818.69 | -0.98 | 7.54 | 7.55 |
| 242 | 1823.16 | 1825.00 | -1.84 | 7.53 | 7.54 |
| 243 | 1828.59 | 1830.03 | -1.44 | 7.53 | 7.53 |
| 244 | 1834.01 | 1836.05 | -2.04 | 7.52 | 7.52 |
| 245 | 1839.40 | 1841.36 | -1.96 | 7.51 | 7.52 |
| 246 | 1844.78 | 1847.82 | -3.04 | 7.50 | 7.51 |
| 247 | 1850.14 | 1852.98 | -2.83 | 7.49 | 7.50 |
| 248 | 1855.48 | 1859.19 | -3.71 | 7.48 | 7.50 |
| 249 | 1860.80 | 1864.02 | -3.22 | 7.47 | 7.49 |
| 250 | 1866.11 | 1869.99 | -3.88 | 7.46 | 7.48 |
| 251 | 1871.39 | 1875.09 | -3.70 | 7.46 | 7.47 |
| 252 | 1876.65 | 1881.27 | -4.61 | 7.45 | 7.47 |
| 253 | 1881.90 | 1886.07 | -4.17 | 7.44 | 7.45 |
| 254 | 1887.13 | 1892.10 | -4.97 | 7.43 | 7.45 |
| 255 | 1892.34 | 1896.64 | -4.30 | 7.42 | 7.44 |
| 256 | 1897.53 | 1902.54 | -5.01 | 7.41 | 7.43 |
| 257 | 1902.70 | 1907.50 | -4.80 | 7.40 | 7.42 |
| 258 | 1907.86 | 1911.69 | -3.84 | 7.39 | 7.41 |
| 259 | 1912.99 | 1906.33 | 6.66 | 7.39 | 7.36 |
| 260 | 1918.11 | 1909.07 | 9.04 | 7.38 | 7.34 |
| 261 | 1923.20 | 1923.93 | -0.72 | 7.37 | 7.37 |
| 262 | 1928.28 | 1923.39 | 4.89 | 7.36 | 7.34 |
| 263 | 1933.34 | 1929.63 | 3.71 | 7.35 | 7.34 |
| 264 | 1938.38 | 1937.23 | 1.15 | 7.34 | 7.34 |
| 265 | 1943.41 | 1943.25 | 0.16 | 7.33 | 7.33 |
| 266 | 1948.41 | 1950.31 | -1.90 | 7.32 | 7.33 |
| 267 | 1953.40 | 1956.31 | -2.91 | 7.32 | 7.33 |
| 268 | 1958.36 | 1963.37 | -5.01 | 7.31 | 7.33 |
| 269 | 1963.31 | 1968.54 | -5.23 | 7.30 | 7.32 |
| 270 | 1968.24 | 1974.78 | -6.54 | 7.29 | 7.31 |
| 271 | 1973.15 | 1979.66 | -6.50 | 7.28 | 7.31 |
| 272 | 1978.04 | 1985.87 | -7.83 | 7.27 | 7.30 |
| 273 | 1982.92 | 1990.44 | -7.53 | 7.26 | 7.29 |
| 274 | 1987.77 | 1994.17 | -6.40 | 7.25 | 7.28 |
| 275 | 1992.61 | 2000.08 | -7.47 | 7.25 | 7.27 |
| 276 | 1997.42 | 2004.86 | -7.44 | 7.24 | 7.26 |
| 277 | 2002.22 | 2009.64 | -7.41 | 7.23 | 7.26 |
| 278 | 2007.00 | 2013.00 | -6.00 | 7.22 | 7.24 |
| 279 | 2011.76 | 2019.40 | -7.64 | 7.21 | 7.24 |
| 280 | 2016.50 | 2023.56 | -7.06 | 7.20 | 7.23 |
| 281 | 2021.23 | 2028.82 | -7.59 | 7.19 | 7.22 |
| 282 | 2025.93 | 2031.81 | -5.88 | 7.18 | 7.21 |
| 283 | 2030.62 | 2038.45 | -7.83 | 7.18 | 7.20 |
| 284 | 2035.29 | 2042.53 | -7.24 | 7.17 | 7.19 |
| 285 | 2039.94 | 2047.73 | -7.79 | 7.16 | 7.19 |
| 286 | 2044.57 | 2050.33 | -5.77 | 7.15 | 7.17 |
| 287 | 2049.18 | 2057.22 | -8.04 | 7.14 | 7.17 |
| 288 | 2053.77 | 2060.64 | -6.87 | 7.13 | 7.16 |
| 289 | 2058.34 | 2066.06 | -7.72 | 7.12 | 7.15 |
| 290 | 2062.90 | 2068.28 | -5.38 | 7.11 | 7.13 |
| 291 | 2067.44 | 2075.12 | -7.69 | 7.10 | 7.13 |
| 292 | 2071.95 | 2078.16 | -6.21 | 7.10 | 7.12 |
| 293 | 2076.45 | 2083.52 | -7.07 | 7.09 | 7.11 |
| 294 | 2080.93 | 2085.34 | -4.41 | 7.08 | 7.09 |
| 295 | 2085.39 | 7.07 | |||
| 296 | 2089.84 | 7.06 | |||
| 297 | 2094.26 | 7.05 | |||
| 298 | 2098.67 | 7.04 | |||
| 299 | 2103.05 | 7.03 | |||
| 300 | 2107.42 | 7.02 | |||
| 301 | 2111.77 | 7.02 | |||
| 302 | 2116.10 | 7.01 | |||
| 303 | 2120.41 | 7.00 | |||
| 304 | 2124.71 | 6.99 | |||
| 305 | 2128.98 | 6.98 | |||
| 306 | 2133.24 | 6.97 | |||
| 307 | 2137.48 | 6.96 | |||
| 308 | 2141.69 | 6.95 | |||
| 309 | 2145.89 | 6.94 | |||
| 310 | 2150.07 | 6.94 | |||
| 311 | 2154.24 | 6.93 | |||
| 312 | 2158.38 | 6.92 | |||
| 313 | 2162.50 | 6.91 | |||
| 314 | 2166.61 | 6.90 | |||
| 315 | 2170.70 | 6.89 | |||
| 316 | 2174.77 | 6.88 | |||
| 317 | 2178.82 | 6.87 | |||
| 318 | 2182.85 | 6.86 | |||
| 319 | 2186.86 | 6.86 | |||
| 320 | 2190.85 | 6.85 | |||
| 321 | 2194.83 | 6.84 | |||
| 322 | 2198.78 | 6.83 | |||
| 323 | 2202.72 | 6.82 | |||
| 324 | 2206.64 | 6.81 | |||
| 325 | 2210.54 | 6.80 | |||
| 326 | 2214.42 | 6.79 | |||
| 327 | 2218.28 | 6.78 | |||
| 328 | 2222.13 | 6.77 | |||
| 329 | 2225.95 | 6.77 | |||
| 330 | 2229.76 | 6.76 | |||
| 331 | 2233.54 | 6.75 | |||
| 332 | 2237.31 | 6.74 | |||
| 333 | 2241.06 | 6.73 | |||
| 334 | 2244.79 | 6.72 | |||
| 335 | 2248.51 | 6.71 | |||
| 336 | 2252.20 | 6.70 | |||
| 337 | 2255.88 | 6.69 | |||
| 338 | 2259.53 | 6.69 | |||
| 339 | 2263.17 | 6.68 | |||
| 340 | 2266.79 | 6.67 |
| Atomic No | Symbol | Element | Atomic Mass | Valence | Estimated ECE (kg/C) |
Estimated ECE (gram/amp-hr) |
|---|---|---|---|---|---|---|
| 1 | H | Hydrogen | 1.00797 | 1 | 1.04469E-08 | 0.03761 |
| 3 | Li | Lithium | 6.941 | 1 | 7.19388E-08 | 0.25898 |
| 4 | Be | Beryllium | 9.01218 | 2 | 4.67026E-08 | 0.16813 |
| 5 | B | Boron | 10.81 | 1 | 1.12038E-07 | 0.40334 |
| 6 | C | Carbon | 12.011 | 2 | 6.22430E-08 | 0.22407 |
| 7 | N | Nitrogen | 14.0067 | 3 | 4.83900E-08 | 0.17420 |
| 8 | O | Oxygen | 15.9994 | 2 | 8.29116E-08 | 0.29848 |
| 9 | F | Fluorine | 18.998403 | 1 | 1.96906E-07 | 0.70886 |
| 11 | Na | Sodium | 22.98977 | 1 | 2.38274E-07 | 0.85779 |
| 12 | Mg | Magnesium | 24.305 | 2 | 1.25953E-07 | 0.45343 |
| 13 | Al | Aluminum | 26.98154 | 3 | 9.32152E-08 | 0.33557 |
| 14 | Si | Silicon | 28.0855 | 4 | 7.27719E-08 | 0.26198 |
| 15 | P | Phosphorus | 30.97376 | 5 | 6.42045E-08 | 0.23114 |
| 16 | S | Sulfur | 32.06 | 4 | 8.30701E-08 | 0.29905 |
| 17 | Cl | Chlorine | 35.453 | 1 | 3.67447E-07 | 1.32281 |
| 19 | K | Potassium | 39.0983 | 1 | 4.05228E-07 | 1.45882 |
| 20 | Ca | Calcium | 40.08 | 2 | 2.07701E-07 | 0.74772 |
| 21 | Sc | Scandium | 44.9559 | 3 | 1.55313E-07 | 0.55913 |
| 22 | Ti | Titanium | 47.9 | 4 | 1.24113E-07 | 0.44681 |
| 23 | V | Vanadium | 50.9415 | 5 | 1.05595E-07 | 0.38014 |
| 24 | Cr | Chromium | 51.996 | 6 | 8.98173E-08 | 0.32334 |
| 25 | Mn | Manganese | 54.938 | 7 | 8.13423E-08 | 0.29283 |
| 26 | Fe | Iron | 55.847 | 2 | 2.89408E-07 | 1.04187 |
| 27 | Co | Cobalt | 58.7 | 2 | 3.04193E-07 | 1.09510 |
| 28 | Ni | Nickel | 58.6934 | 2 | 3.04159E-07 | 1.09497 |
| 29 | Cu | Copper | 63.546 | 2 | 3.29306E-07 | 1.18550 |
| 30 | Zn | Zinc | 65.38 | 2 | 3.38810E-07 | 1.21972 |
| 31 | Ga | Gallium | 69.72 | 3 | 2.40867E-07 | 0.86712 |
| 32 | Ge | Germanium | 72.59 | 4 | 1.88087E-07 | 0.67711 |
| 33 | As | Arsenic | 74.9216 | 3 | 2.58837E-07 | 0.93181 |
| 34 | Se | Selenium | 78.96 | 4 | 2.04592E-07 | 0.73653 |
| 35 | Br | Bromine | 79.904 | 1 | 8.28152E-07 | 2.98135 |
| 37 | Rb | Rubidium | 85.4678 | 1 | 8.85817E-07 | 3.18894 |
| 38 | Sr | Strontium | 87.62 | 2 | 4.54061E-07 | 1.63462 |
| 39 | Y | Yttrium | 88.9059 | 3 | 3.07150E-07 | 1.10574 |
| 40 | Zr | Zirconium | 91.22 | 4 | 2.36359E-07 | 0.85089 |
| 41 | Nb | Niobium | 92.9064 | 5 | 1.92583E-07 | 0.69330 |
| 42 | Mo | Molybdenum | 95.94 | 4 | 2.48589E-07 | 0.89492 |
| 43 | Tc | Technetium | 98 | 7 | 1.45101E-07 | 0.52236 |
| 44 | Ru | Ruthenium | 101.07 | 3 | 3.49174E-07 | 1.25703 |
| 45 | Rh | Rhodium | 102.9055 | 3 | 3.55516E-07 | 1.27986 |
| 46 | Pd | Palladium | 106.4 | 2 | 5.51383E-07 | 1.98498 |
| 47 | Ag | Silver | 107.868 | 1 | 1.11798E-06 | 4.02473 |
| 48 | Cd | Cadmium | 112.41 | 2 | 5.82527E-07 | 2.09710 |
| 49 | In | Indium | 114.82 | 3 | 3.96678E-07 | 1.42804 |
| 50 | Sn | Tin | 118.69 | 4 | 3.07536E-07 | 1.10713 |
| 51 | Sb | Antimony | 121.75 | 3 | 4.20619E-07 | 1.51423 |
| 52 | Te | Tellurium | 126.9045 | 4 | 3.28820E-07 | 1.18375 |
| 53 | I | Iodine | 127.6 | 1 | 1.32249E-06 | 4.76096 |
| 55 | Cs | Cesium | 132.9054 | 1 | 1.37748E-06 | 4.95891 |
| 56 | Ba | Barium | 137.33 | 2 | 7.11667E-07 | 2.56200 |
| 57 | La | Lanthanum | 138.9055 | 3 | 4.79888E-07 | 1.72760 |
| 58 | Ce | Cerium | 140.12 | 3 | 4.84083E-07 | 1.74270 |
| 59 | Pr | Praseodymium | 140.9077 | 3 | 4.86805E-07 | 1.75250 |
| 60 | Nd | Neodymium | 144.24 | 3 | 4.98317E-07 | 1.79394 |
| 61 | Pm | Promethium | 145 | 3 | 5.00943E-07 | 1.80339 |
| 62 | Sm | Samarium | 150.4 | 3 | 5.19599E-07 | 1.87056 |
| 63 | Eu | Europium | 151.96 | 3 | 5.24988E-07 | 1.88996 |
| 64 | Gd | Gadolinium | 157.25 | 3 | 5.43264E-07 | 1.95575 |
| 65 | Tb | Terbium | 158.9254 | 3 | 5.49052E-07 | 1.97659 |
| 66 | Dy | Dysprosium | 162.5 | 3 | 5.61401E-07 | 2.02105 |
| 67 | Ho | Holmium | 164.9304 | 3 | 5.69798E-07 | 2.05127 |
| 68 | Er | Erbium | 167.26 | 3 | 5.77846E-07 | 2.08025 |
| 69 | Tm | Thulium | 168.9342 | 3 | 5.83630E-07 | 2.10107 |
| 70 | Yb | Ytterbium | 173.04 | 3 | 5.97815E-07 | 2.15213 |
| 71 | Lu | Lutetium | 174.967 | 3 | 6.04472E-07 | 2.17610 |
| 72 | Hf | Hafnium | 178.49 | 4 | 4.62482E-07 | 1.66494 |
| 73 | Ta | Tantalum | 180.9479 | 5 | 3.75081E-07 | 1.35029 |
| 74 | W | Tungsten | 183.85 | 6 | 3.17580E-07 | 1.14329 |
| 75 | Re | Rhenium | 186.207 | 7 | 2.75702E-07 | 0.99253 |
| 76 | Os | Osmium | 190.2 | 4 | 4.92824E-07 | 1.77417 |
| 77 | Ir | Iridium | 192.22 | 4 | 4.98058E-07 | 1.79301 |
| 78 | Pt | Platinum | 195.09 | 4 | 5.05494E-07 | 1.81978 |
| 79 | Au | Gold | 196.9665 | 3 | 6.80476E-07 | 2.44971 |
| 80 | Hg | Mercury | 200.59 | 2 | 1.03949E-06 | 3.74217 |
| 81 | Tl | Thallium | 204.37 | 1 | 2.11816E-06 | 7.62537 |
| 82 | Pb | Lead | 207.2 | 2 | 1.07375E-06 | 3.86548 |
| 83 | Bi | Bismuth | 208.9804 | 3 | 7.21981E-07 | 2.59913 |
| 84 | Po | Polonium | 208.9824 | 2 | 1.08298E-06 | 3.89873 |
| 85 | At | Astatine | 209.9871 | 1 | 2.17638E-06 | 7.83496 |
| 87 | Fr | Francium | 223 | 1 | 2.31125E-06 | 8.32049 |
| 88 | Ra | Radium | 226.0254 | 2 | 1.17130E-06 | 4.21668 |
| 89 | Ac | Actinium | 227.0278 | 3 | 7.84331E-07 | 2.82359 |
| 90 | Th | Thorium | 232.0381 | 4 | 6.01230E-07 | 2.16443 |
| 91 | Pa | Protactinium | 231.0359 | 5 | 4.78907E-07 | 1.72406 |
| 92 | U | Uranium | 238.0289 | 6 | 4.11169E-07 | 1.48021 |
| 93 | Np | Neptunium | 237.0482 | 5 | 4.91369E-07 | 1.76893 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





