Submitted:
03 February 2024
Posted:
05 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. History
3. Causes of E. Faecalis bacteremia
3.1. Induced enterococcal colonisation involves cell surface mechanisms. Ultra-large von Willebrand factor and sortase are key players in this process.
3.2. The role of endocardium and enterococcal pathoadaptation
4. Point and Counterpoint
| Gene/locus | Protein/function | Reference |
|---|---|---|
| srtC | Sortase C/an enzyme that anchors surface proteins to the cell wall | Nallapareddy et al. (2006); Ref [46] |
| atn | Autolysin | Mohamed et al. (2004); Ref [191] |
| salB | Secretory antigen-like B/cell-shape determinant | Mohamed et al. (2006); Ref [192] |
| bee | Biofilm enhancer in Enterococcus/a putative cell wall-anchored protein | Tendolkar et al. (2006); Ref [193] |
| salA | Secretory antigen-like A | Mohamed et al. (2006); Ref [192] |
| bop | Biofilm on plastic surface/a putative sugar-binding transcriptional regulator | Hufnagel et al. (2004); Ref [194] |
| gelE | Secretory metalloprotease gelatinase E | Mohamed et al. (2004); Kristich et al. (2004); Hancock & Perego (2004); Ref [191,195,196] |
| dltA | D-alanine lipoteichoic acid/D-alanine-D-alanyl carrier protein ligase | Fabretti et al. (2006); Ref [197] |
| ebpA, ebpB, ebpC | Endocarditis and biofilm-associated pili | Nallapareddy et al. (2006); Ref [46] |
| ebpR | Transcriptional regulator of ebpABC | Bourgogne et al. (2007); Ref [198] |
| epa (orfde4) | Enterococcal polysaccharide antigen/a putative glycosyltransferase involved in polysaccharide synthesis |
Mohamed et al. (2004) ; Ref [191] |
| esp | Enterococcal surface protein | Toledo-Arana et al. (2001); Tendolkar et al. (2004, 2006); Ref [193,199,200] |
| etaR | Enterococcal two-component system regulator | Mohamed et al. (2004); Ref [191] |
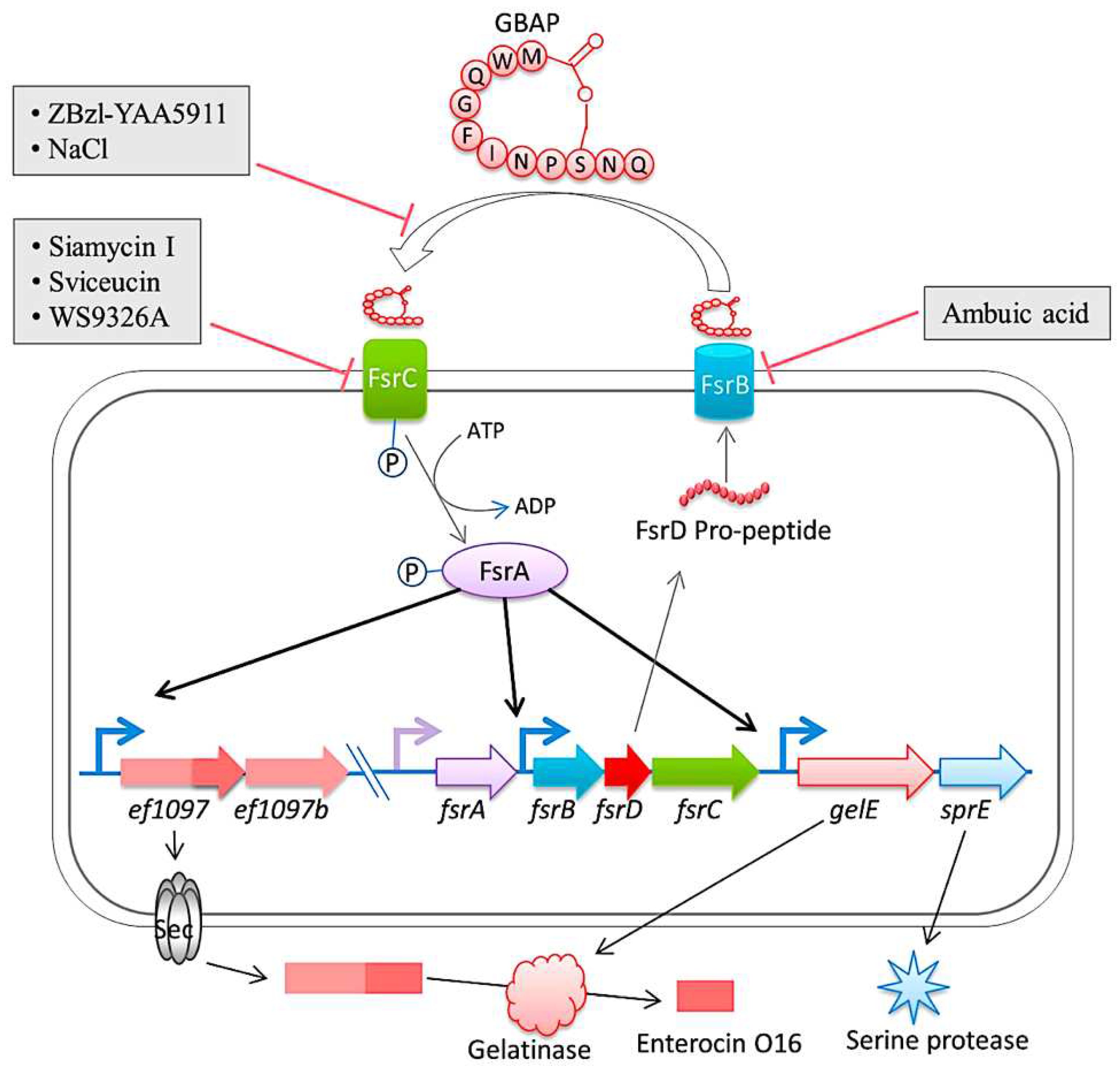
| fsrA, fsrB, fsrC | E. faecalis regulator/two-component quorum-sensing signal transduction system, regulates the expression of gelatinase and serine protease | Mohamed et al. (2004, 2006) ; Pillai et al. (2004); Hancock & Perego (2004) |
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaca, A.O.; Lemos, J.A. Adaptation to Adversity: the Intermingling of Stress Tolerance and Pathogenesis in Enterococci. Microbiol Mol Biol Rev. 2019, 83, e00008–19. [Google Scholar] [CrossRef]
- Fiore, E.; Van Tyne, D.; Gilmore, M.S. Pathogenicity of Enterococci. Microbiol Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Goh, H.M.S.; Yong, M.H.A.; Chong, K.K.L.; Kline, K.A. Model systems for the study of Enterococcal colonization and infection. Virulence 2017, 8, 1525–1562. [Google Scholar] [CrossRef]
- Ramsey, M.; Hartke, A.; Huycke, M. The Physiology and Metabolism of Enterococci. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; Massachusetts Eye and Ear Infirmary: Boston, 2014. [Google Scholar]
- Lebreton, F.; Willems, R.J.L.; Gilmore, M.S. Enterococcus Diversity, Origins in Nature, and Gut Colonization. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; Massachusetts Eye and Ear Infirmary: Boston, 2014. [Google Scholar]
- Boehm, A.B.; Sassoubre, L.M. Enterococci as Indicators of Environmental Fecal Contamination. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; Massachusetts Eye and Ear Infirmary: Boston, 2014. [Google Scholar]
- Nappi, F.; Avtaar Singh, S.S.; Jitendra, V.; Fiore, A. Bridging Molecular and Clinical Sciences to Achieve the Best Treatment of Enterococcus faecalis Endocarditis. Microorganisms 2023, 11, 2604. [Google Scholar] [CrossRef] [PubMed]
- Ch'ng, J.H.; Chong, K.K.L.; Lam, L.N.; Wong, J.J.; Kline, K.A. Biofilm-associated infection by enterococci. Nat Rev Microbiol. 2019, 17, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; Silva, V.; Dapkevicius, M.L.E.; Igrejas, G.; Poeta, P. Enterococci, from Harmless Bacteria to a Pathogen. Microorganisms 2020, 8, 1118. [Google Scholar] [CrossRef] [PubMed]
- Holland, T.L.; Baddour, L.M.; Bayer, A.S.; Hoen, B.; Miro, J.M.; Fowler, V.G., Jr. Infective endocarditis. Nat Rev Dis Primers. 2016, 2, 16059. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Martuscelli, G.; Bellomo, F.; Avtaar Singh, S.S.; Moon, M.R. Infective Endocarditis in High-Income Countries. Metabolites 2022, 12, 682. [Google Scholar] [CrossRef]
- Barnes, A.M.T.; Frank, K.L.; Dale, J.L.; Manias, D.A.; Powers, J.L.; Dunny, G.M. Enterococcus faecalis colonizes and forms persistent biofilm microcolonies on undamaged endothelial surfaces in a rabbit endovascular infection model. FEMS Microbes. 2021, 2, xtab014. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.M.T.; Dale, J.L.; Chen, Y.; Manias, D.A.; Greenwood Quaintance, K.E.; Karau, M.K.; Kashyap, P.C.; Patel, R.; Wells, C.L.; Dunny, G.M. Enterococcus faecalis readily colonizes the entire gastrointestinal tract and forms biofilms in a germ-free mouse model. Virulence 2017, 8, 282–296. [Google Scholar] [CrossRef]
- Mazzantini, D.; Calvigioni, M.; Celandroni, F.; Lupetti, A.; Ghelardi, E. Spotlight on the Compositional Quality of Probiotic Formulations Marketed Worldwide. Front Microbiol. 2021, 12, 693973. [Google Scholar] [CrossRef]
- Barnes, A.M.T.; Frank, K.L.; Dunny, G.M. Enterococcal Endocarditis: Hiding in Plain Sight. Front Cell Infect Microbiol. 2021, 11, 722482. [Google Scholar] [CrossRef]
- Madsen, K.T.; Skov, M.N.; Gill, S.; Kemp, M. Virulence Factors Associated with Enterococcus Faecalis Infective Endocarditis: A Mini Review. Open Microbiol J. 2017, 11, 1–11. [Google Scholar] [CrossRef]
- Kafil, H.S.; Mobarez, A.M. Spread of Enterococcal Surface Protein in Antibiotic Resistant Entero-coccus faecium and Enterococcus faecalis isolates from Urinary Tract Infections. Open Microbiol J. 2015, 9, 14–17. [Google Scholar] [CrossRef]
- Frank, K.L.; Guiton, P.S.; Barnes, A.M.; Manias, D.A.; Chuang-Smith, O.N.; Kohler, P.L.; Spaulding, A.R.; Hultgren, S.J.; Schlievert, P.M.; Dunny, G.M. AhrC and Eep are biofilm infection-associated virulence factors in Enterococcus faecalis. Infect Immun. 2013, 81, 1696–1708. [Google Scholar] [CrossRef]
- Sillanpää, J.; Chang, C.; Singh, K.V.; Montealegre, M.C.; Nallapareddy, S.R.; Harvey, B.R.; Ton-That, H.; Murray, B.E. Contribution of individual Ebp Pilus subunits of Enterococcus faecalis OG1RF to pilus biogenesis, biofilm formation and urinary tract infection. PLoS One 2013, 8, e68813. [Google Scholar] [CrossRef]
- Thurlow, L.R.; Thomas, V.C.; Narayanan, S.; Olson, S.; Fleming, S.D.; Hancock, L.E. Gelatinase contributes to the pathogenesis of endocarditis caused by Enterococcus faecalis. Infect Immun. 2010, 78, 4936–4943. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.V.; Nallapareddy, S.R.; Murray, B.E. Importance of the ebp (endocarditis- and biofilm-associated pilus) locus in the pathogenesis of Enterococcus faecalis ascending urinary tract infection. J Infect Dis. 2007, 195, 1671–1677. [Google Scholar] [CrossRef]
- Rouchon, C.N.; Harris, J.; Zubair-Nizami, Z.; Weinstein, A.J.; Roky, M.; Frank, K.L. The Cationic Antimicrobial Peptide Activity of Lysozyme Reduces Viable Enterococcus faecalis Cells in Biofilms. Antimicrob Agents Chemother. 2022, 66, e0233921. [Google Scholar] [CrossRef]
- Qu, Q.; Chen, T.; He, P.; Geng, H.; Zeng, P.; Luan, G. Isolation and characterization of a novel lytic bacteriophage vB_Efm_LG62 infecting Enterococcus faecium. Virus Genes 2023, 59, 763–774. [Google Scholar] [CrossRef]
- Holmberg, A.; Rasmussen, M. Mature biofilms of Enterococcus faecalis and Enterococcus faecium are highly resistant to antibiotics. Diagn Microbiol Infect Dis. 2016, 84, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Schoell, T.; Spadaccio, C.; Acar, C.; da Costa, F.D.A. A Literature Review on the Use of Aortic Allografts in Modern Cardiac Surgery for the Treatment of Infective Endocarditis: Is There Clear Evidence or Is It Merely a Perception? Life 2023, 13, 1980. [Google Scholar] [CrossRef]
- Andrewes, F.W.; Horder, T.J. A Study of the Streptococci Pathogenic for Man. Lancet 1906, 2, 708–713. [Google Scholar] [CrossRef]
- Geraci, J.E.; Martin, W.J. Antibiotic therapy of bacterial endocarditis. VI. Subacute enterococcal endocarditis; clinical, pathologic and therapeutic consideration of 33 cases. Circulation. 1954, 10, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Toh, C.C.; Ball, K. Natural History of Streptococcus faecalis Endocarditis. Br Med J 1960, 2, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.E. The Experimental Production of Streptococcal Endocarditis in the Pig. J Pathol 1969, 99, 307–318. [Google Scholar] [CrossRef]
- Durack, D.T.; Beeson, P.B.; Petersdorf, R.G. Experimental bacterial endocarditis. 3. Production and progress of the disease in rabbits. Br J Exp Pathol. 1973, 54, 142–151. [Google Scholar]
- Schleifer, K.H.; Kilpper-Bälz, R.; Kraus, J.; Gehring, F. Relatedness and classification of Streptococcus mutans and "mutans-like" streptococci. J Dent Res. 1984, 63, 1047–1050. [Google Scholar] [CrossRef]
- Clewell, D.B. Movable genetic elements and antibiotic resistance in enterococci. Eur J Clin Microbiol Infect Dis. 1990, 9, 90–102. [Google Scholar] [CrossRef]
- Murray, B.E. The life and times of the Enterococcus. Clin Microbiol Rev. 1990, 3, 46–65. [Google Scholar] [CrossRef]
- Donati, L.; Scamazzo, F.; Gervasoni, M.; Magliano, A.; Stankov, B.; Fraschini, F. Infection and antibiotic therapy in 4000 burned patients treated in Milan, Italy, between 1976 and 1988. Burns 1993, 19, 345–348. [Google Scholar] [CrossRef]
- Peng, M.Y.; Young, T.G.; Yang, C.H.; Chou, M.Y. Enterococcal bacteremia in a medical center. Zhonghua Yi Xue Za Zhi 1994, 54, 306–311. [Google Scholar] [PubMed]
- Nicoletti, G.; Stefani, S. Enterococci: susceptibility patterns and therapeutic options. Eur J Clin Microbiol Infect Dis. 1995, 14 (Suppl 1), S33–S37. [Google Scholar] [PubMed]
- de Vera, M.E.; Simmons, R.L. Antibiotic-resistant enterococci and the changing face of surgical infections. Arch Surg. 1996, 131, 338–342. [Google Scholar] [CrossRef]
- Gin, A.S.; Zhanel, G.G. Vancomycin-resistant enterococci. Ann Pharmacother. 1996, 30, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Evers, S.; Quintiliani RJr Courvalin, P. Genetics of glycopeptide resistance in enterococci. Microb Drug Resist. 1996, 2, 219–223. [Google Scholar] [CrossRef]
- Biavasco, F.; Miele, A.; Vignaroli, C.; Manso, E.; Lupidi, R.; Varaldo, P.E. Genotypic characterization of a nosocomial outbreak of VanA Enterococcus faecalis. Microb Drug Resist. 1996, 2, 231–237. [Google Scholar] [CrossRef]
- Shorrock, P.J.; Lambert, P.A.; Aitchison, E.J.; Smith, E.G.; Farrell, I.D.; Gutschik, E. Serological response in Enterococcus faecalis endocarditis determined by enzyme-linked immunosorbent assay. J Clin Microbiol. 1990, 28, 195–200. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, L.; Murray, B.E.; Weinstock, G.M. Enterococcus faecalis antigens in human infections. Infect Immun. 1997, 65, 4207–4215. [Google Scholar] [CrossRef]
- Rich, R.L.; Kreikemeyer, B.; Owens, R.T.; LaBrenz, S.; Narayana, S.V.; Weinstock, G.M.; Murray, B.E.; Höök, M. Ace is a collagen-binding MSCRAMM from Enterococcus faecalis. J Biol Chem. 1999, 274, 26939–26945. [Google Scholar] [CrossRef]
- Teng, F.; Jacques-Palaz, K.D.; Weinstock, G.M.; Murray, B.E. Evidence that the enterococcal polysaccharide antigen gene (epa) cluster is widespread in Enterococcus faecalis and influences resistance to phagocytic killing of E. faecalis. Infect Immun. 2002, 70, 2010–2015. [Google Scholar] [CrossRef]
- Ton-That, H.; Schneewind, O. Assembly of pili in Gram-positive bacteria. Trends Microbiol. 2004, 12, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Nallapareddy, S.R.; Singh, K.V.; Sillanpää, J.; Garsin, D.A.; Höök, M.; Erlandsen, S.L.; Murray, B.E. Endocarditis and biofilm-associated pili of Enterococcus faecalis. J Clin Invest. 2006, 116, 2799–2807. [Google Scholar] [CrossRef] [PubMed]
- Budzik, J.M.; Schneewind, O. Pili prove pertinent to enterococcal endocarditis. J Clin Invest. 2006, 116, 2582–2584. [Google Scholar] [CrossRef] [PubMed]
- Kemp, K.D.; Singh, K.V.; Nallapareddy, S.R.; Murray, B.E. Relative contributions of Enterococcus faecalis OG1RF sortase-encoding genes, srtA and bps (srtC), to biofilm formation and a murine model of urinary tract infection. Infect Immun. 2007, 75, 5399–5404. [Google Scholar] [CrossRef]
- Scott, J.R.; Zähner, D. Pili with strong attachments: Gram-positive bacteria do it differently. Mol Microbiol. 2006, 62, 320–330. [Google Scholar] [CrossRef]
- Galli, D.; Wirth, R.; Wanner, G. Identification of aggregation substances of Enterococcus faecalis cells after induction by sex pheromones. An immunological and ultrastructural investigation. Arch Microbiol. 1989, 151, 486–490. [Google Scholar] [CrossRef]
- Olmsted, S.B.; Kao, S.M.; van Putte, L.J.; Gallo, J.C.; Dunny, G.M. Role of the pheromone-inducible surface protein Asc10 in mating aggregate formation and conjugal transfer of the Enterococcus faecalis plasmid pCF10. J Bacteriol. 1991, 173, 7665–7672. [Google Scholar] [CrossRef]
- Hirt, H.; Wanner, G.; Galli, D.; Wirth, R. Biochemical, immunological and ultrastructural characterization of aggregation substances encoded by Enterococcus faecalis sex-pheromone plasmids. Eur J Biochem. 1993, 211, 711–716. [Google Scholar] [CrossRef]
- Dunny, G.M.; Leonard, B.A.; Hedberg, P.J. Pheromone-inducible conjugation in Enterococcus faecalis: interbacterial and host-parasite chemical communication. J Bacteriol. 1995, 177, 871–876. [Google Scholar] [CrossRef]
- Leonard, B.A.; Bensing, B.A.; Hedberg, P.J.; Ruhfel, R.E.; Chung, J.W.; Dunny, G.M. Pheromone-inducible gene regulation and signalling for the control of aggregation substance expression in the conjugative plasmid pCF10. Dev Biol Stand. 1995, 85, 27–34. [Google Scholar] [PubMed]
- Nakayama, J.; Clewell, D.B.; Suzuki, A. Targeted disruption of the PD78 gene (traF) reduces pheromone-inducible conjugal transfer of the bacteriocin plasmid pPD1 in Enterococcus faecalis. FEMS Microbiol Lett. 1995, 128, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Bae, T.; Kozlowicz, B.; Dunny, G.M. Two targets in pCF10 DNA for PrgX binding: their role in production of Qa and prgX mRNA and in regulation of pheromone-inducible conjugation. J Mol Biol. 2002, 315, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Hidalgo, N.; Escolà-Vergé, L.; Pericàs, J.M. Enterococcus faecalis endocarditis: what's next? Future Microbiol. 2020, 15, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Pericàs, J.M.; Llopis, J.; Muñoz, P.; Gálvez-Acebal, J.; Kestler, M.; Valerio, M.; Hernández-Meneses, M.; Goenaga, M.Á.; Cobo-Belaustegui, M.; Montejo, M.; Ojeda-Burgos, G.; Sousa-Regueiro, M.D.; de Alarcón, A.; Ramos-Martínez, A.; Miró, J.M.; GAMES Investigators. A Contemporary Picture of Enterococcal Endocarditis. J Am Coll Cardiol. 2020, 75, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Escolà-Vergé, L.; Fernández-Hidalgo, N.; Larrosa, M.N.; Fernandez-Galera, R.; Almirante, B. Secular trends in the epidemiology and clinical characteristics of Enterococcus faecalis infective endocarditis at a referral center (2007-2018). Eur J Clin Microbiol Infect Dis. 2021, 40, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Bashore, T.M.; Turner, N.A. Addressing the Menace of Enterococcal Endocarditis. J Am Coll Cardiol. 2020, 75, 495–497. [Google Scholar] [CrossRef]
- Ramos-Martínez, A.; Domínguez, F.; Muñoz, P.; Marín, M.; Pedraz, Á.; Fariñas, M.C.; Tascón, V.; de Alarcón, A.; Rodríguez-García, R.; Miró, J.M.; Goikoetxea, J.; Ojeda-Burgos, G.; Escrihuela-Vidal, F.; Calderón-Parra, J.; GAMES investigators. Clinical presentation, microbiology, and prognostic factors of prosthetic valve endocarditis. Lessons learned from a large prospective registry. PLoS One. 2023, 18, e0290998. [Google Scholar] [CrossRef]
- Herrera-Hidalgo, L.; Fernández-Rubio, B.; Luque-Márquez, R.; López-Cortés, L.E.; Gil-Navarro, M.V.; de Alarcón, A. Treatment of Enterococcus faecalis Infective Endocarditis: A Continuing Challenge. Antibiotics 2023, 12, 704. [Google Scholar] [CrossRef]
- Parsek, M.R.; Fuqua, C. Biofilms 2003: emerging themes and challenges in studies of surface-associated microbial life. J Bacteriol. 2004, 186, 4427–4440. [Google Scholar] [CrossRef]
- Häussler, S.; Parsek, M.R. Biofilms 2009: new perspectives at the heart of surface-associated microbial communities. J Bacteriol. 2010, 192, 2941–2949. [Google Scholar] [CrossRef]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS Suppl. 2013, 136, 1–51. [Google Scholar] [CrossRef]
- Haussler, S.; Fuqua, C. Biofilms 2012: new discoveries and significant wrinkles in a dynamic field. J Bacteriol. 2013, 195, 2947–2958. [Google Scholar] [CrossRef]
- Visick, K.L.; Schembri, M.A.; Yildiz, F.; Ghigo, J.M. Biofilms 2015: Multidisciplinary Approaches Shed Light into Microbial Life on Surfaces. J Bacteriol. 2016, 198, 2553–2563. [Google Scholar] [CrossRef]
- Høiby, N. A short history of microbial biofilms and biofilm infections. APMIS. 2017, 125, 272–275. [Google Scholar] [CrossRef]
- Fuqua, C.; Filloux, A.; Ghigo, J.M.; Visick, K.L. Biofilms 2018: A diversity of microbes and mechanisms. J Bacteriol. 2019, 201, e00118–19. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, R.; Chen, Z.; Cao, P.; Zhou, Q.; Wu, Q. A global bibliometric and visualized analysis of bacterial biofilm eradication from 2012 to 2022. Front Microbiol. 2023, 14, 1287964. [Google Scholar] [CrossRef]
- Săndulescu, O.; Săndulescu, M. Oral biofilms - pivotal role in understanding microbes and their relevance to the human host. Germs. 2023, 13, 7–9. [Google Scholar] [CrossRef]
- Hegstad, K.; Mikalsen, T.; Coque, T.M.; Werner, G.; Sundsfjord, A. Mobile genetic elements and their contribution to the emergence of antimicrobial resistant Enterococcus faecalis and Enterococcus faecium. Clin Microbiol Infect. 2010, 16, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, I.T.; Banerjei, L.; Myers, G.S.; Nelson, K.E.; Seshadri, R.; Read, T.D.; Fouts, D.E.; Eisen, J.A.; Gill, S.R.; Heidelberg, J.F.; Tettelin, H.; Dodson, R.J.; Umayam, L.; Brinkac, L.; Beanan, M.; Daugherty, S.; DeBoy, R.T.; Durkin, S.; Kolonay, J.; Madupu, R.; Nelson, W.; Vamathevan, J.; Tran, B.; Upton, J.; Hansen, T.; Shetty, J.; Khouri, H.; Utterback, T.; Radune, D.; Ketchum, K.A.; Dougherty, B.A.; Fraser, C.M. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science. 2003, 299, 2071–2074. [Google Scholar] [CrossRef] [PubMed]
- Weigel, L.M.; Clewell, D.B.; Gill, S.R.; Clark, N.C.; McDougal, L.K.; Flannagan, S.E.; Kolonay, J.F.; Shetty, J.; Killgore, G.E.; Tenover, F.C. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science. 2003, 302, 1569–1571. [Google Scholar] [CrossRef]
- Bourgogne, A.; Garsin, D.A.; Qin, X.; Singh, K.V.; Sillanpaa, J.; Yerrapragada, S.; Ding, Y.; Dugan-Rocha, S.; Buhay, C.; Shen, H.; Chen, G.; Williams, G.; Muzny, D.; Maadani, A.; Fox, K.A.; Gioia, J.; Chen, L.; Shang, Y.; Arias, C.A.; Nallapareddy, S.R.; Zhao, M.; Prakash, V.P.; Chowdhury, S.; Jiang, H.; Gibbs, R.A.; Murray, B.E.; Highlander, S.K.; Weinstock, G.M. Large scale variation in Enterococcus faecalis illustrated by the genome analysis of strain OG1RF. Genome Biol. 2008, 9, R110. [Google Scholar] [CrossRef]
- Palmer, K.L.; Carniol, K.; Manson, J.M.; Heiman, D.; Shea, T.; Young, S.; Zeng, Q.; Gevers, D.; Feldgarden, M.; Birren, B.; Gilmore, M.S. High-quality draft genome sequences of 28 Enterococcus sp. isolates. J Bacteriol. 2010, 192, 2469–2470. [Google Scholar] [CrossRef]
- Kristich, C.J.; Chandler, J.R.; Dunny, G.M. Development of a host-genotype-independent counterselectable marker and a high-frequency conjugative delivery system and their use in genetic analysis of Enterococcus faecalis. Plasmid. 2007, 57, 131–144. [Google Scholar] [CrossRef]
- Kristich, C.J.; Manias, D.A.; Dunny, G.M. Development of a method for markerless genetic exchange in Enterococcus faecalis and its use in construction of a srtA mutant. Appl Environ Microbiol. 2005, 71, 5837–5849. [Google Scholar] [CrossRef]
- Kristich, C.J.; Nguyen, V.T.; Le, T.; Barnes, A.M.; Grindle, S.; Dunny, G.M. Development and use of an efficient system for random mariner transposon mutagenesis to identify novel genetic determinants of biofilm formation in the core Enterococcus faecalis genome. Appl Environ Microbiol. 2008, 74, 3377–3386. [Google Scholar] [CrossRef]
- Ballering, K.S.; Kristich, C.J.; Grindle, S.M.; Oromendia, A.; Beattie, D.T.; Dunny, G.M. Functional genomics of Enterococcus faecalis: multiple novel genetic determinants for biofilm formation in the core genome. J Bacteriol. 2009, 191, 2806–2814. [Google Scholar] [CrossRef] [PubMed]
- Frank, K.L.; Barnes, A.M.; Grindle, S.M.; Manias, D.A.; Schlievert, P.M.; Dunny, G.M. Use of recombinase-based in vivo expression technology to characterize Enterococcus faecalis gene expression during infection identifies in vivo-expressed antisense RNAs and implicates the protease Eep in pathogenesis. Infect Immun. 2012, 80, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.A.; Huang, D.B. Biofilm formation by enterococci. J Med Microbiol. 2007, 56 Pt 12, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, F.L.; Willems, R.J.; Leavis, H.L. Optimizing future treatment of enterococcal infections: attacking the biofilm? Trends Microbiol. 2012, 20, 40–49. [Google Scholar] [CrossRef]
- Dunny, G.M.; Hancock, L.E.; Shankar, N. Enterococcal Biofilm Structure and Role in Colonization and Disease. 2014. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; Massachusetts Eye and Ear Infirmary: Boston, 2014. [Google Scholar]
- Tan, C.A.Z.; Antypas, H.; Kline, K.A. Overcoming the challenge of establishing biofilms in vivo: a roadmap for Enterococci. Curr Opin Microbiol. 2020, 53, 9–18. [Google Scholar] [CrossRef]
- Frank, K.L.; Vergidis, P.; Brinkman, C.L.; Greenwood Quaintance, K.E.; Barnes, A.M.; Mandrekar, J.N.; Schlievert, P.M.; Dunny, G.M.; Patel, R. Evaluation of the Enterococcus faecalis Biofilm-Associated Virulence Factors AhrC and Eep in Rat Foreign Body Osteomyelitis and In Vitro Biofilm-Associated Antimicrobial Resistance. PLoS One 2015, 10, e0130187. [Google Scholar] [CrossRef] [PubMed]
- Leuck, A.M.; Johnson, J.R.; Dunny, G.M. A widely used in vitro biofilm assay has questionable clinical significance for enterococcal endocarditis. PLoS One 2014, 9, e107282. [Google Scholar] [CrossRef] [PubMed]
- Colomer-Winter, C.; Gaca, A.O.; Chuang-Smith, O.N.; Lemos, J.A.; Frank, K.L. Basal levels of (p)ppGpp differentially affect the pathogenesis of infective endocarditis in Enterococcus faecalis. Microbiology 2018, 164, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Manias, D.A.; Dunny, G.M. Expression of Adhesive Pili and the Collagen-Binding Adhesin Ace Is Activated by ArgR Family Transcription Factors in Enterococcus faecalis. J Bacteriol. 2018, 200, e00269–18. [Google Scholar] [CrossRef] [PubMed]
- Evers, S.; Quintiliani RJr Courvalin, P. Genetics of glycopeptide resist- ance in enterococci. Microb Drug Resist 1996, 2, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Arthur, M.; Reynolds, P.E.; Depardieu, F.; et al. Mechanisms of glycopep- tide resistance in enterococci. J Infect 1996, 32, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Arthur, M.; Depardieu, F.; Gerbaud, G.; Galimand, M.; Leclercq, R.; Cour- valin, P. The VanS sensor negatively controls VanR-mediated transcriptional activation of glycopeptide resistance genes of Tn 1546 and related elements in the absence of induction. J Bacteriol 1997, 179, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Bugg, T.D.H.; Wright, G.D.; Dutka-Malen, S.; Arthur, M.; Courvalin, P.; Walsh, C.T. Molecular basis for vancomycin resistance in Enterococcus faecium BM4147: biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry 1991, 30, 10408–10415. [Google Scholar] [CrossRef]
- Milbrandt, E. A novel source of enterococcal endocarditis. Clin Cardiol 1998, 21, 123–126. [Google Scholar] [CrossRef]
- Khan, Z.; Siddiqui, N.; Saif, M.W. Enterococcus Faecalis Infective Endocarditis and Colorectal Carcinoma: Case of New Association Gaining Ground. Gastroenterology Res. 2018, 11, 238–240. [Google Scholar] [CrossRef]
- Manoil, D.; Cerit, E.E.; Fang, H.; Durual, S.; Brundin, M.; Belibasakis, G.N. Profiling Antibiotic Susceptibility among Distinct Enterococcus faecalis Isolates from Dental Root Canals. Antibiotics 2023, 13, 18. [Google Scholar] [CrossRef]
- Pandova, M.; Kizheva, Y.; Tsenova, M.; Rusinova, M.; Borisova, T.; Hristova, P. Pathogenic Potential and Antibiotic Susceptibility: A Comprehensive Study of Enterococci from Different Ecological Settings. Pathogens 2023, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Midiri, A.; Gerace, E.; Marra, M.; Zummo, S.; Biondo, C. Urinary Tract Infections: The Current Scenario and Future Prospects. Pathogens 2023, 12, 623. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.A.; Murray, B.E. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012, 10, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Jahansepas, A.; Aghazadeh, M.; Rezaee, M.A.; Hasani, A.; Sharifi, Y.; Aghazadeh, T.; Mardaneh, J. Occurrence of Enterococcus faecalis and Enterococcus faecium in Various Clinical Infections: Detection of Their Drug Resistance and Virulence Determinants. Microb Drug Resist. 2018, 24, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Coccitto, S.N.; Cinthi, M.; Simoni, S.; Pocognoli, A.; Zeni, G.; Mazzariol, A.; Morroni, G.; Mingoia, M.; Giovanetti, E.; Brenciani, A.; Vignaroli, C. Genetic analysis of vancomycin-variable Enterococcus faecium clinical isolates in Italy. Eur J Clin Microbiol Infect Dis. 2024. [Google Scholar] [CrossRef]
- Dubin, K.; Pamer, E.G. Enterococci and Their Interactions with the Intestinal Microbiome. Microbiol Spectr. 2014, 5. [Google Scholar] [CrossRef]
- Hendrickx, A.P.; Top, J.; Bayjanov, J.R.; Kemperman, H.; Rogers, M.R.; Paganelli, F.L.; Bonten, M.J.; Willems, R.J. Antibiotic-Driven Dysbiosis Mediates Intraluminal Agglutination and Alternative Segregation of Enterococcus faecium from the Intestinal Epithelium. mBio. 2015, 6, e01346–15. [Google Scholar] [CrossRef]
- Wells, C.L.; Jechorek, R.P.; Erlandsen, S.L. Evidence for the translocation of Enterococcus faecalis across the mouse intestinal tract. J Infect Dis. 1990, 162, 82–90. [Google Scholar] [CrossRef]
- Qin, X.; Singh, K.V.; Weinstock, G.M.; Murray, B.E. Effects of Enterococcus faecalis fsr genes on production of gelatinase and a serine protease and virulence. Infect Immun. 2000, 68, 2579–2586. [Google Scholar] [CrossRef]
- Zeng, J.; Teng, F.; Weinstock, G.M.; Murray, B.E. Translocation of Enterococcus faecalis strains across a monolayer of polarized human enterocyte-like T84 cells. J Clin Microbiol. 2004, 42, 1149–1154. [Google Scholar] [CrossRef]
- Zeng, J.; Teng, F.; Murray, B.E. Gelatinase is important for translocation of Enterococcus faecalis across polarized human enterocyte-like T84 cells. Infect Immun. 2005, 73, 1606–1612. [Google Scholar] [CrossRef]
- Archambaud, C.; Derré-Bobillot, A.; Lapaque, N.; Rigottier-Gois, L.; Serror, P. Intestinal translocation of enterococci requires a threshold level of enterococcal overgrowth in the lumen. Sci Rep. 2019, 9, 8926. [Google Scholar] [CrossRef]
- Manfredo Vieira, S.; Hiltensperger, M.; Kumar, V.; Zegarra-Ruiz, D.; Dehner, C.; Khan, N.; Costa, F.R.C.; Tiniakou, E.; Greiling, T.; Ruff, W.; Barbieri, A.; Kriegel, C.; Mehta, S.S.; Knight, J.R.; Jain, D.; Goodman, A.L.; Kriegel, M.A. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018, 359, 1156–1161. [Google Scholar] [CrossRef]
- Fine, R.L.; Manfredo Vieira, S.; Gilmore, M.S.; Kriegel, M.A. Mechanisms and consequences of gut commensal translocation in chronic diseases. Gut Microbes. 2020, 11, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Little, R.; Wine, E.; Kamath, B.M.; Griffiths, A.M.; Ricciuto, A. Gut microbiome in primary sclerosing cholangitis: A review. World J Gastroenterol. 2020, 26, 2768–2780. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Huang, Y.; Chen, R.; Li, L.; Chen, M.; Zhang, S. Current insights on the roles of gut microbiota in inflammatory bowel disease-associated extra-intestinal manifestations : pathophysiology and therapeutic targets. Gut Microbes. 2023, 15, 2265028. [Google Scholar] [CrossRef]
- Knoop, K.A.; McDonald, K.G.; Kulkarni, D.H.; Newberry, R.D. Antibiotics promote inflammation through the translocation of native commensal colonic bacteria. Gut. 2016, 65, 1100–1109. [Google Scholar] [CrossRef]
- Kulkarni, D.H.; Rusconi, B.; Floyd, A.N.; Joyce, E.L.; Talati, K.B.; Kousik, H.; Alleyne, D.; Harris, D.L.; Garnica, L.; McDonough, R.; Bidani, S.S.; Kulkarni, H.S.; Newberry, E.P.; McDonald, K.G.; Newberry, R.D. Gut microbiota induces weight gain and inflammation in the gut and adipose tissue independent of manipulations in diet, genetics, and immune development. Gut Microbes. 2023, 15, 2284240. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.A.; Casterline, B.W.; Valguarnera, E.; Hecht, A.L.; Shepherd, E.S.; Sonnenburg, J.L.; Bubeck Wardenburg, J. Bacteroides fragilis toxin expression enables lamina propria niche acquisition in the developing mouse gut. Nat Microbiol. 2024, 9, 85–94. [Google Scholar] [CrossRef]
- Brown, A.O.; Singh, K.V.; Cruz, M.R.; Kaval, K.G.; Francisco, L.E.; Murray, B.E.; Garsin, D.A. Cardiac Microlesions Form During Severe Bacteremic Enterococcus faecalis Infection. J Infect Dis. 2021, 223, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Siddiqui, N.; Saif, M.W. Enterococcus Faecalis Infective Endocarditis and Colorectal Carcinoma: Case of New Association Gaining Ground. Gastroenterology Res. 2018, 11, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Cabiltes, I.; Coghill, S.; Bowe, S.J.; Athan, E. Enterococcal bacteraemia 'silent but deadly': a population-based cohort study. Intern Med J. 2020, 50, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Pericàs, J.M.; Ambrosioni, J.; Muñoz, P.; de Alarcón, A.; Kestler, M.; Mari-Hualde, A.; Moreno, A.; Goenaga, M.Á.; Fariñas, M.C.; Rodríguez-Álvarez, R.; Ojeda-Burgos, G.; Gálvez-Acebal, J.; Hidalgo-Tenorio, C.; Noureddine, M.; Miró, J.M.; GAMES Investigators. Prevalence of Colorectal Neoplasms Among Patients With Enterococcus faecalis Endocarditis in the GAMES Cohort (2008-2017). Mayo Clin Proc. 2021, 96, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Pasquereau-Kotula, E.; Martins, M.; Aymeric, L.; Dramsi, S. Significance of Streptococcus gallolyticus subsp. gallolyticus Association With Colorectal Cancer. Front Microbiol. 2018, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Jans, C.; Boleij, A. The Road to Infection: Host-Microbe Interactions Defining the Pathogenicity of Streptococcus bovis/Streptococcus equinus Complex Members. Front Microbiol. 2018, 9, 603. [Google Scholar] [CrossRef] [PubMed]
- Aymeric, L.; Donnadieu, F.; Mulet, C.; du Merle, L.; Nigro, G.; Saffarian, A.; Bérard, M.; Poyart, C.; Robine, S.; Regnault, B.; Trieu-Cuot, P.; Sansonetti, P.J.; Dramsi, S. Colorectal cancer specific conditions promote Streptococcus gallolyticus gut colonization. Proc Natl Acad Sci USA 2018, 115, E283–E291. [Google Scholar] [CrossRef]
- Taylor, J.C.; Gao, X.; Xu, J.; Holder, M.; Petrosino, J.; Kumar, R.; Liu, W.; Höök, M.; Mackenzie, C.; Hillhouse, A.; Brashear, W.; Nunez, M.P.; Xu, Y. A type VII secretion system of Streptococcus gallolyticus subsp. gallolyticus contributes to gut colonization and the development of colon tumors. PLoS Pathog. 2021, 17, e1009182. [Google Scholar] [CrossRef]
- Taylor, J.C.; Kumar, R.; Xu, J.; Xu, Y. A pathogenicity locus of Streptococcus gallolyticus subspecies gallolyticus. Sci Rep. 2023, 13, 6291. [Google Scholar] [CrossRef]
- Stanley, D.; Mason, L.J.; Mackin, K.E.; Srikhanta, Y.N.; Lyras, D.; Prakash, M.D.; Nurgali, K.; Venegas, A.; Hill, M.D.; Moore, R.J.; Wong, C.H. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat Med. 2016, 22, 1277–1284. [Google Scholar] [CrossRef]
- Claes, J.; Liesenborghs, L.; Peetermans, M.; Veloso, T.R.; Missiakas, D.; Schneewind, O.; Mancini, S.; Entenza, J.M.; Hoylaerts, M.F.; Heying, R.; et al. Clumping factor A, von Willebrand factor-binding protein and von Willebrand factor anchor Staphylococcus aureus to the vessel wall. J. Thromb. Haemost. 2017, 15, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Claes, J.; Ditkowski, B.; Liesenborghs, L.; Veloso, T.R.; Entenza, J.M.; Moreillon, P.; Vanassche, T.; Verhamme, P.; Hoylaerts, M.F.; Heying, R. Assessment of the Dual Role of Clumping Factor A in S. Aureus Adhesion to Endothelium in Absence and Presence of Plasma. Thromb. Haemost. 2018, 118, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.P.; Kang, M.; Ganesh, V.K.; Ravirajan, D.; Li, B.; Höök, M. Coagulase and Efb of Staphylococcus aureus Have a Common Fibrinogen Binding Motif. mBio 2016, 7, e01885–15. [Google Scholar] [CrossRef] [PubMed]
- Pappelbaum, K.I.; Gorzelanny, C.; Grässle, S.; Suckau, J.; Laschke, M.W.; Bischoff, M.; Bauer, C.; Schorpp-Kistner, M.; Weidenmaier, C.; Schneppenheim, R.; et al. Ultralarge von Willebrand factor fibers mediate luminal Staphylococcus aureus adhesion to an intact endothelial cell layer under shear stress. Circulation 2013, 128, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwiu, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutro- phil extracellular traps. J Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Thiam, H.R.; Wong, S.L.; Wagner, D.D.; Waterman, C.M. Cellular mechanisms of NETosis. Annu Rev Cell Dev Biol. 2020, 36, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Bellomo, F.; Avtaar Singh, S.S. Insights into the Role of Neutrophils and Neutrophil Extracellular Traps in Causing Cardiovascular Complications in Patients with COVID-19: A Systematic Review. J Clin Med. 2022, 11, 2460. [Google Scholar] [CrossRef]
- Nappi, F.; Iervolino, A.; Avtaar Singh, S.S. Thromboembolic Complications of SARS-CoV-2 and Metabolic Derangements: Suggestions from Clinical Practice Evidence to Causative Agents. Metabolites. 2021, 11, 341. [Google Scholar] [CrossRef]
- Wong, S.L.; Demers, M.; Martinod, K.; Gallant, M.; Wang, Y.; Goldfine, A.B.; Kahn, C.R.; Wagner, D.D. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med. 2015, 21, 815–819. [Google Scholar] [CrossRef]
- Nappi, F.; Bellomo, F.; Avtaar Singh, S.S. Worsening Thrombotic Complication of Atherosclerotic Plaques Due to Neutrophils Extracellular Traps: A Systematic Review. Biomedicines. 2023, 11, 113. [Google Scholar] [CrossRef]
- Nappi, F.; Nappi, P.; Gambardella, I.; Avtaar Singh, S.S. Thromboembolic Disease and Cardiac Thrombotic Complication in COVID-19: A Systematic Review. Metabolites. 2022, 12, 889. [Google Scholar] [CrossRef]
- Morrell, C.N.; Hilt, Z.T.; Pariser, D.N.; Maurya, P. PAD4 and von Willebrand Factor Link Inflammation and Thrombosis. Circ Res. 2019, 125, 520–522. [Google Scholar] [CrossRef]
- Sorvillo, N.; Mizurini, D.M.; Coxon, C.; Martinod, K.; Tilvawala, R.; Cherpokova, D.; Salinger, A.J.; Seward, R.J.; Staudinger, C.; Weerapana, E.; et al. Plasma peptidy- larginine deiminase IV promotes VWF-platelet string formation and accel- erates thrombosis after vessel injury. Circ Res. 2019, 125, 507–519. [Google Scholar] [CrossRef]
- Liberale, L.; Holy, E.W.; Akhmedov, A.; Bonedi, N.R.; Nietlispach, F.; Mader, C.M.; Mach, F.; Montecucco, F.; Beer, J.H.; Paneni, F.; et al. Interleukin-1β mediates arterial thrombus formation via NET-associated tissue factor. J Clin Med. 2019, 8, E2072. [Google Scholar] [CrossRef]
- Wu, R.; Wang, N.; Comish, P.B.; Tang, D.; Kang, R. Inflammasome-dependent coagulation activation in sepsis. Front Immunol. 2021, 12, 641750. [Google Scholar] [CrossRef] [PubMed]
- Franklin, B.S.; Bossaller, L.; De Nardo, D.; Rader, J.M.; Stuu, A.; Engels, G.; Brenker, C.; Nordhoff, M.; Mirandola, S.R.; Al- Amoudi, A.; et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat Immunol. 2014, 15, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Braï, M.A.; Hannachi, N.; El Gueddari, N.; Baudoin, J.P.; Dahmani, A.; Lepidi, H.; Habib, G.; Camoin-Jau, L. The Role of Platelets in Infective Endocarditis. Int J Mol Sci. 2023, 24, 7540. [Google Scholar] [CrossRef] [PubMed]
- Misfeldt, A.M.; Boyle, S.C.; Tompkins, K.L.; Bautch, V.L.; Labosky, P.A.; Baldwin, H.S. Endocardial cells are a distinct endothelial lineage derived from Flk1+ multipotent cardiovascular progenitors. Dev Biol. 2009, 333, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Dyer, L.A.; Patterson, C. Development of the endothelium: an emphasis on heterogeneity. Semin Thromb Hemost. 2010, 36, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Harris, I.S.; Black, B.L. Development of the endocardium. Pediatr Cardiol. 2010, 31, 391–399. [Google Scholar] [CrossRef]
- Milgrom-Hoffman, M.; Harrelson, Z.; Ferrara, N.; Zelzer, E.; Evans, S.M.; Tzahor, E. The heart endocardium is derived from vascular endothelial progenitors. Development. 2011, 138, 4777–4787. [Google Scholar] [CrossRef]
- Borasch, K.; Richardson, K.; Plendl, J. Cardiogenesis with a focus on vasculogenesis and angiogenesis. Anat Histol Embryol. 2020, 49, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Calles-Enríquez, M.; del Rio, B.; Ladero, V.; Martín, M.C.; Fernández, M.; Alvarez, M.A. IS256 abolishes gelatinase activity and biofilm formation in a mutant of the nosocomial pathogen Enterococcus faecalis V583. Can J Microbiol. 2015, 61, 517–519. [Google Scholar] [CrossRef] [PubMed]
- Ali, L.; Goraya, M.U.; Arafat, Y.; Ajmal, M.; Chen, J.L.; Yu, D. Molecular Mechanism of Quorum-Sensing in Enterococcus faecalis: Its Role in Virulence and Therapeutic Approaches. Int J Mol Sci. 2017, 18, 960. [Google Scholar] [CrossRef]
- Kirsch, J.M.; Ely, S.; Stellfox, M.E.; Hullahalli, K.; Luong, P.; Palmer, K.L.; Van Tyne, D.; Duerkop, B.A. Targeted IS-element sequencing uncovers transposition dynamics during selective pressure in enterococci. PLoS Pathog. 2023, 19, e1011424. [Google Scholar] [CrossRef]
- Brown, A.O.; Garsin, D.A. The pathogenesis of cardiac microlesion formation during severe bacteremic infection. PLoS Pathog. 2020, 16, e1009021. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huycke, M.M. Extracellular superoxide production by Enterococcus faecalis promotes chromo- somal instability in mammalian cells. Gastroenterology. 2007, 132, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Reardon-Robinson, M.E.; Ton-That, H. Disulfide-Bond-Forming Pathways in Gram-Positive Bacteria. J Bacteriol. 2015, 198, 746–754. [Google Scholar] [CrossRef]
- McDonald, J. Acute Infective Endocarditis. Infect. Dis. Clin. North Am. 2009, 23, 643–664. [Google Scholar] [CrossRef]
- Dahl, A.; Iversen, K.; Tonder, N.; Hoest, N.; Arpi, M.; Dalsgaard, M.; Chehri, M.; Soerensen, L.L.; Fanoe, S.; Junge, S.; Hoest, U.; Valeur, N.; Lauridsen, T.K.; Fosbol, E.; Hoi-Hansen, T.; Bruun, N.E. Prevalence of Infective Endocarditis in Enterococcus faecalis Bacteremia. J Am Coll Cardiol. 2019, 74, 193–201. [Google Scholar] [CrossRef]
- Keynan, Y.; Rubinstein, E. Pathophysiology of infective endocarditis. Curr Infect Dis Rep. 2013, 15, 342–346. [Google Scholar] [CrossRef]
- Liesenborghs, L.; Meyers, S.; Vanassche, T.; Verhamme, P. Coagulation: At the heart of infective endocarditis. J Thromb Haemost. 2020, 18, 995–1008. [Google Scholar] [CrossRef]
- Bizzini, A.; Beggah-Möller, S.; Moreillon, P.; Entenza, J.M. Lack of in vitro biofilm formation does not attenuate the virulence of Streptococcus gordonii in experimental endocarditis. FEMS Immunol Med Microbiol. 2006, 48, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, D.T., Jr.; Vakilzadeh, J.; Sherwood, B.F.; LeMay, J.C. Experimental bacterial endocarditis in the opossum (Didelphis virginiana). I. Valvular changes following a single injection of bacteria in unmodified adult opossums. Am J Pathol. 1970, 58, 295–304. [Google Scholar] [PubMed]
- Vakilzadeh, J.; Rowlands, D.T., Jr.; Sherwood, B.F.; LeMay, J.C. Experimental bacterial endocarditis in the opossum (Didelphis virginiana). II. Induction of endocarditis with a single injection of Streptococcus viridans. J Infect Dis. 1970, 122, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, B.F.; Rowlands, D.T., Jr.; Vakilzadeh, J.; LeMay, J.C. Experimental bacterial endocarditis in the opossum (Didelphis virginiana). 3. Comparison of spontaneously occurring endocarditis with that induced experimentally by pyogenic bacteria and fungi. Am J Pathol. 1971, 64, 513–520. [Google Scholar] [PubMed]
- Jones, J.E. Experimental bacterial endocarditis in the pig. Proc R Soc Med. 1972, 65, 990–994. [Google Scholar]
- La Regina, M.C.; Lonigro, J.; Woods, L.; Williams, G.A.; Vogler, G.A. Valvular endocarditis associated with experimental Erysipelothrix rhusiopathiae infection in the opossum (Didelphis virginiana). Lab Anim Sci. 1988, 38, 159–161. [Google Scholar] [PubMed]
- Garrison, P.K.; Freedman, L.R. Experimental endocarditis I. Staphylococcal endocarditis in rabbits resulting from placement of a polyethylene catheter in the right side of the heart. Yale J Biol Med. 1970, 42, 394–410. [Google Scholar] [PubMed]
- Perlman, B.B.; Freedman, L.R. Yale Experimental endocarditis. II. Staphylococcal infection of the aortic valve following placement of a polyethylene catheter in the left side of the heart. J Biol Med. 1971, 44, 206–213. [Google Scholar]
- Perlman, B.B.; Freedman, L.R. Experimental endocarditis. 3. Natural history of catheter induced staphylococcal endocarditis following catheter removal. Yale J Biol Med. 1971, 44, 214–224. [Google Scholar]
- Durack, D.T.; Beeson, P.B. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br J Exp Pathol. 1972, 53, 44–49. [Google Scholar]
- Durack, D.T.; Beeson, P.B. Experimental bacterial endocarditis. II. Survival of a bacteria in endocardial vegetations. Br J Exp Pathol. 1972, 53, 50–53. [Google Scholar]
- Durack, D.T.; Petersdorf, R.G.; Beeson, P.B. Penicillin prophylaxis of experimental S. viridans endocarditis. Trans Assoc Am Physicians. 1972, 85, 222–230. [Google Scholar]
- Durack, D.T.; Beeson, P.B.; Petersdorf, R.G. Experimental bacterial endocarditis. 3. Production and progress of the disease in rabbits. Br J Exp Pathol. 1973, 54, 142–151. [Google Scholar]
- Freedman, L.R.; Arnold, S.; Valone, J. Experimental endocarditis. Ann N Y Acad Sci. 1974, 236, 456–465. [Google Scholar] [CrossRef]
- Durack, D.T.; Beeson, P.B. Protective role of complement in experimental Escherichia coli endocarditis. Infect Immun. 1977, 16, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Tunkel, A.; Scheld, W. Experimental Models of Endocarditis. In Infective Endocarditis; Kaye, D., Ed.; Raven Press: New York, 1992; pp. 37–56. [Google Scholar]
- Jamet, A.; Dervyn, R.; Lapaque, N.; Bugli, F.; Perez-Cortez, N.G.; Blottière, H.M.; Twizere, J.C.; Sanguinetti, M.; Posteraro, B.; Serror, P.; Maguin, E. The Enterococcus faecalis virulence factor ElrA interacts with the human Four-and-a-Half LIM Domains Protein 2. Sci Rep. 2017, 7, 4581. [Google Scholar] [CrossRef] [PubMed]
- Huck, V.; Schneider, M.F.; Gorzelanny, C.; Schneider, S.W. The various states of von Willebrand factor and their function in physiology and pathophysiology. Thromb Haemost. 2014, 111, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Steinert, M.; Ramming, I.; Bergmann, S. Front Med (Lausanne). Impact of Von Willebrand Factor on Bacterial. Pathogenesis. 2020, 7, 543. [Google Scholar] [CrossRef]
- Wagner, D.D. Cell biology of von Willebrand factor. Annu Rev Cell Biol. 1990, 6, 217–246. [Google Scholar]
- Journet, A.M.; Saffaripour, S.; Cramer, E.M.; Tenza, D.; Wagner, D.D. von Willebrand factor storage requires intact prosequence cleavage site. Eur J Cell Biol. 1993, 60, 31–41. [Google Scholar]
- Bowman, M.; Casey, L.; Selvam, S.N.; Lima, P.D.A.; Rawley, O.; Hinds, M.; Tuttle, A.; Grabell, J.; Iorio, A.; Walker, I.; Lillicrap, D.; James, P. von Willebrand factor propeptide variants lead to impaired storage and ER retention in patient-derived endothelial colony-forming cells. J Thromb Haemost. 2022, 20, 1599–1609. [Google Scholar] [CrossRef]
- Gaytán, M.O.; Singh, A.K.; Woodiga, S.A.; Patel, S.A.; An, S.S.; Vera-Ponce de León, A.; McGrath, S.; Miller, A.R.; Bush, J.M.; van der Linden, M.; Magrini, V.; Wilson, R.K.; Kitten, T.; King, S.J. A novel sialic acid-binding adhesin present in multiple species contributes to the pathogenesis of Infective endocarditis. PLoS Pathog. 2021, 17, e1009222. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Lee, G.-H.; Bak, H.R.; Park, Y.M.; Lee, S.H.; Hong, S.-J.; Lee, D.-W. Complete genome assembly of Enterococcus faecalis strain HL1, isolated from an infant fecal sample. Microbiol Resour Announc. 2023, 12, e0055823. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Hufnagel, M.; Theilacker, C.; Huebner, J. Enterococcal infections: host response, therapeutic, and prophylactic possibilities. Vaccine 2004, 22, 822–830. [Google Scholar] [CrossRef]
- Rich, R.L.; Kreikemeyer, B.; Owens, R.T.; LaBrenz, S.; Narayana, S.V.; Weinstock, G.M.; Murray, B.E. Höök M, 1.9.9.9. Ace is a collagen binding MSCRAMM from Enterococcus faecalis. J Biol Chem. 1999, 274, 26939–26945. [Google Scholar] [CrossRef]
- Giuliano, S.; Angelini, J.; D'Elia, D.; Geminiani, M.; Barison, R.D.; Giacinta, A.; Sartor, A.; Campanile, F.; Curcio, F.; Cotta, M.O.; Roberts, J.A.; Baraldo, M.; Tascini, C. Ampicillin and Ceftobiprole Combination for the Treatment of Enterococcus faecalis Invasive Infections: "The Times They Are A-Changin". Antibiotics 2023, 12, 879. [Google Scholar] [CrossRef] [PubMed]
- Shankar, V.; Baghdayan, A.S.; Huycke, M.M.; Lindahl, G.; Gilmore, M.S. Infection-derived Enterococcus faecalis strains are enriched in esp, a gene encoding a novel surface protein. Infect Immun. 1999, 67, 193–200. [Google Scholar] [CrossRef]
- El-Telbany, M.; Lin, C.Y.; Abdelaziz, M.N.; Maung, A.T.; El-Shibiny, A.; Mohammadi, T.N.; Zayda, M.; Wang, C.; Zar Chi Lwin, S.; Zhao, J.; Masuda, Y.; Honjoh, K.I.; Miyamoto, T.; El, M. Potential application of phage vB_EfKS5 to control Enterococcus faecalis and its biofilm in food. AMB Express. 2023, 13, 130. [Google Scholar] [CrossRef]
- Galli, D.; Wirth, R. Comparative analysis of Enterococcus faecalis sex pheromone plasmids identifies a single homologous DNA region which codes for aggregation substance. J Bacteriol. 1991, 173, 3029–3033. [Google Scholar] [CrossRef]
- Vlková, B.; Szemes, T.; Minárik, G.; Tóthová, L.; Drahovská, H.; Turňa, J.; Celec, P. Food-borne enterococci and their resistance to oxidative stress. J Microbiol. 2011, 49, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Carniol, K.; Gilmore, M.S. Signal transduction, quorum-sensing, and extracellular protease activity in Enterococcus faecalis biofilm formation. J Bacteriol. 2004, 186, 8161–8163. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.A.; Alorabi, J.A.; Al-Otaibi, L.M.; Ali, S.S.; Elsilk, S.E. Antibiotic Resistance and Biofilm Formation in Enterococcus spp. Isolated from Urinary Tract Infections. Pathogens. 2022, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.A.; Huang, W.; Nallapareddy, S.R.; Teng, F.; Murray, B.E. Influence of origin of isolates, especially endocarditis isolates, and various genes on biofilm formation by Enterococcus faecalis. Infect Immun 2004, 72, 3658–3663. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.A.; Teng, F.; Nallapareddy, S.R.; Murray, B.E. Pleiotrophic effects of 2 Enterococcus faecalis sagA-like genes, salA and salB, which encode proteins that are antigenic during human infection, on biofilm formation and binding to collagen type I and fibronectin. J Infect Dis 2006, 193, 231–240. [Google Scholar] [CrossRef]
- Tendolkar, P.M.; Baghdayan, A.S.; Shankar, N. Putative surface proteins encoded within a novel transferable locus confer a high-biofilm phenotype to Enterococcus faecalis. J Bacteriol 2006, 188, 2063–2072. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, M.; Koch, S.; Creti, R.; Baldassarri, L.; Huebner, J. A putative sugar-binding transcriptional regulator in a novel gene locus in Enterococcus faecalis contributes to production of biofilm and prolonged bacteremia in mice. J Infect Dis 2004, 189, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Kristich, C.J.; Li, Y.H.; Cvitkovitch, D.G.; Dunny, G.M. Esp- independent biofilm formation by Enterococcus faecalis. J Bacteriol 2004, 186, 154–163. [Google Scholar] [CrossRef]
- Hancock, L.E.; Perego, M. The Enterococcus faecalis fsr two- component system controls biofilm development through production of gelatinase. J Bacteriol 2004, 186, 5629–5639. [Google Scholar] [CrossRef]
- Fabretti, F.; Theilacker, C.; Baldassarri, L.; Kaczynski, Z.; Kropec, A.; Holst, O.; Huebner, J. Alanine esters of enterococcal lipoteichoic acid play a role in biofilm formation and resistance to antimicrobial peptides. Infect Immun 2006, 74, 4164–4171. [Google Scholar] [CrossRef]
- Bourgogne, A.; Singh, K.V.; Fox, K.A.; Plughoeft, K.J.; Murray, B.E.; Garsin, D.A. EbpR is important for biofilm formation by activating expression of the endocarditis and biofilm-associated pilus operon (ebpABC) of Enterococcus faecalis OG1RF. J Bacteriol 2007, 189, 6490–6493. [Google Scholar] [CrossRef]
- Toledo-Arana, A.; Valle, J.; Solano, C.; Arrizubieta, M.J.; Cucarella, C.; Lamata, M.; Amorena, B.; Leiva, J.; Penades, J.R.; Lasa, I. The enterococcal surface protein, Esp, is involved in Enterococcus faecalis biofilm formation. Appl Environ Microbiol 2001, 67, 4538–4545. [Google Scholar] [CrossRef] [PubMed]
- Tendolkar, P.M.; Baghdayan, A.S.; Gilmore, M.S.; Shankar, N. Enterococcal surface protein, Esp, enhances biofilm formation by Enterococcus faecalis. Infect Immun 2004, 72, 6032–6039. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. Thebiofilmmatrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, F.A.; Christophersen, L.; Laulund, A.S.; Lundquist, R.; Lerche, C.; Nielsen, P.R.; Bundgaard, H.; Høiby, N.; Moser, C. Novel human in vitro vegetation simulation model for infective endocarditis. APMIS 2021, 129, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Rimoldi, S.G.; Cavallo, I.; D’Agosto, G.; Trento, E.; Cagnoni, G.; Palazzin, A.; Pagani, C.; Romeri, F.; De Vecchi, E.; et al. Microbial biofilm correlates with an increased antibiotic tolerance and poor therapeutic outcome in infective endocarditis. BMC Microbiol. 2019, 19, 228. [Google Scholar] [CrossRef]
- Schwartz, F.A.; Nielsen, L.; Struve Andersen, J.; Bock, M.; Christophersen, L.; Sunnerhagen, T.; Lerche, C.J.; Bay, L.; Bundgaard, H.; Høiby, N.; et al. Dynamics of a Staphylococcus aureus infective endocarditis simulation model. APMIS 2022, 130, 515–523. [Google Scholar] [CrossRef]
- Leeten, K.; Jacques, N.; Lancellotti, P.; Oury, C. Aspirin or Ticagrelor in Staphylococcus aureus Infective Endocarditis :Where Do We Stand? Front. Cell. Dev. Biol. 2021, 9, 716302. [Google Scholar] [CrossRef]
- Ditkowski, B.; Bezulska Ditkowska, M.; Jashari, R.; Baatsen, P.; Moreillon, P.; Rega, F.; Veloso, T.R.; Hoylaerts, M.F.; Heying, R.; Congenital Cardiology and Cardiac Surgery Group. Antiplatelet therapy abrogates platelet-assisted Staphylococcus aureus infectivity of biological heart valve conduits. J. Thorac. Cardiovasc. Surg. 2021, 161, e457–e472. [Google Scholar] [CrossRef] [PubMed]
- Hannachi, N.; Habib, G.; Camoin-Jau, L. Aspirin Effect on Staphylococcus aureus-Platelet Interactions During Infectious Endocarditis. Front. Med. 2019, 6, 217. [Google Scholar] [CrossRef] [PubMed]
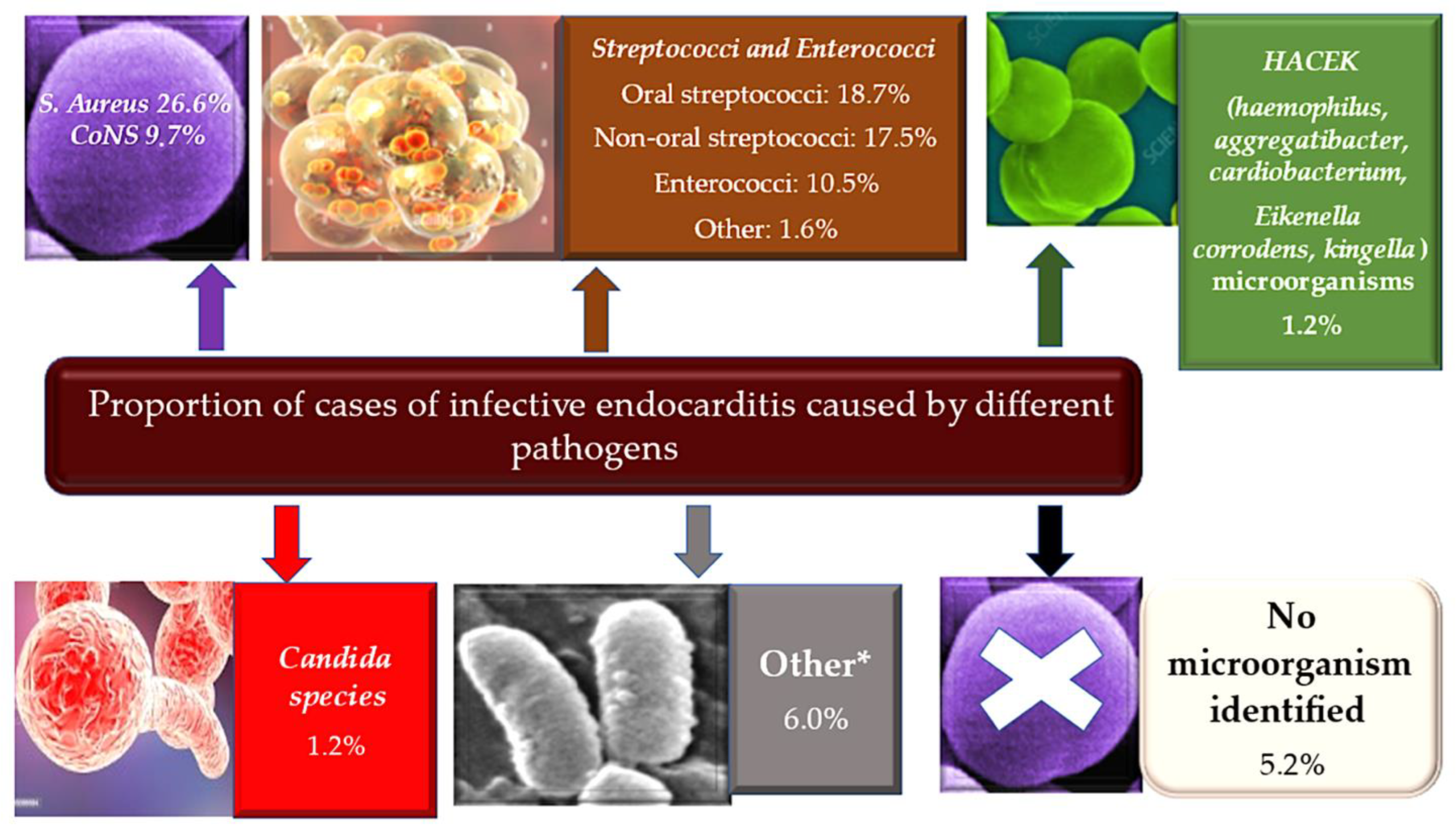
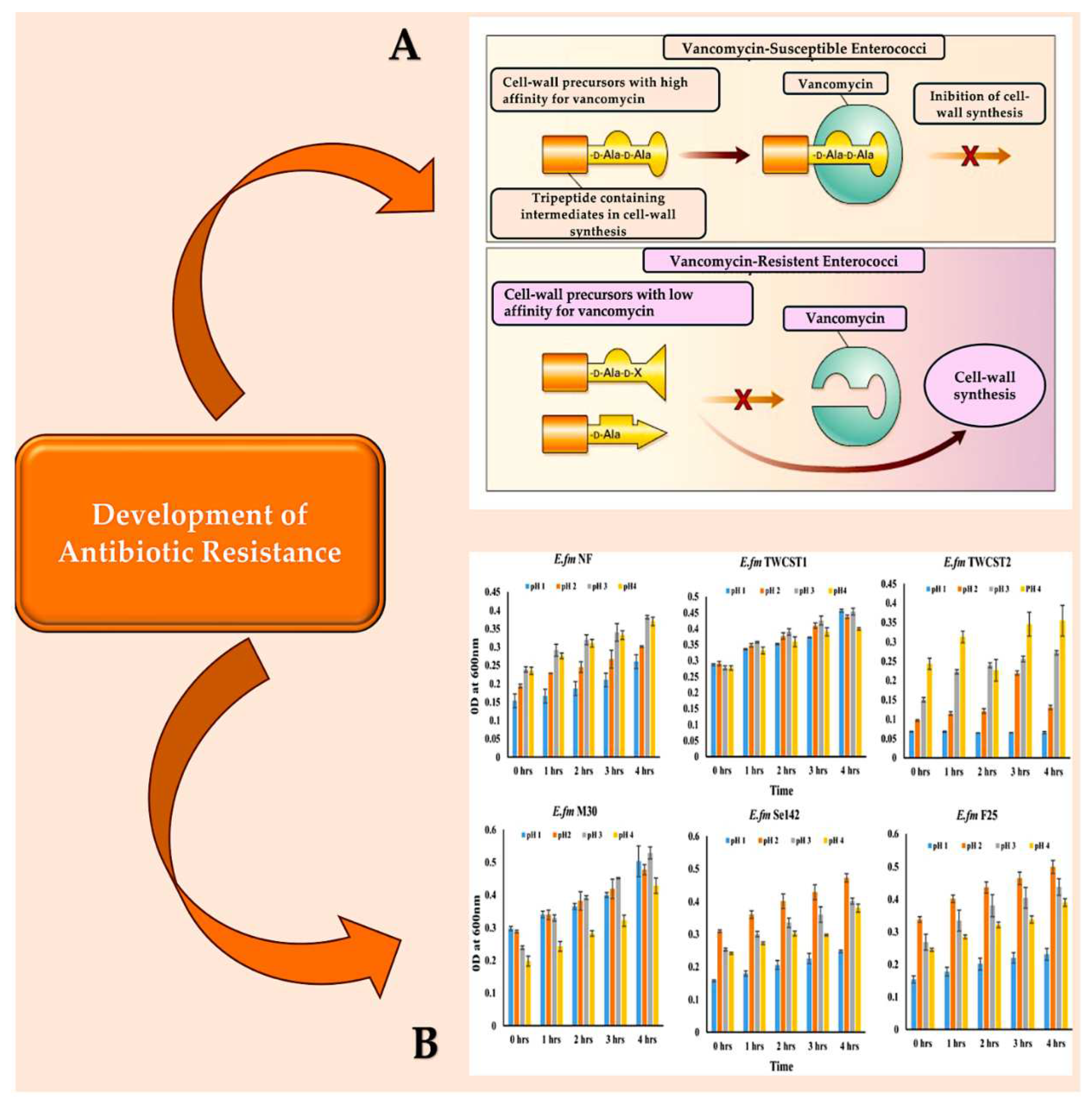

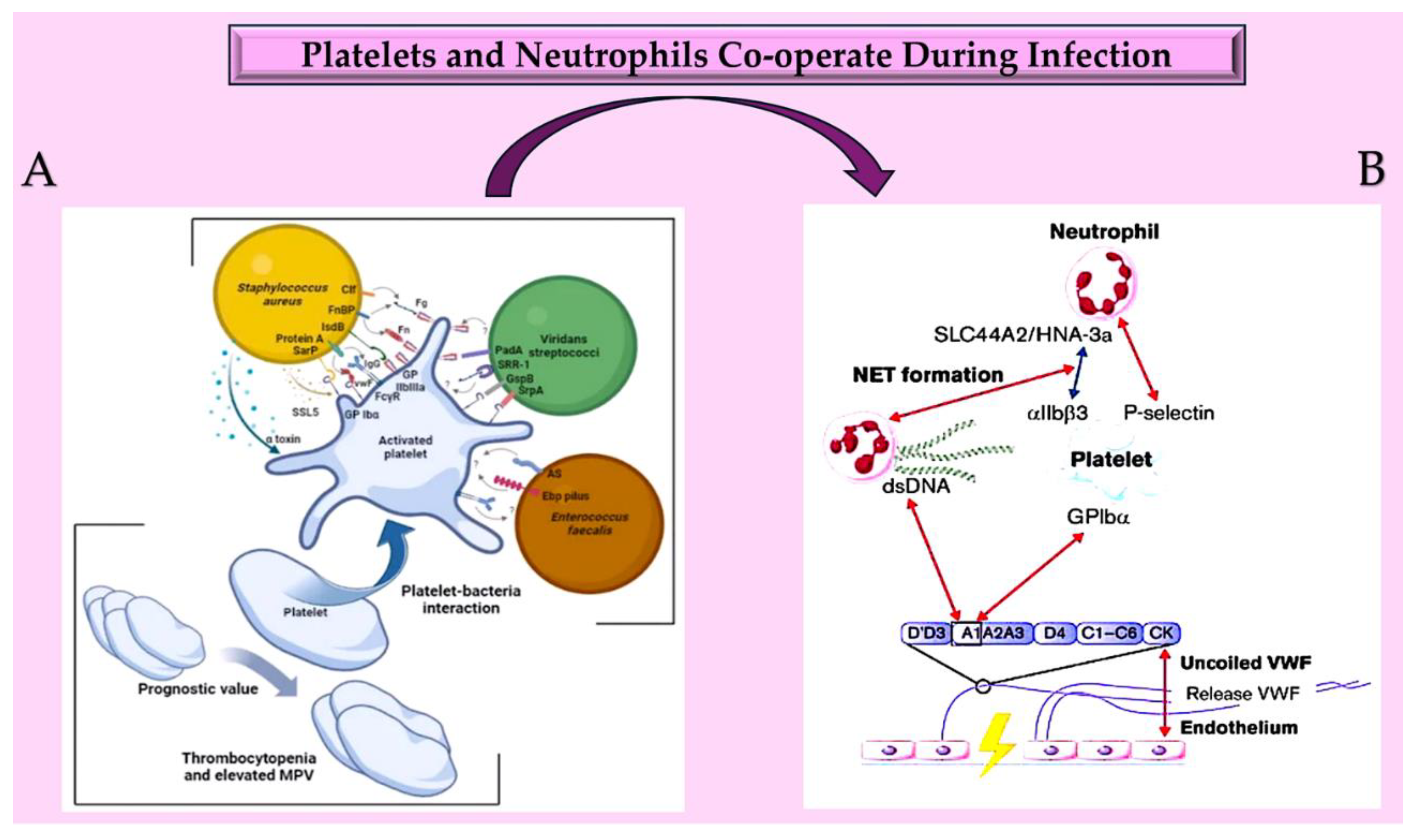
| Pathogen | Non-IE Allograft infection | IE Pathogen at allograft implant |
IE Pathogen at allograft infection |
|||
|---|---|---|---|---|---|---|
| n* 22 |
No.% | nγ 46 |
No.% | nλ 42 |
No.% | |
| Staphylococcus aureus | 0 (0) | 9 (20) | 11 (26) | |||
| CoNS | 0 (0) | 4 (8,7) | 3 (7.1) | |||
| Virdans group strep | 10 (45) | 5 (11) | 7 (17) | |||
| Enterococcus | 0 (0) | 7 (15) | 3 (7.1) | |||
| Others | 3 (14) | 5 (11) | 5 (12) | |||
| Pathogen not identified | 3 (14) | 9 (20) | 5 (12) | |||
| Other GPC | 3 (14) | 4 (8.7) | 4 (9.5) | |||
| Fungus | 3 (14) | 3 (6.5) | 4 (9.5) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





