Submitted:
31 January 2024
Posted:
01 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and evaluation of ostrich oil
2.2.1. Preparation of ostrich oil
2.2.2. Fatty acid composition
2.2.3. Antioxidant activity
DPPH radical scavenging
Copper-chelating activity
2.2.4. Acid value (AV), Peroxide value (PV), iodine value (IV), saponification value (SV), and refractive index (RI)
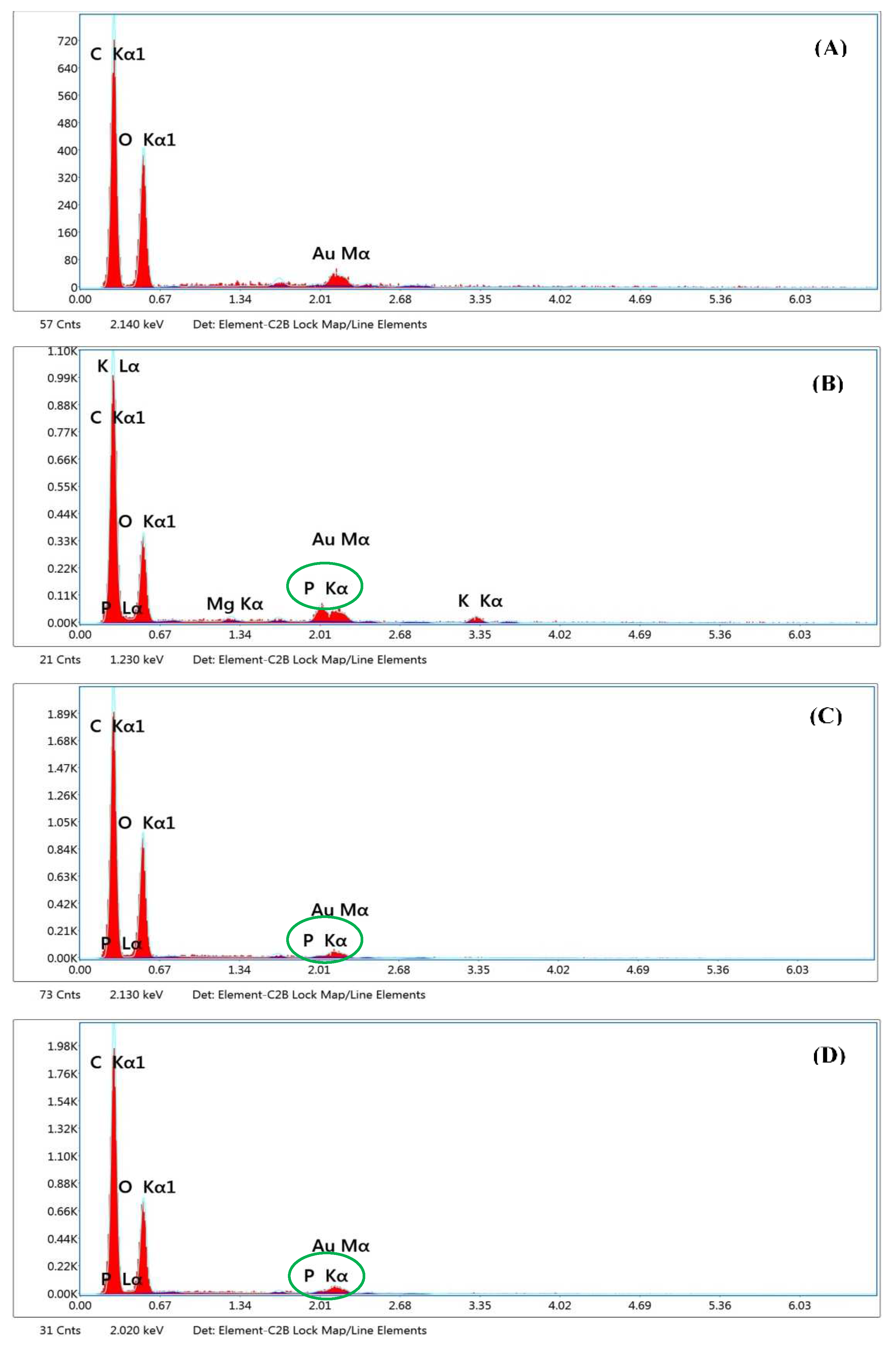
2.2.5. Heavy metal contents
2.2.6. Microbial contamination
2.3. Formulation and evaluation of emulsion containing ostrich oil
2.3.1. Formulation of emulsion
2.3.2. Evaluation of emulsion
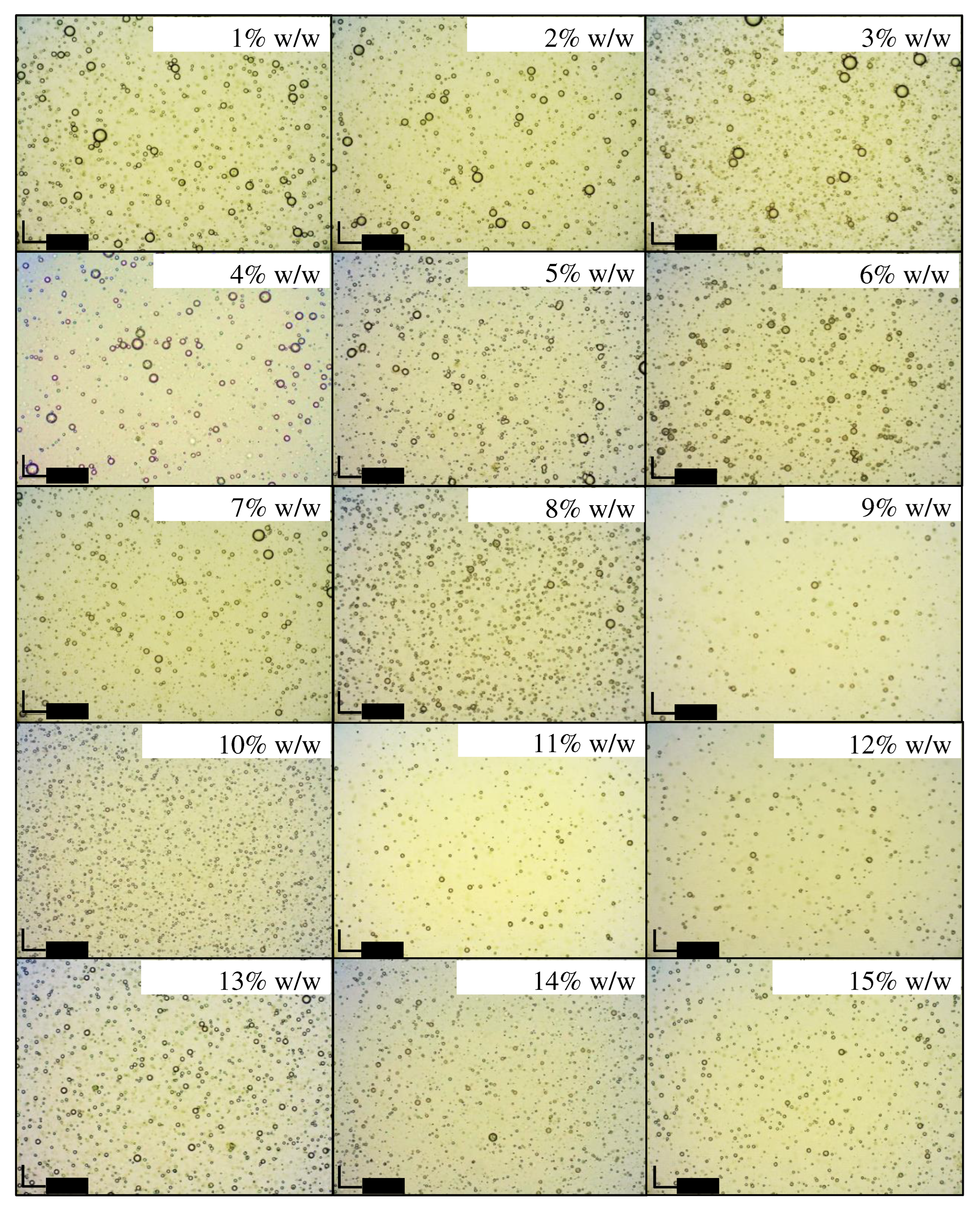
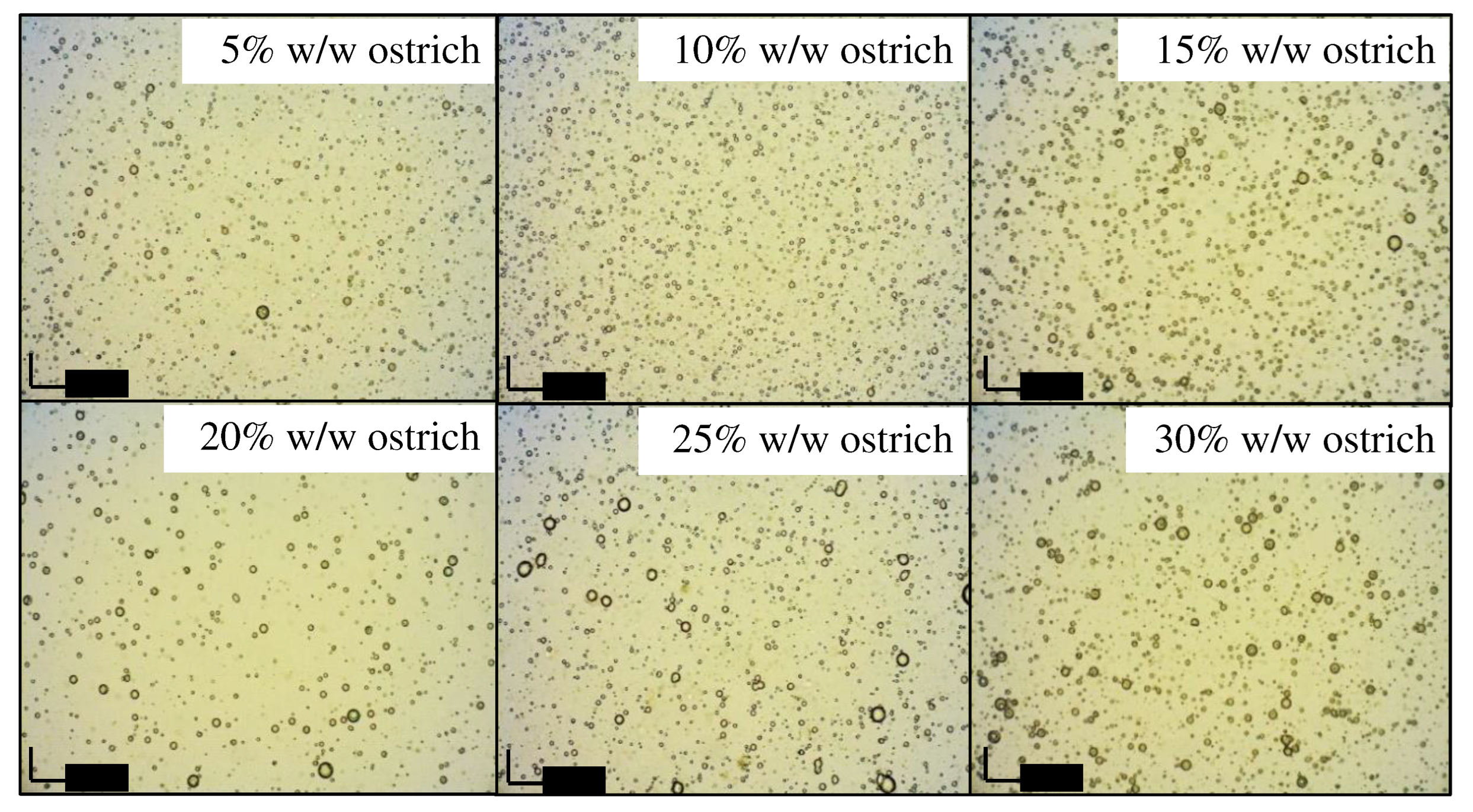
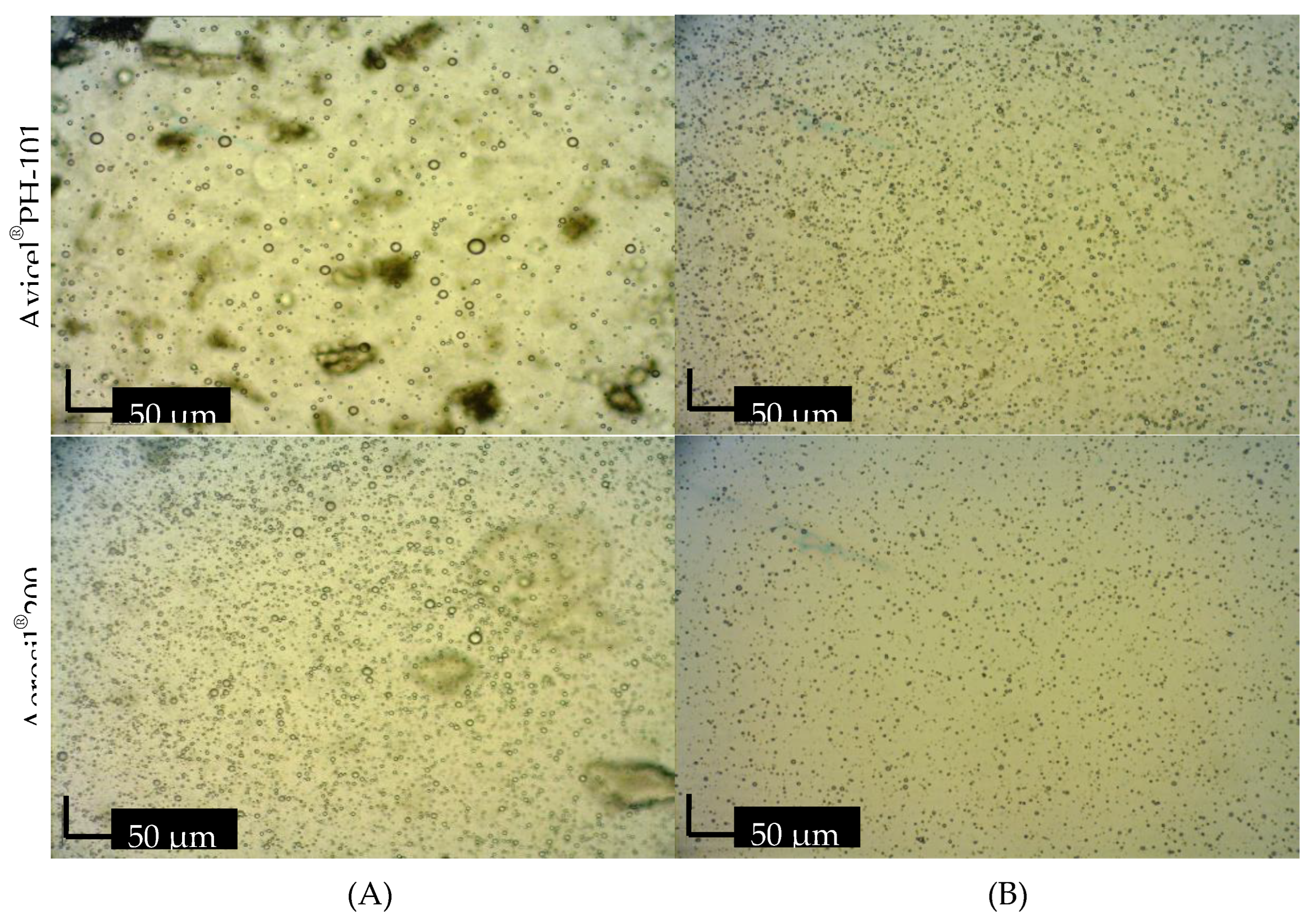
Microscopic examination
Physical characteristics
Droplet size
Zeta potential value
Viscosity
2.4. Fabrication and evaluation of dry emulsions containing ostrich oil
2.4.1. Fabrication of dry emulsions
2.4.2. Evaluation of dry emulsions
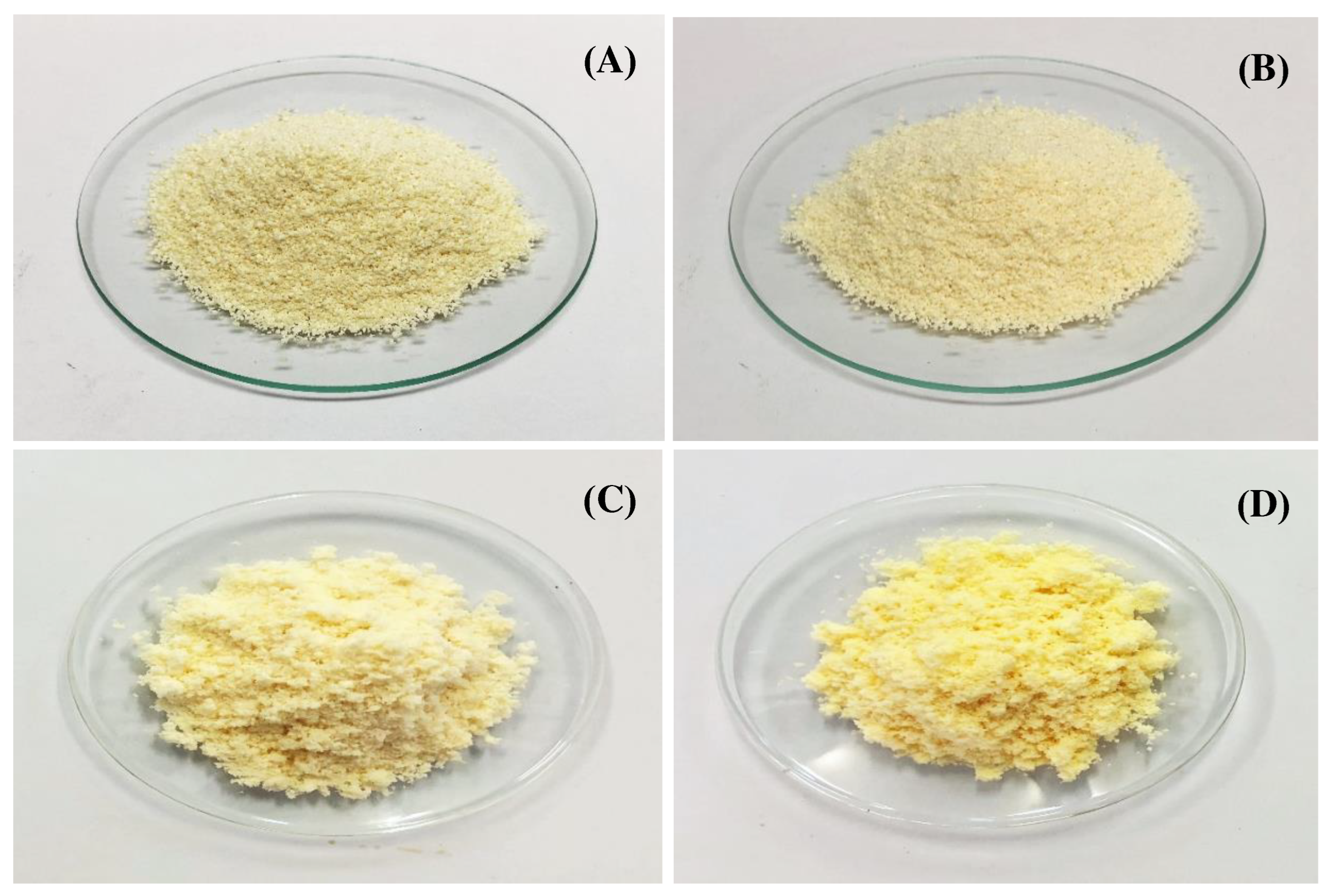
Physical characteristics
Percentage of moisture
Percentage of weight loss after oil release
Microbial contamination
Heavy metal contamination
Particle size analysis
Compressibility index
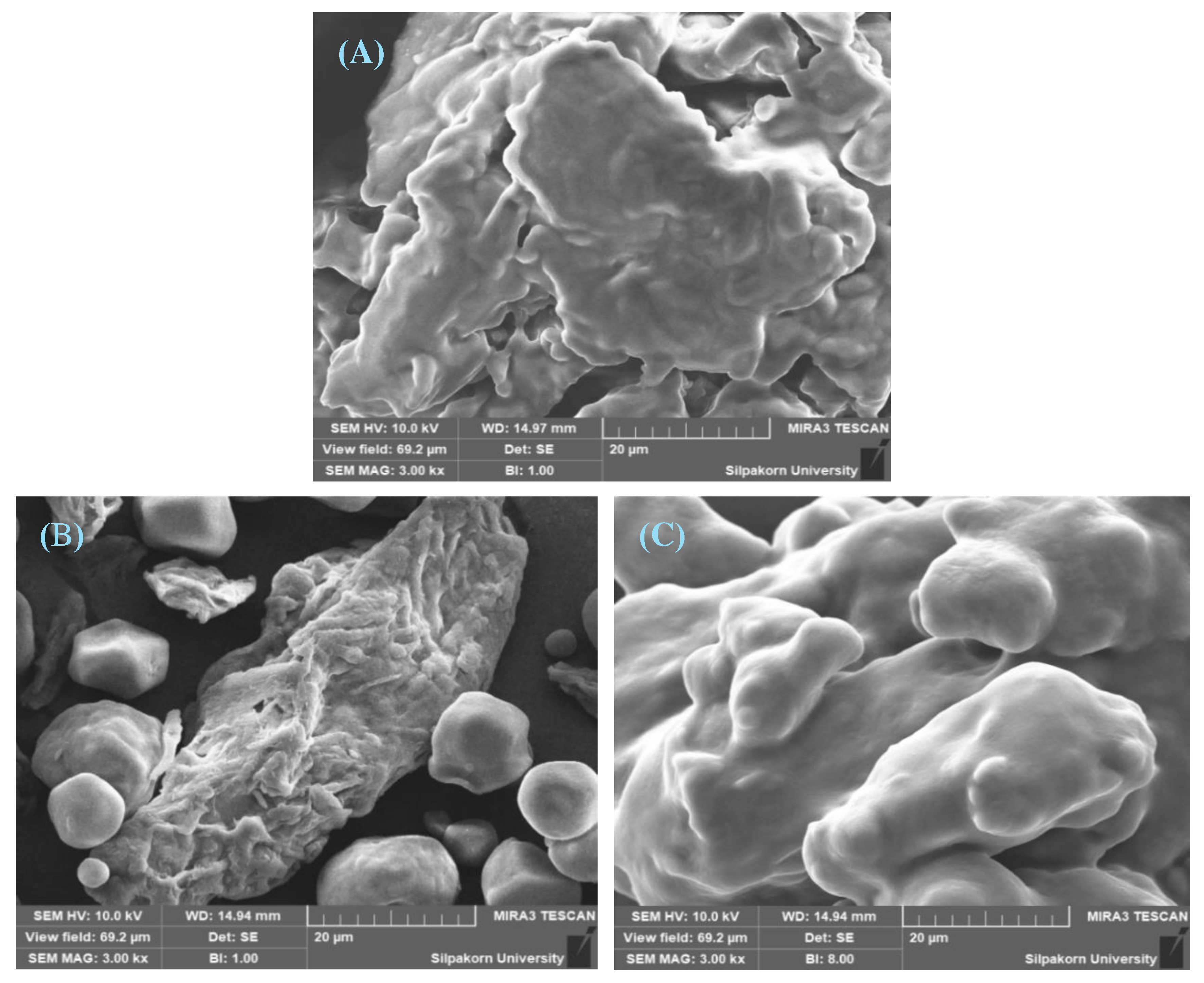
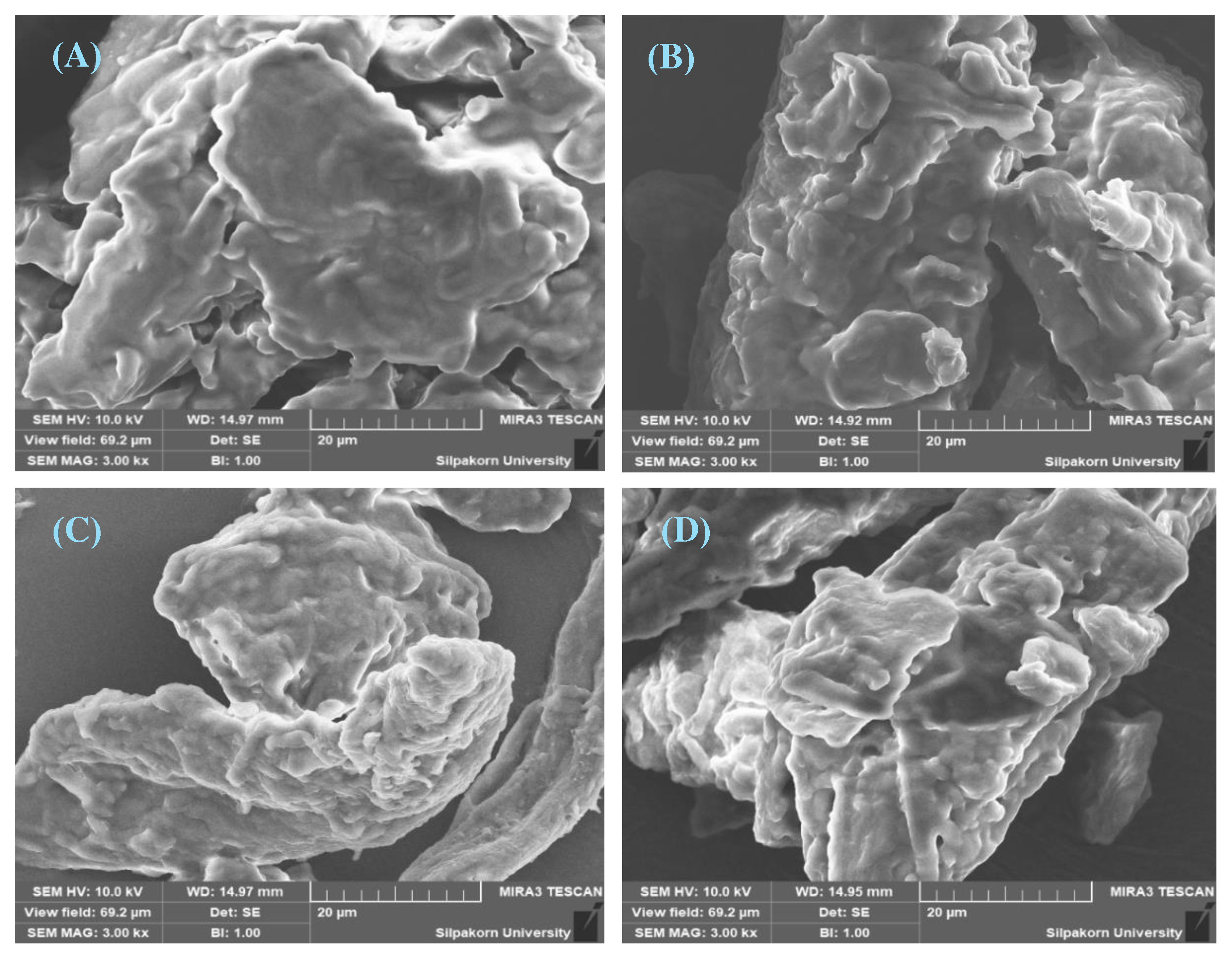
Particle morphology
Disintegration
2.5. Stability
2.5.1. Stability under temperature cycling
2.5.2. Stability under storage at various temperatures
2.6. Statistical analysis
3. Results
3.1. Preparation and evaluation of ostrich oil
3.2. Formulation and evaluation of emulsion containing ostrich oil
3.2.1. Formulation of emulsion
3.2.2. Evaluation of emulsion
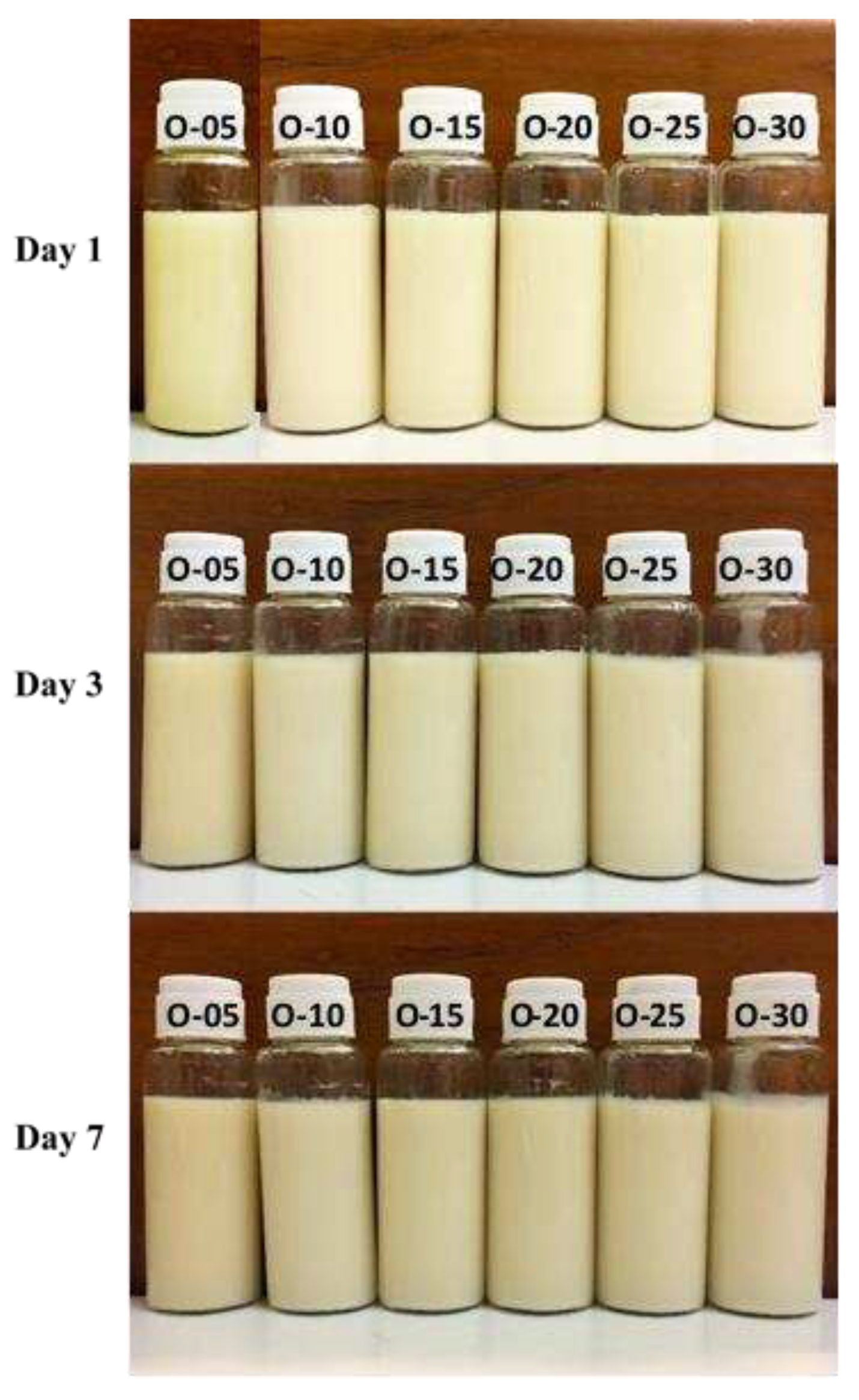
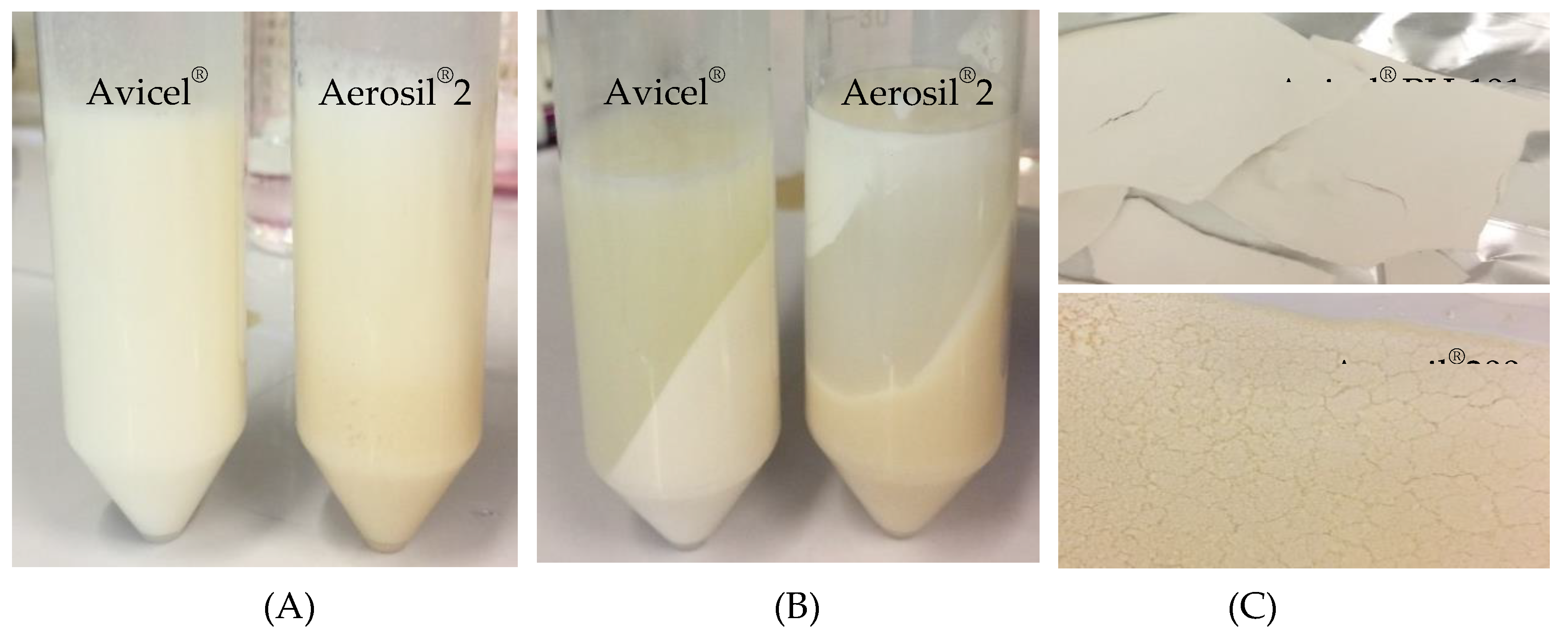
Visual observation and creaming indices
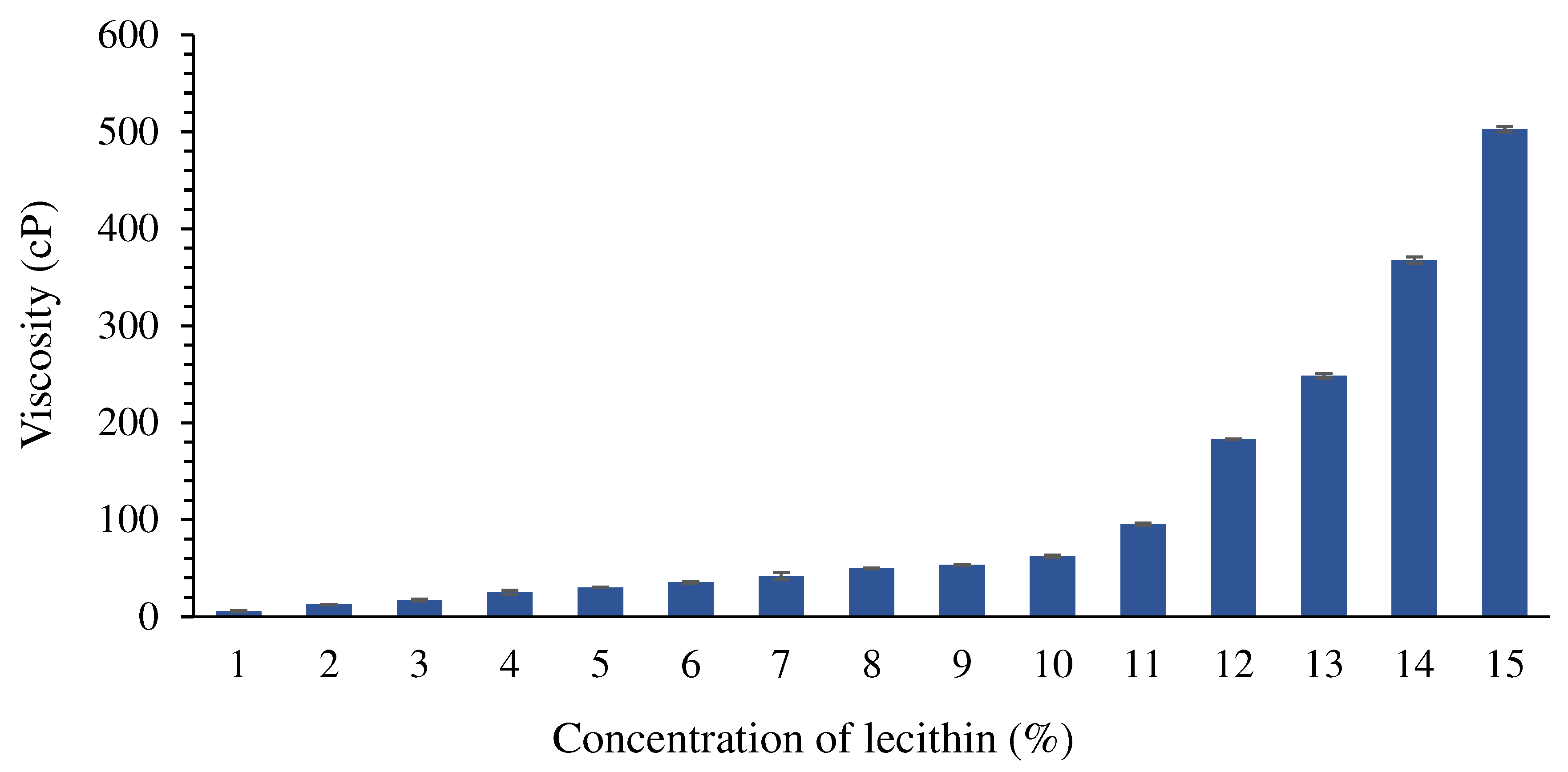
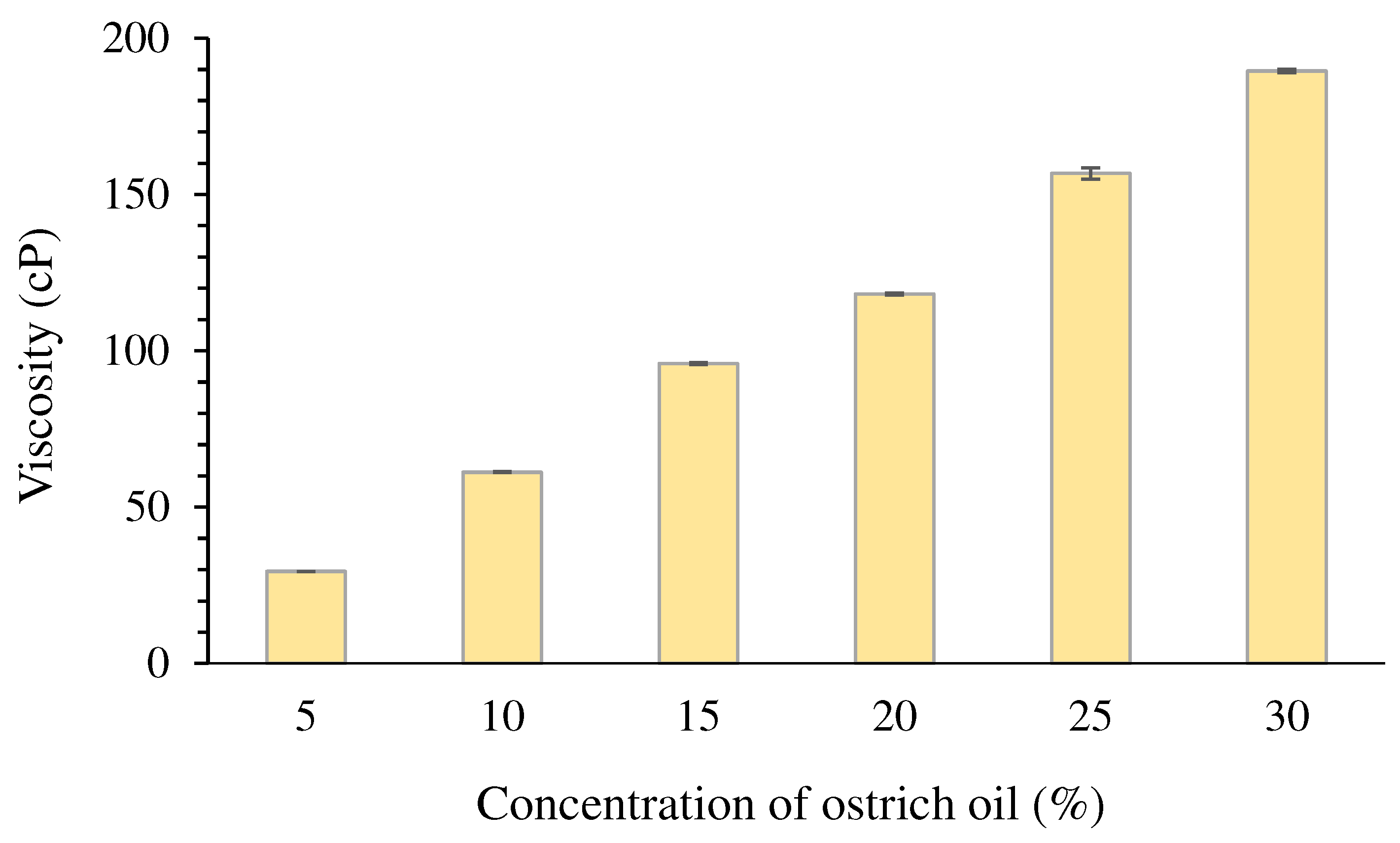
Viscosity
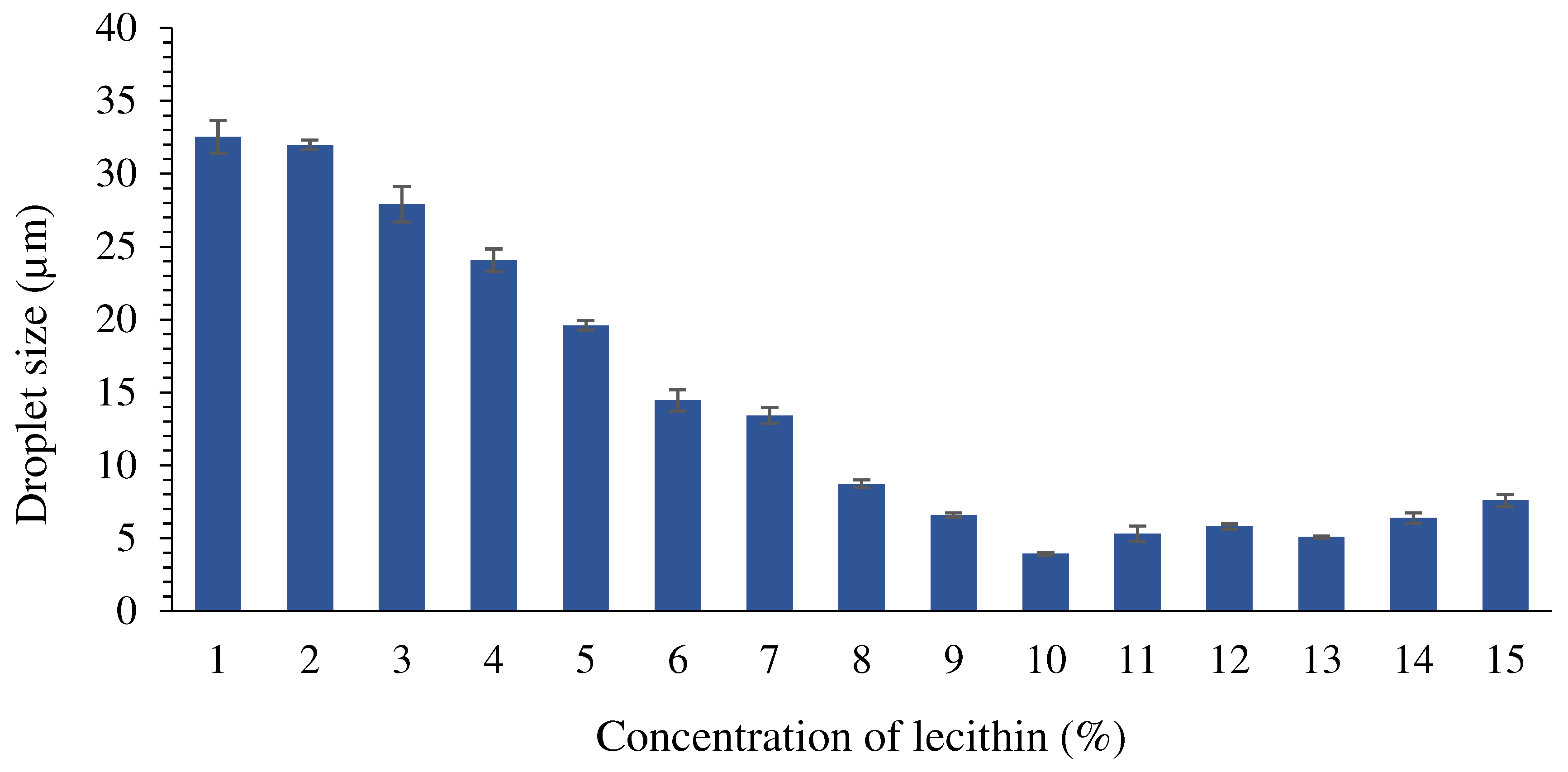
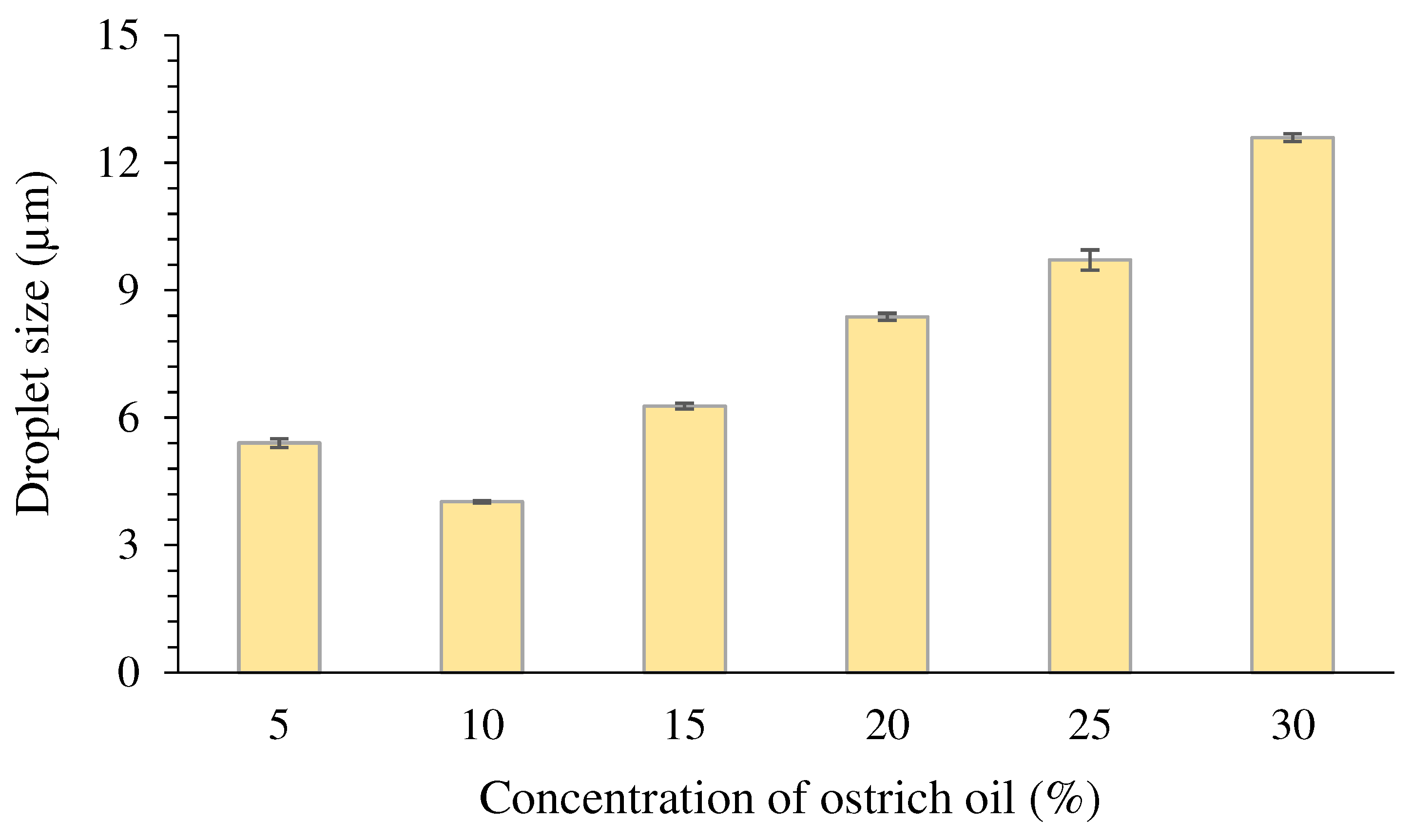
Droplet size
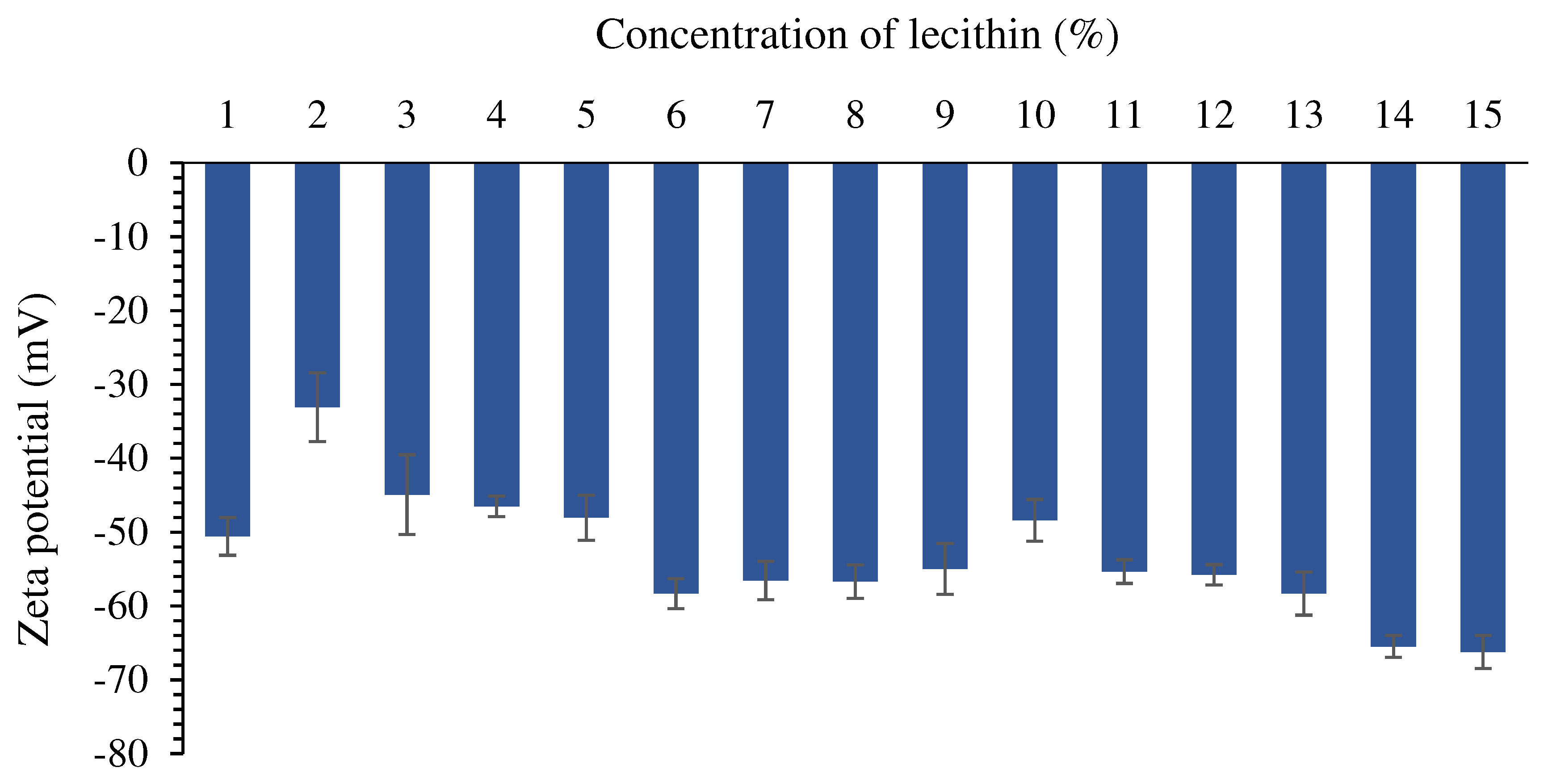
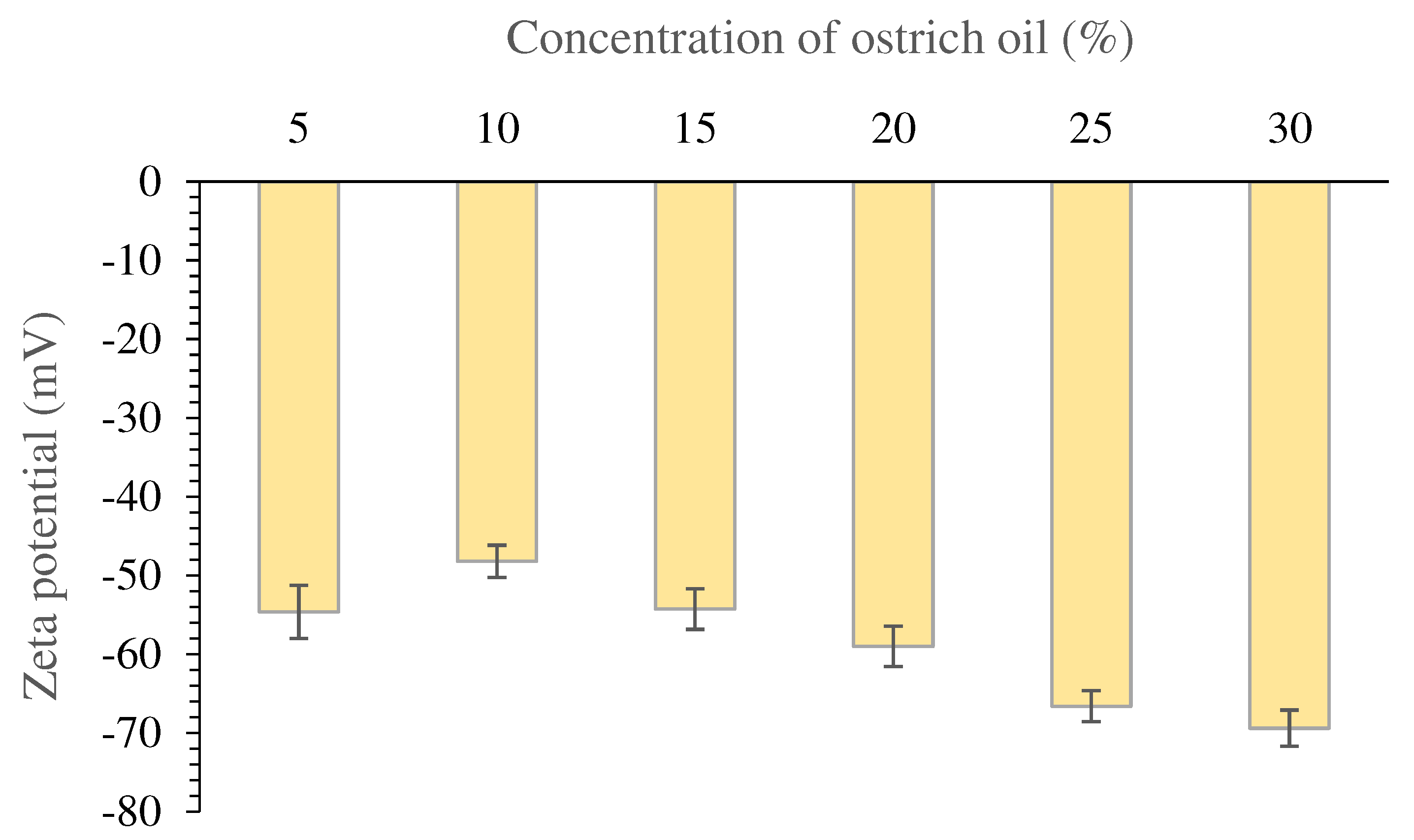
Zeta potential value
3.3. Fabrication and evaluation of dry emulsions containing ostrich oil
3.3.1. Fabrication of dry emulsions
3.3.2. Evaluation of Avicel® PH-101 granules containing ostrich oil emulsion
3.4. Stability
3.4.1. Color and morphological stability
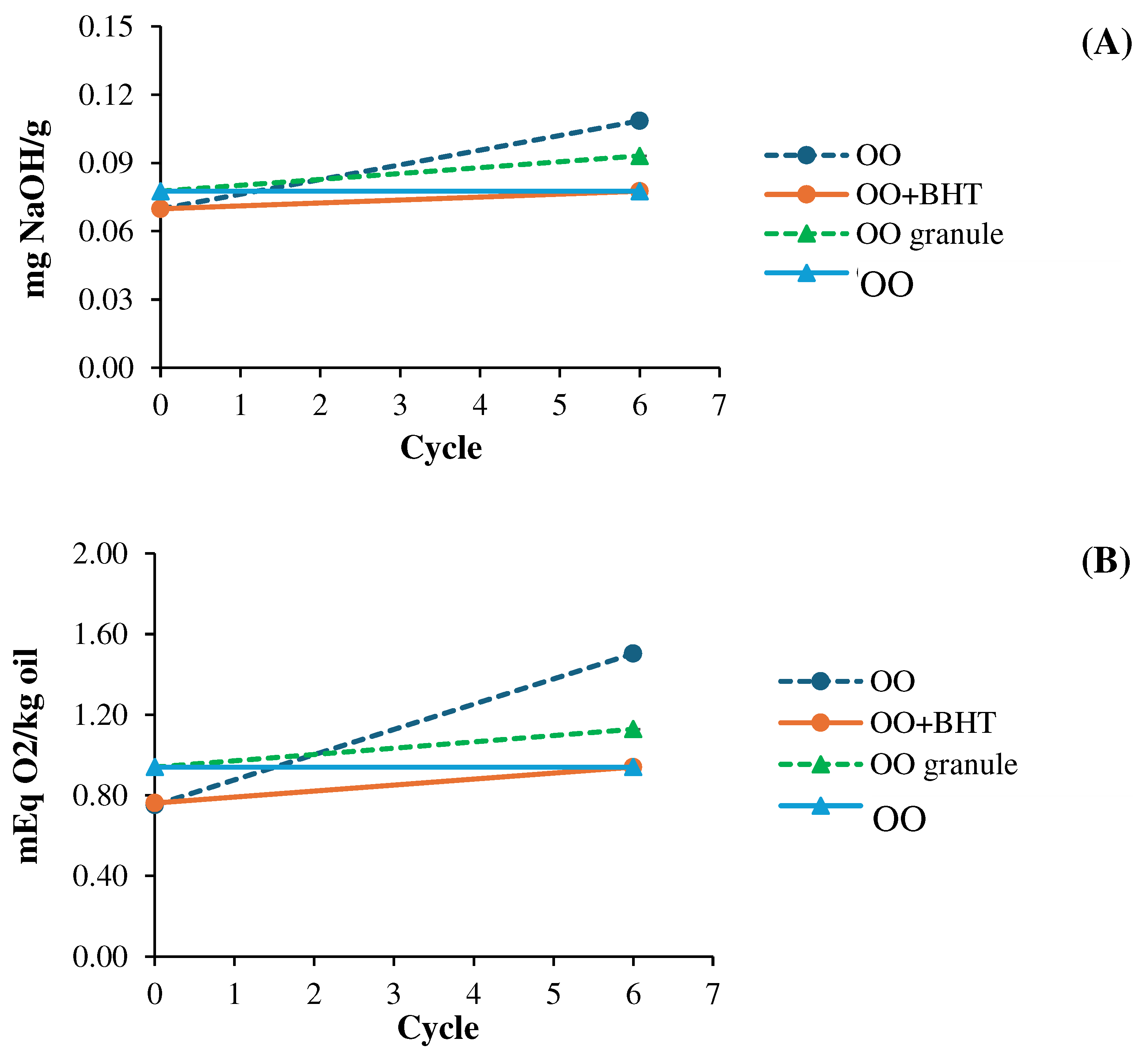
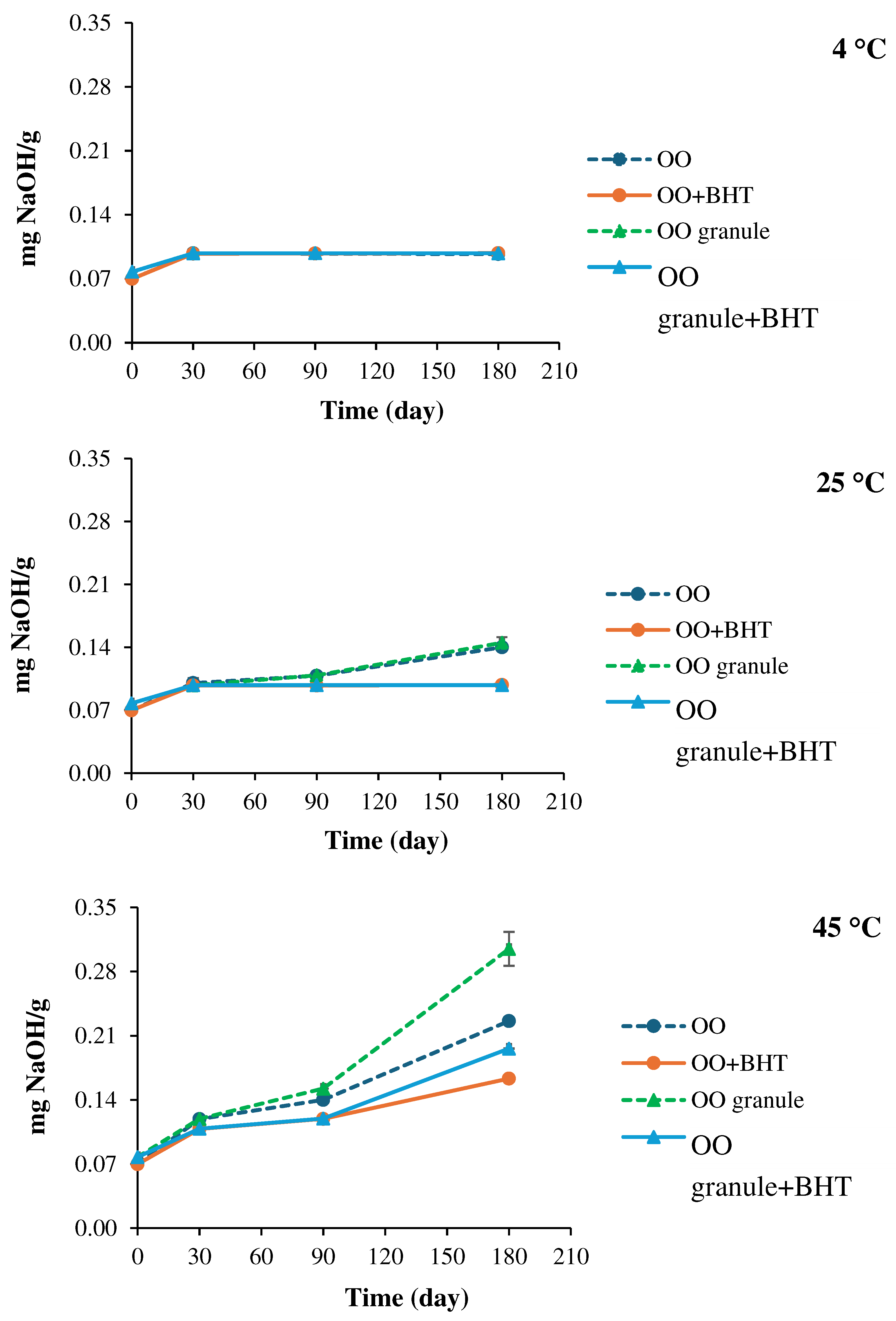
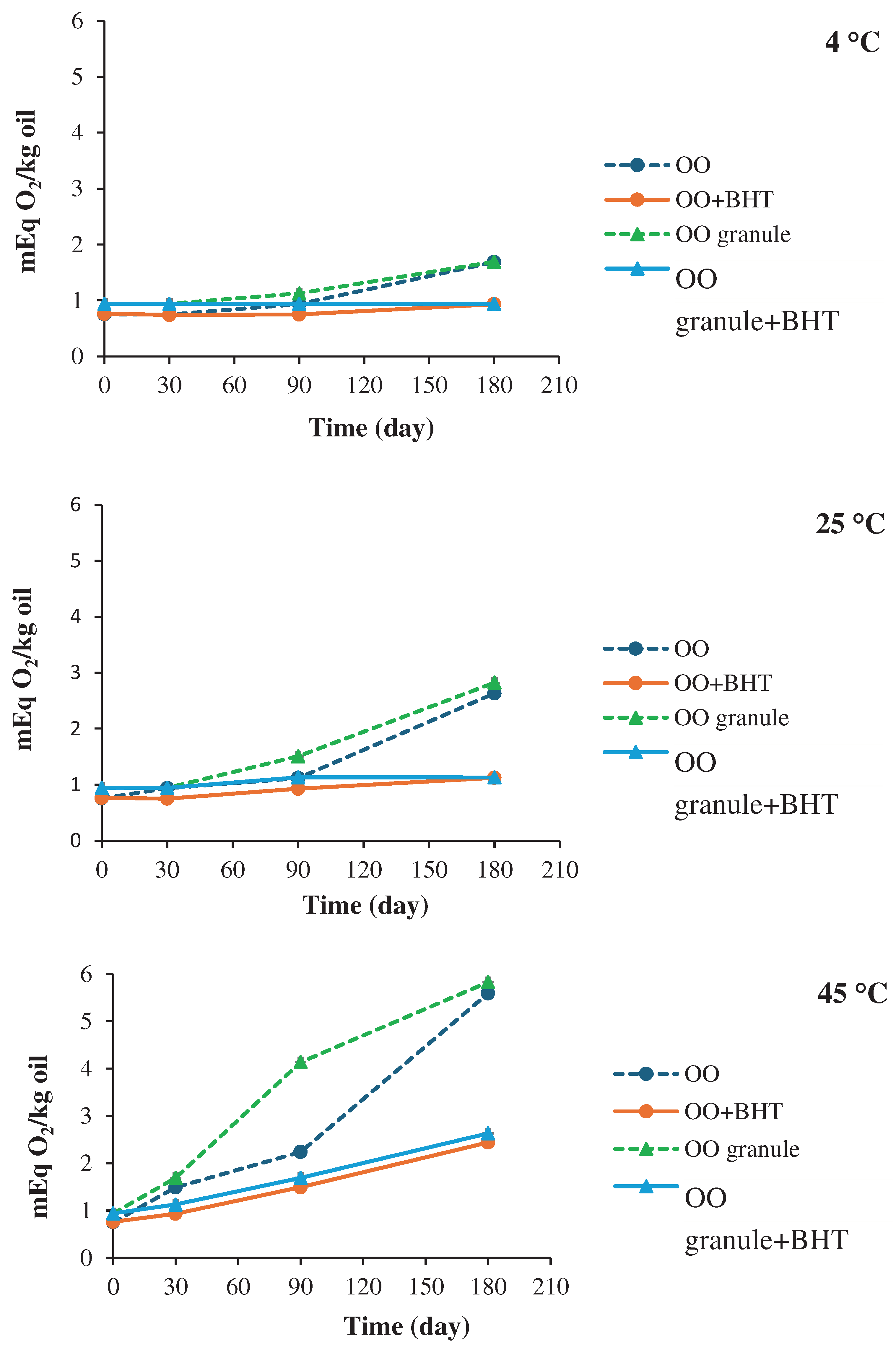
3.4.2. Physicochemical stability
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Brassó, L.D.; Béri, B.; Komlósi, I. Studies on ostrich (Struthio Camelus) – Review. Acta Agrar. Debr. 2020, 1, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Kuzelov, A.; Jordanoski, M.; Gacovski, Z.; Trajcova, D. Economical benefit from Ostrich (Struthio Camelus L.) breeding and primary processing. Maced. J. Anim. Sci. 2012, 2, 89–92. [Google Scholar] [CrossRef]
- Hui, Y. (1996) Bailey’s Industrial Oil and Fat Products. John Wiley and Sons Inc., New York, 281-282.
- Sharma, H.; Giriprasad, R.; Goswami, M. Animal fat-processing and its quality control. J. Food Process. Technol. 2013, 4, 1000252. [Google Scholar] [CrossRef]
- Sugihartono; Rahmawati, D.; Radnawati, E.; Priatni, A. Extraction of fat/oil from fleshing by-product using a wet rendering process and combination with organic solvents. MKKP 2019, 35, 7–16. [Google Scholar] [CrossRef]
- Gavanji, S.; Larki, B.; Taraghian, A.H. A review of application of ostrich oil in pharmacy and diseases treatment. J. Nov. Appl. Sci. 2013, 2, 650–654. [Google Scholar]
- Okparanta, S.; Daminabo, V.; Solomon, L. Assessment of rancidity and other physicochemical properties of edible oils (mustard and corn oils) stored at room temperature. J. Food Nutr. Sci. 2018, 6, 70–75. [Google Scholar] [CrossRef]
- Talbot, G. 24-The stability and shelf life of fats and oils. In Food and Beverage Stability and Shelf Life; Woodhead Publishing Series in Food Science, Technology and Nutrition, 2011, 683-715. [CrossRef]
- Mimica-Dukić, N.; Simin, N.; Svirčev, E.; Orčić, D.; Beara, I.; Lesjak, M.; Božin, B. The effect of plant secondary metabolites on lipid peroxidation and eicosanoid pathway. In Lipid Peroxidation; Catala, A. Ed.; 2012, 193-210. [CrossRef]
- Gresley, A.L.; Ampem, G.; Mars, S.D.; Grootveld, M.; Naughton, D.P. “Real-world” Evaluation of lipid oxidation products and trace metals in french fries from two chain fast-food restaurants. Front Nutr. 2021, 8, 620952. [Google Scholar] [CrossRef]
- Tubino, M.; Aricetti, J.A. A green potentiometric method for determination of the acid number of oils and fats. J. Braz. Chem. Soc. 2013, 24, 1691–1696. [Google Scholar] [CrossRef]
- Sulieman, A.M.E.; Mohammed, M.B.; Ali, A.O. Physicochemical and sensory properties of traditionally and laboratory made ghee (Samin) of the Sudan. Int. J. Food Sci. Nutr. Eng. 2013, 3, 7–11. [Google Scholar]
- Limmatvapirat, C.; Limmatvapirat, S.; Charoenteeraboon, J.; Wessapan, C.; Kumsum, A.; Jenwithayaamornwech, S.; Luangthuwapranit, P. Determination of heavy metals in herbal drinks using ICP-MS. Adv. Mat. Res. 2014, 1060, 199–202. [Google Scholar] [CrossRef]
- Horbańczuk, O.K.; Moczkowska, M.; Marchewka, J.; Atanasov, A.G.; Kurek, M.A. The composition of fatty acids in ostrich meat influenced by the type of packaging and refrigerated storage. Molecules 2019, 24, 4128. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.C.; Leung, G.; Wang, E.; Ma, S.; Lo, B.K.; McElwee, K.J.; Cheng, K.M. Ratite oils promote keratinocyte cell growth and inhibit leukocyte activation. Poult. Sci 2015, 94, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Belichovska, D.; Hajrulai-Musliu, Z.; Uzunov, R.; Belichovska, K.; Arapcheska, M. Fatty acid composition of ostrich (Struthio Camelus) abdominal adipose tissue. Maced. Vet. Rev. 2015, 38, 53–59. [Google Scholar] [CrossRef]
- Tse-Hung Huang; Pei-Wen Wang; Shih-Chun Yang; Wei-Ling Chou; Jia-You Fang. Cosmetic and therapeutic applications of fish oil’s fatty acids on the skin. Mar Drugs. 2018, 16, 256. [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Horbańczuk, J.O.; Malecki, I.; Cooper, R.; Jóźwik, A.; Klewiec, J.; Krzyżewski, J.; Kalifa, H.; Chyliński, W.; Wójcik, A.; Kawka, M. Cholesterol content and fatty acid composition of two fat depots from slaughter ostriches (Struthio camelus) aged 14 months. Anim. Sci. Pap. Rep. 2004, 22, 247–251. [Google Scholar]
- Zhang, W.; Hao, J.; Yuan, Y.; Xum, D. Influence of carboxymethyl cellulose on the stability, rheological property, and in-vitro digestion of soy protein isolate (SPI)-stabilized rice bran oil emulsion. Front. Nutr. 2022, 9, 878725. [Google Scholar] [CrossRef]
- Usaid, A.A.; Premkumar, J.; Rangganathan, T. Emulsion and it’s applications in food processing - a review. Int J Eng Res Appl. 2014, 4, 241–248. [Google Scholar]
- Tian, Y.; Zhou, J.; He, C.; He, L.; Li, X.; Sui, H. The formation, stabilization and separation of oil-water emulsions: A review. Processes 2022, 10, 738. [Google Scholar] [CrossRef]
- Mirzanajafi-Zanjani, M.; Yousefi, M.; Ehsani, A. Challenges and approaches for production of a healthy and functional mayonnaise sauce. Food Sci Nutr. 2019, 7, 2471–2484. [Google Scholar] [CrossRef]
- Nasr, N.E.H.; ElMeshad, A.N.; Fares, A.R. Nanocarrier systems in taste masking. Sci. Pharm. 2022, 90, 20. [Google Scholar] [CrossRef]
- Marhamati, M.; Ranjbar, G.; Rezaie, M. Effects of emulsifiers on the physicochemical stability of oil-in-water nanoemulsions: A critical review. J. Mol. Liq. 2021, 340, 117218. [Google Scholar] [CrossRef]
- Khan, B.A.; Akhtar, N.; Khan, H.M.S.; Waseem, K.; Mahmood, T.A.; Rasul, M.; Iqbal, M.; Khan, H. Basics of pharmaceutical emulsions: A review. Afr. J. Pharm. Pharmacol. 2011, 5, 2715–2725. [Google Scholar] [CrossRef]
- Berton-Carabin, C.C.; Ropers, M.H.; Genot, C. Lipid oxidation in oil-in-water emulsions: involvement of the interfacial layer. Compr. Rev. Food Sci. Food Saf. 2014, 13, 945–977. [Google Scholar] [CrossRef]
- Scholfield, C.R. Composition of soybean lecithin. J. Am. Oil Chem. Soc. 1981, 58, 889–892. [Google Scholar] [CrossRef]
- Honda, K.; Enoshima, T.; Oshikata, T.; Kamiya, K.; Hamamura, M.; Yamaguchi, N.; Nakamura, K.; Oguma, Y.; Fujiwara, S.; Takabe, M. Toxicity studies of Asahi Kasei PI, purified phosphatidylinositol from soy lecithin. J. Toxicol. Sci. 2009, 34, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Turchiuli, C.; Gallottia, F.; Hernandez-Sancheza, M.R.; Cuveliera, M. Improvement of oxidative stability of dry emulsion containing antioxidants by modifying process conditions. Chem. Eng. Trans. 2017, 57, 1915–1920. [Google Scholar]
- Lu, W.; Maidannyk, V.; Kelly, A.L.; Miao, S. Fabrication and characterization of highly re-dispersible dry emulsions. Food Hydrocoll. 2020, 102, 105617. [Google Scholar] [CrossRef]
- Plati, F.; Matsakidou, A.; Kiosseoglou, V.; Paraskevopoulou, A. Development of a dehydrated dressing-type emulsion with instant powder characteristics. Food Struct. 2019, 20, 100110. [Google Scholar] [CrossRef]
- Yacob Baraki, S.; Liu, L.; Li, X.; Debeli, D.K.; Wang, B.; Feng, X.; Mao, Z.; Sui, X. Re-dispersible dry sunflower oil emulsions enabled by regenerated chitin. LWT 2021, 149, 111892. [Google Scholar] [CrossRef]
- Haritha, M.; Priyanka, M.; Aqther, A.; Neeharika, R.; Kumar, P.B. Dry emulsion: a promising dosage form to deliver lipophilic drug molecules with improved stability and effectiveness. IJRPB 2013, 1, 119–121. [Google Scholar]
- Shaw, L.A.; McClements, D.J.; Decker, E.A. Spray-dried multilayered emulsions as a delivery method for ω-3 fatty acids into food systems. J. Agric. Food Chem 2007, 55, 3112–3119. [Google Scholar] [CrossRef]
- Kaur, A.; Chand, B.; Kamal, A.S. Development and evaluation of dry adsorbed emulsion for extended release of niacinamide. Int. J. Adv. Pharm., Biol. Chem. 2013, 2, 291–306. [Google Scholar]
- Vidya, D.; Shubhangi, S.; Magdum, C.; Mohite, S.; Nitalikar, M. A review: Dry emulsion. Asian J. Pharm. Res. 2015, 5, 208–210. [Google Scholar] [CrossRef]
- Vehovec, T.; Gartner, A.; Planinšek, O.; Obreza, A. Influence of different types of commercially available microcrystalline cellulose on degradation of perindopril erbumine and enalapril maleate in binary mixtures. Acta Pharm. 2012, 62, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Hindi, S.S.Z. Microcrystalline cellulose: The inexhaustible treasure for pharmaceutical industry. Nanosci. Nanotechnol. Res. 2017, 4, 17–24. [Google Scholar]
- Saigal, N.; Baboota, S.; Ahuja, A.; Ali, J. Microcrystalline cellulose as a versatile excipient in drug research. J. Young Pharm. 2009, 1, 6–12. [Google Scholar]
- Krstić, M.; Medarević, D.; Duriš, J.; Ibrić, S. Chapter 12 - Self-nanoemulsifying drug delivery systems (SNEDDS) and self-microemulsifying drug delivery systems (SMEDDS) as lipid nanocarriers for improving dissolution rate and bioavailability of poorly soluble drugs. Lipid Nanocarriers for Drug Targeting 2018, pp. 473-508. [CrossRef]
- Albertini, B.; Passerini, N.; González-Rodríguez, M. L.; Perissutti, B.; Rodriguez, L. Effect of Aerosil® on the properties of lipid controlled release microparticles. J. Control. Release 2004, 100, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Patel, C.; Patel, B.; Thakkar, H. Formulation and evaluation of raloxifene hydrochloride dry emulsion tablet using solid carrier adsorption technique. Ther. Deliv. 2021, 12, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.R.; Belhekar, S.N.; Mehetre, J.S. Enhancement of aqueous solubility and oral bioavailability of Bcs class II drug by dry emulsion. Int. J. Pharmacogn. Chinese Med. 2021, 5, 000218. [Google Scholar] [CrossRef]
- Dixit, R.P.; Nagarsenker, M.S. Dry adsorbed emulsion of simvastatin: Optimization and in vivo advantage. Pharm. Dev. Technol. 2007, 12, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Dange, V.; Shid, S.; Magdum, C.S.; Mohite, S.K.; Nitalikar, M.M. A review: Dry emulsion. Asian J. Pharm. Res. 2015, 5, 208–210. [Google Scholar] [CrossRef]
- Anwar, S.H.; Kunz, B. The influence of drying methods on the stabilization of fish oil microcapsules: Comparison of spray granulation, spray drying, and freeze drying. J. Food Eng. 2011, 105, 367–378. [Google Scholar] [CrossRef]
- Poullain-Termeau, S.; Crauste-Manciet, S.; Brossard, D.; Muhamed, S.; Nicolaos, G. Effect of oil-in-water submicron emulsion surface chargeon oral absorption of a poorly water-soluble drug in rats. Drug Deliv. 2008, 15, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Teixé-Roig, J.; Oms-Oliu, G.; Odriozola-Serrano, I.; Martín-Belloso, O. Emulsion-based delivery systems to enhance the functionality of bioactive compounds: Towards the use of ingredients from natural, sustainable sources. Foods 2023, 12, 1502. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.; Holmes, P.; Schultz, K. Process characteristics and compaction of spray dried emulsions containing a drug dissolved in lipid. Int. J. Pharm. 2004, 287, 55–66. [Google Scholar] [CrossRef]
- Palanisamy, U.D.; Sivanathan, M.; Subramaniam, T.; Radhakrishnan, A.K.; Haleagrahara, N.; Sundralingam, U.; Chiew, G.S. Refining ostrich oil and its stabilization with curcumin. J. Nutr. Health Food Eng. 2015, 2, 63–69. [Google Scholar] [CrossRef]
- Khan, B. A.; Akhtar, N.; Khan, H.M.S.; Waseem, K.; Mahmood, T.; Rasul, A.; Iqbal, M.; Khan, H. Basics of pharmaceutical emulsions: A review. Afr. J. Pharm. Pharmacol. 2011, 5, 2715–2725. [Google Scholar] [CrossRef]
- Fuentes, K.; Matamala, C.; Martínez, N.; Zúñiga, R.N.; Troncoso, E. Comparative study of physicochemical properties of nanoemulsions fabricated with natural and synthetic surfactants. Processes 2021, 9, 2002. [Google Scholar] [CrossRef]
- Joseph, C.; Savoire, R.; Harscoat-Schiavo, C.; Pintori, D.; Monteil, J.; Faure, C.; Leal-Calderon, F. Redispersible dry emulsions stabilized by plant material: Rapeseed press-cake or cocoa powder. LWT-Food Sci. Technol. 2019, 113, 108311. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A.; Weiss, J. Emulsion-based delivery systems for lipophilic bioactive components. J. Food Sci. 2007, 72, R109–R124. [Google Scholar] [CrossRef] [PubMed]
- Ghelichi, S.; Hajfathalian, M.; Yesiltas, B.; Sørensen, A.M.; García-Moreno, P.J.; Jacobsen, C. Oxidation and oxidative stability in emulsions. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1864–1901. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, H.C.; Tonon, R.V.; Grosso, C.R.; Hubinger, M.D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef]
- Chambin, O.; Bellone, C.; Champion, D.; Rochat-Gonthier, M.H.; Pourcelot, Y. Dry adsorbed emulsion: 1 Characterization of an intricate physicochemical structure. J. Pharm. Sci. 2000, 89, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Ponphaiboon, J.; Limmatvapirat, S.; Chaidedgumjorn, A.; Limmatvapirat, C. Physicochemical property, fatty acid composition, and antioxidant activity of ostrich oils using different rendering methods. LWT-Food Sci. Technol. 2018, 93, 45–50. [Google Scholar] [CrossRef]
- Limmatvapirat, C.; Limmatvapirat, S.; Chansatidkosol, S.; Krongrawa, W.; Liampipat, N.; Leechaiwat, S.; Lamaisri, P.; Siangjong, L.; Meetam, P.; Tiankittumrong, K. Preparation and properties of anti-nail-biting lacquers containing shellac and bitter herbal extract. International J. Polym. Sci. 2021, 8537544. [Google Scholar] [CrossRef]
- Limmatvapirat, C.; Limmatvapirat, S.; Krongrawa, W.; Ponphaiboon, J.; Witchuchai, T.; Jiranuruxwong, P.; Theppitakpong, P.; Pathomcharoensukchai, P. Beef tallow: Extraction, physicochemical property, fatty acid composition, antioxidant activity, and formulation of lotion bars. J. Appl. Pharm. Sci. 2021, 11, 018–028. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, J.; Tang, X.; Xiong, Y.L. Reducing, radical scavenging, and chelation properties of in vitro digests of alcalase-treated zein hydrolysate. J. Agric. Food Chem. 2008, 56, 2714–2721. [Google Scholar] [CrossRef]
- AOCS. (2017). Method Cd 3d-63: Acid value of fats and oils. Official methods and recommended practices of the AOCS, American Oil Chemists’ Society, American Oil Chemists’ Society.
- AOCS. (2017). Method Cd 8b-90: Peroxide value of fats and oils, Acetic acid, isooctane method. Official methods and recommended practices of the AOCS, American Oil Chemists’ Society, American Oil Chemists’ Society.
- AOCS. (2017). Method Cd 1d-92: Iodine value of fats and oils, Cyclohexane-acetic acid method. Official methods and recommended practices of the AOCS, American Oil Chemists’ Society, American Oil Chemists’ Society.
- AOCS. (2017). Method Cd 3-25: Saponification value of fats and oils. Official methods and recommended practices of the AOCS, American Oil Chemists’ Society, American Oil Chemists’ Society.
- Limmatvapirat, C.; Limmatvapirat, S.; Charoenteeraboon, J.; Wessapan, C.; Kumsum, A.; Jenwithayaamornwech, S.; Luangthuwapranit, P. Comparison of eleven heavy metals in Moringa oleifera Lam. products. Indian J. Pharm. Sci. 2015, 77, 485–490. [Google Scholar] [CrossRef]
- The United States Pharmacopeial Convention. 〈61〉 Microbiological examination of nonsterile products: Microbial enumeration tests. The United States Pharmacopeia 43 and the National Formulary 38 (USP 43-NF 38), 2023. [CrossRef]
- Miller, D.J.; Henning, T.; Grünbein, W. Phase inversion of W/O emulsions by adding hydrophilic surfactant - a technique for making cosmetics products. Colloids Surf. A: Physicochem. Eng. Asp. 2001, 183, 681–688. [Google Scholar] [CrossRef]
- The United States Pharmacopeial Convention. 〈616〉 Bulk density and tapped density of powders. The United States Pharmacopeia 43 and the National Formulary 38 (USP 43-NF 38), 2023. [CrossRef]
- The United States Pharmacopeial Convention. 〈701〉 Disintegration. The United States Pharmacopeia 43 and the National Formulary 38 (USP 43-NF 38), 2023. [CrossRef]
- FAO/WHO. (2001). Codex standard for fats and oils from animal sources, Codex Standard for Named Animal Fats (CODEX-STAN 211-1999), Codex Alimentarius: Fats, oils and related products, Food and Agriculture Organization of the United Nations, Rome, Italy.
- Abdelhadi, O.M.A.; Babiker, S.A.; Bauchart, D.; Listrat, A.; Remond, D.; Hocquette, J.F.; Faye, B. Effect of gender on quality and nutritive value of dromedary camel (Camelus dromedarius) longissimus lumborum muscle. J. Saudi Soc. Agric. Sci. 2017, 16, 242–249. [Google Scholar] [CrossRef]
- Cabezas, D.M.; Diehl, B.W.K.; Tomás, M.C. Emulsifying properties of hydrolysed and low HLB sunflower lecithin mixtures. Eur J Lipid Sci Technol. 2016, 118, 975–983. [Google Scholar] [CrossRef]
- Fedotova, Y.; Lencki, R.W. The effect of phospholipids on butter physical and sensory properties. J. Am. Oil Chem. Soc. 2010, 87, 75–82. [Google Scholar] [CrossRef]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; Leblanc, J.; Lindtner, O.; Moldeus, P.; Mosesso, P.; Oskarsson, A.; Parent-Massin, D.; Stankovic, I.; Waalkens-Berendsen, I.; Woutersen, R. A.; Wright, M.; Younes, M.; Brimer, L.; Altieri, A.; Christodoulidou, A.; Lodi, F.; Dusemund, B. Re-evaluation of lecithins (E 322) as a food additive. EFSA Journal 2017, 15, 1–74. [Google Scholar] [CrossRef]
- Sui X, Bi S, Qi B, Wang Z, Zhang M, Li Y et al., Impact of ultrasonic treatment on an emulsion system stabilized with soybean protein isolate and lecithin: its emulsifying property and emulsion stability. Food Hydrocoll. 2017, 63, 727–734. [CrossRef]
- Preziosi, V.; Perazzo, A.; Caserta, S.; Tomaiuolo, G.; Guido, S. Phase inversion emulsification. Chem. Eng. Trans. 2013, 32, 1585–1590. [Google Scholar] [CrossRef]
- Sherman, P. The influence of emulsifying agent concentration on emulsion viscosity. Colloid Polym. Sci. 1959, 165, 156–161. [Google Scholar] [CrossRef]
- Ramisetty, K.A.; Shyamsunder, R. Effect of ultrasonication on stability of oil in water emulsions. Int. J. Drug Deliv. 2011, 3, 133–142. [Google Scholar] [CrossRef]
- Bhatt, N.; Prasad, R.K.; Singh, K.; Panpalia, G.M. Stability study of O/W emulsions using zeta potential. J. Chem. Pharm. Res. 2010, 2, 512–527. [Google Scholar]
- Fatfat, Z.; Karam, M.; Maatouk, B.; Fahs, D.; Gali-Muhtasib, H. Chapter 7 - Nanoliposomes as safe and efficient drug delivery nanovesicles. Advanced and Modern Approaches for Drug Delivery. 2023, pp.159-197. [CrossRef]
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrard, B. Microcrystalline cellulose, a direct compression binder in a quality by design environment - A review. Int. J. Pharm. 2014, 473, 64–72. [Google Scholar] [CrossRef]
- Van Eerdenbrugh, B.; Froyen, L.; Van Humbeeck, J.; Martens, J.A.; Augustijns, P.; Van Den Mooter, G. Alternative matrix formers for nanosuspension solidification: dissolution performance and X-ray microanalysis as an evaluation tool for powder dispersion. Eur. J. Pharm. Sci. 2008, 35, 344–353. [Google Scholar] [CrossRef]
- Cho, S.; Lee, J.; Yoo, Y.; Cho, M.; Sohn, S.; Lee, B. Improved manufacturability and in vivo comparative pharmacokinetics of dapagliflozin cocrystals in beagle dogs and human volunteers. Pharmaceutics 2021, 13, 70. [Google Scholar] [CrossRef]
- Ali, H.H.; Hussein, A.A. Oral solid self-nanoemulsifying drug delivery systems of candesartan citexetil: formulation, characterization and in vitro drug release studies. AAPS 2017, 3, 6. [Google Scholar] [CrossRef]
- Pedersen, G.P.; Fäldt, P.; Bergenståhl, B.; Kristensen, H.G. Solid state characterisation of a dry emulsion: a potential drug delivery system. Int. J. Pharm. 1998, 171, 257–270. [Google Scholar] [CrossRef]
- Taboada, M.L.; Heiden-Hecht, T.; Brückner-Gühmann, M.; Karbstein, H.P.; Drusch, S.; Gaukel, V. Spray drying of emulsions: Influence of the emulsifier system on changes in oil droplet size during the drying step. J. Food Process. Preserv. 2021, 45, e15753. [Google Scholar] [CrossRef]
- Pohlen, M.; Pirker, L.; Dreu, R. The potential of macroporous silica-nanocrystalline cellulose combination for formulating dry emulsion systems with improved flow properties: A DoE study. Pharmaceutics 2021, 13, 1177. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.L.; Pedersen, G.P.; Kristensen, H.G. Preparation of redispersible dry emulsions by spray drying. Int. J. Pharm. 2001, 212, 187–194. [Google Scholar] [CrossRef]
- Patel, V.; Patel, C.; Patel, B.; Thakkar, H. Formulation and evaluation of raloxifene hydrochloride dry emulsion tablet using solid carrier adsorption technique. Ther. Deliv. 2021, 12, 539–552. [Google Scholar] [CrossRef]
- Whitby, C.P. Structuring edible oils with fumed silica particles. Front. Sustain. Food Syst. 2020, 4, 585160. [Google Scholar] [CrossRef]
- The United States Pharmacopeial Convention. 〈1174〉 Powder flow. The United States Pharmacopeia 43 and the National Formulary 38 (USP 43-NF 38), 2023. [CrossRef]
- The United States Pharmacopeial Convention. 〈2040〉 Disintegration and dissolution of dietary supplements. The United States Pharmacopeia 43 and the National Formulary 38 (USP 43-NF 38), 2023. [CrossRef]
- The United States Pharmacopeial Convention. 〈2232〉 Element contaminants in dietary supplements. The United States Pharmacopeia 43 and the National Formulary 38 (USP 43-NF 38), 2023. [CrossRef]
- The United States Pharmacopeial Convention. 〈2023〉 Microbiological attributes of nonsterile nutritional and dietary supplements. The United States Pharmacopeia 43 and the National Formulary 38 (USP 43-NF 38), 2023. [CrossRef]
- Lin, S.S.; Hsieh, A.L.; Min, D.B.S.; Chang, S.S. A study of the color stability of commercial oleic acid. JAOCS 1976, 53, 157–161. [Google Scholar] [CrossRef]
- Belović, M.M.; Mastilović, J.S.; Kevrešan, Ž.S. Change of surface colour parameters during storage of paprika (Capsicum annuum L.). Food Feed Res. 2014, 41, 85–92. [Google Scholar] [CrossRef]
- Pignitter, M.; Somoza, V. Critical evaluation of methods for the measurement of oxidative rancidity in vegetable oils. J. Food Drug Anal. 2012, 20, 772–777. [Google Scholar] [CrossRef]
- Pragasam, A.; Prithvi, J.; Majalikar, P.; Tallur, P.N.; Naik, V.M. Secondary anti-oxidative effect of soya lecithin in bulk soya bean oil. Chem. Sci. Rev. Lett. 2018, 7, 892–899. [Google Scholar]
- United States Pharmacopeial Convention. 〈2800〉 Multi-ingredient dietary supplement products-product quality tests. The United States Pharmacopeia 43 and the National Formulary 38 (USP 43-NF 38), 2023. [CrossRef]
- The United States Pharmacopeial Convention. Fish oil containing omega-3 acids capsules. The United States Pharmacopeia 43 and the National Formulary 38 (USP 43-NF 38), 2023. [CrossRef]
- The United States Pharmacopeial Convention. Cod liver oil capsules. The United States Pharmacopeia 43 and the National Formulary 38 (USP 43-NF 38), 2023. [CrossRef]
- Global Organization for EPA and DHA (GOED). Voluntary Monograph; Version 8.1, Salt Lake City, Utah, USA, 2022.
- Moustiés, C.; Bourlieu-Lacanal, C.; Hemery, Y.M.; Baréa, B.; Villeneuve, P.; Servent, A.; Alter, P.; Lebrun, M.; Laillou, A.; Wieringa, F.T.; Avallone, S. Nutritional quality of ready-to-use therapeutic foods: focus on lipid composition and vitamin content. OCL 2022, 29, 13. [Google Scholar] [CrossRef]
- Sales, J.; Marais, D.; Kruger, M. Fat content, caloric value, cholesterol content, and fatty acid composition of raw and cooked ostrich meat. J. Food Compost. Anal. 1996, 9, 85–89. [Google Scholar] [CrossRef]
- Horbanczuk, J.O.; Malecki, I.; Cooper, R.G.; Józwik, A.; Klewiec, J.; Krzyzewski, J.; Khalifa, H.; Chylinski, W.; Wójcik, A.; Kawka, M. Cholesterol content and fatty acid composition of two fat depots from slaughter ostriches (Struthio camelus) aged 14 months. Anim. Sci. Pap. Rep. 2004, 22, 247–251. [Google Scholar]
- Carvalho-Filho, E.V.; Costa, M.J.C.; Bion, F.M.; Silva, J.A. Effect of the daily consumption of ostrich and bovine meat on the lipid metabolism in rats. Cienc. Tecnol. Aliment. 2011, 31, 72–77. [Google Scholar] [CrossRef]
- Basuny, A.; Arafat, S.; Soliman, H. Biological evaluation of ostrich oil and its using for production of biscuit. Egypt. J. Chem. 2017, 60, 1091–1099. [Google Scholar] [CrossRef]
- Dalvi-Isfahan, M.; Moammernezhad, Z.; Tavakoli, J. Ostrich oil as a fat substitute in milk-based infant formula. Food Sci Nutr. 2023, 11, 1872–1881. [Google Scholar] [CrossRef]
- Ryan-Harshman, M.; Aldoori, W. New dietary reference intakes for macronutrients and fibre. Can. Fam. Physician. 2006, 52, 177–179. [Google Scholar]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. J. Am. Diet Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.G.; Ford, N.A.; Hu, F.B.; Zelman, K.M.; Mozaffarian, D.; Kris-Etherton, P.M. A healthy approach to dietary fats: understanding the science and taking action to reduce consumer confusion. Nutr. J. 2017, 16, 53. [Google Scholar] [CrossRef]
- FAO (2010) Fats and fatty acids in human nutrition. Report of an Expert Consultation in Food and Nutrition Paper 91. FAO, Rome.
- Gil, A.; Serra-Majem, L.; Calder, P.C.; Uauy, R. Systematic reviews of the role of omega-3 fatty acids in the prevention and treatment of disease. Br. J. Nutr. 2012, 107, S1–S2. [Google Scholar] [CrossRef]
- Sioen, I.; van Lieshout, L.; Eilander, A.; Fleith, M.; Lohner, S.; Szommer, A.; Petisca, C.; Eussen, S.; Forsyth, S.; Calder, P.C.; Campoy, C.; Mensink, R.P. Systematic review on N-3 and N-6 polyunsaturated fatty acid intake in European countries in light of the current recommendations - Focus on specific population groups. Ann. Nutr. Metab. 2017, 70, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Li, D. Omega-3 polyunsaturated fatty acids and non-communicable diseases: Meta-analysis based systematic review. Asia Pac J Clin. Nutr. 2015, 24, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Szostak-Wegierek, D.; Klosiewicz-Latoszek, L.; Szostak, W.B.; Cybulska, B. The role of dietary fats for preventing cardiovascular disease. A review. Rocz Panstw Zakl Hig. 2013, 64, 263–269. [Google Scholar]
- Flachs, P.; Rossmeisl, M.; Kopecky, J. The effect of n-3 fatty acids on glucose homeostasis and insulin sensitivity. Physiol Res. 2014, 63, S93–S118. [Google Scholar] [CrossRef]
- Roberts, H.C.; Lim, S.E.R.; Cox, N.J.; Ibrahim, K. The challenge of managing undernutrition in older people with frailty. Nutrients 2019, 11, 808. [Google Scholar] [CrossRef]
- Jéquier, E. Response to and range of acceptable fat intake in adults. Eur. J. Clin. Nutr. 1999, 53, S84–S88. [Google Scholar] [CrossRef]
- Uauy, R. Dietary fat quality for optimal health and well-being: Overview of recommendations. Ann. Nutr. Metab. 2009, 54, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Stupin, M.; Kibel, A.; Stupin, A.; Selthofer-Relatić, K.; Matić, A.; Mihalj, M.; Mihaljević, Z.; Jukić, I.; Drenjančević, I. The physiological effect of n-3 polyunsaturated fatty acids (n-3 PUFAs) intake and exercise on hemorheology, microvascular function, and physical performance in health and cardiovascular diseases; Is there an interaction of exercise and dietary n-3 PUFA Intake? Front. Physiol. 2019, 10, 1129. [Google Scholar] [CrossRef] [PubMed]
- Santin, J.R.; Kopp, M.A.T.; Correa, T.P.; Melato, J.; Benvenutti, L.; Nunes, R.; Goldoni, F.C.; Patel, Y.B.K.; Souza, J.A.; Soczek, S.H.S.; Fernandes, E.S.; Pastor, M.V.D.; Junior, L.C.K.; Apel, M.A.; Henriques, A.T.; Quintão, N.L.M. Neuroinflammation and hypersensitivity evidenced by the acute and 28-day repeated dose toxicity tests of ostrich oil in mice. Food Chem. Toxicol. 2023, 177, 113852. [Google Scholar] [CrossRef] [PubMed]





| Fomulation |
Lecithin (% w/w) |
Distilled water (% w/w) |
Ostrich oil (% w/w) |
% Creaming index (% CI) | ||
| Day 1 | Day 3 | Day 7 | ||||
| L01 | 1 | 89 | 10 | 78.38 | 78.38 | 77.78 |
| L02 | 2 | 88 | 10 | 80.56 | 80.56 | 80.56 |
| L03 | 3 | 87 | 10 | 77.78 | 75.68 | 72.22 |
| L04 | 4 | 86 | 10 | 0.00 | 78.38 | 76.32 |
| L05 | 5 | 85 | 10 | 0.00 | 0.00 | 0.00 |
| L06 | 6 | 84 | 10 | 0.00 | 0.00 | 0.00 |
| L07 | 7 | 83 | 10 | 0.00 | 0.00 | 0.00 |
| L08 | 8 | 82 | 10 | 0.00 | 0.00 | 0.00 |
| L09 | 9 | 81 | 10 | 0.00 | 0.00 | 0.00 |
| L10 | 10 | 80 | 10 | 0.00 | 0.00 | 0.00 |
| L11 | 11 | 79 | 10 | 0.00 | 0.00 | 0.00 |
| L12 | 12 | 78 | 10 | 0.00 | 0.00 | 0.00 |
| L13 | 13 | 77 | 10 | 0.00 | 0.00 | 0.00 |
| L14 | 14 | 76 | 10 | 0.00 | 0.00 | 0.00 |
| L15 | 15 | 75 | 10 | 0.00 | 0.00 | 0.00 |
| Fomulation |
Lecithin (% w/w) |
Distilled water (% w/w) |
Ostrich oil (% w/w) |
% Creaming index (% CI) | ||
| Day 1 | Day 3 | Day 7 | ||||
| O-05 | 10 | 85 | 5 | 0.00 | 0.00 | 0.00 |
| O-10 | 10 | 80 | 10 | 0.00 | 0.00 | 0.00 |
| O-15 | 10 | 75 | 15 | 0.00 | 0.00 | 0.00 |
| O-20 | 10 | 70 | 20 | 0.00 | 0.00 | 0.00 |
| O-25 | 10 | 65 | 25 | 0.00 | 0.00 | 0.00 |
| O-30 | 10 | 60 | 30 | 0.00 | 0.00 | 0.00 |
| Color components | Dry emulsions | |
|---|---|---|
| Avicel® PH-101 | Aerosil® 200 | |
| Granule color L* a* b* |
92.48 ± 0.43 -0.32 ± 0.07 18.44 ± 0.16 |
92.55 ± 0.37 -0.47 ± 0.10 18.60 ± 0.12 |
| Avicel® PH-101 granules containing ostrich oil emulsion | Cycle | L* | a* | b* | ∆E |
|---|---|---|---|---|---|
| With BHT | 0 6 |
92.37 ± 0.47 92.23 ± 0.27 |
-0.64 ± 0.17 -0.63 ± 0.10 |
18.32 ± 0.26 18.38 ± 0.62 |
0.15 |
| Without BHT | 0 6 |
92.34 ± 0.43 92.17 ± 0.45 |
-0.68 ± 0.10 -0.62 ± 0.07 |
18.29 ± 0.16 18.24 ± 0.37 |
0.19 |
| Ostrich oil - Avicel®101 granules | Day | L* | a* | b* | ∆E |
|---|---|---|---|---|---|
| With BHT Stored at 4 °C |
0 30 90 180 |
92.37 ± 0.47 92.37 ± 0.42 92.37 ± 0.39 92.37 ± 0.70 |
-0.67 ± 0.18 -0.64 ± 0.08 -0.67 ± 0.11 -0.63 ± 0.12 |
18.32 ± 0.26 18.10 ± 0.24 18.10 ± 0.32 18.14 ± 0.21 |
0.22 0.22 0.18 |
| With BHT Stored at 25 °C |
0 30 90 180 |
92.37 ± 0.47 92.30 ± 0.72 92.13 ± 0.16 92.00 ± 0.17 |
-0.64 ± 0.17 -0.67 ± 0.07 -0.62 ± 0.03 -0.62 ± 0.09 |
18.32 ± 0.26 18.09 ± 0.19 18.06 ± 0.24 18.22 ± 0.11 |
0.24 0.35 0.38 |
| With BHT Stored at 45 °C |
0 30 90 180 |
92.37 ± 0.47 91.66 ± 0.10 89.88 ± 0.34 88.51 ± 0.21 |
-0.64 ± 0.17 0.58 ± 0.07 1.48 ± 0.13 2.36 ± 0.17 |
18.32 ± 0.26 19.15 ± 0.38 20.92 ± 0.26 23.95 ± 0.06 |
1.64 4.18 7.46 |
| Without BHT Stored at 4 °C |
0 30 90 180 |
92.34 ± 0.43 92.31 ± 0.78 92.38 ± 0.17 92.09 ± 0.19 |
-0.68 ± 0.10 -0.64 ± 0.13 -0.64 ± 0.16 -0.62 ± 0.13 |
18.29 ± 0.16 18.23 ± 0.32 18.10 ± 0.15 18.13 ± 0.07 |
0.08 0.20 0.30 |
| Without BHT Stored at 25 °C |
0 30 90 180 |
92.34 ± 0.43 92.22 ± 0.69 91.96 ± 0.15 91.93 ± 0.08 |
-0.64 ± 0.11 -0.42 ± 0.14 -0.48 ± 0.15 -0.44 ± 0.17 |
18.29 ± 0.16 18.10 ± 0.11 18.12 ± 0.26 18.01 ± 0.14 |
0.31 0.45 0.54 |
| Without BHT Stored at 45 °C |
0 30 90 180 |
92.34 ± 0.43 91.07 ± 0.18 87.76 ± 0.67 83.13 ± 0.22 |
-0.64 ± 0.11 0.62 ± 0.45 3.64 ± 0.17 6.41 ± 0.06 |
18.29 ± 0.16 21.38 ± 0.05 27.55 ± 0.13 34.55 ± 0.23 |
3.57 11.18 19.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





