Submitted:
01 February 2024
Posted:
02 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2.1. SOURCES
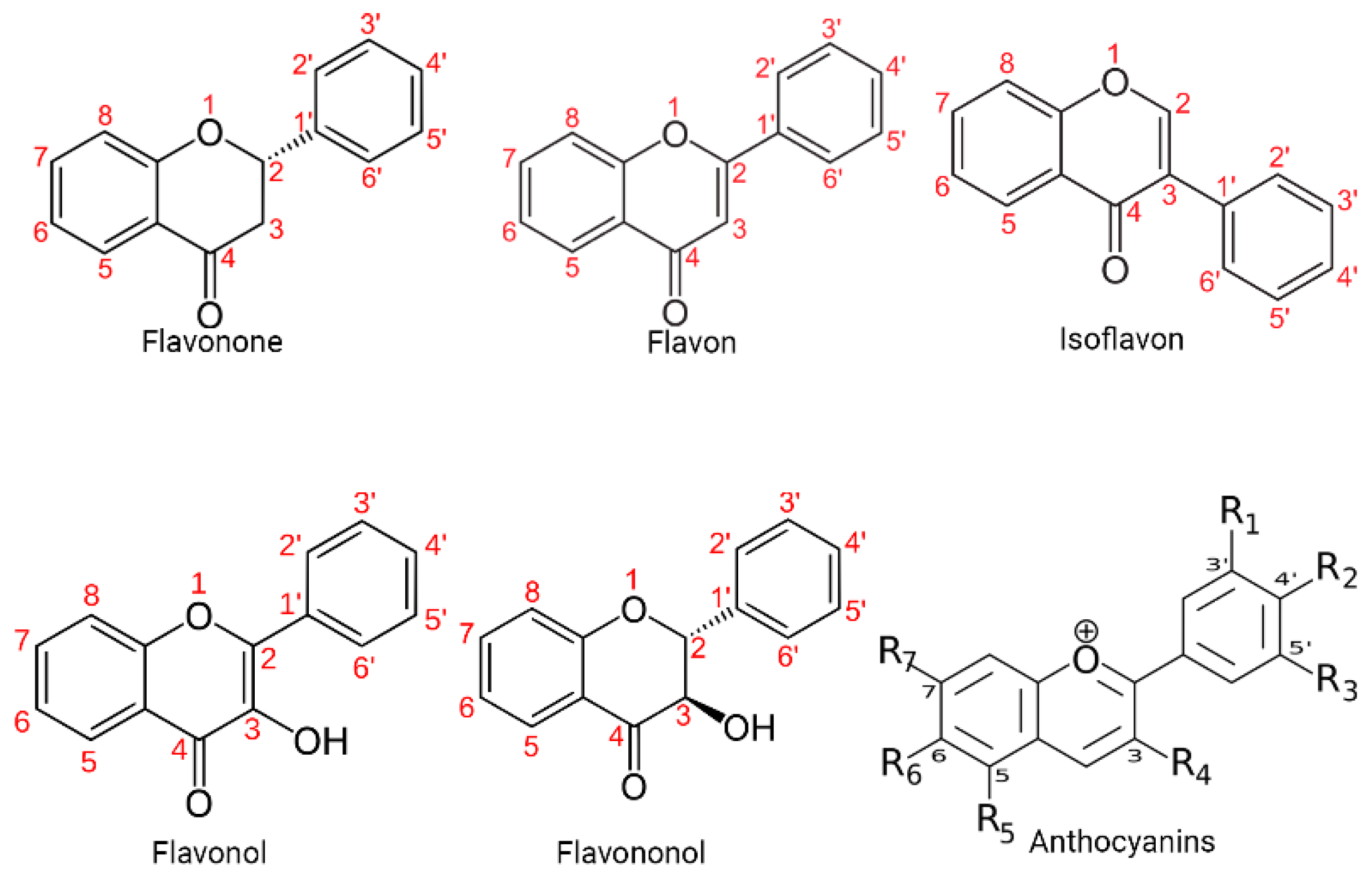
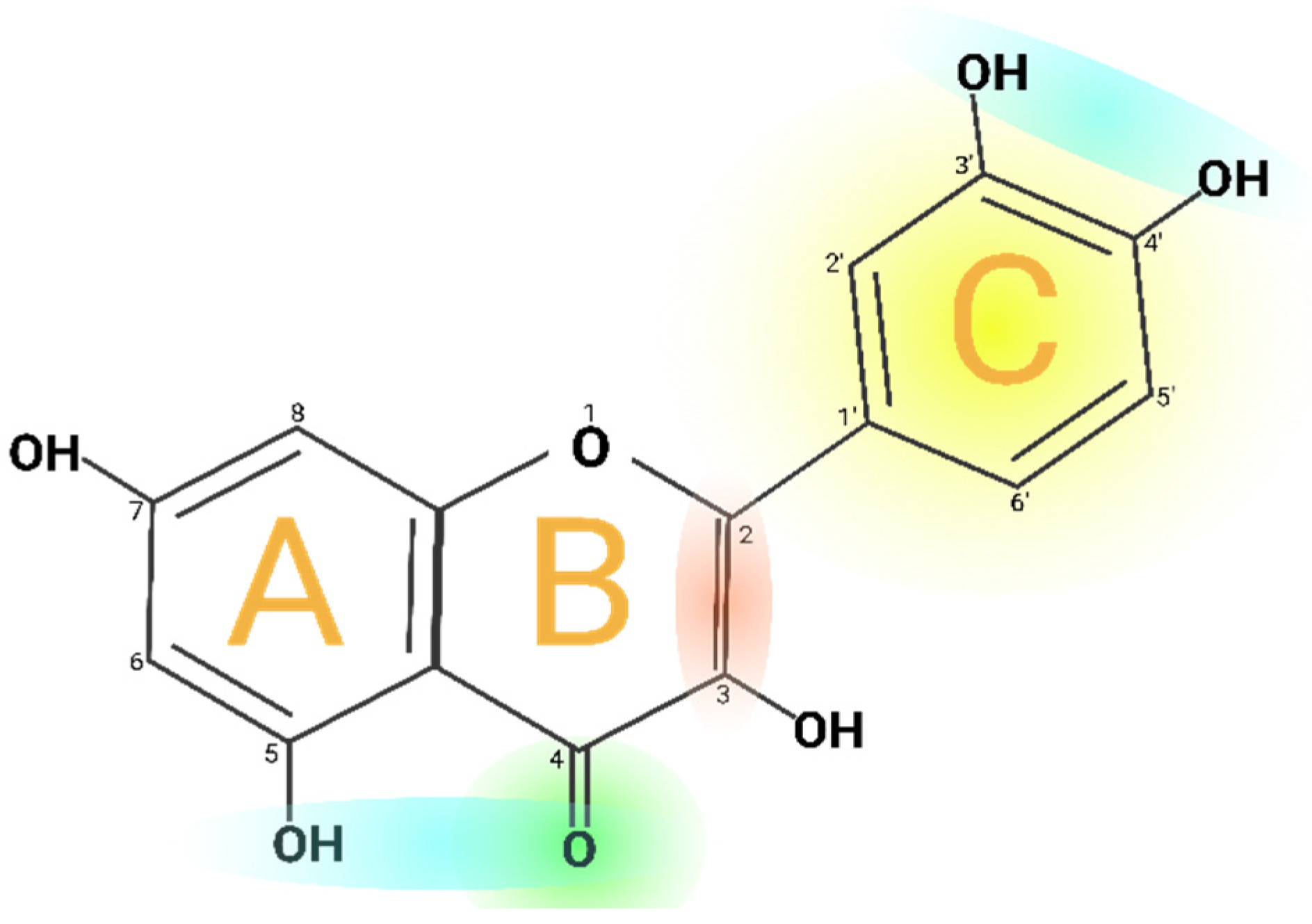
2.2. PHYSICOCHEMICAL PROPERTIES
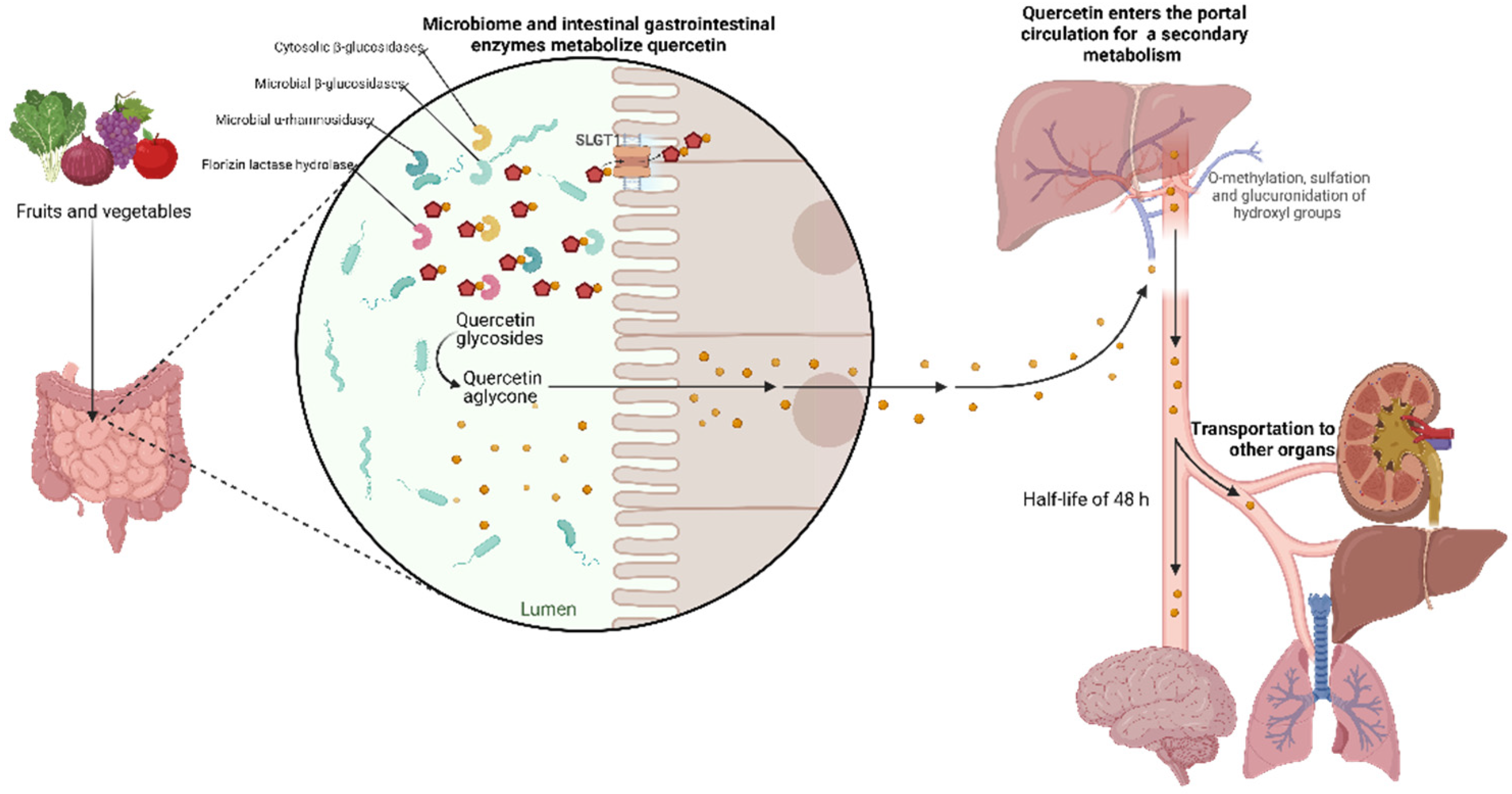
2.3. BIOAVAILABILITY AND PHARMACOKINETICS
2.4. HUMAN ABSORPTION AND METABOLISM OF QUERCETIN
2.5. QUERCETIN EXCRETION
2.6. PHARMACOLOGICAL PROPERTIES
2.6.1. Antioxidant
2.6.2. Hypertensive
2.6.3. Cardiovascular
2.6.4. Alzheimer’s disease
2.6.5. Antimicrobial
2.6.6. Antiviral
2.6.7. Hepatoprotective
2.6.8. Oxidative stress
2.6.9. Inflammation
2.6.10. Fibrosis
2.6.11. Cirrhosis
2.6.12. Diabetes
2.6.13. Arthritis
2.6.14. Cancer
2.6.14.1. Synergistic effect
2.6.14.1.1. Synergistic effect against breast cancer
2.6.14.1.2. Synergistic effect against prostate cancer
2.6.14.1.3. Synergistic effect against Leukemia
2.6.14.1.4. Synergistic effect in other types of cancer
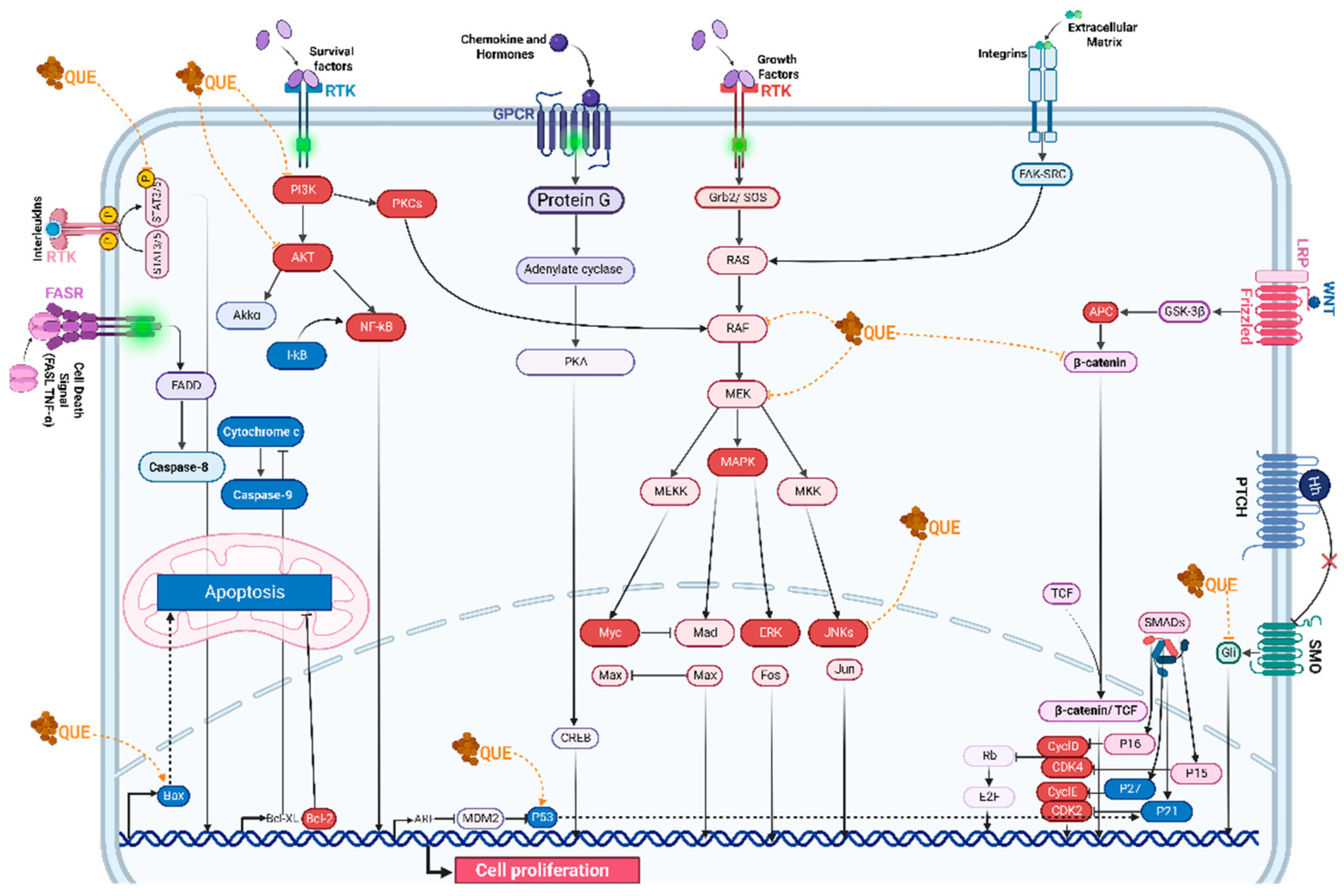
2.7. MOLECULAR PATHWAYS TARGETED BY QUERCETIN
2.7.1. Cell cycle
2.7.2. Apoptosis
2.7.3. Wnt/β-Catenin signaling.
2.7.4. p53 activity
2.7.5. Ras expression
2.7.6. PI3K signaling pathway
2.7.7. NF-κB signaling pathway
2.7.8. Autophagy.
2.8. NANOPARTICLES AS THERAPY
2.8.1. Liposomes
2.8.2. Lipid nanoparticles
2.8.3. Polymeric nanoparticles
2.8.4. PLGA nanoparticles
2.8.5. Inorganic nanoparticles
2.8.6. Silica nanoparticles
2.8.7. Magnetic nanoparticles
2.8.8. Extracellular vesicles
2.9. CONCLUSIONS AND FUTURE PROSPECTS
3. Conclusions
Funding
Conflicts of Interest
Apendix A Abbreviations
References
- Flores, G.P. Relaciones entre el Estrés Oxidativo y la Salud. Sociedades Rurales, Producción y Medio Ambiente 2019, 19, 34–34. [Google Scholar]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: an overview. Futur J Pharm Sci 2021, 7, 25. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. Journal of Food Biochemistry 2020, 44, e13394. [Google Scholar] [CrossRef]
- Terao, J. Potential Role of Quercetin Glycosides as Anti-Atherosclerotic Food-Derived Factors for Human Health. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: from its metabolism to possible mechanisms of its biological activities. Critical Reviews in Food Science and Nutrition 2020, 60, 3290–3303. [Google Scholar] [CrossRef]
- Heřmánková, E.; Zatloukalová, M.; Biler, M.; Sokolová, R.; Bancířová, M.; Tzakos, A.G.; Křen, V.; Kuzma, M.; Trouillas, P.; Vacek, J. Redox properties of individual quercetin moieties. Free Radical Biology and Medicine 2019, 143, 240–251. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Cires, M.J.; Gotteland, M. Quercetin and epigallocatechin gallate in the prevention and treatment of obesity: from molecular to clinical studies. Journal of medicinal food 2019, 22, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Ye, H.; Kamaraj, R.; Zhang, T.; Zhang, J.; Pavek, P. A review on pharmacological activities and synergistic effect of quercetin with small molecule agents. Phytomedicine 2021, 92, 153736. [Google Scholar] [CrossRef]
- Cattivelli, A.; Conte, A.; Martini, S.; Tagliazucchi, D. Influence of Cooking Methods on Onion Phenolic Compounds Bioaccessibility. Foods 2021, 10, 1023. [Google Scholar] [CrossRef]
- Mourabit, Y.; El Hajjaji, S.; Taha, D.; Badaoui, B.; El Yadini, M.; Rusu, M.E.; Lee, L.-H.; Bouyahya, A.; Bourais, I. HPLC-DAD-ESI/MS phytochemical investigation, antioxidant, and antidiabetic activities of Moroccan Rosa canina L. extracts. Biocatalysis and Agricultural Biotechnology 2023, 52, 102817. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. Journal of AOAC INTERNATIONAL 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. Biomed Res Int 2022, 2022, 5445291. [Google Scholar] [CrossRef] [PubMed]
- Tronina, T.; Łużny, M.; Dymarska, M.; Urbaniak, M.; Kozłowska, E.; Piegza, M.; Stępień, Ł.; Janeczko, T. Glycosylation of Quercetin by Selected Entomopathogenic Filamentous Fungi and Prediction of Its Products’ Bioactivity. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Shorobi, F.M.; Nisa, F.Y.; Saha, S.; Chowdhury, M.A.H.; Srisuphanunt, M.; Hossain, K.H.; Rahman, M.A. Quercetin: A Functional Food-Flavonoid Incredibly Attenuates Emerging and Re-Emerging Viral Infections through Immunomodulatory Actions. Molecules 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Russo, C.; Statti, G.; Argentieri, M.P.; Meleleo, D.; Mallamaci, R.; Avato, P.; Conforti, F. Phytochemical and biological characterization of dry outer scales extract from Tropea red onion (Allium cepa L. var. Tropea)–A promising inhibitor of pancreatic lipase. Phytomedicine Plus 2022, 2, 100235. [Google Scholar] [CrossRef]
- Roszkowski, S. Application of Polyphenols and Flavonoids in Oncological Therapy. Molecules 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Rajesh R, U.; Dhanaraj, S. A critical review on quercetin bioflavonoid and its derivatives: Scope, synthesis, and biological applications with future prospects. Arabian Journal of Chemistry 2023, 16, 104881. [Google Scholar] [CrossRef]
- Kooshki, L.; Zarneshan, S.N.; Fakhri, S.; Moradi, S.Z.; Echeverria, J. The pivotal role of JAK/STAT and IRS/PI3K signaling pathways in neurodegenerative diseases: Mechanistic approaches to polyphenols and alkaloids. Phytomedicine 2023, 112, 154686. [Google Scholar] [CrossRef]
- George, J.; Edwards, D.; Pun, S.; Williams, D. Evaluation of Antioxidant Capacity (ABTS and CUPRAC) and Total Phenolic Content (Folin-Ciocalteu) Assays of Selected Fruit, Vegetables, and Spices. Int J Food Sci 2022, 2022, 2581470. [Google Scholar] [CrossRef]
- Golmohammadi, M.; Elmaghraby, D.A.; Ramírez-Coronel, A.A.; Rakhimov, N.; Mohammed, S.S.; Romero-Parra, R.M.; Jawad, M.A.; Zamanian, M.Y.; Soltani, A.; Taheri, N.; et al. A comprehensive view on the quercetin impact on bladder cancer: Focusing on oxidative stress, cellular, and molecular mechanisms. Fundamental and Clinical Pharmacology 2023, 37, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.H.; Lin, S.Y.; Chen, L.L.; Ouyang, K.H.; Wang, W.J. The Interaction between Flavonoids and Intestinal Microbes: A Review. Foods 2023, 12. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Chen, H.Y.; Tang, Q.Q.; Li, Y.F.; Liu, X.S.; Lu, F.H.; Gu, Y.Y. Protective effect of quercetin on kidney diseases: From chemistry to herbal medicines. Front Pharmacol 2022, 13, 968226. [Google Scholar] [CrossRef]
- Kandemir, K.; Tomas, M.; McClements, D.J.; Capanoglu, E. Recent advances on the improvement of quercetin bioavailability. Trends in Food Science & Technology 2022, 119, 192–200. [Google Scholar] [CrossRef]
- Almeida, A.F.; Borge, G.I.A.; Piskula, M.; Tudose, A.; Tudoreanu, L.; Valentová, K.; Williamson, G.; Santos, C.N. Bioavailability of Quercetin in Humans with a Focus on Interindividual Variation. Compr Rev Food Sci Food Saf 2018, 17, 714–731. [Google Scholar] [CrossRef] [PubMed]
- Owczarek-Januszkiewicz, A.; Magiera, A.; Olszewska, M.A. Enzymatically Modified Isoquercitrin: Production, Metabolism, Bioavailability, Toxicity, Pharmacology, and Related Molecular Mechanisms. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Billowria, K.; Ali, R.; Rangra, N.K.; Kumar, R.; Chawla, P.A. Bioactive Flavonoids: A Comprehensive Review on Pharmacokinetics and Analytical Aspects. Critical Reviews in Analytical Chemistry 2022. [Google Scholar] [CrossRef]
- Ullah, H.; Minno, A.D.; Santarcangelo, C.; Tantipongpiradet, A.; Dacrema, M.; Matteo, R.D.; El-Seedi, H.R.; Khalifa, S.A.M.; Baldi, A.; Rossi, A.; et al. In Vitro Bioaccessibility and Anti-Inflammatory Activity of a Chemically Characterized Allium cepa L. Extract Rich in Quercetin Derivatives Optimized by the Design of Experiments. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Rha, C.-S.; Choi, J.-M.; Jung, Y.S.; Kim, E.-R.; Ko, M.J.; Seo, D.-H.; Kim, D.-O.; Park, C.-S. High-efficiency enzymatic production of α-isoquercitrin glucosides by amylosucrase from Deinococcus geothermalis. Enzyme and Microbial Technology 2019, 120, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mu, T.; Deng, X.; Guo, R.; Xia, B.; Jiang, L.; Wu, Z.; Liu, M. New Insights of Biological Functions of Natural Polyphenols in Inflammatory Intestinal Diseases. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-H.; Lee, J.H.; Lee, Y.-J. Evaluation of the Mrp2-mediated flavonoid-drug interaction potential of quercetin in rats and in vitro models. Asian Journal of Pharmaceutical Sciences 2019, 14, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.H.; Eslami, A.; Jelodar, S.k.; Ranjbar, M.; Hasantabar, V. Preventive effect of quercetin-Loaded nanophytosome against autistic-like damage in maternal separation model: The possible role of Caspase-3, Bax/Bcl-2 and Nrf2. Behavioural Brain Research 2023, 441, 114300. [Google Scholar] [CrossRef]
- Hedayati, N.; Yaghoobi, A.; Salami, M.; Gholinezhad, Y.; Aghadavood, F.; Eshraghi, R.; Aarabi, M.H.; Homayoonfal, M.; Asemi, Z.; Mirzaei, H.; et al. Impact of polyphenols on heart failure and cardiac hypertrophy: clinical effects and molecular mechanisms. Front Cardiovasc Med 2023, 10, 1174816. [Google Scholar] [CrossRef]
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Lyu, Y.L.; Zhou, H.F.; Yang, J.; Wang, F.X.; Sun, F.; Li, J.Y. Biological Activities Underlying the Therapeutic Effect of Quercetin on Inflammatory Bowel Disease. Mediators Inflamm 2022, 2022, 5665778. [Google Scholar] [CrossRef]
- Cheng, S.C.; Huang, W.C.; JH, S.P.; Wu, Y.H.; Cheng, C.Y. Quercetin Inhibits the Production of IL-1β-Induced Inflammatory Cytokines and Chemokines in ARPE-19 Cells via the MAPK and NF-κB Signaling Pathways. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef]
- Li, F.; Liu, J.; Tang, S.; Yan, J.; Chen, H.; Li, D.; Yan, X. Quercetin regulates inflammation, oxidative stress, apoptosis, and mitochondrial structure and function in H9C2 cells by promoting PVT1 expression. Acta Histochemica 2021, 123, 151819. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed]
- Pan, E.; Feng, H.; Yang, Z.; Xin, Y.; Ji, X.; Ping, K.; Sun, Y.; Dong, J. Quercetin dietary supplementation protects against difenoconazole-induced carp spleen inflammatory damage via regulating ROS/NF-κB/NLRP3 inflammasome axis. Aquaculture 2024, 579. [Google Scholar] [CrossRef]
- Wuputra, K.; Tsai, M.H.; Kato, K.; Ku, C.C.; Pan, J.B.; Yang, Y.H.; Saito, S.; Wu, C.C.; Lin, Y.C.; Cheng, K.H.; et al. Jdp2 is a spatiotemporal transcriptional activator of the AhR via the Nrf2 gene battery. Inflammation and Regeneration 2023, 43. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, E.V.; Falchetti, F.; Pernomian, L.; De Mello, M.M.B.; Parente, J.M.; Nogueira, R.C.; Gomes, B.Q.; Bertozi, G.; Sanches-Lopes, J.M.; Tanus-Santos, J.E. Quercetin decreases cardiac hypertrophic mediators and maladaptive coronary arterial remodeling in renovascular hypertensive rats without improving cardiac function. Naunyn-Schmiedeberg’s Archives of Pharmacology 2023, 396, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Abdelghffar, E.A.; Obaid, W.A.; Elgamal, A.M.; Daoud, R.; Sobeh, M.; El Raey, M.A. Pea (Pisum sativum) peel extract attenuates DOX-induced oxidative myocardial injury. Biomedicine & Pharmacotherapy 2021, 143, 112120. [Google Scholar] [CrossRef]
- Popiolek-Kalisz, J.; Fornal, E. The Effects of Quercetin Supplementation on Blood Pressure – Meta-Analysis. Current Problems in Cardiology 2022, 47, 101350. [Google Scholar] [CrossRef]
- Das, M.; Devi, K.P.; Belwal, T.; Devkota, H.P.; Tewari, D.; Sahebnasagh, A.; Nabavi, S.F.; Khayat Kashani, H.R.; Rasekhian, M.; Xu, S.; et al. Harnessing polyphenol power by targeting eNOS for vascular diseases. Critical Reviews in Food Science and Nutrition 2023, 63, 2093–2118. [Google Scholar] [CrossRef]
- Ra, J.-E.; Woo, S.-Y.; Jin, H.; Lee, M.J.; Kim, H.Y.; Ham, H.; Chung, I.-M.; Seo, W.D. Evaluation of antihypertensive polyphenols of barley (Hordeum vulgare L.) seedlings via their effects on angiotensin-converting enzyme (ACE) inhibition. Applied Biological Chemistry 2020, 63, 1–9. [Google Scholar] [CrossRef]
- Fikriah, I.; Ismail, S.; Kosala, K. In vitro evaluation of the vasodilatory activity of ethanol extracts of Eleutherine bulbosa bulbs and leaves. Journal of Applied Pharmaceutical Science 2021, 11, 135–140. [Google Scholar]
- Solfaine, R.; Muniroh, L.; Irawan, A. Roles of averrhoa bilimbi extract in increasing serum nitric oxide concentration and vascular dilatation of ethanol-induced hypertensive rats. Preventive Nutrition and Food Science 2021, 26, 186. [Google Scholar] [CrossRef]
- Chekalina, N.; Burmak, Y.; Petrov, Y.; Borisova, Z.; Manusha, Y.; Kazakov, Y.; Kaidashev, I. Quercetin reduces the transcriptional activity of NF-kB in stable coronary artery disease. Indian Heart Journal 2018, 70, 593–597. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Zhang, J.; Zhang, X.; Yang, G. Molecular mechanism of autophagy: Its role in the therapy of Alzheimer’s disease. Current neuropharmacology 2020, 18, 720–739. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zhu, F.; Qiu, W.; Qiao, G.; Law, B.Y.-K.; Yu, L.; Wu, J.; Tang, Y.; Yu, C.; Qin, D.; et al. High-throughput screening for amyloid-β binding natural small-molecules based on the combinational use of biolayer interferometry and UHPLC−DAD-Q/TOF-MS/MS. Acta Pharmaceutica Sinica B 2022, 12, 1723–1739. [Google Scholar] [CrossRef] [PubMed]
- Zaplatic, E.; Bule, M.; Shah, S.Z.A.; Uddin, M.S.; Niaz, K. Molecular mechanisms underlying protective role of quercetin in attenuating Alzheimer’s disease. Life Sciences 2019, 224, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. Journal of Food Protection 2018, 81, 68–78. [Google Scholar] [CrossRef]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Shorobi, F.M.; Uddin, M.N.; Saha, S.; Hossain, M.A. Quercetin attenuates viral infections by interacting with target proteins and linked genes in chemicobiological models. In Silico Pharmacology 2022, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Parvez, M.K.; Al-Dosari, M.S.; Arbab, A.H.; Al-Rehaily, A.J.; Abdelwahid, M.A.S. Bioassay-guided isolation of anti-hepatitis B virus flavonoid myricetin-3-O-rhamnoside along with quercetin from Guiera senegalensis leaves. Saudi Pharmaceutical Journal 2020, 28, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.C.F.d.; Salatino, A.; Motta, L.B.d.; Negri, G.; Salatino, M.L.F. Chemical characterization, antioxidant and anti-HIV activities of a Brazilian propolis from Ceará state. Revista Brasileira de Farmacognosia 2019, 29, 309–318. [Google Scholar] [CrossRef]
- Upadhyay, R.; Tiwari, K.N. The antiviral potential of Phyllanthus species: a systematic review. Archives of Virology 2023, 168, 177. [Google Scholar] [CrossRef]
- Shao, C.; Xu, H.; Sun, X.; Huang, Y.; Guo, W.; He, Y.; Ye, L.; Wang, Z.; Huang, J.; Liang, X.; et al. New Perspectives on Chinese Medicine in Treating Hepatic Fibrosis: Lipid Droplets in Hepatic Stellate Cells. The American Journal of Chinese Medicine 2023, 51, 1413–1429. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, G.M.; Al-Qahtani, W.H.; AlFaris, N.A.; Alzahrani, N.S.; Alkhateeb, M.A.; Yahya, M.A. Quercetin prevents cadmium chloride-induced hepatic steatosis and fibrosis by downregulating the transcription of miR-21. BioFactors 2021, 47, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Shakerian, E.; Akbari, R.; Mohammadtaghvaei, N.; Mohammadi Gahrooie, M.; Afarin, R. Quercetin Reduces Hepatic Fibrogenesis by Inhibiting TGF-β/Smad3 Signaling Pathway in LX-2 Cell Line. Jundishapur J Nat Pharm Prod 2022, 17, e113484. [Google Scholar] [CrossRef]
- Serna-Salas, S.A.; Arroyave-Ospina, J.C.; Zhang, M.; Damba, T.; Buist-Homan, M.; Muñoz-Ortega, M.H.; Ventura-Juárez, J.; Moshage, H. α-1 Adrenergic receptor antagonist doxazosin reverses hepatic stellate cells activation via induction of senescence. Mechanisms of Ageing and Development 2022, 201, 111617. [Google Scholar] [CrossRef]
- Owen, T.; Carpino, G.; Chen, L.; Kundu, D.; Wills, P.; Ekser, B.; Onori, P.; Gaudio, E.; Alpini, G.; Francis, H.; et al. Endothelin Receptor-A Inhibition Decreases Ductular Reaction, Liver Fibrosis, and Angiogenesis in a Model of Cholangitis. Cellular and Molecular Gastroenterology and Hepatology 2023, 16, 513–540. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Choudhury, S.T.; Seidel, V.; Rahman, A.B.; Aziz, M.A.; Richi, A.E.; Rahman, A.; Jafrin, U.H.; Hannan, J.M.A.; Abdel-Wahab, Y.H.A. Therapeutic Potential of Quercetin in the Management of Type-2 Diabetes Mellitus. Life 2022, 12, 1146. [Google Scholar] [CrossRef]
- Shi, G.-J.; Li, Y.; Cao, Q.-H.; Wu, H.-X.; Tang, X.-Y.; Gao, X.-H.; Yu, J.-Q.; Chen, Z.; Yang, Y. In vitro and in vivo evidence that quercetin protects against diabetes and its complications: A systematic review of the literature. Biomedicine & Pharmacotherapy 2019, 109, 1085–1099. [Google Scholar]
- Hamilton, K.E.; Rekman, J.F.; Gunnink, L.K.; Busscher, B.M.; Scott, J.L.; Tidball, A.M.; Stehouwer, N.R.; Johnecheck, G.N.; Looyenga, B.D.; Louters, L.L. Quercetin inhibits glucose transport by binding to an exofacial site on GLUT1. Biochimie 2018, 151, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- Borghi, S.M.; Mizokami, S.S.; Pinho-Ribeiro, F.A.; Fattori, V.; Crespigio, J.; Clemente-Napimoga, J.T.; Napimoga, M.H.; Pitol, D.L.; Issa, J.P.; Fukada, S.Y. The flavonoid quercetin inhibits titanium dioxide (TiO2)-induced chronic arthritis in mice. The Journal of Nutritional Biochemistry 2018, 53, 81–95. [Google Scholar] [CrossRef]
- Guo, H.; Yin, W.; Zou, Z.; Zhang, C.; Sun, M.; Min, L.; Yang, L.; Kong, L. Quercitrin alleviates cartilage extracellular matrix degradation and delays ACLT rat osteoarthritis development: An in vivo and in vitro study. J Adv Res 2021, 28, 255–267. [Google Scholar] [CrossRef]
- Córdoba-Moreno, M.O.; Mendes, M.T.; Markus, R.P.; Fernandes, P.A. Rat resistance to rheumatoid arthritis induction as a function of the early-phase adrenal–pineal crosstalk. The Journal of Physiology 2023, 601, 535–549. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomedicine 2021, 90, 153554. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Gao, Y.; Liu, S.; Li, K.; Wang, S.; Gao, L.; Shi, M.; Liu, Z.; Han, Z.; et al. Effect of a Drug Delivery System Made of Quercetin Formulated into PEGylation Liposomes on Cervical Carcinoma <i>In Vitro</i> and <i>In Vivo</i>. Journal of Nanomaterials 2021, 2021, 9389934. [Google Scholar] [CrossRef]
- Tang, S.-M.; Deng, X.-T.; Zhou, J.; Li, Q.-P.; Ge, X.-X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomedicine & Pharmacotherapy 2020, 121, 109604. [Google Scholar] [CrossRef]
- Joyner, P.M. Protein Adducts and Protein Oxidation as Molecular Mechanisms of Flavonoid Bioactivity. Molecules 2021, 26, 5102. [Google Scholar] [CrossRef]
- Shah, S.; Narang, R.; Singh, V.J.; Pilli, G.; Nayak, S.K. A Review on Anticancer Profile of Flavonoids: Sources, Chemistry, Mechanisms, Structure-activity Relationship and Anticancer Activity. Current Drug Research Reviews Formerly: Current Drug Abuse Reviews 2023, 15, 122–148. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, W.; Bi, L.; Qin, F.; Li, J.; Zeng, H.; Lu, L. [Quercetin induces apoptosis of human breast cancer cells by activiting PTEN and inhibiting PI3K/AKT and JNK signaling pathways]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2022, 38, 714–720. [Google Scholar] [PubMed]
- Mohammadinejad, S.; Jafari-Gharabaghlou, D.; Zarghami, N. Development of PEGylated PLGA Nanoparticles Co-Loaded with Bioactive Compounds: Potential Anticancer Effect on Breast Cancer Cell Lines. Asian Pac J Cancer Prev 2022, 23, 4063–4072. [Google Scholar] [CrossRef] [PubMed]
- Svitina, H.; Hamman, J.H.; Gouws, C. Molecular mechanisms and associated cell signalling pathways underlying the anticancer properties of phytochemical compounds from <em>Aloe</em> species (Review). Exp Ther Med 2021, 22, 852. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.F.; Saleem, A.; Rasul, A.; Faran Ashraf Baig, M.M.; Bin-Jumah, M.; Abdel Daim, M.M. Anticancer natural medicines: An overview of cell signaling and other targets of anticancer phytochemicals. European Journal of Pharmacology 2020, 888, 173488. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, P.T.; Bashir, S.M.; Rather, M.A.; Dar, K.B.; Taban, Q.; Sajood, S.; Ali, A.; Rather, Z.A.; Amin, I.; Dar, M.A. Antiproliferative and Apoptotic Activities of Natural Honey. In Therapeutic Applications of Honey and its Phytochemicals: Vol.1; Rehman, M.U., Majid, S., Eds.; Springer Singapore: Singapore, 2020; pp. 345–360. [Google Scholar]
- Boccellino, M.; Quagliuolo, L.; D’Angelo, S. Annurca Apple Biophenols’ Effects in Combination with Cisplatin on A549 Cells. Current Nutrition & Food Science 2021, 17, 111–120. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alsahli, M.A.; Almatroudi, A.; Verma, A.K.; Aloliqi, A.; Allemailem, K.S.; Khan, A.A.; Rahmani, A.H. Potential Therapeutic Targets of Quercetin, a Plant Flavonol, and Its Role in the Therapy of Various Types of Cancer through the Modulation of Various Cell Signaling Pathways. Molecules 2021, 26, 1315. [Google Scholar] [CrossRef] [PubMed]
- Kicinska, A.; Jarmuszkiewicz, W. Flavonoids and Mitochondria: Activation of Cytoprotective Pathways? Molecules 2020, 25, 3060. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, R.; Qian, J.; Sun, J.; Li, G.; Shen, J.; Xie, Y. Combination therapy of doxorubicin and quercetin on multidrug-resistant breast cancer and their sequential delivery by reduction-sensitive hyaluronic acid-based conjugate/d-α-tocopheryl poly (ethylene glycol) 1000 succinate mixed micelles. Molecular pharmaceutics 2020, 17, 1415–1427. [Google Scholar] [CrossRef]
- Kantapan, J.; Paksee, S.; Chawapun, P.; Sangthong, P.; Dechsupa, N. Pentagalloyl Glucose- and Ethyl Gallate-Rich Extract from Maprang Seeds Induce Apoptosis in MCF-7 Breast Cancer Cells through Mitochondria-Mediated Pathway. Evidence-Based Complementary and Alternative Medicine 2020, 2020, 5686029. [Google Scholar] [CrossRef]
- Gupta, P.; Neupane, Y.R.; Aqil, M.; Kohli, K.; Sultana, Y. Lipid-based nanoparticle-mediated combination therapy for breast cancer management: a comprehensive review. Drug Delivery and Translational Research 2023, 13, 2739–2766. [Google Scholar] [CrossRef]
- Maugeri, A.; Calderaro, A.; Patanè, G.T.; Navarra, M.; Barreca, D.; Cirmi, S.; Felice, M.R. Targets Involved in the Anti-Cancer Activity of Quercetin in Breast, Colorectal and Liver Neoplasms. International Journal of Molecular Sciences 2023, 24, 2952. [Google Scholar] [CrossRef]
- Sharma, N.; Raut, P.W.; Baruah, M.M.; Sharma, A. Combination of quercetin and 2-methoxyestradiol inhibits epithelial–mesenchymal transition in PC-3 cell line via Wnt signaling pathway. Future Science OA 2021, 7, FSO747. [Google Scholar] [CrossRef]
- Shi, Y.; Su, X.; Cui, H.; Yu, L.; Du, H.; Han, Y. Combination of quercetin and Adriamycin effectively suppresses the growth of refractory acute leukemia. Oncology Letters 2019, 18, 153–160. [Google Scholar] [CrossRef]
- Chen, K.T.J.; Anantha, M.; Leung, A.W.Y.; Kulkarni, J.A.; Militao, G.G.C.; Wehbe, M.; Sutherland, B.; Cullis, P.R.; Bally, M.B. Characterization of a liposomal copper(II)-quercetin formulation suitable for parenteral use. Drug Delivery and Translational Research 2020, 10, 202–215. [Google Scholar] [CrossRef]
- Jaisamut, P.; Wanna, S.; Limsuwan, S.; Chusri, S.; Wiwattanawongsa, K.; Wiwattanapatapee, R. Enhanced Oral Bioavailability and Improved Biological Activities of a Quercetin/Resveratrol Combination Using a Liquid Self-Microemulsifying Drug Delivery System. Planta Med 2020, 87, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Feriotto, G.; Tagliati, F.; Giriolo, R.; Casciano, F.; Tabolacci, C.; Beninati, S.; Khan, M.T.H.; Mischiati, C. Caffeic Acid Enhances the Anti-Leukemic Effect of Imatinib on Chronic Myeloid Leukemia Cells and Triggers Apoptosis in Cells Sensitive and Resistant to Imatinib. International Journal of Molecular Sciences 2021, 22, 1644. [Google Scholar] [CrossRef] [PubMed]
- Maleki Dana, P.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The role of polyphenols in overcoming cancer drug resistance: a comprehensive review. Cellular & Molecular Biology Letters 2022, 27, 1. [Google Scholar] [CrossRef]
- Mahbub, A.A.; Maitre, C.L.L.; Haywood-Small, S.; Cross, N.A.; Jordan-Mahy, N. Polyphenols enhance the activity of alkylating agents in leukaemia cell lines. Oncotarget 2019, 10, 4570–4586. [Google Scholar] [CrossRef]
- Cione, E.; La Torre, C.; Cannataro, R.; Caroleo, M.C.; Plastina, P.; Gallelli, L. Quercetin, Epigallocatechin Gallate, Curcumin, and Resveratrol: From Dietary Sources to Human MicroRNA Modulation. Molecules 2020, 25, 63. [Google Scholar] [CrossRef]
- Doghish, A.S.; Hegazy, M.; Ismail, A.; El-Mahdy, H.A.; Elsakka, E.G.E.; Elkhawaga, S.Y.; Elkady, M.A.; Yehia, A.M.; Abdelmaksoud, N.M.; Mokhtar, M.M. A spotlight on the interplay of signaling pathways and the role of miRNAs in osteosarcoma pathogenesis and therapeutic resistance. Pathology - Research and Practice 2023, 245, 154442. [Google Scholar] [CrossRef] [PubMed]
- Tavana, E.; Mollazadeh, H.; Mohtashami, E.; Modaresi, S.M.S.; Hosseini, A.; Sabri, H.; Soltani, A.; Javid, H.; Afshari, A.R.; Sahebkar, A. Quercetin: A promising phytochemical for the treatment of glioblastoma multiforme. BioFactors 2020, 46, 356–366. [Google Scholar] [CrossRef]
- Rather, R.A.; Bhagat, M. Quercetin as an innovative therapeutic tool for cancer chemoprevention: Molecular mechanisms and implications in human health. Cancer Medicine 2020, 9, 9181–9192. [Google Scholar] [CrossRef] [PubMed]
- Soofiyani, S.R.; Hosseini, K.; Forouhandeh, H.; Ghasemnejad, T.; Tarhriz, V.; Asgharian, P.; Reiner, Ž.; Sharifi-Rad, J.; Cho, W.C. Quercetin as a Novel Therapeutic Approach for Lymphoma. Oxidative Medicine and Cellular Longevity 2021, 2021, 3157867. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Garg, V.K.; Tuli, H.S.; Yerer, M.B.; Sak, K.; Sharma, A.K.; Kumar, M.; Aggarwal, V.; Sandhu, S.S. Fisetin and Quercetin: Promising Flavonoids with Chemopreventive Potential. Biomolecules 2019, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.; Dey, D.; Biswas, P.K.; Rahaman, T.I.; Saha, S.; Parvez, A.; Khan, D.A.; Lily, N.J.; Saha, K.; Sohel, M.; et al. A Comprehensive Analysis and Anti-Cancer Activities of Quercetin in ROS-Mediated Cancer and Cancer Stem Cells. International Journal of Molecular Sciences 2022, 23, 11746. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cheng, M.; Zhang, W.; He, R.; Yang, H. New Insights into the Mechanisms of Polyphenol from Plum Fruit Inducing Apoptosis in Human Lung Cancer A549 Cells Via PI3K/AKT/FOXO1 Pathway. Plant Foods for Human Nutrition 2021, 76, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Vinayak, M.; Maurya, A.K. Quercetin Loaded Nanoparticles in Targeting Cancer: Recent Development. Anti-Cancer Agents in Medicinal Chemistry- Anti-Cancer Agents) 2019, 19, 1560–1576. [Google Scholar] [CrossRef]
- Zhang, C.; Hao, Y.; Sun, Y.; Liu, P. Quercetin suppresses the tumorigenesis of oral squamous cell carcinoma by regulating microRNA-22/WNT1/β-catenin axis. Journal of Pharmacological Sciences 2019, 140, 128–136. [Google Scholar] [CrossRef]
- Ezzati, M.; Yousefi, B.; Velaei, K.; Safa, A. A review on anti-cancer properties of Quercetin in breast cancer. Life Sciences 2020, 248, 117463. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Deng, Y.; Liao, L.; Zhou, M.; Peng, C.; Li, Y. Quercetin as a protective agent for liver diseases: A comprehensive descriptive review of the molecular mechanism. Phytotherapy Research 2021, 35, 4727–4747. [Google Scholar] [CrossRef]
- Rizzotto, D.; Englmaier, L.; Villunger, A. At a Crossroads to Cancer: How p53-Induced Cell Fate Decisions Secure Genome Integrity. International Journal of Molecular Sciences 2021, 22, 10883. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, S.-D.; Jiao, J.; Wang, W.; Yu, L.; Zhao, X.-L.; Wang, L.-T.; Meng, D.; Fu, Y.-J. ROS -mediated p53 activation by juglone enhances apoptosis and autophagy in vivo and in vitro. Toxicology and Applied Pharmacology 2019, 379, 114647. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Y.M.A.; Lotfy, G.; Said, M.M.; El Ashry, E.S.H.; El Tamany, E.S.H.; Soliman, S.M.; Abu-Serie, M.M.; Teleb, M.; Yousuf, S.; Dömling, A.; et al. Design, Synthesis, Chemical and Biochemical Insights Into Novel Hybrid Spirooxindole-Based p53-MDM2 Inhibitors With Potential Bcl2 Signaling Attenuation. Frontiers in Chemistry 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lee, J.; Moon, H.; Ryu, C.H.; Seok, J.; Jung, Y.-S.; Ryu, J.; Baek, S.J. Quercetin Induces Anticancer Activity by Upregulating Pro-NAG-1/GDF15 in Differentiated Thyroid Cancer Cells. Cancers 2021, 13, 3022. [Google Scholar] [CrossRef]
- Tisi, R.; Gaponenko, V.; Vanoni, M.; Sacco, E. Natural Products Attenuating Biosynthesis, Processing, and Activity of Ras Oncoproteins: State of the Art and Future Perspectives. Biomolecules 2020, 10, 1535. [Google Scholar] [CrossRef] [PubMed]
- Cháirez-Ramírez, M.H.; de la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Frontiers in Pharmacology 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, X.; Zhu, G.; Liu, H.; Chen, J.; Wang, Y.; He, X. Quercetin inhibits TNF-α induced HUVECs apoptosis and inflammation via downregulating NF-kB and AP-1 signaling pathway in vitro. Medicine (Baltimore) 2020, 99, e22241. [Google Scholar] [CrossRef]
- Sul, O.J.; Ra, S.W. Quercetin Prevents LPS-Induced Oxidative Stress and Inflammation by Modulating NOX2/ROS/NF-kB in Lung Epithelial Cells. Molecules 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ding, H.; Tang, X.; Liang, M.; Li, S.; Zhang, J.; Cao, J. Quercetin induces pro-apoptotic autophagy via SIRT1/AMPK signaling pathway in human lung cancer cell lines A549 and H1299 in vitro. Thoracic Cancer 2021, 12, 1415–1422. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef]
- Feynman, R. There’s plenty of room at the bottom. In Feynman and computation; CRC Press, 2018; pp. 63–76. [Google Scholar]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- McGuckin, M.B.; Wang, J.; Ghanma, R.; Qin, N.; Palma, S.D.; Donnelly, R.F.; Paredes, A.J. Nanocrystals as a master key to deliver hydrophobic drugs via multiple administration routes. Journal of Controlled Release 2022, 345, 334–353. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Cheng, M.; Zhang, X.; Chen, X. Quercetin nanoformulations: a promising strategy for tumor therapy. Food & Function 2021, 12, 6664–6681. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: current progress and perspectives. Journal of Hematology & Oncology 2021, 14, 85. [Google Scholar] [CrossRef]
- Patel, G.; Thakur, N.S.; Kushwah, V.; Patil, M.D.; Nile, S.H.; Jain, S.; Kai, G.; Banerjee, U.C. Mycophenolate co-administration with quercetin via lipid-polymer hybrid nanoparticles for enhanced breast cancer management. Nanomedicine: Nanotechnology, Biology and Medicine 2020, 24, 102147. [Google Scholar] [CrossRef]
- Zhou, H.; Yuan, Y.; Wang, Z.; Ren, Z.; Hu, M.; Lu, J.; Gao, H.; Pan, C.; Zhao, W.; Zhu, B. Co-delivery of doxorubicin and quercetin by Janus hollow silica nanomotors for overcoming multidrug resistance in breast MCF-7/Adr cells. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2023, 658, 130654. [Google Scholar] [CrossRef]
- Pinheiro, R.G.R.; Pinheiro, M.; Neves, A.R. Nanotechnology Innovations to Enhance the Therapeutic Efficacy of Quercetin. Nanomaterials (Basel) 2021, 11. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Hussain, A.; Sameen, A.; Sahar, A.; Khan, S.; Siddique, R.; Aadil, R.M.; Xu, B. Novel extraction, rapid assessment and bioavailability improvement of quercetin: A review. Ultrasonics Sonochemistry 2021, 78, 105686. [Google Scholar] [CrossRef]
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Fallahi, F.; Taghavipour, M.; Ghasemi, Y.; Akbari, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; et al. Quercetin and cancer: new insights into its therapeutic effects on ovarian cancer cells. Cell & Bioscience 2020, 10, 32. [Google Scholar] [CrossRef]
- Nasirizadeh, S.; Malaekeh-Nikouei, B. Solid lipid nanoparticles and nanostructured lipid carriers in oral cancer drug delivery. Journal of Drug Delivery Science and Technology 2020, 55, 101458. [Google Scholar] [CrossRef]
- Marques, M.P.; Varela, C.; Mendonça, L.; Cabral, C. Nanotechnology-Based Topical Delivery of Natural Products for the Management of Atopic Dermatitis. Pharmaceutics 2023, 15, 1724. [Google Scholar] [CrossRef] [PubMed]
- Molani Gol, R.; Kheirouri, S. The Effects of Quercetin on the Apoptosis of Human Breast Cancer Cell Lines MCF-7 and MDA-MB-231: A Systematic Review. Nutrition and Cancer 2022, 74, 405–422. [Google Scholar] [CrossRef]
- Lohan, S.; Sharma, T.; Saini, S.; Singh, A.; Kumar, A.; Raza, K.; Kaur, J.; Singh, B. Galactosylated nanoconstructs of Berberine with enhanced Biopharmaceutical and cognitive potential: A preclinical evidence in Alzheimer ‘s disease. Journal of Drug Delivery Science and Technology 2021, 66, 102695. [Google Scholar] [CrossRef]
- Guo, S.; Liang, Y.; Liu, L.; Yin, M.; Wang, A.; Sun, K.; Li, Y.; Shi, Y. Research on the fate of polymeric nanoparticles in the process of the intestinal absorption based on model nanoparticles with various characteristics: size, surface charge and pro-hydrophobics. Journal of Nanobiotechnology 2021, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Das, S.S.; Verma, P.R.P.; Kar, S.; Singh, S.K. Quercetin-Loaded Nanomedicine as Oncotherapy. In Nanomedicine for Bioactives: Healthcare applications; Rahman, M., Beg, S., Kumar, V., Ahmad, F.J., Eds.; Springer Singapore: Singapore, 2020; pp. 155–183. [Google Scholar]
- Gu, L.-Q.; Cui, P.-F.; Xing, L.; He, Y.-J.; Chang, X.; Zhou, T.-J.; Liu, Y.; Li, L.; Jiang, H.-L. An energy-blocking nanoparticle decorated with anti-VEGF antibody to reverse chemotherapeutic drug resistance. RSC Advances 2019, 9, 12110–12123. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Xu, X.; Liu, J.; Jia, Q.; Ke, C.; Zhang, H.; Xu, C.; Ou, E.; Tan, W.; Zhao, Y. Mitochondria-Targeted Triphenylphosphonium Conjugated C-3 Modified Betulin: Synthesis, Antitumor Properties and Mechanism of Action. ChemMedChem 2022, 17, e202100659. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front Pharmacol 2018, 9, 1260. [Google Scholar] [CrossRef]
- Ersoz, M.; Erdemir, A.; Derman, S.; Arasoglu, T.; Mansuroglu, B. Quercetin-loaded nanoparticles enhance cytotoxicity and antioxidant activity on C6 glioma cells. Pharm Dev Technol 2020, 25, 757–766. [Google Scholar] [CrossRef]
- Jha, N.K.; Arfin, S.; Jha, S.K.; Kar, R.; Dey, A.; Gundamaraju, R.; Ashraf, G.M.; Gupta, P.K.; Dhanasekaran, S.; Abomughaid, M.M.; et al. Re-establishing the comprehension of phytomedicine and nanomedicine in inflammation-mediated cancer signaling. Seminars in Cancer Biology 2022, 86, 1086–1104. [Google Scholar] [CrossRef] [PubMed]
- Gulin-Sarfraz, T.; Kalantzopoulos, G.N.; Kvalvåg Pettersen, M.; Wold Åsli, A.; Tho, I.; Axelsson, L.; Sarfraz, J. Inorganic Nanocarriers for Encapsulation of Natural Antimicrobial Compounds for Potential Food Packaging Application: A Comparative Study. Nanomaterials (Basel) 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomedicine & Pharmacotherapy 2019, 109, 1100–1111. [Google Scholar] [CrossRef]
- Mishra, S.; Manna, K.; Kayal, U.; Saha, M.; Chatterjee, S.; Chandra, D.; Hara, M.; Datta, S.; Bhaumik, A.; Das Saha, K. Folic acid-conjugated magnetic mesoporous silica nanoparticles loaded with quercetin: a theranostic approach for cancer management. RSC Advances 2020, 10, 23148–23164. [Google Scholar] [CrossRef] [PubMed]
- Zaharudin, N.S.; Mohamed Isa, E.D.; Ahmad, H.; Abdul Rahman, M.B.; Jumbri, K. Functionalized mesoporous silica nanoparticles templated by pyridinium ionic liquid for hydrophilic and hydrophobic drug release application. Journal of Saudi Chemical Society 2020, 24, 289–302. [Google Scholar] [CrossRef]
- Otieno, E.; Huang, Y.; Li, N.; Li, T.; Wang, M.; Qiu, X.; Xiao, X. Utilization of superparamagnetic iron oxide nanoparticles (SPIONs) as a vector for drug delivery. Applied Nanoscience 2023, 13, 6191–6216. [Google Scholar] [CrossRef]
- Zandieh, M.A.; Farahani, M.H.; Daryab, M.; Motahari, A.; Gholami, S.; Salmani, F.; Karimi, F.; Samaei, S.S.; Rezaee, A.; Rahmanian, P.; et al. Stimuli-responsive (nano)architectures for phytochemical delivery in cancer therapy. Biomedicine & Pharmacotherapy 2023, 166, 115283. [Google Scholar] [CrossRef]
- Ghosh, M.; Godderis, L.; Hoet, P. Epigenetic Mechanisms in Understanding Nanomaterial-Induced Toxicity. In Nanotoxicology in Safety Assessment of Nanomaterials; Louro, H., Silva, M.J., Eds.; Springer International Publishing: Cham, 2022; pp. 195–223. [Google Scholar]
- Kondath, S.; Rajaram, R.; Anantanarayanan, R. Curcumin reduced gold nanoparticles synergistically induces ROS mediated apoptosis in MCF-7 cancer cells. Inorganic and Nano-Metal Chemistry 2020, 51, 601–613. [Google Scholar] [CrossRef]
- Raghav, A.; Giri, R.; Agarwal, S.; Kala, S.; Jeong, G.-B. Protective role of engineered extracellular vesicles loaded quercetin nanoparticles as anti-viral therapy against SARS-CoV-2 infection: A prospective review. Frontiers in Immunology 2022, 13. [Google Scholar] [CrossRef]
- Qi, Y.; Guo, L.; Jiang, Y.; Shi, Y.; Sui, H.; Zhao, L. Brain delivery of quercetin-loaded exosomes improved cognitive function in AD mice by inhibiting phosphorylated tau-mediated neurofibrillary tangles. Drug Delivery 2020, 27, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Huang, Z.; Huang, L.; Liang, J.; Wang, P.; Zhao, L.; Shi, Y. Surface-modified engineered exosomes attenuated cerebral ischemia/reperfusion injury by targeting the delivery of quercetin towards impaired neurons. Journal of Nanobiotechnology 2021, 19, 141. [Google Scholar] [CrossRef] [PubMed]
- Donoso-Quezada, J.; Guajardo-Flores, D.; González-Valdez, J. Enhanced exosome-mediated delivery of black bean phytochemicals (Phaseolus vulgaris L.) for cancer treatment applications. Biomedicine & Pharmacotherapy 2020, 131, 110771. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Chan, C.-Y.; Chou, I.T.; Lien, C.-H.; Hung, H.-C.; Lee, M.-F. Quercetin induces growth arrest through activation of FOXO1 transcription factor in EGFR-overexpressing oral cancer cells. The Journal of Nutritional Biochemistry 2013, 24, 1596–1603. [Google Scholar] [CrossRef]
| Food source | Quercetin content (mg/100 g) |
|---|---|
| Fruits | |
| Apple with skin (Malus domestica) | 4,42 |
| Acerola (Malpighia emarginata) | 4,74 |
| Arctic bramble (Rubus arcticus) | 9.1 |
| Blue berries (Vaccinium caesariense) | 7,67 |
| Cranberries (Vaccinium macrocarpon) | 14.84 |
| Elderberries (Sambucus spp.) | 26,77 |
| Figs (Ficus carica) | 5.47 |
| Plums (Prunus domestica) | 12.45 |
| Sea buckthorn (Hippophae rhamnoides) | 7.4 |
| Wolfberry (Lycium barbarum) | 13.6 |
| Common juniper (Juniperus communis) | 46.61 |
| Prickly pear (Opuntia spp.) | 4.86 |
| Vegetable products | |
| Sowthistle (Sonchus oleraceus) | 16 |
| Arugula (Eruca sativa) | 7,92 |
| Sparrow grass (Asparagus officinalis) | 13,98 |
| Swiss chard (Beta vulgaris) | 7.5 |
| Green chicory (Cichorium intybus) | 6.49 |
| Coriander (Coriandrum sativum) | 52.9 |
| Golden poppy (Eschscholzia californica) | 26.3 |
| Drumstick tree (Moringa oleifera) | 16,65 |
| Fennel (Foeniculum vulgare) | 48,80 |
| Eaf cabbage (Brassica oleracea) | 7,71 |
| Red lettuce (Lactuca sativa) | 7,61 |
| Mustard greens (Brassica juncea) | 8.8 |
| Okra (Abelmoschus esculentus) | 20,97 |
| Onions (Allium cepa) | 20.3 |
| Perennial Wall-rocket (Diplotaxis tenuifolia) | 66.19 |
| New Mexico chile (Capsicum annuum) | 15 |
| Sweet potato (Ipomoea batatas) | 16.94 |
| Spices and herbs | |
| Caper bush (Capparis spinosa) | 180.77 |
| Dill (Anethum graveolens) | 55.15 |
| Oregano(Origanum vulgare) | 7.3 |
| Tarragon (Artemisia dracunculus) | 11 |
| Turmeric (Curcuma longa) | 4,92 |
| Buckwheat (Fagopyrum esculentum) | 15.38 |
| Malignant cell line | Reference |
|---|---|
| HepG2 hepatocellular carcinoma cells | Parvez, Al-Dosari, Arbab, Al-Rehaily and Abdelwahid [54] |
| PC-3, LNCaP, and DU-145 human prostate cancer cells. | Sharma, Raut, Baruah and Sharma [86] |
| MDA-MB-231 and AU565 human breast cancer cells. | Molani Gol and Kheirouri [127] |
| Caco-2, DLD1, HT-29, SW620, HKE-3, HCT-116, FHC, DKO-4 and HKE-3 human colon cancer cells. | Aziz, Lotfy, Said, El Ashry, El Tamany, Soliman, Abu-Serie, Teleb, Yousuf, Dömling, Domingo and Barakat [107] |
| HGC-27, NUGC-2, MKN-7, and MKN-28 human gastric cancer cells. | SDF |
| 143B highly metastatic human osteosarcoma cell line. | Doghish, Hegazy, Ismail, El-Mahdy, Elsakka, Elkhawaga, Elkady, Yehia, Abdelmaksoud and Mokhtar [94] |
| HPB-ALL chronic leukemia B-cells | Shi, Su, Cui, Yu, Du, and Han [87] |
| H460 Lung cancer cell lines | Sul and Ra [112] |
| MNT1, M10, and M14 human melanoma cells. | Córdoba-Moreno, Mendes, Markus and Fernandes [68] |
| A2780s and A2780cp ovarian cancer cell lines | Vafadar, Shabaninejad, Movahedpour, Fallahi, Taghavipour, Ghasemi, Akbari, Shafiee, Hajighadimi, Moradizarmehri, Razi, Savardashtaki and Mirzaei [124] |
| HeLa cervical cancer cell lines | Shorobi, Nisa, Saha, Chowdhury, Srisuphanunt, Hossain and Rahman [14] |
| HSC-3 and TW206 head and neck squamous cell carcinoma cells. | Huang et al. [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





