Submitted:
31 January 2024
Posted:
01 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant material
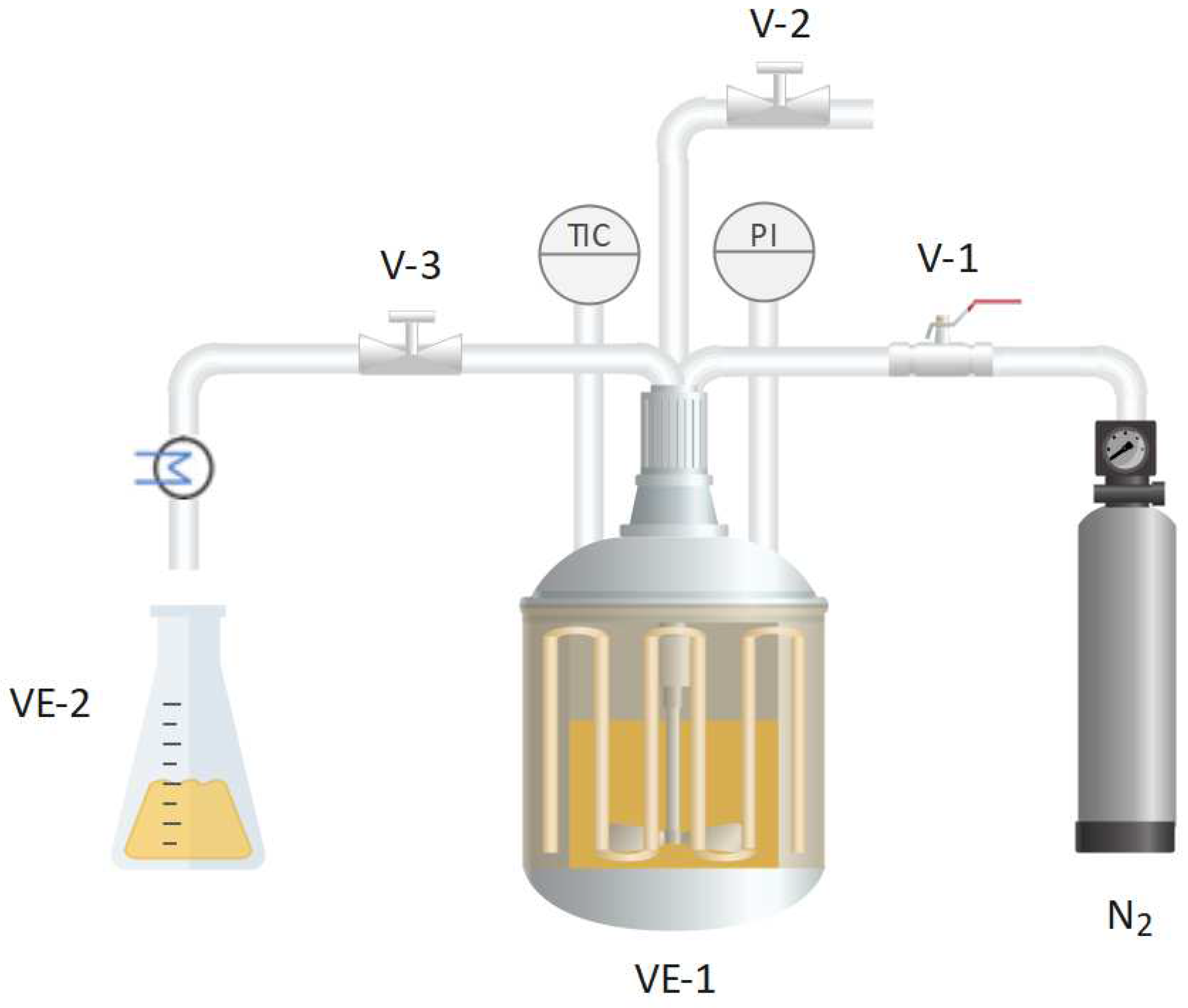
2.3. Subcritical water extraction
2.4. Chemical characterization
2.4.1. HPLC-DAD analysis
2.4.2. Total flavonoids content (TFC)
2.4.3. Total soluble sugars
2.4.4. Total protein content
2.4.5. Total free amino acids content
2.5. Radical scavenging activity
2.5.1. Nitric Oxide (●NO) scavenging activity
2.5.2. Superoxide (O2●-) scavenging activity
2.6. In vitro enzymatic assays
2.6.1. α-Amylase inhibition assay
2.6.2. α-Glucosidase inhibition assay
2.6.3. Aldose reductase inhibition assay
2.7. Cellular assays
2.7.1. Cell culture
2.7.2. Cell viability assay
2.7.3. Inhibition of α-glucosidase in Caco-2 cells
2.8. Statistical analysis
3. Results and Discussion
3.1. Chemical characterization
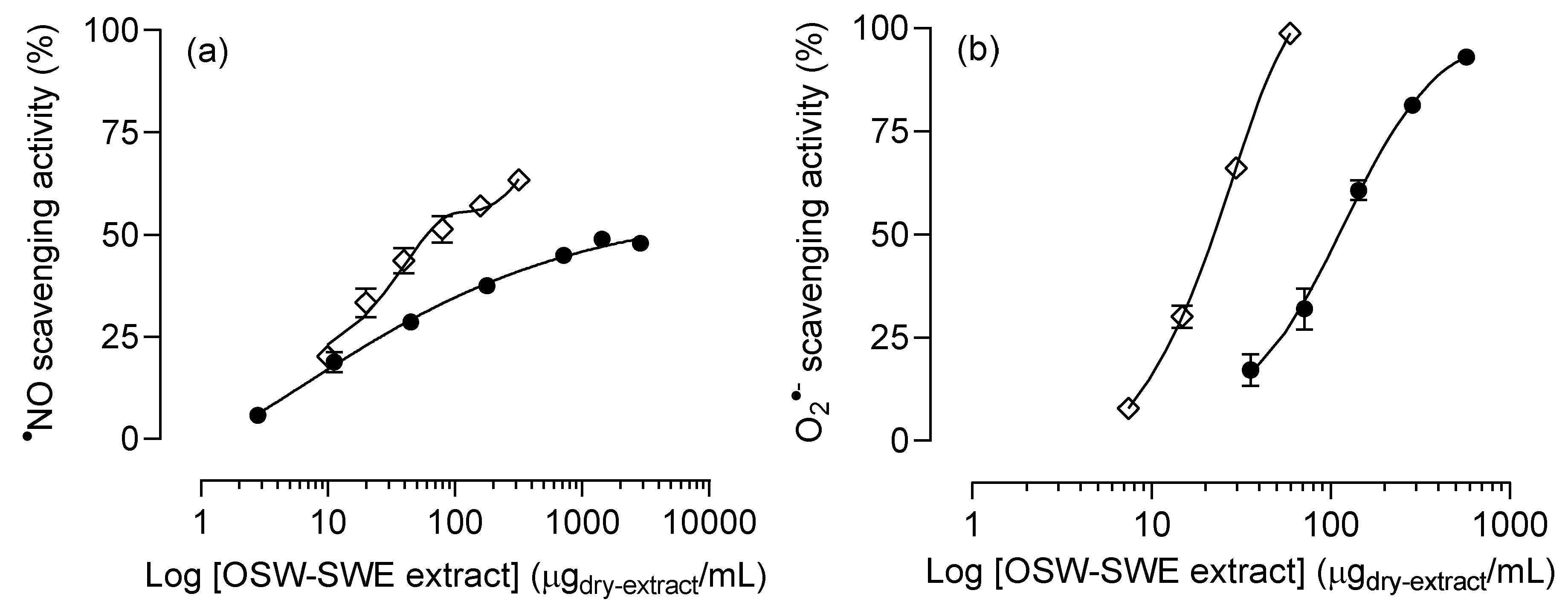
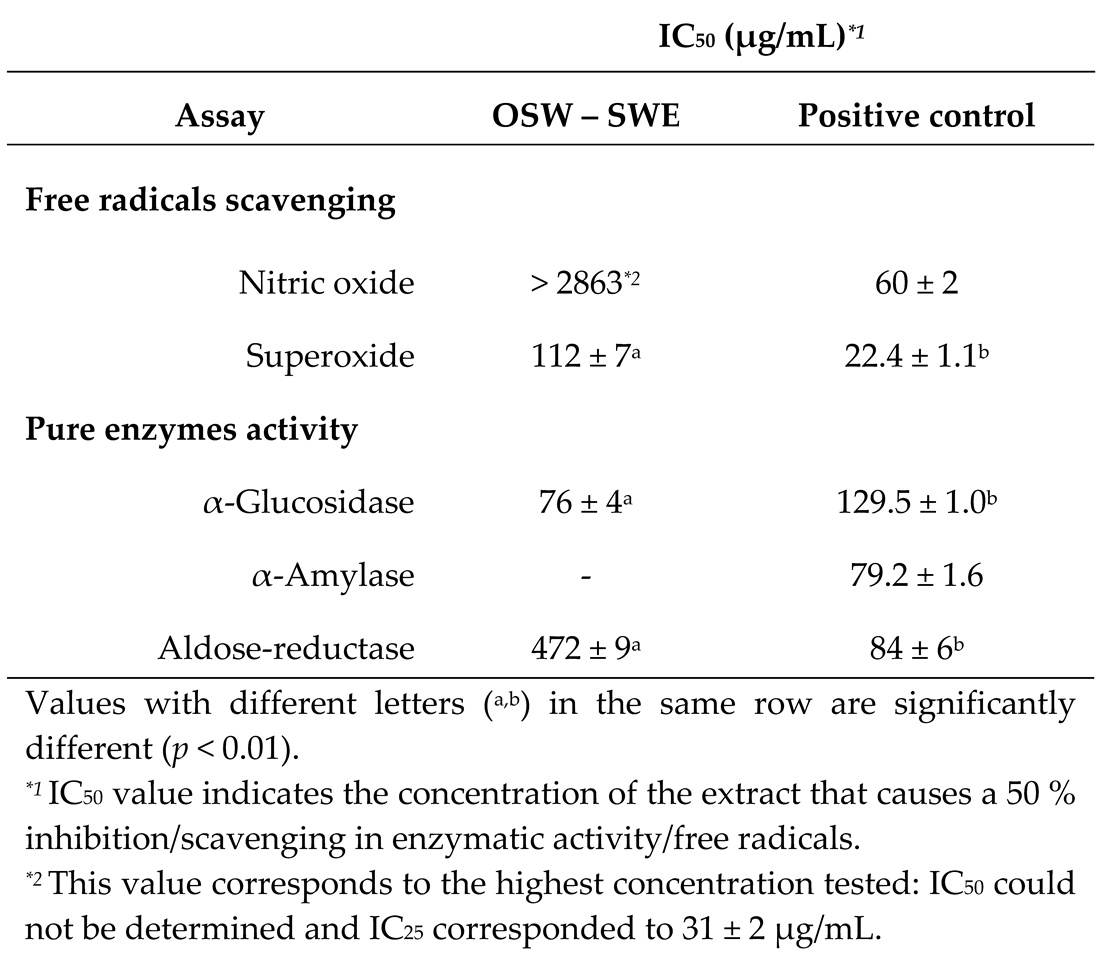
3.2. Effects on protection against radicals
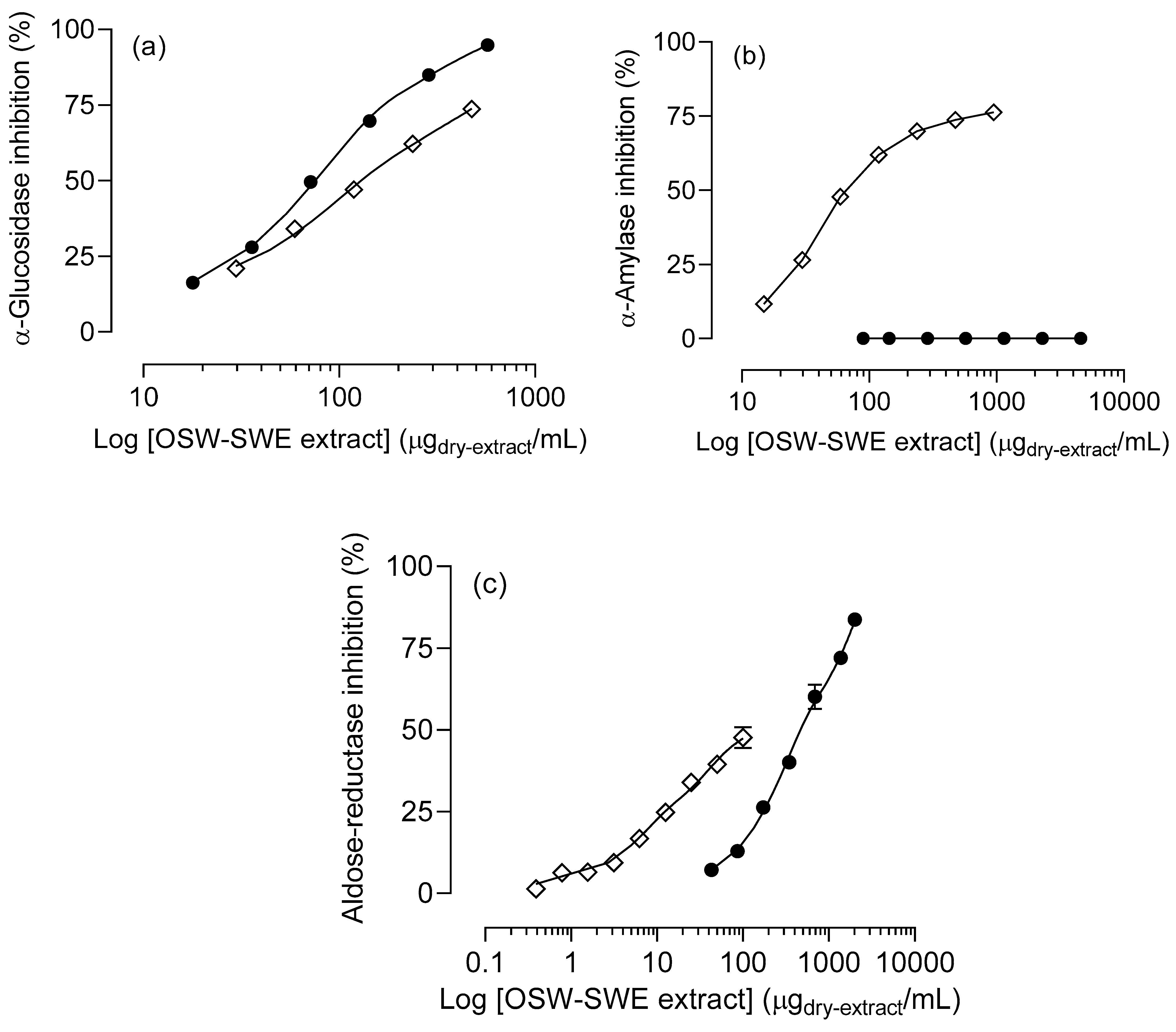
3.3. Effects on enzymatic digestion of carbohydrates
3.3.1. α-Amylase and α-glucosidase inhibition
3.3.2. Aldose-reductase inhibition
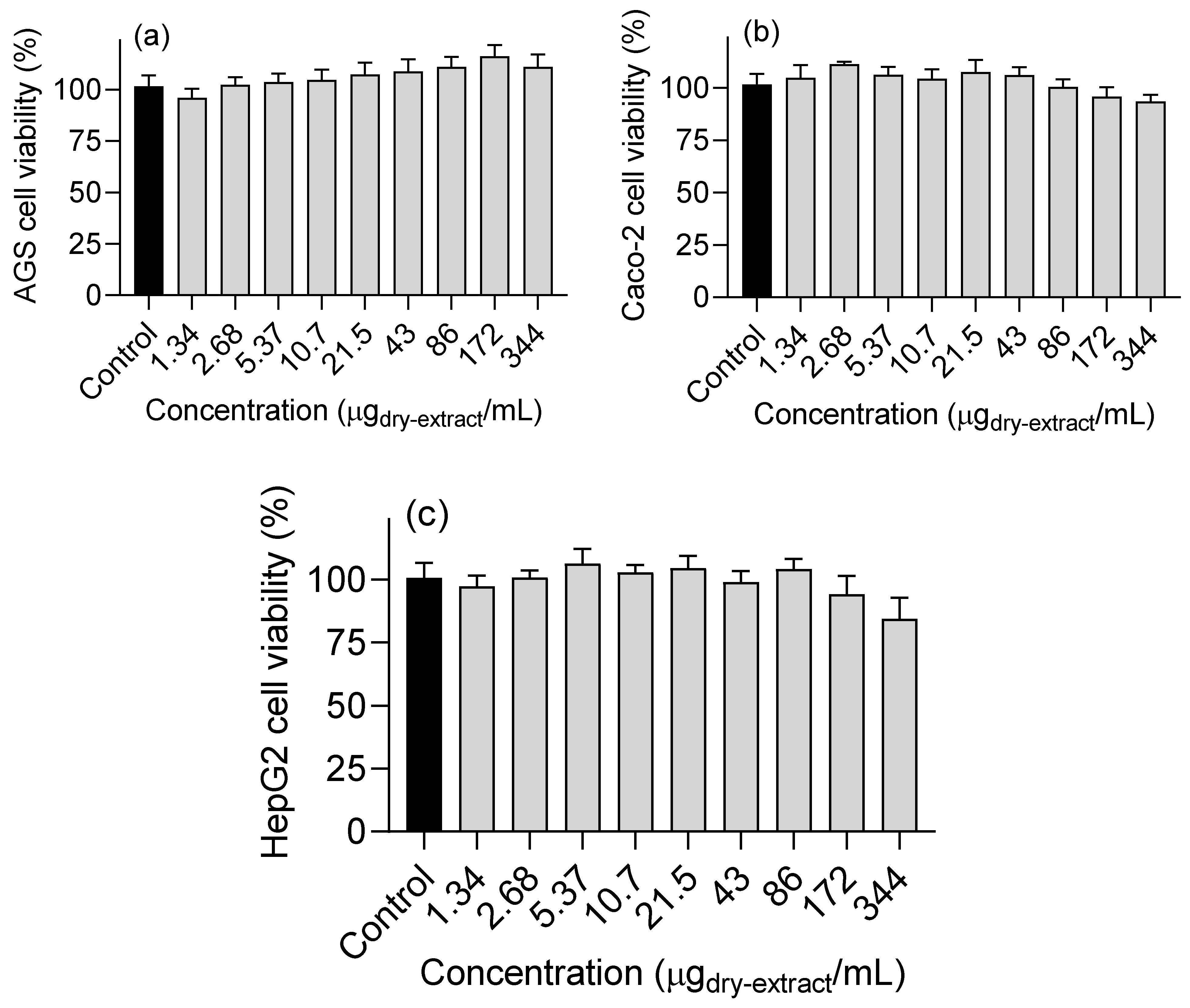
3.4. Effects on cell viability
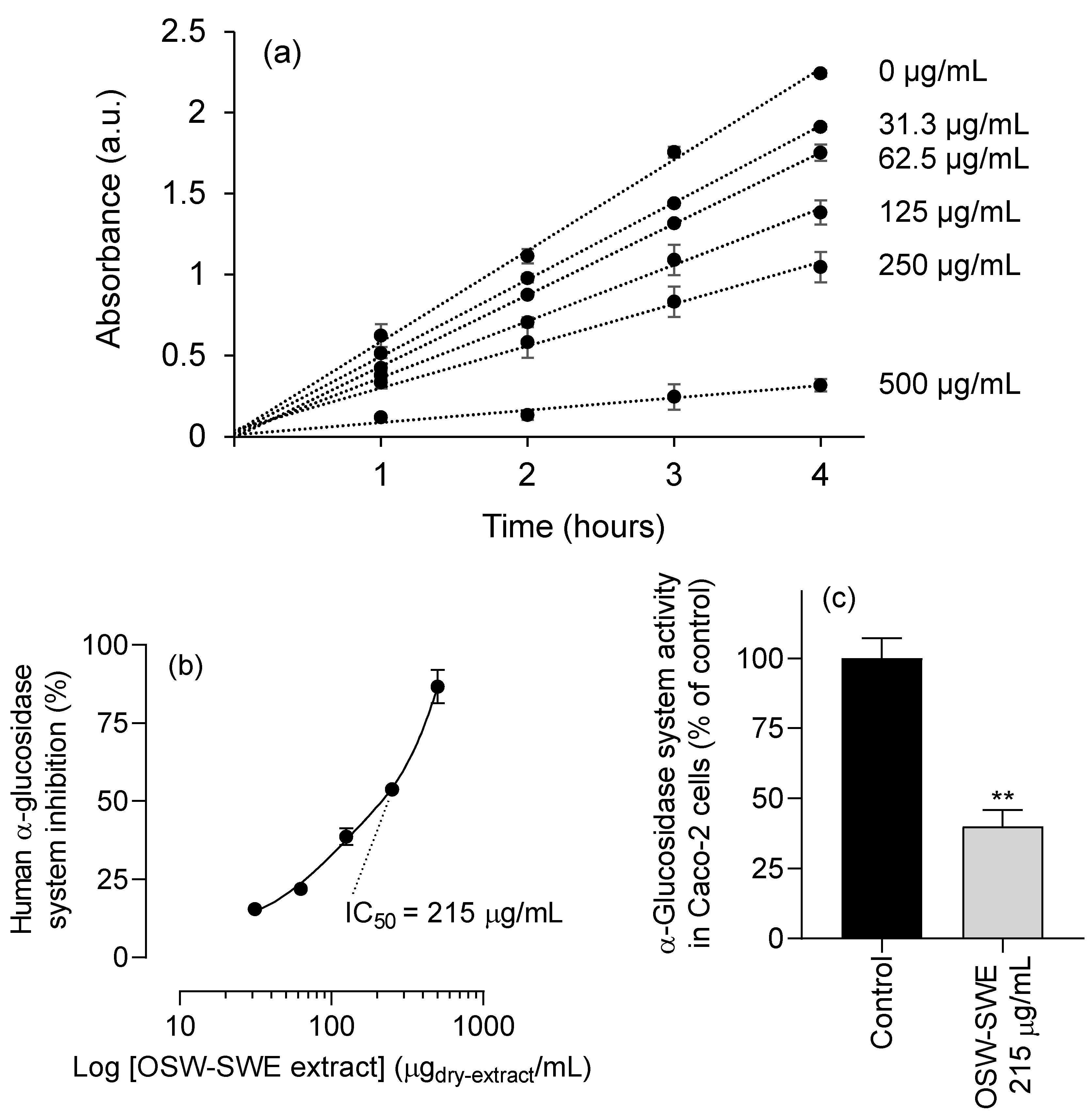
3.5. α-Glucosidase inhibition in Caco-2 cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar; Barbhai, M.D.; Hasan, M.; Punia, S.; Dhumal, S.; Radha; Rais, N.; Chandran, D.; Pandiselvam, R.; Kothakota, A.; et al. Onion (Allium Cepa L.) Peels: A Review on Bioactive Compounds and Biomedical Activities. Biomed. Pharmacother. 2022, 146, 112498. [Google Scholar] [CrossRef]
- Črnivec, I.G.; Skrt, M.; Šeremet, D.; Sterniša, M.; Farčnik, D.; Štrumbelj, E.; Poljanšek, A.; Cebin, N.; Pogačnik, L.; Smole Možina, S.; et al. Waste Streams in Onion Production: Bioactive Compounds, Quercetin and Use of Antimicrobial and Antioxidative Properties. Waste Manag. 2021, 126, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Marefati, N.; Ghorani, V.; Shakeri, F.; Boskabady, M.; Kianian, F.; Rezaee, R.; Boskabady, M.H. A Review of Anti-Inflammatory, Antioxidant, and Immunomodulatory Effects of Allium Cepa and Its Main Constituents. Pharm. Biol. 2021, 59, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Zhou, S. Phenolic Components and Health Beneficial Properties of Onions. Agriculture 2021, 11, 872. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S. Antimicrobial Assessment of Polyphenolic Extracts from Onion (Allium Cepa L.) Skin of Fifteen Cultivars by Sonication-Assisted Extraction Method. Heliyon 2020, 6, e05478. [Google Scholar] [CrossRef]
- Neeland, I.J.; Patel, K. V. Diabetes: Key Markers of Injury and Prognosis. In Biomarkers in Cardiovascular Disease; Elsevier Inc.,, 2019; pp. 41–51. ISBN 9780323548359. [Google Scholar]
- Santosh, H.N.; David, C.M. Role of Ascorbic Acid in Diabetes Mellitus: A Comprehensive Review. J. Med. Radiol. Pathol. Surg. 2017, 4, 1–3. [Google Scholar] [CrossRef]
- IDF IDF Diabetes Atlas 2021 - 10th Edition; 2021; ISBN 9782930229980.
- Mishra, V.; Nayak, P.; Sharma, M.; Albutti, A.; Alwashmi, A.S.S.; Aljasir, M.A.; Alsowayeh, N.; Tambuwala, M.M. Emerging Treatment Strategies for Diabetes Mellitus and Associated Complications: An Update. Pharmaceutics 2021, 13, 1568. [Google Scholar] [CrossRef]
- Kim, K.T.; Rioux, L.E.; Turgeon, S.L. Alpha-Amylase and Alpha-Glucosidase Inhibition Is Differentially Modulated by Fucoidan Obtained from Fucus Vesiculosus and Ascophyllum Nodosum. Phytochemistry 2014, 98, 27–33. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Ibrahim, A.; Dahiru, N.J.; Mohammed, H.S. Alpha Amylase Inhibitory Potential and Mode of Inhibition of Oils from Allium Sativum (Garlic) and Allium Cepa (Onion). Clin. Med. Insights Endocrinol. Diabetes 2020, 13, 1–5. [Google Scholar] [CrossRef]
- Kim, M.H.; Jo, S.H.; Jang, H.D.; Lee, M.S.; Kwon, Y.I. Antioxidant Activity and α-Glucosidase Inhibitory Potential of Onion (Allium Cepa L.) Extracts. Food Sci. Biotechnol. 2010, 19, 159–164. [Google Scholar] [CrossRef]
- Benito-Román; Blanco, B.; Sanz, M.T.; Beltrán, S. Freeze-Dried Extract from Onion (Allium Cepa Cv. Horcal) Skin Wastes: Extraction Intensification and Flavonoids Identification. Food Bioprod. Process. 2021, 130, 92–105. [Google Scholar] [CrossRef]
- Trigueros, E.; Sanz, M.T.; Alonso-Riaño, P.; Beltrán, S.; Ramos, C.; Melgosa, R. Recovery of the Protein Fraction with High Antioxidant Activity from Red Seaweed Industrial Solid Residue after Agar Extraction by Subcritical Water Treatment. J. Appl. Phycol. 2020. [Google Scholar] [CrossRef]
- Atanasova, A.; Petrova, A.; Teneva, D.; Ognyanov, M.; Georgiev, Y.; Nenov, N.; Denev, P. Subcritical Water Extraction of Rosmarinic Acid from Lemon Balm (Melissa Officinalis L.) and Its Effect on Plant Cell Wall Constituents. Antioxidants 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.; Silva, A.M.; Moreira, M.M.; Salazar, M.; Švarc-Gajić, J.; Brezo-Borjan, T.; Cádiz-Gurrea, M. de la L.; Carretero, A.S.; Loschi, F.; Dall’Acqua, S.; et al. Salicornia Ramosissima: A New Green Cosmetic Ingredient with Promising Skin Effects. Antioxidants 2022, 11, 1–18. [Google Scholar] [CrossRef]
- Mandura Jarić, A.; Čikoš, A.; Pocrnić, M.; Aladić, K.; Jokić, S.; Šeremet, D.; Vojvodić Cebin, A.; Komes, D. Teucrium Montanum L.—Unrecognized Source of Phenylethanoid Glycosides: Green Extraction Approach and Elucidation of Phenolic Compounds via NMR and UHPLC-HR MS/MS. Antioxidants 2023, 12. [Google Scholar] [CrossRef]
- Boateng, I.D. Mechanisms, Capabilities, Limitations, and Economic Stability Outlook for Extracting Phenolics from Agro-Byproducts Using Emerging Thermal Extraction Technologies and Their Combinative Effects. Food Bioprocess Technol. 2023. [Google Scholar] [CrossRef]
- Cravotto, C.; Grillo, G.; Binello, A.; Gallina, L.; Olivares-vicente, M.; Herranz-lópez, M.; Micol, V.; Barrajón-catalán, E.; Cravotto, G. Bioactive Antioxidant Compounds from Chestnut Peels through Semi-Industrial Subcritical Water Extraction. Antioxidants 2022, 11. [Google Scholar] [CrossRef]
- Dunford, N.T.; Gumus, Z.P.; Gur, C.S. Chemical Composition and Antioxidant Properties of Pecan Shell Water Extracts. Antioxidants 2022, 11, 1–13. [Google Scholar] [CrossRef]
- Benito-Román, Ó.; Blanco, B.; Sanz, M.T.; Beltrán, S. Subcritical Water Extraction of Phenolic Compounds from Onion Skin Wastes (Allium Cepa Cv. Horcal): Effect of Temperature and Solvent Properties. Antioxidants 2020, 9, 1233. [Google Scholar] [CrossRef]
- Trigueros, E.; Ramos, C.; Alonso-Riaño, P.; Beltrán, S.; Sanz, M.T. Subcritical Water Treatment for Valorization of the Red Algae Residue after Agar Extraction: Scale-Up from Laboratory to Pilot Plant. Ind. Eng. Chem. Res. 2023, 62, 3503–3514. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colometric Methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Nielsen, S.S. Phenol-Sulfuric Acid Method for Total Carbohydrates. In Food Analysis Laboratory Manual; Nielsen, S.S., Ed.; Springer, 2010; pp. 47–53. ISBN 9781441914620. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dry Bingin. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ferreres, F.; Andrade, C.; Gomes, N.G.M.; Andrade, P.B.; Gil-Izquierdo, A.; Pereira, D.M.; Suksungworn, R.; Duangsrisai, S.; Videira, R.A.; Valentão, P. Valorisation of Kitul, an Overlooked Food Plant: Phenolic Profiling of Fruits and Inflorescences and Assessment of Their Effects on Diabetes-Related Targets. Food Chem. 2021, 342, 128323. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Kim, K.T.; Kim, H.J.; Chung, M.S.; Chang, P.S.; Park, H.; Pai, H.D. Antioxidant Activities of Onion (Allium Cepa L.) Peel Extracts Produced by Ethanol, Hot Water, and Subcritical Water Extraction. Food Sci. Biotechnol. 2014, 23, 615–621. [Google Scholar] [CrossRef]
- Kim; Ko, M.J.; Park, C.H.; Chung, M.S. Application of Pulsed Electric Field as a Pre-Treatment for Subcritical Water Extraction of Quercetin from Onion Skin. Foods 2022, 11, 1069. [Google Scholar] [CrossRef]
- Dhanya, R. Quercetin for Managing Type 2 Diabetes and Its Complications, an Insight into Multitarget Therapy. Biomed. Pharmacother. 2022, 146, 112560. [Google Scholar] [CrossRef]
- Rocha, L.; Neves, D.; Valentão, P.; Andrade, P.B.; Videira, R.A. Adding Value to Polyvinylpolypyrrolidone Winery Residue: A Resource of Polyphenols with Neuroprotective Effects and Ability to Modulate Type 2 Diabetes-Relevant Enzymes. Food Chem. 2020, 329, 127168. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Chen, D.; Chen, S. Antioxidant Activity and Mechanism of Protocatechuic Acid in Vitro. Funct. Foods Heal. Dis. 2011, 7, 232–244. [Google Scholar] [CrossRef]
- Kumari, N.; Kumar, M.; Radha; Lorenzo, J.M.; Sharma, D.; Puri, S.; Pundir, A.; Dhumal, S.; Bhuyan, D.J.; Jayanthy, G.; et al. Onion and Garlic Polysaccharides: A Review on Extraction, Characterization, Bioactivity, and Modifications. Int. J. Biol. Macromol. 2022, 219, 1047–1061. [Google Scholar] [CrossRef]
- Khalili, S.; Saeidi Asl, M.R.; Khavarpour, M.; Vahdat, S.M.; Mohammadi, M. Comparative Study on the Effect of Extraction Solvent on Total Phenol, Flavonoid Content, Antioxidant and Antimicrobial Properties of Red Onion (Allium Cepa). J. Food Meas. Charact. 2022, 16, 3578–3588. [Google Scholar] [CrossRef]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, Vegetables, and Mushrooms for the Preparation of Extracts with α-Amylase and α-Glucosidase Inhibition Properties: A Review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding Phenolic Acids Inhibition of α-Amylase and α-Glucosidase and Influence of Reaction Conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef]
- Ruivo Da Silva, M.G.; Skrt, M.; Komes, D.; Poklar Ulrih, N.; Pogačnik, L. Enhanced Yield of Bioactivities from Onion (Allium Cepa L.) Skin and Their Antioxidant and Anti-α-Amylase Activities. Int. J. Mol. Sci. 2020, 21, 2909. [Google Scholar] [CrossRef]
- Sun, L.; Warren, F.J.; Gidley, M.J. Natural Products for Glycaemic Control: Polyphenols as Inhibitors of Alpha-Amylase. Trends Food Sci. Technol. 2019, 91, 262–273. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Zhang, J.; Yan, L.; Liu, S.; Taha, A.A.; Wang, J.; Ma, C. Subcritical Water Extraction of Phenolic Antioxidants with Improved α-Amylase and α-Glucosidase Inhibitory Activities from Exocarps of Castanea Mollissima Blume. J. Supercrit. Fluids 2020, 158, 104747. [Google Scholar] [CrossRef]
- Ti, Y.; Wang, W.; Wang, X.; Ban, Y.; Wang, P.; Zhang, Y.; Song, Z. Pumpkin Polysaccharide Extracted by Subcritical Water: Physicochemical Characterization and Anti-Diabetic Effects in T2DM Rats. Mol. Nutr. Food Res. 2022, 66, 2200160. [Google Scholar] [CrossRef]
- Wang, S.T.; Dan, Y.Q.; Zhang, C.X.; Lv, T.T.; Qin, Z.; Liu, H.M.; Ma, Y.X.; He, J.R.; Wang, X. De Structures and Biological Activities of Proanthocyanidins Obtained from Chinese Quince by Optimized Subcritical Water-Ethanol Extraction. J. Food Meas. Charact. 2022, 17, 1703–1713. [Google Scholar] [CrossRef]
- Wu, T.; Luo, J.; Xu, B. In Vitro Antidiabetic Effects of Selected Fruits and Vegetables against Glycosidase and Aldose Reductase. Food Sci. Nutr. 2015, 3, 495–505. [Google Scholar] [CrossRef]
- Yang, J.; Meyers, K.J.; Van Der Heide, J.; Rui, H.L. Varietal Differences in Phenolic Content and Antioxidant and Antiproliferative Activities of Onions. J. Agric. Food Chem. 2004, 52, 6787–6793. [Google Scholar] [CrossRef]
- Jocković, N.; Fischer, W.; Brandsch, M.; Brandt, W.; Dräger, B. Inhibition of Human Intestinal α-Glucosidases by Calystegines. J. Agric. Food Chem. 2013, 61, 5550–5557. [Google Scholar] [CrossRef]
| Phenolic Standard | Wavelength = 280 nm | ||||
| Regression equation | R2 | Concentration range (µg) | LOD (µg) | LOQ (µg) | |
| Protocatechuic acid | y = 1721.4x + 61.2 | 0.9984 | 0.072-8.582 | 0.022 | 0.067 |
| p-Coumaric acid | y = 5839.2x +187.2 | 0.9990 | 0.034-13.0 | 0.010 | 0.030 |
| Quercetin-3-O-glucoside | y = 1218.3x - 8.6 | 0.9998 | 0.020-2.61 | 0.006 | 0.018 |
| Quercetin-4'-O-glucoside | y = 1310.3x + 14.9 | 0.9998 | 0.050-5.94 | 0.015 | 0.045 |
| Myricetin | y = 1535.4x - 70.9 | 0.9994 | 0.268-2.679 | 0.030 | 0.090 |
| Quercetin | y = 1578.5x + 165.1 | 0.9990 | 0.127-15.81 | 0.030 | 0.090 |
| Kaempferol | y = 2036.4x - 3.4 | 0.9999 | 0.073-2.273 | 0.020 | 0.060 |
| Isorhamnetin | y = 3877x + 106.0 | 0.9980 | 0.063-3.136 | 0.006 | 0.018 |
| Phenolic Standard | Wavelength = 330 nm | ||||
| Regression equation | R2 | Concentration range (µg) | LOD (µg) | LOQ (µg) | |
| Protocatechuic acid | nd | nd | nd | nd | nd |
| p-Coumaric acid | y = 4923.4x + 362.1 | 0.9983 | 0.037-13 | 0.010 | 0.030 |
| Quercetin-3-O-glucoside | y = 1992x - 16.6 | 0.9999 | 0.02-2.61 | 0.006 | 0.018 |
| Quercetin-4'-O-glucoside | y = 1793.7x + 29.5 | 0.9998 | 0.0397-5.94 | 0.010 | 0.030 |
| Myricetin | y = 2099.4x - 131.3 | 0.9994 | 0.268-2.679 | 0.030 | 0.090 |
| Quercetin | y = 2012.4x + 322.9 | 0.9969 | 0.067-15.81 | 0.020 | 0.060 |
| Kaempferol | y = 2792.7x - 6.3 | 0.9999 | 0.033-2.273 | 0.010 | 0.030 |
| Isorhamnetin | y = 4892.2x + 233.9 | 0.9956 | 0.063-6.272 | 0.006 | 0.018 |
| Phenolic Standard | Wavelength = 370 nm | ||||
| Regression equation | R2 | Concentration range (µg) | LOD (µg) | LOQ (µg) | |
| Protocatechuic acid | nd | nd | nd | nd | nd |
| p-Coumaric acid | nd | nd | nd | nd | nd |
| Quercetin-3-O-glucoside | y = 2199.6x -7.4 | 0.9965 | 0.099-2.61 | 0.003 | 0.010 |
| Quercetin-4'-O-glucoside | y = 2778.2x + 132.3 | 0.9994 | 0.030-5.94 | 0.006 | 0.018 |
| Myricetin | y = 4440.6x - 112.9 | 0.9999 | 0.268-2.679 | 0.010 | 0.030 |
| Quercetin | y = 3024.4x + 1677.8 | 0.9900 | 0.022-15.81 | 0.006 | 0.018 |
| Kaempferol | y = 4897.9x + 24.1 | 0.9999 | 0.023-2.273 | 0.005 | 0.015 |
| Isorhamnetin | y = 9324.1x + 462.2 | 0.9943 | 0.063-1.568 | 0.002 | 0.006 |
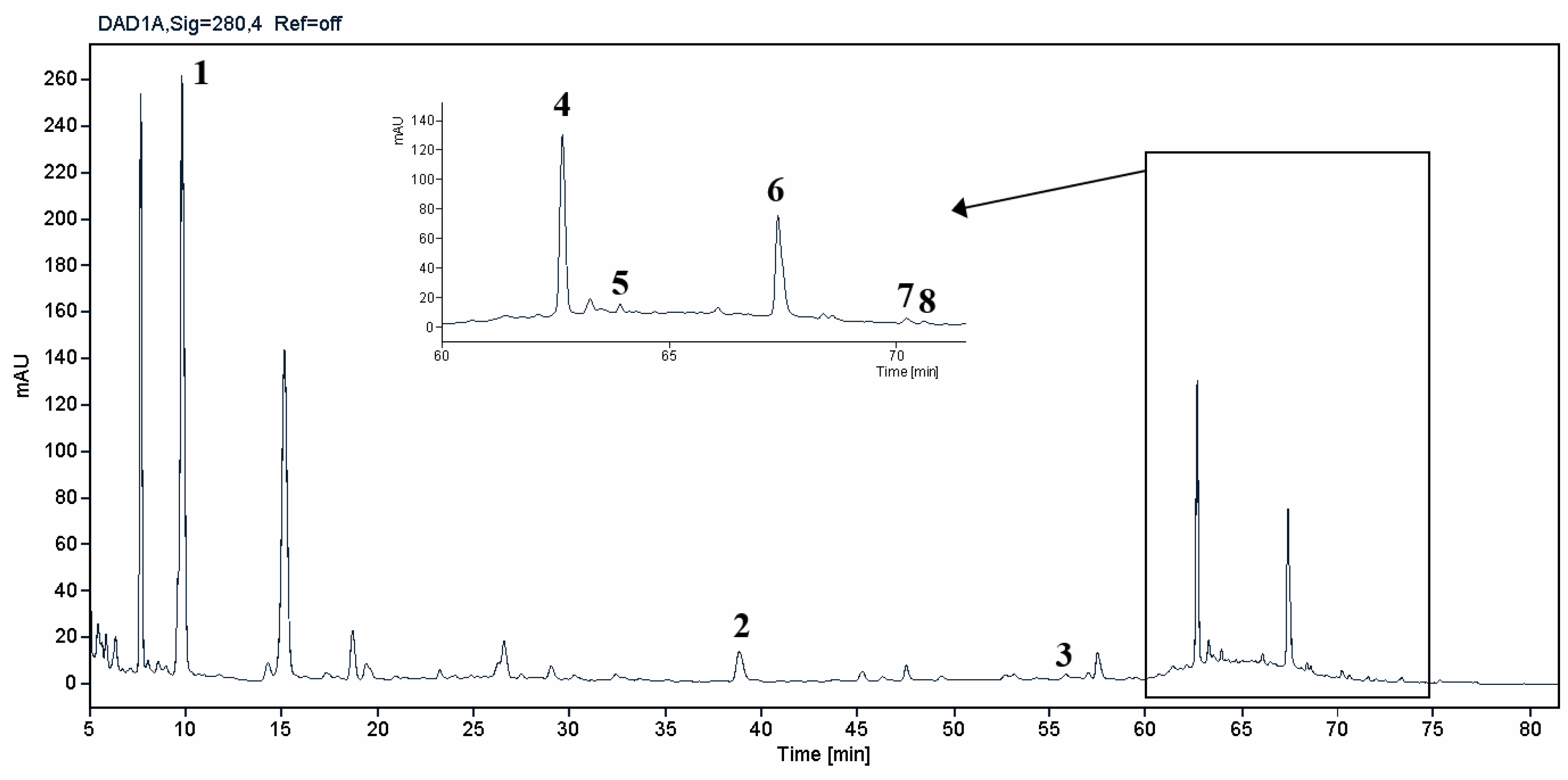
| Phenolic compounds: | OSW-SWE | |||||
| Rt (min) | (mg/gdry-extract)* | |||||
| 1 | Protocatechuic acid | 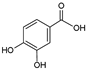 |
9.7 | 20.3i | ±i | 2.5 |
| 2 | p-Coumaric acid | 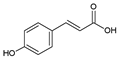 |
39.9 | 0.10i | ±i | 0.05 |
| 3 | Quercetin-3-O-glucoside | 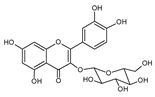 |
55.5 | 0.10i | ±i | 0.05 |
| 4 | Quercetin-4´-O-glucoside | 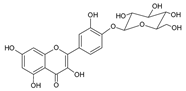 |
62.6 | 7.5i | ±i | 0.2 |
| 5 | Myricetin | 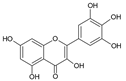 |
63.8 | 0.7i | ±i | 0.1 |
| 6 | Quercetin | 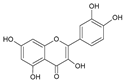 |
67.5 | 3.2i | ±i | 0.6 |
| 7 | Kaempferol | 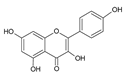 |
70.1 | 0.20i | ±i | 0.05 |
| 8 | Isorhamnetin | 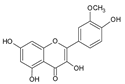 |
70.6 | 0.20i | ±i | 0.05 |
| ∑ phenolic compounds (mg/gdry-extract) | 32.3 | ±i | 2.6 | |||
| TFC (mgQEq/gdry-extract) (colorimetric assay) | 26.0 | ± | 3.0 | |||
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





