Submitted:
31 January 2024
Posted:
31 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
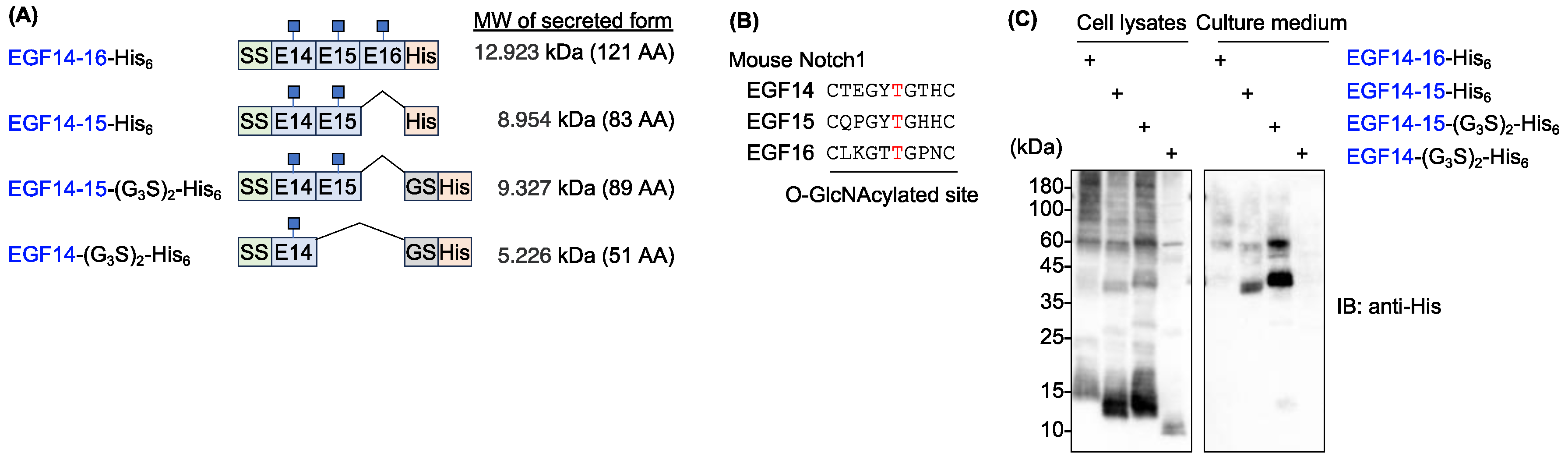
2.1. Mouse Notch1 fragments containing EGF14-16 tended to aggregate
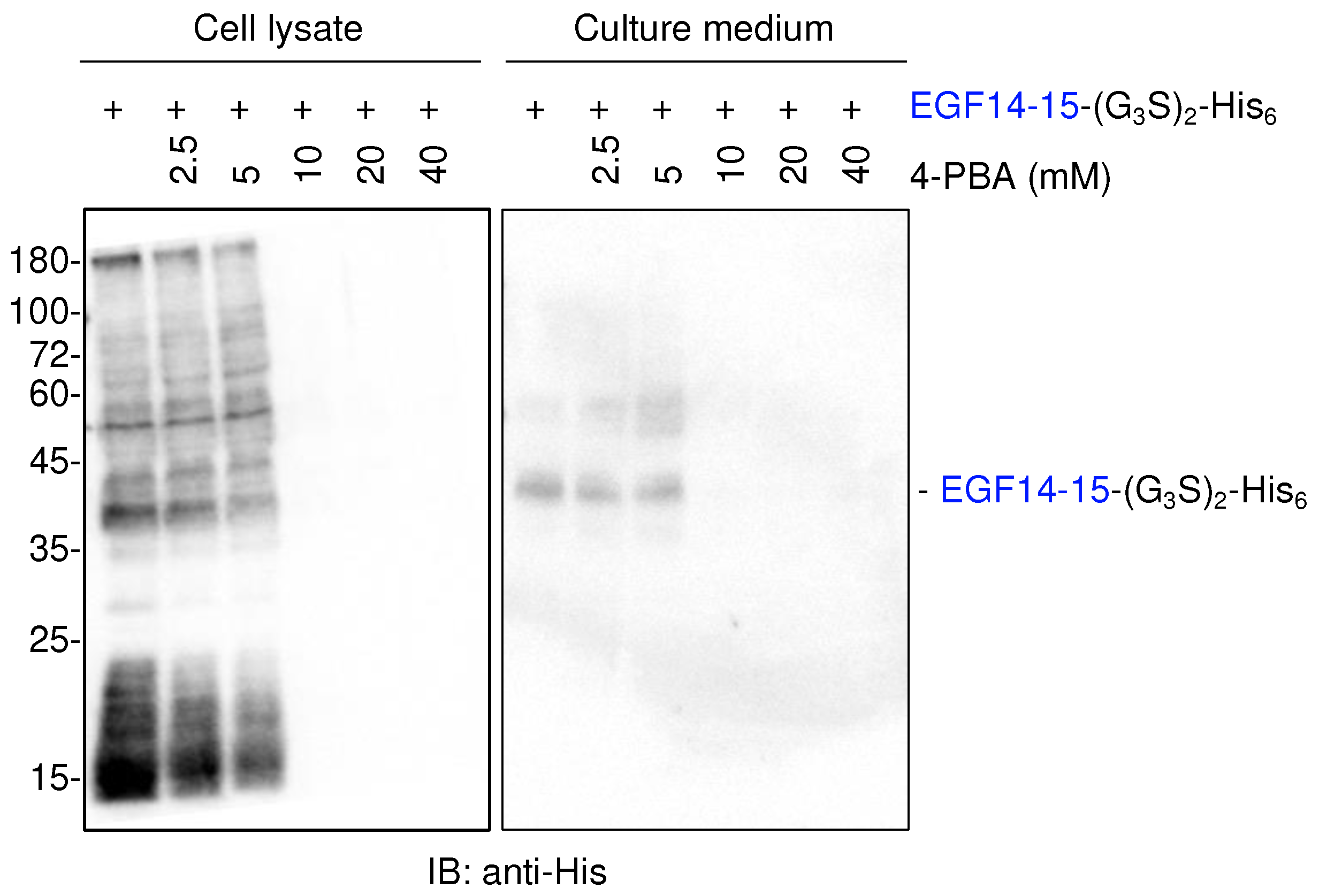
2.2. Chemical chaperone 4-PBA does not enhance the secretion of EGF14-15-(G3S)2-His6
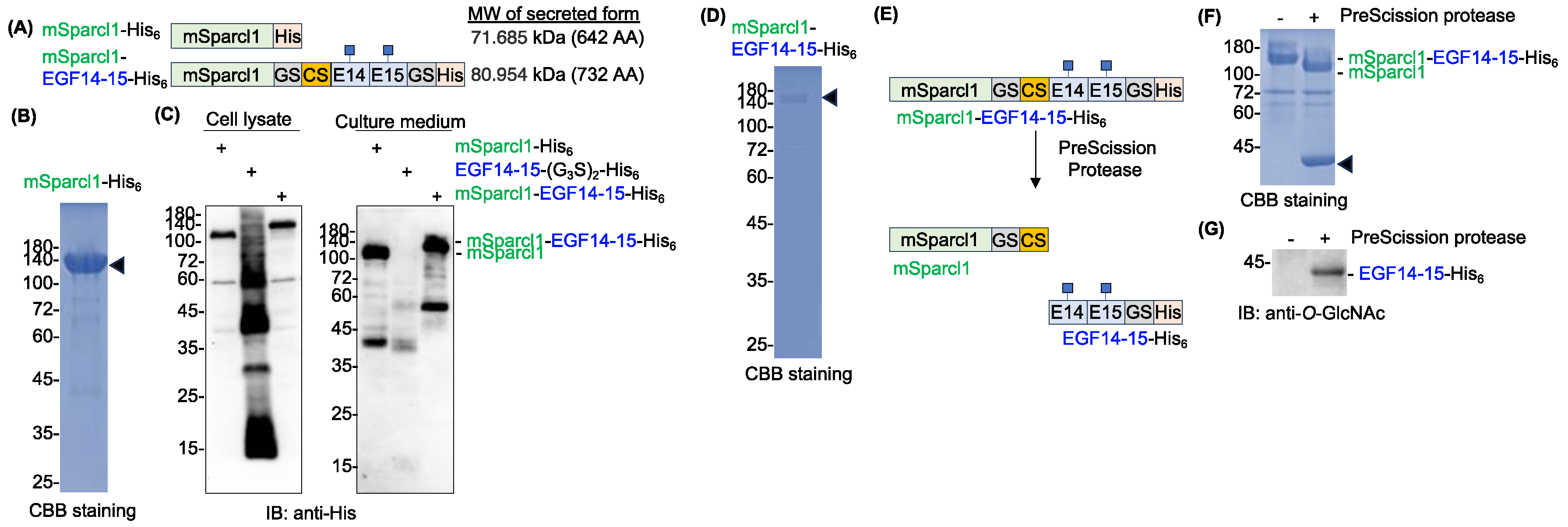
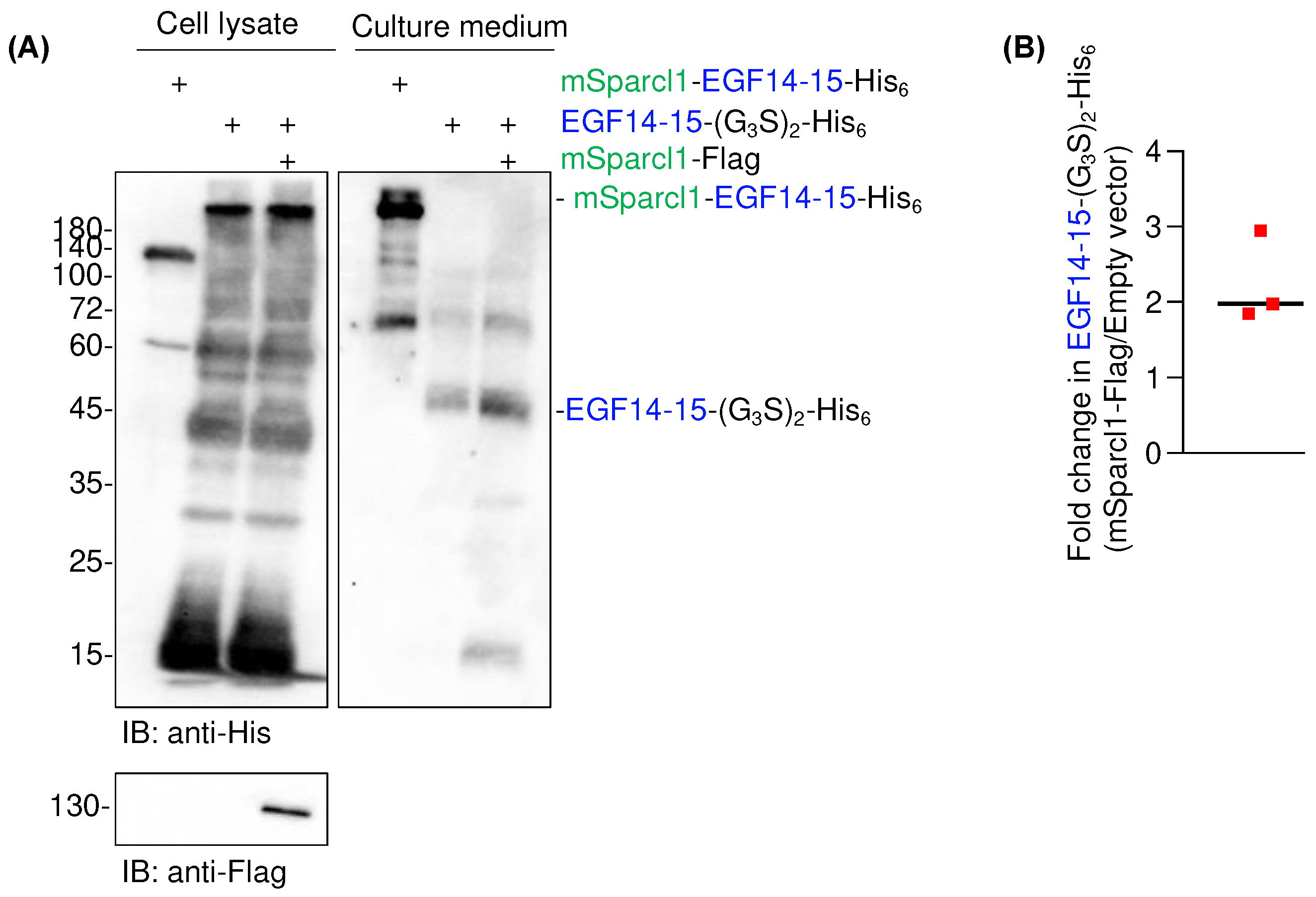
2.3. Sparcl1 fusion facilitates extracellular secretion of EGF14-15-(G3S)2-His6
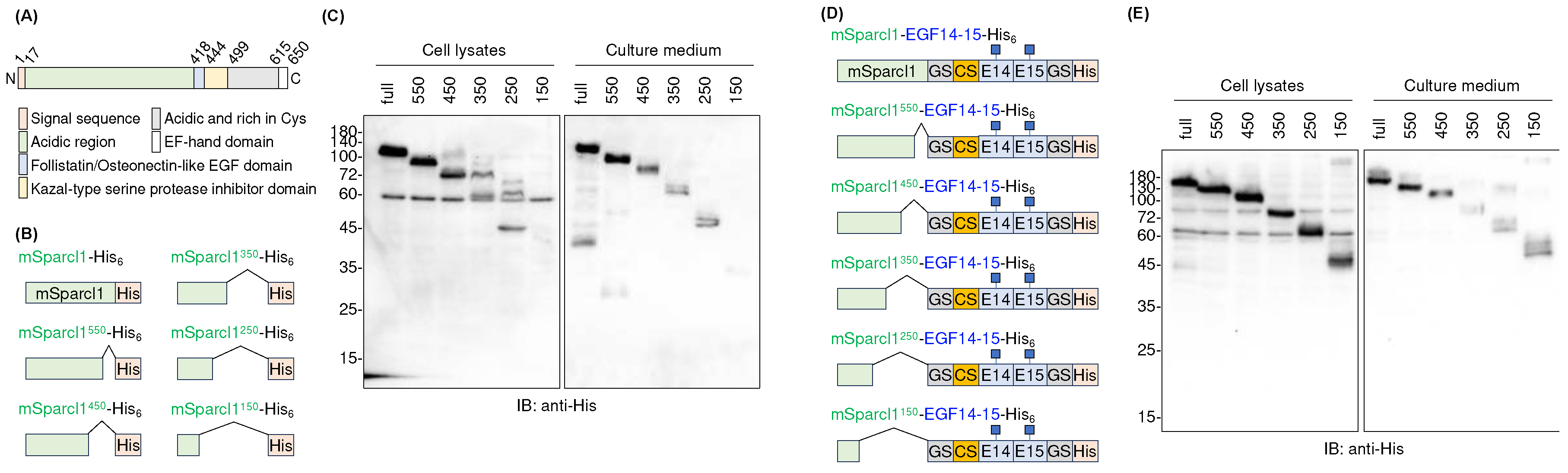
2.4. Deletion mutants of mSparcl1 and their effects on the secretion of EGF14-15-His6
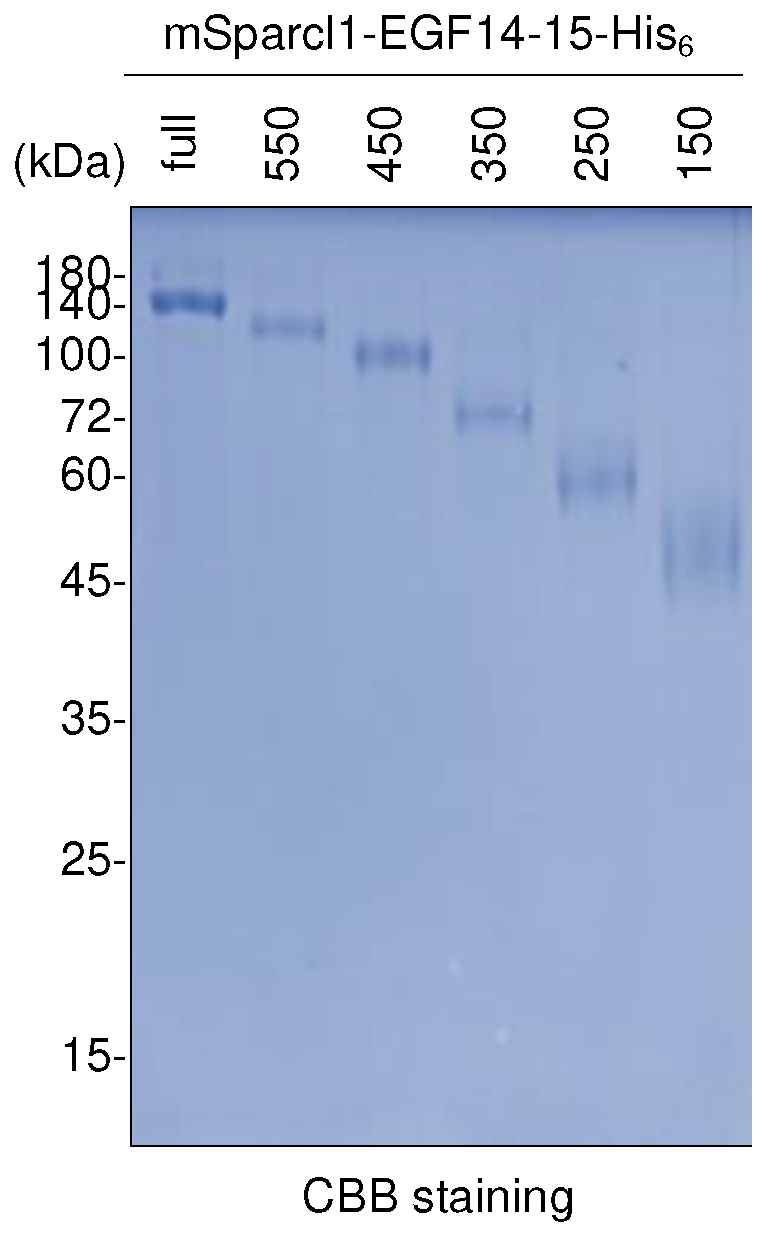
2.5. Purification of deletion mutants of mSparcl1-EGF14-15-His6
2.6. Co-transfection of mSparcl1 slightly enhances secretion of EGF14-15-(G3S)2-His6
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Plasmids
4.3. SDS-PAGE, Immunoblotting, and CBB Staining
4.4. Recombinant Protein Expression and Purification
4.5. Data Analysis and Statistics
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saiki, W.; Ma, C.; Okajima, T.; Takeuchi, H. Current Views on the Roles of O-Glycosylation in Controlling Notch-Ligand Interactions. Biomolecules 2021, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Haltiwanger, R.S. Significance of glycosylation in Notch signaling. Biochem. Biophys. Res. Commun. 2014, 453, 235–242. [Google Scholar] [CrossRef]
- Takeuchi, H.; Yu, H.; Hao, H.; Takeuchi, M.; Ito, A.; Li, H.; Haltiwanger, R.S. O-Glycosylation modulates the stability of epidermal growth factor-like repeats and thereby regulates Notch trafficking. J. Biol. Chem. 2017, 292, 15964–15973. [Google Scholar] [CrossRef]
- Tsukamoto, Y.; Ogawa, M.; Yogi, K.; Tashima, Y.; Takeuchi, H.; Okajima, T. Glycoproteomics of NOTCH1 EGF repeat fragments overexpressed with different glycosyltransferases in HEK293T cells reveals insights into O-GlcNAcylation of NOTCH1. Glycobiology 2022, 32, 616–628. [Google Scholar] [CrossRef]
- Sakaidani, Y.; Ichiyanagi, N.; Saito, C.; Nomura, T.; Ito, M.; Nishio, Y.; Nadano, D.; Matsuda, T.; Furukawa, K.; Okajima, T. O-linked-N-acetylglucosamine modification of mammalian Notch receptors by an atypical O-GlcNAc transferase Eogt1. Biochem Biophys Res Commun 2012, 419, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Stanley, P. Protein O -fucosyltransferase 1 is an essential component of Notch signaling pathways. Proc. Natl. Acad. Sci. USA 2003, 100, 5234–5239. [Google Scholar] [CrossRef]
- Fernandez-Valdivia, R.; Takeuchi, H.; Samarghandi, A.; Lopez, M.; Leonardi, J.; Haltiwanger, R. S.; Jafar-Nejad, H. Regulation of mammalian Notch signaling and embryonic development by the protein O-glucosyltransferase Rumi. Development 2011, 138, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Sawaguchi, S.; Varshney, S.; Ogawa, M.; Sakaidani, Y.; Yagi, H.; Takeshita, K.; Murohara, T.; Kato, K.; Sundaram, S.; Stanley, P.; Okajima, T. O-GlcNAc on NOTCH1 EGF repeats regulates ligand-induced Notch signaling and vascular development in mammals. eLife 2017, 6, e24419. [Google Scholar] [CrossRef]
- Tashima, Y.; Okajima, T. Congenital diseases caused by defective O-glycosylation of Notch receptors. Nagoya J Med Sci 2018, 80, 299–307. [Google Scholar] [CrossRef]
- Cortez, L.; Sim, V. The therapeutic potential of chemical chaperones in protein folding diseases. Prion 2014, 8, 197–202. [Google Scholar] [CrossRef]
- Delic, M.; Göngrich, R.; Mattanovich, D.; Gasser, B. Engineering of protein folding and secretion—Strategies to overcome bottlenecks for efficient production of recombinant proteins. Antioxid Redox Signal. 2014, 21, 414–437. [Google Scholar] [CrossRef]
- Andersen, E.; Chollet, M.E.; Baroni, M.; Pinotti, M.; Bernardi, F.; Skarpen, E.; Sandset, P.M.; Skretting, G. The effect of the chemical chaperone 4-phenylbutyrate on secretion and activity of the p.Q160R missense variant of coagulation factor FVII. Cell Biosci. 2019, 9, 69. [Google Scholar] [CrossRef]
- Besio, R.; Iula, G.; Garibaldi, N.; Cipolla, L.; Sabbioneda, S.; Biggiogera, M.; Marini, J.C.; Rossi, A.; Forlino, A. 4-PBA ameliorates cellular homeostasis in fibroblasts from osteogenesis imperfecta patients by enhancing autophagy and stimulating protein secretion. Biochim Biophys Acta Mol Basis Dis 2018, 1864 (5 Pt A), 1642–1652. [Google Scholar] [CrossRef]
- Iwayama, T.; Iwashita, M.; Miyashita, K.; Sakashita, H.; Matsumoto, S.; Tomita, K.; Bhongsatiern, P.; Kitayama, T.; Ikegami, K.; Shimbo, T.; et al. Plap-1 lineage tracing and single-cell transcriptomics reveal cellular dynamics in the periodontal ligament. Development 2022, 149. [Google Scholar] [CrossRef]
- Klingler, A.; Regensburger, D.; Tenkerian, C.; Britzen-Laurent, N.; Hartmann, A.; Stürzl, M.; Naschberger, E. Species-, organ- and cell-type-dependent expression of SPARCL1 in human and mouse tissues. PLoS ONE 2020, 15, e0233422. [Google Scholar] [CrossRef] [PubMed]
- Viloria, K.; Munasinghe, A.; Asher, S.; Bogyere, R.; Jones, L.; Hill, N.J. A holistic approach to dissecting SPARC family protein complexity reveals FSTL-1 as an inhibitor of pancreatic cancer cell growth. Sci. Rep. 2016, 6, 37839. [Google Scholar] [CrossRef]
- Bradshaw, A.D. Diverse biological functions of the SPARC family of proteins. Int. J. Biochem. Cell Biol. 2012, 44, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Gangwar, S.P.; Machius, M.; Rudenko, G. Interplay between hevin, SPARC, and MDGAs: Modulators of neurexin-neuroligin transsynaptic bridges. Structure 2021, 29, 664–678.e6. [Google Scholar] [CrossRef] [PubMed]
- Naschberger, E.; Liebl, A.; Schellerer, V.S.; Schütz, M.; Britzen-Laurent, N.; Kölbel, P.; Schaal, U.; Haep, L.; Regensburger, D.; Wittmann, T.; et al. Matricellular protein SPARCL1 regulates tumor microenvironment–dependent endothelial cell heterogeneity in colorectal carcinoma. J. Clin. Investig. 2016, 126, 4187–4204. [Google Scholar] [CrossRef]
- Kalucka, J.; de Rooij, L.P.M.H.; Goveia, J.; Rohlenova, K.; Dumas, S.J.; Meta, E.; Conchinha, N.V.; Taverna, F.; Teuwen, L.-A.; Veys, K.; et al. Single-Cell Transcriptome Atlas of Murine Endothelial Cells. Cell 2020, 180, 764–779.e20. [Google Scholar] [CrossRef]
- Bae, J.-H.; Sung, B.H.; Kim, H.-J.; Park, S.-H.; Lim, K.-M.; Kim, M.-J.; Lee, C.-R.; Sohn, J.-H. An Efficient Genome-Wide Fusion Partner Screening System for Secretion of Recombinant Proteins in Yeast. Sci. Rep. 2015, 5, 12229. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, C.; Pan, Y.; Qin, L.; Zheng, L.; Zhao, M.; Huang, M. Optimization of Protein Folding for Improved Secretion of Human Serum Albumin Fusion Proteins in Saccharomyces cerevisiae. J. Agric. Food Chem. 2023, 71, 18414–18423. [Google Scholar] [CrossRef]
- Bruijn, L.I.; Houseweart, M.K.; Kato, S.; Anderson, K.L.; Anderson, S.D.; Ohama, E.; Reaume, A.G.; Scott, R.W.; Cleveland, D.W. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science 1998, 281, 1851–1854. [Google Scholar] [CrossRef]
- DiFiglia, M.; Sapp, E.; Chase, K.O.; Davies, S.W.; Bates, G.P.; Vonsattel, J.P.; Aronin, N. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 1997, 277, 1990–1993. [Google Scholar] [CrossRef]
- Narhi, L.; Wood, S. J.; Steavenson, S.; Jiang, Y.; Wu, G. M.; Anafi, D.; Kaufman, S. A.; Martin, F.; Sitney, K.; Denis, P.; Louis, J. C.; Wypych, J.; Biere, A. L.; Citron, M. Both familial Parkinson's disease mutations accelerate alpha-synuclein aggregation. J Biol Chem 1999, 274, 9843–9846. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.A.; Poirier, M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004, 10 (Suppl. S7), S10–S17. [Google Scholar] [CrossRef]
- Risher, W. C.; Patel, S.; Kim, I. H.; Uezu, A.; Bhagat, S.; Wilton, D. K.; Pilaz, L. J.; Singh Alvarado, J.; Calhan, O. Y.; Silver, D. L.; Stevens, B.; Calakos, N.; Soderling, S. H.; Eroglu, C. Astrocytes refine cortical connectivity at dendritic spines. eLife 2014, 3, e04047. [Google Scholar] [CrossRef] [PubMed]
- Scalabrini, D.; Fenoglio, C.; Scarpini, E.; De Riz, M.; Comi, C.; Venturelli, E.; Cortini, F.; Piola, M.; Villa, C.; Naldi, P.; et al. Candidate gene analysis of SPARCL1 gene in patients with multiple sclerosis. Neurosci. Lett. 2007, 425, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Taketomi, T.; Yasuda, T.; Morita, R.; Kim, J.; Shigeta, Y.; Eroglu, C.; Harada, R.; Tsuruta, F. Autism-associated mutation in Hevin/Sparcl1 induces endoplasmic reticulum stress through structural instability. Sci. Rep. 2022, 12, 11891. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




