1. Introduction
Lithium-ion batteries are widely used in electronic products, scale energy storage, military industry, aerospace and other fields due to the advantages of high specific energy, long cycle life and no memory effect [
1,
2,
3]. Currently, the organic electrolytes used in lithium-ion batteries are flammable and explosive, which poses a major safety hazard at high power densities [
4,
5,
6,
7]. Ionic liquids (ILs), with high ionic conductivity, low viscosity, excellent thermal stability, negligible vapor pressure, and a wide electrochemical window [
8,
9,
10] are expected to solve the safety problems of lithium-ion batteries and improve its electrochemical performance [
11,
12,
13]. In addition, ILs can also broaden the operating voltage of the battery [
14] and increase its temperature range. However, ILs are expensive due to high production costs, resulting in a high threshold for their application [
15,
16,
17]. On the other hand, a large amount of waste liquid containing low concentration of ionic liquids will be generated, which needs to be properly disposed prior to discharge. Otherwise the potential toxicity of ionic liquids and their low natural degradation property will increase environmental pollution risk [
18,
19,
20].
Lithium mainly exists in lithium ores, salt lake brines and seawater. After years of development, lithium ore resources have been on the verge of depletion. Therefore, obtaining lithium resources from salt lake brine, geothermal brine, seawater and lithium containing industrial wastewater is of strategic importance [
21,
22,
23].
Currently, the separation and recovery of lithium ions mainly focuses on the separation of Li
+ from divalent cations such as Mg
2+, Ca
2+, Co
2+, Ni
2+, Mn
2+, and Fe
2+ [
24,
25,
26,
27], and there are also some reports on the separation of Li
+ from Na
+ and K
+ [
28,
29,
30]. [Bmim]
+(1-Butyl-3-methylimidazolium) is an ionic liquid cation with promising applications in high-voltage electrolytes for lithium-ion batteries, but there are few reports on the separation of Li
+ and [Bmim]
+ cation. Because of same charge properties and charge numbers, the separation between monovalent cations is difficult. The research on the separation of Li
+ and [Bmim]
+ cation can provide technical support for the recovery of new high-efficiency lithium ion batteries using ionic liquid as electrolyte in the future, and can also provide reference for the separation between other monovalent cations.
Considering the size and structural differences between Li+ and [Bmim]+ cation, there exists a competitive migration phenomenon when they pass through the cation exchange membrane. Driven by an electric field, this work explores a green separation method of Li+ and [Bmim]+ cation without the addition of external reagents.
2. Experimental
2.1. Equipment and Methods
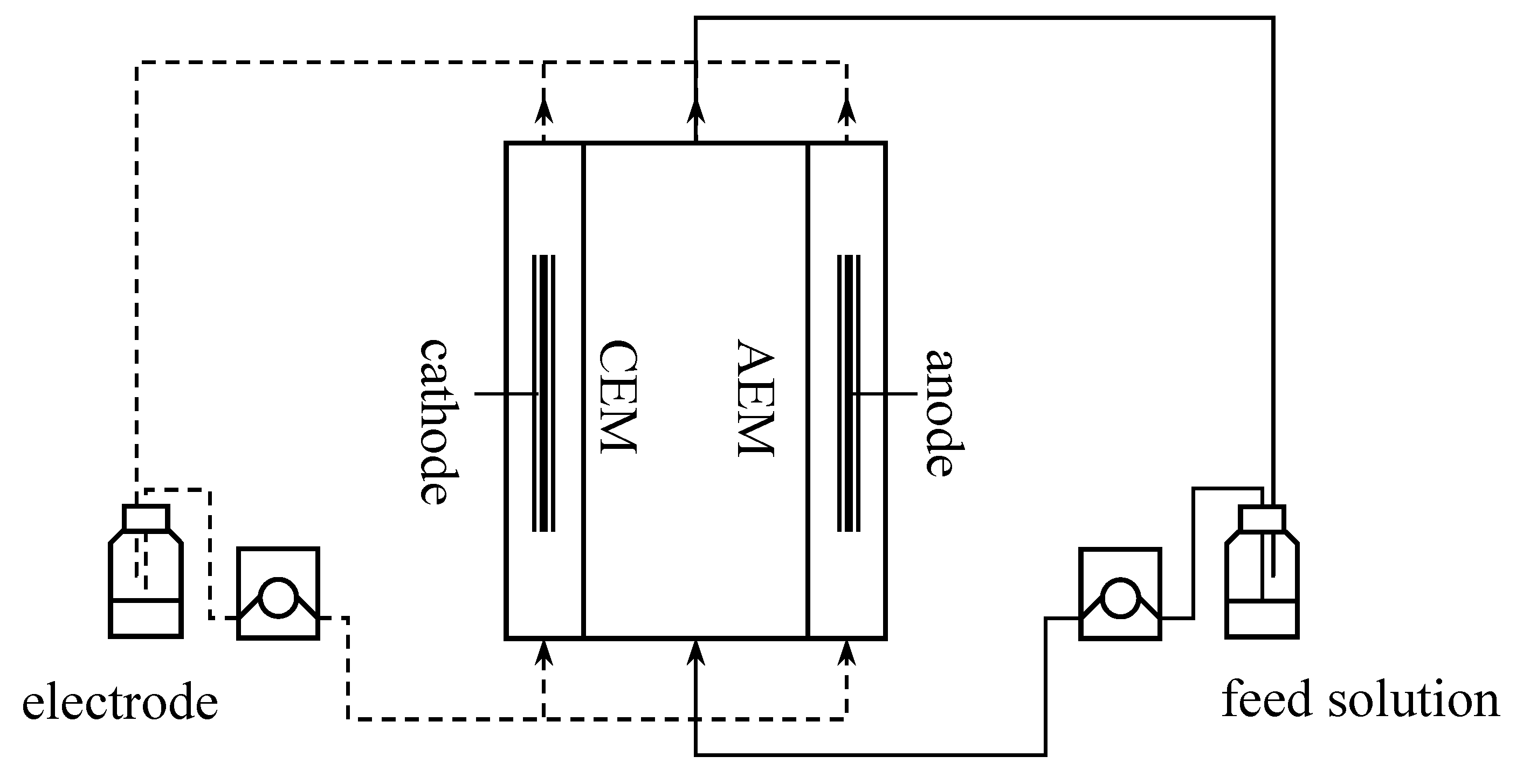
The laboratory-scale electrically driven membrane separation unit used is schematically shown in
Figure 1. It consists of an electrodialysis chamber (desalination chamber) and two electrode chambers; the two chambers are connected to share a common electrode solution to maintain the pH value of the solution; both the electrode solution and the feed solution are circulated by a peristaltic pump. The anode and cathode were made of ruthenium coated titanium and titanium respectively. The separation unit was powered by a constant-current DC power supply (omitted from the figure). The electrode solution used in the experiments was 150 mL 0.1 mol/L LiNO
3 solution, and the feed solution was 150 mL LiCl and [Bmim]Cl solution; the flow rate of the peristaltic pump was 100 mL/min.
The selective transport coefficient was used to evaluate the separation effect of the process, which could be calculated as follows:
Style:
ΔQt(Li+)--Decrease in Li+ in the electrodialysis chamber at moment t, mol;
ΔQt([Bmim]+)--Decrease in [Bmim]+ of the electrodialysis chamber at moment t, mol;
Q0(Li+)--The amount of Li+ in the electrodialysis chamber at the initial time, mol;
Q0([Bmim]+)--The amount of [Bmim]+in the electrodialysis chamber at the initial time, mol.
2.2. Cation Exchange Membranes and Modification
Five types of cation exchange membranes were studied. Among them, three types are commercial cation exchange membranes, namely N-117 (Jiangsu Thinkre Membrane Materials Co., Ltd.), CMB, and CMX (Astom Corportation). CMX is a standard model, so Modified membrane A and B are obtained by modifying CMX. The properties of cation exchange membranes are shown in
Table 1. Pretreatment is required before modification, and the specific process is as follows: Firstly, the cation exchange membrane CMX is immersed in deionized water for 4 hours, then immersed in 1 mol/L sulfuric acid for 4 h, rinsed and immersed in 1 mol/L sodium hydroxide for 4 h, and immersed in deionized water for 4 h again, then rinsed repeatedly with a large amount of deionized water to remove surface residual ions. The next step of modified membrane A was to immerse the CMX membrane into 0.5 mol/L polypyrrole solution at a constant temperature of 25°C, stirred for 8 h, stood for 24 h, and then rinsed with deionized water, thus obtained the Modified membrane A. The modification step of the Modified membrane B was to immerse the CMX membrane in methanol solution at 25 °C for 24 h, and rinsed it with deionized water, and then immersed it in a solution of 0.5 mol/L polypyrrole and 0.1 mol/L hydrogen peroxide at 25°C, stirred for 8 h, stood for 24 h, then immersed in 0.05 mol/L sulfuric acid solution for 2 h, finally removed it and rinsed with deionized water, thus obtained the Modified membrane B.
2.3. Analytical Methods
2.3.1. Analytical Methods of [Bmim]+
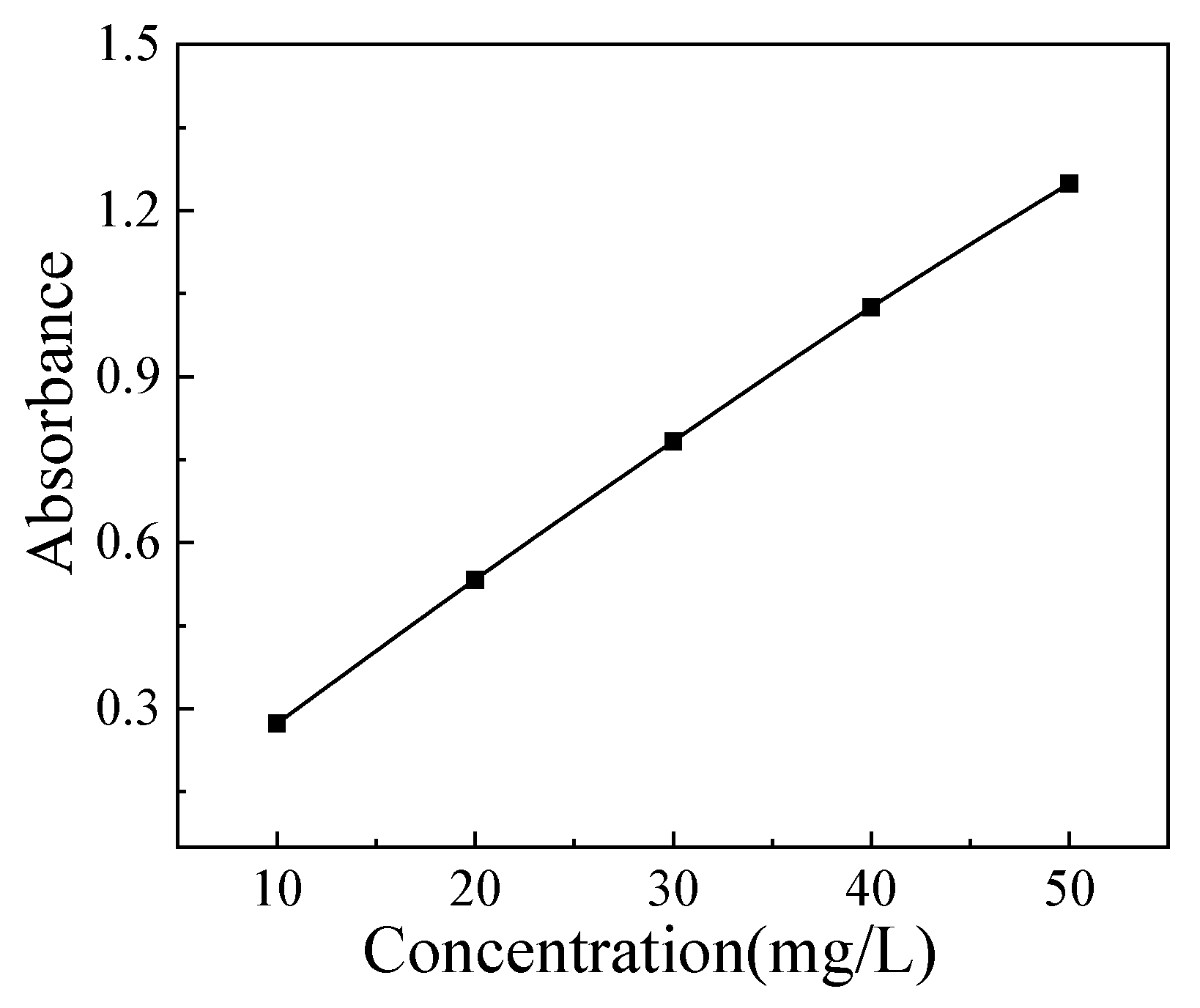
The concentration of [Bmim]
+ was determined by UV spectrophotometry. In this study, the maximum absorption wavelength of [Bmim]
+ is 211 nm. Weigh several grams of [Bmim]Cl, then dissovle it in 500 mL deioned water. Then dilute it to obtain solutions with concentrations of 10, 20, 30, 40, and 50 mg/L, respectively. The standard curve was got from these solutions, and it is shown in
Figure 2. The standard curve equation is: y=0.095+3.981x, r
2=0.999.
2.3.2. Analytical Methods of Lithium Ion
The concentration of Li+ was analyzed by atomic absorption spectrometry (ICP-AES). The analysis method is the standard curve method, which is mainly suitable for samples with coexisting components that do not interfere with each other. Measure the absorbance of a series of standard solutions under the same conditions and obtain a standard curve. Then, measure the absorbance of the sample under the same conditions to obtain the lithium content of the sample to be tested.
3. Results and Discussion
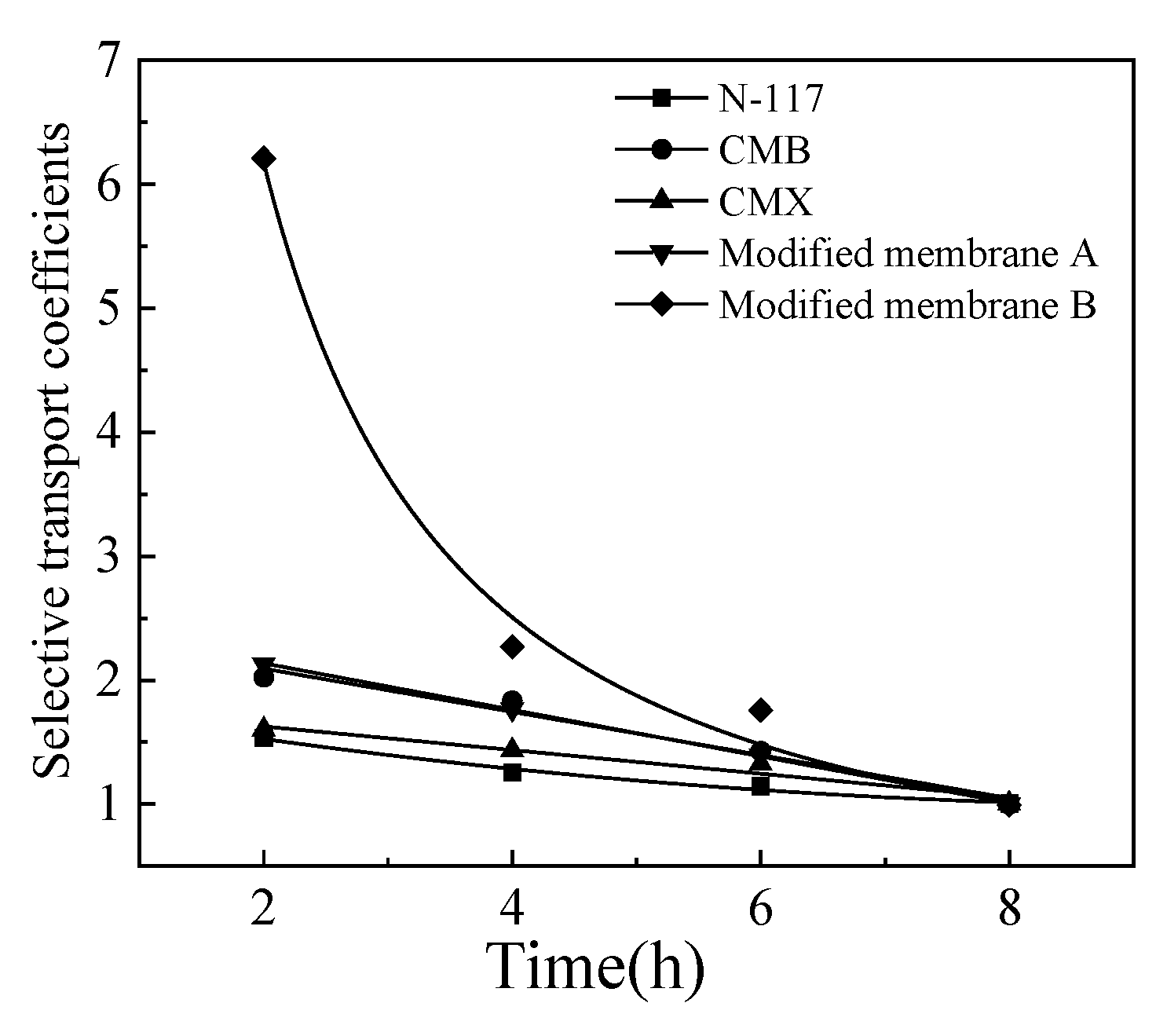
3.1. Comparison of Cation Exchange Membranes
The current density of was set to 5 mA/cm
2, and the feed concentrations of Li
+ and [Bmim]
+ were 0.3 mol/L and 0.2 mol/L, respectively. The separation performances of the five types of selected membranes for Li
+ and [Bmim]
+ were compared. The results are shown in
Figure 3. All the tested five membranes showed selective transport of Li
+ from [Bmim]
+, and the modified membrane B showing the most excellent selective performance, with the selective transport coefficients of up to 6.2 in the first 120 minutes of the process. The selectivity performance of N-117 and CMX is not satisfactory, with the maximum selection transport coefficients during the experiment being 1.53 and 1.60, respectively. The selectivity performance of CMB and modified membrane A has slightly improved, with the maximum selectivity transmittance coefficients of 2.02 and 2.12, respectively. By modifying CMX with polypyrrole solution, the pore size of the cation exchange membrane is reduced, thereby enhancing the effect of pore size screening on ion separation. However, the performance of the cation exchange membrane modified by impregnation method is not stable enough, and the surface modification layer is prone to detachment. The modified membrane B has improved the stability of the modified layer through hydrogen peroxide oxidation, therefore, the modified membrane B showed the best selectivity performance.
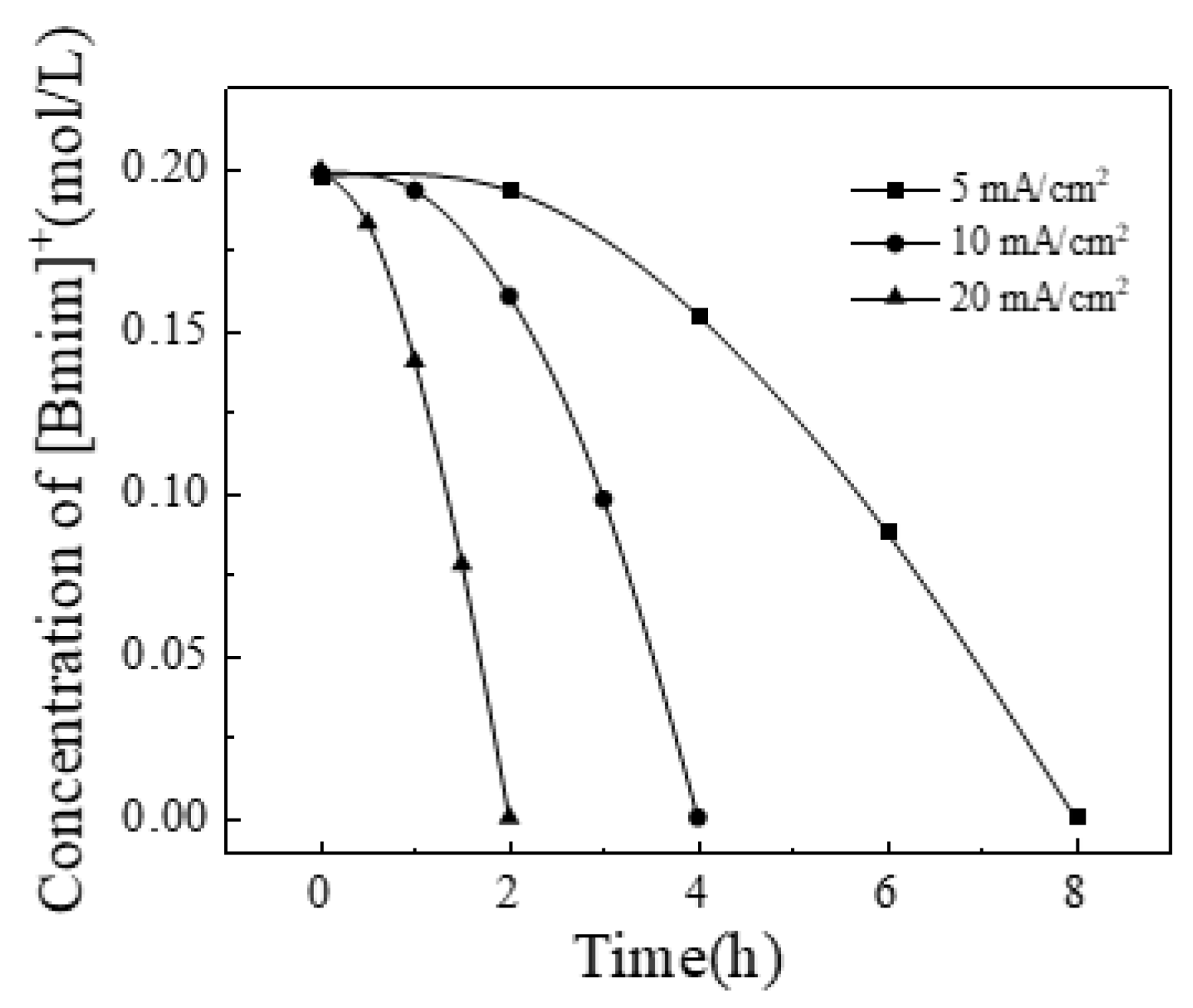
3.2. The Effect of Current Density
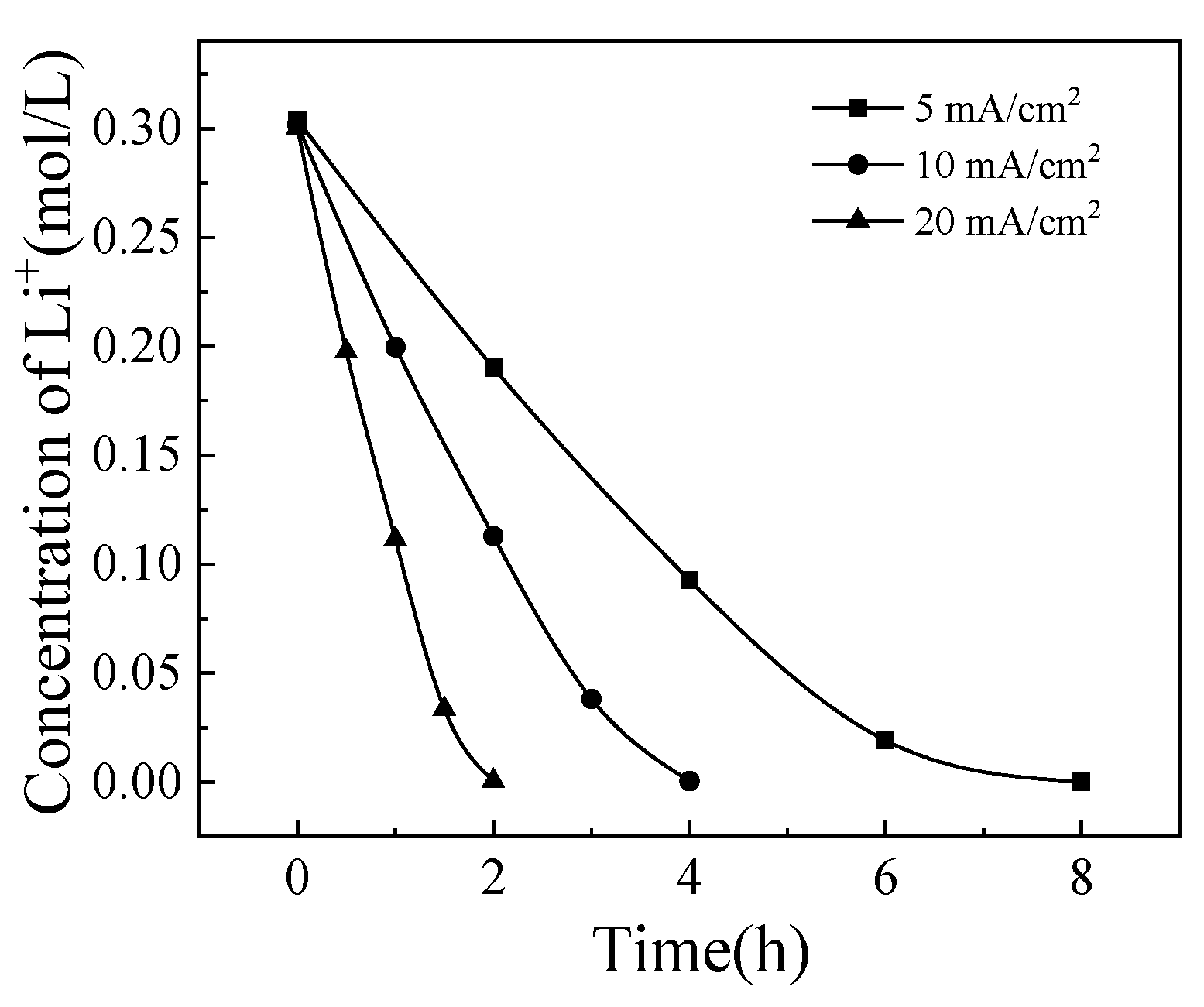
Since the modified membrane B showed best separation performance, the experiments were carried out using the modified membrane B in all subsequent studies. The concentrations of Li
+ and [Bmim]
+ were 0.3 and 0.2 mol/L. Constant current densities of 5, 10, and 20 mA/cm
2 were applied to investigate its effect on the process. The changes in Li
+ and [Bmim]
+ concentrations over time were shown in
Figure 4 and
Figure 5. It can be seen that the decreasing rate of [Bmim]
+ concentration increased gradually, while the decreasing rate of Li
+ concentration slows down gradually. In the initial stage of the process, due to the membrane selectivity, Li
+ preferentially transmembrane transport. As the process progresses, the Li
+ content in the solution gradually decreases, and the total amount of cations migrated is fixed during the constant current process. More [Bmim]
+ replace Li
+ for transmembrane migration, resulting in this phenomenon.
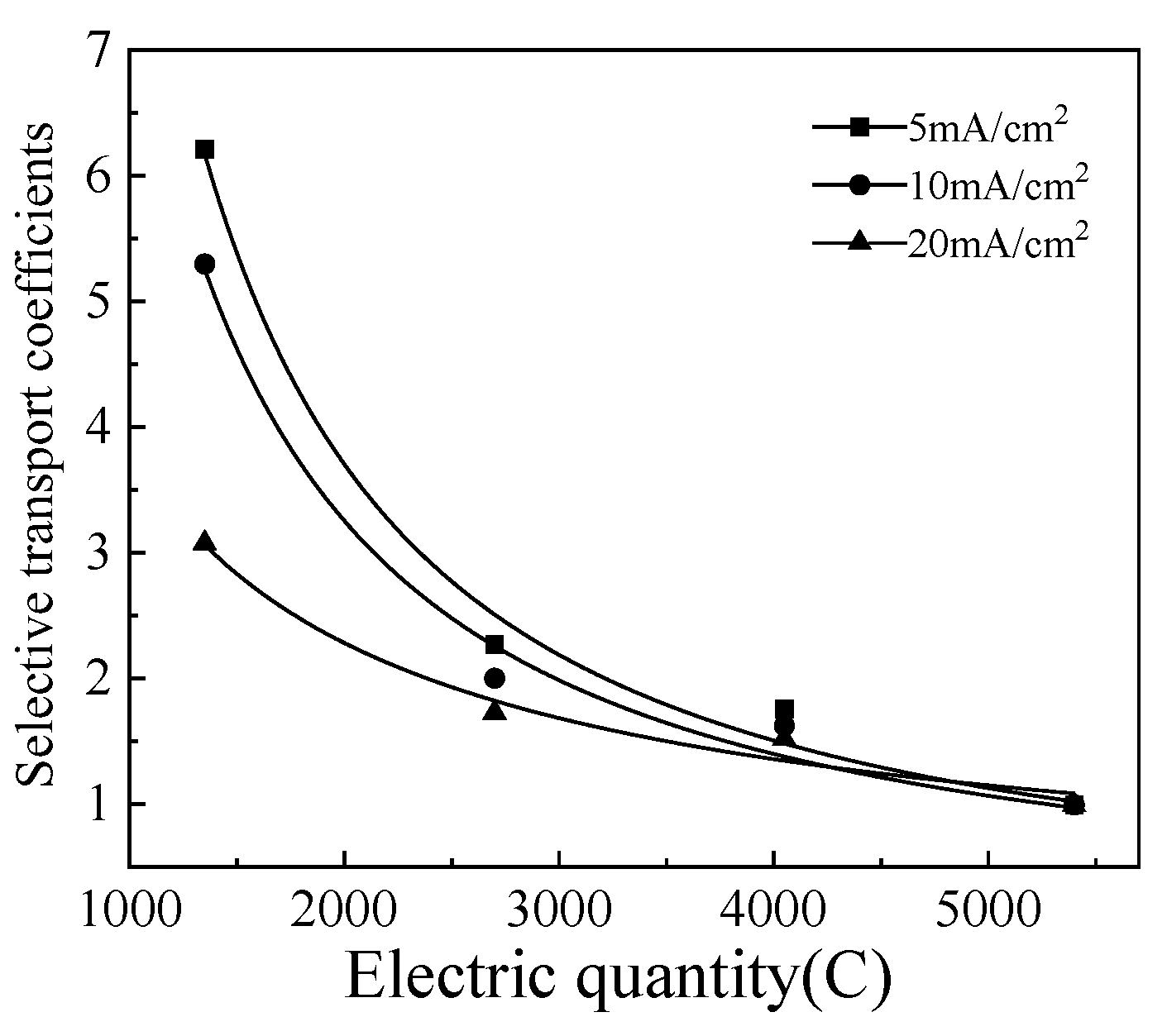
Different current densities result in different separation times for the same solution. It is more convenient to study the effect of current density on separation process under the same electric quantity passing by the membrane. The selective transport coefficients of Li
+ and [Bmim]
+ with the electric quantity was shown in
Figure 6. It can be seen that when the current density is 5 mA/cm
2, the selective transport coefficients is the highest, and Li
+ is more likely to pass through the cation exchange membrane. Therefore, under the conditions of these tests, a current density of 5 mA/cm
2 is the optimal condition for separating Li
+ and [Bmim]
+.
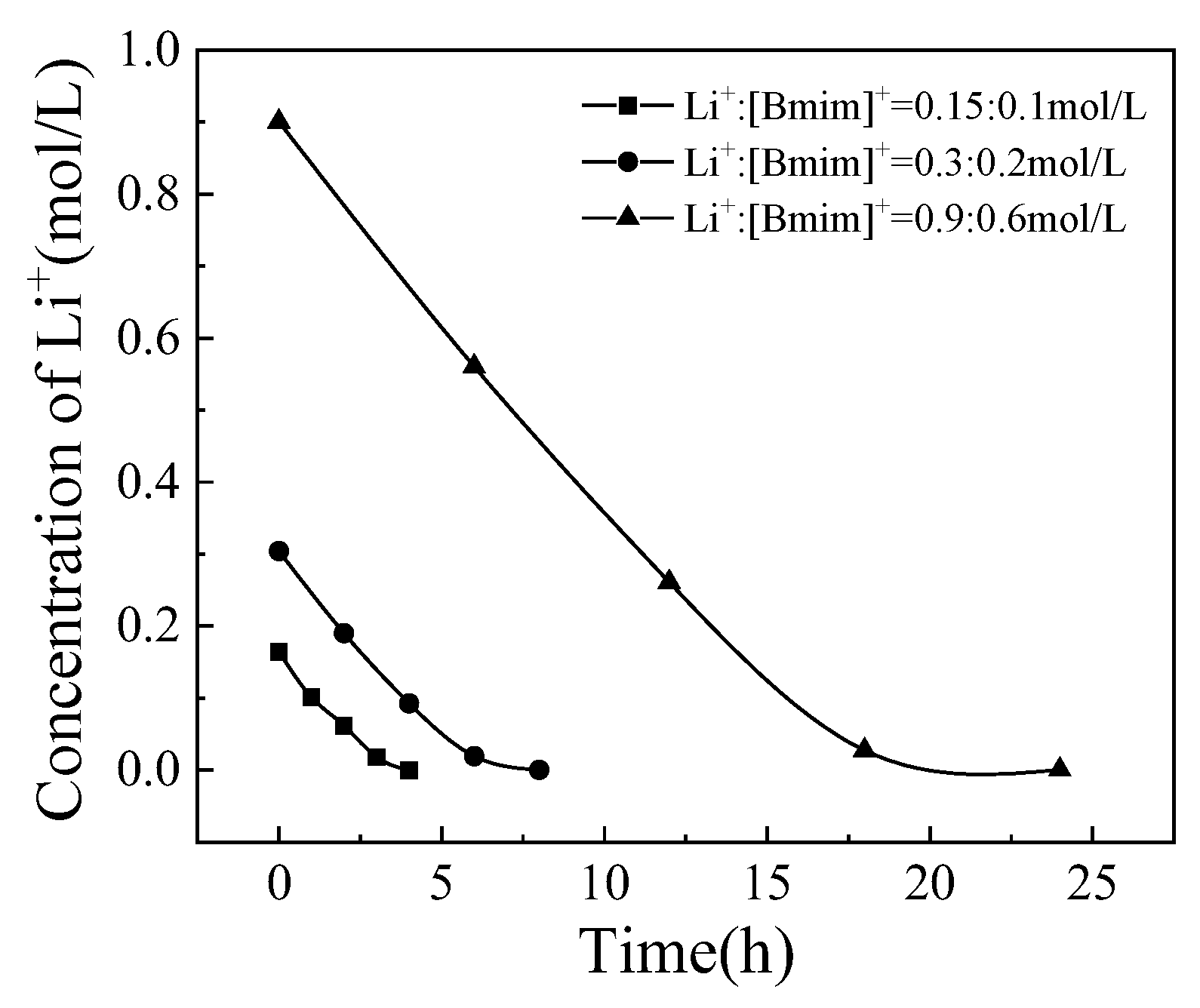
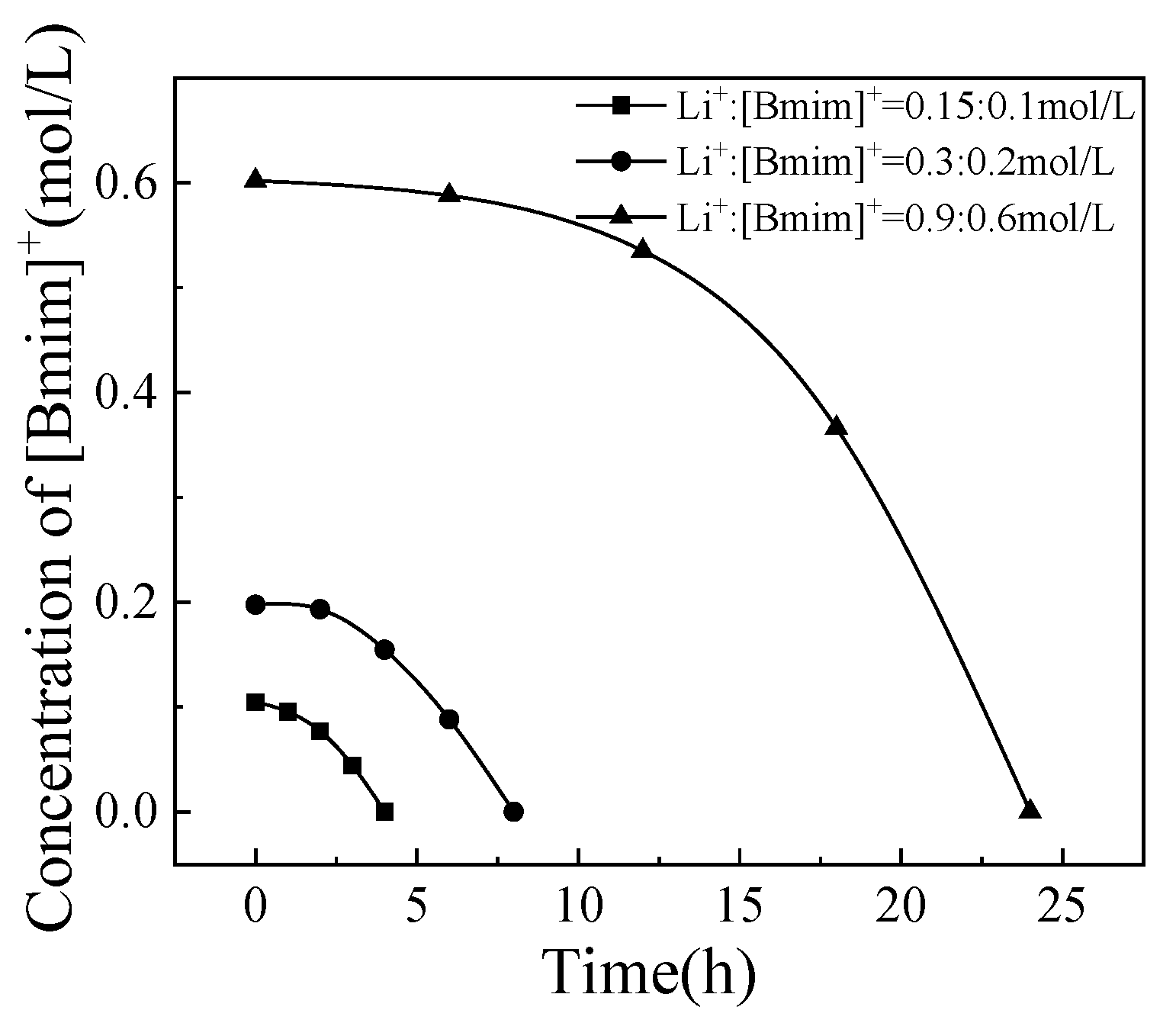
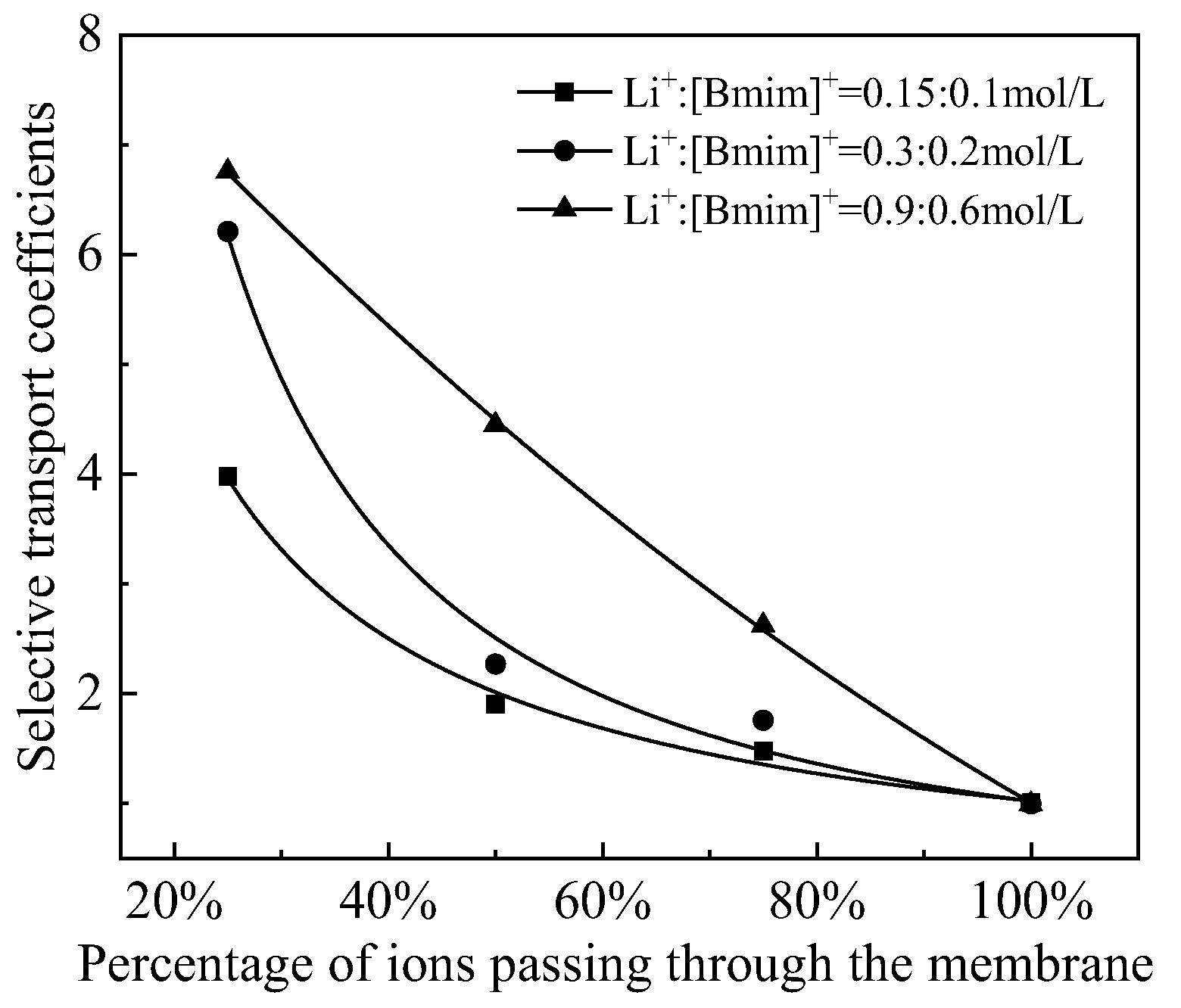
3.3. The Effect of Total Cation Concentration
The concentration ratio of Li
+:[Bmim]
+ was fixed at 3:2, and the total cation concentrations were 0.25 mol/L, 0.5 mol/L, and 1.5 mol/L, respectively, to investigate the effect of total cation concentration on the separation process. The changes in Li
+ and [Bmim]
+ concentrations over time are shown in
Figure 7 and
Figure 8. Similar to the experimental phenomenon of current density mentioned earlier, under all three conditions, there was a trend of gradually increasing [Bmim]
+ concentration reduction rate and gradually slowing down Li
+ concentration reduction rate. The total cation concentration is different, but the selective transport coefficient with the same percentage of ions passing through the membrane can indicate the separation effect. The variation of selective transport coefficients with the percentage of ions passing through the membrane was shown in
Figure 9. It can be seen from
Figure 9 that selective transport coefficients increases with the increase of total cation concentration. When the total concentration of Li
+ and [Bmim]
+ is 1.5 mol/L, the maximum selective transport coefficients is 6.75. The reason may be that when the total cation concentration in the feed increases, there will be more cations that binding with the ion exchange groups in the cation exchange membrane, thereby providing a stronger shielding effect on the double layer on the membrane surface. Therefore, pore size screening may be the dominant factor during the separation process.
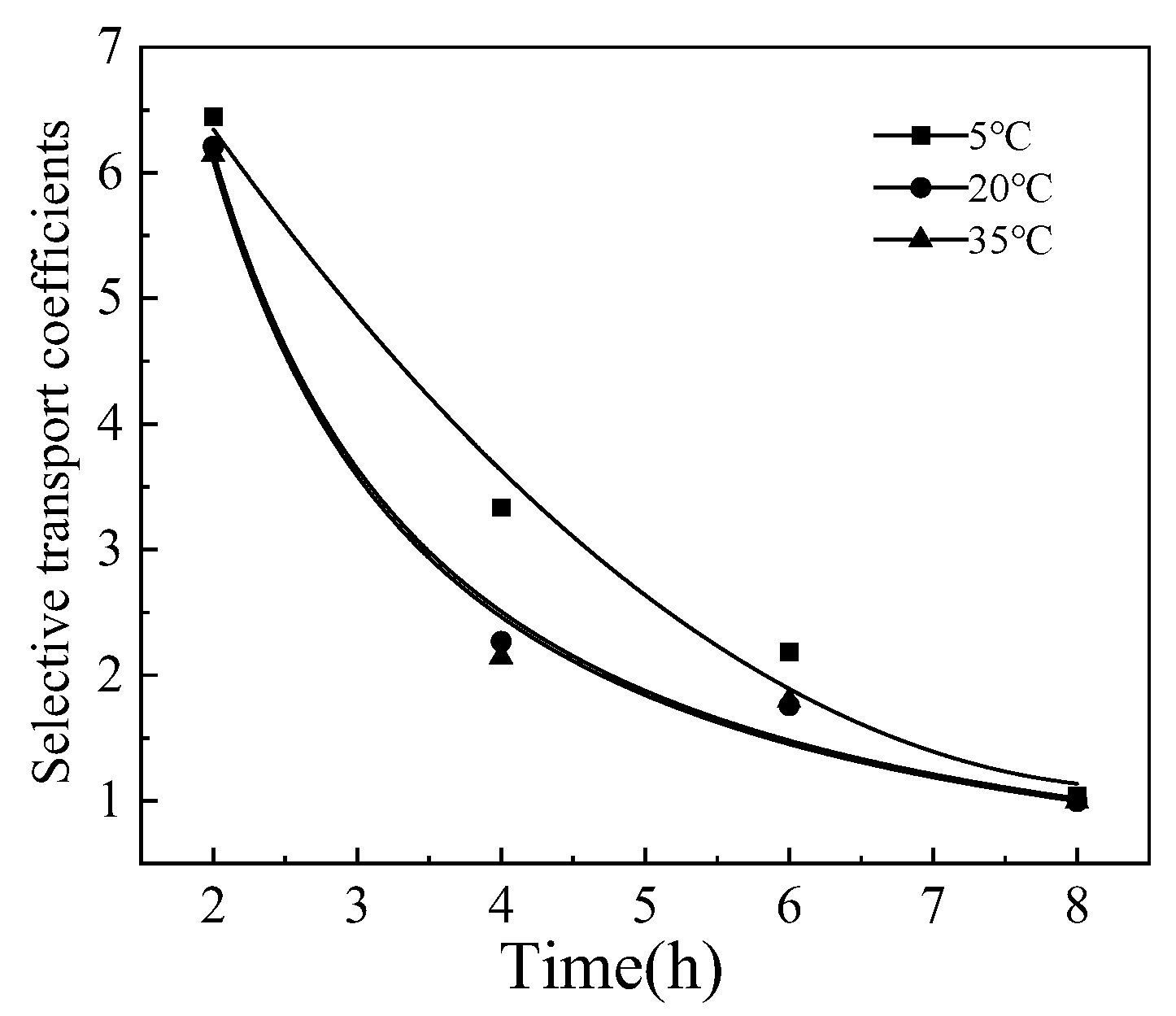
3.4. The Effect of Temperature
The concentrations of Li+ and [Bmim]+ were 0.3 and 0.2mol/L, at 5mA/cm2, the effect of feed temperature on the process was shown in Figure 9. It can be seen that compared to the feed temperature of 20 and 35 °C, when the material liquid temperature was set to 5 °C, the selective transport coefficients is higher. However, the improvement of the separation effect by reducing the temperature is limited. The reason may be that the swelling degree of the membrane increases with the increase of the feed temperature, leading to an increase in the membrane pore size. Therefore, Li+ and [Bmim]+ in the solution both are more likely to transmembrane transport.
3.5. The Effect of Anion Types
The concentrations of Li
+ and [Bmim]
+ were 0.3 and 0.2 mol/L, at 5 mA/cm
2, changing the anion of Cl
- to CH
3COO
- (acetate), the effect of anion types on separation efficiency was investigated. The change in the selective transport coefficients with time is negligible, as shown in
Figure 10. Changing the chloride ion in the system to acetate ion has almost no effect on the separation effect. Acetate and chloride ions do not complex with imidazole cations, and the two ionic liquids dissociate completely in aqueous solution. Therefore, changing the chloride ion in the system to ion has almost no effect on separation effect.
4. Conclusions
- (1)
It is feasible to utilize the selectivity of cation exchange membranes to realize the efficient separation of Li+ and [Bmim]+ under the driving of electric field. The modified membrane B showed best selective for Li+ and [Bmim]+, with the selective transport coefficients up to 6.2 under the test conditions.
- (2)
Compared with 10 and 20mA/cm2, a smaller current density of 5mA/cm2 has better separation efficiency for Li+ and [Bmim]+.
- (3)
Increasing the total cation concentration in the solution is beneficial for the separation of Li+ and [Bmim]+. When the total concentration of Li+ and [Bmim]+ is 1.5 mol/L, the maximum selective transport coefficients is 6.75.
- (4)
Compared to 20 and 35°C, when the feed temperature was set to 5°C, the selective transport coefficients is higher. However, the improvement degree by reducing the temperature is limited.
- (5)
Changing the chloride ion to acetate ion has almost no effect on the separation.
References
- CHEN Gangxin, SUN Xianzhong, ZHANG Xiong, WANG Kai, MA Yan-wei. Progress of high-power lithium-ion batteries[J]. Progress of high-power lithium-ion batteries[J]. Chinese Journal of Engineering, 2022, 44(4): 612-624. [CrossRef]
- LUO Guogang. Application of High Performance Li ion Battery[J]. Yunnan Chemical Technology, 2021, 48(2): 105-107.
- WU Guoqing. Research on the application of aviation lithium-ion batteries[J]. Chinese Scientific and Technological Journal Database, 2021, (3): 223-226.
- Maryam Golozar,Andrea Paolella,Hendrix Demers, et al. In situ observation of solid electrolyte interphase evolution in a lithium metal battery[J]. . Communications Chemistry, 2019, (No.1): 1-9. [CrossRef]
- Eliana Quartarone,Piercarlo Mustarelli. Electrolytes for solid-state lithium rechargeable batteries: recent advances and perspectives[J]. Chemical Society Reviews, 2011, (No.5): 2525-2540. [CrossRef]
- JIN Yang, XUE Zhiye, JIANG Xin LYU Nawei.Research Progress of Safety Protection of Lithium-ion Energy Storage Power Station[J]. J. Zhengzhou Univ. (Nat. Sci. Ed.), 2023, 55(3): 1-13.
- Thermal runaway caused fire and explosion of lithium ion battery[J]. Journal of Power Sources, 2012: 210-224. [CrossRef]
- Beatrice Garcia,Gerald Perron,Michel Armand, et al. Room temperature molten salts as lithium battery electrolyte[J]. Electrochimica-acta, 2004, (No.26): 4583-4588. [CrossRef]
- Cheng Hu, Nie Xiaoyan, Shen Yedan. Performance of Piperidine Ionic Liquid Based Mixed Electrolyte in Li/LiCoO2 Cell[J]. Journal of Electrochemistry, 2017, 23(1): 59-63. [CrossRef]
- YANG Shu-juan, ZHAO Di-shun, ZHAO Yu, ZHAI Jian-hua. Research progress of ionic liquid in lithium-ion batteries electrolyte[J]. Chinese Journal of Power Sources, 2016, 40(4): 912-914.
- Kexin Liu,Zhuyi Wang,Liyi Shi, et al. Ionic liquids for high performance lithium metal batteries[J]. Journal of Energy Chemistry, 2021, 59(No.8): 320-333. [CrossRef]
- F (Ahmed Faiz) Ahmed,MM (Rahman Md. Mahbubur) Rahman,SC (Sutradhar Sabuj Chandra) Sutradhar, et al. Synthesis of an imidazolium functionalized imide based electrolyte salt and its electrochemical performance enhancement with additives in li-ion batteries.[J]. Journal of Industrial & Engineering Chemistry, 2019: 178-185. [CrossRef]
- N Cvjetićanin Slobodan Gadžurić M Bešter-Rogač M Vraneš S Papović. Physicochemical and Electrochemical Characterisation of Imidazolium Based IL + GBL Mixtures as Electrolytes for Lithium-Ion Batteries[J]. Physical Chemistry Chemical Physics : Pccp, 2017, (No.41): 28139-28152. [CrossRef]
- Asier (AUTHOR) Fdz De Anastro,Nerea (AUTHOR) Lago,Carlos (AUTHOR) Berlanga, et al. Poly(ionic liquid) iongel membranes for all solid-state rechargeable sodium battery.[J]. Journal of Membrane Science, 2019: 435-441. [CrossRef]
- LIU Yadi; Hedin Niklas; JIA Lina, et al. JIAStudies on reaction kinetics and phase changes during the synthesis of ionic liquids using an in-situ low field MRI spectrometer[J]. Chinese Journal of Process Engineering, 2020, (7): 807-821. [CrossRef]
- WANG Mei, ZHANG Liqi, YANG Ming, HU Wenyun. Synthesis and Preparation of [NH2 e-mim][BF4] [J]. Journal of Wuhan Polytechnic University, 2014, (2): 41-45.
- LIU Huan, WEI Xiaoyi, LI Jihua, et al. Review of Ionic Liquids[J]. Recycling Journal of Cellulose Science and Technology, 2013, 21(2): 63-69.
- K. M. Docherty, C. F. Kulpa. Toxicity and antimicrobial activity of imidazolium and pyridinium ionic liquids.[J]. Green Chemistry, 2005, (No.4): 185-189. [CrossRef]
- AI(Horowitz ArielI.) Horowitz,YS(Wang Yushi) Wang,MJ(Panzer MatthewJ.) Panzer.Reclamation and reuse of ionic liquids from silica-based ionogels using spontaneous water-driven separation[J]. Green Chemistry, 2013, (No.12): 3414-3420. [CrossRef]
- Sylvie Viboud, Nicolas Papaiconomou, Auré Cortesi, et al. Correlating the structure and composition of ionic liquids with their toxicity on Vibrio fischeri: A systematic study[J]. Journal of Hazardous Materials, 2012: 40-48. [CrossRef]
- SU Hui, ZHU Zhaowu, WANG Lina, QI Tao. research progress in extraction and recovery of lithium from hard-rock ores[J]. CIESC Journal, 2019, 70(1): 10-23. [CrossRef]
- SHANG Xi, MENG Yuhang, ZHANG Qian, YANG Huaming. Lithium Extraction and Strategic Application of Lithium-rich Minerals[J]. Conservation and Utilization of Mineral Resources, 2019, 39(6): 152-158.
- Qin X, Gao X, He Y, et al. Recent advances in lithium extraction from salt lake brine using Ti-based ion sieve absorbent[J]. Journal of Salt Lake Research, 2023, 31(2):91-101. [CrossRef]
- Miao S S, Shi L, Song Z P,et al. Preparation of nanofiltration membranes with dually charged separation layer for Mg2+ /Li+ separation[J]. Membrane Science and Technology (Chinese), 2023, 43(4): 37-43.
- Luo Q, Dong M, Li J, et al. Research Progress of Lithium Separation from Salt Lake Brine by adsorption method[J]. Research Progress of Lithium Separation from Salt Lake Brine by adsorption method[J]. loumal of Salt Lake Reserch, 2023, 31(1): 106-117. [CrossRef]
- Xianhui (AUTHOR) ( lixianhui2006@hotmail.com) Li,Yinghui (AUTHOR) Mo,Weihua (AUTHOR) Qing, et al. Membrane-based technologies for lithium recovery from water lithium resources: a review.[J]. Journal of Membrane Science, 2019, (No.0): 117317. [CrossRef]
- Junxiang Zhang,Zeyu Cheng,Xinbo Qin, et al. Recent advances in lithium extraction from salt lake brine using coupled and tandem technologies.[J]. Desalination, 2023. [CrossRef]
- Zhang Xukun, Zhang Jian, Su Hui, Qi Tao, Wang Lina. Recovery of Lithium from Lithium Sulfate Precipitation Mother Liquor [J]. Rare Metals, 2022,46 (1): 67-77.
- Qin Yaru, Shi Chenglong, Wang Xingquan, et al. Study on scrubbing process of lithium extraction from brine in ionic liquid system[J] Inorganic Salt Industry, 2020, 52 (3): 55-58, 106. [CrossRef]
- Weiwen Xin, Jingru Fu, Yongchao Qian, et al. Biomimetic KcsA channels with ultra-selective K+ transport for monovalent ion sieving[J]. Nature Communications, 2022, (No.1): 1-11. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).