Submitted:
29 January 2024
Posted:
31 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Demographic characteristics of the COVID-19 patients
2.2. Demographic characteristics of the healthy subjects y subjects
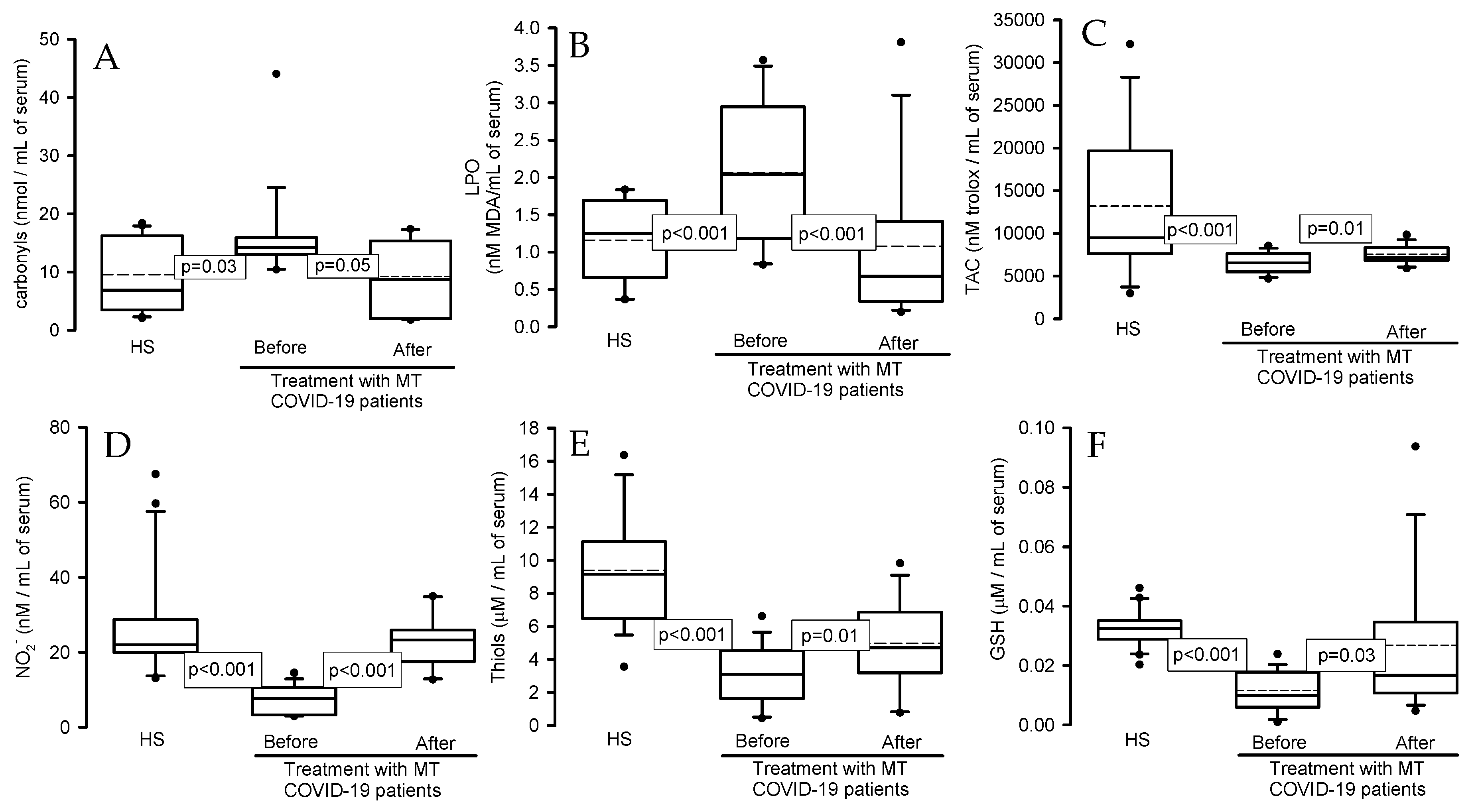
2.3. Oxidative stress markers
2.4. Activities of the enzymes that employ the GSH
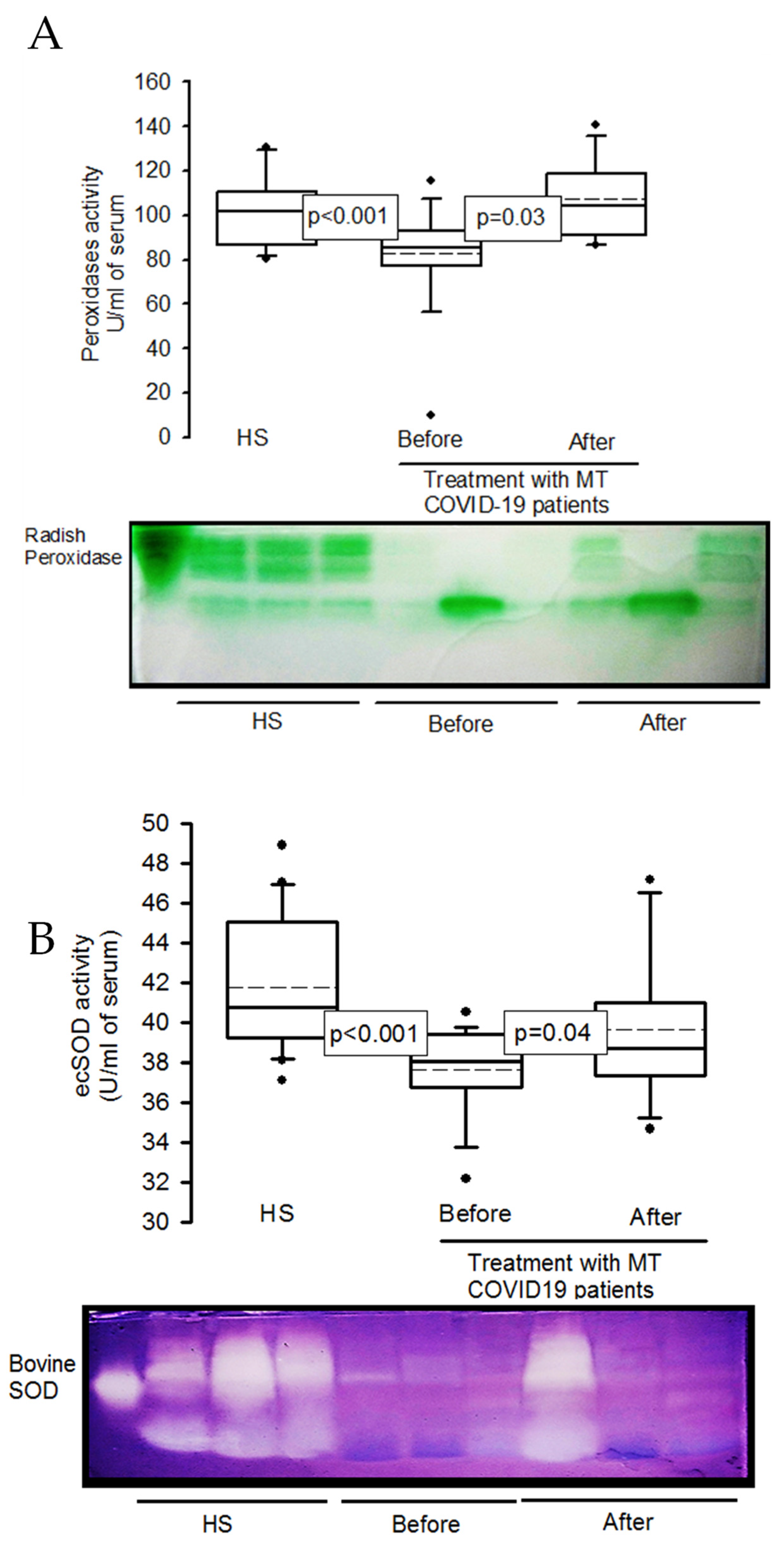
2.5. Activities of the enzymes peroxidases and ecSOD in native gels
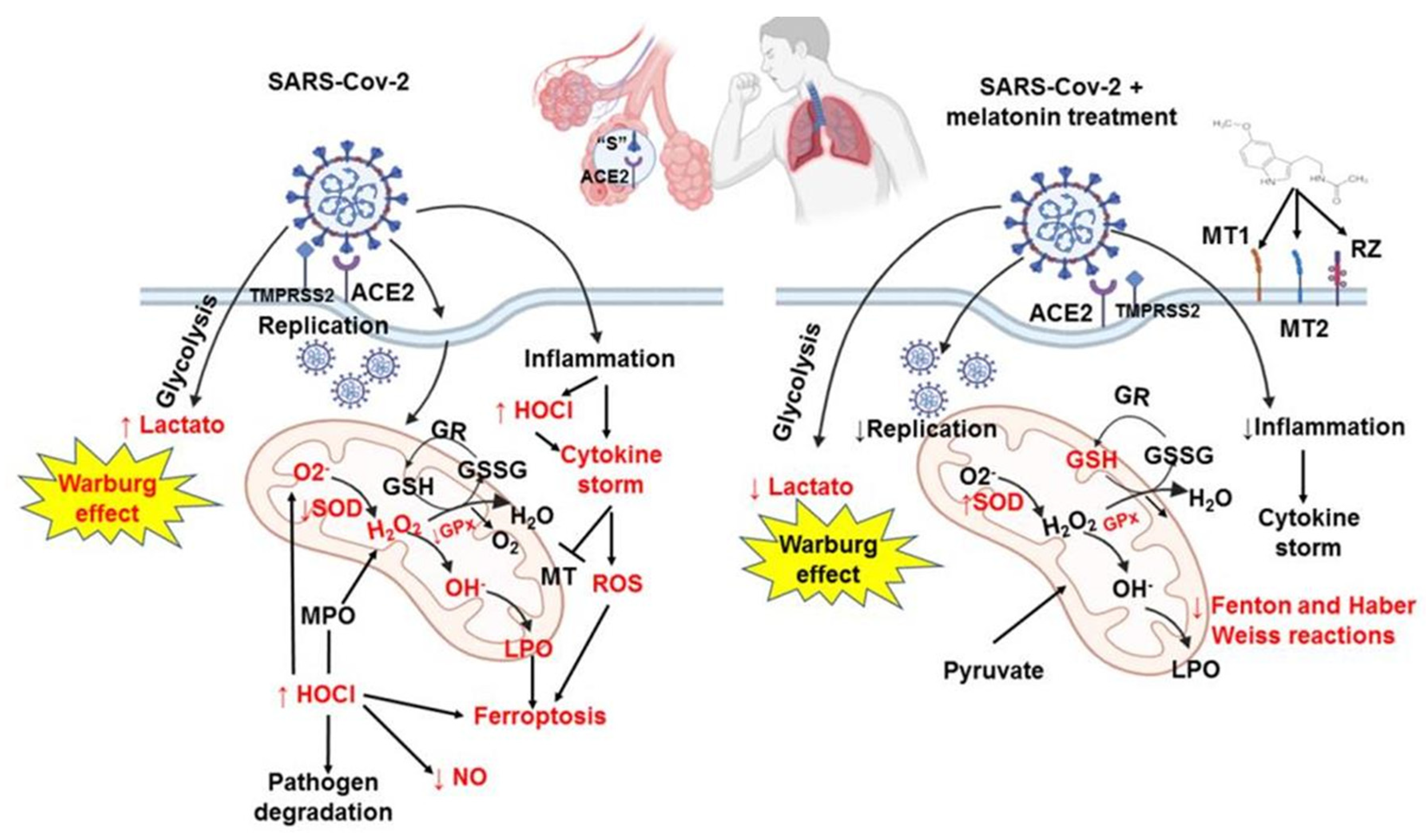
3. Discussion
4. Materials and Methods
4.1. Population that comprised the study
4.2. Detection of SARS-CoV-2 by real-time reverse transcriptase polymerase chain reaction
4.3. Healthy subjects
4.4. Therapeutic Management
4.5. Peripheral blood samples
4.6. Oxidative stress markers
4.6.1. Carbonyls protein
4.6.2. Evaluation of total antioxidant capacity
4.6.3. Determination of lipid peroxidation (LPO) levels
4.6.4. Nitrites
4.6.5. Thiols and glutathione levels
4.6.6. Determinations of antioxidant enzymes that employ GSH
4.6.7. Interleukin-6 Concentration
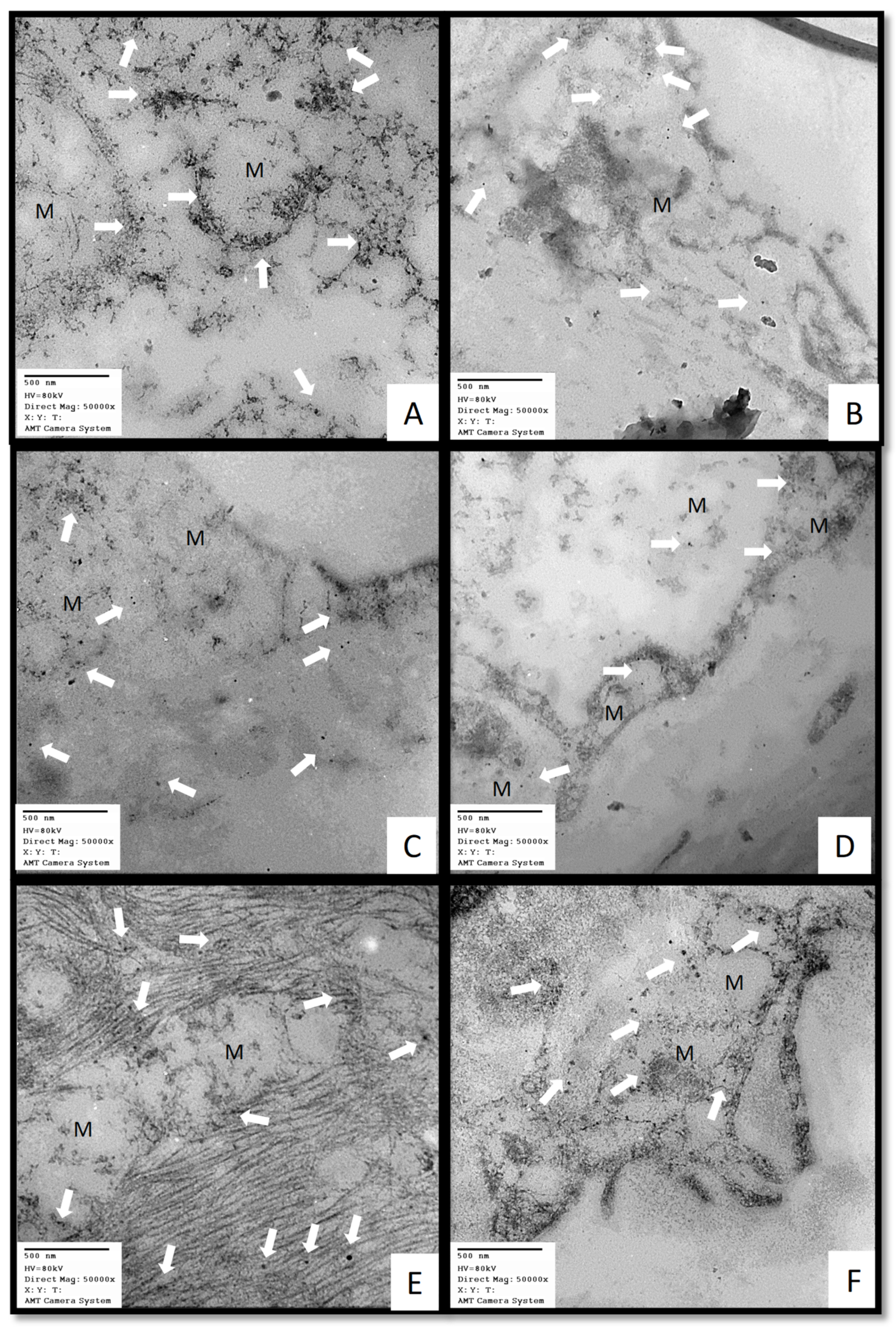
4.6.8. Obtainment of the post-mortem biopsies
4.6.10. Immune colloidal gold technique
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Soto, M.E.; Guarner-Lans, V.; Soria-Castro, E.; Manzano-Pech, L.; Pérez-Torres, I. Is antioxidant therapy a useful complementary measure for Covid-19 treatment? An algorithm for its application. Medicina. (Kaunas). 2020, 56, 386–415. [Google Scholar] [CrossRef] [PubMed]
- Soria-Castro, E.; Soto, M.E.; Guarner-Lans, V.; Rojas, G.; Perezpeña-Diazconti, M.; Críales-Vera, S.A.; Manzano-Pech, L.; Pérez-Torres, I. The kidnapping of mitochondrial function associated with the SARS-CoV-2 infection. Histol. Histopathol. 2021, 36, 947–965. [Google Scholar]
- Zhang, D.; Kukkar, D.; Kim, K.H.; Bhatt, P. A comprehensive review on immunogen and immune-response proteins of SARS-CoV-2 and their applications in prevention, diagnosis, and treatment of COVID-19. Int. J. Biol. Macromol. 2024, 259, 129284. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Guarner-Lans, V.; Soria-Castro, E.; Manzano-Pech, L.; Palacios-Chavarría, A.; Valdez-Vázquez, R.R.; Domínguez-Cherit, J.G.; Herrera-Bello, H.; Castillejos-Suastegui, H.; Moreno-Castañeda, L.; Alanís-Estrada, G.; Hernández, F.; González-Marcos, O.; Márquez-Velasco, R.; Soto, M.E. Alteration in the lipid profile and the desaturases activity in patients with severe pneumonia by SARS-CoV-2. Front Physiol. 2021, 12, 667024. [Google Scholar] [CrossRef] [PubMed]
- Negru, P.A.; Radu, A.F.; Vesa, C.M.; Behl, T.; Abdel-Daim, M.M.; Nechifor, A.C.; Endres, L.; Stoicescu, M.; Pasca, B.; Tit, D.M.; Bungau, S.G. Therapeutic dilemmas in addressing SARS-CoV-2 infection: Favipiravir versus Remdesivir. Biomed. Pharmacother. 2022, 147, 112700. [Google Scholar] [CrossRef] [PubMed]
- Molina-Carballo, A.; Palacios-López, R.; Jerez-Calero, A.; Augustín-Morales, M.C.; Agil, A.; Muñoz-Hoyos, A.; Muñoz-Gallego, A. Protective effect of melatonin administration against SARS-CoV-2 infection: A systematic review. Curr. Issues. Mol. Biol. 2022, 44, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Boutin, J.A.; Liberelle, M.; Yous, S.; Ferry, G.; Nepveu, F. Melatonin facts: Lack of evidence that melatonin is a radical scavenger in living systems. J. Pineal. Res. 2023, e12926. [Google Scholar] [CrossRef]
- Reppert, S.M. Melatonin receptors: Molecular biology of a new family of G protein-coupled receptors. J. Biol. Rhythm. 1997, 12, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Mehrzadi, S.; Karimi, M.Y.; Fatemi, A.; Reiter, R.J. Hosseinzadeh A. SARS-CoV-2 and other coronaviruses negatively influence mitochondrial quality control: beneficial effects of melatonin. Pharmacol. Ther. 2021, 224, 107825. [Google Scholar] [CrossRef]
- Pontes, G.N.; Cardoso, E.C.; Carneiro-Sampaio, M.M.; Markus, R.P. Pineal melatonin and the innate immune response: the TNF-alpha increase after cesarean section suppresses nocturnal melatonin production. J. Pineal. Res. 2007, 43, 365–371. [Google Scholar] [CrossRef]
- Markus, R.P.; Fernandes, P.A.; Kinker, G.S.; da Silveira, C.M.S.; Marçola, M. Immune-pineal axis—acute inflammatory responses coordinate melatonin synthesis by pinealocytes and phagocytes. Br. J. Pharmacol. 2018, 175, 3239–3250. [Google Scholar] [CrossRef] [PubMed]
- Pablos, M.I.; Guerrero, J.M.; Ortiz, G.G.; Agapito, M.T., Reiter, R.J. Both melatonin and a putative nuclear melatonin receptor agonist CGP 52608 stimulate glutathione peroxidase and glutathione reductase activities in mouse brain in vivo. Neuroendocrinol. Lett. 1997, 18, 49–58.
- Terron, M.P.; Flores, L.J.; Czarnocki, Z. Melatonin and its metabolites: new findings regarding their production and their radical scavenging actions. Acta. Biochim. Pol. 2007, 54, 1–9. [Google Scholar]
- Gulcin, I.; Buyukokuroglu, M.E.; Kufrevioglu, O.I. Metal chelating and hydrogen peroxide scavenging effects of melatonin. J. Pineal. Res. 2003, 34, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin and related compounds: Chemical insights into their protective effects against oxidative stress. Curr. Org. Chem. 2017, 21, 2077–2095. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, W.; Han, D.; Shi, X.; Xu, S. New insights into how melatonin ameliorates bisphenol A-induced colon damage: Inhibition of NADPH oxidase. J. Agric. Food. Chem. 2023, 71, 2566–2578. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Antioxidant protection by melatonin: Multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine. 2005, 27, 119–130. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal. Res. 2014, 57, 131–146. [Google Scholar] [CrossRef]
- Budkowska, M.; Cecerska-Heryć, E.; Marcinowska, Z.; Siennicka, A.; Dołęgowska, B. The influence of circadian rhythm on the activity of oxidative stress enzymes. Int. J. Mol. Sci. 2022, 23, 14275. [Google Scholar] [CrossRef]
- Soto, M.E.; Guarner-Lans, V.; Díaz-Díaz, E.; Manzano-Pech, L.; Palacios-Chavarría, A.; Valdez-Vázquez, R.R.; Aisa-Álvarez, A.; Saucedo-Orozco, H.; Pérez-Torres, I. Hyperglycemia and loss of redox homeostasis in COVID-19 Patients. Cells. 2022, 11, 932. [Google Scholar] [CrossRef]
- Nakao, T.; Morita, H.; Maemura, K.; Amiya, E.; Inajima, T.; Saito, Y.; Watanabe, M.; Manabe, I.; Kurabayashi, M.; Nagai, R. , Nagai, R.; Komuro, I. Melatonin ameliorates Angiotensin II-induced vascular endothelial damage via its antioxidative properties. J. Pineal. Res. 2013, 55, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, E.L.; Júnior, F.T.D.S.S.; NeryNeto, J.A.O.; Matos, L.F.L.; Moura, M.H.S.; Rosales, T.O.; De Freitas, G.B.L. COVID-19: Rational discovery of the therapeutic potential of melatonin as a SARS-CoV-2 main protease inhibitor. Int. J. Med. Sci. 2020, 17, 2133–2146. [Google Scholar] [CrossRef] [PubMed]
- Boga, J.A.; Coto-Montes, A.; Rosales-Corral, S.A.; Tan, D.X.; Reiter, R.J. Beneficial actions of melatonin in the management of viral infections: a new use for this molecular handyman. Rev. Med. Virol. 2012, 22, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Reiter, R.J. Melatonin: Roles in influenza, Covid-19, and other viral infections. Rev. Med. Virol. 2020, 30, e2109. [Google Scholar] [CrossRef] [PubMed]
- Bahrampour, J.K.; Pourhanifeh, M.H.; Hosseinzadeh, A.; Hemati, K.; Mehrzadi, S. Melatonin potentials against viral infections including COVID-19: Current evidence and new findings. Virus. Res. 2020, 287, 198108. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.E.; Manzano-Pech, L.; Palacios-Chavarría, A.; Valdez-Vázquez, R.R.; Guarner-Lans, V.; Pérez-Torres, I. N-acetyl cysteine restores the diminished activity of the antioxidant enzymatic system caused by SARS-CoV-2 infection: Preliminary findings. Pharmaceuticals. Basel. 2023, 16, 591. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F. Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal. Res. 2004, 36, 1–9. [Google Scholar] [CrossRef]
- Öztürk, G.; Akbulut, G.K.; Güney, S.; Acuna-Castroviejo, D. Age-related changes in the rat brain mitochondrial antioxidative enzyme ratios: modulation by melatonin. Exp. Gerontol. 2012, 47, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, G.; Özturk, G.; Güney, S.; Sinanoğlu, O.; Tunçdemir, M. Protective effect of melatonin and 1,25-dihydroxyvitamin D3 on renal ischemia-reperfusion injury in rats. Ren. Fail. 2013, 35, 374–379. [Google Scholar] [CrossRef]
- Crespo, I.; Miguel, B.S.; Laliena, A.; Alvarez, M.; Culebras, J.M.; González-Gallego, J.; Tuñón, M.J. Melatonin prevents the decreased activity of antioxidant enzymes and activates nuclear erythroid 2-related factor 2 signaling in an animal model of fulminant hepatic failure of viral origin. J. Pineal. Res. 2010, 49, 193–200. [Google Scholar] [CrossRef]
- Muhammad, Y.; Kani, Y.A.; Iliya, S.; Muhammad, J.B.; Binji, A.; El-Fulaty, A.A.; Kabir, M.B.; Bindawa, K.U.; Ahmed, A. Deficiency of antioxidants and increased oxidative stress in COVID-19 patients: A cross-sectional comparative study in Jigawa, Northwestern Nigeria. SAGE. Open. Med. 2021, 9, 2050312121991246. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Comish, P.; Kang, R. The hallmarks of COVID-19 disease. PLoS. Pathog. 2020, 16, e1008536. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, C.H.; Ryu, J.H.; Kim, M.J.; Park, C.Y.; Lee, J.M. Holtzman, M.J.; Joo-Heon Y. Reactive oxygen species induce antiviral innate immune response through IFN-lambda regulation in human nasal epithelial cells. Am. J. Respir. Cell. Mol. Biol. 2013, 49, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, I.I. Peroxidase activity of human hemoproteins: Keeping the fire under control. Molecules. 2018, 23, 2561. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Cheng, G. Recombinant myeloperoxidase as a New class of antimicrobial agents. Microbiol. Spectr. 2022, 10, e0052221. [Google Scholar] [CrossRef] [PubMed]
- Camp, O.G.; Bai, D.; Gonullu, D.C.; Nayak, N.; Abu-Soud, H.M. Melatonin interferes with COVID-19 at several distinct ROS-related steps. J. Inorg. Biochem. 2021, 223, 111546. [Google Scholar] [CrossRef] [PubMed]
- Goud, P.T.; Bai, D.; Abu-Soud. H.M. A Multiple-hit hypothesis involving reactive oxygen species and myeloperoxidase explains clinical deterioration and fatality in COVID-19. Int. J. Biol. Sci. 2021, 17, 62–72. [CrossRef] [PubMed]
- Abu-Soud, H.M.; Hazen, S.L. Nitric oxide is a physiological substrate for mammalian peroxidases. J. Biol. Chem. 2000, 275, 37524–37532. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H. Blood nitrate and nitrite modulating nitric oxide bioavailability: potential therapeutic functions in COVID-19. Nitric. Oxide. 2020, 103, 29–30. [Google Scholar] [CrossRef]
- Kouhpayeh, S.; Shariati, L.; Boshtam, M.; Rahimmanesh, I.; Mirian, M.; Esmaeili, Y.; Najaflu, M.; Khanahmad, N.; Zeinalian, M.; Trovato, M., Tay, F.R.; Khanahmad, H.; Makvandi, P. The molecular basis of COVID-19 pathogenesis, conventional and nanomedicine therapy. Int. J. Mol. Sci. 2021, 22, 5438. [CrossRef]
- Wang, J.; Mei, F.; Bai, L.; Zhou, S.; Liu, D.; Yao, L.; Ahluwalia, A.; Ghiladi, R.A.; Su, L.; Shu, T.; Gong, M.; Wang, X.; Zhu, L.; Cai, K.; Zhang, X. Serum nitrite and nitrate: A potential biomarker for post-COVID-19 complications? Free. Radic. Biol. Med. 2021, 175, 216–225. [Google Scholar] [CrossRef]
- Corrao, S.; Bocchio M.R.; Monaco M.L.; Natoli, G.; Cavezzi, A.; Troiani, E.; Argano, C. Does evidence exist to blunt inflammatory response by nutraceutical supplementation during COVID-19 pandemic? An overview of systematic reviews of vitamin D, vitamin C, melatonin, and Zinc. Nutrients. 2021, 13, 1261. [CrossRef]
- Ghaleh, H.E.G.; Hosseini, A.; Aghamollaei, H.; Fasihi-Ramandi, M.; Alishiri, G.; Saeedi-Boroujeni, A.; Hassanpour, K.; Mahmoudian-Sani, M.R.; Farnoosh, G. NLRP3 inflammasome activation and oxidative stress status in the mild and moderate SARS-CoV-2 infected patients: Impact of melatonin as a medicinal supplement. Z. Naturforsch. C. J. Biosci. 2022, 77, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Zarezadeh, M.; Khorshidi, M.; Emami, M.; Janmohammadi, P.; Kord-Varkaneh, H.; Mousavi, S.M.; Mohammed, S.H.; Saedisomeolia, A.; Alizadeh, S. Melatonin supplementation and pro-inflammatory mediators: A systematic review and meta-analysis of clinical trials. Eur. J. Nutr. 2020, 59, 1803–1813. [Google Scholar] [CrossRef]
- Farnoosh, G.; Akbariqomi, M.; Badri, T.; Bagheri, M.; Izadi, M.; Saeedi-Boroujeni, A.; Rezaie, E.; Ghaleh, H.E.G.; Aghamollaei, H.; Fasihi-Ramandi, M., Hassanpour, K.; Alishiri. G. Efficacy of a low dose of melatonin as an adjunctive therapy in hospitalized patients with COVID-19: A randomized, double-blind clinical Trial. Arch. Med. Res. 2021, 53, 79–85. [CrossRef]
- Alizadeh, N.; Dianatkhah, M.; Alimohamadi, Y.; Moradi, H.; Akbarpour, S.; Akrami, M.; Mansouri, F.; Faraji, N.; Rezaie, Z.; Alizadeh, M.; Hosamirudsari, H. High dose melatonin as an adjuvant therapy in intubated patients with COVID-19: A randomized clinical trial. J. Taibah Univ. Med. Sci. 2022, 17, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Can Melatonin be a potential "Silver Bullet" in treating COVID-19 patients? Diseases. 2020, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.M.; Zhao, C.C.; Xie, Q.M.; Xu, J.; Fei, G.H. TLR2-melatonin feedback loop regulates the activation of NLRP3 inflammasome in murine allergic airway inflammation. Front. Immunol. 2020, 11, 172. [Google Scholar] [CrossRef]
- Owino, S.; Buonfiglio, D.D.C.; Tchio, C.; Tosini, G. Melatonin signaling a key regulator of glucose homeostasis and energy metabolism. Front. Endocrinol. 2019, 10, 488. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Rosales-Corral S. Anti-Warburg effect of melatonin: A proposed mechanism to explain its inhibition of multiple diseases Int. J. Mol. Sci. 2021, 22, 764. [CrossRef]
- Gomez-Pastora, J.; Weigand, M.; Kim, J.; Wu, X.; Strayer, J.; Palmer, A.F.; Zborowski, M.; Yazer, M.; Chalmers, J.J. Hyperferritinemia in critically ill COVID-19 patients—Is ferritin the product of inflammation or a pathogenic mediator? Clin. Chim. Acta. 2020, 509, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Sayed, R.K.; Fernández-Ortiz, M.; Diaz-Casado, M.E.; Rusanova, I.; Rahim, I.; Escames, G.; López, L.C.; Mokhtar, D.M.; Acuña-Castroviejo, D. The protective effect of melatonin against age-associated, sarcopenia-dependent tubular aggregate formation, lactate depletion, and mitochondrial changes. J. Gerontol. A. Biol. Sci. Med. Sci. 2018, 73, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Dun.-Xian, T.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a potent and inducible endogenous antioxidant: Synthesis and metabolism. Molecules. 2015, 20, 18886–18906. [CrossRef] [PubMed]
- Tarocco, A.; Caroccia, N.; Morciano, G.; Wieckowski, M.R.; Ancora, G.; Garani, G.; Pinton, P. Melatonin as a master regulator of cell death and inflammation: Molecular mechanisms and clinical implications for newborn care. Cell. Death. Dis. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Juybari, K.B.; Pourhanifeh, M.H.; Hosseinzadeh, A.; Hemati, K.; Mehrzadi, S. Melatonin potentials against viral infections including COVID-19: Current evidence and new findings. Virus. Res. 2020, 287, 198108. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Reiter, R.J. Melatonin: Roles in influenza, COVID-19, and other viral infections. Rev. Med. Virol. 2020, 30, e2109. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.; López-Muñoz, F.; Gil-Martín, E.; Egea, J.; Álvarez-Merz, I.; Painuli, S.; Semwal, P.; Martins, N.; Hernández-Guijo, J.M.; Romero, A. The coronavirus disease 2019 (COVID-19): Key emphasis on melatonin safety and therapeutic efficacy. Antioxidants. Basel. 2021, 10, 1152. [Google Scholar] [CrossRef] [PubMed]
- Camp, O.G.; Bai, D.; Gonullu, D.C.; Nayak, N.; Abu-Soud, H.M. Melatonin interferes with COVID-19 at several distinct ROS-related steps. J. Inorg. Biochem. 2021, 223, 111546. [Google Scholar] [CrossRef] [PubMed]
- Olson, G.; Andrew, M.D. Diagnosis and treatment of adults with community-acquired pneumonia. JAMA. 2020, 323, 885–886. [Google Scholar] [CrossRef]
- Lambden, S.; Laterre, P.F.; Levy, M.M.; Francois, B. The SOFA score-development, utility, and challenges of accurate assessment in clinical trials. Crit. Care. 2019, 23. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012, 307, 2526–2533. [Google Scholar] [PubMed]
- Chavarría, A.P.; Vázquez, R.R.V.; Cherit, J.G.D.; Bello, H.H.; Suastegui, H.C.; Moreno-Castañeda, L.; Estrada, A.G.; Hernández. F.; González-Marcos, O.; Saucedo-Orozco, H.; Manzano-Pech, L.; Márquez-Velasco, R.; Guarner-Lans, V.; Pérez-Torres, I.; Soto, M.E. Antioxidants and pentoxifylline as coadjuvant measures to standard therapy to improve prognosis of patients with pneumonia by COVID-19. Comput. Struct. Biotechnol. J. 2021, 19, 1379–1390.
- Brower, R.G., Lanken P.N., MacIntyre N., Matthay M.A., Morris A., Marek A. National heart, lung, and blood institute ARDS clinical trials network higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N. Engl. J. Med. 2004, 351, 327–336. [CrossRef] [PubMed]
- Thachil, J.; Tang, N.; Gando, S.; Falanga, A.; Cattaneo, M.; Levi, M. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020, 18, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, H.P.; Timerman, S.; Rodrigues, R.D.R.; Corrêa, T.D.; Schubert, D.U.C.; Freitas, A.P. Position statement: cardiopulmonary resuscitation of patients with confirmed or suspected COVID-19-2020. Arq. Bras. Cardiol. 2020, 114, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.E.; Pérez-Torres, I.; Manzano-Pech, L.; Soria-Castro, E.; Morales-Marín, A.; Ramírez-Marroquín, E.S.; Martínez-Hernández, H.; Herrera-Alarcón, V.; Guarner-Lans, V. Reduced levels of selenium and thioredoxin reductase in the thoracic aorta could contribute to aneurysm formation in patients with Marfan syndrome. Int. J. Mol. Sci. 2023, 24, 10429. [Google Scholar] [CrossRef] [PubMed]
- Erel, O.; Neşelioğlu, S. Erel, O.; Neşelioğlu, S.; Tunçay,M.E.; Oğuz, E.F.; Eren, F.; Akkuş, M.S.; Güner, H.R.; Ateş, İ.. A sensitive indicator for the severity of COVID-19: thiol. Turk. J. Med. Sci. 2021, 51, 921–928. [Google Scholar] [CrossRef]
- Anderson, G.; Reiter, R.J. Melatonin: Roles in influenza, Covid-19, and other viral infections Rev. Med. Virol. 2020, 30, e2109. [Google Scholar] [CrossRef]

| Variables | Median and (Min–Max range) |
|---|---|
| Age | 62 (34-84) |
| Body mass index | 30 (20-37) |
| Comorbidities (%) | |
| Diabetes Mellitus | 11 (65) |
| Hypertension | 7 (41) |
| Dyslipidemia | 10 (59) |
| Chronic obstructive pulmonary disease | 1 (6) |
| Normal Weight | 6 (35) |
| Overweight | 3 (18) |
| Obesity | 8 (47) |
| Gasometry and blood biochemistry median and (Min–Max range) | |
| PaO2 | 79 (58-152) |
| PCO2 | 31 (27-36) |
| PaO2/FIO2 (mmHg) | 145 (49-243) |
| SPO2/FIO2 (mmHg) | 160 (50-280) |
| Heart Rate lpm | 84 (58-106) |
| Mean arterial pressure (mmHg) | 82 (65-99) |
| Temperature °C | 36.5 (35.9- 37.2) |
| Creatinine in serum mg/dL | 0.80 (0.60-2.5) |
| Blood urea nitrogen mg/dL | 18.6 (5.8-77.7) |
| Leukocytes 10ˆ3/µL | 10.1 (5.3-22.6) |
| Lymphocytes 10ˆ3/µL | 0.66 (0.39-1.29) |
| Platelets 10ˆ3/µL | 275 (180-576) |
| Ferritin ng/mL | 665.7 (175-2354) |
| D-dimer ng/dL | 655 (27-35,200) |
| C reactive protein mg/dL | 145 (37-308) |
| Procalcitonin ng/dL | 0.40 (0.06-34.7) |
| Score median and (Min-Max range) | |
| SOFA | 2 (1-8) |
| APACHE | 5 (4-7) |
| SAPS | 26.5 (13-31) |
| Days with mechanic ventilation | 2 (1-2) |
| Days in ICU | 14.5 (6-20) |
| Variable | Median (Min–Max range) |
|---|---|
| Gender | 15 men and 5 female |
| Age | 57 (27–87) |
| Glucose (mg/dL) | 105.50 (32.00–148.00) |
| Uric acid (mg/dL) | 6.65 (2.95–9.60) |
| Cholesterol (mg/dL) | 166.50 (108.00–276.00) |
| HDL (mg/dL) | 37.00 (24.20–54.60) |
| LDL (mg/dL) | 93.35 (54.20–146.00) |
| TG (mg/dL) | 122.50 (67.50–369.00) |
| C reactive protein (mg/dL) | 1.43 (1.18–9.02) |
| Atherogenic index | 2.60 (0.26–4.48) |
| Variable | BeforeMedian (min – max range) | AfterMedian (min – max range) |
|---|---|---|
| Glucose (mg/dL) | 204.38 (96.49–460.52)* 1.30 (0.10–10.10) 9.71 (1.67–100.15) | 139.47 (79.82–321.05) 1.30 (0.20–3.70) 8.63 (0.82–64.18) |
| Insulin (ng/mL) | ||
| HOMA-Index | ||
| IL-6 (pg/mL) | 43.28 (7.8–388)** | 7.8 (7.8–128.28) |
| HDL (mg/dL) | 29.9 (14.5–51.30)† | 57.8 (23–90) |
| LDL (mg/dL) | 83.1 (40.7–108.20) | 86.1 (65.5–132) |
| Total cholesterol (mg/dL) | 141 (76–196) | 146 (130–227) |
| TG (mg/dL) | 140 (37–325) | 205 (79–295) |
| Before vs. after of the treatment with MT *p=0.05, **p=0.02, † NS (0.09). Abbreviations: IL= Interleukin, HDL= High-density lipoproteins, LDL= Low-density lipoproteins, TG= Triglycerides | ||
| Activities enzymes | HS | SARS-CoV-2 infection | |
|---|---|---|---|
| (min/mL of serum) | Before of the treatment with MTMedian (Min-Max range) | After of the treatment with MTMedian (Min-Max range) | |
| GPx (nmol NADPH) | 0.68 (0.08–2.03) | 0.21 (0.05–0.91)*** | 1.15 (0.8–1.44)*** |
| Q1, Q3, IQR | 0.49, 1.64, 1.14 | 0.15, 0.56, 0.41 | 1.05, 1.20, 0.14 |
| TxrR (TNB nmol) | 6.91–3 (–4.4–3–0.02) | 3.14–3 (–1.5–3–8.8–3)*** | 3.30–3 (9.4–3–8.4–3) |
| Q1, Q3, IQR | 3.14–3, 1.42–2, 1.10–2 | 2.20–3, 4.79–3, 2.59–3 | 2.83–3, 5.89–3, 3.06–3 |
| GST (GS-DNB μmol) | 3.37–4 (7.95–5–7.95–4) | 7.95–5 (–1.59–4–1.59–4)*** | 1.59–4 (3.9–5–4.77–4)* |
| Q1, Q3, IQR | 1.39–4, 4.37–4, 2.98–4 | 7.95–5, 1.19–4, 3.97–5 | 9.96–5, 2.9–4, 2.38–4 |
| GR (units) | 1.93 (1.16–10.24) | 1.64 (0.19–2.70)* | 2.32 (0.96–5.60)*** |
| Q1, Q3, IQR | 1.40, 2.61, 1.20 | 0.48, 2.03, 1.54 | 1.45, 3.48, 2.03 |
| HS vs. Before SARS-CoV-2 ***p=0.001, before of the MT vs. after of the MT *p=0.01. Abbreviations: GPx= Glutathione peroxidase, TrxR= Thioredoxin reductase, GST= Glutathione-S-transferase, GR= gluthatione reductase, Q1= first quartile, Q2= second quartile, IQR= interquartile range. The results shown median. First quartile, second quartile, interquartile range and min and max range. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





