1. Introduction
Abnormalities in the processes of epigenetic regulation undoubtedly disrupt physiological growth progression of the human brain contributing to the aetiology of neurodevelopmental disorders.
ZMYND11 encodes a zinc finger MYND domain-containing protein 11 that acts as a transcriptional co-repressor which inhibits the elongation phase of RNA polymerase II by recognition of the histone modification of transcribed regions. The
ZMYND11 gene has been reported to play a crucial role in the 10p15.3 microdeletion syndrome, associated with neurodevelopmental disorder, dysmorphic features, hypotonia and seizures [
1,
2]. Heterozygous pathogenic variants in the
ZMYND11 gene has been subsequently identified as the cause of autosomal dominant intellectual developmental disorder-30 with speech delay and behavioural abnormalities, MRD30 (OMIM 616083, ORPHA 178469). Additional features may include various types of seizures, hypotonia, feeding difficulties, and craniofacial dysmorphism results in a phenotype resembling 10p15.3 microdeletion syndrome [
2,
3]. However, in 2012, in a group of 343 children with the autism spectrum disorder (ASD), splice variant of the
ZMYND11 gene was identified in the patient with ASD, but without intellectual disability and obvious dysmorphism [
4,
5]. In 2020 Yates et al. described 27 patients (including 16 previously unreported individuals) with pathogenic variants in the
ZMYND11 gene, suggesting a genotype-phenotype correlation [
6]. In 2021 Oates et al. identified 47 people (including 16 previously unreported) with pathogenic variants in the
ZMYND11 and described in detail 20 patients with epilepsy (including 11 previously unreported). It was found that neurodevelopmental disorders are common in the people with epilepsy (intellectual disability: mild to moderate in 16/20; severe in 4/20 individuals). Dysmorphic features were variable and occurred in only 12 patients (12/20) [
7].
Here, we present 2 previously unreported paediatric patients with neurodevelopmental dysmorphic syndrome due to the pathogenic variants in ZMYND11. In addition, one of the patient had uncommon feature of this syndrome such as hyperinsulinemic hypoglycaemia (HH) not described in other ZMYND11 cases. The cause of HH in our case has not been clarified. It can be connected with the specific feeding problem, but there is a possibility that hyperinsulinemic hypoglycaemia belongs to a broad spectrum of this syndrome. However, the number of patients described in the literature is still too small.
2. Clinical Report
Patient 1
First patient is currently a 5-and-a-half-old boy born from non-consanguineous, healthy Caucasian parents. His main clinical features include developmental delay (especially affecting speech) dysmorphic features and short stature.
He was born at 37th week of gestation by spontaneous vaginal delivery with birth weight of 4400 g (SDS 2.39), length of 57 cm (SDS 4.03), head circumference of 37 cm (SDS 2.14) and 10 points in Apgar scale. In the neonatal period an episode of transient hypoglycaemia (31 mg/dl and 40 mg/dl) occurred and therefore, intravenous intake of 10% glucose was required for three days. Hearing assessment was normal. In general, recurrent respiratory infections occurred repeatedly throughout childhood. Otitis media occurred at 18 months of age. His development appeared delayed. The boy was undergoing rehabilitation. He was able to sit at the age of 24 months, and walk at the age of 4 years. Tooth eruption was slightly delayed.
At the age of 6 months, due to delayed psychomotor development, the boy was hospitalized in the the department of pediatric neurology. It was noted that hypotonia occurred since 2 months of life. From the 3rd month of age an episode of absent staring was noted. EEG examination showed no deviations. In the 14th month of age, the patient was hospitalized due to fever and an episode of seizures where he was diagnosed with meningitis and encephalitis in the course of Streptococcus pneumoniae infection. Levetiracetam and sodium valproate were recommended to treat seizures. He was treated with sodium valproate only for 3 months. The levetiracetam was discontinued when he was 4 years old. He had no seizures while taking levetiracetam but the history of anxiety attacks with awakenings at night was noted. Until the age of 5, he had severe sleep problems and woke up restless. The patient stays under ophthalmological care due to strabismus and nystagmus.
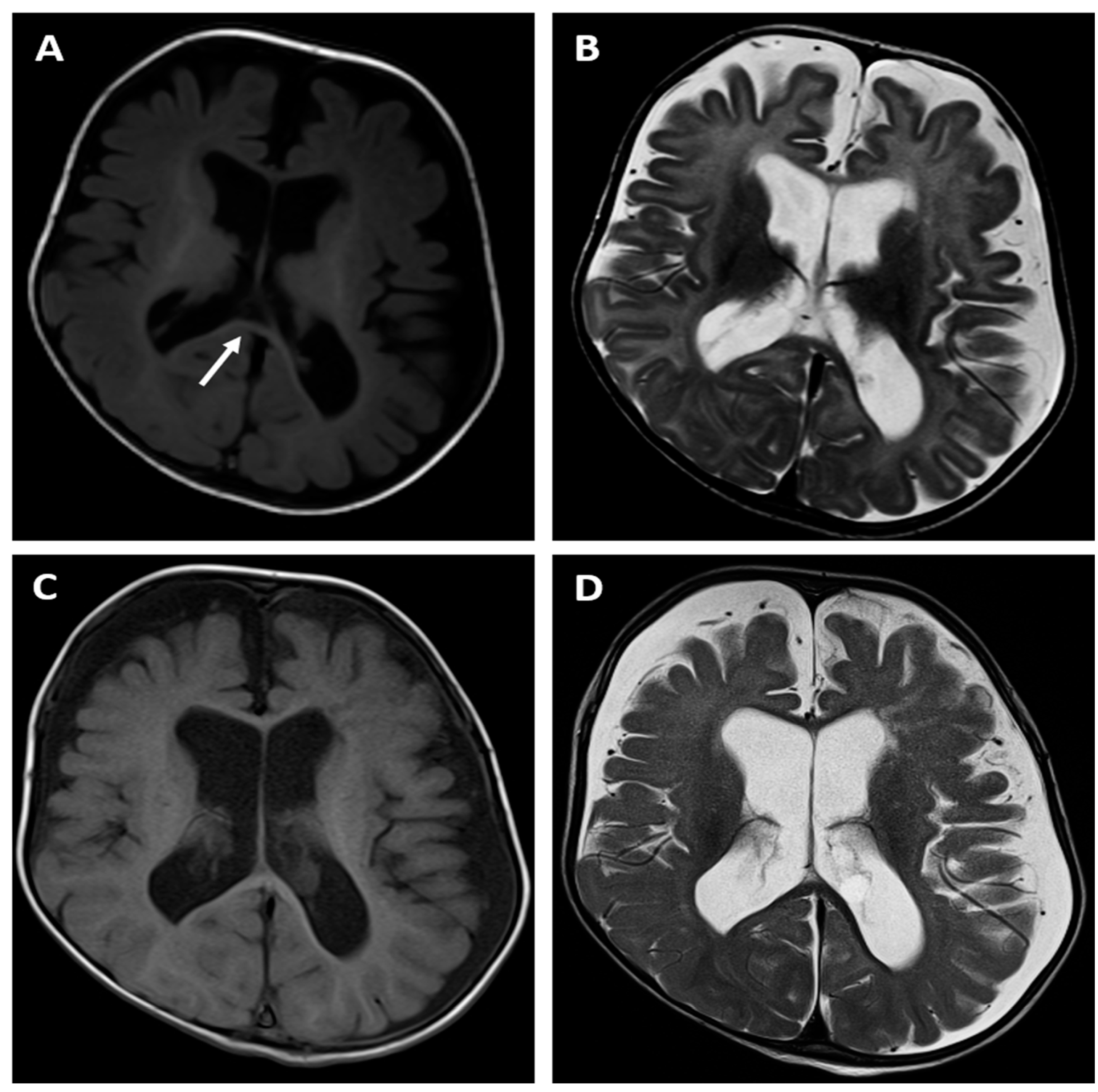
The brain MRI examination performed at the age of 6 months (
Figure 1A,B) revealed significantly enlarged cisterna magna, moderate dilatation of sulci in the frontal lobes as well as moderate dilatation of the lateral and third ventricles as a feature of cortico-subcortical atrophy with adjacent enlargement of the subarachnoid space in the fronto-temporal areas. There was also delayed myelination of the white matter indicating the typical pattern for the age of 4 months (high signal intensity of the splenium of the corpus callosum on T1-weithed image). The follow-up MRI examination was done at the age of 14 months (
Figure 1C,D) and showed progression of the myelination process, however there were still features of cortico-subcortical atrophy. Moreover the cortex/white matter differentiation was blurred, especially on T2-weighted image instead of being clearly visible at this age, which could indicated some demyelination disorder.
From the moment of delivery, sucking and swallowing disorders, regurgitation and anxiety attacks were observed. Up to 4 months of age the patient was breastfed. In the infancy period, based on the clinical picture (abdominal pain, bloating, regurgitation, anxiety, constipation), allergy to cow proteins was diagnosed and amino acid-based hypoallergenic formula was applied. Due to constipation, he was treated with macrogol. From the age of 2 he has remained on a low fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet (including gluten-free).
At the age of 3 years and 2 months the boy was hospitalized in the department of pediatric endocrinology due to hypoglycemia with transient hyperinsulinemia (the outpatient laboratory tests showed: glycemia 54 mg/dl with insulin 75.1 uIU/ml and fasting glycemia 49 mg/dl with insulin 6.8 uIU/ml). No hypoglycemia was observed during hospitalization. The HbA1c level was 4.6%. It was concluded that the cause of lower glycaemia could possibly be too restrictive diet (a low FODMAP diet).
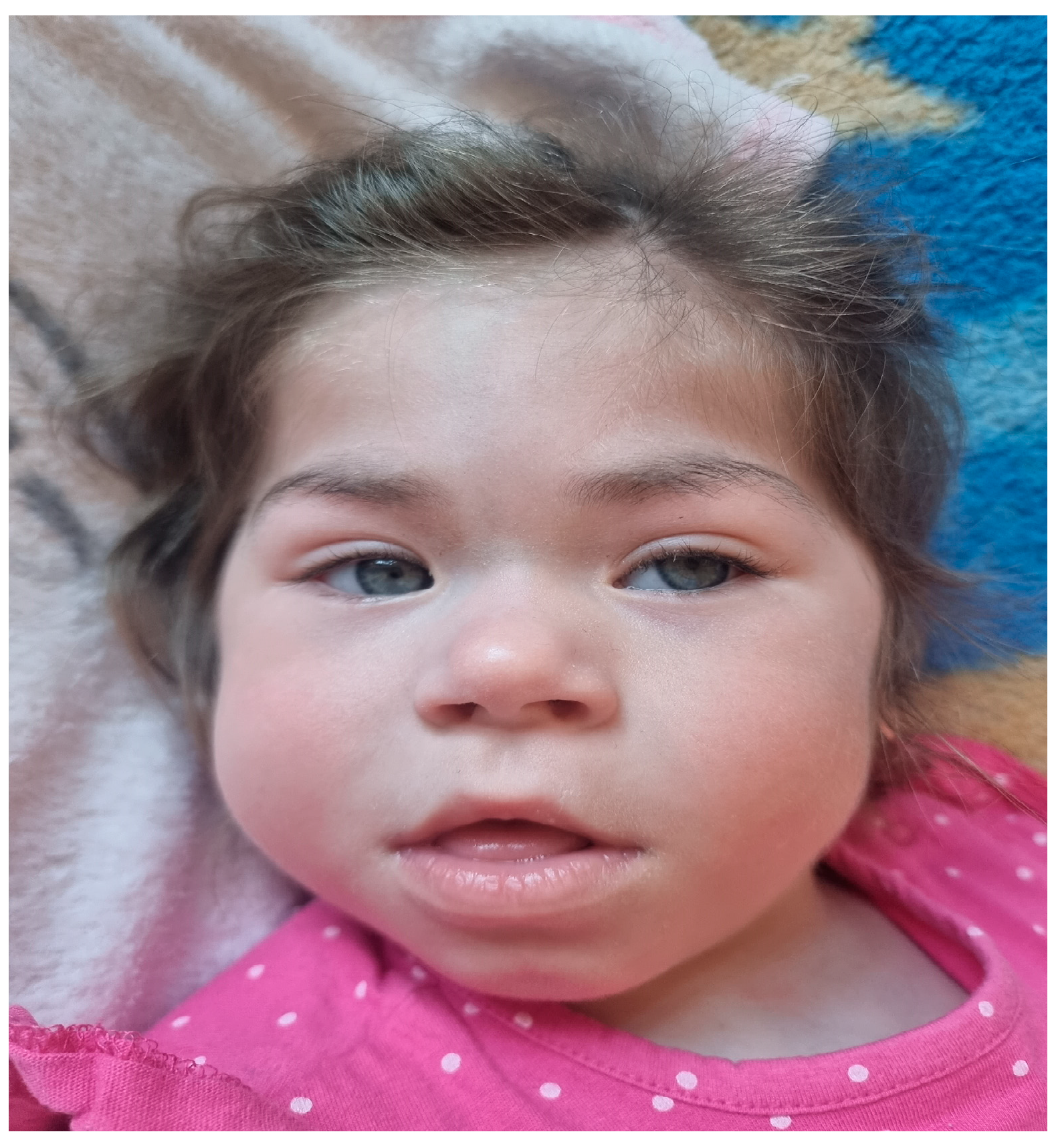
At the age of 5-and-a-half a physical examination, performed by a clinical geneticist, revealed facial-cranial dysmorphic features including asymmetry of the skull with bitemporal narrowing and prominent frontal tubercles, long and thick eyebrows, deep-set eyes, strabismus, low-set ears with prominent auricles and consistently open mouth. Additionally, short nose, low hairline, hyperelastic skin, short neck, prominent pectus carinatum, raised shoulder blades and kyphosis. Moreover hypotonia, abnormal a wide-based gait and short stature are note. The patient gives the general impression of an overactive child with increased mobility. He is nonverbal but makes sounds. His weight at 5 year of age is 17.3 kg, height 101.7 cm (<3 percentile, HSDS -3.43), BMI 16.7 kg/m2 (75-90 percentile) and occipito-frontal circumference (OFC) 53.8 cm (75-90 percentile).
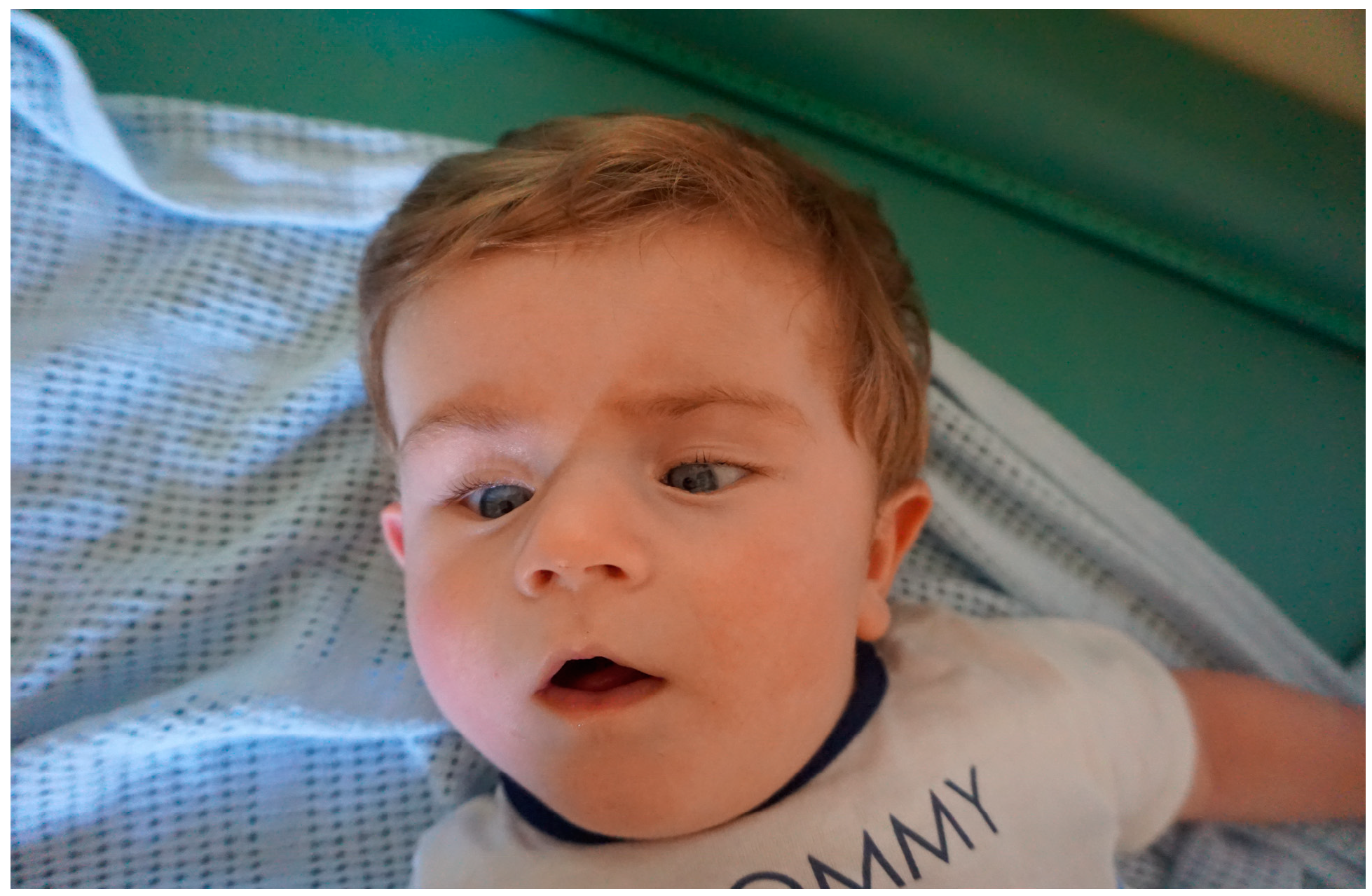
His first genetic consultation was at the age of 6 months because of developmental delay and dysmorphic features. Facial phenotype of patient 1 is presented in
Figure 2. His array-CGH did not reveal any copy number changes. At the age of 12 months further genetic diagnostics was performed using NGS-based whole exome sequencing (WES) in mono scheme (only proband) with Sanger verification in proband and parents.
Figure 1.
Brain MR examinations of patient 1: axial T1-weighted images (A, C), axial T2-weighted images (B,D) performed at the age of 6 months (upper row) and at the age of 14 months (bottom row). The images showed a feature of cortico-subcortical atrophy with adjacent enlargement of the subarachnoid space in the fronto-temporal areas (A,B). There was also delayed myelination of the white matter indicating the typical pattern for the age of 4 months (high signal intensity of the splenium of the corpus callosum on T1-weithed image - arrow) (A). The follow-up MRI examination (C,D) revealed progression of the myelination process, however the cortex/white matter differentiation was blurred, especially on T2-weighted image (D) instead of being clearly visible at this age, which could indicated some demyelination disorder.
Figure 1.
Brain MR examinations of patient 1: axial T1-weighted images (A, C), axial T2-weighted images (B,D) performed at the age of 6 months (upper row) and at the age of 14 months (bottom row). The images showed a feature of cortico-subcortical atrophy with adjacent enlargement of the subarachnoid space in the fronto-temporal areas (A,B). There was also delayed myelination of the white matter indicating the typical pattern for the age of 4 months (high signal intensity of the splenium of the corpus callosum on T1-weithed image - arrow) (A). The follow-up MRI examination (C,D) revealed progression of the myelination process, however the cortex/white matter differentiation was blurred, especially on T2-weighted image (D) instead of being clearly visible at this age, which could indicated some demyelination disorder.
Figure 2.
Facial phenotype of patient 1 with ZMYND11 in 6 months of age.
Figure 2.
Facial phenotype of patient 1 with ZMYND11 in 6 months of age.
Patient 2
Second patient is currently a 30-month-old girl born from non-consanguineous, healthy Caucasian parents. Her main clinical features include severe global developmental delay, feeding problem, dysmorphic features and hyperinsulinemic hypoglycaemia.
She was born at 38th week of gestation by spontaneous vaginal delivery with birth weight of 4200 g (SDS 1.94), length of 58 cm (SDS 4.77), head circumference of 34 cm (SDS 0) and 6/7 points in Apgar scale. Umbilical cord gas analysis showed pH 7.4 and BE 0.6. In the physical examination of the newborn numerous petechiae on the head and trunk were observed. In the neonatal period there were pneumonia, respiratory failure and thrombocytopenia. An episode of transient hypoglycaemia (12 mg/dl) occurred 16 hours after delivery. On the 3rd day of life, a CT scan of the abdomen and head was performed and it led to the suspicion of a spleen injury and hypoxic and post-traumatic lesions of the brain (blood/hematoma on the surface of the cerebellar tentorium on the right side, suspicion of indentation injuries to the skull bones). Moreover, transfontanella ultrasound showed calcifications along the course of the lenticulostriatal vessels. At 3 months of age, the blood test for CMV IgM and IgG antibodies was positive and the genetic material of the virus was present also in the urine (detected by PCR). Nevertheless, the test was performed in the 3rd month of the child's life that made it impossible to determine whether the infection was intrauterine or postnatal. At 8 months of age, a hearing test based on wave V yielded a bilateral threshold of 30 dBnHL, for 2-4kHz (mild hearing loss). Recurrent lower respiratory tract infections were occurring throughout the first year of life, often presenting with shortness of breath and respiratory failure. For this reason, the hospitalization in the intensive care unit was required twice. A noticeable gross motor and fine motor delay is observed (she is able to roll over, but yet not achieving the ability to sit). Speech is absent. There was a significant delay in the tooth eruption (first teeth at 12 months of age).
From the age of 2 months onwards, seizure incidents of various morphologies without fever and episodes of unconsciousness were observed. A sleep EEG performed at the age of 2.5 months showed bifocal seizure activity which led to the applying of the phenobarbital treatment. No epileptic seizures were observed from the commencing of the medication administration up till the 13th month of life. Afterward several incidents of immobility with eye rotation and increased muscle tone were noted and an epileptic episode with a morphology of limb tremor with eye rotation to the right and mouth open occurred. A sleep EEG performed at the age of 14 months showed background disorganization and epileptiform discharges. Seizures remained intractable, despite treatment with antiepileptic drugs such as lamotrigine and phenobarbital.
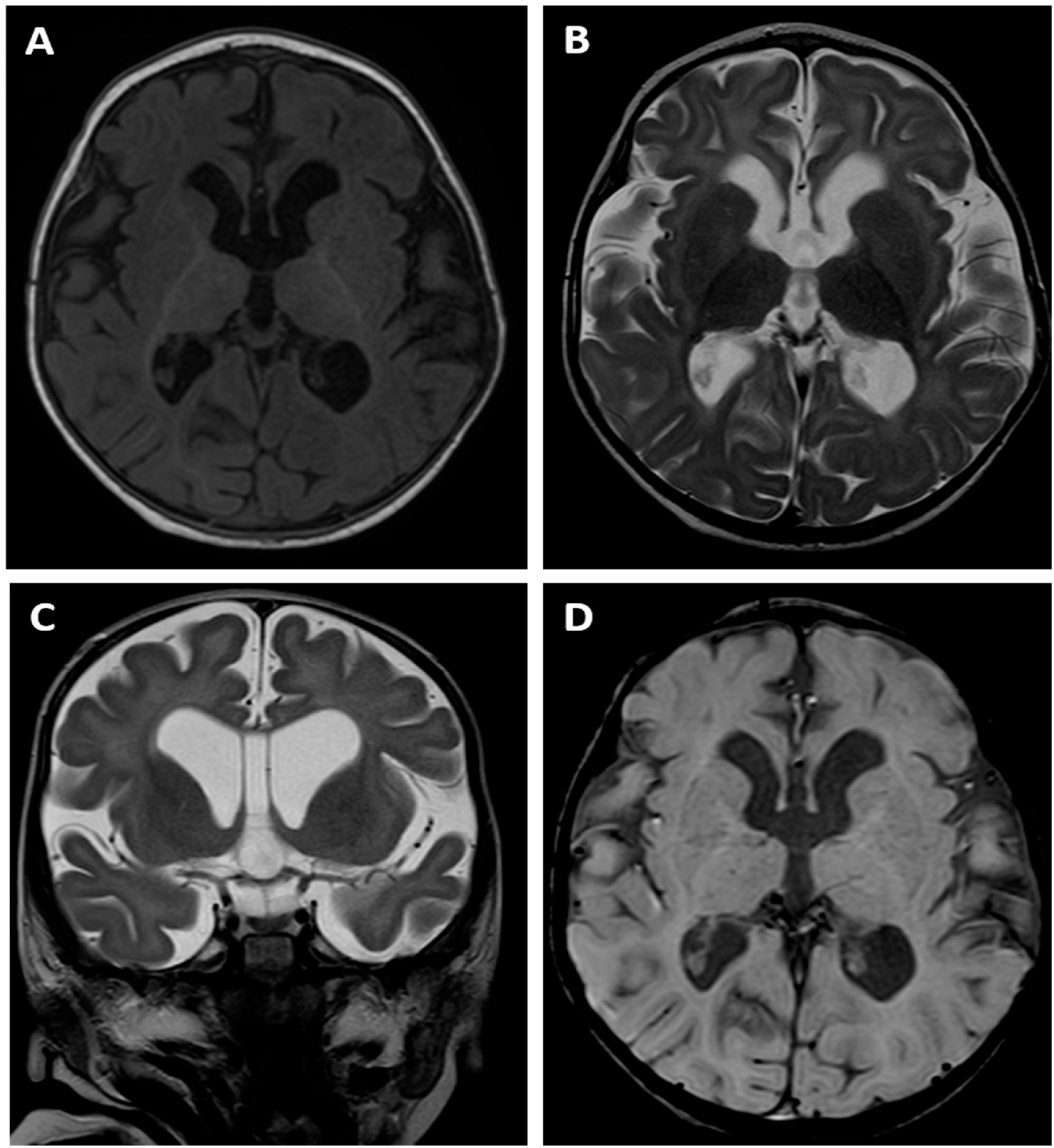
The brain MRI examination performed at the age of 14 months revealed an absence of normal myelination according to the age of the child. T1- and T2-weighted images (
Figure 3A, B) suggested delayed myelination of the cerebral white matter. The MRI appearance indicated the stage of myelination typical for the age of 8 months as there was a pronounced difference between the „anterior” and „posterior” part of the brain visible on T1 image with lower signal within the former one. However the cortex/white matter differentiation could be appreciated instead of being blurred that is a typical finding of a normal myelination process which means that a dysmyelination disorder should be suspected in this child.
There was also dilatation of the lateral ventricles, third ventricle and cerebral sulci as a sign of the cerebral cortico-subcortical atrophy. The MRI also showed a cavum septum pellucidum that is a normal variant of the CSF space between the leaflets of the septum pellucidum (
Figure 3C). SWI sequence (
Figure 3D) did not reveal any low signal of the hemosiderin depositions which meant that there were no signs of any previous intracranial bleeding, including intraventricular haemorrhage (IVH).
The patient was initially fed by a nasogastric tube with breast milk, then with a first infant formula. Abdominal pain and constipation suggested food allergy. After modifying the diet (hydrolysed whey protein) and pharmacological treatment (macrogol, trimebutine, omeprazole), a partially improvement was observed. At the age of 11 months, the patient was admitted to the gastroenterological ward due to feeding problems such as food retraction, spitting up and gag reflex. The patient’s weight was 10.26 kg, length 74 cm, (weight-to-length ratio 90-97 percentile). At 12 months of age due to the gastroesophageal reflux disease (GERD) as well as recurrent aspiration pneumonia, a Nissen fundoplication was performed and percutaneous endoscopic gastrostomy (PEG) was placed. Until now the patient is under the care of the nutrition ward. Currently, she is fed through a gastrostomy with a liquid, high-calorie diet (hydrolysed whey protein). Due to orofacial disorders, neurologopedic rehabilitation was recommended. In the second year of life, reduction in the incidence of lower respiratory tract infections was observed (pneumonia and bronchitis), whereas she suffered from purulent otitis three times during this period.
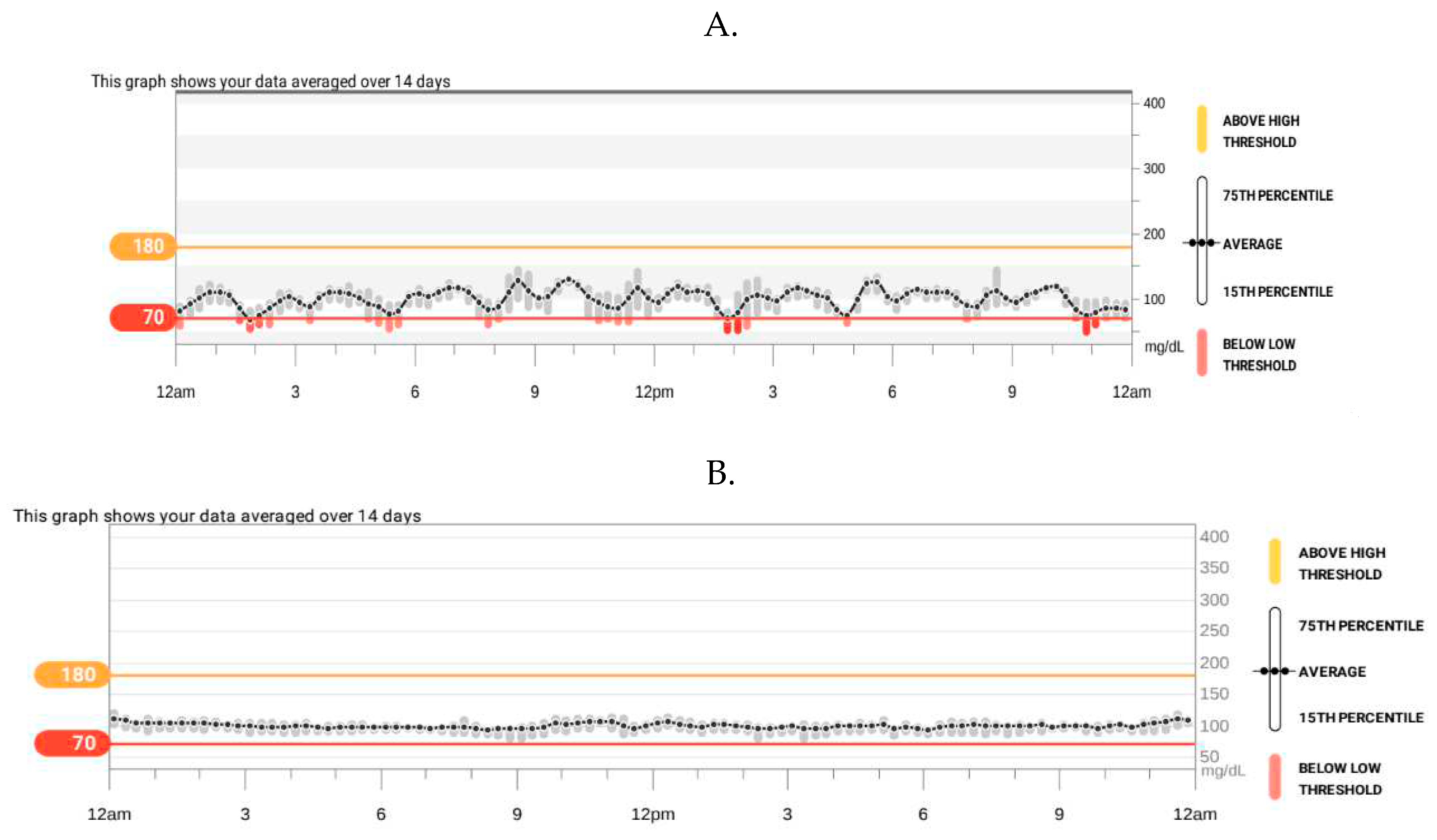
Hypoglycemia of 11 mg/dl was noted during a seizure attack in the 15 months of age. Therefore she was diagnosed in the department of pediatric endocrinology. Glucose levels were being tested successively from the moment of birth and except for the first day of life the results were normal. Hypoglycaemia reappeared 2 days before percutaneous gastrostomy placement at the age of 12 months (glycemia 30 mg/dl and 32 mg/dl, control 121 mg/dl). Thereafter, until 15 months of age glycemia was not being checked. On admission the physical examination revealed moderate macroglossia, the presence of 2 teeth, generalized hypotonia, a noticeable gross and fine motor delay. Auxological examination found: weight 11.5 kg, length 83.5 cm (>90 percentile, HSDS 1.76), weight-to-length ratio 75 percentile. Hyperinsulinemic hypoglycaemia was confirmed (critical sample at the time of hypoglycemia: glycemia 21 mg/dl, insulin 21.9 uIU/ml N<29, normal value during hypoglycaemia: < 2 uIU/ml, C-peptide 2.2 ng/ml, normal value during hypoglycaemia < 0.5 ng/ml, urine ketones were not detected, additionally appropriate counterregulatory hormone response: growth hormone 10.7 ng/ml and cortisol 20 ug/dl). HbA1c 4.7% (N: 4.5-6.2%). IGF1 33.2 ng/ml Normal range: 55-327, IGFBP-3 2.21 ug/ml N: 0.7-3.6. Abdominal ultrasonography showed hepatomegaly. Echocardiography was normal. The patient has been fed via gastrostomy regularly every 3 hours with extensively hydrolysed whey protein suitable as a sole source of nutrition, unable to take solid food. The girl was being assessed with continuous glucose monitoring. On the first glucose readings, a certain rhythmicity of its fluctuations, including hypoglycaemia, were observed (
Figure 4A). The diazoxide was initiated ( 5 mg/kg/day) and the dose was titrated to 10.8 mg/kg/day divided into three doses. Afterwards, no seizure or episodes of hypoglycaemia (
Figure 4B) were observed so the patient was responsive to diazoxide. From the 18
th month of age an increase in the frequency of gag reflexes is noticeable. There is a tendency to a mild hyperkalemia (before treatment with diazoxide potassium from 4.1 to 6.9, after introduction of diazoxide: potassium from 4.2 to 6.1 mmol/l N: 3.8-5.5).
A physical examination at the age of 22 months performed by a clinical geneticist revealed facial-skull dysmorphic features resembling Cornelia de Lange syndrome such as small teeth, consistently open mouth, low hairline, prominent eyebrows, increased hairiness on lower legs, thighs and forearms/ Facial phenotype of patient 2 is presented in
Figure 5. Excessive skin between the fingers of the hands, proximal displacement of the thumb, strabismus, nystagmus, hypotonia and increased excitability were also observed. Patient’s breathing is accompanied by wheezing. Her weight was 13.2 kg, height 88 cm (HSDS 1.36) and OFC 45.5cm (<3 percentile). At the age of 30 months her current weight is 11.9 kg, height 90 cm (25-50 percentile, HSDS -0.53), BMI 14.69kg/m
2 (15-25 percentile WHO).
Due to the suggestive features of Beckwith-Wiedemann syndrome (BWS), such as HH and macroglossia, molecular testing was performed (MS-MLPA), with the negative result. Further analysis of the phenotype by the clinical geneticist did not incline continuing the diagnosis towards BWS. At the age of 20 months further genetic diagnostics was performed using NGS-based whole exome sequencing (WES) in trio scheme (proband and both parents).
Figure 3.
Brain MR examination of patient 2: axial T1-weighted image (A), axial (B) and coronal (C) T2-weighted images as well as SWI image (D). T1- and T2-weighted images (A, B, C) suggested delayed myelination of the cerebral white matter indicating the stage of myelination typical for the age of 8 months. There was also dilatation of the ventricular system and cerebral sulci as a sign of the cerebral cortico-subcortical atrophy. The MRI also showed a cavum septum pellucidum (C). SWI sequence (D) did not reveal any low signal of the hemosiderin depositions.
Figure 3.
Brain MR examination of patient 2: axial T1-weighted image (A), axial (B) and coronal (C) T2-weighted images as well as SWI image (D). T1- and T2-weighted images (A, B, C) suggested delayed myelination of the cerebral white matter indicating the stage of myelination typical for the age of 8 months. There was also dilatation of the ventricular system and cerebral sulci as a sign of the cerebral cortico-subcortical atrophy. The MRI also showed a cavum septum pellucidum (C). SWI sequence (D) did not reveal any low signal of the hemosiderin depositions.
Figure 4.
Results of continuous glucose monitoring in a patient 2: at diagnosis of hyperinsulinemic hypoglycaemia (A); during treatment with diazoxide (B).
Figure 4.
Results of continuous glucose monitoring in a patient 2: at diagnosis of hyperinsulinemic hypoglycaemia (A); during treatment with diazoxide (B).
Figure 5.
Facial phenotype of patient 2 with ZMYND11 in 22 months of age.
Figure 5.
Facial phenotype of patient 2 with ZMYND11 in 22 months of age.
3. Genetic Studies
DNA form both probands and their relatives was extracted from blood using standard protocol. Both patients were subjected into exome sequencing (ES), for the proband 1 mono analysis was performed using SureSelectXT Human All Exon v7 (Agilent Technologies, Cedar Creek, TX, USA), while for the proband 2 trio analysis was performed using Twist Human Core Exome 2.0 + Comp Spike-in + Twist mtDNA Panel (Twist Bioscience, South San Francisco, CA, USA), respectively. Enriched libraries were pair-end sequenced (2x100 bp) on NovaSeq 6000 (Illumina, San Diego, CA, USA). Bioinformatics analysis of raw WES data and variants prioritization were performed as previously described (PMID: 32668698). Identified variants were further annotated with functional information, frequency in population (including gnomAD database
http://gnomad.broadinstitute.org/, and an in-house database of > 8500 Polish exomes), and known association with clinical phenotypes, based on both ClinVar (
https://www.ncbi.nlm.nih.gov/clinvar/) and HGMD (
http://www.hgmd.c HYPERLINK "http://www.hgmd.cf.ac.uk/"f.ac.uk) databases.
In silico pathogenicity prediction was performed based on Varsome provided pathogenicity and conservation scores (PMID:30376034) and American College of Medical Genetics and Genomics (ACMG) guidelines (PMID:25741868). For the proband 1 variant considered as disease causative were further subjected into family study and were analyzed in the proband, his heathy parents and brother by amplicon deep sequencing (ADS) performed using Nextera XT Kit (Illumina) and sequenced) as described above. While for the proband 2 the inheritance of plausible disease-causing variants was determined directly based on trio ES analysis.
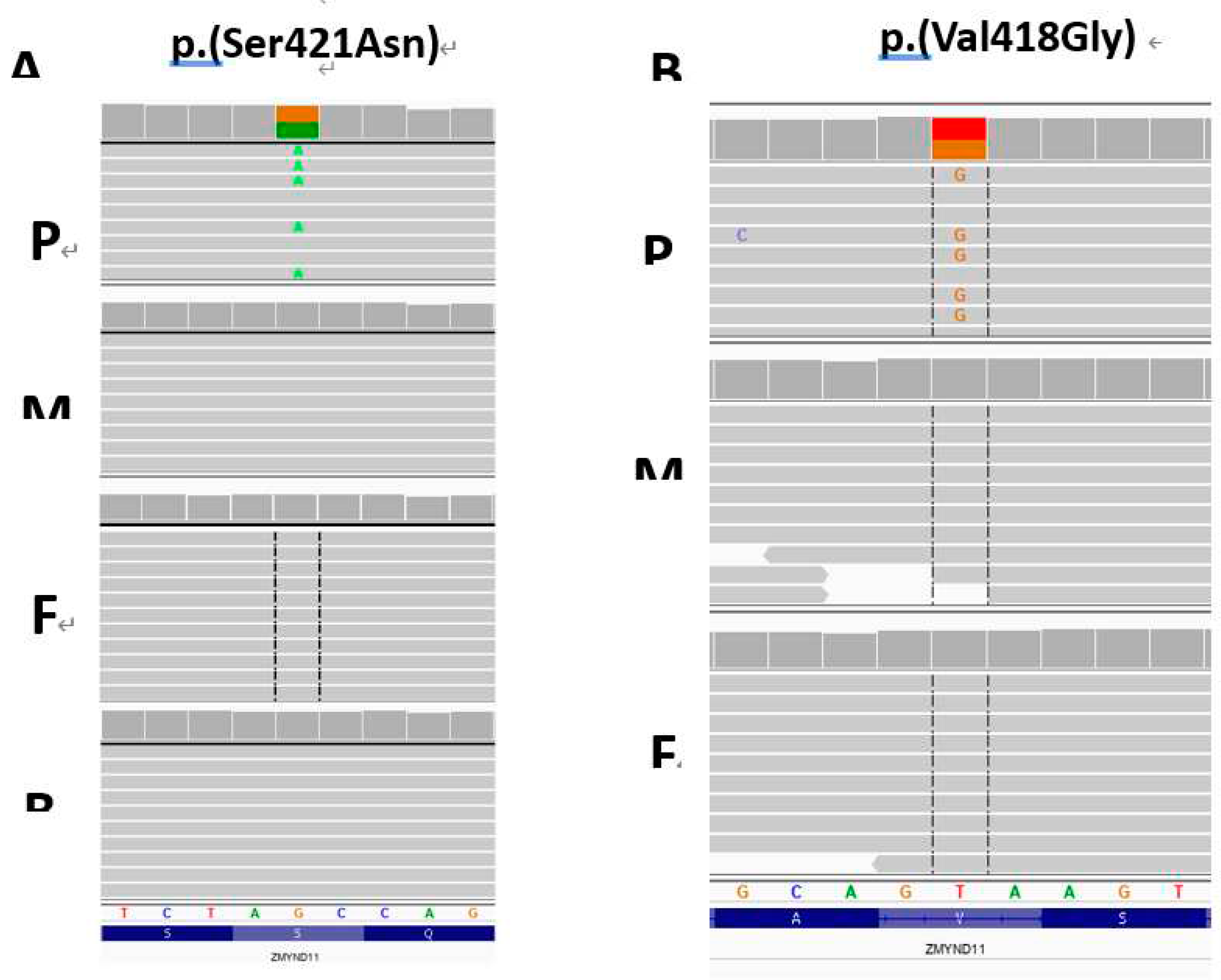
In both probands missense variants in
ZMYND11 gene were identified and prioritized as causative: NM_001370100.5:c.1262G>A, NP_001357029.1:p.Ser421Asn, rs869320713 located in exon 13 (position 35 of 273) in the proband 1 and a novel NM_001370100.5: c.1253T>G, NP_001357029.1:p.Val418Gly located in exon 13 (position 26 of 273) in the proband 2, respectively. Both, p.Ser421Asn and p.Val418Gly,
ZMYND11 variants were absent in probands’ parents and were considered as likely
de novo events (
Figure 6A and B, respectively).
The p.(Ser421Asn) variant has 0 frequency in gnomAD v3.1.2 database (accessed 10 August 2023) and is classified as variant of uncertain significance (VUS) according to ACMG guidelines (score: 3 points, PP5 Moderate, PM1 Supporting, PM2 Supporting, BP4 Supporting), however when the de novo status is taking into account the variant is classified as likely pathogenic (score 7 points, PP5 Moderate, PM1 Supporting, PM2 Supporting, BP4 Supporting, PS2 Strong). Results of an in silico pathogenicity prediction are as follows: CADD score 24.1, six meta scores predictors indicate p.Ser421Asn as “benign”, six individual predictions as “pathogenic”, six as VUS and 13 as “benign. The p.Ser421Asn variant is located in a highly constrained coding region (CCRS=92,33) and the c.1262G is highly conserved (phyloP100: 9.646). Moreover, the p.(Ser421Asn) variant is registered in ClinVar database (Version: 31-Jul-2023) and classified as “likely pathogenic” (accession no. VCV000225254.2), in HGMD database has confidence high”, and was reported as disease-causing in patients with neurodevelopmental disorders (PMID: 27626064, 28135719, 31785789).
The p.Val418Gly variant has 0 frequency in gnomAD v3.1.2 database (accessed 10 August 2023) and is classified as VUS according to ACMG guidelines (score: 3 points, PM1 Moderate, PM2 Supporting) however when the de novo status is taking into account the variant is classified as likely pathogenic (score 7 points, PM1 Moderate, PM2 Supporting, PS2 Strong). Results of an in silico pathogenicity prediction are as follows: CADD score 28.5, three meta scores predictors indicate p.(Ser421Asn) as “pathogenic”, one as VUS, five as “benign”, three individual predictions as “pathogenic”, eight as VUS and ten as “benign”. The p. Val418Gly variant is located in a highly constrained coding region (CCRS=92,33) and the c.1253T is highly conserved (phyloP100: 7.736).
Figure 6.
Results of molecular study. A – amplicon deep sequencing-based family study and ZMYND11 p.(Ser421Asn) variant validation for the proband 1. B – TRIO exome sequencing in the proband 2 revealed ZMYND11 p.(Val418Gly). Both identified ZMYND11 variants were considered as likely de novo events. P – proband, M – mother, F – father, B – brother. The IGV screen shots are present.
Figure 6.
Results of molecular study. A – amplicon deep sequencing-based family study and ZMYND11 p.(Ser421Asn) variant validation for the proband 1. B – TRIO exome sequencing in the proband 2 revealed ZMYND11 p.(Val418Gly). Both identified ZMYND11 variants were considered as likely de novo events. P – proband, M – mother, F – father, B – brother. The IGV screen shots are present.
4. Discussion
Histone modifications (acetylation, methylation, and others) affect chromatin structure, provide binding platforms for diverse transcription factors and therefore play important roles in many cellular events, including gene expression, DNA repair, and cell cycle control [
8]. Misregulation of histone modifications is associated with developmental defects [
9]. Zinc finger MYND domain-containing protein 11 is epigenetic reader protein that bind to specific methylation histone (H3.3K36me3) to regulated pre-mRNA [
10]. Disorders of transcriptional regulations associated with histone modification and chromatin remodeling such as Coffin-Siris syndrome (disruption of histone phosphorylation), Rubinstein-Taybi syndrome (disruption of histone acetylation) and Sotos syndrome (disruption of histone methylation) or Cornelia de Lange syndrome display similar clinical features to
ZMYND11 disease such as developmental delay/intellectual disability, facial dysmorphisms and growth problems [
11]. In a group of 57 patients with a clinical suspicion of Cornelia de Lange syndrome one
ZMYND11 frameshift variant was identified [
12].
ZMYND11 gene is expressed in many human tissues, including brain. Pathogenic variants in the
ZMYND11 gene can lead to a broad spectrum of signs and symptoms. There is considerable variability in the degree of psychomotor and speech developmental delay and intellectual disability in patients with this syndrome. Presented patients in this paper were referred to genetic examination due to severe developmental delay, hypotonia and dysmorphic features and they have been identified through a whole exome sequencing test.
Pathogenic variants in the Z
MYND11 gene as a specific dysmorphic syndrome was first described in 2014 [
2,
5]. Further cases were reported by Moskowitz et al. in 2016 [
13] and by Popp et al. in 2017 [
14]. Yates at al. [
6] summarize 27 patients with pathogenic variants in the
ZMYND11 gene. Among them, all of them had intellectual disabilities including 4 with severe intellectual disability (21/21 except for 6 in whom no information was provided), 38 % had epilepsy and 48 % hypotonia. Growth abnormalities like short stature (proband 1) or microcephaly (proband 2) are additional findings in this disorder.
It was noticed that patients with truncating variants in ZMYND gene have a similar phenotype to 10p15.3 microdeletion syndrome, but in some missense variants there is more severe phenotype (for example cases described by Moskowitz et al or Cobben et al [5, 6, 13].
Presented first patient and a 24-year-old female described by Moskowitz et al. have the same variant in the
ZMYND11 gene. Both patients had global developmental delay especially affecting speech, hypotonia and in MRI delayed myelination. However, in the patient described by us there is no basis for the diagnosis of epilepsy, unlike the patient described by Moskowitz et al. [
13], although our one patient had been receiving antiepileptic drug for several years. Clinical characteristics of presented patients and referred to the literature are shown in
Table 1.
Feeding problems is another issue affecting a significant proportion of people with the
ZMYND11 variant, a finding that may be partially associated with global hypotonia. In the second proband presented in this paper the following symptoms appeared in this matter: choking during and after a meal, food retraction, spitting up and increased gag reflex with solid foods. A similar clinical picture has already been described in the literature. Yates et al. [
6] report that problems with feeding, including excess vomiting after feeds and/or bottle feeding requiring more than 1 hour, were present in 59% of all patients described in his work. However, their manifestation was usually mild - only three patients required supplementary feeding by nasogastric tube [
6]. Interestingly, two patients described in the literature presented with tooth enamel hypoplasia, a problem that we did observe in our patient is delayed eruption of the teeth, a finding not extensively discussed in literature. When compared to literature, it can be noticed that no patients presented with hypoglycemia.
HH is characterized by hypoglycaemia with inadequate suppression of insulin, detectable C-peptide, suppressed or low level of ketones (3-β-hydroxybutyrate <2 mmol/L) and absence of met abolic acidosis [
15]. The time of onset of hypoglycaemia, its correlation with the type of meals and the presence of dysmorphic features are essential because HH is heterogenous disorder divided into: a transient form related to perinatal stress or following gastrointestinal procedures; congenital, monogenic HH related to variants in fifteen genes implicated in pancreatic development and function (due to genetic defect insulin release is independent from glucose level) and associated with syndromes (such as Beckwith-Wiedemann syndrome) [
16]. Considering the known mechanisms monogenic HH can be grouped into categories: channel defects (ATP-sensitive potassium channel), metabolic defects (the importance of ATP level in the control of insulin secretion indicates that mitochondria and oxidative metabolism are important) and transcription factors defects (HNF4A and HNF1A) [
17]. Although the loss of function of
ZMYND11 gene is a recognized cause of hypotonia and feeding problems, it has not previously been noted as a risk factor for HH. We have studied exome sequencing data of presented patients and have not found any pathogenic or likely pathogenic variants that could cause HH. However, this is not a rare case because, a large number of HH cases remain unexplained. In approximately 50% of patients with the persistent form of HH a known underlying genetic basis may explain the molecular mechanism for dysregulated insulin secretion [
15]. In presented proband 2 noteworthy is the rather late onset of hyperinsulinemic hypoglycemia (in the 15th months of life ) and unnatural way of feeding via a gastrostomy tube. Postprandial hypoglycemia is a common complication of Nissen fundoplication in children as well. The mechanism responsible for the hypoglycemia involves an exaggerated secretion of the glucagon-like peptide 1 (GLP-1) which may contribute to the excessive insulin secretion and subsequent hypoglycemia [
18]. The fact that the proband received only liquid meals after the surgery may further exacerbate the problem because it is known that a liquid meal results in significantly more GLP-1 release than a solid meal of identical composition [
19]. However, hypoglycaemia (30 mg/dl) was diagnosed 2 days before gastrostomy insertion but diagnostics were not continued at that time. The question remains whether in the presented patients, pathogenic variants in the hitherto unknown genes responsible for HH caused the disorder, whether it is the result of environmental or epigenetic factors. A successful attempt to return to the natural way of feeding could provide the answer, but so far this is not possible. As a result of inserting gastrostomy the frequency of recurrent pneumonia with respiratory failure was reduced. The non-physiological way of feeding could, however, contribute to the appearance or aggravation of HH. This was the reason for the introduction of diazoxide, which in turn may have exacerbated the hypotonia [
20]. Diazoxide is the first-line agent for treatment of congenital HH. If the patient is responsive to diazoxide, then they could be treated with the medicine for a long time. Common side effects of diazoxide are mild-to-severe hypertrichosis (as in our patient), the fluid retention, pulmonary hypertension, neutropenia or trombocythopenia. Diazoxide inhibits insulin release, but also can causes smooth muscle relaxation by opening K ATP channels, which leads to cell hyperpolarization and thus to blockage of calcium channels [
21]. However, it is worth noting that the KATP channel has a less significant impact on regulating electrical activity of muscle and nerve cells which results from contribution of additional channels to the resting membrane potential and membrane resistance in those tissues. Thus, equivalent changes in KATP channel activity are expected to have less dramatic effects in these cell types. It is noteworthy that K-ATP channels are normally open in pancreatic beta cells, whereas in other tissues they are closed and only open under conditions of metabolic stress. Still, we are taking into consideration that diazoxide during use may have contributed to hypotonia to some extent by opening potassium channels in skeletal muscles, which led to their excessive relaxation [
22]. Nevertheless, we believe that the ZMYND11 mutation plays the most significant role in the girl's development of severe hypotonia and feeding problem.
One of the most severe issues of the presented proband 2 are the pathologies in her brain revealed in MRI examination performed at the age of 14 months. The brain MRI showed an absence of normal myelination according to the age of the child that suggested delayed myelination of the white matter indicating the stage of myelination typical for the age of 8 months. There was also dilatation of the lateral ventricles, third ventricle and cerebral sulci as a sign of the cerebral cortico-subcortical atrophy.
The clinical picture suggests two main causes of these disorders - hypoglycemia and the presence of the
ZMYND11 mutation. In the case of both diagnoses, CNS abnormalities of various morphologies are very common. In the case of hypoglycemia, disturbances in the structure of the cerebral cortex are described in the literature, including advanced destructive changes in the white matter and dilatation of the ventricular system [23, 24], which is consistent with the MRI image of our patient. In addition, intraventricular haemorrhage (IVH) reported by two centers [25, 26], both occurring within the first 10 days of life, seems to be associated with hyperinsulinemic hypoglycemia, but not in the proband presented in this paper as we did not observe any low signal of the hemosiderin depositions in SWI sequence. The correlation of HH with dysmyelination changes draws our attention to cases of vanishing syndrome, which have been described as accompanying glucose fluctuations of a similar nature. Vanishing syndrome (VWM, OMIM 603896) is a childhood ataxia with central nervous system hypomyelination (CACH) is one of the most common leukodystrophies [
27]. Although VWM is associated with the occurrence of mutations in the
EIF2B1, EIF2B2, EIF2B3, EIF2B4, EIF2B5 genes, which were not detected in the WES examination in presented patients, considering the incompletely known etiology of HH, we believe that VWM is worth mentioning in the context of the described proband although the typical MR appearance of white matter alterations in VHM syndrome is completely different.
Characteristic MRI findings of VWM include diffuse, symmetric cerebral white matter involvement with T2 and FLAIR high signal intensity areas, extending from periventricular regions to the subcortical arcuate fibers. Over time, the white matter vanishes and is replaced by characteristic areas presenting almost CSF signal intensity which means low signal changes on FLAIR images. Cerebellar atrophy is also present in VWM being the only similar feature regarding the white matter MR appearance in VWM and in our patient.
Moving on to neurological disorders in the ZMYND11 pathological variants cases, their range is immensely wide and includes generalized developmental delay, seizures, autism and behavioural abnormalities [6, 7, 13]. Among them, presented patient 2 suffers from generalized developmental delay and seizures diagnosed at 2 months of age. We believe that the
ZMYND11 mutation also seems to have a significant impact on dysmyelination changes in the presented probands, which is confirmed by scientific reports. Cortico-subcortical atrophy of the cerebral cortex [
1], cerebral atrophy, delayed myelination, compression of myelin [5, 7, 13] are MRI findings that have appeared in the papers published hitherto. These vastly coincide with the changes exhibited by our proband.
Table 1.
Clinical characteristics of presented patients and referred to the literature.
Table 1.
Clinical characteristics of presented patients and referred to the literature.
| |
Proband 1 |
Proband 2 |
Moskovitz et al [13] |
| age reported / gender |
5.6 yr/male |
2,5yr/female |
24 yr/female |
Birth weight (SDS)
length (SDS) |
4400 g (+2.39)
57 cm (+4,03) |
4200 g (+1.94)
58 cm (+4.77) |
3740 g
52 cm |
| HBD |
37 |
38 |
born full term |
| birth OFC (cm) |
37 (2.14) |
34 (0) |
- |
| OFC (age reported) |
53.8 cm (75-90c) |
45.5 c <3 centile (1.8 year) |
Microcephaly
51.5 cm |
| height/weight |
short stature |
normal height |
No information |
| feeding problems |
mild |
Severe nasogastric tube feeding, at 12 months PEG was placed. |
Severe eosinophilic esophagitis |
| development: gross motor |
sitting at 24 mo walking: 4-5 yr |
sitting, walking not yet achieved |
sitting at 18mo walking: 4-5 yr |
| development: speech/communicate |
nonverbal makes sounds. |
nonverbal |
Nonverbal makes sounds/ communicate using a few signs |
| hearing impairment |
no |
mild |
mild |
| hypotonia |
yes |
yes |
yes |
| epilepsy |
no |
yes (dgn 2 mo) |
yes (dgn 9 mo) |
| brain MRI (age at which the test was performed) |
cerebral atrophy, delayed myelination (6 and 14 mo) |
cerebral atrophy, delayed myelination (14 mo) |
cerebral atrophy and delayed myelination (8 years) |
| behavioural difficulties |
hyperactivity sleeping problems |
|
hyperactivity happy disposition |
| dysmorphic |
deep-set eyes, long and thick eyebrows, strabismus, consistently open mouth, low-set ears with prominent auricles, |
prominent eyebrows, strabismus, consistently open mouth, small teeth, low hairline, |
deep -set eyes , prominent jaw, wide mouth, broad nasal root and bulbous nasal tip, |
| frequent infections |
recurrent respiratory infections otitis media
encephalitis in the course of Streptococcus pneumoniae infection |
recurrent lower respiratory chronic otitis media |
chronic otitis media, sinusitis, and recurrent lower respiratory infections |
| allergy |
allergy to cow proteins was suspected. |
allergy to cow proteins was suspected |
eosinophilic esophagitis, asthma, atopic dermatitis, drug and food allergies |
| variant in ZMYND11 gene |
c.1262G>A |
c.1253T>G |
c.1262G>A |
| variant mechanism |
missense |
missense |
missense |
| predicted effect on protein |
p.Ser421Asn |
p.Val418Gly |
p.Ser421Asn |