Submitted:
26 January 2024
Posted:
29 January 2024
You are already at the latest version
Abstract
Keywords:
Introduction
- Irregular R-R intervals (when atrioventricular conduction is not impaired),
- Absence of distinct repeating P waves, and
- Irregular atrial activations.” (Hindricks et al. 2021)
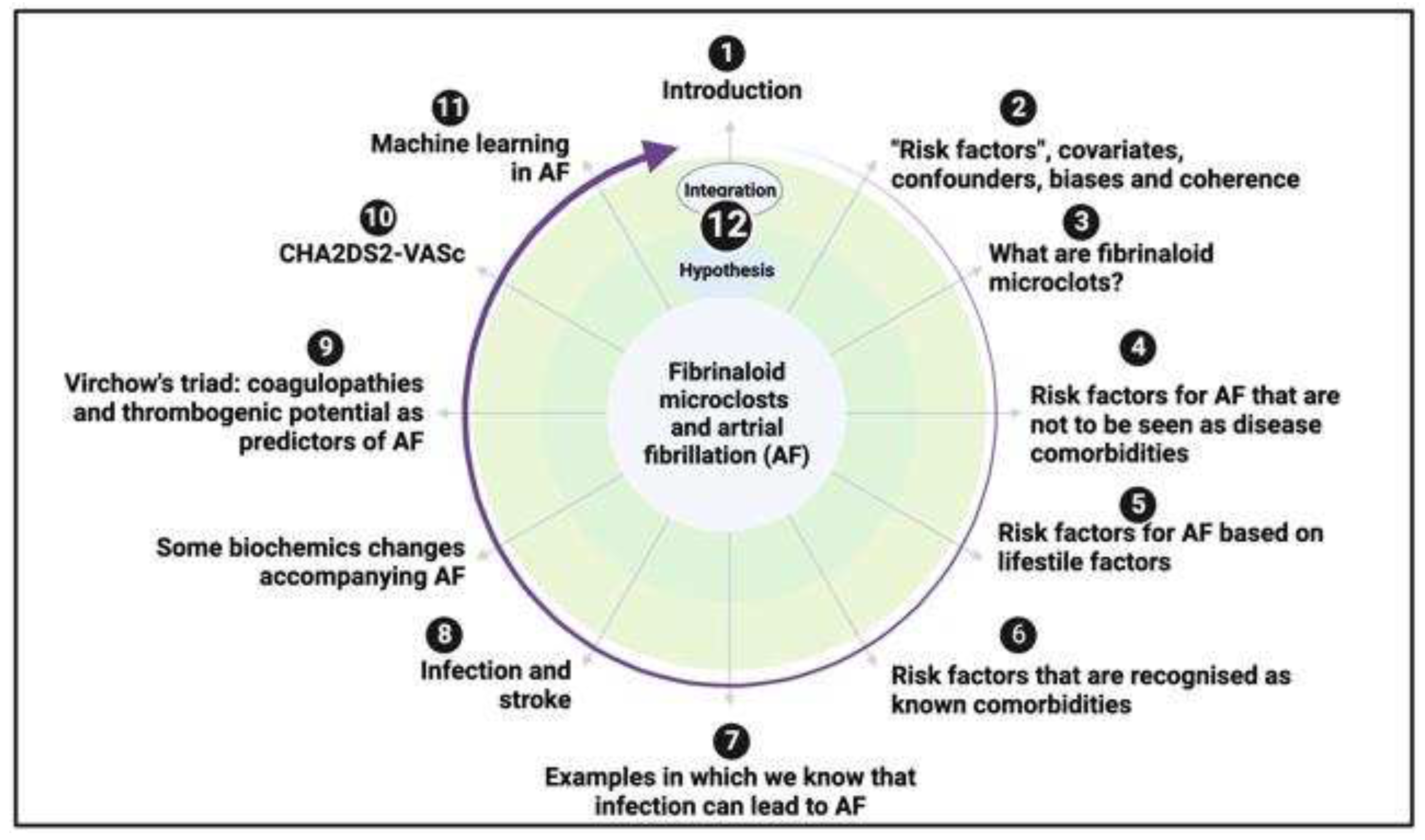
“Risk factors”, covariates, confounders, biases and coherence
What are fibrinaloid microclots?
Risk factors for AF that are not to be seen as disease comorbidities
| Risk factor | Comments | Selected references |
|---|---|---|
| Age | Normally a significant increase in AF with age | (Hindricks et al. 2021; Lowres et al. 2019; Marinigh et al. 2010; Zhang et al. 2021a) |
| In some cases the opposite can be true for athletes. | (Ayinde et al. 2018; Goudis et al. 2015) | |
| May involve age-dependent Na+ channel expression | (Isaac et al. 2020) | |
| BMI | Obesity is sometimes a clear risk factor (Benjamin et al. 1998) (as for many metabolic diseases), but there are also (and more commonly) a variety of so-called ‘obesity paradoxes’ where the hazard ratios for acquiring or manifesting AF, and in particular suffering disease sequelae therefrom, are actually significantly greater for those with a lower BMI. | (Li et al. 2022; Liu et al. 2020; Overvad et al. 2013; Panchal et al. 2019b; Proietti and Boriani 2020; Proietti et al. 2017; Rodríguez-Reyes et al. 2021; Sandhu et al. 2016; Sandhu et al. 2018; Wanahita et al. 2008; Wang et al. 2004; Wu et al. 2023b; Zhu et al. 2016) |
| Obesity may induce sleep apnoea, which is a known risk factor for AF | (Al-Falahi et al. 2017; Benjafield et al. 2019; Khan et al. 2018; Zhang et al. 2021b) | |
| Ethnicity | More prevalent among Caucasians; not entirely clear how much is genetics, culture/lifestyle, or GxE, and as with genetics no studies really seek to deconvolve these factors | (Zhang et al. 2021a) |
| Familial associations/ Genetics | Mostly less significant than lifestyle factors and co-morbidities, apart from some particular and relatively uncommon ion channelopathies | (Feghaly et al. 2018); |
| Monozygotic:dizygotic ratio does predict a role for genetics, so not purely cultural associations | (Christophersen et al. 2013; Christophersen et al. 2009) | |
| Highly polygenic, with genes involved in developmental, contractile, and electrophysiological functions. Necessarily convolved with GxE association that cannot be interpreted from GWAS studies alone. | (Kalstø et al. 2019; Lee et al. 2023; Roselli et al. 2018; Selewa et al. 2023; Weng et al. 2017) | |
| Gender | More prevalent in males, though outcomes can be worse for females (so being female contributes to the CHA2DS2-VASc score (Friberg et al. 2012; Lip et al. 2010)). Less important than age for asymptomatic AF. | (Li et al. 2019; Xiong et al. 2015; Zhang et al. 2021a) |
Risk factors for AF based on lifestyle factors
Risk factors that are recognised as known disease comorbidities
Examples in which we know that infection can lead to atrial fibrillation
- 7.6% of cases of community acquired pneumonia led to new-onset AF (Corica et al. 2023b)
- AF is a common occurrence following infection with SARS-CoV-2 (COVID-19) (Abbasi 2022; Al-Abbas et al. 2021; Bagnato et al. 2022; Bhatla et al. 2020; Duckheim and Schreieck 2021; García-Granja et al. 2021; Kanuri et al. 2023; Mouram et al. 2022; Niehues et al. 2022; Rav-Acha et al. 2021; Wu et al. 2023a)
- The Odds Ratio OR for AF 365 days after COVID-19 compared to a well-established control group was 1.83 in a large study (Berman et al. 2023).
- A previous use of DOACs is protective against AF following SARS-CoV-2 infection (Azaña Gómez et al. 2022). Note that AF increased the bleeding risk of those on anticoagulants (Rubini-Costa et al. 2022).
- There was an increased mortality from acute COVID-19 in patients with AF (Musikantow et al. 2021; Pardo Sanz et al. 2021; Zuin et al. 2021), especially older ones.
- The same applies to Long COVID (Huseynov et al. 2023) (which is not surprising giving its incidence following acute COVID). Incidence after covid and refs
- Cardiac arrhythmias also seem to be caused by COVID-19 vaccination (Pari et al. 2023) (which of course includes spike protein or RNA coding for it) and spike protein is known to cause microclots (Grobbelaar et al. 2022; Grobbelaar et al. 2021).
- New-onset AF is a common occurrence in sepsis (which of course convolves e.g. infection and inflammation), increasing as sepsis leads to severe sepsis and then septic shock, and leading to poorer outcomes (Aibar and Schulman 2021; Bashar et al. 2020; Bosch et al. 2019; Corica et al. 2022; Downes et al. 2023; Honorato et al. 2023; Induruwa et al. 2022; Klein Klouwenberg et al. 2017; Kuipers et al. 2014; Proietti and Romiti 2021; Walkey et al. 2013; Xiao et al. 2021).
- Sepsis (we ignore subtypes (Cano-Gamez et al. 2022; van Amstel et al. 2023)) likely involves microclots (Kell and Pretorius 2018b), which can be induced experimentally in the presence of cell-surface components of infectious agents such as bacterial lipopolysaccharide (Pretorius et al. 2018a; Pretorius et al. 2016b; Pretorius et al. 2018c; Pretorius et al. 2018d) or lipoteichoic acid (Pretorius et al. 2018c), or the spike protein of SARS-CoV-2 (Grobbelaar et al. 2022; Grobbelaar et al. 2021; Pretorius and Kell 2023)
- Coagulation, in the worst case disseminated intravascular coagulation, is a frequent accompaniment of sepsis (Allen et al. 2015; Gando 2010; Iba et al. 2013; Levi 2010; Levi et al. 1997; Li and Ma 2017; Murao and Yamakawa 2019; Yu et al. 2022)
- Anticoagulants are significantly protective against the complications of sepsis when timed properly (Meziani et al. 2017; Scarlatescu et al. 2017) and especially in the presence of disseminated intravascular coagulation (Qi et al. 2023; Umemura et al. 2023; Umemura and Yamakawa 2018)
Infection and Stroke
Some biochemical changes accompanying AF
Virchow’s triad: coagulopathies and thrombogenic potential as predictors of AF
Clinical risk scores, e.g. CHA2DS2-VASc
Machine learning in AF
Discussion, conclusions, and a forward look
Funding
References
- Abbasi J: The COVID Heart-One Year After SARS-CoV-2 Infection, Patients Have an Array of Increased Cardiovascular Risks. JAMA 2022; 327:1113-1114. [CrossRef]
- Aguzzi A, De Cecco E: Shifts and drifts in prion science. Science 2020; 370:32-34. [CrossRef]
- Aguzzi A, Lakkaraju AKK: Cell Biology of Prions and Prionoids: A Status Report. Trends Cell Biol 2016; 26:40-51. [CrossRef]
- Ahmed S, Zimba O, Gasparyan AY: Thrombosis in Coronavirus disease 2019 (COVID-19) through the prism of Virchow's triad. Clin Rheumatol 2020; 39:2529-2543. [CrossRef]
- Aibar J, Schulman S: New-Onset Atrial Fibrillation in Sepsis: A Narrative Review. Semin Thromb Hemost 2021; 47:18-25. [CrossRef]
- Aizer A, Gaziano JM, Cook NR, Manson JE, Buring JE, Albert CM: Relation of vigorous exercise to risk of atrial fibrillation. Am J Cardiol 2009; 103:1572-1577. [CrossRef]
- Al-Abbas O, Alshaikhli A, Amran HA: New-Onset Atrial Fibrillation and Multiple Systemic Emboli in a COVID-19 Patient. Cureus 2021; 13:e12917. [CrossRef]
- Al-Falahi Z, Williamson J, Dimitri H: Atrial Fibrillation and Sleep Apnoea: Guilt by Association? Heart Lung Circ 2017; 26:902-910. [CrossRef]
- Ali S, Saadat V, Co ML, Contractor T, Mandapati R, Parwani P: Permanent left atrial thrombi after multiple atrial fibrillation ablations: Acquired Virchow's triad? HeartRhythm Case Rep 2022; 8:715-718. [CrossRef]
- Alijla F, Buttia C, Reichlin T, Razvi S, Minder B, Wilhelm M, Muka T, Franco OH, Bano A: Association of diabetes with atrial fibrillation types: a systematic review and meta-analysis. Cardiovasc Diabetol 2021; 20:230. [CrossRef]
- Allen KS, Sawheny E, Kinasewitz GT: Anticoagulant modulation of inflammation in severe sepsis. World J Crit Care Med 2015; 4:105-115. [CrossRef]
- Allotey J, Snell KIE, Smuk M, Hooper R, Chan CL, Ahmed A, Chappell LC, von Dadelszen P, Dodds J, Green M, Kenny L, Khalil A, Khan KS, Mol BW, Myers J, Poston L, Thilaganathan B, Staff AC, Smith GCS, Ganzevoort W, Laivuori H, Odibo AO, Ramírez JA, Kingdom J, Daskalakis G, Farrar D, Baschat AA, Seed PT, Prefumo F, da Silva Costa F, Groen H, Audibert F, Masse J, Skråstad RB, Salvesen KÅ, Haavaldsen C, Nagata C, Rumbold AR, Heinonen S, Askie LM, Smits LJM, Vinter CA, Magnus PM, Eero K, Villa PM, Jenum AK, Andersen LB, Norman JE, Ohkuchi A, Eskild A, Bhattacharya S, McAuliffe FM, Galindo A, Herraiz I, Carbillon L, Klipstein-Grobusch K, Yeo S, Teede HJ, Browne JL, Moons KGM, Riley RD, Thangaratinam S: Validation and development of models using clinical, biochemical and ultrasound markers for predicting pre-eclampsia: an individual participant data meta-analysis. Health Technol Assess 2020; 24:72. [CrossRef]
- Alon U: An introduction to systems biology: design principles of biological circuits. London: Chapman and Hall/CRC, 2006.
- Alshehri AM: Stroke in atrial fibrillation: Review of risk stratification and preventive therapy. J Family Community Med 2019; 26:92-97. [CrossRef]
- Alsheikh AJ, Wollenhaupt S, King EA, Reeb J, Ghosh S, Stolzenburg LR, Tamim S, Lazar J, Davis JW, Jacob HJ: The landscape of GWAS validation; systematic review identifying 309 validated non-coding variants across 130 human diseases. BMC Med Genomics 2022; 15:74. [CrossRef]
- Altieri C, Pisano C, Vincenzo L, Ferrante MS, Pellerito V, Nardi P, Bassano C, Buioni D, Greco E, Ruvolo G, Balistreri CR: Circulating Levels of Ferritin, RDW, PTLs as Predictive Biomarkers of Postoperative Atrial Fibrillation Risk after Cardiac Surgery in Extracorporeal Circulation. Int J Mol Sci 2022; 23:14800. [CrossRef]
- Alves M, Abrantes AM, Portugal G, Cruz MM, Reimao S, Caldeira D, Ferro JM, Ferreira JJ: Does Parkinson's Disease Increase the Risk of Atrial Fibrillation? Insights From Electrocardiogram and Risk Scores From a Case-Control Study. Front Neurol 2021; 12:633900. [CrossRef]
- Antoniak S, Boltzen U, Riad A, Kallwellis-Opara A, Rohde M, Dorner A, Tschope C, Noutsias M, Pauschinger M, Schultheiss HP, Rauch U: Viral myocarditis and coagulopathy: increased tissue factor expression and plasma thrombogenicity. J Mol Cell Cardiol 2008; 45:118-126. [CrossRef]
- Appelman B, Charlton BT, Goulding RP, Kerkhoff TJ, Breedveld EA, Noort W, Offringa C, Bloemers FW, van Weeghel M, Schomakers BV, Coelho P, Posthuma JJ, Aronica E, Wiersinga WJ, van Vugt M, Wüst RCI: Muscle abnormalities worsen after postexertional malaise in long COVID. Nat Commun 2024; 15:17. [CrossRef]
- Arvanitis P, Johansson AK, Frick M, Malmborg H, Gerovasileiou S, Larsson EM, Blomström-Lundqvist C: Recent-onset atrial fibrillation: a study exploring the elements of Virchow's triad after cardioversion. J Interv Card Electrophysiol 2022; 64:49-58. [CrossRef]
- Ayinde H, Schweizer ML, Crabb V, Ayinde A, Abugroun A, Hopson J: Age modifies the risk of atrial fibrillation among athletes: A systematic literature review and meta-analysis. Int J Cardiol Heart Vasc 2018; 18:25-29. [CrossRef]
- Azaña Gómez J, Pérez-Belmonte LM, Rubio-Rivas M, Bascuñana J, Quirós-López R, Martínez MLT, Hernandez EM, Roque-Rojas F, Méndez-Bailón M, Gómez-Huelgas R, SEMI-COVID-19 Group: Mortality risk factors in patients with SARS-CoV-2 infection and atrial fibrillation: Data from the SEMI-COVID-19 registry. Med Clin (Engl Ed) 2022; 159:457-464. [CrossRef]
- B J: Historical aspects and current understanding of the connections and implications of viruses and diabetes: A narrative review. Clin Epidemiol Global Health 2022; 16:101110. [CrossRef]
- Badve MS, Zhou Z, Anderson CS, Hackett ML: Effectiveness and Safety of Antibiotics for Preventing Pneumonia and Improving Outcome after Acute Stroke: Systematic Review and Meta-analysis. J Stroke Cerebrovasc Dis 2018; 27:3137-3147. [CrossRef]
- Badve MS, Zhou Z, van de Beek D, Anderson CS, Hackett ML: Frequency of post-stroke pneumonia: Systematic review and meta-analysis of observational studies. Int J Stroke 2019; 14:125-136. [CrossRef]
- Bagnato G, Imbalzano E, Aragona CO, Ioppolo C, Di Micco P, La Rosa D, Costa F, Micari A, Tomeo S, Zirilli N, Sciacqua A, D'Angelo T, Cacciola I, Bitto A, Irrera N, Russo V, Roberts WN, Gangemi S, Versace AG: New-Onset Atrial Fibrillation and Early Mortality Rate in COVID-19 Patients: Association with IL-6 Serum Levels and Respiratory Distress. Medicina (Kaunas) 2022; 58:530. [CrossRef]
- Bagot CN, Arya R: Virchow and his triad: a question of attribution. Br J Haematol 2008; 143:180-190. [CrossRef]
- Bai Y, Guo SD, Liu Y, Ma CS, Lip GYH: Relationship of troponin to incident atrial fibrillation occurrence, recurrence after radiofrequency ablation and prognosis: a systematic review, meta-analysis and meta-regression. Biomarkers 2018; 23:512-517. [CrossRef]
- Baker M: Genomics: The search for association. Nature 2010; 467:1135-1138. [CrossRef]
- Bao J, Gao Z, Hu Y, Liu W, Ye L, Wang L: Serum fibrinogen-to-albumin ratio predicts new-onset atrial fibrillation risk during hospitalization in patients with acute myocardial infarction after percutaneous coronary intervention: a retrospective study. BMC Cardiovasc Disord 2023; 23:432. [CrossRef]
- Bartsch E, Medcalf KE, Park AL, Ray JG, High Risk of Pre-eclampsia Identification G: Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ 2016; 353:i1753. [CrossRef]
- Bashar SK, Hossain MB, Ding E, Walkey AJ, McManus DD, Chon KH: Atrial Fibrillation Detection During Sepsis: Study on MIMIC III ICU Data. IEEE J Biomed Health Inform 2020; 24:3124-3135. [CrossRef]
- Belcher HA, Guthold M, Hudson NE: What is the diameter of a fibrin fiber? Res Pract Thromb Haemost 2023; 7:100285. [CrossRef]
- Benenati S, Canale C, De Marzo V, Della Bona R, Rosa GM, Porto I: Atrial fibrillation and Alzheimer's disease: A conundrum. Eur J Clin Invest 2021; 51:e13451. [CrossRef]
- Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, Nunez CM, Patel SR, Penzel T, Pepin JL, Peppard PE, Sinha S, Tufik S, Valentine K, Malhotra A: Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med 2019; 7:687-698. [CrossRef]
- Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D: Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998; 98:946-952. [CrossRef]
- Benjamini Y, Hochberg Y: Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met 1995; 57:289-300. [CrossRef]
- Bennett PC, Silverman SH, Gill PS, Lip GYH: Peripheral arterial disease and Virchow's triad. Thromb Haemost 2009; 101:1032-1040. [CrossRef]
- Berman A, Iglesias M, Khanna R, Beaulieu T: The association between COVID-19 infection and incident atrial fibrillation: results from a retrospective cohort study using a large US commercial insurance database. Open Heart 2023; 10. [CrossRef]
- Bezuidenhout J, Venter C, Roberts T, Tarr G, Kell D, Pretorius E: Detection of citrullinated fibrin in plasma clots of RA patients and its relation to altered structural clot properties, disease-related inflammation and prothrombotic tendency. Front Immunol 2020; 11:577523. [CrossRef]
- Bhatla A, Mayer MM, Adusumalli S, Hyman MC, Oh E, Tierney A, Moss J, Chahal AA, Anesi G, Denduluri S, Domenico CM, Arkles J, Abella BS, Bullinga JR, Callans DJ, Dixit S, Epstein AE, Frankel DS, Garcia FC, Kumareswaram R, Nazarian S, Riley MP, Santangeli P, Schaller RD, Supple GE, Lin D, Marchlinski F, Deo R: COVID-19 and cardiac arrhythmias. Heart Rhythm 2020; 17:1439-1444. [CrossRef]
- Bilano VL, Ota E, Ganchimeg T, Mori R, Souza JP: Risk factors of pre-eclampsia/eclampsia and its adverse outcomes in low- and middle-income countries: a WHO secondary analysis. PLoS One 2014; 9:e91198. [CrossRef]
- Blaszczyk RT, Gorlo A, Dukacz M, Konopka A, Glowniak A: Association between exposure to air pollution and incidence of atrial fibrillation. Ann Agric Environ Med 2023; 30:15-21. [CrossRef]
- Bohne LJ, Johnson D, Rose RA, Wilton SB, Gillis AM: The Association Between Diabetes Mellitus and Atrial Fibrillation: Clinical and Mechanistic Insights. Front Physiol 2019; 10:135. [CrossRef]
- Bommasani R, Hudson DA, Adeli E, Altman R, Arora S, Arx Sv, Bernstein MS, Bohg J, Bosselut A, Brunskill E, Brynjolfsson E, Buch S, Card D, Castellon R, Chatterji N, Chen A, Creel K, Davis JQ, Demszky D, Donahue C, Doumbouya M, Durmus E, Ermon S, Etchemendy J, Ethayarajh K, Fei-Fei L, Finn C, Gale T, Gillespie L, Goel K, Goodman N, Grossman S, Guha N, Hashimoto T, Henderson P, Hewitt J, Ho DE, Hong J, Hsu K, Huang J, Icard T, Jain S, Jurafsky D, Kalluri P, Karamcheti S, Keeling G, Khani F, Khattab O, Kohd PW, Krass M, Krishna R, Kuditipudi R, Kumar A, Ladhak F, Lee M, Lee T, Leskovec J, Levent I, Li XL, Li X, Ma T, Malik A, Manning CD, Mirchandani S, Mitchell E, Munyikwa Z, Nair S, Narayan A, Narayanan D, Newman B, Nie A, Niebles JC, Nilforoshan H, Nyarko J, Ogut G, Orr L, Papadimitriou I, Park JS, Piech C, Portelance E, Potts C, Raghunathan A, Reich R, Ren H, Rong F, Roohani Y, Ruiz C, Ryan J, Ré C, Sadigh D, Sagawa S, Santhanam K, Shih A, Srinivasan K, Tamkin A, Taori R, Thomas AW, Tramèr F, Wang RE, Wang W, Wu B, Wu J, Wu Y, Xie S, Yasunaga M, You J, Zaharia M, Zhang M, Zhang T, Zhang X, Zhang Y, Zheng L, Zhou K, Liang P: On the Opportunities and Risks of Foundation Models. arXiv 2021. arXiv:2108.07258.
- Bosch NA, Cohen DM, Walkey AJ: Risk Factors for New-Onset Atrial Fibrillation in Patients With Sepsis: A Systematic Review and Meta-Analysis. Crit Care Med 2019; 47:280-287. [CrossRef]
- Broadhurst D, Kell DB: Statistical strategies for avoiding false discoveries in metabolomics and related experiments. Metabolomics 2006; 2:171-196. [CrossRef]
- Bryk AH, Natorska J, Zabczyk M, Zettl K, Wiśniewski JR, Undas A: Plasma fibrin clot proteomics in patients with acute pulmonary embolism: Association with clot properties. J Proteomics 2020; 229:103946. [CrossRef]
- Bustamante A, Garcia-Berrocoso T, Penalba A, Giralt D, Simats A, Muchada M, Zapata E, Rubiera M, Montaner J: Sepsis biomarkers reprofiling to predict stroke-associated infections. J Neuroimmunol 2017; 312:19-23. [CrossRef]
- Buzan T: How to mind map. London: Thorsons, 2002.
- Caggiu E, Arru G, Hosseini S, Niegowska M, Sechi G, Zarbo IR, Sechi LA: Inflammation, Infectious Triggers, and Parkinson's Disease. Front Neurol 2019; 10. [CrossRef]
- Calderón-Garcidueñas L, Solt AC, Henriquez-Roldán C, Torres-Jardon R, Nuse B, Herritt L, Villarreal-Calderón R, Osnaya N, Stone I, Garcia R, Brooks DM, González-Maciel A, Reynoso-Robles R, Delgado-Chávez R, Reed W: Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol 2008; 36:289-310. [CrossRef]
- Camacho EM, Lip GYH: Estimating the impact of implementing an integrated care management approach with Atrial fibrillation Better Care (ABC) pathway for patients with atrial fibrillation in England from 2020-2040. European Heart Journal - Quality of Care and Clinical Outcomes 2023. [CrossRef]
- Camino JD, Gracia P, Cremades N: The role of water in the primary nucleation of protein amyloid aggregation. Biophys Chem 2021; 269:106520. [CrossRef]
- Cannon T, Gruenheid S: Microbes and Parkinson's disease: from associations to mechanisms. Trends Microbiol 2022; 30:749-760. [CrossRef]
- Cano-Gamez E, Burnham KL, Goh C, Allcock A, Malick ZH, Overend L, Kwok A, Smith DA, Peters-Sengers H, Antcliffe D, GAinS Investigators, McKechnie S, Scicluna BP, van der Poll T, Gordon AC, Hinds CJ, Davenport EE, Knight JC, Webster N, Galley H, Taylor J, Hall S, Addison J, Roughton S, Tennant H, Guleri A, Waddington N, Arawwawala D, Durcan J, Short A, Swan K, Williams S, Smolen S, Mitchell-Inwang C, Gordon T, Errington E, Templeton M, Venatesh P, Ward G, McCauley M, Baudouin S, Higham C, Soar J, Grier S, Hall E, Brett S, Kitson D, Wilson R, Mountford L, Moreno J, Hall P, Hewlett J, McKechnie S, Garrard C, Millo J, Young D, Hutton P, Parsons P, Smiths A, Faras-Arraya R, Soar J, Raymode P, Thompson J, Bowrey S, Kazembe S, Rich N, Andreou P, Hales D, Roberts E, Fletcher S, Rosbergen M, Glister G, Cuesta JM, Bion J, Millar J, Perry EJ, Willis H, Mitchell N, Ruel S, Carrera R, Wilde J, Nilson A, Lees S, Kapila A, Jacques N, Atkinson J, Brown A, Prowse H, Krige A, Bland M, Bullock L, Harrison D, Mills G, Humphreys J, Armitage K, Laha S, Baldwin J, Walsh A, Doherty N, Drage S, Ortiz-Ruiz de Gordoa L, Lowes S, Higham C, Walsh H, Calder V, Swan C, Payne H, Higgins D, Andrews S, Mappleback S, Hind C, Garrard C, Watson D, McLees E, Purdy A, Stotz M, Ochelli-Okpue A, Bonner S, Whitehead I, Hugil K, Goodridge V, Cawthor L, Kuper M, Pahary S, Bellingan G, Marshall R, Montgomery H, Ryu JH, Bercades G, Boluda S, Bentley A, McCalman K, Jefferies F, Knight J, Davenport E, Burnham K, Maugeri N, Radhakrishnan J, Mi Y, Allcock A, Goh C: An immune dysfunction score for stratification of patients with acute infection based on whole-blood gene expression. Sci Transl Med 2022; 14:eabq4433. [CrossRef]
- Carbillon L, Fermaut M, Benbara A, Boujenah J: COVID-19, Virchow's triad and thromboembolic risk in obese pregnant women. Clin Cardiol 2021; 44:593-594. [CrossRef]
- Carr ME, Jr. Carr ME, Jr., Alving BM: Effect of fibrin structure on plasmin-mediated dissolution of plasma clots. Blood Coag Fibrinol 1995; 6:567-573. [CrossRef]
- Cawood EE, Karamanos TK, Wilson AJ, Radford SE: Visualizing and trapping transient oligomers in amyloid assembly pathways. Biophys Chem 2021; 268:106505. [CrossRef]
- Cereja F, Alves M, Ferreira JJ, Caldeira D: Atrial fibrillation risk on Parkinson's disease - a systematic review and meta-analysis. J Thromb Thrombolysis 2023; 55:747-750. [CrossRef]
- Cerqueira E, Marinho DA, Neiva HP, Lourenco O: Inflammatory Effects of High and Moderate Intensity Exercise-A Systematic Review. Front Physiol 2019; 10:1550. [CrossRef]
- Chao TF, Joung B, Takahashi Y, Lim TW, Choi EK, Chan YH, Guo Y, Sriratanasathavorn C, Oh S, Okumura K, Lip GYH: 2021 Focused Update Consensus Guidelines of the Asia Pacific Heart Rhythm Society on Stroke Prevention in Atrial Fibrillation: Executive Summary. Thromb Haemost 2022; 122:20-47. [CrossRef]
- Chi EY, Frey SL, Winans A, Lam KL, Kjaer K, Majewski J, Lee KY: Amyloid-beta fibrillogenesis seeded by interface-induced peptide misfolding and self-assembly. Biophys J 2010; 98:2299-2308. [CrossRef]
- Chiner E, Llombart M, Valls J, Pastor E, Sancho-Chust JN, Andreu AL, Sanchez-de-la-Torre M, Barbe F: Association between Obstructive Sleep Apnea and Community-Acquired Pneumonia. PLoS One 2016; 11:e0152749. [CrossRef]
- Choi J, Lee SR, Choi EK, Lee H, Han M, Ahn HJ, Kwon S, Lee SW, Han KD, Oh S, Lip GYH: Accumulated hypertension burden on atrial fibrillation risk in diabetes mellitus: a nationwide population study. Cardiovasc Diabetol 2023; 22:12. [CrossRef]
- Christophersen IE, Budtz-Jorgensen E, Olesen MS, Haunso S, Christensen K, Svendsen JH: Familial atrial fibrillation predicts increased risk of mortality: a study in Danish twins. Circ Arrhythm Electrophysiol 2013; 6:10-15. [CrossRef]
- Christophersen IE, Ravn LS, Budtz-Jørgensen E, Skytthe A, Haunsø S, Svendsen JH, Christensen K: Familial aggregation of atrial fibrillation: a study in Danish twins. Circ Arrhythm Electrophysiol 2009; 2:378-383. [CrossRef]
- Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Jr., Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ: Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014; 129:837-847. [CrossRef]
- Chung I, Lip GYH: Virchow's triad revisited: blood constituents. Pathophysiol Haemost Thromb 2003; 33:449-454. [CrossRef]
- Collet JP, Park D, Lesty C, Soria J, Soria C, Montalescot G, Weisel JW: Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: dynamic and structural approaches by confocal microscopy. Arterioscler Thromb Vasc Biol 2000; 20:1354-1361. [CrossRef]
- Conway EM, Mackman N, Warren RQ, Wolberg AS, Mosnier LO, Campbell RA, Gralinski LE, Rondina MT, van de Veerdonk FL, Hoffmeister KM, Griffin JH, Nugent D, Moon K, Morrissey JH: Understanding COVID-19-associated coagulopathy. Nat Rev Immunol 2022; 22:639-649. [CrossRef]
- Corica B, Bonini N, Imberti JF, Romiti GF, Vitolo M, Attanasio L, Basili S, Freedman B, Potpara TS, Boriani G, Lip GYH, Proietti M: Yield of diagnosis and risk of stroke with screening strategies for atrial fibrillation: a comprehensive review of current evidence. Eur Heart J Open 2023a; 3:oead031. [CrossRef]
- Corica B, Romiti GF, Basili S, Proietti M: Prevalence of New-Onset Atrial Fibrillation and Associated Outcomes in Patients with Sepsis: A Systematic Review and Meta-Analysis. J Pers Med 2022; 12:547. [CrossRef]
- Corica B, Tartaglia F, Oliva A, Raparelli V, Cangemi R, Basili S, Lip GYH, Proietti M, Romiti GF: Prevalence of new-onset atrial fibrillation in hospitalized patients with community-acquired pneumonia: a systematic review and meta-analysis. Intern Emerg Med 2023b; 18:127-135. [CrossRef]
- Cortés, M. Cortés, M., Arbucci R, Lambardi F, Furmento J, Muñoz F, Viruel M, Alexander B, Baranchuk A, Costabel JP: High-Sensitivity Troponin T For The Risk Assessment Of Patients With Acute Atrial Fibrillation. Curr Probl Cardiol 2022; 47:101079. [CrossRef]
- Costabel JP, Burgos LM, Trivi M: The Significance Of Troponin Elevation In Atrial Fibrillation. J Atr Fibrillation 2017; 9:1530. [CrossRef]
- Craighead JE: The role of viruses in the pathogenesis of pancreatic disease and diabetes mellitus. Prog Med Virol 1975; 19:161-214.
- Crystal AS, Giasson BI, Crowe A, Kung MP, Zhuang ZP, Trojanowski JQ, Lee VM: A comparison of amyloid fibrillogenesis using the novel fluorescent compound K114. J Neurochem 2003; 86:1359-1368. [CrossRef]
- da Rocha AL, Pinto AP, Kohama EB, Pauli JR, de Moura LP, Cintra DE, Ropelle ER, da Silva ASR: The proinflammatory effects of chronic excessive exercise. Cytokine 2019; 119:57-61. [CrossRef]
- Dai H, Zhang Q, Much AA, Maor E, Segev A, Beinart R, Adawi S, Lu Y, Bragazzi NL, Wu J: Global, regional, and national prevalence, incidence, mortality, and risk factors for atrial fibrillation, 1990-2017: results from the Global Burden of Disease Study 2017. Eur Heart J Qual Care Clin Outcomes 2021; 7:574-582. [CrossRef]
- Danese E, Montagnana M, Cervellin G, Lippi G: Hypercoagulability, D-dimer and atrial fibrillation: an overview of biological and clinical evidence. Ann Med 2014; 46:364-371. [CrossRef]
- Dardiotis E, Tsouris Z, Mentis AA, Siokas V, Michalopoulou A, Sokratous M, Dastamani M, Bogdanos DP, Deretzi G, Kountouras J: H. pylori and Parkinson's disease: Meta-analyses including clinical severity. Clin Neurol Neurosurg 2018; 175:16-24. [CrossRef]
- Darlington A, McCauley MD: Atrial Cardiomyopathy: An Unexplored Limb of Virchow's Triad for AF Stroke Prophylaxis. Front Cardiovasc Med 2020; 7:11. [CrossRef]
- De S, Klenerman D: Imaging individual protein aggregates to follow aggregation and determine the role of aggregates in neurodegenerative disease. Biochim Biophys Acta Proteins Proteom 2019; 1867:870-878. [CrossRef]
- de Waal GM, Engelbrecht L, Davis T, de Villiers WJS, Kell DB, Pretorius E: Correlative Light-Electron Microscopy detects lipopolysaccharide and its association with fibrin fibres in Parkinson's Disease, Alzheimer's Disease and Type 2 Diabetes Mellitus. Scientific reports 2018; 8:16798. [CrossRef]
- Di Lecce VN, Loffredo L, Fimognari FL, Cangemi R, Violi F: Fibrinogen as predictor of ischemic stroke in patients with non-valvular atrial fibrillation. J Thromb Haemost 2003; 1:2453-2455. [CrossRef]
- Ding WY, Gupta D, Lip GYH: Atrial fibrillation and the prothrombotic state: revisiting Virchow's triad in 2020. Heart 2020; 106:1463-1468. [CrossRef]
- Ding WY, Kotalczyk A, Boriani G, Marin F, Blomström-Lundqvist C, Potpara TS, Fauchier L, Lip GYH, ESC-EHRA EORP-AF Long-Term General Registry Investigators: Impact of diabetes on the management and outcomes in atrial fibrillation: an analysis from the ESC-EHRA EORP-AF Long-Term General Registry. Eur J Intern Med 2022a; 103:41-49. [CrossRef]
- Ding WY, Protty MB, Davies IG, Lip GYH: Relationship between lipoproteins, thrombosis, and atrial fibrillation. Cardiovasc Res 2022b; 118:716-731. [CrossRef]
- Djoussé L, Levy D, Benjamin EJ, Blease SJ, Russ A, Larson MG, Massaro JM, D'Agostino RB, Wolf PA, Ellison RC: Long-term alcohol consumption and the risk of atrial fibrillation in the Framingham Study. Am J Cardiol 2004; 93:710-713. [CrossRef]
- Domek M, Li YG, Gumprecht J, Asaad N, Rashed W, Alsheikh-Ali A, Nabrdalik K, Gumprecht J, Zubaid M, Lip GYH: One-year all-cause mortality risk among atrial fibrillation patients in Middle East with and without diabetes: The Gulf SAFE registry. Int J Cardiol 2020; 302:47-52. [CrossRef]
- Downes M, Welters ID, Johnston BW: Study protocol: A systematic review and meta-analysis regarding the influence of coagulopathy and immune activation on new onset atrial fibrillation in patients with sepsis. PLoS One 2023; 18:e0290963. [CrossRef]
- Drabik L, Konieczynska M, Undas A: Clot Lysis Time Predicts Stroke During Anticoagulant Therapy in Patients with Atrial Fibrillation. Can J Cardiol 2020; 36:119-126. [CrossRef]
- Drabik L, Wolkow P, Undas A: Denser plasma clot formation and impaired fibrinolysis in paroxysmal and persistent atrial fibrillation while on sinus rhythm: association with thrombin generation, endothelial injury and platelet activation. Thromb Res 2015; 136:408-414. [CrossRef]
- Drabik L, Wolkow P, Undas A: Fibrin Clot Permeability as a Predictor of Stroke and Bleeding in Anticoagulated Patients With Atrial Fibrillation. Stroke 2017; 48:2716-2722. [CrossRef]
- Duckheim M, Schreieck J: COVID-19 and Cardiac Arrhythmias. Hamostaseologie 2021; 41:372-378. [CrossRef]
- Durand F, Raberin A: Exercise-Induced Hypoxemia in Endurance Athletes: Consequences for Altitude Exposure. Front Sports Act Living 2021; 3:663674. [CrossRef]
- Eaker ED, Sullivan LM, Kelly-Hayes M, D'Agostino RB, Sr., Benjamin EJ: Anger and hostility predict the development of atrial fibrillation in men in the Framingham Offspring Study. Circulation 2004; 109:1267-1271. [CrossRef]
- Ebringer A: Rheumatoid arthritis and Proteus. London: Springer, 2012.
- Ebringer A, Rashid T: Rheumatoid arthritis is caused by a Proteus urinary tract infection. APMIS 2014; 122:363-368. [CrossRef]
- Ebringer A, Rashid T: Rheumatoid arthritis is caused by Proteus: the molecular mimicry theory and Karl Popper. Front Biosci (Elite Ed) 2009; 1:577-586. [CrossRef]
- Ebringer A, Rashid T, Wilson C: Rheumatoid arthritis, Proteus, anti-CCP antibodies and Karl Popper. Autoimmun Rev 2010; 9:216-223. [CrossRef]
- Elawad T, Scott G, Bone JN, Elwell H, Lopez CE, Filippi V, Green M, Khalil A, Kinshella MW, Mistry HD, Pickerill K, Shanmugam R, Singer J, Townsend R, Tsigas EZ, Vidler M, Volvert ML, von Dadelszen P, Magee LA, Network P: Risk factors for pre-eclampsia in clinical practice guidelines: Comparison with the evidence. BJOG 2024; 131:46-62. [CrossRef]
- Emdin CA, Wong CX, Hsiao AJ, Altman DG, Peters SAE, Woodward M, Odutayo AA: Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. BMJ 2016; 532:h7013. [CrossRef]
- Essien UR, Chiswell K, Kaltenbach LA, Wang TY, Fonarow GC, Thomas KL, Turakhia MP, Benjamin EJ, Rodriguez F, Fang MC, Magnani JW, Yancy CW, Piccini JP, Sr.: Association of Race and Ethnicity With Oral Anticoagulation and Associated Outcomes in Patients With Atrial Fibrillation: Findings From the Get With The Guidelines-Atrial Fibrillation Registry. JAMA Cardiol 2022; 7:1207-1217. [CrossRef]
- Fagihi MHA, Bhattacharjee S: Amyloid Fibrillation of Insulin: Amelioration Strategies and Implications for Translation. ACS Pharmacol Transl Sci 2022; 5:1050-1061. [CrossRef]
- Falsetti L, Viticchi G, Buratti L, Grigioni F, Capucci A, Silvestrini M: Interactions between Atrial Fibrillation, Cardiovascular Risk Factors, and ApoE Genotype in Promoting Cognitive Decline in Patients with Alzheimer's Disease: A Prospective Cohort Study. J Alzheimers Dis 2018; 62:713-725. [CrossRef]
- Farrell DH: gamma' Fibrinogen as a novel marker of thrombotic disease. Clin Chem Lab Med 2012; 50:1903-1909. [CrossRef]
- Feghaly J, Zakka P, London B, MacRae CA, Refaat MM: Genetics of Atrial Fibrillation. J Am Heart Assoc 2018; 7:e009884. [CrossRef]
- Ferri S, Kojima K, Sode K: Review of glucose oxidases and glucose dehydrogenases: a bird's eye view of glucose sensing enzymes. J Diabetes Sci Technol 2011; 5:1068-1076. [CrossRef]
- Flaumenhaft R, Enjyoji K, Schmaier AA: Vasculopathy in COVID-19. Blood 2022; 140:222-235. [CrossRef]
- Freestone B, Chong AY, Nuttall S, Lip GY: Impaired flow mediated dilatation as evidence of endothelial dysfunction in chronic atrial fibrillation: relationship to plasma von Willebrand factor and soluble E-selectin levels. Thromb Res 2008a; 122:85-90. [CrossRef]
- Freestone B, Gustafsson F, Chong AY, Corell P, Kistorp C, Hildebrandt P, Lip GYH: Influence of atrial fibrillation on plasma von willebrand factor, soluble E-selectin, and N-terminal pro B-type natriuretic peptide levels in systolic heart failure. Chest 2008b; 133:1203-1208. [CrossRef]
- Freestone B, Lip GYH: The endothelium and atrial fibrillation. The prothrombotic state revisited. Hamostaseologie 2008; 28:207-212.
- Friberg L, Rosenqvist M, Lip GYH: Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J 2012; 33:1500-1510. [CrossRef]
- Frontzek K, Bardelli M, Senatore A, Henzi A, Reimann RR, Bedir S, Marino M, Hussain R, Jurt S, Meisl G, Pedotti M, Mazzola F, Siligardi G, Zerbe O, Losa M, Knowles T, Lakkaraju A, Zhu C, Schwarz P, Hornemann S, Holt MG, Simonelli L, Varani L, Aguzzi A: A conformational switch controlling the toxicity of the prion protein. Nat Struct Mol Biol 2022; 29:831-840. [CrossRef]
- Gando S: Microvascular thrombosis and multiple organ dysfunction syndrome. Crit Care Med 2010; 38:S35-S42. [CrossRef]
- García-Granja PE, Veras C, Aparisi Á, Amat-Santos IJ, Catalá P, Marcos M, Cabezón G, Candela J, Gil JF, Uribarri A, Revilla A, Carrasco M, Gómez I, San Román JA: Atrial fibrillation in patients with SARS-CoV-2 infection. Med Clin (Engl Ed) 2021; 157:58-63. [CrossRef]
- Giannakou K, Evangelou E, Papatheodorou SI: Genetic and non-genetic risk factors for pre-eclampsia: umbrella review of systematic reviews and meta-analyses of observational studies. Ultrasound Obstet Gynecol 2018; 51:720-730. [CrossRef]
- Gillis J, Pavlidis P: "Guilt by association" is the exception rather than the rule in gene networks. PLoS Comput Biol 2012; 8:e1002444. [CrossRef]
- Gonzalez-Gonzalez FJ, Ziccardi MR, McCauley MD: Virchow's Triad and the Role of Thrombosis in COVID-Related Stroke. Front Physiol 2021; 12:769254. [CrossRef]
- Goudis CA, Ketikoglou DG: Obstructive sleep and atrial fibrillation: Pathophysiological mechanisms and therapeutic implications. Int J Cardiol 2017; 230:293-300. [CrossRef]
- Goudis CA, Ntalas IV, Ketikoglou DG: Atrial Fibrillation in Athletes. Cardiol Rev 2015; 23:247-251. [CrossRef]
- Grant PJ: Diabetes mellitus as a prothrombotic condition. J Intern Med 2007; 262:157-172. [CrossRef]
- Grobbelaar LM, Kruger A, Venter C, Burger EM, Laubscher GJ, Maponga TG, Kotze MJ, Kwaan HC, Miller JB, Fulkerson D, Huff W, Chang E, Wiarda G, Bunch CM, Walsh MM, Raza S, Zamlut M, Moore HB, Moore EE, Neal MD, Kell DB, Pretorius E: Relative hypercoagulopathy of the SARS-CoV-2 Beta and Delta variants when compared to the less severe Omicron variants is related to TEG parameters, the extent of fibrin amyloid microclots, and the severity of clinical illness. Semin Thromb Haemost 2022; 48:858-868. [CrossRef]
- Grobbelaar LM, Venter C, Vlok M, Ngoepe M, Laubscher GJ, Lourens PJ, Steenkamp J, Kell DB, Pretorius E: SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: implications for microclot formation in COVID-19. Biosci Rep 2021; 41:BSR20210611. [CrossRef]
- Grobler C, Maphumulo SC, Grobbelaar LM, Bredenkamp` J, Laubscher J, Lourens PJ, Steenkamp J, Kell DB, Pretorius E: COVID-19: The Rollercoaster of Fibrin(ogen), D-dimer, von Willebrand Factor, P-selectin and Their Interactions with Endothelial Cells, Platelets and Erythrocytes. Int J Mol Sci 2020; 21:5168. [CrossRef]
- Grobler C, van Tongeren M, Gettemans J, Kell D, Pretorius E: Alzheimer-type dementia: a systems view provides a unifying explanation of its development. J Alz Dis 2023; 91:43-70. [CrossRef]
- Gundersen E: Is Diabetes of Infectious Origin? . J Infect Dis 1927; 41:197-202. [CrossRef]
- Gunning M, Pavlidis P: "Guilt by association" is not competitive with genetic association for identifying autism risk genes. Scientific reports 2021; 11:15950. [CrossRef]
- Guthold M, Liu W, Stephens B, Lord ST, Hantgan RR, Erie DA, Taylor RM, Jr., Superfine R: Visualization and mechanical manipulations of individual fibrin fibers suggest that fiber cross section has fractal dimension 1.3. Biophys J 2004; 87:4226-4236. [CrossRef]
- Haak BW, Westendorp WF, van Engelen TSR, Brands X, Brouwer MC, Vermeij JD, Hugenholtz F, Verhoeven A, Derks RJ, Giera M, Nederkoorn PJ, de Vos WM, van de Beek D, Wiersinga WJ: Disruptions of Anaerobic Gut Bacteria Are Associated with Stroke and Post-stroke Infection: a Prospective Case-Control Study. Transl Stroke Res 2021; 12:581-592. [CrossRef]
- Han S, Moon I, Choi EK, Han KD, Cho HC, Lee SY, Yang S, Kwon S, Choi YJ, Lee HJ, Lee E, Lee SR, Oh S: Increased atrial fibrillation risk in Parkinson's disease: A nationwide population-based study. Ann Clin Transl Neurol 2021; 8:238-246. [CrossRef]
- Hariyanto TI, Kurniawan A: Obstructive sleep apnea (OSA) and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: a systematic review and meta-analysis. Sleep Med 2021; 82:47-53. [CrossRef]
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL, Group ESCSD: 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021; 42:373-498. [CrossRef]
- Hong CT, Chan L, Wu D, Chen WT, Chien LN: Association Between Parkinson's Disease and Atrial Fibrillation: A Population-Based Study. Front Neurol 2019; 10:22. [CrossRef]
- Honorato MO, Sousa Filho JT, Honorato Junior LFB, Watanabe N, Goulart GM, Prado RRD: Atrial Fibrillation and Sepsis in Elderly Patients and Their Associaton with In-Hospital Mortality. Arq Bras Cardiol 2023; 120:e20220295. [CrossRef]
- Huang HK, Wang JH, Lei WY, Chen CL, Chang CY, Liou LS: Helicobacter pylori infection is associated with an increased risk of Parkinson's disease: A population-based retrospective cohort study. Parkinsonism Relat Disord 2018; 47:26-31. [CrossRef]
- Hull D, Pettifer SR, Kell DB: Defrosting the digital library: bibliographic tools for the next generation web. PLoS Comput Biol 2008; 4:e1000204. [CrossRef]
- Huseynov A, Akin I, Duerschmied D, Scharf RE: Cardiac Arrhythmias in Post-COVID Syndrome: Prevalence, Pathology, Diagnosis, and Treatment. Viruses 2023; 15. [CrossRef]
- Iaccarino L, La Joie R, Lesman-Segev OH, Lee E, Hanna L, Allen IE, Hillner BE, Siegel BA, Whitmer RA, Carrillo MC, Gatsonis C, Rabinovici GD: Association Between Ambient Air Pollution and Amyloid Positron Emission Tomography Positivity in Older Adults With Cognitive Impairment. JAMA Neurol 2021; 78:197-207. [CrossRef]
- Iba T, Nagaoka I, Boulat M: The anticoagulant therapy for sepsis-associated disseminated intravascular coagulation. Thromb Res 2013; 131:383-389. [CrossRef]
- Ihara M, Washida K: Linking Atrial Fibrillation with Alzheimer's Disease: Epidemiological, Pathological, and Mechanistic Evidence. J Alzheimers Dis 2018; 62:61-72. [CrossRef]
- Induruwa I, Hennebry E, Hennebry J, Thakur M, Warburton EA, Khadjooi K: Sepsis-driven atrial fibrillation and ischaemic stroke. Is there enough evidence to recommend anticoagulation? Eur J Intern Med 2022; 98:32-36. [CrossRef]
- Invernizzi A, Schiuma M, Parrulli S, Torre A, Zicarelli F, Colombo V, Marini S, Villella E, Bertoni A, Antinori S, Rizzardini G, Galli M, Meroni L, Giacomelli A, Staurenghi G: Retinal vessels modifications in acute and post-COVID-19. Scientific reports 2021; 11:19373. [CrossRef]
- Isaac E, Cooper SM, Jones SA, Loubani M: Do age-associated changes of voltage-gated sodium channel isoforms expressed in the mammalian heart predispose the elderly to atrial fibrillation? World J Cardiol 2020; 12:123-135. [CrossRef]
- Itzhaki RF: Corroboration of a Major Role for Herpes Simplex Virus Type 1 in Alzheimer's Disease. Front Aging Neurosci 2018; 10:324. [CrossRef]
- Itzhaki RF, Cosby SL, Wozniak MA: Herpes simplex virus type 1 and Alzheimer's disease: the autophagy connection. J Neurovirol 2008; 14:1-4. [CrossRef]
- Itzhaki RF, Lathe R: Herpes Viruses and Senile Dementia: First Population Evidence for a Causal Link. J Alzheimers Dis 2018; 64:363-366. [CrossRef]
- Itzhaki RF, Lathe R, Balin BJ, Ball MJ, Braak H, Bearer EL, Bullido MJ, Carter C, Clerici M, Cosby SL, Del Tredici K, Field H, Fulop T, Grassi C, Griffin WST, Haas J, Hudson AP, Kamer A, Kell DB, Licastro F, Letenneur L, Lövheim H, Mancuso R, Miklossy J, Otth C, Palamara AT, Perry G, Preston C, Pretorius E, Strandberg T, Tabet N, Taylor-Robinson SD, Whittum-Hudson JA: Microbes and Alzheimer's Disease. J Alzheimers Dis 2016; 51:979-984. [CrossRef]
- Jackson JJ, Kropp H: beta-Lactam antibiotic-induced release of free endotoxin: in vitro comparison of penicillin-binding protein (PBP) 2-specific imipenem and PBP 3-specific ceftazidime. J Infect Dis 1992; 165:1033-1041. [CrossRef]
- Jang S, Kim EW, Zhang Y, Lee J, Cho SY, Ha J, Kim H, Kim E: Particulate matter increases beta-amyloid and activated glial cells in hippocampal tissues of transgenic Alzheimer's mouse: Involvement of PARP-1. Biochem Biophys Res Commun 2018; 500:333-338. [CrossRef]
- Jean-Baptiste VSE, Xia CQ, Clare-Salzler MJ, Horwitz MS: Type 1 Diabetes and Type 1 Interferonopathies: Localization of a Type 1 Common Thread of Virus Infection in the Pancreas. EBioMedicine 2017; 22:10-17. [CrossRef]
- Jing H, Wu X, Xiang M, Liu L, Novakovic VA, Shi J: Pathophysiological mechanisms of thrombosis in acute and long COVID-19. Front Immunol 2022; 13:992384. [CrossRef]
- Joseph PG, Healey JS, Raina P, Connolly SJ, Ibrahim Q, Gupta R, Avezum A, Dans AL, Lopez-Jaramillo P, Yeates K, Teo K, Douma R, Bahonar A, Chifamba J, Lanas F, Dagenais GR, Lear SA, Kumar R, Kengne AP, Keskinler M, Mohan V, Mony P, Alhabib KF, Huisman H, Iype T, Zatonska K, Ismail R, Kazmi K, Rosengren A, Rahman O, Yusufali A, Wei L, Orlandini A, Islam S, Rangarajan S, Yusuf S, Investigators P: Global variations in the prevalence, treatment, and impact of atrial fibrillation in a multi-national cohort of 153 152 middle-aged individuals. Cardiovasc Res 2021; 117:1523-1531. [CrossRef]
- Kalarus Z, Mairesse GH, Sokal A, Boriani G, Sredniawa B, Casado-Arroyo R, Wachter R, Frommeyer G, Traykov V, Dagres N, Lip GYH, Boersma L, Peichl P, Dobrev D, Bulava A, Blomstrom-Lundqvist C, de Groot NMS, Schnabel R, Heinzel F, Van Gelder IC, Carbuccichio C, Shah D, Eckardt L: Searching for atrial fibrillation: looking harder, looking longer, and in increasingly sophisticated ways. An EHRA position paper. Europace 2023; 25:185-198. [CrossRef]
- Kalra L, Irshad S, Hodsoll J, Simpson M, Gulliford M, Smithard D, Patel A, Rebollo-Mesa I, Investigators S-I: Prophylactic antibiotics after acute stroke for reducing pneumonia in patients with dysphagia (STROKE-INF): a prospective, cluster-randomised, open-label, masked endpoint, controlled clinical trial. Lancet 2015; 386:1835-1844. [CrossRef]
- Kalstø SM, Siland JE, Rienstra M, Christophersen IE: Atrial Fibrillation Genetics Update: Toward Clinical Implementation. Front Cardiovasc Med 2019; 6:127. [CrossRef]
- Kamath S, Blann AD, Chin BS, Lanza F, Aleil B, Cazenave JP, Lip GYH: A study of platelet activation in atrial fibrillation and the effects of antithrombotic therapy. Eur Heart J 2002; 23:1788-1795. [CrossRef]
- Kannel WB, Abbott RD, Savage DD, McNamara PM: Coronary heart disease and atrial fibrillation: the Framingham Study. Am Heart J 1983; 106:389-396. [CrossRef]
- Kanuri SH, Jayesh Sirrkay P, Ulucay AS: COVID-19 HEART unveiling as atrial fibrillation: pathophysiology, management and future directions for research. Egypt Heart J 2023; 75:36. [CrossRef]
- Karaban K, Slupik D, Reda A, Gajewska M, Rolek B, Borovac JA, Papakonstantinou PE, Bongiovanni D, Ehrlinder H, Parker WAE, Siniarski A, Gasecka A: Coagulation Disorders and Thrombotic Complications in Heart Failure With Preserved Ejection Fraction. Curr Probl Cardiol 2024; 49:102127. [CrossRef]
- Kaura A, Arnold AD, Panoulas V, Glampson B, Davies J, Mulla A, Woods K, Omigie J, Shah AD, Channon KM, Weber JN, Thursz MR, Elliott P, Hemingway H, Williams B, Asselbergs FW, O'Sullivan M, Lord GM, Melikian N, Lefroy DC, Francis DP, Shah AM, Kharbanda R, Perera D, Patel RS, Mayet J: Prognostic significance of troponin level in 3121 patients presenting with atrial fibrillation (The NIHR Health Informatics Collaborative TROP-AF study). J Am Heart Assoc 2020; 9:e013684. [CrossRef]
- Kazemi S, Pourgholaminejad A, Saberi A: Stroke Associated with SARS-CoV-2 Infection and its Pathogenesis: A Systematic Review. Basic Clin Neurosci 2021; 12:569-586. [CrossRef]
- Kell DB: Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med Genom 2009; 2:2. [CrossRef]
- Kell DB: Metabolites do social networking. Nat Chem Biol 2011; 7:7-8.
- Kell DB: Towards a unifying, systems biology understanding of large-scale cellular death and destruction caused by poorly liganded iron: Parkinson’s, Huntington’s, Alzheimer’s, prions, bactericides, chemical toxicology and others as examples. Arch Toxicol 2010; 577:825-889. . [CrossRef]
- Kell DB, Heyden EL, Pretorius E: The biology of lactoferrin, an iron-binding protein that can help defend against viruses and bacteria Frontiers Immunol 2020a; 11:1221. [CrossRef]
- Kell DB, Heyden EL, Pretorius E: The biology of lactoferrin, an iron-binding protein that defends against viruses and bacteria. OSF Preprints 2020b:https://osf.io/fxudj/.
- Kell DB, Kenny LC: A dormant microbial component in the development of pre-eclampsia. Front Med Obs Gynecol 2016; 3:60. [CrossRef]
- Kell DB, Knowles JD: The role of modeling in systems biology. In Szallasi Z, Stelling J, Periwal V (eds.): System modeling in cellular biology: from concepts to nuts and bolts. Cambridge: MIT Press, 2006:3-18.
- Kell DB, Laubscher GJ, Pretorius E: A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications. Biochem J 2022; 479:537-559. [CrossRef]
- Kell DB, Oliver SG: Here is the evidence, now what is the hypothesis? The complementary roles of inductive and hypothesis-driven science in the post-genomic era. Bioessays 2004; 26:99-105. [CrossRef]
- Kell DB, Pretorius E: Are fibrinaloid microclots a cause of autoimmunity in Long Covid and other post-infection diseases? Biochem J 2023; 480:1217-1240. [CrossRef]
- Kell DB, Pretorius E: No effects without causes. The Iron Dysregulation and Dormant Microbes hypothesis for chronic, inflammatory diseases. Biol Rev 2018a; 93:1518-1557. [CrossRef]
- Kell DB, Pretorius E: The potential role of ischaemia-reperfusion injury in chronic, relapsing diseases such as rheumatoid arthritis, long COVID and ME/CFS: evidence, mechanisms, and therapeutic implications. Biochem J 2022; 479:1653-1708. [CrossRef]
- Kell DB, Pretorius E: Proteins behaving badly. Substoichiometric molecular control and amplification of the initiation and nature of amyloid fibril formation: lessons from and for blood clotting. Progr Biophys Mol Biol 2017; 123:16-41. [CrossRef]
- Kell DB, Pretorius E: Serum ferritin is an important disease marker, and is mainly a leakage product from damaged cells. Metallomics 2014; 6:748-773. [CrossRef]
- Kell DB, Pretorius E: The simultaneous occurrence of both hypercoagulability and hypofibrinolysis in blood and serum during systemic inflammation, and the roles of iron and fibrin(ogen). Integr Biol 2015; 7:24-52. [CrossRef]
- Kell DB, Pretorius E: To what extent are the terminal stages of sepsis, septic shock, SIRS, and multiple organ dysfunction syndrome actually driven by a toxic prion/amyloid form of fibrin? Semin Thromb Hemost 2018b; 44:224-238. [CrossRef]
- Kenny LC, Kell DB: Immunological tolerance, pregnancy and pre-eclampsia: the roles of semen microbes and the father. Front Med Obs Gynecol 2018; 4:239. [CrossRef]
- Kerola AM, Rollefstad S, Semb AG: Atherosclerotic Cardiovascular Disease in Rheumatoid Arthritis: Impact of Inflammation and Antirheumatic Treatment. Eur Cardiol 2021; 16:e18. [CrossRef]
- Khan AA, Lip GYH: The prothrombotic state in atrial fibrillation: pathophysiological and management implications. Cardiovasc Res 2019; 115:31-45. [CrossRef]
- Khan SU, Duran CA, Rahman H, Lekkala M, Saleem MA, Kaluski E: A meta-analysis of continuous positive airway pressure therapy in prevention of cardiovascular events in patients with obstructive sleep apnoea. Eur Heart J 2018; 39:2291-2297. [CrossRef]
- Kim HW, Han M, Jung I, Ahn SS: New-onset atrial fibrillation in seropositive rheumatoid arthritis: association with disease-modifying anti-rheumatic drugs treatment. Rheumatology (Oxford) 2023. [CrossRef]
- Kishore AK, Vail A, Jeans AR, Chamorro A, Di Napoli M, Kalra L, Langhorne P, Roffe C, Westendorp W, Nederkoorn PJ, Garau J, van de Beek D, Montaner J, Woodhead M, Meisel A, Smith CJ, Pneumonia in Stroke Consensus G: Microbiological Etiologies of Pneumonia Complicating Stroke: A Systematic Review. Stroke 2018; 49:1602-1609. [CrossRef]
- Klein Klouwenberg PMC, Frencken JF, Kuipers S, Ong DSY, Peelen LM, van Vught LA, Schultz MJ, van der Poll T, Bonten MJ, Cremer OL, MARS Consortium: Incidence, Predictors, and Outcomes of New-Onset Atrial Fibrillation in Critically Ill Patients with Sepsis. A Cohort Study. Am J Respir Crit Care Med 2017; 195:205-211. [CrossRef]
- Klipp E, Herwig R, Kowald A, Wierling C, Lehrach H: Systems biology in practice: concepts, implementation and clinical application. Berlin: Wiley/VCH, 2005.
- Kollman JM, Pandi L, Sawaya MR, Riley M, Doolittle RF: Crystal Structure of Human Fibrinogen. Biochemistry 2009; 48:3877-3886. [CrossRef]
- Konieczynska M, Fil K, Bazanek M, Undas A: Prolonged duration of type 2 diabetes is associated with increased thrombin generation, prothrombotic fibrin clot phenotype and impaired fibrinolysis. Thromb Haemost 2014; 111:685-693. [CrossRef]
- Korantzopoulos P, Letsas KP, Tse G, Fragakis N, Goudis CA, Liu T: Inflammation and atrial fibrillation: A comprehensive review. J Arrhythm 2018; 34:394-401. [CrossRef]
- Kruger A, Vlok M, Turner S, Venter C, Laubscher GJ, Kell DB, Pretorius E: Proteomics of fibrin amyloid microclots in Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) shows many entrapped pro-inflammatory molecules that may also contribute to a failed fibrinolytic system. Cardiovasc Diabetol 2022; 21:190. [CrossRef]
- Kuipers S, Klein Klouwenberg PMC, Cremer OL: Incidence, risk factors and outcomes of new-onset atrial fibrillation in patients with sepsis: a systematic review. Crit Care 2014; 18:688. [CrossRef]
- Kuo CT, Chen YL, Hsu WT, How SC, Cheng YH, Hsueh SS, Liu HS, Lin TH, Wu JW, Wang SS: Investigating the effects of erythrosine B on amyloid fibril formation derived from lysozyme. Int J Biol Macromol 2017; 98:159-168. [CrossRef]
- Kwon S, Lee SR, Choi EK, Ahn HJ, Lee SW, Jung JH, Han KD, Oh S, Lip GYH: Association Between Atrial Fibrillation and Diabetes-Related Complications: A Nationwide Cohort Study. Diabetes Care 2023; 46:2240-2248. [CrossRef]
- Lappalainen T, Scott AJ, Brandt M, Hall IM: Genomic Analysis in the Age of Human Genome Sequencing. Cell 2019; 177:70-84. [CrossRef]
- Laubscher GJ, Lourens PJ, Venter C, Kell DB, Pretorius E: TEG®, Microclot and Platelet Mapping for Guiding Early Management of Severe COVID-19 Coagulopathy. J Clin Med 2021; 10:5381. [CrossRef]
- Lee DSM, Damrauer SM, Levin MG: Genetics of atrial fibrillation. Curr Opin Cardiol 2023; 38:162-168. [CrossRef]
- Lee K, Brayboy L, Tripathi A: Pre-eclampsia: a Scoping Review of Risk Factors and Suggestions for Future Research Direction. Regen Eng Transl Med 2022; 8:394-406. [CrossRef]
- Leta V, Urso D, Batzu L, Lau YH, Mathew D, Boura I, Raeder V, Falup-Pecurariu C, van Wamelen D, Ray Chaudhuri K: Viruses, parkinsonism and Parkinson's disease: the past, present and future. J Neural Transm (Vienna) 2022; 129:1119-1132. [CrossRef]
- Levi M: The coagulant response in sepsis and inflammation. Hamostaseologie 2010; 30:10-12, 14-16.
- Levi M, van der Poll T, ten Cate H, van Deventer SJ: The cytokine-mediated imbalance between coagulant and anticoagulant mechanisms in sepsis and endotoxaemia. Eur J Clin Invest 1997; 27:3-9. [CrossRef]
- Li-Saw-Hee FL, Blann AD, Gurney D, Lip GY: Plasma von Willebrand factor, fibrinogen and soluble P-selectin levels in paroxysmal, persistent and permanent atrial fibrillation. Effects of cardioversion and return of left atrial function. Eur Heart J 2001; 22:1741-1747. [CrossRef]
- Li Q, Lai Y, Gao X, Li X, Deng CY, Guo H, Zhao J, Yang H, Xu Y, Wu S, Xue Y, Rao F: Involvement of plasminogen activator inhibitor-1 and its related molecules in atrial fibrosis in patients with atrial fibrillation. PeerJ 2021; 9:e11488. [CrossRef]
- Li W, Sigley J, Pieters M, Helms CC, Nagaswami C, Weisel JW, Guthold M: Fibrin Fiber Stiffness Is Strongly Affected by Fiber Diameter, but Not by Fibrinogen Glycation. Biophys J 2016; 110:1400-1410. [CrossRef]
- Li X, Gianoulis TA, Yip KY, Gerstein M, Snyder M: Extensive in vivo metabolite-protein interactions revealed by large-scale systematic analyses. Cell 2010; 143:639-650. [CrossRef]
- Li X, Ma X: The role of heparin in sepsis: much more than just an anticoagulant. Br J Haematol 2017; 179:389-398. [CrossRef]
- Li X, Snyder M: Metabolites as global regulators: a new view of protein regulation: systematic investigation of metabolite-protein interactions may help bridge the gap between genome-wide association studies and small molecule screening studies. Bioessays 2011; 33:485-489. [CrossRef]
- Li YG, Miyazawa K, Wolff A, Zubaid M, Alsheikh-Ali AA, Sulaiman K, Lip GYH: One-year risks of stroke and mortality in patients with atrial fibrillation from different clinical settings: The Gulf SAFE registry and Darlington AF registry. Int J Cardiol 2019; 274:158-162. [CrossRef]
- Li YG, Xie PX, Alsheikh-Ali AA, AlMahmeed W, Sulaiman K, Asaad N, Liu SW, Zubaid M, Lip GYH: The "obesity paradox" in patients with atrial fibrillation: Insights from the Gulf SAFE registry. Front Cardiovasc Med 2022; 9:1032633. [CrossRef]
- Limphaibool N, Iwanowski P, Holstad MJV, Kobylarek D, Kozubski W: Infectious Etiologies of Parkinsonism: Pathomechanisms and Clinical Implications. Front Neurol 2019; 10:652. [CrossRef]
- Link MS, Luttmann-Gibson H, Schwartz J, Mittleman MA, Wessler B, Gold DR, Dockery DW, Laden F: Acute exposure to air pollution triggers atrial fibrillation. J Am Coll Cardiol 2013; 62:816-825. [CrossRef]
- Lip GYH, Genaidy A, Tran G, Marroquin P, Estes C: Incident atrial fibrillation and its risk prediction in patients developing COVID-19: A machine learning based algorithm approach. Eur J Intern Med 2021; 91:53-58. [CrossRef]
- Lip GYH, Genaidy A, Tran G, Marroquin P, Estes C, Sloop S: Improving Stroke Risk Prediction in the General Population: A Comparative Assessment of Common Clinical Rules, a New Multimorbid Index, and Machine-Learning-Based Algorithms. Thromb Haemost 2022a; 122:142-150. [CrossRef]
- Lip GYH, Lane DA, Lenarczyk R, Boriani G, Doehner W, Benjamin LA, Fisher M, Lowe D, Sacco RL, Schnabel R, Watkins C, Ntaios G, Potpara T: Integrated care for optimizing the management of stroke and associated heart disease: a position paper of the European Society of Cardiology Council on Stroke. Eur Heart J 2022b; 43:2442-2460. [CrossRef]
- Lip GYH, Lenarczyk R, Pastori D, Ntaios G, Doehner W, Schnabel RB: Poststroke Cardiovascular Management: Current Concepts, Integrated Care, and Future Developments. Curr Probl Cardiol 2023; 48:101738. [CrossRef]
- Lip GYH, Lip PL, Zarifis J, Watson RDS, Bareford D, Lowe GDO, Beevers DG: Fibrin D-dimer and beta-thromboglobulin as markers of thrombogenesis and platelet activation in atrial fibrillation. Effects of introducing ultra-low-dose warfarin and aspirin. Circulation 1996a; 94:425-431. [CrossRef]
- Lip GYH, Lowe GD, Rumley A, Dunn FG: Fibrinogen and fibrin D-dimer levels in paroxysmal atrial fibrillation: evidence for intermediate elevated levels of intravascular thrombogenesis. Am Heart J 1996b; 131:724-730. [CrossRef]
- Lip GYH, Lowe GD, Rumley A, Dunn FG: Increased markers of thrombogenesis in chronic atrial fibrillation: effects of warfarin treatment. Br Heart J 1995; 73:527-533. [CrossRef]
- Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM: Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010; 137:263-272. [CrossRef]
- Lipinski B, Pretorius E: Novel pathway of iron-induced blood coagulation: implications for diabetes mellitus and its complications. Pol Arch Med Wewn 2012; 122:115-122.
- Lipinski B, Pretorius E, Oberholzer HM, van der Spuy WJ: Interaction of fibrin with red blood cells: the role of iron. Ultrastruct Pathol 2012; 36:79-84. [CrossRef]
- Liu X, Guo L, Xiao K, Zhu W, Liu M, Wan R, Hong K: The obesity paradox for outcomes in atrial fibrillation: Evidence from an exposure-effect analysis of prospective studies. Obes Rev 2020; 21:e12970. [CrossRef]
- Lorenzo-Almorós A, Casado Cerrada J, Álvarez-Sala Walther LA, Méndez Bailón M, Lorenzo González Ó: Atrial Fibrillation and Diabetes Mellitus: Dangerous Liaisons or Innocent Bystanders? J Clin Med 2023; 12. [CrossRef]
- Lowres N, Olivier J, Chao TF, Chen SA, Chen Y, Diederichsen A, Fitzmaurice DA, Gomez-Doblas JJ, Harbison J, Healey JS, Hobbs FDR, Kaasenbrood F, Keen W, Lee VW, Lindholt JS, Lip GYH, Mairesse GH, Mant J, Martin JW, Martin-Rioboó E, McManus DD, Muniz J, Munzel T, Nakamya J, Neubeck L, Orchard JJ, Pérula de Torres LÁ, Proietti M, Quinn FR, Roalfe AK, Sandhu RK, Schnabel RB, Smyth B, Soni A, Tieleman R, Wang J, Wild PS, Yan BP, Freedman B: Estimated stroke risk, yield, and number needed to screen for atrial fibrillation detected through single time screening: a multicountry patient-level meta-analysis of 141,220 screened individuals. PLoS medicine 2019; 16:e1002903. [CrossRef]
- Lucrecia Maria B, Marcelo T, Andreina GR, Juan Pablo C: Prognostic Value of Troponin in Patients with Atrial Fibrillation Admitted to an Emergency Department: Review and Meta-Analysis. J Atr Fibrillation 2020; 13:2346. [CrossRef]
- Luyendyk JP, Schoenecker JG, Flick MJ: The multifaceted role of fibrinogen in tissue injury and inflammation. Blood 2019; 133:511-520. [CrossRef]
- Ma Y, Su B, Li D, Cui F, Tang L, Wang J, Tian Y, Zheng X: Air pollution, genetic susceptibility, and the risk of atrial fibrillation: A large prospective cohort study. Proc Natl Acad Sci U S A 2023; 120:e2302708120. [CrossRef]
- Machano MM, Joho AA: Prevalence and risk factors associated with severe pre-eclampsia among postpartum women in Zanzibar: a cross-sectional study. BMC Public Health 2020; 20:1347. [CrossRef]
- Mandaglio-Collados D, López-Gálvez R, Ruiz-Alcaraz AJ, López-García C, Roldán V, Lip GYH, Marín F, Rivera-Caravaca JM: Impact of particulate matter on the incidence of atrial fibrillation and the risk of adverse clinical outcomes: A review. Sci Total Environ 2023; 880:163352. [CrossRef]
- Mandel HL, Colleen G, Abedian S, Ammar N, Charles Bailey L, Bennett TD, Daniel Brannock M, Brosnahan SB, Chen Y, Chute CG, Divers J, Evans MD, Haendel M, Hall MA, Hirabayashi K, Hornig M, Katz SD, Krieger AC, Loomba J, Lorman V, Mazzotti DR, McMurry J, Moffitt RA, Pajor NM, Pfaff E, Radwell J, Razzaghi H, Redline S, Seibert E, Sekar A, Sharma S, Thaweethai T, Weiner MG, Jae Yoo Y, Zhou A, Thorpe LE: Risk of post-acute sequelae of SARS-CoV-2 infection associated with pre-coronavirus disease obstructive sleep apnea diagnoses: an electronic health record-based analysis from the RECOVER initiative. Sleep 2023; 46. [CrossRef]
- Manemann SM, Chamberlain AM, Bielinski SJ, Jiang R, Weston SA, Roger VL: Predicting Alzheimer's Disease and Related Dementias in Heart Failure and Atrial Fibrillation. Am J Med 2023; 136:302-307. [CrossRef]
- Marín F, Roldan V, Lip GYH: Fibrinolytic function and atrial fibrillation. Thromb Res 2003; 109:233-240. [CrossRef]
- Marín F, Roldan V, Monmeneu JV, Bodí V, Fernández C, Garía de Burgos F, Marco P, Sogorb F: Prothrombotic state and elevated levels of plasminogen activator inhibitor-1 in mitral stenosis with and without atrial fibrillation. Am J Cardiol 1999; 84:862-864, A869. [CrossRef]
- Marini C, De Santis F, Sacco S, Russo T, Olivieri L, Totaro R, Carolei A: Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population-based study. Stroke 2005; 36:1115-1119. [CrossRef]
- Marinigh R, Lip GY, Fiotti N, Giansante C, Lane DA: Age as a risk factor for stroke in atrial fibrillation patients: implications for thromboprophylaxis. J Am Coll Cardiol 2010; 56:827-837. [CrossRef]
- Martinez-Naharro A, Baksi AJ, Hawkins PN, Fontana M: Diagnostic imaging of cardiac amyloidosis. Nat Rev Cardiol 2020; 17:413-426. [CrossRef]
- Martinez-Naharro A, Gonzalez-Lopez E, Corovic A, Mirelis JG, Baksi AJ, Moon JC, Garcia-Pavia P, Gillmore JD, Hawkins PN, Fontana M: High Prevalence of Intracardiac Thrombi in Cardiac Amyloidosis. J Am Coll Cardiol 2019; 73:1733-1734. [CrossRef]
- Martinez-Naharro A, Hawkins PN, Fontana M: Cardiac amyloidosis. Clin Med (Lond) 2018; 18:s30-s35. [CrossRef]
- Mehta JL, Calcaterra G, Bassareo PP: COVID-19, thromboembolic risk, and Virchow's triad: Lesson from the past. Clin Cardiol 2020; 43:1362-1367. [CrossRef]
- Mélé N, Turc G: Stroke Associated With Recent Mycoplasma pneumoniae Infection: A Systematic Review of Clinical Features and Presumed Pathophysiological Mechanisms. Front Neurol 2018; 9:1109. [CrossRef]
- Meng L, Shen L, Ji HF: Impact of infection on risk of Parkinson's disease: a quantitative assessment of case-control and cohort studies. J Neurovirol 2019; 25:221-228. [CrossRef]
- Meziani F, Gando S, Vincent JL: Should all patients with sepsis receive anticoagulation? Yes. Intensive Care Med 2017; 43:452-454. [CrossRef]
- Michaels TCT, Liu LX, Meisl G, Knowles TPJ: Physical principles of filamentous protein self-assembly kinetics. J Phys Condens Matter 2017; 29:153002. [CrossRef]
- Michaels TCT, Qian D, Šarić A, Vendruscolo M, Linse S, Knowles TPJ: Amyloid formation as a protein phase transition. Nature Rev Phys 2023; 5:379-397. [CrossRef]
- Mickiewicz KM, Kawai Y, Drage L, Gomes MC, Davison F, Pickard R, Hall J, Mostowy S, Aldridge PD, Errington J: Possible role of L-form switching in recurrent urinary tract infection. Nat Commun 2019; 10:4379. [CrossRef]
- Mikkelsen LF, Nordestgaard BG, Schnohr P, Ellervik C: Increased Ferritin Concentration and Risk of Atrial Fibrillation and Heart Failure in Men and Women: Three Studies of the Danish General Population Including 35799 Individuals. Clin Chem 2019; 65:180-188. [CrossRef]
- Miller MA, Cappuccio FP: A systematic review of COVID-19 and obstructive sleep apnoea. Sleep Med Rev 2021; 55:101382. [CrossRef]
- Mouram S, Pannone L, Gauthey A, Sorgente A, Vergara P, Bisignani A, Monaco C, Mojica J, Al Housari M, Miraglia V, Del Monte A, Paparella G, Ramak R, Overeinder I, Bala G, Almorad A, Stroker E, Sieira J, Brugada P, La Meir M, Chierchia GB, de Asmundis C: Incidence and Predictors of Cardiac Arrhythmias in Patients With COVID-19. Front Cardiovasc Med 2022; 9:908177. [CrossRef]
- Mukamal KJ, Tolstrup JS, Friberg J, Gronbaek M, Jensen G: Fibrinogen and albumin levels and risk of atrial fibrillation in men and women (the Copenhagen City Heart Study). Am J Cardiol 2006; 98:75-81. [CrossRef]
- Mukamal KJ, Tolstrup JS, Friberg J, Jensen G, Gronbaek M: Alcohol consumption and risk of atrial fibrillation in men and women: the Copenhagen City Heart Study. Circulation 2005; 112:1736-1742. [CrossRef]
- Murao S, Yamakawa K: A Systematic Summary of Systematic Reviews on Anticoagulant Therapy in Sepsis. J Clin Med 2019; 8:1869. [CrossRef]
- Musikantow DR, Turagam MK, Sartori S, Chu E, Kawamura I, Shivamurthy P, Bokhari M, Oates C, Zhang C, Pumill C, Malick W, Hashemi H, Ruiz-Maya T, Hadley MB, Gandhi J, Sperling D, Whang W, Koruth JS, Langan MN, Sofi A, Gomes A, Harcum S, Cammack S, Ellsworth B, Dukkipati SR, Bassily-Marcus A, Kohli-Seth R, Goldman ME, Halperin JL, Fuster V, Reddy VY: Atrial Fibrillation in Patients Hospitalized With COVID-19: Incidence, Predictors, Outcomes, and Comparison to Influenza. JACC Clin Electrophysiol 2021; 7:1120-1130. [CrossRef]
- Nakase T, Tatewaki Y, Thyreau B, Odagiri H, Tomita N, Yamamoto S, Takano Y, Muranaka M, Taki Y: Impact of atrial fibrillation on the cognitive decline in Alzheimer's disease. Alzheimers Res Ther 2023; 15:15. [CrossRef]
- Naveed Z, Velasquez Garcia HA, Wong S, Wilton J, McKee G, Mahmood B, Binka M, Rasali D, Janjua NZ: Association of COVID-19 Infection With Incident Diabetes. JAMA Netw Open 2023; 6:e238866. [CrossRef]
- Niehues P, Wegner FK, Wolfes J, Willy K, Ellermann C, Vollenberg R, Reinecke H, Rosenow F, Lepper J, Sackarnd J, Eckardt L: Incidence and predictors of cardiac arrhythmias in patients with COVID-19 induced ARDS. J Cardiol 2022; 80:298-302. [CrossRef]
- Nobs SP, Kolodziejczyk AA, Adler L, Horesh N, Botscharnikow C, Herzog E, Mohapatra G, Hejndorf S, Hodgetts RJ, Spivak I, Schorr L, Fluhr L, Kviatcovsky D, Zacharia A, Njuki S, Barasch D, Stettner N, Dori-Bachash M, Harmelin A, Brandis A, Mehlman T, Erez A, He Y, Ferrini S, Puschhof J, Shapiro H, Kopf M, Moussaieff A, Abdeen SK, Elinav E: Lung dendritic-cell metabolism underlies susceptibility to viral infection in diabetes. Nature 2023; 624:645-652. [CrossRef]
- Notkins AL: Virus-induced diabetes mellitus. Arch Virol 1977; 54:1-17. [CrossRef]
- Nunes JM, Kell DB, Pretorius E: Cardiovascular and haematological pathology in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): a role for Viruses. Blood Rev 2023; 60:101075. [CrossRef]
- Nunes JM, Kruger A, Proal A, Kell DB, Pretorius E: The Occurrence of Hyperactivated Platelets and Fibrinaloid Microclots in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Pharmaceuticals (Basel) 2022; 15:931. [CrossRef]
- Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA: Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ 2016; 354:i4482. [CrossRef]
- Okafor C, Byrnes J, Stewart S, Scuffham P, Afoakwah C: Cost Effectiveness of Strategies to Manage Atrial Fibrillation in Middle- and High-Income Countries: A Systematic Review. Pharmacoeconomics 2023; 41:913-943. [CrossRef]
- Oliver SG: Guilt-by-association goes global. Nature 2000; 403:601-603.
- Opal SM, Horn DL, Palardy JE, Parejo N, Jhung J, Bhattacharjee A, Young LD: The in vivo significance of antibiotic-induced endotoxin release in experimental Gram-negative sepsis. J Endotox Res 1996; 3:245-252. [CrossRef]
- Overvad TF, Rasmussen LH, Skjøth F, Overvad K, Lip GYH, Larsen TB: Body mass index and adverse events in patients with incident atrial fibrillation. Am J Med 2013; 126:640 e649-617. [CrossRef]
- Ozdemir H, Sagris D, Lip GYH, Abdul-Rahim AH: Stroke in Atrial Fibrillation and Other Atrial Dysrhythmias. Curr Cardiol Rep 2023; 25:357-369. [CrossRef]
- Palladini G, Merlini G: Systemic amyloidoses: what an internist should know. Eur J Intern Med 2013; 24:729-739. [CrossRef]
- Palsson BØ: Systems biology: properties of reconstructed networks. Cambridge: Cambridge University Press, 2006.
- Pan Y, Wang Y, Wang Y: Investigation of Causal Effect of Atrial Fibrillation on Alzheimer Disease: A Mendelian Randomization Study. J Am Heart Assoc 2020; 9:e014889. [CrossRef]
- Panchal G, Mahmood M, Lip GYH: Revisiting the risks of incident atrial fibrillation: a narrative review. Part 1. Kardiol Pol 2019a; 77:430-436. [CrossRef]
- Panchal G, Mahmood M, Lip GYH: Revisiting the risks of incident atrial fibrillation: a narrative review. Part 2. Kardiol Pol 2019b; 77:515-524. [CrossRef]
- Papanastasiou CA, Theochari CA, Zareifopoulos N, Arfaras-Melainis A, Giannakoulas G, Karamitsos TD, Palaiodimos L, Ntaios G, Avgerinos KI, Kapogiannis D, Kokkinidis DG: Atrial Fibrillation Is Associated with Cognitive Impairment, All-Cause Dementia, Vascular Dementia, and Alzheimer's Disease: a Systematic Review and Meta-Analysis. J Gen Intern Med 2021; 36:3122-3135. [CrossRef]
- Paquissi FC: The Predictive Role of Inflammatory Biomarkers in Atrial Fibrillation as Seen through Neutrophil-Lymphocyte Ratio Mirror. J Biomark 2016; 2016:8160393. [CrossRef]
- Pardo Sanz A, Salido Tahoces L, Ortega Pérez R, González Ferrer E, Sánchez Recalde Á, Zamorano Gómez JL: New-onset atrial fibrillation during COVID-19 infection predicts poor prognosis. Cardiol J 2021; 28:34-40. [CrossRef]
- Pari B, Babbili A, Kattubadi A, Thakre A, Thotamgari S, Gopinathannair R, Olshansky B, Dominic P: COVID-19 Vaccination and Cardiac Arrhythmias: A Review. Curr Cardiol Rep 2023; 25:925-940. [CrossRef]
- Petsko GA: Guilt by association. Genome Biol 2009; 10:104. [CrossRef]
- Pollock KG, Sekelj S, Johnston E, Sandler B, Hill NR, Ng FS, Khan S, Nassar A, Farooqui U: Application of a machine learning algorithm for detection of atrial fibrillation in secondary care. Int J Cardiol Heart Vasc 2020; 31:100674. [CrossRef]
- Powers SK, Deminice R, Ozdemir M, Yoshihara T, Bomkamp MP, Hyatt H: Exercise-induced oxidative stress: Friend or foe? J Sport Health Sci 2020; 9:415-425. [CrossRef]
- Pretorius E: The use of a desktop scanning electron microscope as a diagnostic tool in studying fibrin networks of thrombo-embolic ischemic stroke. Ultrastruct Pathol 2011; 35:245-250. [CrossRef]
- Pretorius E, Akeredolu O-O, Soma P, Kell DB: Major involvement of bacterial components in rheumatoid arthritis and its accompanying oxidative stress, systemic inflammation and hypercoagulability. Exp Biol Med 2017a; 242:355-373. [CrossRef]
- Pretorius E, Bester J, Kell DB: A bacterial component to Alzheimer-type dementia seen via a systems biology approach that links iron dysregulation and inflammagen shedding to disease J Alzheimers Dis 2016a; 53:1237-1256. [CrossRef]
- Pretorius E, Bester J, Page MJ, Kell DB: The potential of LPS-binding protein to reverse amyloid formation in plasma fibrin of individuals with Alzheimer-type dementia. Frontiers Aging Neurosci 2018a; 10:257. [CrossRef]
- Pretorius E, Bester J, Page MJ, Kell DB: Reversal of amyloid formation in the plasma fibrin of individuals with Alzheimer-type dementia using LPS-binding protein J Alzheimers Dis 2018b.
- Pretorius E, Bester J, Vermeulen N, Alummoottil S, Soma P, Buys AV, Kell DB: Poorly controlled type 2 diabetes is accompanied by significant morphological and ultrastructural changes in both erythrocytes and in thrombin-generated fibrin: implications for diagnostics. Cardiovasc Diabetol 2015; 13:30. [CrossRef]
- Pretorius E, Bronkhorst P, Briedenhann S, Smit E, Franz RC: Comparisons of the fibrin networks during pregnancy, nonpregnancy and pregnancy during dysfibrinogenaemia using the scanning electron microscope. Blood Coag Fibrinol 2009; 20:12-16. [CrossRef]
- Pretorius E, Kell DB: Diagnostic morphology: biophysical indicators for iron-driven inflammatory diseases. Integrative Biol 2014; 6:486-510. [CrossRef]
- Pretorius E, Kell DB: A perspective on how microscopy imaging of fibrinaloid microclots and platelet pathology may be applied in clinical investigations. Semin Thromb Haemost 2023:DOI: 10.1055/s-0043-1774796. [CrossRef]
- Pretorius E, Mbotwe S, Bester J, Robinson C, Kell DB: Acute induction of anomalous blood clotting by highly substoichiometric levels of bacterial lipopolysaccharide (LPS). bioRxiv version. bioRxiv 2016b:2016-053538v053531. [CrossRef]
- Pretorius E, Mbotwe S, Bester J, Robinson CJ, Kell DB: Acute induction of anomalous and amyloidogenic blood clotting by molecular amplification of highly substoichiometric levels of bacterial lipopolysaccharide. J R Soc Interface 2016c; 123:20160539. [CrossRef]
- Pretorius E, Mbotwe S, Kell DB: Lipopolysaccharide-binding protein (LBP) reverses the amyloid state of fibrin seen in plasma of type 2 diabetics with cardiovascular comorbidities. Scientific reports 2017b; 7:9680.
- Pretorius E, Oberholzer HM, van der Spuy WJ, Meiring JH: The changed ultrastructure of fibrin networks during use of oral contraception and hormone replacement. J Thromb Thrombolysis 2010; 30:502-506. [CrossRef]
- Pretorius E, Oberholzer HM, van der Spuy WJ, Swanepoel AC, Soma P: Qualitative scanning electron microscopy analysis of fibrin networks and platelet abnormalities in diabetes. Blood Coagul Fibrinol 2011a; 22:463-467. [CrossRef]
- Pretorius E, Oberholzer HM, van der Spuy WJ, Swanepoel AC, Soma P: Scanning electron microscopy of fibrin networks in rheumatoid arthritis: a qualitative analysis. Rheumatol Int 2012; 32:1611-1615. [CrossRef]
- Pretorius E, Page MJ, Engelbrecht L, Ellis GC, Kell DB: Substantial fibrin amyloidogenesis in type 2 diabetes assessed using amyloid-selective fluorescent stains. Cardiovasc Diabetol 2017c; 16:141. [CrossRef]
- Pretorius E, Page MJ, Hendricks L, Nkosi NB, Benson SR, Kell DB: Both lipopolysaccharide and lipoteichoic acids potently induce anomalous fibrin amyloid formation: assessment with novel Amytracker™ stains. J R Soc Interface 2018c; 15:20170941. [CrossRef]
- Pretorius E, Page MJ, Mbotwe S, Kell DB: Lipopolysaccharide-binding protein (LBP) can reverse the amyloid state of fibrin seen or induced in Parkinson’s disease. PlosOne 2018d; 13:e0192121. [CrossRef]
- Pretorius E, Steyn H, Engelbrecht M, Swanepoel AC, Oberholzer HM: Differences in fibrin fiber diameters in healthy individuals and thromboembolic ischemic stroke patients. Blood Coagul Fibrinolysis 2011b; 22:696-700. [CrossRef]
- Pretorius E, Swanepoel AC, Oberholzer HM, van der Spuy WJ, Duim W, Wessels PF: A descriptive investigation of the ultrastructure of fibrin networks in thrombo-embolic ischemic stroke. J Thromb Thrombolysis 2011c; 31:507-513. [CrossRef]
- Pretorius E, Venter C, Laubscher GJ, Kotze MJ, Oladejo S, Watson LR, Rajaratnam K, Watson BW, Kell DB: Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) Cardiovasc Diabetol 2022; 21:148. [CrossRef]
- Pretorius E, Venter C, Laubscher GJ, Lourens PJ, Steenkamp J, Kell DB: Prevalence of readily detected amyloid blood clots in ‘unclotted’ Type 2 Diabetes Mellitus and COVID-19 plasma: A preliminary report. Cardiovasc Diabetol 2020; 19:193. [CrossRef]
- Pretorius E, Vermeulen N, Bester J, Lipinski B, Kell DB: A novel method for assessing the role of iron and its functional chelation in fibrin fibril formation: the use of scanning electron microscopy. Toxicol Mech Methods 2013; 23:352-359. [CrossRef]
- Pretorius E, Vlok M, Venter C, Bezuidenhout JA, Laubscher GJ, Steenkamp J, Kell DB: Persistent clotting protein pathology in Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol 2021; 20:172. [CrossRef]
- Pretorius M, Donahue BS, Yu C, Greelish JP, Roden DM, Brown NJ: Plasminogen activator inhibitor-1 as a predictor of postoperative atrial fibrillation after cardiopulmonary bypass. Circulation 2007; 116:I1-7. [CrossRef]
- Proietti M, Boriani G: Obesity Paradox in Atrial Fibrillation: Implications for Outcomes and Relationship with Oral Anticoagulant Drugs. Am J Cardiovasc Drugs 2020; 20:125-137. [CrossRef]
- Proietti M, Guiducci E, Cheli P, Lip GY: Is There an Obesity Paradox for Outcomes in Atrial Fibrillation? A Systematic Review and Meta-Analysis of Non-Vitamin K Antagonist Oral Anticoagulant Trials. Stroke 2017; 48:857-866. [CrossRef]
- Proietti M, Romiti GF: Management of Ventricular Heart Rate in Atrial Fibrillation Patients With Sepsis. Chest 2021; 159:1315-1316. [CrossRef]
- Proietti R, AlTurki A, Vio R, Licchelli L, Rivezzi F, Marafi M, Russo V, Potpara TS, Kalman JM, de Villers-Sidani E, Bunch TJ: The association between atrial fibrillation and Alzheimer's disease: fact or fallacy? A systematic review and meta-analysis. J Cardiovasc Med (Hagerstown) 2020; 21:106-112. [CrossRef]
- Qi W, Liu J, Li A: Effect of Anticoagulant Versus Non-Anticoagulant Therapy on Mortality of Sepsis-Induced Disseminated Intravascular Coagulation: A Systematic Review and Meta-Analysis. Clin Appl Thromb Hemost 2023; 29:10760296231157766. [CrossRef]
- Qureshi A, Lip GYH, Nordsletten DA, Williams SE, Aslanidi O, de Vecchi A: Imaging and biophysical modelling of thrombogenic mechanisms in atrial fibrillation and stroke. Front Cardiovasc Med 2022; 9:1074562. [CrossRef]
- Qureshi WT, Alirhayim Z, Blaha MJ, Juraschek SP, Keteyian SJ, Brawner CA, Al-Mallah MH: Cardiorespiratory Fitness and Risk of Incident Atrial Fibrillation: Results From the Henry Ford Exercise Testing (FIT) Project. Circulation 2015; 131:1827-1834. [CrossRef]
- Rafaqat S, Afzal S, Khurshid H, Safdar S, Rafaqat S, Rafaqat S: The Role of Major Inflammatory Biomarkers in the Pathogenesis of Atrial Fibrillation. J Innov Card Rhythm Manag 2022; 13:5265-5277. [CrossRef]
- Rajsfus BF, Mohana-Borges R, Allonso D: Diabetogenic viruses: linking viruses to diabetes mellitus. Heliyon 2023; 9:e15021. [CrossRef]
- Rav-Acha M, Orlev A, Itzhaki I, Zimmerman SF, Fteiha B, Bohm D, Kurd R, Samuel TY, Asher E, Helviz Y, Glikson M, Michowitz Y: Cardiac arrhythmias amongst hospitalised Coronavirus 2019 (COVID-19) patients: Prevalence, characterisation, and clinical algorithm to classify arrhythmic risk. Int J Clin Pract 2021; 75:e13788. [CrossRef]
- Rizwan A, Zoha A, Mabrouk IB, Sabbour HM, Al-Sumaiti AS, Alomainy A, Imran MA, Abbasi QH: A Review on the State of the Art in Atrial Fibrillation Detection Enabled by Machine Learning. IEEE Rev Biomed Eng 2021; 14:219-239. [CrossRef]
- Rodríguez-Reyes H, Lara-Vaca S, Ochoa-Guzmán A, Chiquete E, Registro Mexicano de Fibrilación Auricular Study Group: Obesity Paradox and 12 Month Outcome in Patients with Atrial Fibrillation. Arch Med Res 2021; 52:233-239. [CrossRef]
- Romiti GF, Guo Y, Corica B, Proietti M, Zhang H, Lip GYH, mAF-App II trial investigators: Mobile Health-Technology-Integrated Care for Atrial Fibrillation: A Win Ratio Analysis from the mAFA-II Randomized Clinical Trial. Thromb Haemost 2023; 123:1042-1048. [CrossRef]
- Romiti GF, Pastori D, Rivera-Caravaca JM, Ding WY, Gue YX, Menichelli D, Gumprecht J, Koziel M, Yang PS, Guo Y, Lip GYH, Proietti M: Adherence to the 'Atrial Fibrillation Better Care' Pathway in Patients with Atrial Fibrillation: Impact on Clinical Outcomes-A Systematic Review and Meta-Analysis of 285,000 Patients. Thromb Haemost 2022a; 122:406-414. [CrossRef]
- Romiti GF, Proietti M, Bonini N, Ding WY, Boriani G, Huisman MV, Lip GYH, Investigators G-A: Clinical Complexity Domains, Anticoagulation, and Outcomes in Patients with Atrial Fibrillation: A Report from the GLORIA-AF Registry Phase II and III. Thromb Haemost 2022b; 122:2030-2041. [CrossRef]
- Rong JC, Chen XD, Jin NK, Hong J: Exploring the causal association of rheumatoid arthritis with atrial fibrillation: a Mendelian randomization study. Clin Rheumatol 2023. [CrossRef]
- Roselli C, Chaffin MD, Weng LC, Aeschbacher S, Ahlberg G, Albert CM, Almgren P, Alonso A, Anderson CD, Aragam KG, Arking DE, Barnard J, Bartz TM, Benjamin EJ, Bihlmeyer NA, Bis JC, Bloom HL, Boerwinkle E, Bottinger EB, Brody JA, Calkins H, Campbell A, Cappola TP, Carlquist J, Chasman DI, Chen LY, Chen YI, Choi EK, Choi SH, Christophersen IE, Chung MK, Cole JW, Conen D, Cook J, Crijns HJ, Cutler MJ, Damrauer SM, Daniels BR, Darbar D, Delgado G, Denny JC, Dichgans M, Dörr M, Dudink EA, Dudley SC, Esa N, Esko T, Eskola M, Fatkin D, Felix SB, Ford I, Franco OH, Geelhoed B, Grewal RP, Gudnason V, Guo X, Gupta N, Gustafsson S, Gutmann R, Hamsten A, Harris TB, Hayward C, Heckbert SR, Hernesniemi J, Hocking LJ, Hofman A, Horimoto A, Huang J, Huang PL, Huffman J, Ingelsson E, Ipek EG, Ito K, Jimenez-Conde J, Johnson R, Jukema JW, Kääb S, Kahonen M, Kamatani Y, Kane JP, Kastrati A, Kathiresan S, Katschnig-Winter P, Kavousi M, Kessler T, Kietselaer BL, Kirchhof P, Kleber ME, Knight S, Krieger JE, Kubo M, Launer LJ, Laurikka J, Lehtimaki T, Leineweber K, Lemaitre RN, Li M, Lim HE, Lin HJ, Lin H, Lind L, Lindgren CM, Lokki ML, London B, Loos RJF, Low SK, Lu Y, Lyytikainen LP, Macfarlane PW, Magnusson PK, Mahajan A, Malik R, Mansur AJ, Marcus GM, Margolin L, Margulies KB, Marz W, McManus DD, Melander O, Mohanty S, Montgomery JA, Morley MP, Morris AP, Muller-Nurasyid M, Natale A, Nazarian S, Neumann B, Newton-Cheh C, Niemeijer MN, Nikus K, Nilsson P, Noordam R, Oellers H, Olesen MS, Orho-Melander M, Padmanabhan S, Pak HN, Pare G, Pedersen NL, Pera J, Pereira A, Porteous D, Psaty BM, Pulit SL, Pullinger CR, Rader DJ, Refsgaard L, Ribases M, Ridker PM, Rienstra M, Risch L, Roden DM, Rosand J, Rosenberg MA, Rost N, Rotter JI, Saba S, Sandhu RK, Schnabel RB, Schramm K, Schunkert H, Schurman C, Scott SA, Seppala I, Shaffer C, Shah S, Shalaby AA, Shim J, Shoemaker MB, Siland JE, Sinisalo J, Sinner MF, Slowik A, Smith AV, Smith BH, Smith JG, Smith JD, Smith NL, Soliman EZ, Sotoodehnia N, Stricker BH, Sun A, Sun H, Svendsen JH, Tanaka T, Tanriverdi K, Taylor KD, Teder-Laving M, Teumer A, Theriault S, Trompet S, Tucker NR, Tveit A, Uitterlinden AG, Van Der Harst P, Van Gelder IC, Van Wagoner DR, Verweij N, Vlachopoulou E, Völker U, Wang B, Weeke PE, Weijs B, Weiss R, Weiss S, Wells QS, Wiggins KL, Wong JA, Woo D, Worrall BB, Yang PS, Yao J, Yoneda ZT, Zeller T, Zeng L, Lubitz SA, Lunetta KL, Ellinor PT: Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet 2018; 50:1225-1233. [CrossRef]
- Rousseau BA, Bhaduri-McIntosh S: Inflammation and Epstein-Barr Virus at the Crossroads of Multiple Sclerosis and Post-Acute Sequelae of COVID-19 Infection. Viruses 2023; 15:949. [CrossRef]
- Roy M, Nath AK, Pal I, Dey SG: Second Sphere Interactions in Amyloidogenic Diseases. Chem Rev 2022; 122:12132-12206. [CrossRef]
- Rubini-Costa R, Bermúdez-Jiménez F, Rivera-López R, Sola-García E, Nagib-Raya H, Moreno-Escobar E, López-Zúñiga MÁ, Briones-Través A, Sanz-Herrera F, Sequí-Sabater JM, Romero-Cabrera JL, Maíllo-Seco J, Fernández-Vázquez F, Rivadeneira-Ruiz M, López-Valero L, Gómez-Navarro C, Aparicio-Gómez JA, López MÁ, Tercedor L, Molina-Jiménez M, Macías-Ruiz R, Jiménez-Jáimez J: Prevalence of bleeding secondary to anticoagulation and mortality in patients with atrial fibrillation admitted with SARS-CoV-2 infection. Med Clin (Barc) 2022; 158:569-575. [CrossRef]
- Ruddox V, Sandven I, Munkhaugen J, Skattebu J, Edvardsen T, Otterstad JE: Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure: A systematic review and meta-analysis. Eur J Prev Cardiol 2017; 24:1555-1566. [CrossRef]
- Sahu B, Mackos AR, Floden AM, Wold LE, Combs CK: Particulate Matter Exposure Exacerbates Amyloid-beta Plaque Deposition and Gliosis in APP/PS1 Mice. J Alzheimers Dis 2021; 80:761-774. [CrossRef]
- Sandhu RK, Ezekowitz J, Andersson U, Alexander JH, Granger CB, Halvorsen S, Hanna M, Hijazi Z, Jansky P, Lopes RD, Wallentin L: The 'obesity paradox' in atrial fibrillation: observations from the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial. Eur Heart J 2016; 37:2869-2878. [CrossRef]
- Sandhu RK, Ezekowitz JA, Hijazi Z, Westerbergh J, Aulin J, Alexander JH, Granger CB, Halvorsen S, Hanna MS, Lopes RD, Siegbahn A, Wallentin L: Obesity paradox on outcome in atrial fibrillation maintained even considering the prognostic influence of biomarkers: insights from the ARISTOTLE trial. Open Heart 2018; 5:e000908. [CrossRef]
- Scarlatescu E, Tomescu D, Arama SS: Anticoagulant Therapy in Sepsis. The Importance of Timing. J Crit Care Med (Targu Mures) 2017; 3:63-69. [CrossRef]
- Schnabel RB, Haeusler KG, Healey JS, Freedman B, Boriani G, Brachmann J, Brandes A, Bustamante A, Casadei B, Crijns H, Doehner W, Engstrom G, Fauchier L, Friberg L, Gladstone DJ, Glotzer TV, Goto S, Hankey GJ, Harbison JA, Hobbs FDR, Johnson LSB, Kamel H, Kirchhof P, Korompoki E, Krieger DW, Lip GYH, Lochen ML, Mairesse GH, Montaner J, Neubeck L, Ntaios G, Piccini JP, Potpara TS, Quinn TJ, Reiffel JA, Ribeiro ALP, Rienstra M, Rosenqvist M, Themistoclakis S, Sinner MF, Svendsen JH, Van Gelder IC, Wachter R, Wijeratne T, Yan B: Searching for Atrial Fibrillation Poststroke: A White Paper of the AF-SCREEN International Collaboration. Circulation 2019; 140:1834-1850. [CrossRef]
- Schönrich G, Abdelaziz MO, Raftery MJ: Epstein-Barr virus, interleukin-10 and multiple sclerosis: A ménage à trois. Front Immunol 2022; 13:1028972. [CrossRef]
- Sekelj S, Sandler B, Johnston E, Pollock KG, Hill NR, Gordon J, Tsang C, Khan S, Ng FS, Farooqui U: Detecting undiagnosed atrial fibrillation in UK primary care: Validation of a machine learning prediction algorithm in a retrospective cohort study. Eur J Prev Cardiol 2021; 28:598-605. [CrossRef]
- Selewa A, Luo K, Wasney M, Smith L, Sun X, Tang C, Eckart H, Moskowitz IP, Basu A, He X, Pott S: Single-cell genomics improves the discovery of risk variants and genes of atrial fibrillation. Nat Commun 2023; 14:4999. [CrossRef]
- Semb AG, Rollefstad S, Sexton J, Ikdahl E, Crowson CS, van Riel P, Kitas G, Graham I, Kerola AM, Surf-RA: Oral anticoagulant treatment in rheumatoid arthritis patients with atrial fibrillation results of an international audit. Int J Cardiol Heart Vasc 2022; 42:101117. [CrossRef]
- Semczuk-Kaczmarek K, Platek AE, Rys A, Adamowicz J, Legosz P, Kotkowski M, Dudzik-Plocica A, Gorko D, Szymanski FM, Filipiak KJ: CHA2DS2-VASc score and fibrinogen concentration in patients with atrial fibrillation. Adv Clin Exp Med 2019; 28:1451-1457. [CrossRef]
- Sepehri Shamloo A, Arya A, Darma A, Nedios S, Doring M, Bollmann A, Dagres N, Hindricks G: Atrial fibrillation: is there a role for cardiac troponin? Diagnosis (Berl) 2021; 8:295-303. [CrossRef]
- Seuma M, Faure AJ, Badia M, Lehner B, Bolognesi B: The genetic landscape for amyloid beta fibril nucleation accurately discriminates familial Alzheimer's disease mutations. Elife 2021; 10:e63364. [CrossRef]
- Seuma M, Lehner B, Bolognesi B: An atlas of amyloid aggregation: the impact of substitutions, insertions, deletions and truncations on amyloid beta fibril nucleation. Nat Commun 2022; 13:7084. [CrossRef]
- Shao Q, Liu T, Korantzopoulos P, Zhang Z, Zhao J, Li G: Association between air pollution and development of atrial fibrillation: A meta-analysis of observational studies. Heart Lung 2016; 45:557-562. [CrossRef]
- Shim R, Wong CHY: Ischemia, Immunosuppression and Infection--Tackling the Predicaments of Post-Stroke Complications. Int J Mol Sci 2016; 17:64. [CrossRef]
- Siontis KC, Yao X, Pirruccello JP, Philippakis AA, Noseworthy PA: How Will Machine Learning Inform the Clinical Care of Atrial Fibrillation? Circ Res 2020; 127:155-169. [CrossRef]
- Sluis WM, Westendorp WF, van de Beek D, Nederkoorn PJ, van der Worp HB: Preventive ceftriaxone in patients at high risk of stroke-associated pneumonia. A post-hoc analysis of the PASS trial. PLoS One 2022; 17:e0279700. [CrossRef]
- Smeyne RJ, Noyce AJ, Byrne M, Savica R, Marras C: Infection and Risk of Parkinson's Disease. J Parkinsons Dis 2021; 11:31-43. [CrossRef]
- Sohail MU, Mashood F, Oberbach A, Chennakkandathil S, Schmidt F: The role of pathogens in diabetes pathogenesis and the potential of immunoproteomics as a diagnostic and prognostic tool. Front Microbiol 2022; 13:1042362. [CrossRef]
- Sohara H, Amitani S, Kurose M, Miyahara K: Atrial fibrillation activates platelets and coagulation in a time-dependent manner: a study in patients with paroxysmal atrial fibrillation. J Am Coll Cardiol 1997; 29:106-112. [CrossRef]
- Soma P, Pretorius E: Interplay between ultrastructural findings and atherothrombotic complications in type 2 diabetes mellitus. Cardiovasc Diabetol 2015; 14:96. [CrossRef]
- Son MK, Lim NK, Kim HW, Park HY: Risk of ischemic stroke after atrial fibrillation diagnosis: A national sample cohort. PLoS One 2017; 12:e0179687. [CrossRef]
- Sposato LA, Cipriano LE, Saposnik G, Ruiz Vargas E, Riccio PM, Hachinski V: Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 2015; 14:377-387. [CrossRef]
- Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH: Atrial Fibrillation: Epidemiology, Pathophysiology, and Clinical Outcomes. Circ Res 2017; 120:1501-1517. [CrossRef]
- Staff AC, Redman CW, Williams D, Leeson P, Moe K, Thilaganathan B, Magnus P, Steegers EA, Tsigas EZ, Ness RB, Myatt L, Poston L, Roberts JM, Global Pregnancy Collaboration: Pregnancy and Long-Term Maternal Cardiovascular Health: Progress Through Harmonization of Research Cohorts and Biobanks. Hypertension 2016; 67:251-260. [CrossRef]
- Su VY, Liu CJ, Wang HK, Wu LA, Chang SC, Perng DW, Su WJ, Chen YM, Lin EY, Chen TJ, Chou KT: Sleep apnea and risk of pneumonia: a nationwide population-based study. CMAJ 2014; 186:415-421. [CrossRef]
- Sun T, Ye M, Lei F, Qin JJ, Liu YM, Chen Z, Chen MM, Yang C, Zhang P, Ji YX, Zhang XJ, She ZG, Cai J, Jin ZX, Li H: Prevalence and trend of atrial fibrillation and its associated risk factors among the population from nationwide health check-up centers in China, 2012-2017. Front Cardiovasc Med 2023; 10:1151575. [CrossRef]
- Swanepoel AC, Lindeque BG, Swart PJ, Abdool Z, Pretorius E: Estrogen causes ultrastructural changes of fibrin networks during the menstrual cycle: a qualitative investigation. Microsc Res Tech 2014; 77:594-601. [CrossRef]
- Swanepoel AC, Visagie A, de Lange Z, Emmerson O, Nielsen VG, Pretorius E: The clinical relevance of altered fibrinogen packaging in the presence of 17beta-estradiol and progesterone. Thromb Res 2016; 146:23-34. [CrossRef]
- Szymanski T, Ashton R, Sekelj S, Petrungaro B, Pollock KG, Sandler B, Lister S, Hill NR, Farooqui U: Budget impact analysis of a machine learning algorithm to predict high risk of atrial fibrillation among primary care patients. Europace 2022; 24:1240-1247. [CrossRef]
- Taylor CG, Meisl G, Horrocks MH, Zetterberg H, Knowles TPJ, Klenerman D: Extrinsic Amyloid-Binding Dyes for Detection of Individual Protein Aggregates in Solution. Analytical chemistry 2018; 90:10385-10393. [CrossRef]
- Thagard P (ed.): The cognitive science of science: explanation, discovery, and conceptual change. Cambridge, MA: MIT Press, 2012.
- Thagard P: Coherence, truth, and the development of scientific knowledge. Philosophy of Science 2007; 74:28-47.
- Thagard P: How scientists explain disease. Princeton, NJ: Princeton University Press, 1999.
- Thakur M, Alsinbili A, Chattopadhyay R, Warburton EA, Khadjooi K, Induruwa I: Identifying the optimal time period for detection of atrial fibrillation after ischaemic stroke and TIA: An updated systematic review and meta-analysis of randomized control trials. Int J Stroke 2023:17474930231215277. [CrossRef]
- Thilaganathan B, Kalafat E: Cardiovascular System in Preeclampsia and Beyond. Hypertension 2019; 73:522-531. [CrossRef]
- Thirupathi A, Pinho RA, Ugbolue UC, He Y, Meng Y, Gu Y: Effect of Running Exercise on Oxidative Stress Biomarkers: A Systematic Review. Front Physiol 2020; 11:610112. [CrossRef]
- Tian W, Zhang LV, Tasan M, Gibbons FD, King OD, Park J, Wunderlich Z, Cherry JM, Roth FP: Combining guilt-by-association and guilt-by-profiling to predict Saccharomyces cerevisiae gene function. Genome Biol 2008; 9 Suppl 1:S7. [CrossRef]
- Tilly MJ, Geurts S, Pezzullo AM, Bramer WM, de Groot NMS, Kavousi M, de Maat MPM: The association of coagulation and atrial fibrillation: a systematic review and meta-analysis. Europace 2023; 25:28-39. [CrossRef]
- Todros T, Vasario E, Cardaropoli S: Preeclampsia as an infectious disease. Exp Rev Obs Gynecol 2007; 2:735-741. [CrossRef]
- Todros T, Verdiglione P, Oggè G, Paladini D, Vergani P, Cardaropoli S: Low incidence of hypertensive disorders of pregnancy in women treated with spiramycin for toxoplasma infection. Br J Clin Pharmacol 2006; 61:336-340. [CrossRef]
- Treewaree S, Lip GYH, Krittayaphong R: Non-vitamin K Antagonist Oral Anticoagulant, Warfarin, and ABC Pathway Adherence on Hierarchical Outcomes: Win Ratio Analysis of the COOL-AF Registry. Thromb Haemost 2024; 124:69-79. [CrossRef]
- Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Beaton AZ, Boehme AK, Buxton AE, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Fugar S, Generoso G, Heard DG, Hiremath S, Ho JE, Kalani R, Kazi DS, Ko D, Levine DA, Liu J, Ma J, Magnani JW, Michos ED, Mussolino ME, Navaneethan SD, Parikh NI, Poudel R, Rezk-Hanna M, Roth GA, Shah NS, St-Onge MP, Thacker EL, Virani SS, Voeks JH, Wang NY, Wong ND, Wong SS, Yaffe K, Martin SS, American Heart Association Council on E, Prevention Statistics C, Stroke Statistics S: Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 2023; 147:e93-e621. [CrossRef]
- Tseng AS, Noseworthy PA: Prediction of Atrial Fibrillation Using Machine Learning: A Review. Front Physiol 2021; 12:752317. [CrossRef]
- Turner S, Laubscher GJ, Khan MA, Kell DB, Pretorius E: Accelerating discovery: A novel flow cytometric method for detecting fibrin(ogen) amyloid microclots using long COVID as a model Heliyon 2023; 9:e19605. [CrossRef]
- Umemura Y, Nishida T, Yamakawa K, Ogura H, Oda J, Fujimi S: Anticoagulant therapies against sepsis-induced disseminated intravascular coagulation. Acute Med Surg 2023; 10:e884. [CrossRef]
- Umemura Y, Yamakawa K: Optimal patient selection for anticoagulant therapy in sepsis: an evidence-based proposal from Japan. J Thromb Haemost 2018; 16:462-464. [CrossRef]
- Undas A: Fibrin clot properties and their modulation in thrombotic disorders. Thromb Haemost 2014; 112:32-42. [CrossRef]
- van Amstel RBE, Kennedy JN, Scicluna BP, Bos LDJ, Peters-Sengers H, Butler JM, Cano-Gamez E, Knight JC, Vlaar APJ, Cremer OL, Angus DC, van der Poll T, Seymour CW, van Vught LA, Consortium M: Uncovering heterogeneity in sepsis: a comparative analysis of subphenotypes. Intensive Care Med 2023; 49:1360-1369. [CrossRef]
- van den Oord EJ: Controlling false discoveries in genetic studies. Am J Med Genet B Neuropsychiatr Genet 2008; 147B:637-644. [CrossRef]
- van Langevelde P, Kwappenberg KM, Groeneveld PH, Mattie H, van Dissel JT: Antibiotic-induced lipopolysaccharide (LPS) release from Salmonella typhi: delay between killing by ceftazidime and imipenem and release of LPS. Antimicrob Agents Chemother 1998; 42:739-743. [CrossRef]
- Vaswani A, Shazeer N, Parmar N, Uszkoreit J, Jones L, Gomez AN, Kaiser L, Polosukhin I: Attention Is All You Need. arXiv 2017. arXiv:1706.03762.
- Vermeij FH, Scholte op Reimer WJ, de Man P, van Oostenbrugge RJ, Franke CL, de Jong G, de Kort PL, Dippel DW, Netherlands Stroke Survey I: Stroke-associated infection is an independent risk factor for poor outcome after acute ischemic stroke: data from the Netherlands Stroke Survey. Cerebrovasc Dis 2009; 27:465-471. [CrossRef]
- Vermeij JD, Westendorp WF, Dippel DWJ, van de Beek D, Nederkoorn PJ: Antibiotic therapy for preventing infections in people with acute stroke. Cochrane Database Syst Rev 2018; 1:CD008530. [CrossRef]
- Voy BH, Scharff JA, Perkins AD, Saxton AM, Borate B, Chesler EJ, Branstetter LK, Langston MA: Extracting gene networks for low-dose radiation using graph theoretical algorithms. PLoS Comput Biol 2006; 2:e89. [CrossRef]
- Wadowski PP, Panzer B, Jozkowicz A, Kopp CW, Gremmel T, Panzer S, Koppensteiner R: Microvascular Thrombosis as a Critical Factor in Severe COVID-19. Int J Mol Sci 2023; 24:2492. [CrossRef]
- Walkey AJ, Greiner MA, Heckbert SR, Jensen PN, Piccini JP, Sinner MF, Curtis LH, Benjamin EJ: Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J 2013; 165:949-955 e943. [CrossRef]
- Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS: Atrial fibrillation and obesity--results of a meta-analysis. Am Heart J 2008; 155:310-315. [CrossRef]
- Wang A, Green JB, Halperin JL, Piccini JP, Sr.: Atrial Fibrillation and Diabetes Mellitus: JACC Review Topic of the Week. J Am Coll Cardiol 2019; 74:1107-1115. [CrossRef]
- Wang F, Ahat X, Liang Q, Ma Y, Sun M, Lin L, Li T, Duan J, Sun Z: The relationship between exposure to PM(2.5) and atrial fibrillation in older adults: A systematic review and meta-analysis. Sci Total Environ 2021; 784:147106. [CrossRef]
- Wang H, Liu X, Tan C, Zhou W, Jiang J, Peng W, Zhou X, Mo L, Chen L: Bacterial, viral, and fungal infection-related risk of Parkinson's disease: Meta-analysis of cohort and case-control studies. Brain Behav 2020; 10:e01549. [CrossRef]
- Wang TJ, Parise H, Levy D, D'Agostino RB, Sr., Wolf PA, Vasan RS, Benjamin EJ: Obesity and the risk of new-onset atrial fibrillation. JAMA 2004; 292:2471-2477. [CrossRef]
- Watson T, Shantsila E, Lip GYH: Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet 2009; 373:155-166. [CrossRef]
- Weisel JW: Fibrinogen and fibrin. Adv Protein Chem 2005; 70:247-299. [CrossRef]
- Weisel JW, Litvinov RI: Fibrin Formation, Structure and Properties. Subcell Biochem 2017; 82:405-456. [CrossRef]
- Wekerle H: Epstein-Barr virus sparks brain autoimmunity in multiple sclerosis. Nature 2022; 603:230-232. [CrossRef]
- Weng LC, Choi SH, Klarin D, Smith JG, Loh PR, Chaffin M, Roselli C, Hulme OL, Lunetta KL, Dupuis J, Benjamin EJ, Newton-Cheh C, Kathiresan S, Ellinor PT, Lubitz SA: Heritability of Atrial Fibrillation. Circ Cardiovasc Genet 2017; 10. [CrossRef]
- Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D: Post-stroke infection: a systematic review and meta-analysis. BMC Neurol 2011; 11:110. [CrossRef]
- Westendorp WF, Vermeij JD, Hilkens NA, Brouwer MC, Algra A, van der Worp HB, Dippel DWJ, van de Beek D, Nederkoorn PJ, PASS-Investigators: Development and internal validation of a prediction rule for post-stroke infection and post-stroke pneumonia in acute stroke patients. Eur Stroke J 2018; 3:136-144. [CrossRef]
- Westendorp WF, Vermeij JD, Smith CJ, Kishore AK, Hodsoll J, Kalra L, Meisel A, Chamorro A, Chang JJ, Rezaei Y, Amiri-Nikpour MR, DeFalco FA, Switzer JA, Blacker DJ, Dijkgraaf MG, Nederkoorn PJ, van de Beek D: Preventive antibiotic therapy in acute stroke patients: A systematic review and meta-analysis of individual patient data of randomized controlled trials. Eur Stroke J 2021; 6:385-394. [CrossRef]
- Westendorp WF, Vermeij JD, Zock E, Hooijenga IJ, Kruyt ND, Bosboom HJLW, Kwa VI, Weisfelt M, Remmers MJM, ten Houten R, Tobien Schreuder AHCM, Vermeer SE, van Dijk EJ, Dippel DW, Dijkgraaf MGW, Spanjaard L, Vermeulen M, van der Poll T, Prins JM, Vermeij FH, Roos YB, Kleyweg RP, Kerkhoff H, Brouwer MC, Zwinderman AH, van de Beek D, Nederkoorn PJ, PASS investigators: The Preventive Antibiotics in Stroke Study (PASS): a pragmatic randomised open-label masked endpoint clinical trial. Lancet 2015; 385:1519-1526. [CrossRef]
- Weymann A, Sabashnikov A, Ali-Hasan-Al-Saegh S, Popov AF, Jalil Mirhosseini S, Baker WL, Lotfaliani M, Liu T, Dehghan H, Yavuz S, de Oliveira Sa MP, Jang JS, Zeriouh M, Meng L, D'Ascenzo F, Deshmukh AJ, Biondi-Zoccai G, Dohmen PM, Calkins H, Surgery C, Cardiology-Group (IMSRC-Group): Predictive Role of Coagulation, Fibrinolytic, and Endothelial Markers in Patients with Atrial Fibrillation, Stroke, and Thromboembolism: A Meta-Analysis, Meta-Regression, and Systematic Review. Med Sci Monit Basic Res 2017; 23:97-140. [CrossRef]
- Wolf PA, Abbott RD, Kannel WB: Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22:983-988. [CrossRef]
- Writing Committee Members, Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, Cronin EM, Deswal A, Eckhardt LL, Goldberger ZD, Gopinathannair R, Gorenek B, Hess PL, Hlatky M, Hogan G, Ibeh C, Indik JH, Kido K, Kusumoto F, Link MS, Linta KT, Marcus GM, McCarthy PM, Patel N, Patton KK, Perez MV, Piccini JP, Russo AM, Sanders P, Streur MM, Thomas KL, Times S, Tisdale JE, Valente AM, Van Wagoner DR: 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2024; 83:109-279. [CrossRef]
- Wu L, Jiang Z, Meulendijks ER, Baylan U, Waas ISE, Bugiani M, Tuinman PR, Fronczek J, Heunks LMA, de Groot JR, van Rossum AC, Niessen HWM, Krijnen PAJ: Atrial inflammation and microvascular thrombogenicity are increased in deceased COVID-19 patients. Cardiovasc Pathol 2023a; 64:107524. [CrossRef]
- Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew-Graham CA, Mamas MA: Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ Cardiovasc Qual Outcomes 2017; 10. [CrossRef]
- Wu S, Huang N, Chen X, Jiang S, Zhang W, Hu W, Su J, Dai H, Gu P, Huang X, Du X, Li R, Zheng Q, Lin X, Zhang Y, Zou L, Liu Y, Zhang M, Liu X, Zhu Z, Sun J, Hong S, She W, Zhang J: Association between Body Mass Index and Clinical Outcomes in Patients with Non-valvular Atrial Fibrillation Receiving Direct Oral Anticoagulants: A New Piece of Evidence on the Obesity Paradox from China. Cardiovasc Drugs Ther 2023b; 37:715-727. [CrossRef]
- Xiao FP, Chen MY, Wang L, He H, Jia ZQ, Kuai L, Zhou HB, Liu M, Hong M: Outcomes of new-onset atrial fibrillation in patients with sepsis: A systematic review and meta-analysis of 225,841 patients. Am J Emerg Med 2021; 42:23-30. [CrossRef]
- Xiong Q, Proietti M, Senoo K, Lip GYH: Asymptomatic versus symptomatic atrial fibrillation: A systematic review of age/gender differences and cardiovascular outcomes. Int J Cardiol 2015; 191:172-177. [CrossRef]
- Xiong Z, Liu T, Tse G, Gong M, Gladding PA, Smaill BH, Stiles MK, Gillis AM, Zhao J: A Machine Learning Aided Systematic Review and Meta-Analysis of the Relative Risk of Atrial Fibrillation in Patients With Diabetes Mellitus. Front Physiol 2018; 9:835. [CrossRef]
- Xue Y, Yang N, Gu X, Wang Y, Zhang H, Jia K: Risk Prediction Model of Early-Onset Preeclampsia Based on Risk Factors and Routine Laboratory Indicators. Life (Basel) 2023; 13:1648. [CrossRef]
- Yamashita T: Virchow triad and beyond in atrial fibrillation. Heart Rhythm 2016; 13:2377-2378. [CrossRef]
- Yamauchi K, Furui H, Taniguchi N, Sotobata I: Plasma beta-thromboglobulin and platelet factor 4 concentrations in patients with atrial fibrillation. Jpn Heart J 1986; 27:481-487. [CrossRef]
- Yang XM, Rao ZZ, Gu HQ, Zhao XQ, Wang CJ, Liu LP, Liu C, Wang YL, Li ZX, Xiao RP, Wang YJ: Atrial Fibrillation Known Before or Detected After Stroke Share Similar Risk of Ischemic Stroke Recurrence and Death. Stroke 2019; 50:1124-1129. [CrossRef]
- Yoon JW, Notkins AL: Virus-induced diabetes in mice. Metabolism 1983; 32:37-40. [CrossRef]
- Yu S, Ma X, Li X: Phenotype-oriented anticoagulant therapy for sepsis: still a work in progress. Int J Hematol 2022; 116:48-54. [CrossRef]
- Yue C, Yang F, Li F, Chen Y: Association between air pollutants and atrial fibrillation in general population: A systematic review and meta-analysis. Ecotoxicol Environ Saf 2021; 208:111508. [CrossRef]
- Zamolodchikov D, Strickland S: Abeta delays fibrin clot lysis by altering fibrin structure and attenuating plasminogen binding to fibrin. Blood 2012; 119:3342-3351. [CrossRef]
- Zanini G, Selleri V, Roncati L, Coppi F, Nasi M, Farinetti A, Manenti A, Pinti M, Mattioli AV: Vascular "Long COVID": A New Vessel Disease? Angiology 2024; 75:8-14. [CrossRef]
- Zhang J, Chen G, Xia H, Wang X, Wang C, Cai M, Gao Y, Lip GYH, Lin H: Associations of Life's Essential 8 and fine particulate matter pollution with the incidence of atrial fibrillation. J Hazard Mater 2023; 459:132114. [CrossRef]
- Zhang J, Johnsen SP, Guo Y, Lip GYH: Epidemiology of Atrial Fibrillation: Geographic/Ecological Risk Factors, Age, Sex, Genetics. Card Electrophysiol Clin 2021a; 13:1-23. [CrossRef]
- Zhang L, Ye K, Xiaokereti J, Ma M, Guo Y, Zhou X, Tang B: Histopathological substrate of the atrial myocardium in the progression of obstructive sleep apnoea-related atrial fibrillation. Sleep Breath 2021b; 25:807-818. [CrossRef]
- Zheng H, Stergiopoulos K, Wang L, Chen L, Cao J: COVID-19 Presenting as Major Thromboembolic Events: Virchow's Triad Revisited and Clinical Considerations of Therapeutic Anticoagulation. Cureus 2020; 12:e10137. [CrossRef]
- Zheng Y, Li S, Liu X, Lip GYH, Guo L, Zhu W: Effect of Oral Anticoagulants in Atrial Fibrillation Patients with Polypharmacy: A Meta-analysis. Thromb Haemost 2023. [CrossRef]
- Zhong C, Xin M, He L, Sun G, Shen F: Prognostic value of von Willebrand factor in patients with atrial fibrillation: A meta-analysis. Medicine (Baltimore) 2018; 97:e11269. [CrossRef]
- Zhu W, Wan R, Liu F, Hu J, Huang L, Li J, Hong K: Relation of Body Mass Index With Adverse Outcomes Among Patients With Atrial Fibrillation: A Meta-Analysis and Systematic Review. J Am Heart Assoc 2016; 5. [CrossRef]
- Zuin M, Rigatelli G, Bilato C, Zanon F, Zuliani G, Roncon L: Pre-existing atrial fibrillation is associated with increased mortality in COVID-19 Patients. J Interv Card Electrophysiol 2021; 62:231-238. [CrossRef]
- Zulkifly H, Lip GYH, Lane DA: Epidemiology of atrial fibrillation. Int J Clin Pract 2018; 72:e13070. [CrossRef]
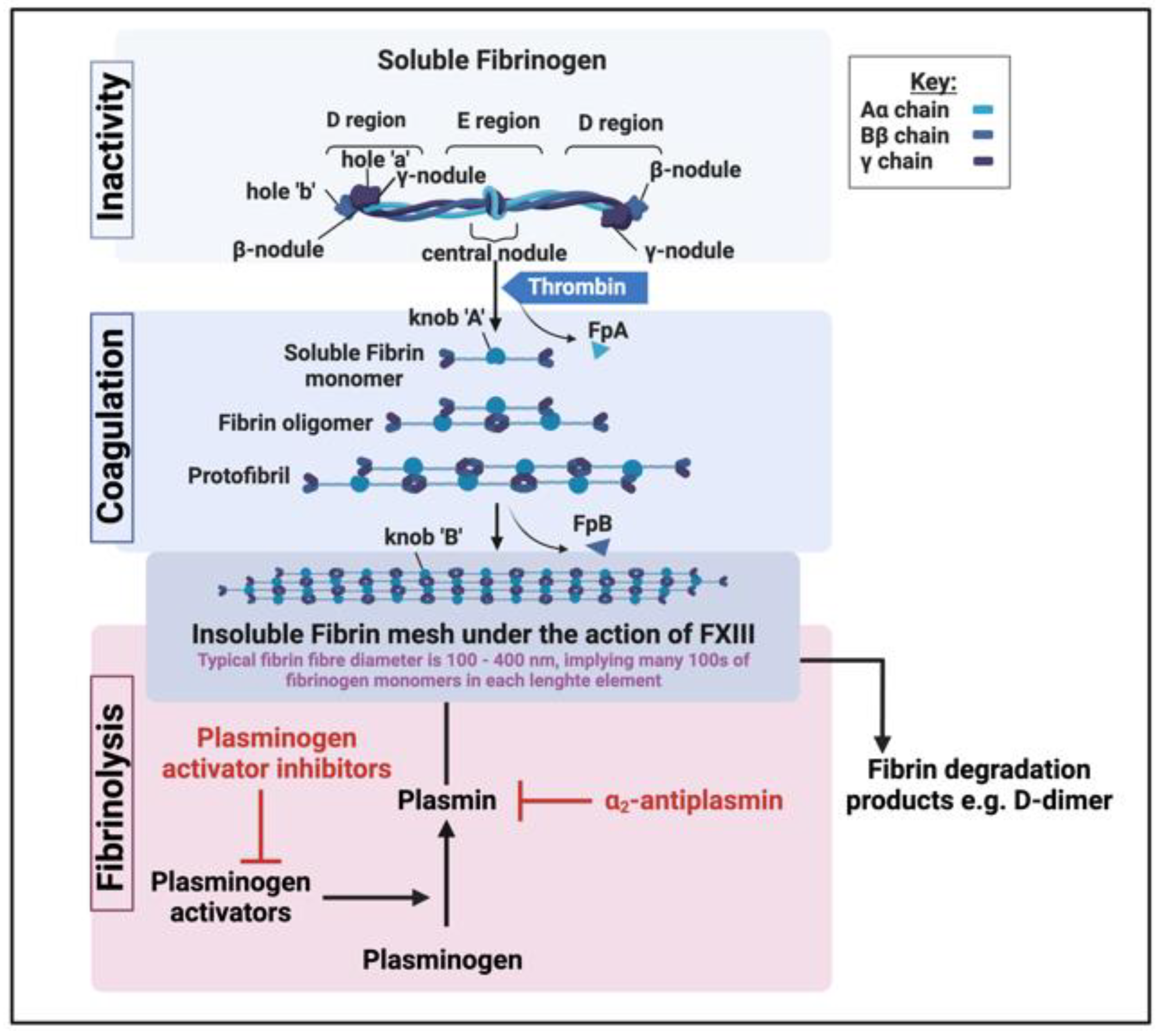
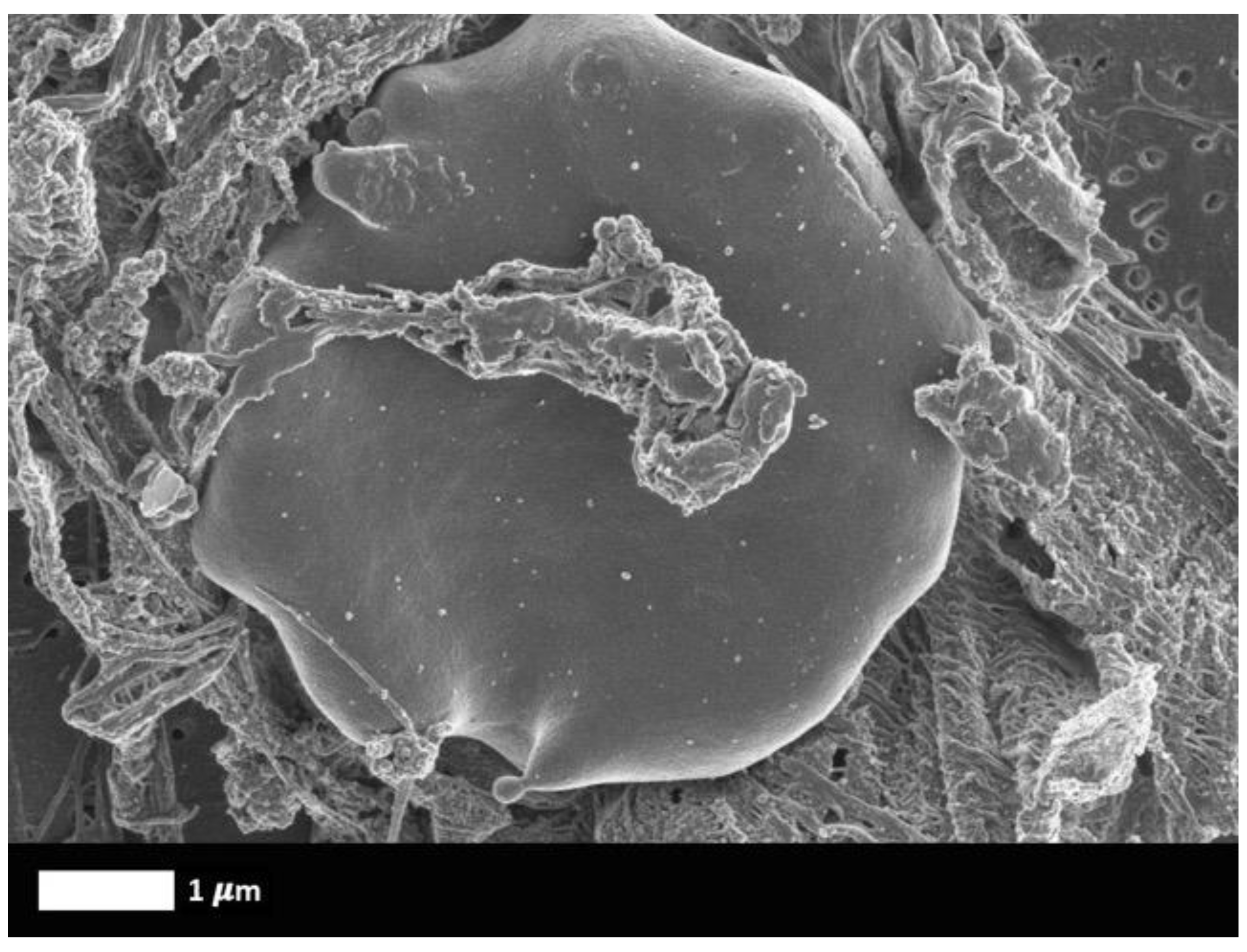
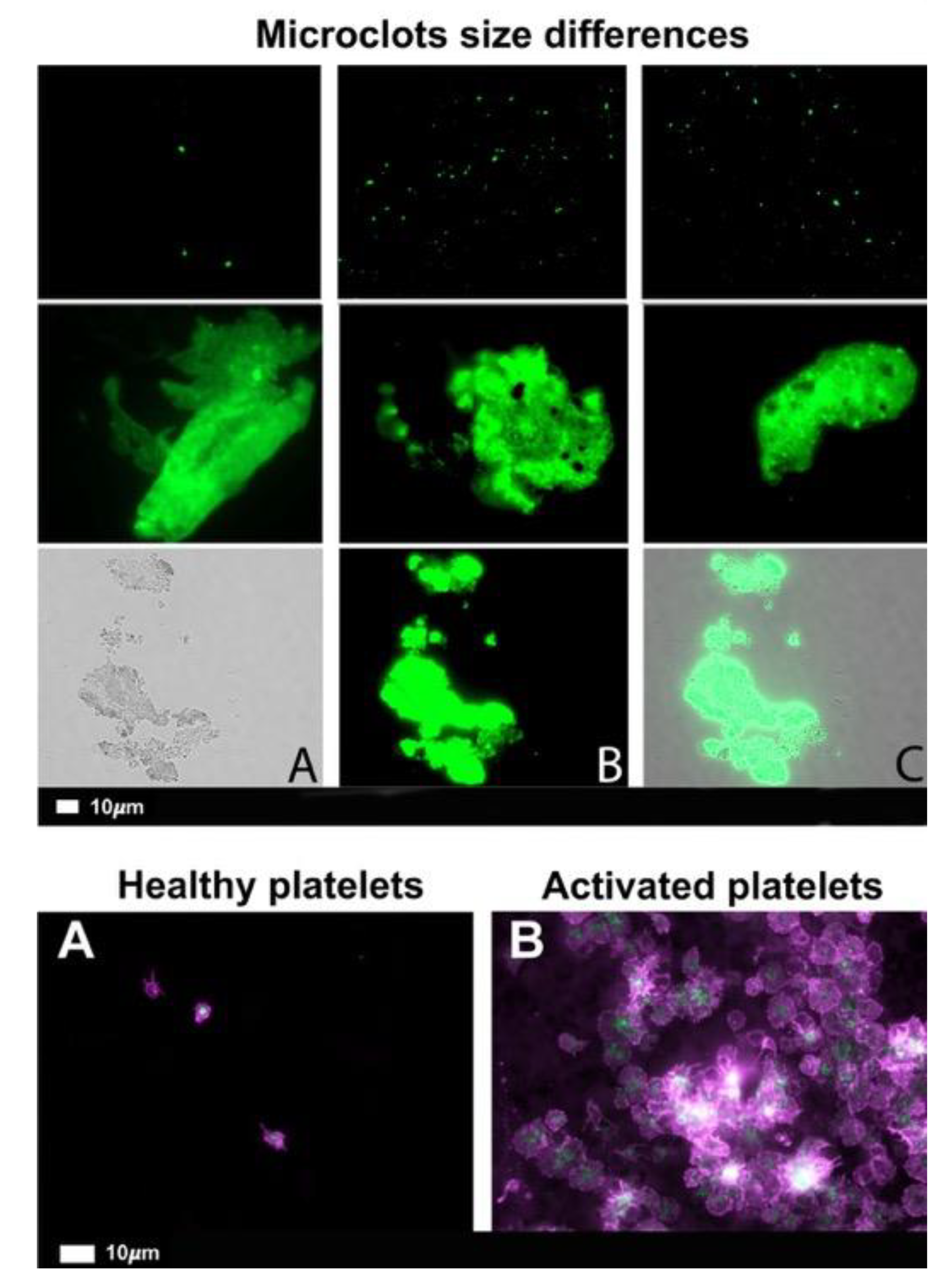
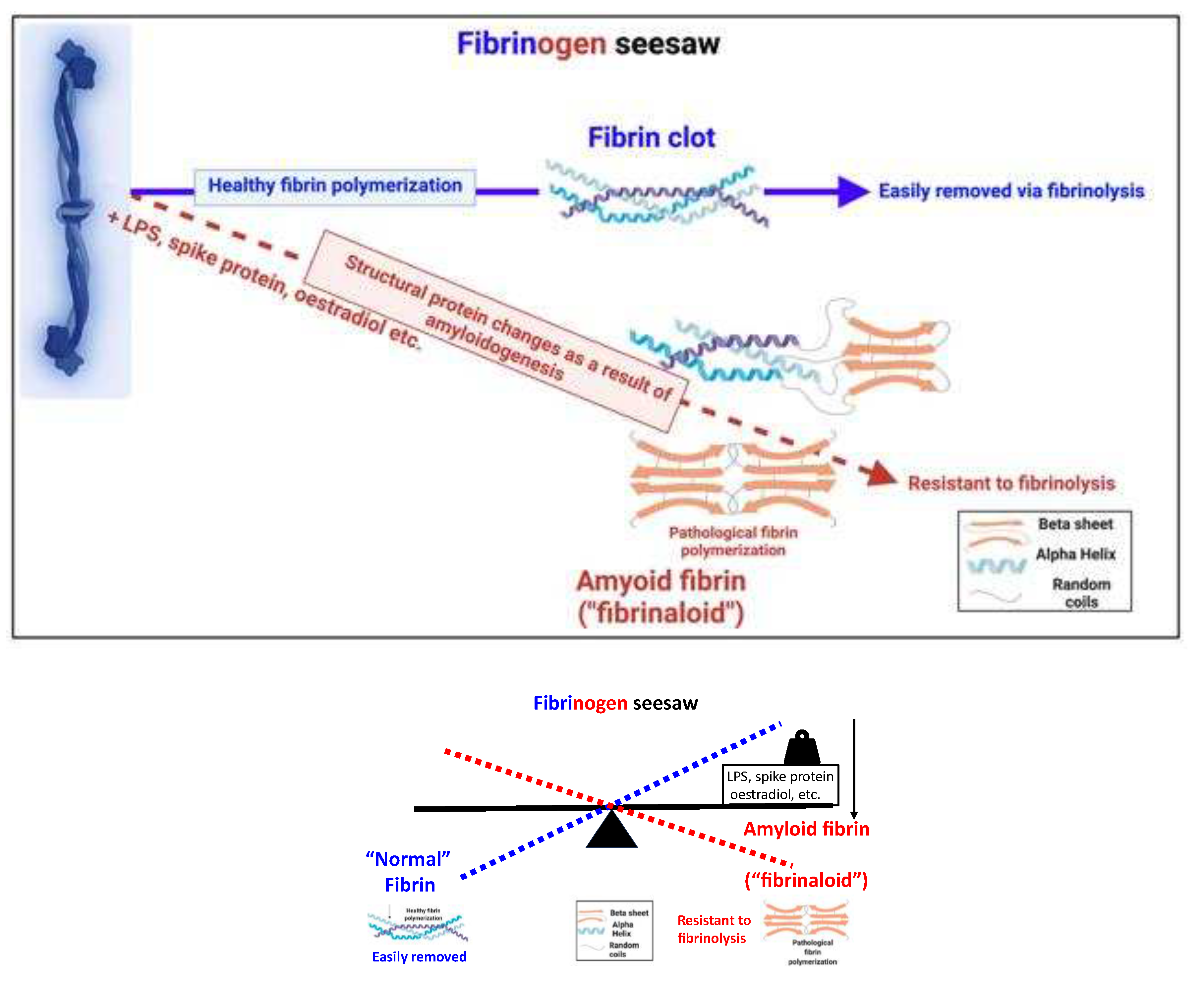
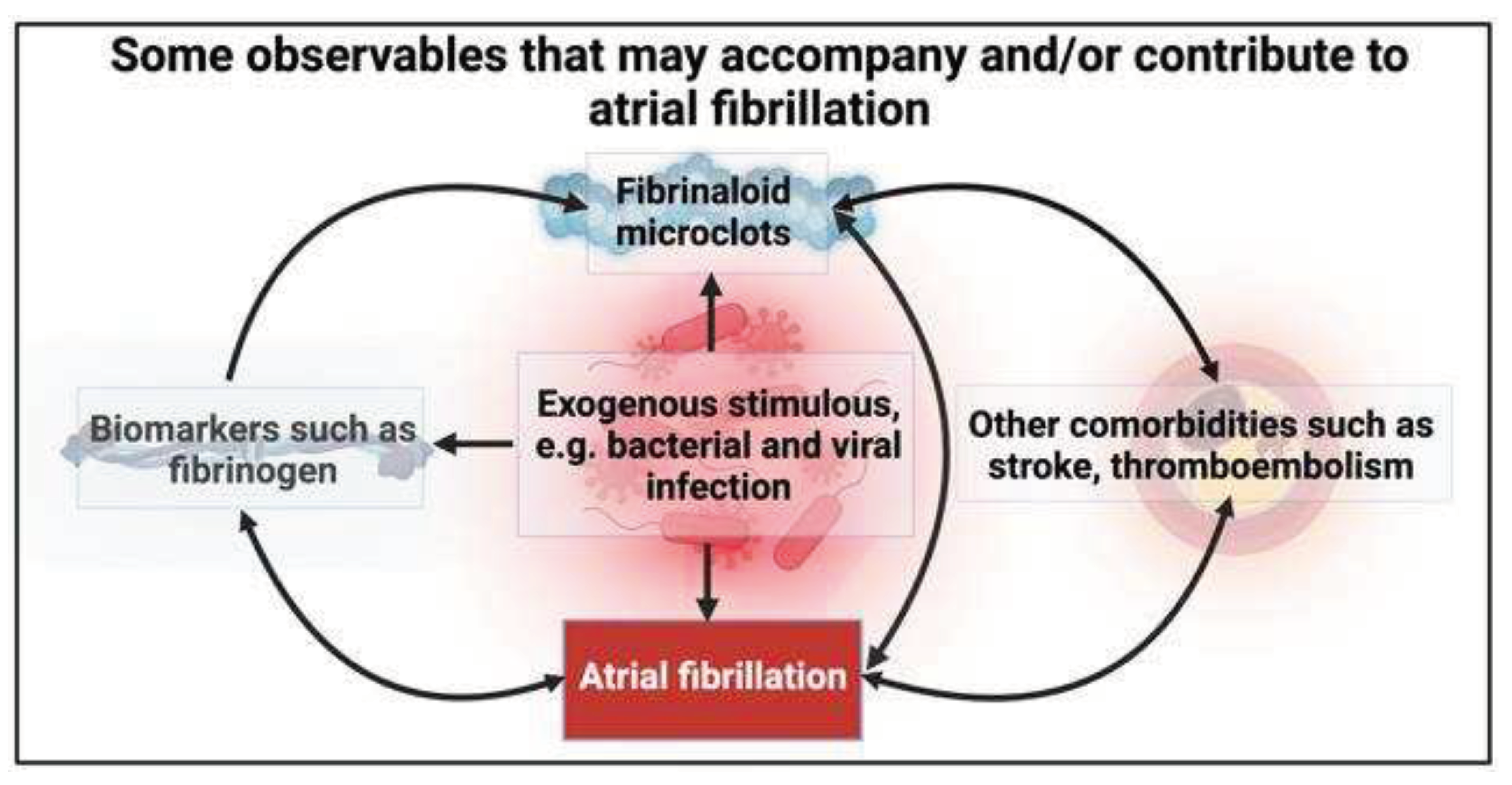
| Risk factor | Comments | Selected references |
|---|---|---|
| Alcohol consumption | Some increase in AF risk as a function of alcohol intake; greater in men; studies mainly not controlled for BMI. Not a huge effect for moderate levels of consumption. | (Djoussé et al. 2004; Mukamal et al. 2005; Panchal et al. 2019b) |
| Exercise | As with BMI, the relationship is nonlinear, with moderate exercise and general cardio-respiratory fitness being beneficial but excess exercise (which could cause oxidative stress (Powers et al. 2020; Thirupathi et al. 2020), inflammation (Cerqueira et al. 2019; da Rocha et al. 2019), hypoxaemia (Durand and Raberin 2021), etc) having negative effects. | (Aizer et al. 2009; Panchal et al. 2019b; Qureshi et al. 2015) |
| Particulate matter exposure | New-onset AF can follow exposure to particulate matter.This can be an acute occurenceIncreases with known genetic risk factors Meta-analyses.Particulate matter is also amyloidogenic | (Blaszczyk et al. 2023; Link et al. 2013; Mandaglio-Collados et al. 2023; Zhang et al. 2023)(Ma et al. 2023) (Shao et al. 2016; Wang et al. 2021; Yue et al. 2021) (Calderón-Garcidueñas et al. 2008; Iaccarino et al. 2021; Jang et al. 2018; Sahu et al. 2021) |
| Psychosocial Stress | As estimated by surrogates reflecting anger and hostility, can be a minor risk factor in men but not women, even after controlling for hypertension. | (Eaker et al. 2004; Panchal et al. 2019b) |
| Smoking | Although important to other cardiovascular diseases, for AF seemingly a marginal risk, and probably dwarfed by other risks of smoking such as lung cancer | (Panchal et al. 2019a) |
| Disease or syndrome | Comments | References regarding an infectious origin | References illustrating anomalous clotting/ microclots |
|---|---|---|---|
| Alzheimer’s | Many references, not least from Ruth Itzhaki focusing on HSV, imply this strongly. Other organisms have also been implicated. | (Grobler et al. 2023; Itzhaki 2018; Itzhaki et al. 2008; Itzhaki and Lathe 2018; Itzhaki et al. 2016) | (de Waal et al. 2018; Pretorius et al. 2016a; Pretorius et al. 2018a) |
| Diabetes, type 2 | Originally asked by Gundersen in 1927. Even greater evidence for type 1 (Jean-Baptiste et al. 2017), not covered here. | (Craighead 1975; Gundersen 1927; Notkins 1977; Rajsfus et al. 2023; Yoon and Notkins 1983) | (Pretorius et al. 2011a) (de Waal et al. 2018; Pretorius et al. 2015; Pretorius et al. 2017c; Pretorius et al. 2020) (Pretorius et al. 2022; Soma and Pretorius 2015) |
| Many reviews (also those with T2D are more susceptible to infections; this direction is not discussed here). | (B 2022; Nobs et al. 2023; Sohail et al. 2022) | ||
| Increased diabetes prevalence following COVID-19 infection | (Naveed et al. 2023) | ||
| Multiple sclerosis | Now recognised as being caused by Epstein-Barr virus | (Rousseau and Bhaduri-McIntosh 2023; Schönrich et al. 2022; Wekerle 2022) | Not yet studied |
| Myalgic encephalitis/ chronic fatigue syndrome (ME/CFS) | Clear infectious origin, likely viral and most likely a herpes virus | (Nunes et al. 2023) | (Nunes et al. 2023; Nunes et al. 2022) |
| Parkinson’s | Induction of disease progression by bacterial LPS and by viruses | (Cannon and Gruenheid 2022; Leta et al. 2022) | (de Waal et al. 2018) |
| Reviews | (Caggiu et al. 2019; Limphaibool et al. 2019) | ||
| Rheumatoid arthritis | Absolutely clear evidence for Proteus spp. as the infectious agent | (Ebringer 2012; Ebringer and Rashid 2014, 2009; Ebringer et al. 2010) | (Bezuidenhout et al. 2020; Kell and Pretorius 2022; Pretorius et al. 2017a; Pretorius et al. 2012) |
| Sleep Apnoea | Obstructive Sleep Apnoea is a strong risk factor or comorbidity of AF, also associated with obesity (Al-Falahi et al. 2017; Goudis and Ketikoglou 2017; Staerk et al. 2017; Zhang et al. 2021b) and both acute (Miller and Cappuccio 2021) and Long COVID (Mandel et al. 2023) | (Chiner et al. 2016; Hariyanto and Kurniawan 2021; Su et al. 2014) | Not yet studied |
| Disease | Comments | Selected references |
|---|---|---|
| Alzheimer’s | AF is of course related to age, as is AD. Stroke is also related to vascular dementia. Strong comorbidities between cardiovascular disease and AD, | (Ihara and Washida 2018)(Benenati et al. 2021) |
| Some indications that AF is associated with exacerbation of the onset of AD and related dementias, but not causally | (Falsetti et al. 2018; Manemann et al. 2023; Nakase et al. 2023; Pan et al. 2020; Papanastasiou et al. 2021; Proietti et al. 2020) | |
| Diabetes, type 2 | Very strong association of AF with diabetic complications, and of diabetes increasing the risk of AF | (Alijla et al. 2021; Bohne et al. 2019; Choi et al. 2023; Ding et al. 2022a; Domek et al. 2020; Kwon et al. 2023; Lorenzo-Almorós et al. 2023; Wang et al. 2019; Xiong et al. 2018) |
| Parkinson’s | Some evidence of an association of AF with early PD, much less so if PD diagnosed later (i.e. evidence either way there is relatively weak) | (Alves et al. 2021; Cereja et al. 2023; Han et al. 2021; Hong et al. 2019) |
| Rheumatoid arthritis | Small, significant association,but confounded with use of small molecule drugsAlso associated with greater risk of cardiovascular disease | (Rong et al. 2023)(Kim et al. 2023)(Kerola et al. 2021; Semb et al. 2022) |
| Biochemical marker | Comments | Selected references |
|---|---|---|
| Ferritin | Serum ferritin is a marker of cell death (Kell and Pretorius 2014), whose accompanying release of free iron can cause microclots and may itself be induced by them or other traumas. It is significantly raised in AF | (Altieri et al. 2022; Mikkelsen et al. 2019) |
| Fibrinogen | Fibrinogen (including g’ (Farrell 2012)) fibrinogen levels are commonly raised in inflammatory diseases (Luyendyk et al. 2019). Fibrinogen levels are higher in individuals with AF (and in those having a higher CHA2DS2-VASc score and likelihood of stroke), consistent with a role of microclots in the onset of AF | (Bao et al. 2023; Di Lecce et al. 2003; Li-Saw-Hee et al. 2001; Lip et al. 1996b, 1995; Mukamal et al. 2006; Rafaqat et al. 2022; Semczuk-Kaczmarek et al. 2019; Tilly et al. 2023; Weymann et al. 2017) |
| Fibrin clot properties also relate to stroke likelihood/severity in AF, though no amyloid measurements were yet made | (Drabik et al. 2020; Drabik et al. 2017) | |
| Inflammation | Occurs (by definition) in all kinds of chronic, inflammatory disease (Kell and Pretorius 2018a), but is certainly associated with AF. An accompaniment to all syndromes involving microclots. | (Korantzopoulos et al. 2018; Paquissi 2016; Rafaqat et al. 2022) |
| Plasminogen Activator Inhibitor-1 (PAI-1) | Significantly raised in AF, potentially reducing the rate at which fibrinaloid microclots might be removed | (Li et al. 2021; Marín et al. 1999 ; Pretorius et al. 2007 ) |
| Platelet Factor-4 and platelet activation | Platelet activation is another key feature of chronic, inflammatory diseases accompanied by microclots | (Drabik et al. 2015; Sohara et al. 1997; Yamauchi et al. 1986) |
| b-Thromboglobulin | Raised in AF | (Kamath et al. 2002; Lip et al. 1996a; Yamauchi et al. 1986 ) |
| Troponin (cardiac isoforms) | Probably more a metric of severity of cardiac events, but as a measure of cell death (like ferritin) may have predictive value | (Bai et al. 2018; Cortés et al. 2022; Costabel et al. 2017; Kaura et al. 2020; Lucrecia Maria et al. 2020; Sepehri Shamloo et al. 2021) |
| Von Willebrand Factor | Note that it is unlikely to be a simple function, as too much or too little can be bad (Grobler et al. 2020) | (Freestone et al. 2008b; Weymann et al. 2017; Zhong et al. 2018) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





