Submitted:
25 January 2024
Posted:
29 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
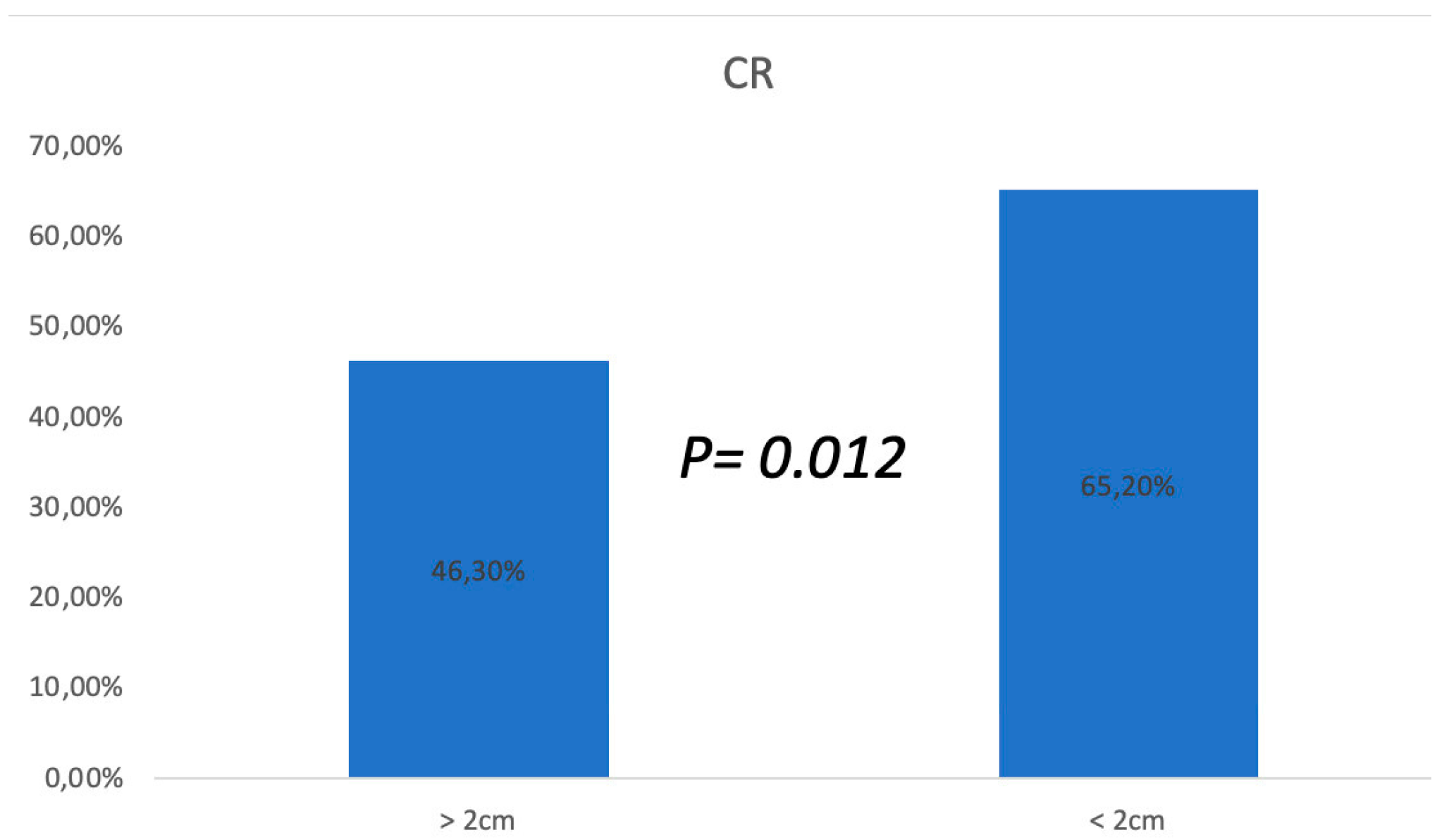
3.1. ECT Treatments
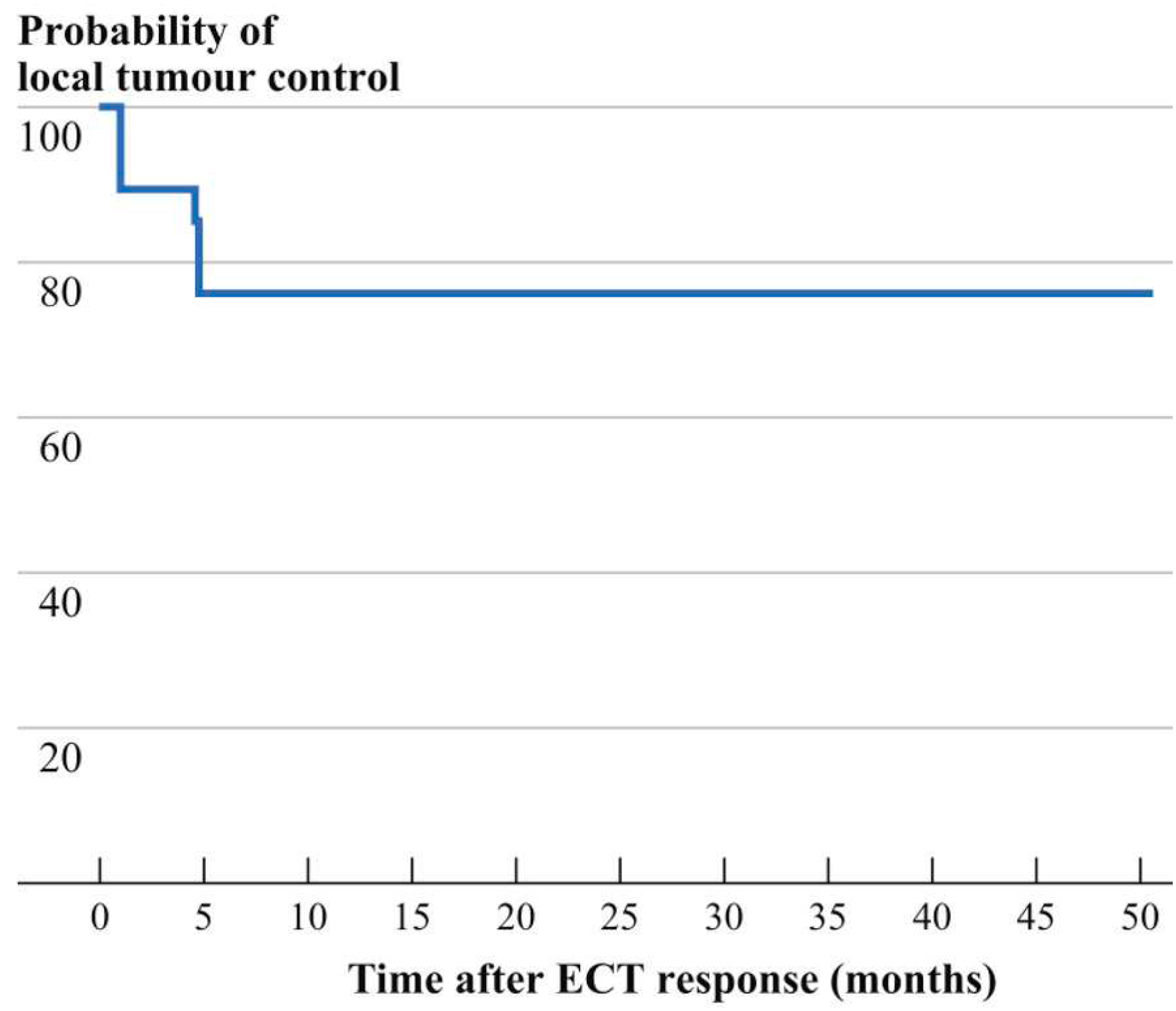
3.2. Follow-up
3.3. Side Effects and Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Etemad SA, Dewan AK. Kaposi Sarcoma Updates. Dermatol Clin. 2019, 37, 505–517. [Google Scholar] [CrossRef]
- Brambilla L, Genovese G, Berti E, et al. Diagnosis and treatment of classic and iatrogenic Kaposi’s sarcoma: Italian recommendations. Ital J Dermatol Venerol. 2021, 156, 356–365. [Google Scholar] [CrossRef] [PubMed]
- COX FH, HELWIG EB. Kaposi’s sarcoma. Cancer. 1959, 12, 289–298. [Google Scholar] [CrossRef]
- Dupin, N. Update on oncogenesis and therapy for Kaposi sarcoma. Curr Opin Oncol. 2020, 32, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Chak LY, Gill PS, Levine AM, et al. Radiation therapy for AIDS-related Kaposi’s sarcoma. J Clin Oncol 1998, 5, 863–867. [Google Scholar]
- Toschi E, Sgadari C, Monini P, et al. Treatment of Kaposi sar- coma-an update. Anticancer Drugs. 2002, 13, 977–87. [Google Scholar] [CrossRef]
- Campana LG, Testori A, Curatolo P, et al. Treatment efficacy with electrochemotherapy: A multi-institutional prospective observational study on 376 patients with superficial tumors. Eur J Surg Oncol. 2016, 42, 1914–1923. [Google Scholar] [CrossRef] [PubMed]
- Curatolo P, Quaglino P, Marenco F, et al. Electrochemotherapy in the treatment of Kaposi sarcoma cutaneous lesions: a two-center prospective phase II trial. Ann Surg Oncol. 2012, 19, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Testori A, Tosti G, Martinoli C, et al. Electrochemotherapy for cutaneous and subcutaneous tumor lesions: a novel therapeutic approach. Dermatol Ther 2010, 23, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Garbay JR, Billard V, Bernat C, Mir LM, Morsli N, Robert C. Successful repetitive treatments by electrochemotherapy of multiple unresectable Kaposi sarcoma nodules. Eur J Cancer 2006, 4 (Suppl. 4), 29–31. [Google Scholar] [CrossRef]
- Latini A, Bonadies A, Trento E, et al. Effective treatment of Kaposi’s sarcoma by electrochemotherapy and intravenous bleomycin administration. Dermatol Ther. 2012, 25, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Giardino R, Fini M, Bonazzi V, Cadossi R, Nicolini A, Carpi A. Electrochemotherapy a novel approach to the treatment of metastatic nodules on the skin and subcutaneous tissues. Biomed Pharmacother. 2006, 60, 458–462. [Google Scholar] [CrossRef]
- Mir LM, Gehl J, Sersa G, et al. Standard operating procedures of the electrochemotherapy: instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the CliniporatorTM by means of invasive or non-invasive electrodes. Eur J Cancer Suppl. 2006, 4, 14–25. [Google Scholar] [CrossRef]
- Solari N, Spagnolo F, Ponte E, et al. Electrochemotherapy for the management of cutaneous and subcutaneous metastasis: a series of 39 patients treated with palliative intent. J Surg Oncol. 2014, 109, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Miklavcic D, Snoj M, Zupanic A, et al. Towards treatment planning and treatment of deep-seated solid tumors by electrochemotherapy. Biomed Eng Online. 2010, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Gehl, J. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand. 2003, 177, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Chachowa A, Kriegel R, Lafleur F,Ostreicher R, Speer M, Lauben- stein L, Wernz G, Rubenstein P, Zang E, Friedman-Kien A. Prognostic factors and staging classification of patients with epidemic Kaposi’s sarcoma. J Clin Oncol 1989, 7, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Brambilla L, Boneschi V, Taglioni M, Ferrucci S. Staging of classic Kaposi’s sarcoma: a useful tool for therapeutic choices. Eur J Dermatol. 2003, 13, 83–86. [Google Scholar]
- Marty MSG, Garbay JR, Gehl J, et al. Electrochemotherapy an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur J Cancer Suppl 2006, 4, 3–13. [Google Scholar] [CrossRef]
- DeVita, V.T. et al, Cancer: Principles and Practice of Oncology, 6th Ed. Bonadonna. G. et al, Medicina Oncologica, Settima Edizione.
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Pincus T, Bergman M, Sokka T, Roth J, Swearingen C, Yazici Y. Visual analog scales in formats other than a 10 centimeter horizontal line to assess pain and other clinical data. J Rheumatol. 2008, 35, 1550–1558. [Google Scholar]
- Sullivan RJ, Pantanowitz L. New drug targets in Kaposi sarcoma. Expert Opin Ther Targets. 2010, 14, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Brambilla L, Miedico A, Ferrucci S, et al. Combination of vinblastine and bleomycin as first line therapy in advanced classic Kaposi’s sarcoma. J Eur Acad Dermatol Venereol. 2006, 20, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013, 137, 289–294. [Google Scholar] [CrossRef]
- Krigel RL, Laubenstein LJ, Muggia FM. Kaposi’s sarcoma: a new staging classification. Cancer Treat Rep. 1983, 67, 531–534. [Google Scholar]
- Fabrizio T, Cagiano L, De Terlizzi F, Grieco MP. Neoadjuvant treatment by ECT in cutaneous malignant neoplastic lesions. J Plast Reconstr Aesthet Surg. 2020, 73, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Mali B, Jarm T, Snoj M, Sersa G, Miklavcic D. Antitumor effectiveness of electrochemotherapy: a systematic review and meta-analysis. Eur J Surg Oncol. 2013, 39, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Di Monta G, Caracò C, Benedetto L, et al. Electrochemotherapy as “new standard of care” treatment for cutaneous Kaposi’s sarcoma. Eur J Surg Oncol. 2014, 40, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Heller R, Jaroszeski MJ, Reintgen DS, et al. Treatment of cutaneous and subcutaneous tumors with electrochemotherapy using intralesional bleomycin. Cancer 1998, 83, 148–57. [Google Scholar] [CrossRef]
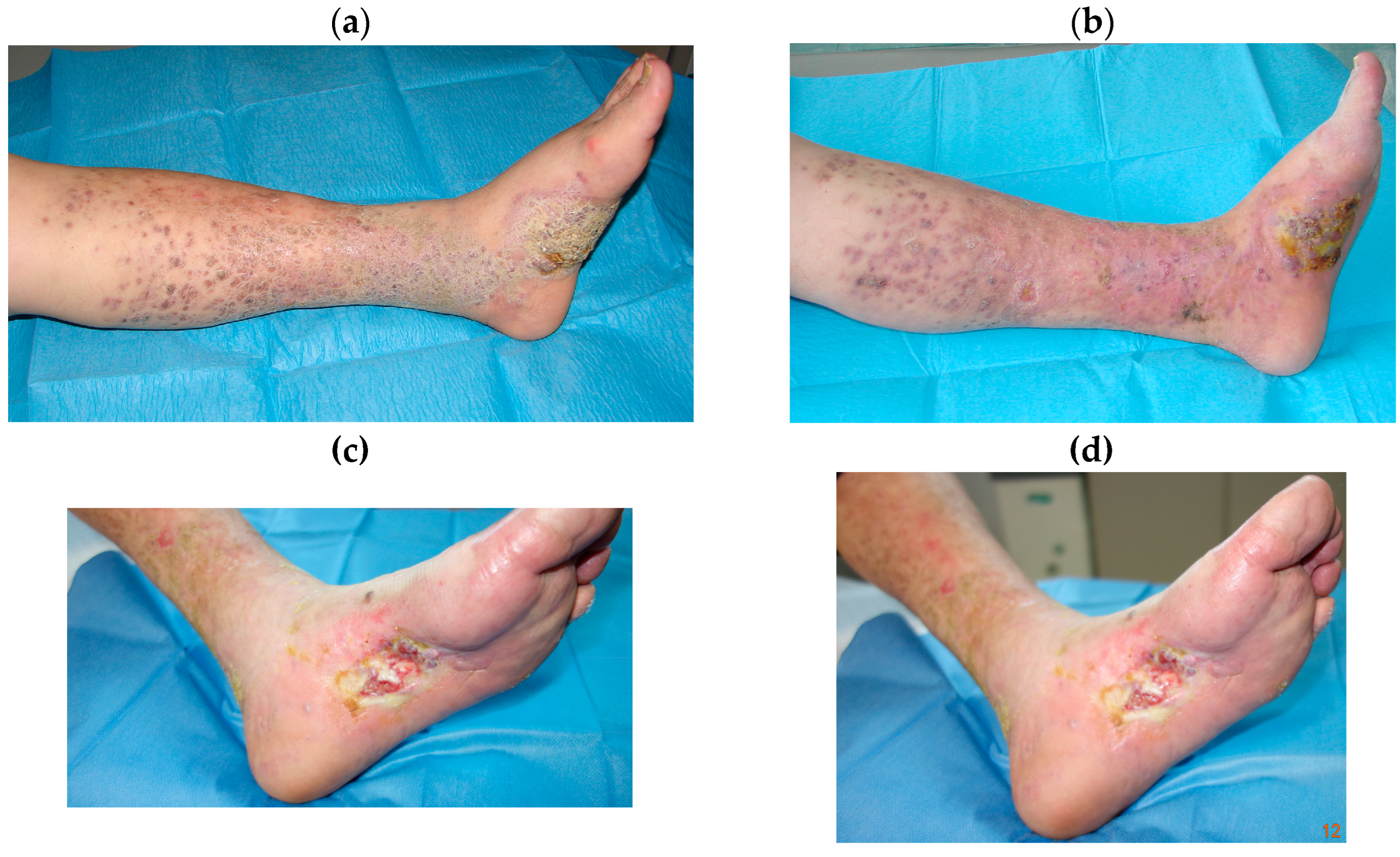
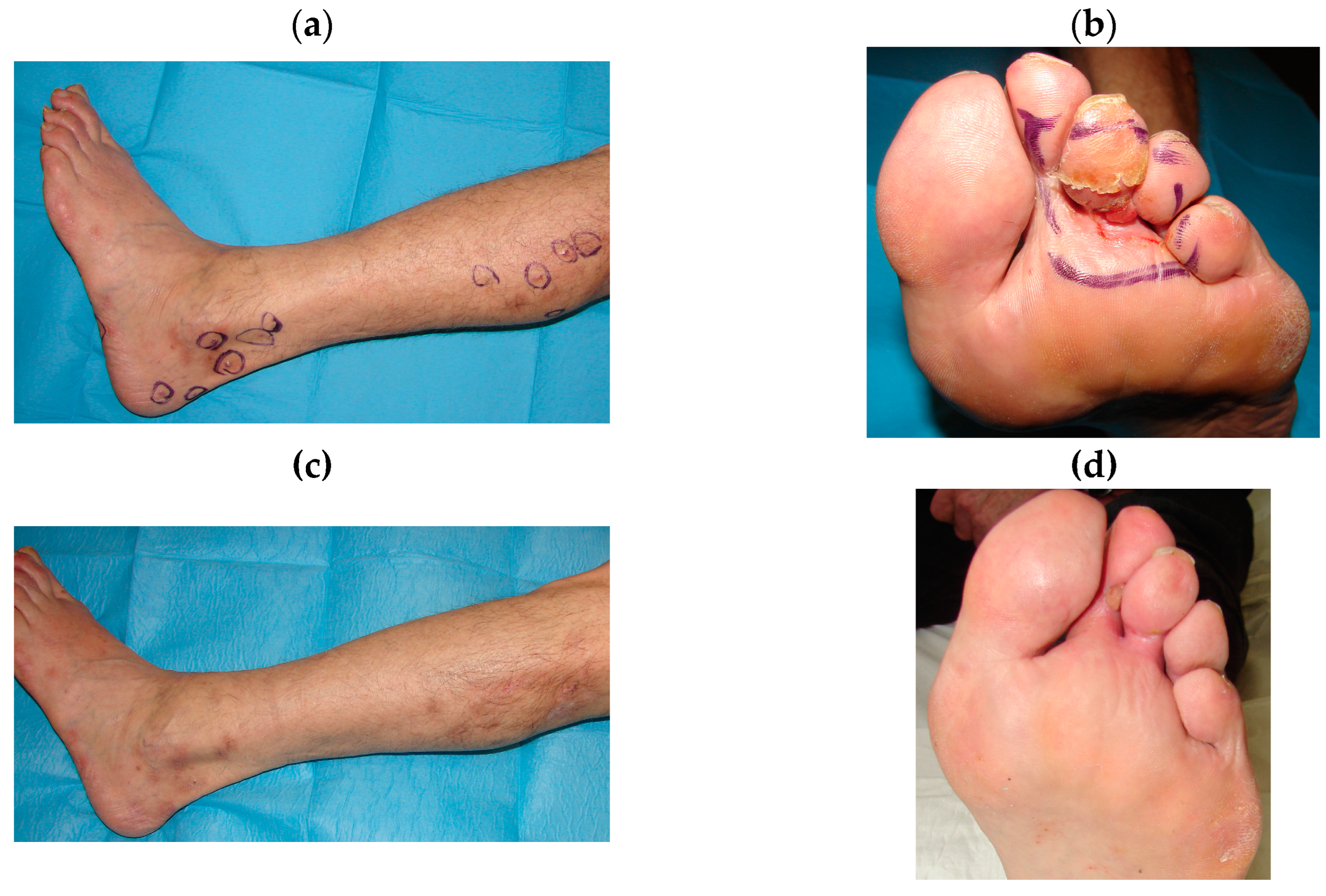
| Patients n° | Sex, Age | Localization | Clinical Response | Response | Stage |
|---|---|---|---|---|---|
| 1 | F, 85 | Right foot | Present | CR | I |
| 2 | F, 74 | Lower limb | Present | CR at second ECT | I |
| 3 | F, 63 | Lower limbs bilateral Foot | Present | PR after third ECT | II |
| 4 | F, 55 | Foot | Present | CR | I |
| 5 | F,70 | Lower limbs bilateral Foot | Present | CR | I |
| 6 | M, 72 | Foot | Present | CR at second ECT | II |
| 7 | M,68 | Foot | Present | CR | I |
| 8 | M,60 | Foot | Present | CR | I |
| 9 | M,80 | Lower limbs | Present | CR | I |
| 10 | M, 75 | Right foot | Present | CR | I |
| 11 | M, 71 | Bilateral foot | Present | PR after third ECT | II |
| 12 | M, 78 | Lower limbs | Present | CR | I |
| 13 | M, 77 | Lower limbs | Present | CR at second ECT | I |
| 14 | M, 68 | Foot | Present | CR | I |
| 15 | M, 70 | Lower limbs | Present | PR after third ECT | II |
| 16 | M, 76 | Left limb | Present | CR | I |
| 17 | M,74 | Right limb | Present | CR | I |
| 18 | M, 84 | Foot | Present | CR at second ECT | I |
| 19 | M, 69 | Bilateral foot | Present | PR after third ECT | II |
| 20 | M, 71 | Excluded for comorbidities. |
| Stage | Prevalent cutaneous lesions | Progression | Visceral involvement |
|---|---|---|---|
| I macular-nodular | macules and/or nodules predominantly on the lower limbs | A, slow B, rapid | ±V |
| II Infiltrative | Plaques predominantly on the lower limbs | A, slow B, rapid | ±V |
| III florid | exuberant angiomatous nodules predominantly on the lower limbs | A, slow B, rapid | ±V |
| IV Disseminate | angiomatous lesions on the head, trunk, and mucosae | A, slow B, rapid | ±V |
| Standard | Personalized |
|---|---|
| Blood tests: full blood count, kidney and liver function, iron, protein electrophoresis, lymphocyte subpopulation analysis, HIV test, anti-HHV8 antibodies and HHV8 Dna | |
| Fecal occult blood test on three samples, if positive ◊ | Colonoscopy |
| Esophagogastroduodenoscopy (EGDS) | Esophagogastroduodenoscopy (EGDS) |
| Abdomen and lymph nodes ultrasound | Total body computerized tomography |
| Ears, nose, and throat (ent) visit |
| Staging | Standard | Personalized |
|---|---|---|
| I | Clinical observation Intralesional vincristine Curettage | Elastocompression for edema or bullous Kaposi’s sarcoma Silver nitrate for soft, fungating and/or oozing lesions |
| II | Elastocompression | Radiotherapy for florid and localized lesions |
| III | Systemic chemotherapy* | Elastocompression for edema or bullous Kaposi’s sarcoma |
| IV | Systemic chemotherapy* | Elastocompression for edema or bullous Kaposi’s sarcoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




