Submitted:
25 January 2024
Posted:
26 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell cultures
2.3. Transendothelial electrical resistance measurement
2.4. Western Blot
2.5. Transendothelial Migration
2.5.1. Basic Experiment
2.5.2. Physiological stimulation experiment
2.6. Analysis and Statistics
3. Results
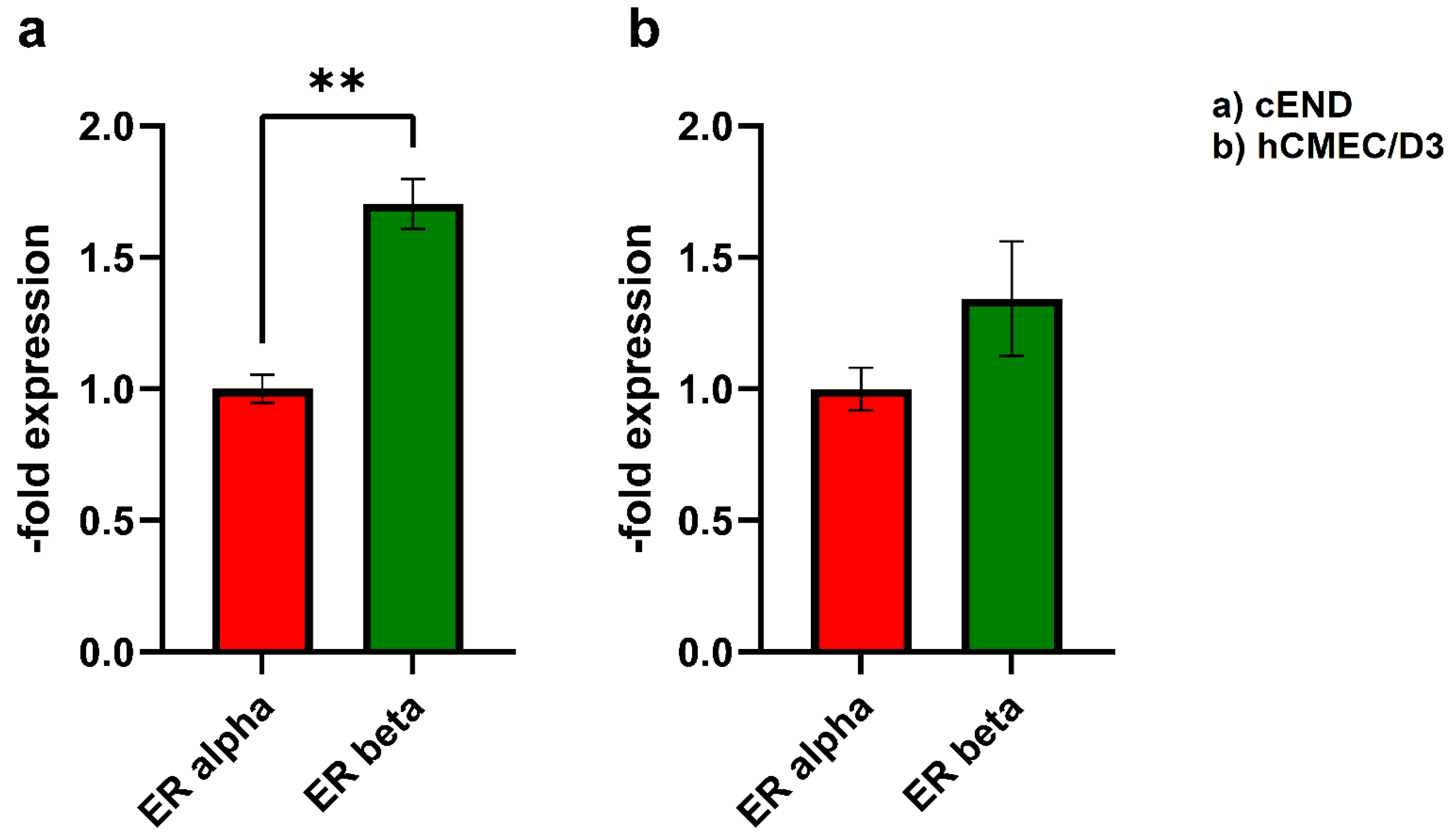
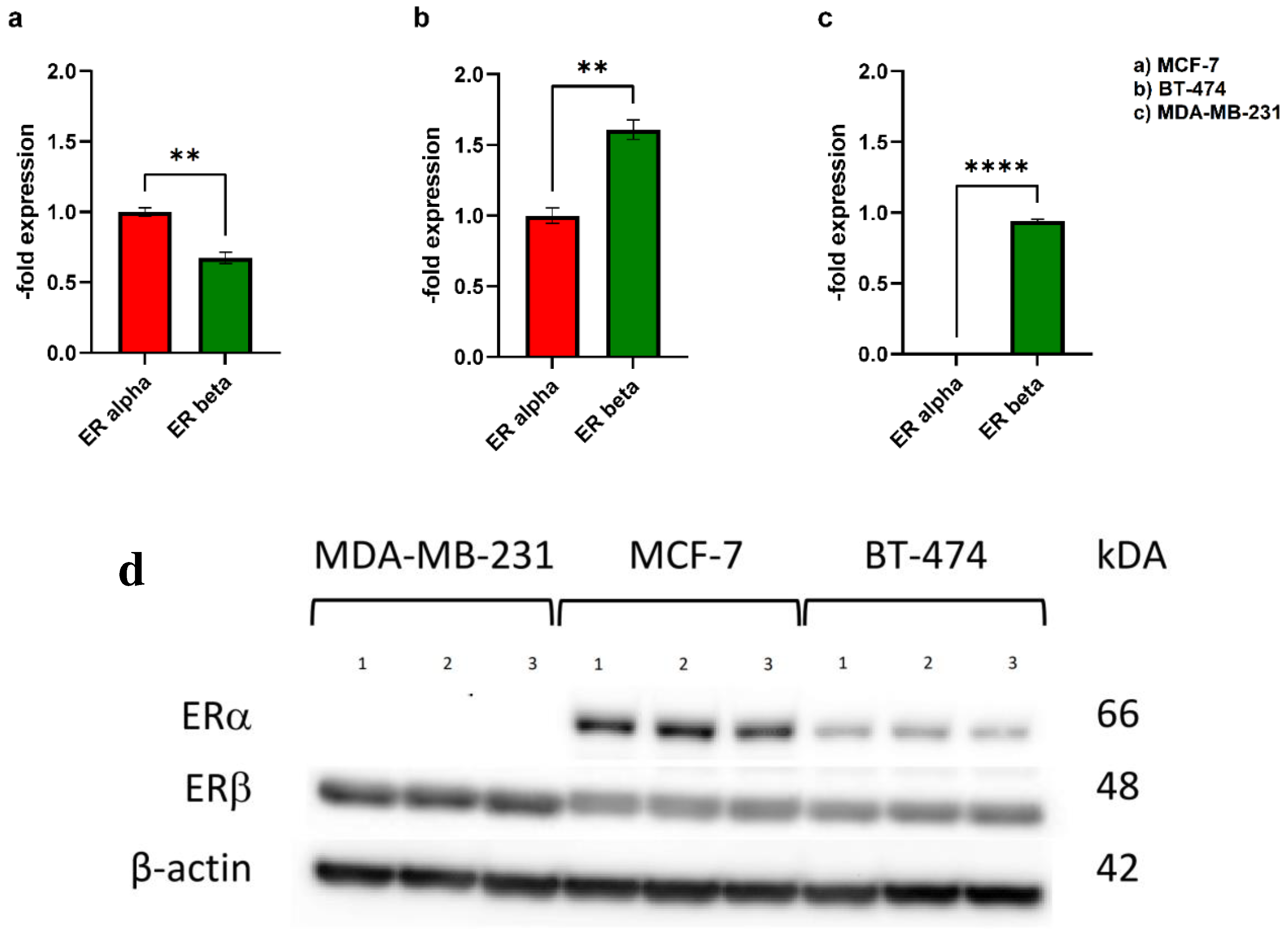
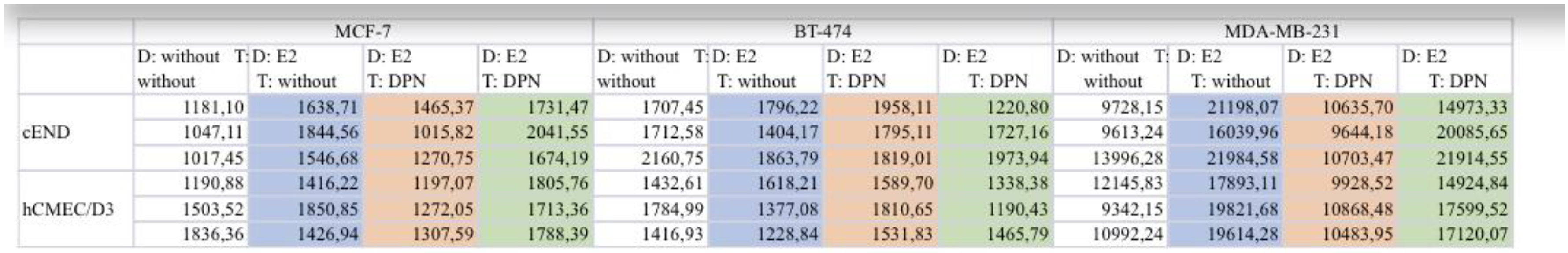
3.1. Western Blot
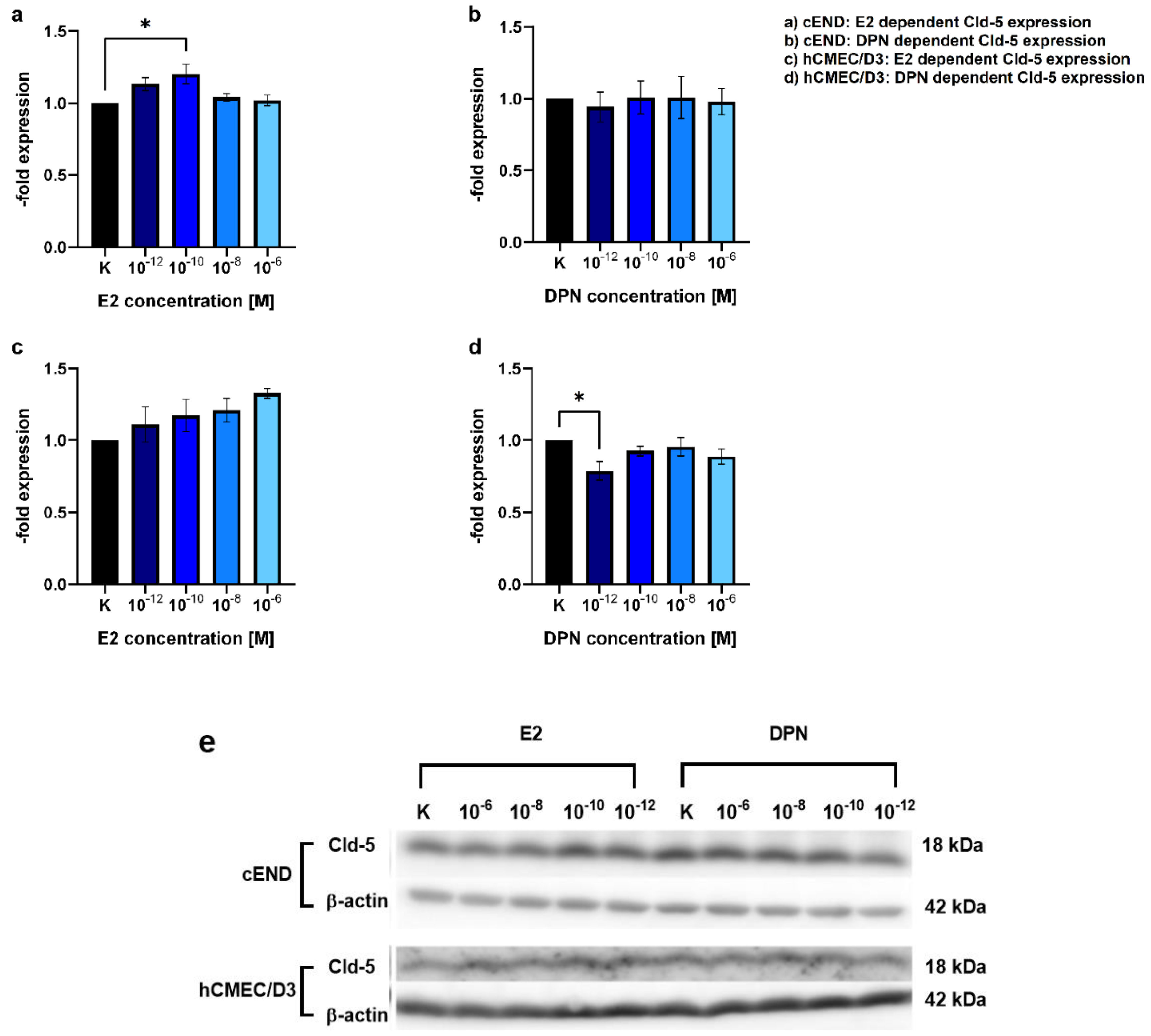
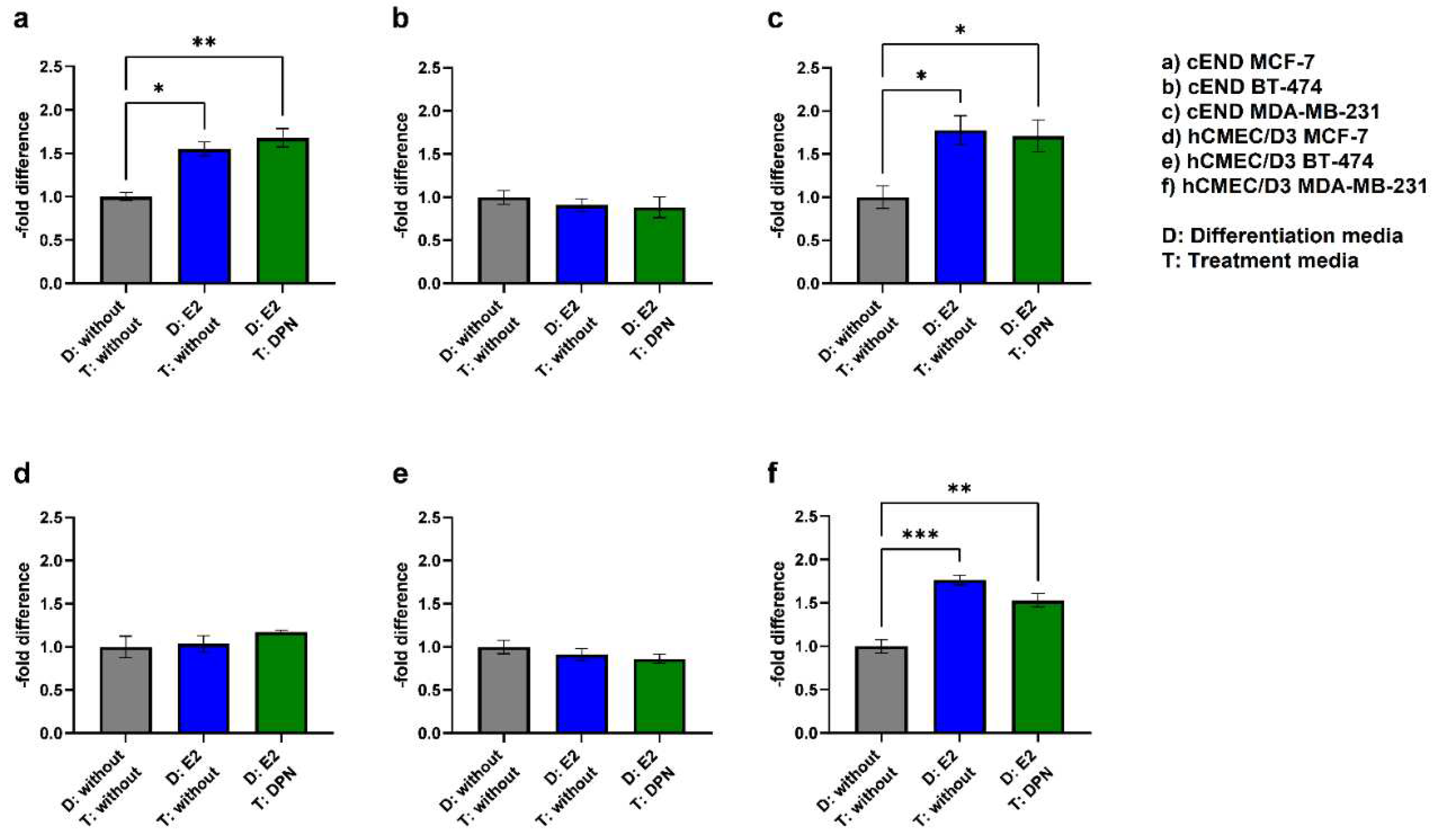
3.2. Transendothelial migration
3.2.1. Basic experiment
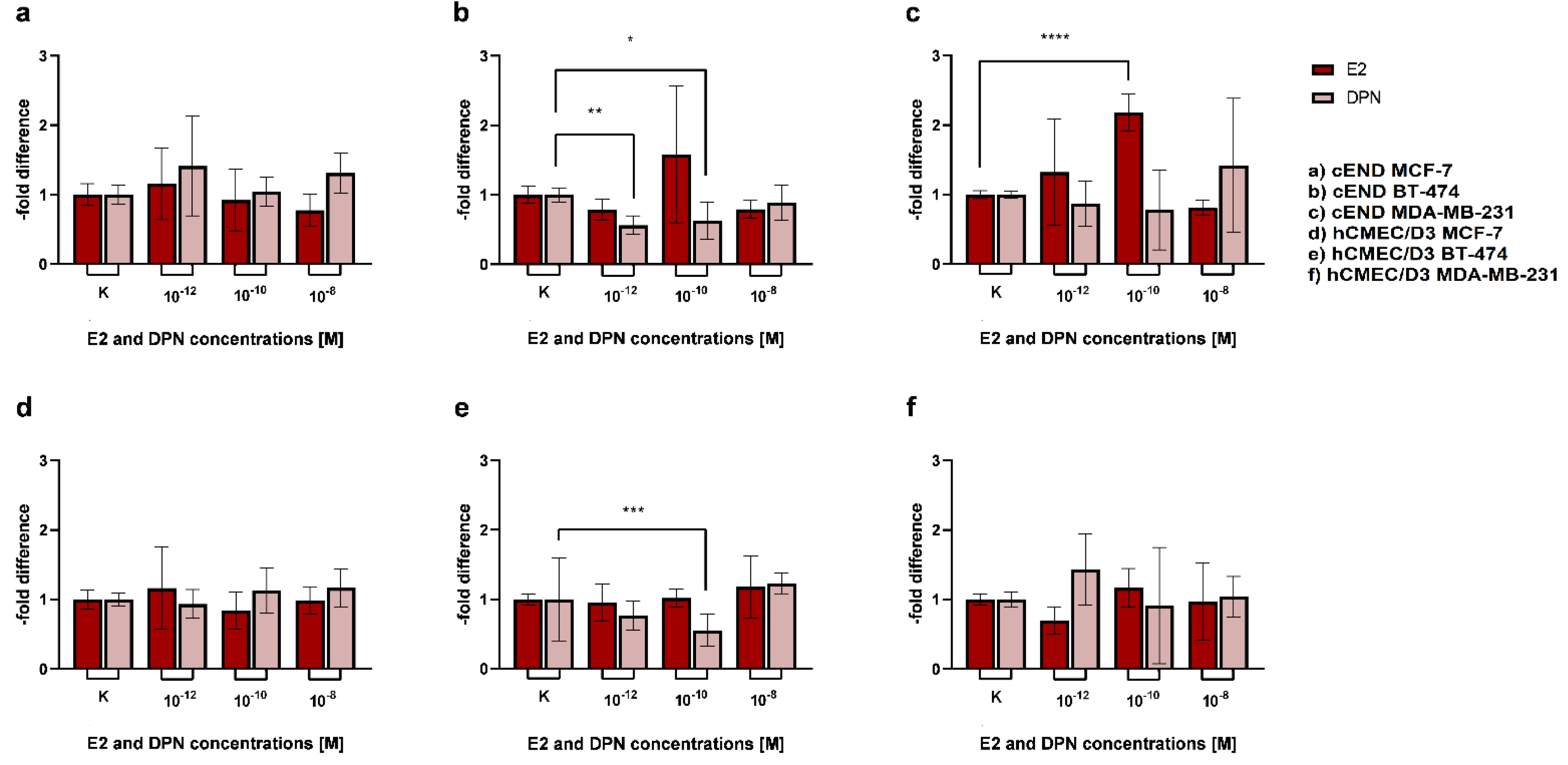
3.2.2. Physiological stimulation experiment
4. Discussion
4.1. and 4.2. Effect of ERligands on TEER in cEND and hCMEC/D3 cells
4.3. Transmigration experiment – effect of different ER agonist administrations
4.3.1. Basic experiment
4.3.2. Physiological stimulation experiment
4.1. ER expression status
4.2. Modulation of TJ protein claudin-5 by ER agonists
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pedrosa, R.M.S.M.; Mustafa, D.A.; Soffietti, R.; Kros, J.M. Breast cancer brain metastasis: molecular mechanisms and directions for treatment. Neuro-Oncology 2018, 20, 1439–1449. [Google Scholar] [CrossRef]
- Weidle, U.H.; Birzele, F.; Kollmorgen, G.; Rüger, R. Dissection of the Process of Brain Metastasis Reveals Targets and Mechanisms for Molecular-based Intervention. Cancer Genomics Proteomics 2016, 13, 245–58. [Google Scholar]
- Helms, H.C.; Abbott, N.J.; Burek, M.; Cecchelli, R.; Couraud, P.-O.; Deli, M.A.; Förster, C.; Galla, H.J.; Romero, I.A.; Shusta, E.V.; et al. In vitro models of the blood–brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J. Cereb. Blood Flow Metab. 2016, 36, 862–890. [Google Scholar] [CrossRef]
- Wilhelm, I.; Molnár, J.; Fazakas, C.; Haskó, J.; Krizbai, I.A. Role of the Blood-Brain Barrier in the Formation of Brain Metastases. Int. J. Mol. Sci. 2013, 14, 1383–1411. [Google Scholar] [CrossRef]
- Förster, C. Tight junctions and the modulation of barrier function in disease. Histochem. Cell Biol. 2008, 130, 55–70. [Google Scholar] [CrossRef]
- Salvador, E.; Burek, M.; Förster, C.Y. Tight Junctions and the Tumor Microenvironment. Curr. Pathobiol. Rep. 2016, 4, 135–145. [Google Scholar] [CrossRef]
- Hosonaga, M.; Saya, H.; Arima, Y. Molecular and cellular mechanisms underlying brain metastasis of breast cancer. Cancer Metastasis Rev. 2020, 39, 711–720. [Google Scholar] [CrossRef]
- Izraely, S.; Witz, I.P. Site-specific metastasis: A cooperation between cancer cells and the metastatic microenvironment. Int. J. Cancer 2021, 148, 1308–1322. [Google Scholar] [CrossRef]
- Warner, M.; Huang, B.; Gustafsson, J.-A. Estrogen Receptor β as a Pharmaceutical Target. Trends Pharmacol. Sci. 2017, 38, 92–99. [Google Scholar] [CrossRef]
- Sandoval, K.E.; Witt, K.A. Age and 17β-estradiol effects on blood–brain barrier tight junction and estrogen receptor proteins in ovariectomized rats. Microvasc. Res. 2011, 81, 198–205. [Google Scholar] [CrossRef]
- C. Förster, S. Mäkela, A. Wärri, S. Kietz, D. Becker, K. Hultenby, M. Warner, J.A. Gustafsson, Involvement of estrogen receptor beta in terminal differentiation of mammary gland epithelium. Proc Natl Acad Sci U S A 2002, 99, 15578–15583. [CrossRef]
- Heldring, N.; Pike, A.; Andersson, S.; Matthews, J.; Cheng, G.; Hartman, J.; Tujague, M.; Ström, A.; Treuter, E.; Warner, M.; et al. Estrogen Receptors: How Do They Signal and What Are Their Targets. Physiol. Rev. 2007, 87, 905–931. [Google Scholar] [CrossRef]
- S.-Y. Wu, S. Sharma, K. Wu, A. Tyagi, D. Zhao, R.P. Deshpande, K. Watabe, Tamoxifen suppresses brain metastasis of estrogen receptor-deficient breast cancer by skewing microglia polarization and enhancing their immune functions. Breast Cancer Research 2021, 23, 35. [CrossRef] [PubMed]
- Mishra, A.; Srivastava, A.; Pateriya, A.; Tomar, M.S.; Mishra, A.K.; Shrivastava, A. Metabolic reprograming confers tamoxifen resistance in breast cancer. Chem. Interactions 2021, 347, 109602. [Google Scholar] [CrossRef] [PubMed]
- Burek, M.; Arias-Loza, P.A.; Roewer, N.; Förster, C.Y.; S, C.; J, S.; A, B.; K, K.; D, T.; J, A.; et al. Claudin-5 as a Novel Estrogen Target in Vascular Endothelium. Arter. Thromb. Vasc. Biol. 2010, 30, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Burek, M.; Steinberg, K.; Förster, C.Y. Mechanisms of transcriptional activation of the mouse claudin-5 promoter by estrogen receptor alpha and beta. Mol. Cell. Endocrinol. 2014, 392, 144–151. [Google Scholar] [CrossRef]
- Weksler, B.B.; Subileau, E.A.; Perrière, N.; Charneau, P.; Holloway, K.; Leveque, M.; Tricoire-Leignel, H.; Nicotra, A.; Bourdoulous, S.; Turowski, P.; et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005, 19, 1872–1874. [Google Scholar] [CrossRef]
- Weksler, B.; A Romero, I.; Couraud, P.-O. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 2013, 10, 16–10. [Google Scholar] [CrossRef]
- Soule, H.D.; Vazquez, J.; Long, A.; Albert, S.; Brennan, M. A Human Cell Line From a Pleural Effusion Derived From a Breast Carcinoma 2. JNCI J. Natl. Cancer Inst. 1973, 51, 1409–1416. [Google Scholar] [CrossRef]
- Cailleau, R.; Young, R.; Olivé, M.; Reeves, W.J., Jr. Breast Tumor Cell Lines From Pleural Effusions2. J. Natl. Cancer Inst. 1974, 53, 661–674. [Google Scholar] [CrossRef]
- R.R. R, P.K. K, O.G. V, V.U. E, V.F. V, I.K. A, S.S. D, E.C. G, Low-Temperature In-Induced Holes Formation in Native-SiOx/Si(111) Substrates for Self-Catalyzed MBE Growth of GaAs Nanowires. Materials (Basel) 2020, 13.
- Tarasov, S.A.; Gorbunov, E.A.; Don, E.S.; Emelyanova, A.G.; Kovalchuk, A.L.; Yanamala, N.; Schleker, A.S.S.; Klein-Seetharaman, J.; Groenestein, R.; Tafani, J.-P.; et al. Insights into the Mechanism of Action of Highly Diluted Biologics. J. Immunol. 2020, 205, 1345–1354. [Google Scholar] [CrossRef]
- Contreras-Zárate, M.J.; Cittelly, D.M. Sex steroid hormone function in the brain niche: Implications for brain metastatic colonization and progression. Cancer Rep. 2022, 5, e1241. [Google Scholar] [CrossRef]
- Chambliss, K.L.; Yuhanna, I.S.; Mineo, C.; Liu, P.; German, Z.; Sherman, T.S.; Mendelsohn, M.E.; Anderson, R.G.W.; Shaul, P. Estrogen Receptor α and Endothelial Nitric Oxide Synthase Are Organized Into a Functional Signaling Module in Caveolae. Circ. Res. 2000, 87, e44–e52. [Google Scholar] [CrossRef]
- Haynes, M.P.; Li, L.; Sinha, D.; Russell, K.S.; Hisamoto, K.; Baron, R.; Collinge, M.; Sessa, W.C.; Bender, J.R. Src Kinase Mediates Phosphatidylinositol 3-Kinase/Akt-dependent Rapid Endothelial Nitric-oxide Synthase Activation by Estrogen. J. Biol. Chem. 2003, 278, 2118–2123. [Google Scholar] [CrossRef]
- Segura-Bautista, D.; Olivares, A.; Bonilla, E.; Salazar, Z.; Casas-González, P.; Pérez-Solis, M.A. GPR30 expression and function in breast cancer cells are induced through a cis-acting element targeted by ETS factors. Oncol. Rep. 2020, 43, 1669–1682. [Google Scholar] [CrossRef]
- Al-Bader, M.; Ford, C.; Al-Ayadhy, B.; Francis, I. Analysis of estrogen receptor isoforms and variants in breast cancer cell lines. Exp. Ther. Med. 2011, 2, 537–544. [Google Scholar] [CrossRef]
- M.M. Liu, C. Albanese, C.M. Anderson, K. Hilty, P. Webb, R.M. Uht, R.H. Price, Jr., R.G. Pestell, P.J. Kushner, Opposing action of estrogen receptors alpha and beta on cyclin D1 gene expression. J Biol Chem 2002, 277, 24353–24360.
- Khaitan, D.; Sankpal, U.T.; Weksler, B.; Meister, E.A.; Romero, I.A.; Couraud, P.-O.; Ningaraj, N.S. Role of KCNMA1gene in breast cancer invasion and metastasis to brain. BMC Cancer 2009, 9, 258. [Google Scholar] [CrossRef]
- R. Harati, M.G. Mohammad, A. Tlili, R.A. El-Awady, R. Hamoudi, Loss of miR-101-3p Promotes Transmigration of Metastatic Breast Cancer Cells through the Brain Endothelium by Inducing COX-2/MMP1 Signaling. Pharmaceuticals (Basel) 2020, 13.
- J. Molnár, C. Fazakas, J. Haskó, O. Sipos, K. Nagy, Á. Nyúl-Tóth, A.E. Farkas, A.G. Végh, G. Váró, P. Galajda, I.A. Krizbai, I. Wilhelm, Transmigration characteristics of breast cancer and melanoma cells through the brain endothelium: Role of Rac and PI3K. Cell Adhes. Migr. 2016, 10, 269–281. [CrossRef]
- Harati, R.; Hafezi, S.; Mabondzo, A.; Tlili, A. Silencing miR-202-3p increases MMP-1 and promotes a brain invasive phenotype in metastatic breast cancer cells. PLOS ONE 2020, 15, e0239292. [Google Scholar] [CrossRef]
- Tourovskaia, A.; Fauver, M.; Kramer, G.; Simonson, S.; Neumann, T. Tissue-engineered microenvironment systems for modeling human vasculature. Exp. Biol. Med. 2014, 239, 1264–1271. [Google Scholar] [CrossRef]
- Drolez, A.; Vandenhaute, E.; Julien, S.; Gosselet, F.; Burchell, J.; Cecchelli, R.; Delannoy, P.; Dehouck, M.-P.; Mysiorek, C. Selection of a Relevant In Vitro Blood-Brain Barrier Model to Investigate Pro-Metastatic Features of Human Breast Cancer Cell Lines. PLOS ONE 2016, 11, e0151155. [Google Scholar] [CrossRef]
- Fan, J.; Fu, B.M. Quantification of Malignant Breast Cancer Cell MDA-MB-231 Transmigration Across Brain and Lung Microvascular Endothelium. Ann. Biomed. Eng. 2016, 44, 2189–2201. [Google Scholar] [CrossRef]
- Torres, C.G.; Iturriaga, M.P.; Cruz, P. Hormonal Carcinogenesis in Canine Mammary Cancer: Molecular Mechanisms of Estradiol Involved in Malignant Progression. Animals 2021, 11, 608. [Google Scholar] [CrossRef]
- Chang, E.C.; Frasor, J.; Komm, B.; Katzenellenbogen, B.S. Impact of Estrogen Receptor β on Gene Networks Regulated by Estrogen Receptor α in Breast Cancer Cells. Endocrinology 2006, 147, 4831–4842. [Google Scholar] [CrossRef]
- Ström, J. Hartman, J.S. Foster, S. Kietz, J. Wimalasena, J.A. Gustafsson, Estrogen receptor beta inhibits 17beta-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc Natl Acad Sci U S A 2004, 101, 1566–1571. [CrossRef]
- J. Datta, N. Willingham, J.M. Manouchehri, P. Schnell, M. Sheth, J.J. David, M. Kassem, T.A. Wilson, H.S. Radomska, C.C. Coss, C.E. Bennett, R.K. Ganju, S.D. Sardesai, M. Lustberg, B. Ramaswamy, D.G. Stover, M.A. Cherian, Activity of Estrogen Receptor beta Agonists in Therapy-Resistant Estrogen Receptor-Positive Breast Cancer. Frontiers in oncology 2022, 12, 857590.
- Motylewska, E.; Stasikowska, O.; Mełeń-Mucha, G. The inhibitory effect of diarylpropionitrile, a selective agonist of estrogen receptor beta, on the growth of MC38 colon cancer line. Cancer Lett. 2009, 276, 68–73. [Google Scholar] [CrossRef]
- A.M. Hartz, A. Mahringer, D.S. Miller, B. Bauer, 17-beta-Estradiol: a powerful modulator of blood-brain barrier BCRP activity. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism 2010, 30, 1742–1755. [CrossRef] [PubMed]
- H.C. Helms, N.J. Abbott, M. Burek, R. Cecchelli, P.O. Couraud, M.A. Deli, C. Forster, H.J. Galla, I.A. Romero, E.V. Shusta, M.J. Stebbins, E. Vandenhaute, B. Weksler, B. Brodin, In vitro models of the blood-brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism 2016, 36, 862–890.
- Burek, M.; Arias-Loza, P.A.; Roewer, N.; Förster, C.Y.; S, C.; J, S.; A, B.; K, K.; D, T.; J, A.; et al. Claudin-5 as a Novel Estrogen Target in Vascular Endothelium. Arter. Thromb. Vasc. Biol. 2010, 30, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Burek, M.; Förster, C.Y. Cloning and characterization of the murine claudin-5 promoter. Mol. Cell. Endocrinol. 2009, 298, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Burek, M.; Steinberg, K.; Förster, C.Y. Mechanisms of transcriptional activation of the mouse claudin-5 promoter by estrogen receptor alpha and beta. Mol. Cell. Endocrinol. 2014, 392, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Fan, P.; Wang, M.; Zhang, C.; Jiang, Y.; Huang, S.; Fang, M.; He, Z.; Wu, A. Elevated estrogen receptor β expression in triple negative breast cancer cells is associated with sensitivity to doxorubicin by inhibiting the PI3K/AKT/mTOR signaling pathway. Exp. Ther. Med. 2020, 20, 1630–1636. [Google Scholar] [CrossRef]
- Blecharz, K.G.; Haghikia, A.; Stasiolek, M.; Kruse, N.; Drenckhahn, D.; Gold, R.; Roewer, N.; Chan, A.; Förster, C.Y. Glucocorticoid effects on endothelial barrier function in the murine brain endothelial cell line cEND incubated with sera from patients with multiple sclerosis. Mult. Scler. J. 2010, 16, 293–302. [Google Scholar] [CrossRef] [PubMed]
- C. Forster, M. Burek, I.A. Romero, B. Weksler, P.O. Couraud, D. Drenckhahn, Differential effects of hydrocortisone and TNFalpha on tight junction proteins in an in vitro model of the human blood-brain barrier. The Journal of physiology 2008, 586, 1937–1949. [CrossRef]
- C. Forster, C. Silwedel, N. Golenhofen, M. Burek, S. Kietz, J. Mankertz, D. Drenckhahn, Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. The Journal of physiology 2005, 565, 475–486. [CrossRef]
- Förster, C.; Waschke, J.; Burek, M.; Leers, J.; Drenckhahn, D. Glucocorticoid effects on mouse microvascular endothelial barrier permeability are brain specific. J. Physiol. 2006, 573, 413–425. [Google Scholar] [CrossRef]
- Gerhartl, A.; Hahn, K.; Neuhoff, A.; Friedl, H.-P.; Förster, C.Y.; Wunder, C.; Schick, M.; Burek, M.; Neuhaus, W. Hydroxyethylstarch (130/0.4) tightens the blood-brain barrier in vitro. Brain Res. 2020, 1727, 146560. [Google Scholar] [CrossRef]
- C. Hagemann, M. Löhr, A.F. Kessler, M. Burek, C. Förster, Using alternating electric fields to increase permeability of the blood brain barrier. Patentiert US16548,514; US16/548,537; PCT/IB2019/057091. (2016).
- Ittner, C.; Burek, M.; Störk, S.; Nagai, M.; Förster, C.Y. Increased Catecholamine Levels and Inflammatory Mediators Alter Barrier Properties of Brain Microvascular Endothelial Cells in vitro. Front. Cardiovasc. Med. 2020, 7, 73. [Google Scholar] [CrossRef]
- C. Kleinschnitz, K. Blecharz, T. Kahles, T. Schwarz, P. Kraft, K. Gobel, S.G. Meuth, M. Burek, T. Thum, G. Stoll, C. Forster, Glucocorticoid insensitivity at the hypoxic blood-brain barrier can be reversed by inhibition of the proteasome. Stroke 2011, 42, 1081–1089. [CrossRef] [PubMed]
- Neuhaus, W.; Burek, M.; Djuzenova, C.S.; Thal, S.C.; Koepsell, H.; Roewer, N.; Förster, C.Y. Addition of NMDA-receptor antagonist MK801 during oxygen/glucose deprivation moderately attenuates the upregulation of glucose uptake after subsequent reoxygenation in brain endothelial cells. Neurosci. Lett. 2012, 506, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Salvador, E.; Burek, M.; Förster, C.Y. Stretch and/or oxygen glucose deprivation (OGD) in an in vitro traumatic brain injury (TBI) model induces calcium alteration and inflammatory cascade. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Salvador, E.; Burek, M.; Löhr, M.; Nagai, M.; Hagemann, C.; Förster, C.Y. Senescence and associated blood–brain barrier alterations in vitro. Histochem. 2021, 156, 283–292. [Google Scholar] [CrossRef]
- Förster, C.; Kahles, T.; Kietz, S.; Drenckhahn, D. Dexamethasone induces the expression of metalloproteinase inhibitor TIMP-1 in the murine cerebral vascular endothelial cell line cEND. J. Physiol. 2007, 580, 937–949. [Google Scholar] [CrossRef] [PubMed]
- S. Shityakov, M. Smetak, C. Bär, N. Schlegel, T. Thum, M. Nagai, F. C.Y., Blood biomarkers in Takotsubo syndrome and their role in diagnosis and management. Frontiers Cardiovasc. Med. 2022, submitted.
- Thal, S.C.; Smetak, M.; Hayashi, K.; Förster, C.Y. Hemorrhagic Cerebral Insults and Secondary Takotsubo Syndrome: Findings in a Novel In Vitro Model Using Human Blood Samples. Int. J. Mol. Sci. 2022, 23, 11557. [Google Scholar] [CrossRef]



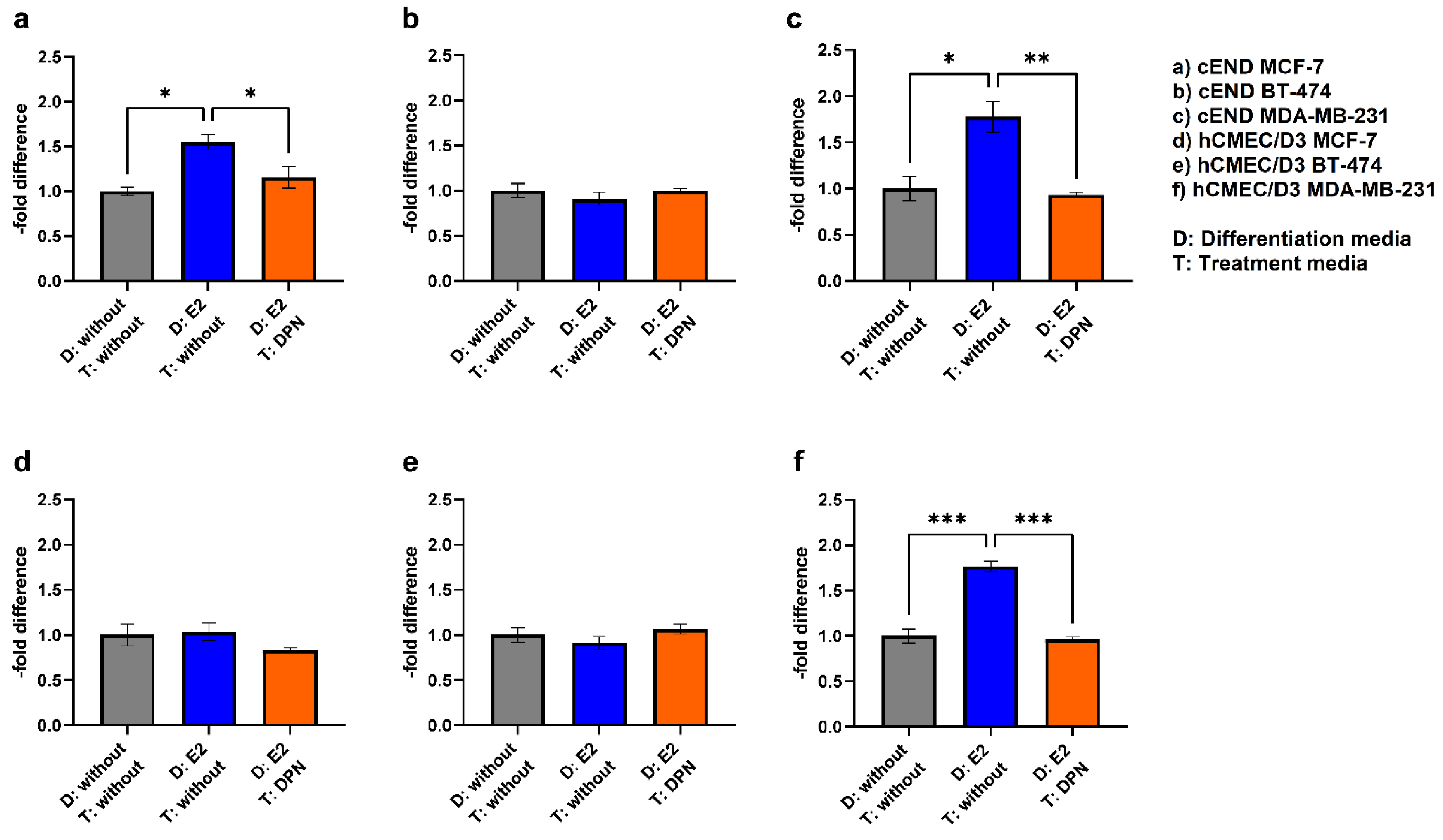
| Cell lines | E2 | DPN |
|---|---|---|
| cEND MCF-7 | ||
| cEND BT-474 | -- | |
| cEND MDA-MB-231 | ++++ | |
| hCMEC/D3 MCF-7 | --- | |
| hCMEC/D3 BT-474 | ||
| hCMEC/D3 MDA-MB-231 |
| Cell Line | D: E2 (C+E) T: without (2) |
D: E2 (E) T: DPN (C+E) (3) |
D: E2 (C+E) T: DPN (C+E) (4) |
|---|---|---|---|
| cEND MCF-7 | + | - | ++ |
| cEND BT-474 | |||
| cEND MDA-MB-231 | + | -- | + |
| hCMEC/D3 MCF-7 | |||
| hCMEC/D3 BT-474 | |||
| hCMEC/D3 MDA-MB-231 | +++ | --- | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





