Submitted:
22 January 2024
Posted:
23 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Trial design
2.2. Participants
2.3. Intervention
2.4. Outcome measures
2.5. Statistical analysis
3. Results
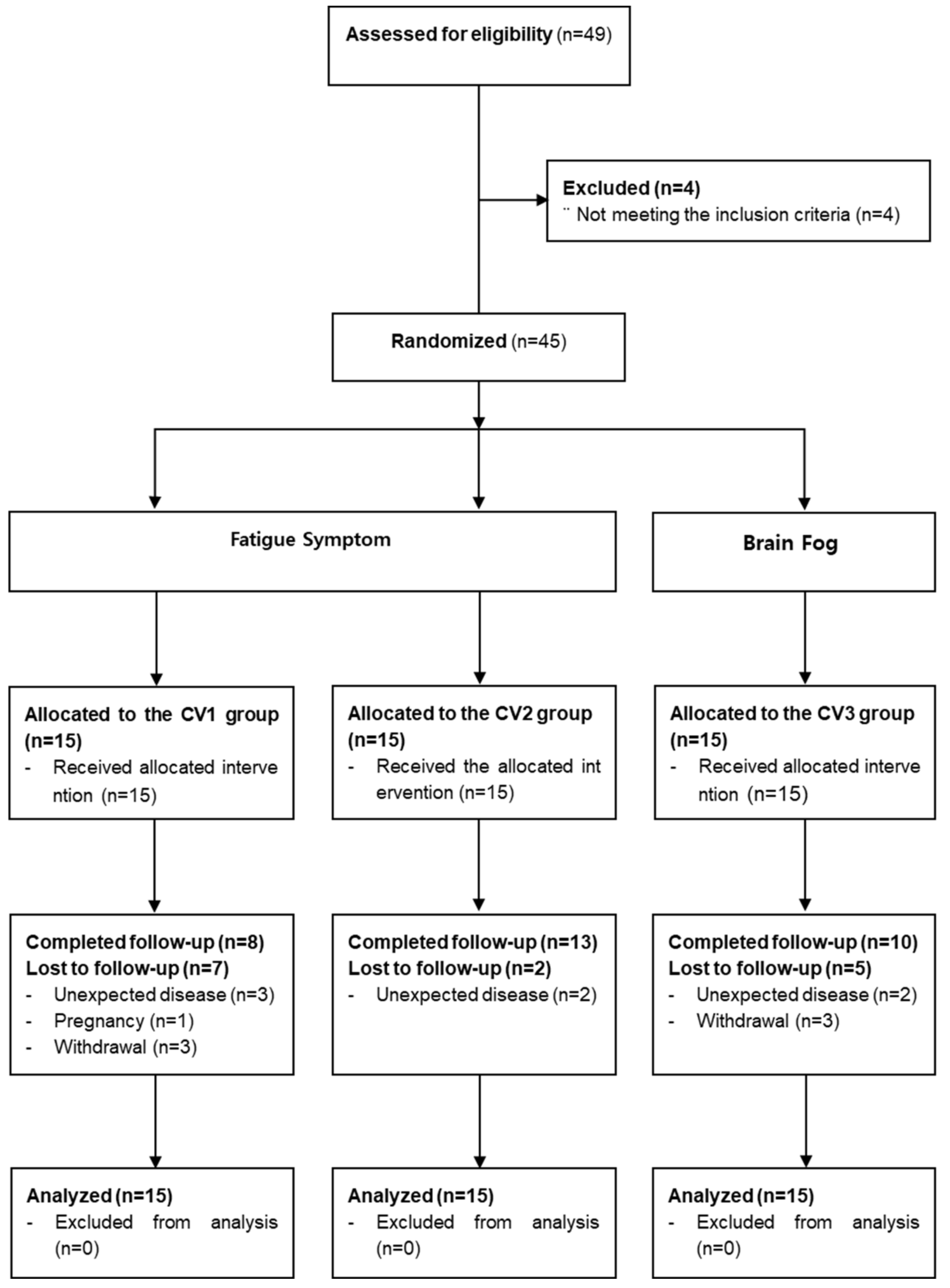
3.1. Participants
3.2. Baseline characteristics
3.3. Feasibility assessment
3.4. Assessments of fatigue and “brain fog” symptoms
3.5. Other outcome and safety assessments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgements
Conflicts of Interest
Abbreviations
References
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, S.E.; Kim, T.; Yun, K.W.; Lee, S.H.; Lee, E.; Seo, J.-W.; Jung, Y.H.; Chong, Y.P. Preliminary Guidelines for the Clinical Evaluation and Management of Long COVID. Infect. Chemother. 2022, 54, 566–597. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) Condition or Long COVID: A Meta-Analysis and Systematic Review. J. Infect. Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef]
- Marshall, M. The lasting misery of coronavirus long-haulers. Nature 2020, 585, 339–341. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re'Em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Ceban, F.; Ling, S.; Lui, L.M.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain, Behav. Immun. 2021, 101, 93–135. [Google Scholar] [CrossRef]
- Subramanian, A.; Nirantharakumar, K.; Hughes, S.; Myles, P.; Williams, T.; Gokhale, K.M.; Taverner, T.; Chandan, J.S.; Brown, K.; Simms-Williams, N.; et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat. Med. 2022, 28, 1706–1714. [Google Scholar] [CrossRef]
- D. Kim, Who Gets Long COVID and Suffers its Mental Health and Socioeconomic Consequences in the United States? Preliminary Findings from a Large Nationwide Study, medRxiv (2023) 2023.2001. 2006.23284199.
- Fowler-Davis, S.; Platts, K.; Thelwell, M.; Woodward, A.; Harrop, D. A mixed-methods systematic review of post-viral fatigue interventions: Are there lessons for long Covid? PLOS ONE 2021, 16, e0259533. [Google Scholar] [CrossRef]
- Jiang, L.; An, X.; Duan, Y.; Lian, F.; Jin, D.; Zhang, Y.; Yang, C.; Zhang, Y.; Kang, X.; Sun, Y. The pathological mechanism of the COVID-19 convalescence and its treatment with traditional Chinese medicine. Front. Pharmacol. 2023, 13, 1054312. [Google Scholar] [CrossRef]
- L.L.-D. Zhong, Y.-P. Wong, C.-Y. Leung, et al., Effects of Chinese medicine for COVID-19 rehabilitation: a multicenter observational study, Chin Med 17(1) (2022) 99. [CrossRef]
- W. Pang, F. Yang, Y. Zhao, et al., Qingjin Yiqi granules for post-COVID-19 condition: A randomized clinical trial, Journal of Evidence-Based Medicine 15(1) (2022) 30-38. [CrossRef]
- Kim, T.-H.; Jeon, S.-R.; Kang, J.W.; Kwon, S. Complementary and Alternative Medicine for Long COVID: Scoping Review and Bibliometric Analysis. Evidence-Based Complement. Altern. Med. 2022, 2022, 1–7. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, H.J.; Jang, E.S.; Jung, H.J.; Hwang, M.W.; Nam, D.H. Survey on Pattern Identification and Treatment of Chronic Fatigue in Korea Medicine. J. Physiol. Pathol. Korean Med. 2018, 32, 126–133. [Google Scholar] [CrossRef]
- Tokumasu, K.; Ueda, K.; Honda, H.; Sunada, N.; Sakurada, Y.; Matsuda, Y.; Nakano, Y.; Hasegawa, T.; Otsuka, Y.; Obika, M.; et al. Application of Kampo Medicines for Treatment of General Fatigue Due to Long COVID. Medicina 2022, 58, 730. [Google Scholar] [CrossRef]
- Jang, S.; Kim, D.; Yi, E.; Choi, G.; Song, M.; Lee, E.-K. Telemedicine and the Use of Korean Medicine for Patients With COVID-19 in South Korea: Observational Study. Psychopharmacol. 2021, 7, e20236–200. [Google Scholar] [CrossRef]
- Jeon, W.-Y.; Jin, S.E.; Sohn, E.; Jo, K.; Ha, H.; Shin, H.-K.; Lee, M.-Y. Anti-inflammatory and anti-allergic effects of Cheonwangbosim-dan water extract: An in vitro and in vivo study. Heliyon 2023, 9, e16172. [Google Scholar] [CrossRef]
- K.H. Jegal, J. Yoon, S. Kim, et al. (2022). Herbal Medicines for Post-Acute Sequelae (Fatigue or Cognitive Dysfunction) of SARS-CoV-2 Infection: A Phase 2 Pilot Clinical Study Protocol. In 10. (MDPI), pp. 1839. [CrossRef]
- Vercoulen, J.H.M.M.; Swanink, C.M.A.; Fennis, J.F.M.; Galama, J.M.D.; van der Meer, J.W.M.; Bleijenberg, G. Dimensional assessment of chronic fatigue syndrome. J. Psychosom. Res. 1994, 38, 383–392. [Google Scholar] [CrossRef]
- Ha, H.; Jeong, D.; Hahm, B.-J.; Shim, E.-J. Cross-Cultural Validation of the Korean Version of the Chalder Fatigue Scale. Int. J. Behav. Med. 2017, 25, 351–361. [Google Scholar] [CrossRef]
- Kim, S.-H.; Ahn, J.; Ock, M.; Shin, S.; Park, J.; Luo, N.; Jo, M.-W. The EQ-5D-5L valuation study in Korea. Qual. Life Res. 2016, 25, 1845–1852. [Google Scholar] [CrossRef]
- Sohn, S.I.; Kim, D.H.; Lee, M.Y.; Cho, Y.W. The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath. 2011, 16, 803–812. [Google Scholar] [CrossRef]
- Y.I. Jung, E.H. Jeong, H. Lee, et al., Validation of MoCA-MMSE conversion scales in Korean patients with cognitive impairments, Dementia and Neurocognitive Disorders 17(4) (2018) 148-155. [CrossRef]
- Broadbent, D.E.; Cooper, P.F.; FitzGerald, P.; Parkes, K.R. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br. J. Clin. Psychol. 1982, 21, 1–16. [Google Scholar] [CrossRef]
- A.T. Beck, C.H. Ward, M. Mendelson, et al., An inventory for measuring depression, Arch Gen Psychiatry 4(6) (1961) 561-571.
- H. Kim, and T. Park, Korean norm for the difference between digits forward and digits backward, The Korean Journal of Clinical Psychology 22(3) (2003) 599-613.
- Kim, H.; Kim, S.R. Development of Short Form of the Korean Version- the Boston Naming Test (K-BNT-15) Based on Item Response Theory. J. Korea Contents Assoc. 2013, 13, 321–327. [Google Scholar] [CrossRef]
- J.-H. Kim, J.-K. Lee, and H.-K. Shin, Analysis of studies on Bojungikgi-tang (Buzhongyiqi-tang) to establish the fundament for Evidence Based Medicine (EBM), Korean Journal of Oriental Medicine 17(2) (2011) 135-167.
- Nam, D. The Effectiveness of Bojungikgi-tang and its modifications on Chronic Fatigue Syndrome: A Systematic Review And Meta-analysis. J. Korean Med. 2020, 41, 93–106. [Google Scholar] [CrossRef]
- Satoh, N.; Sakai, S.; Kogure, T.; Tahara, E.; Origasa, H.; Shimada, Y.; Kohoda, K.; Okubo, T.; Terasawa, K. A randomized double blind placebo-controlled clinical trial of Hochuekkito, a traditional herbal medicine, in the treatment of elderly patients with weakness N of one and responder restricted design. Phytomedicine 2005, 12, 549–554. [Google Scholar] [CrossRef]
- Oka, T. A patient who recovered from post-COVID myalgic encephalomyelitis/chronic fatigue syndrome: a case report. Biopsychosoc. Med. 2023, 17, 1–7. [Google Scholar] [CrossRef]
- E.-S. Lee, B.-I. Seo, J.-U. Lee, et al., The immunological activities of kyungohkgo and prescription of modified kyungohkgo, The Korea Journal of Herbology 17(2) (2002) 95-95.
- Y. Kim, S. Jin, S. Kim, et al., Anti-fatigue effect of Kyung-ok-Ko, Korean Journal of Pharmacognosy 47(3) (2016) 258-263.
- D.-G. Kim, W.-H. Park, and Y.-Y. Cha, Effect of Kyungohkgo on aerobic capacity and anti-fatigue in high school soccer players, Journal of Physiology & Pathology in Korean Medicine 25(5) (2011) 934-944.
- B.Y. Shin, Y.H. B.Y. Shin, Y.H. Lee, D.-h. Kim, et al., Ameliorating effect of a herbal medicinal prescription, Kyung-Ok-Ko, on scopolamine-induced memory impairment in mice, Journal of Traditional Medicines 26(1) (2009) 35-43. [CrossRef]
- M. Park, J.-S. Kim, A. Lee, et al., Inhibition of Inflammation by Kyeongok-go with Black Ginseng in LPS-induced RAW 264.7 Macrophages, The Korea Journal of Herbology 32(3) (2017) 19-27. [CrossRef]
- K.-S. Lee, G.-H. Kim, H.-H. Kim, et al., Qualities and anti-inflammatory activity of Kyungokgos sold in local markets, Journal of the Korean Society of Food Science and Nutrition 42(3) (2013) 335-341. [CrossRef]
- Jang, M.; Lee, M.J.; Lee, J.M.; Bae, C.-S.; Kim, S.-H.; Ryu, J.H.; Cho, I.-H. Oriental Medicine Kyung-Ok-Ko Prevents and Alleviates Dehydroepiandrosterone-Induced Polycystic Ovarian Syndrome in Rats. PLOS ONE 2014, 9, e87623. [Google Scholar] [CrossRef]
- Kim, J.-W.; Geum, J.-H.K.; Ha, W.-B.K.; Woo, H.-J.K.; Han, Y.-H.K.; Park, S.-H.K.; Lee, J.-H.K. The efficacy, effectiveness, and safety of Kyung-ok-ko: A narrative review. Medicine 2022, 101, e31311. [Google Scholar] [CrossRef]
- I.-C. Jung, S.-R. Lee, and J.-Y. Lee, Effects of ChenWhangBosimDan (CWBD) on Inhibition of Impairment of Learning and Memory, and Acetylcholinesterase in Amnesia mice, Journal of Oriental Neuropsychiatry 13(2) (2002) 149-171.
- O.-S. Kim, Y. Kim, S.-R. Yoo, et al., Cheonwangbosimdan, a traditional herbal formula, inhibits inflammatory responses through inactivation of NF-κB and induction of heme oxygenase-1 in raw264. 7 murine macrophages, Int J Clin Exp Med 9(2) (2016) 1692-1699.
- Kim, M.-J.; Bose, S.; Shin, N.-R.; Park, S.; Kwon, O.; Song, E.-J.; Nam, Y.-D.; Koo, B.-S.; Nam, D.-H.; Lee, J.-H.; et al. The Herbal Formula CWBSD Improves Sleep Quality Dependent on Oral Microbial Type and Tongue Diagnostic Features in Insomnia. J. Pers. Med. 2021, 11, 325. [Google Scholar] [CrossRef]
- S.-H. Kim, J.-W. Kim, C.-H. Kang, et al., The effects of Chenwangbosim-dan and herbs on mouse neuroblastoma 2a cells damaged by hypoxia-reoxygenation, Journal of Oriental Neuropsychiatry 17(2) (2006) 15-36.
- Kim, B.; Jo, C.; Choi, H.-Y.; Lee, K. Vasorelaxant and Hypotensive Effects of Cheonwangbosimdan in SD and SHR Rats. Evidence-Based Complement. Altern. Med. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- K.-W. Choi, and I.-C. Jung, The effects of Chenwhangbosindan (CBD) hot water extract & ultra-fine powder on the Alzheimer's disease model, Journal of Oriental Neuropsychiatry 19(2) (2008) 77-93.
- I.-C. Jung, Effects of Chenwhangbosim-dan and Sungsimjihwang-tang on protecting microglia and inhibiting acetylcholinesterase and oxidants, Journal of Physiology & Pathology in Korean Medicine 22(1) (2008) 120-125.
- Lin, S.-K.; Yan, S.-H.; Lai, J.-N.; Tsai, T.-H. Patterns of Chinese medicine use in prescriptions for treating Alzheimer’s disease in Taiwan. Chin. Med. 2016, 11, 12. [Google Scholar] [CrossRef]
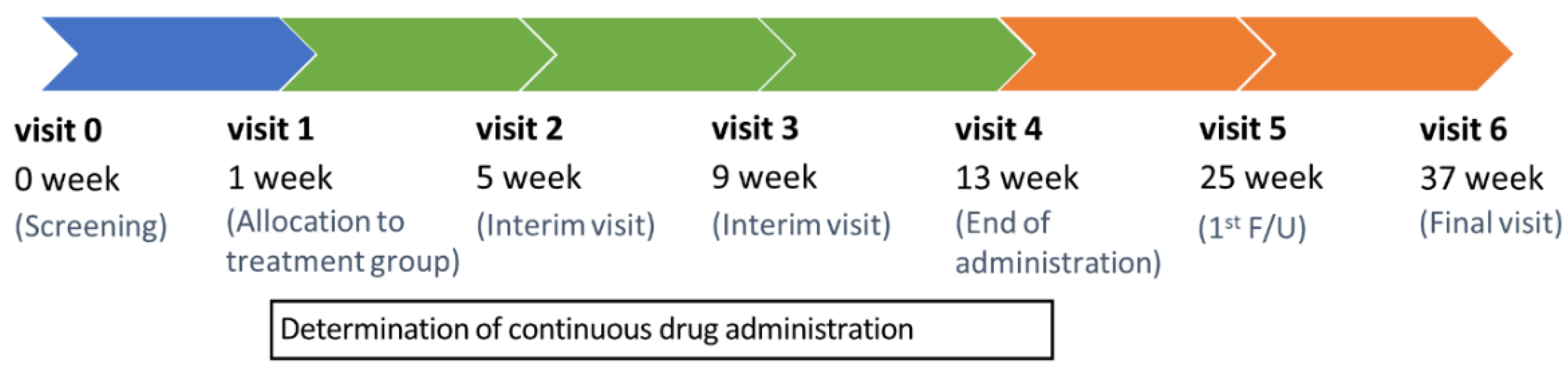
| Visit | 0 | V1 | V2 | V3 | V4 | V5 | V6 | UV * |
|---|---|---|---|---|---|---|---|---|
| Week | −7 | 1 | 5 | 9 | 13 | 25 | 37 | - |
| Visit Window (±Days) | - | 2 | ±7 | ±7 | ±7 | ±7 | ±7 | - |
| Informed consent for the study | ● | |||||||
| Demographic information | ● | |||||||
| Participation in other clinical trials | ● | |||||||
| Medical history | ● | |||||||
| Confirmation of diagnosis of COVID-19 infection | ● | |||||||
| Medication history | ● | |||||||
| Vital signs | ● | ● | ● | ● | ● | ● | ● | ● |
| Electrocardiogram | ● | ● | ● | |||||
| Laboratory test | ● | ● | ● | |||||
| Blood collection for immune response and metabolite analysis | ● | ● | ● | ● | ● | |||
| Pregnancy ** | ● | |||||||
| Evaluation of fatigue or “brain fog” | ● | |||||||
| CIS | ● | ● | ● | ● | ● | ● | ● | |
| VAS (0–100) score for fatigue or “brain fog” | ● | ● | ● | ● | ● | ● | ● | |
| Inclusion/exclusion criteria | ● | |||||||
| KM syndrome differentiation | ● | |||||||
| Prescription of herbal medicine *** | ● | ● | ● | |||||
| Drug adherence | ● | ● | ● | ● | ||||
| Check for combination therapy | ● | ● | ● | ● | ● | ● | ||
| ChFQ | ● | ● | ● | ● | ● | ● | ● | |
| EQ-5D-5L | ● | ● | ● | ● | ● | ● | ● | |
| PSQI-K | ● | ● | ● | ● | ● | ● | ● | |
| K-MoCA | ● | ● | ● | ● | ● | ● | ● | |
| CFQ | ● | ● | ● | |||||
| BDI | ● | ● | ● | ● | ● | ● | ● | |
| Digit span test in K-WAIS (DF, DB, and DF-DB) | ● | ● | ● | ● | ||||
| K-BNT-15 | ● | ● | ● | ● | ||||
| Adverse events check | ● | ● | ● | ● | ● | ● | ● |
| Fatigue | Cognitive Dysfunction | ||
|---|---|---|---|
| Code | BIT | KOG | CBD |
| Syndrome differentiation classification | Lung-Spleen Qi Deficiency | Dual Deficiency of Qi and Yin | Heart Yin Deficiency Heat |
| Symptom | Fatigue, appetite loss, cold sweat, shortness of breath, chest tightness, anxiety and others | Fatigue, dry cough and others | Forgetfulness, fever, insomnia, heart palpitation, stomatitis, tongue needles and others |
| Tongue diagnosis | Pale tongue, thin white fur | Dry mouth, dry tongue | Red tongue and low tongue coated |
| Pulse diagnosis | Vacuous, large, weak pulse/surging, large pulse | Fine pulse/vacuous, weak pulse | Fine, rapid pulse |
| Urine/feces | Difficult stool to pass/sloppy stool | Dry stool | Inhibited stool/sloppy stool |
| Fomula / Product name |
Composition | Manufacturer / License Code |
Group / KM syndrome differentiation |
|
|---|---|---|---|---|
| Bojungikgi-tang (BIT, CV1) Kracie Bojungikgi-tang Extract Fine Granule |
Ginseng Radix | 4.0g | Kyungbang Pharmaceutical Co.,Ltd 201507212 |
Fatigue lung-spleen qi deficiency |
| Panax ginseng C. A. Meyer | ||||
| Atractylodis Rhizoma Alba | 4.0g | |||
| Atractylodes japonica Koidzumi | ||||
| Astragali Radix | 4.0g | |||
| Astragalus membranaceus Bunge | ||||
| Angelicae Gigantis Radix | 3.0g | |||
| Angelica gigas Nakai | ||||
| Zizyphi Fructus | 2.0g | |||
| Zizyphus jujuba Miller var. inermis Rehder | ||||
| Bupleuri Radix | 2.0g | |||
| Bupleurum falcatum Linné | ||||
| Citri Unshius Pericarpium | 2.0g | |||
| Citrus unshiu Markovich | ||||
| Glycyrrhizae Radix et Rhizoma | 1.5g | |||
| Glycyrrhiza uralensis Fischer | ||||
| Cimicifugae Rhizoma | 1.0g | |||
| Cimicifuga heracleifolia Komarov | ||||
| Zingiberis Rhizoma Recens | 0.5g | |||
| Zingiber officinale Roscoe | ||||
| Kyungok-go (KOG, CV2) Kyungbang Kyungokgo |
Rehmanniae Radix | 16.0g | Kyungbang Pharmaceutical Co.,Ltd 201708619 |
Fatigue dual deficiency of qi and yin |
| Rehmannia glutinosa Liboschitz ex Steudel | ||||
| Ginseng Radix | 2.5g | |||
| Panax ginseng C. A. Meyer | ||||
| Poria Sclerotium | 5.0g | |||
| Poria cocos Wolf | ||||
| Mel Honey | 16.6g | |||
| Cheonwang-bosim-dan (CBD, CV3) Soonsimhwan |
Rehmanniae Radix | 500.0mg | Hanpoong Pharmaceutical Co.,Ltd 200100075 |
“brain fog” heart yin deficiency |
| Rehmannia glutinosa Liboschitz ex Steudel | ||||
| Ginseng Radix | 62.5mg | |||
| Panax ginseng C. A. Meyer | ||||
| Scrophulariae radix | 62.5mg | |||
| Scrophularia buergeriana Miquel | ||||
| Salviae Miltiorrhizae Radix | 62.5mg | |||
| Salvia miltiorrhiza Bunge | ||||
| Polygalae Radix | 62.5mg | |||
| Polygala tenuifolia Willdenow | ||||
| Platycodonis Radix | 62.5mg | |||
| Platycodon grandiflorum A. De Candolle | ||||
| Poria Sclerotium | 62.5mg | |||
| Poria cocos Wolf | ||||
| Schisandrae Fructus | 125.0mg | |||
| Schisandra chinensis (Turcz.) Baillon | ||||
| Angelicae Gigantis Radix | 125.0mg | |||
| Angelica gigas Nakai | ||||
| Asparagi Radix | 125.0mg | |||
| Asparagus cochinchinensis Merrill | ||||
| Liriopis seu Ophiopogonis Tuber | 125.0mg | |||
| Liriope platyphylla Wang et Tang | ||||
| Thujae Orientalis Semen | 125.0mg | |||
| Thuja orientalis Linné | ||||
| Zizyphi Semen | 125.0mg | |||
| Zizyphus jujuba Miller var. spinosa Hu ex H. F. Chou | ||||
| Coptidis Rhizoma | 250.0mg | |||
| Coptis japonica Makino | ||||
| BIT (n = 15) | KOG (n = 15) | CBD (n = 15) | P-value | ||
| Age (mean, SD) | 43.80 (14.31) | 45.33 (12.57) | 51.33 (15.71) | 0.3656* | |
| Sex (M/F) | 9/6 | 8/7 | 6/9 | 0.6548** | |
| Body mass index (mean, SD) | 24.21 (3.56) | 23.17 (3.67) | 23.41 (3.07) | 0.8233* | |
| Final level of education | |||||
| Less than elementary school | 0 | 0 | 0 | 0.1742** | |
| Elementary school | 0 | 0 | 0 | ||
| Middle school | 0 | 0 | 0 | ||
| High school | 2 (13.33 %) | 5 (33.33 %) | 7 (46.67 %) | ||
| University or college | 13 (86.67 %) | 10 (66.67 %) | 8 (53.33 %) | ||
| Occupation | |||||
| Physical labor | 0 | 1(6.67 %) | 6(40.00 %) | 0.6692** | |
| Non-physical labor | 10 (66.67 %) | 11(73.33 %) | 8(53.33 %) | ||
| Others (unemployed, etc) | 5 (33.33 %) | 3(20.00 %) | 1(6.67 %) | ||
| Time duration since initial infection of COVID-19 (days, SD) | 8.87 (3.46) | 10.53 (4.61) | 10.20 (8.08) | 0.3310* | |
| NEWS* (SD) | 1.00 (2.54) | 2.47 (4.26) | 2.07 (4.03) | 0.3540* | |
| Symptom durations | |||||
| Fatigue (days, SD) | 89.47 (33.25) | 114.80 (92.11) | |||
| “Brain fog” (days, SD) | 119.13 (97.95) | ||||
| Baseline CIS score (mean, SD) | 100.60 (10.66) | 96.60 (15.36) | 105.4 (17.93) | 0.3331* | |
| Baseline VAS for fatigue symptom (mean, SD) | 76.87 (14.58) | 73.27 (9.05) | 70.33 (20.34) | 0.4900* | |
| Baseline VAS for “brain fog” (mean, SD) | 49.27 (24.86) | 40.60 (26.08) | 62.00 (24.65) | 0.1039* |
| BIT (n = 15) | KOG (n = 15) | CBD (n = 15) | P-value*** | |||
| Outcomes for feasibility assessment | ||||||
| Treatment success rate of VAS for fatigue (%)* | 12 (80) | 8 (53.33) | 7 (46.67) | 0.1431 | ||
| Treatment success rate of VAS for “brain fog” (%)* | 6(40) | 7 (46.67) | 2 (13.33) | 0.1225 | ||
| (n= 13) | (n= 14) | (n= 14) | ||||
| Medication adherence (%)** | 92.27(16.28) | 96.26 (15.75) | 85.66 (23.61) | |||
| Outcomes for fatigue symptoms | ||||||
| CIS (mean difference from baseline with 95% CI)** | ||||||
| At 5 weeks | -18.4, 95% CI[-26.89, -9.91] | -23, 95% CI[-33.03, -12.97] | -8.93, 95% CI[-16.27, -1.6] | 0.0181 | ||
| At 9 weeks | -27.2, 95% CI[-36.15, -18.25] | -25.93, 95% CI[-35.46, -16.41] | -15.53, 95% CI[-24.8, -6.27] | 0.0394 | ||
| At 13 weeks | -29.53, 95% CI[-40.96, -18.1] | -31.47, 95% CI[-43.47, -19.46] | -23.4, 95% CI[-34.79, -12.01] | 0.3391 | ||
| At 25 weeks | -30.2, 95% CI[-40.68, -19.72] | -25.33, 95% CI[-35.78, -14.89] | -26.87, 95% CI[-41, -12.73] | 0.7643 | ||
| At 37 weeks | -29.6, 95% CI[-39.11, -20.09] | -29.2, 95% CI[-39.72, -18.68] | -26.07, 95% CI[-40.95, -11.18] | 0.6119 | ||
| VAS for fatigue (mean difference from baseline with 95% CI)** | ||||||
| At 5 weeks | -28.87, 95% CI[-40.27, -17.47] | -22.27, 95% CI[-36.95, -7.59] | -6.47, 95% CI[-13.12, 0.18] | 0.0275 | ||
| At 9 weeks | -30.07, 95% CI[-42.12, -18.01] | -27.67, 95% CI[-43.02, -12.32] | -14, 95% CI[-25.25, -2.75] | 0.2353 | ||
| At 13 weeks | -32.53, 95% CI[-46.07, -19] | -30.13, 95% CI[-47.11, -13.16] | -16.73, 95% CI[-27.48, -5.98] | 0.2509 | ||
| At 25 weeks | -28.6, 95% CI[-40.93, -16.27] | -28.73, 95% CI[-43.44, -14.03] | -15.2, 95% CI[-24.56, -5.84] | 0.2537 | ||
| At 37 weeks | -32.27, 95% CI[-46.45, -18.08] | -20.07, 95% CI[-36.12, -4.01] | -15.67, 95% CI[-26.24, -5.1] | 0.2784 | ||
| ChFQ (mean difference from baseline with 95% CI)** | ||||||
| At 5 weeks | Total score | -12.73, 95% CI[-21.48, -3.99] | -11.33, 95% CI[-18.93, -3.73] | -5.27, 95% CI[-9.66, -0.87] | 0.1198 | |
| Physical health score | -9.13, 95% CI[-14.11, -4.15] | -7.2, 95% CI[-12.11, -2.29] | -1.53, 95% CI[-4.78, 1.71] | 0.0461 | ||
| Mental health score | -3.6, 95% CI[-8.53, 1.33] | -4.13, 95% CI[-7.42, -0.85] | -3.73, 95% CI[-5.44, -2.03] | 0.3220 | ||
| At 9 weeks | Total score | -20.07, 95% CI[-29, -11.13] | -15.2, 95% CI[-25.83, -4.57] | -5.6, 95% CI[-12.08, 0.88] | 0.0199 | |
| Physical health score | -13.8, 95% CI[-19.62, -7.98] | -9.87, 95% CI[-16.27, -3.46] | -2.27, 95% CI[-6.42, 1.89] | 0.0139 | ||
| Mental health score | -6.27, 95% CI[-10.36, -2.17] | -5.33, 95% CI[-9.86, -0.8] | -3.33, 95% CI[-6.34, -0.32] | 0.0174 | ||
| At 13 weeks | Total score | -23.67, 95% CI[-35.09, -12.24] | -21.27, 95% CI[-32.91, -9.62] | -10.93, 95% CI[-19.93, -1.93] | 0.0991 | |
| Physical health score | -17.27, 95% CI[-25.38, -9.15] | -14.2, 95% CI[-21.41, -6.99] | -6.27, 95% CI[-11.99, -0.54] | 0.0808 | ||
| Mental health score | -6.4, 95% CI[-10.98, -1.82] | -7.07, 95% CI[-11.98, -2.16] | -4.67, 95% CI[-8.3, -1.03] | 0.0469 | ||
| At 25 weeks | Total score | -22.4, 95% CI[-30.36, -14.44] | -17, 95% CI[-28.89, -5.11] | -14.6, 95% CI[-25, -4.2] | 0.4634 | |
| Physical health score | -16.53, 95% CI[-22.48, -10.59] | -11.93, 95% CI[-19.19, -4.67] | -8.73, 95% CI[-15.56, -1.91] | 0.2541 | ||
| Mental health score | -5.87, 95% CI[-9.23, -2.5] | -5.07, 95% CI[-9.97, -0.16] | -5.87, 95% CI[-10.13, -1.6] | 0.5669 | ||
| At 37 weeks | Total score | -25.53, 95% CI[-33.1, -17.97] | -18.27, 95% CI[-30.04, -6.49] | -14.93, 95% CI[-25.55, -4.32] | 0.2411 | |
| Physical health score | -19, 95% CI[-24.43, -13.57] | -12.6, 95% CI[-19.66, -5.54] | -8.27, 95% CI[-15.26, -1.28] | 0.0717 | ||
| Mental health score | -6.53, 95% CI[-10.45, -2.62] | -5.67, 95% CI[-10.59, -0.74] | -6.67, 95% CI[-10.75, -2.58] | 0.5402 | ||
| Outcomes for “brain fog” symptoms | ||||||
| VAS for “brain fog” (mean difference from baseline with 95% CI)** | ||||||
| At 5 weeks | -11.13, 95% CI[-32.3, 10.04] | -4.4, 95% CI[-22.91, 14.11] | -0.4, 95% CI[-9.84, 9.04] | 0.0477 | ||
| At 9 weeks | -7.73, 95% CI[-27.2, 11.73] | -9.33, 95% CI[-29.15, 10.48] | -3.87, 95% CI[-17.04, 9.3] | 0.0358 | ||
| At 13 weeks | -6.27, 95% CI[-26.71, 14.17] | -5.4, 95% CI[-27.98, 17.18] | 1.4, 95% CI[-10.8, 13.6] | 0.0472 | ||
| At 25 weeks | -6.73, 95% CI[-26.23, 12.77] | -7.27, 95% CI[-25.25, 10.71] | -5.87, 95% CI[-14.48, 2.75] | 0.2454 | ||
| At 37 weeks | -12.73, 95% CI[-32.8, 7.33] | -7.13, 95% CI[-24.32, 10.06] | -7.13(-15.81, 1.54] | 0.2193 | ||
| K-MoCA (mean difference from baseline with 95% CI)** | ||||||
| At 5 weeks | -0.53, 95% CI[-2.34, 1.28] | 0.20, 95% CI[-0.79, 1.19] | 0.87, 95% CI[-0.77, 2.50] | 0.5767 | ||
| At 9 weeks | 0.60, 95% CI[-1.10, 2.30] | 1.33, 95% CI[0.43, 2.24] | 1.27, 95% CI[-1.40, 3.93] | 0.5382 | ||
| At 13 weeks | 1.00, 95% CI[-0.51, 2.51] | 1.80, 95% CI[0.77, 2.83] | 1.13, 95% CI[-1.96, 4.22] | 0.3176 | ||
| At 25 weeks | 1.00, 95% CI[-0.29, 2.29] | 1.87, 95% CI[1.03, 2.70] | 0.80, 95% CI[-2.09, 3.69] | 0.1525 | ||
| At 37 weeks | 1.07, 95% CI[-0.30, 2.43] | 1.47, 95% CI[0.53, 2.40] | 1.00, 95% CI[-1.89, 3.89] | 0.4059 | ||
| CFQ (mean difference from baseline with 95% CI)** | ||||||
| At 37 weeks | -4.40, 95% CI[-7.57, -1.23] | -3.93, 95% CI[-11.57, 3.70] | -11.67, 95% CI[-21.07, -2.26] | 0.9368 | ||
| DF-forward (mean difference from baseline with 95% CI)** | ||||||
| At 13 weeks | 0.27, 95% CI[-0.27, 0.8] | 0, 95% CI[-0.47, 0.47] | -0.07, 95% CI[-0.46, 0.32] | 0.4532 | ||
| At 25 weeks | 0.07, 95% CI[-0.42, 0.56] | 0.47, 95% CI[0.11, 0.82] | -0.07, 95% CI[-0.86, 0.73] | 0.3573 | ||
| At 37 weeks | 0.33, 95% CI[-0.01, 0.68] | 0.47, 95% CI[0.11, 0.82] | -0.2, 95% CI[-0.96, 0.56] | 0.1230 | ||
| DF-backward (mean difference from baseline with 95% CI)** | ||||||
| At 13 weeks | 1.4, 95% CI[0.4, 2.4] | 0.4, 95% CI[-0.26, 1.06] | 0.8, 95% CI[-0.21, 1.81] | 0.2095 | ||
| At 25 weeks | 0.87, 95% CI[-0.04, 1.78] | 0.47, 95% CI[-0.31, 1.25] | 0.8, 95% CI[-0.23, 1.83] | 0.8091 | ||
| At 37 weeks | 1.13, 95% CI[0.13, 2.13] | 1.07, 95% CI[0.36, 1.78] | 0.8, 95% CI[-0.21, 1.81] | 0.4459 | ||
| K-BNT-15 (mean difference from baseline with 95% CI)** | ||||||
| At 13 weeks | 0.13, 95% CI[-0.06, 0.33] | 0.13, 95% CI[-0.06, 0.33] | 0.33, 95% CI[-0.21, 0.87] | 0.5933 | ||
| At 25 weeks | 0.13, 95% CI[-0.06, 0.33] | 0.2, 95% CI[-0.11, 0.51] | 0.07, 95% CI[-1.14, 1.28] | 0.6532 | ||
| At 37 weeks | 0.2, 95% CI[-0.11, 0.51] | 0.27, 95% CI[-0.06, 0.6] | 0.13, 95% CI[-1.07, 1.33] | 0.6417 | ||
| Other outcomes | ||||||
| EQ-5D-5L (mean difference from baseline with 95% CI)** | ||||||
| At 5 weeks | 0.01, 95% CI[-0.03, 0.04] | -0.03, 95% CI[-0.07, 0.02] | -0.04, 95% CI[-0.09, 0.01] | 0.1040 | ||
| At 9 weeks | 0.03, 95% CI[0, 0.06] | 0.01, 95% CI[-0.05, 0.07] | -0.01, 95% CI[-0.05, 0.04] | 0.2034 | ||
| At 13 weeks | 0.06, 95% CI[0.02, 0.11] | 0.02, 95% CI[-0.04, 0.07] | -0.01, 95% CI[-0.11, 0.08] | 0.1417 | ||
| At 25 weeks | 0.06, 95% CI[0.02, 0.11] | -0.01, 95% CI[-0.05, 0.03] | 0.03, 95% CI[-0.06, 0.11] | 0.3256 | ||
| At 37 weeks | 0.07, 95% CI[0.03, 0.11] | 0, 95% CI[-0.04, 0.05] | 0.03, 95% CI[-0.06, 0.12] | 0.4582 | ||
| PSQI-K (mean difference from baseline with 95% CI)** | ||||||
| At 5 weeks | 0.14, 95% CI[-1.16, 1.44] | -2.14, 95% CI[-3.10, -1.19] | 0.07, 95% CI[-1.14, 1.28] | 0.0364 | ||
| At 9 weeks | -0.85, 95% CI[-1.92, 0.22] | -3.07, 95% CI[-4.10, -2.05] | -0.64, 95% CI[-2.19, 0.90] | 0.0557 | ||
| At 13 weeks | -1.15, 95% CI[-2.96, 0.66] | -2.71, 95% CI[-3.76, -1.67] | -1.50, 95% CI[-3.35, 0.35] | 0.6978 | ||
| At 25 weeks | -1.25, 95% CI[-3.40, 0.90] | -2.08, 95% CI[-3.28, -0.89] | -1.55, 95% CI[-4.72, 1.63] | 0.9725 | ||
| At 37 weeks | -1.64, 95% CI[-3.72, 0.45] | -2.82, 95% CI[-3.94, -1.70] | -2.33, 95% CI[-4.51, -0.16] | 0.9540 | ||
| BDI (mean difference from baseline with 95% CI)** | ||||||
| At 5 weeks | -3.40, 95% CI[-5.50, -1.30] | -2.20, 95% CI[-3.77, -0.63] | -1.27, 95% CI[-4.87, 2.34] | 0.1882 | ||
| At 9 weeks | -3.27, 95% CI[-5.76, -0.77] | -3.40, 95% CI[-5.14, -1.66] | -4.13, 95% CI[-8.05, -0.21] | 0.8568 | ||
| At 13 weeks | -3.80, 95% CI[-6.86, -0.74] | -4.73, 95% CI[-6.87, -2.59] | -5.53, 95% CI[-11.21, 0.14] | 0.8669 | ||
| At 25 weeks | -4.60, 95% CI[-7.82, -1.38] | -3.80, 95% CI[-6.20, -1.40] | -6.53, 95% CI[-13.15, 0.08] | 0.9885 | ||
| At 37 weeks | -4.47, 95% CI[-7.38, -1.55] | -4.00, 95% CI[-6.62, -1.38] | -6.33, 95% CI[-11.20, -1.47] | 0.9212 | ||
| BIT (n = 15) | KOG (n = 15) | CBD (n = 15) | P-value** | ||
| Number participants with adverse events (%)* | 5(33.33) | 1(6.67) | 4(26.67) | 0.2805 | |
| Type of AEs (n)* | |||||
| Back pain | 1 | 0 | 0 | ||
| Hypothyroidism | 1 | 0 | 0 | ||
| Common cold | 0 | 1 | 0 | ||
| High blood glucocorticoids level | 0 | 0 | 1 | ||
| Hypertension | 0 | 0 | 1 | ||
| Arthritis | 0 | 0 | 1 | ||
| Animal hair allergy | 1 | 0 | 0 | ||
| Insomnia | 1 | 0 | 0 | ||
| Rhinitis | 1 | 0 | 0 | ||
| Mucous cyst | 0 | 0 | 1 | ||
| Severity of AEs (n)* | |||||
| Mild | 5 | 1 | 4 | ||
| Moderate | 0 | 0 | 0 | ||
| Severe | 0 | 0 | 0 | ||
| Causality (n)* | |||||
| Drug-related AEs | 0 | 0 | 0 | ||
| Non-related AEs | 5 | 1 | 4 | ||
| Number of participants with normal laboratory test results at 13 weeks (n, %)* | BIT (n=13)*** | KOG (n=14)*** | CBD (n=14)*** | ||
| BUN | 10(76.92) | 9(64.29) | 12(85.71) | 0.4423 | |
| Creatinine | 11(84.62) | 14(100) | 14(100) | 0.0951 | |
| AST | 12(92.31) | 14(100) | 14(100) | 0.3171 | |
| ALT | 12(92.31) | 13(92.86) | 13(92.86) | 1.0000 | |
| ECG | 12(92.31) | 14(100) | 14(100) | 0.3171 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





