Submitted:
17 January 2024
Posted:
18 January 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Enterococus Species Isolation
2.3. DNA Isolation Protocols
2.4. Screening for Confirmation and Virulence Genes
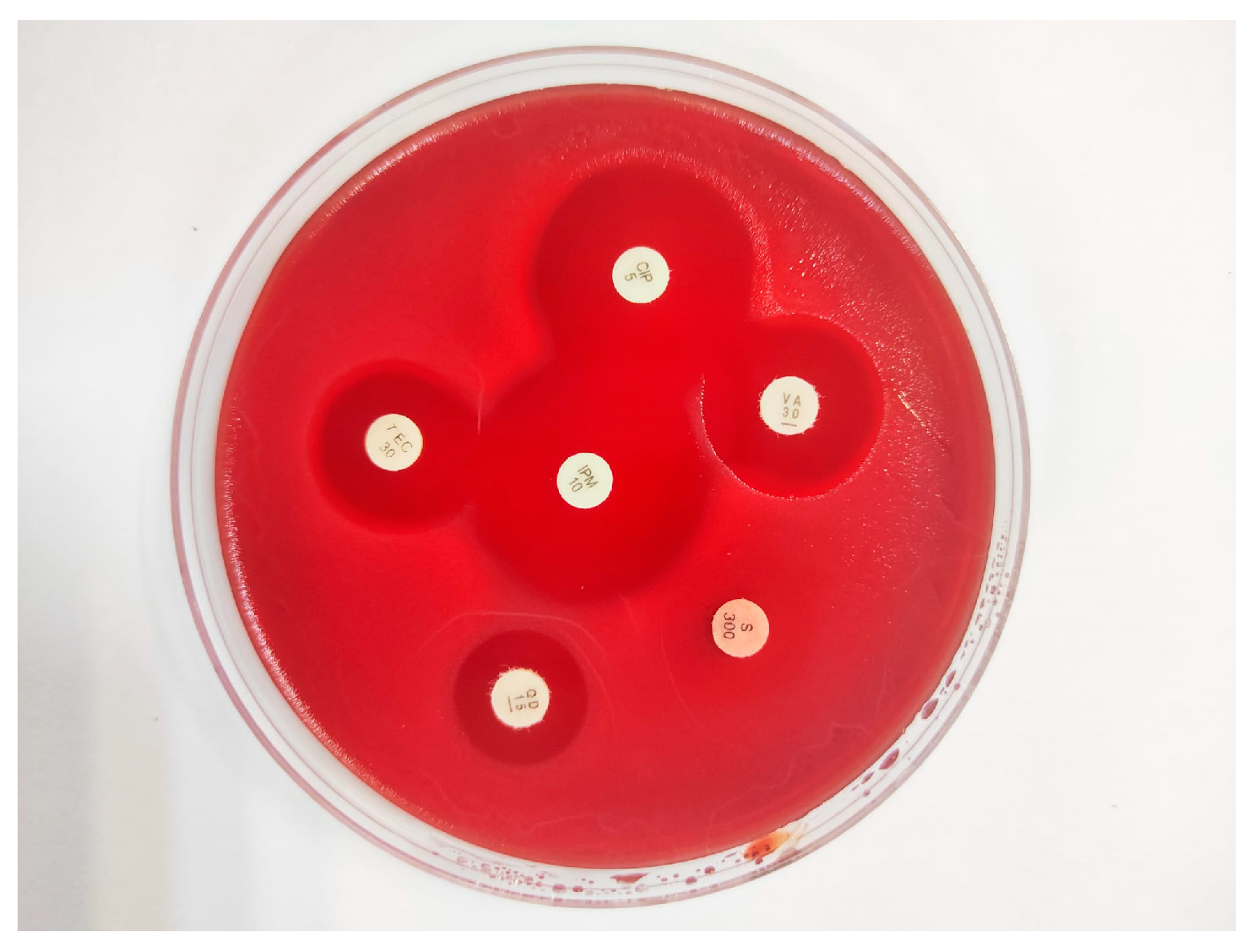
2.5. Antimicrobial Susceptibility Testing
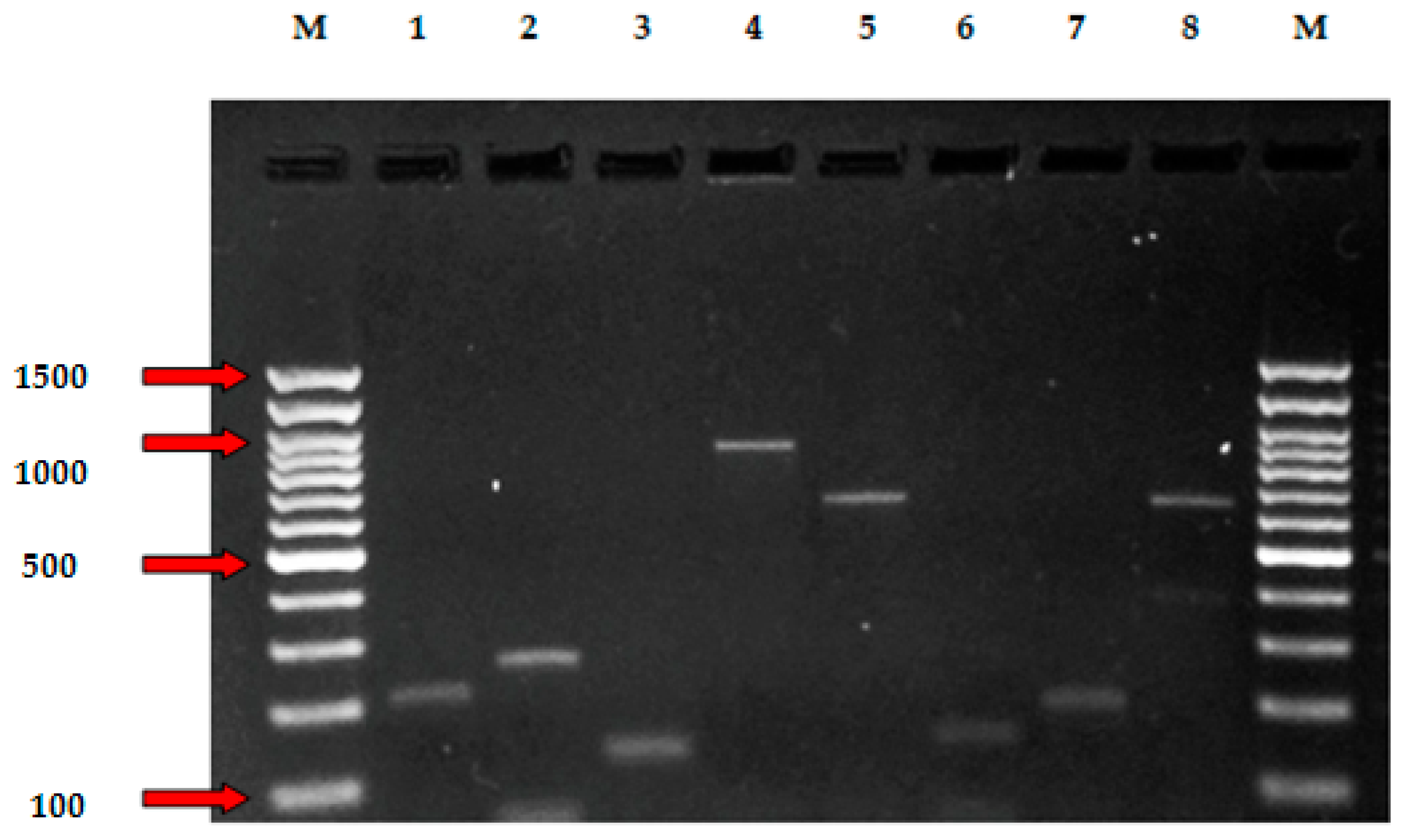
2.6. PCR Detection of Genes for Antimicrobial Resistance
3. Results
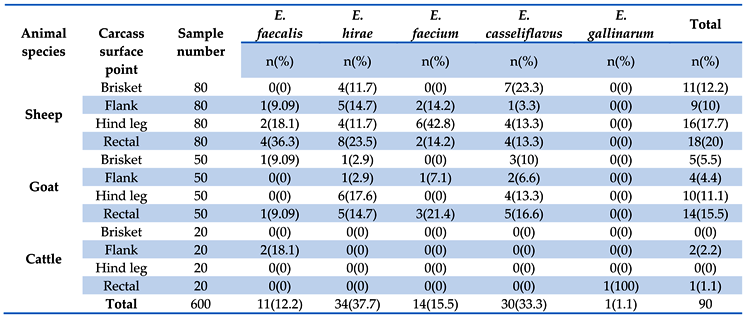
3.1. Prevalence of Enterococci
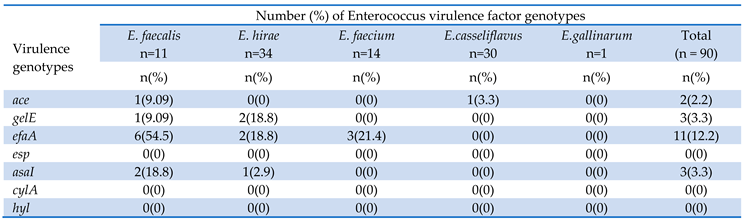
3.2. Virulence of Enterococci
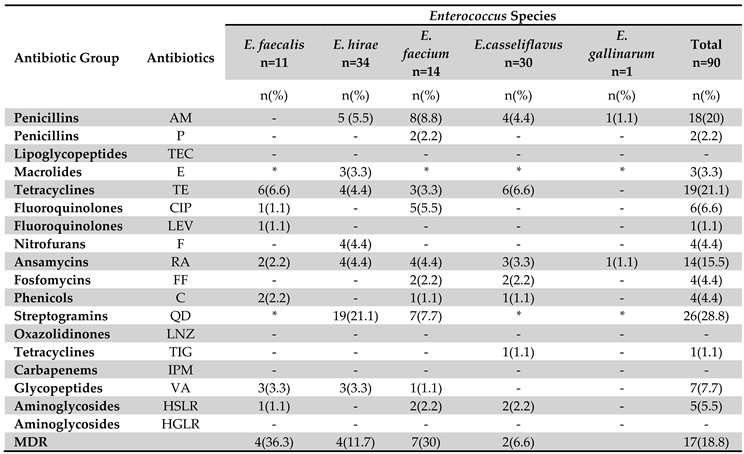
3.3. Phenotyping of Antibiotic Resistance
3.4. Genotyping of Antibiotic Resistance
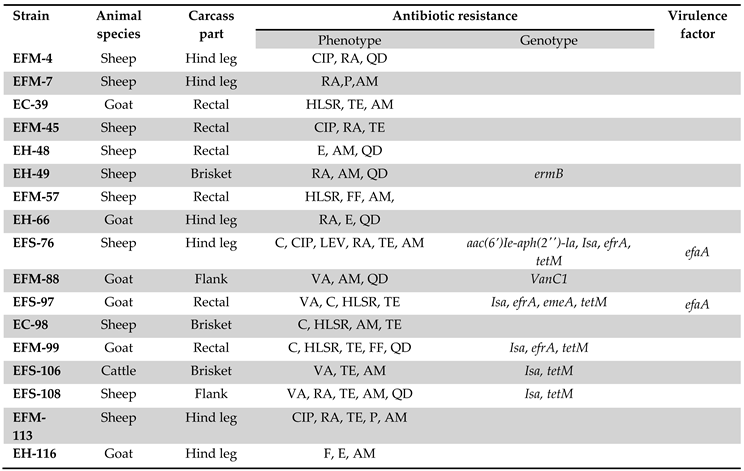
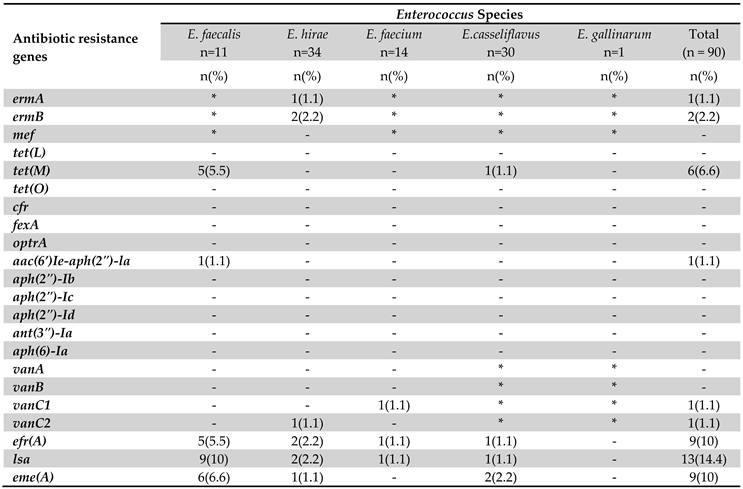
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
References
- Ramos, S.; Igrejas, G.; Capelo-Martinez, J. L.; Poeta, P. Antibiotic resistance and mechanisms implicated in fecal enterococci recovered from pigs, cattle and sheep in a Portuguese slaughterhouse. Annals of microbiology 2012, 62, 1485–1494. [Google Scholar] [CrossRef]
- Angulo, F. J.; Heuer, O. E.; Hammerum, A. M.; Collignon, P.; Wegener, H. C. Human health hazard from antimicrobial-resistant enterococci in animals and food. Clinical Infectious Diseases 2006, 43, 911–916. [Google Scholar]
- Na, S. H.; Moon, D. C.; Choi, M. J.; Oh, S. J.; Jung, D. Y.; Kang, H. Y.; Hyun, S. J.; Lim, S. K. Detection of oxazolidinone and phenicol resistant enterococcal isolates from duck feces and carcasses. International journal of food microbiology 2019, 293, 53–59. [Google Scholar] [CrossRef]
- Kim, M. H.; Moon, D. C.; Kim, S. J.; Mechesso, A. F.; Song, H. J.; Kang, H. Y.; Choi, J. Y.; Yoon, S. S.; Lim, S. K. Nationwide surveillance on antimicrobial resistance profiles of Enterococcus faecium and Enterococcus faecalis isolated from healthy food animals in South Korea, 2010 to 2019. Microorganisms 2021, 9, 925. [Google Scholar] [CrossRef]
- Smoglica, C.; Vergara, A.; Angelucci, S.; Festino, A. R.; Antonucci, A.; Marsilio, F.; Di Francesco, C. E. Evidence of linezolid resistance and virulence factors in Enterococcus spp. isolates from wild and domestic ruminants, Italy. Antibiotics 2022, 11, 223. [Google Scholar] [CrossRef]
- Desire, O. E.; Larson, B.; Richard, O.; Rolande, M. M.; Serge, K. B. Investigating antibiotic resistance in enterococci in Gabonese livestock. Veterinary World 2022, 15, 714. [Google Scholar] [CrossRef]
- Strateva, T.; Atanasova, D.; Savov, E.; Petrova, G.; Mitov, I. Incidence of virulence determinants in clinical Enterococcus faecalis and Enterococcus faecium isolates collected in Bulgaria. Brazilian Journal of Infectious Diseases 2016, 20, 127–133. [Google Scholar] [CrossRef]
- Song, H.; Bae, Y.; Jeon, E.; Kwon, Y.; Joh, S. Multiplex PCR analysis of virulence genes and their influence on antibiotic resistance in Enterococcus spp. isolated from broiler chicken. Journal of veterinary science 2019, 20. [Google Scholar] [CrossRef]
- Alzahrani, O. M.; Fayez, M.; Alswat, A. S.; Alkafafy, M.; Mahmoud, S. F.; Al-Marri, T.; Almuslem, A.; Ashfaq, H.; Yusuf, S. Antimicrobial resistance, biofilm formation, and virulence genes in Enterococcus species from small backyard chicken flocks. Antibiotics 2022, 11, 380. [Google Scholar] [CrossRef]
- Beukers, A. G.; Zaheer, R.; Goji, N.; Amoako, K. K.; Chaves, A. V.; Ward, M. P.; McAllister, T. A. Comparative genomics of Enterococcus spp. isolated from bovine feces. BMC microbiology 2017, 17, 1–18. [Google Scholar] [CrossRef]
- Ribeiro, J.; Silva, V.; Monteiro, A.; Vieira-Pinto, M.; Igrejas, G.; Reis, F. S.; Barros, L.; Poeta, P. Antibiotic Resistance among Gastrointestinal Bacteria in Broilers: A Review Focused on Enterococcus spp. and Escherichia coli. Animals 2023, 13, 1362. [Google Scholar] [CrossRef]
- Klibi, N.; Aouini, R.; Borgo, F.; Ben Said, L.; Ferrario, C.; Dziri, R. , Boudabous, A.; Torres, C.; Ben Slama, K. Antibiotic resistance and virulence of faecal enterococci isolated from food-producing animals in Tunisia. Annals of Microbiology 2015, 65, 695–702. [Google Scholar] [CrossRef]
- Zaheer, R.; Cook, S. R.; Barbieri, R.; Goji, N.; Cameron, A.; Petkau, A.; Polo, R. O.; Thymensen, L.; Stamm, C.; Song, J.; Hannon, S.; Jones, T.; Church, D.; Booker, C. W.; Amoako, K.; Domselaar, G. V.; Read, R. R.; McAllister, T. A. Surveillance of Enterococcus spp. reveals distinct species and antimicrobial resistance diversity across a One-Health continuum. Sci Reports 2020, 10 (1), 1–16. [Google Scholar]
- Dafale, N. A.; Srivastava, S.; Purohit, H. J. Zoonosis: an emerging link to antibiotic resistance under “one health approach”. Indian journal of microbiology 2020, 60, 139–152. [Google Scholar] [CrossRef]
- Bortolaia, V.; Guardabassi, L. Zoonotic Transmission of Antimicrobial-Resistant Enterococci: A Threat to Public Health or an Overemphasized Risk? In Zoonoses: Infections Affecting Humans and Animals, Sing, A. Ed.; Cham: Springer International Publishing, Germany, 2023; pp. 1–33. [Google Scholar]
- Wambui, J.; Tasara, T.; Njage, P. M. K.; Stephan, R. Species distribution and antimicrobial profiles of Enterococcus spp. isolates from Kenyan small and medium enterprise slaughterhouses. Journal of food protection 2018, 81, 1445–1449. [Google Scholar] [CrossRef]
- European Food Safety Authority Panel on Biological Hazards-BIOHAZ. Scientific Opinion on the public health hazards to be covered by inspection of meat (bovine animals). EFSA Journal 2013, 11, 3266. [Google Scholar] [CrossRef]
- European Food Safety Authority Panel on Biological Hazards-BIOHAZ. Scientific Opinion on the public health hazards to be covered by inspection of meat from sheep and goats. EFSA Journal 2013, 11, 3265. [Google Scholar] [CrossRef]
- Wardhana, D. K. Risk factors for bacterial contamination of bovine meat during slaughter in ten Indonesian abattoirs. Veterinary medicine international 2019. [Google Scholar]
- Microbiology of the food chain − Carcass sampling for microbiological analysis, ISO 17604; International Organization for Standardization: Geneva, Switzerland, 2015.
- Cebeci, T. Listeria monocytogenes in Ruminants at an Abattoir: Prevalence, Virulence Characteristics, Serotypes and Antibiotic Resistance in Eastern Türkiye. Israel Journal of Veterinary Medicine 2022, 77, 4. [Google Scholar]
- Pesavento, G.; Calonico, C.; Ducci, B.; Magnanini, A.; Nostro, A. L. Prevalence and antibiotic resistance of Enterococcus spp. isolated from retail cheese, ready-to-eat salads, ham, and raw meat. Food microbiology 2014, 41, 1–7. [Google Scholar] [CrossRef]
- Quintela-Baluja, M.; Böhme, K.; Fernández-No, I. C.; Morandi, S.; Alnakip, M. E.; Caamaño-Antelo, S.; Barros-Velázquez, J.; Calo-Mata, P. Characterization of different food-isolated Enterococcus strains by MALDI-TOF mass fingerprinting. Electrophoresis 2013, 34, 2240–2250. [Google Scholar] [CrossRef]
- Chotinantakul, K.; Chansiw, N.; Okada, S. Antimicrobial resistance of Enterococcus spp. isolated from Thai fermented pork in Chiang Rai Province, Thailand. Journal of Global Antimicrobial Resistance 2018, 12, 143–148. [Google Scholar] [CrossRef]
- Billström, H.; Lund, B.; Sullivan, Å.; Nord, C. E. Virulence and antimicrobial resistance in clinical Enterococcus faecium. International journal of antimicrobial agents 2008, 32, 374–377. [Google Scholar] [CrossRef]
- Performance standards for antimicrobial susceptibility testing, CLSI supplement M100, 33rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023.
- The European Committee on Antimicrobial Susceptibility Testing, EUCAST supplement 2023, version 13; Breakpoint tables for interpretation of MICs and zone diameters,, The European Committee: Växjö, Sweden, 2023.
- Kim, Y. B.; Seo, K. W.; Son, S. H.; Noh, E. B.; Lee, Y. J. Genetic characterization of high-level aminoglycoside-resistant Enterococcus faecalis and Enterococcus faecium isolated from retail chicken meat. Poultry Science 2019, 98, 5981–5988. [Google Scholar] [CrossRef]
- Ben Braiek, O.; Smaoui, S. Enterococci: between emerging pathogens and potential probiotics. BioMed research international 2019, 2019, 5938210. [Google Scholar] [CrossRef]
- Efstratiou, A.; Lamagni, T.; Turner, C. E. Streptococci and Enterococci. In Infectious Diseases, 4th ed.; Cohen, J., Powderly, W. G., Opal, S. M., Eds.; Elsevier: China, 2017; Volume 2, pp. 1523–1536. [Google Scholar]
- Holman, D. B.; Gzyl, K. E.; Zaheer, R.; Jones, T. H.; McAllister, T. A. Draft genome sequences of 43 Enterococcus faecalis and Enterococcus faecium isolates from a commercial beef processing plant and retail ground beef. Microbiology Resource Announcements 2019, 8(42), 10–1128. [Google Scholar] [CrossRef]
- Holman, D. B.; Klima, C. L.; Gzyl, K. E.; Zaheer, R.; Service, C.; Jones, T. H.; McAllister, T. A. A Longitudinal Study of Antimicrobial Resistance in Enterococcus spp. Isolated from a Beef Processing Plant and Retail Ground Beef. bioRxiv 2021, 2021-05. [Google Scholar]
- Telli, N.; Telli, A. E.; Biçer, Y.; Turkal, G.; Uçar, G. Isolation and antimicrobial resistance of vancomycin resistant Enterococcus spp.(VRE) and methicillin-resistant S. aureus (MRSA) on beef and chicken meat, and workers hands from slaughterhouses and retail shops in Turkey. Journal of the Hellenic Veterinary Medical Society 2021, 72, 3345–3354. [Google Scholar] [CrossRef]
- Messele, Y. E.; Hasoon, M. F.; Trott, D. J.; Veltman, T.; McMeniman, J. P.; Kidd, S. P.; Low, W. Y.; Petrovski, K. R. Longitudinal Analysis of Antimicrobial Resistance among Enterococcus Species Isolated from Australian Beef Cattle Faeces at Feedlot Entry and Exit. Animals 2022, 12, 2690. [Google Scholar] [CrossRef]
- Guzman Prieto, A. M.; van Schaik, W.; Rogers, M. R.; Coque, T. M.; Baquero, F.; Corander, J.; Willems, R. J. Global emergence and dissemination of enterococci as nosocomial pathogens: attack of the clones? Frontiers in microbiology 2016, 7, 788. [Google Scholar] [CrossRef]
- Igbinosa, E. O.; Beshiru, A. Antimicrobial resistance, virulence determinants, and biofilm formation of Enterococcus species from ready-to-eat seafood. Frontiers in Microbiology 2019, 10, 728. [Google Scholar] [CrossRef]
- Golob, M.; Pate, M.; Kušar, D.; Dermota, U.; Avberšek, J.; Papić, B.; Zdovc, I. Antimicrobial resistance and virulence genes in Enterococcus faecium and Enterococcus faecalis from humans and retail red meat. BioMed research international 2019.
- Fiore, E.; Van Tyne, D.; Gilmore, M. S. Pathogenicity of enterococci. Microbiology spectrum 2019, 7, 7–4. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, M.; Wan, C.; Chen, X.; Chen, X.; Tao, X.; Shah, N. P.; Wei, H. Screening probiotic strains for safety: Evaluation of virulence and antimicrobial susceptibility of enterococci from healthy Chinese infants. Journal of dairy science 2016, 99(6), 4282–4290. [Google Scholar] [CrossRef]
- Mohanty, S.; Behera, B. Antibiogram Pattern and Virulence Trait Characterization of Enterococcus Species Clinical Isolates in Eastern India: A Recent Analysis. Journal of Laboratory Physicians 2022, 14, 237–246. [Google Scholar] [CrossRef]
- Ahmed, M. O.; Baptiste, K. E. Vancomycin-resistant enterococci: a review of antimicrobial resistance mechanisms and perspectives of human and animal health. Microbial Drug Resistance 2018, 24, 590–606. [Google Scholar] [CrossRef]
- Papich, M. G. Oxytetracycline. In Saunders Handbook of Veterinary Drug Small and Large Animal, 4th ed.; Papich, M. G., Ed.; Sounders: USA, 2016; pp. 595–598. [Google Scholar]
- Ayeni, F. A.; Odumosu, B. T.; Oluseyi, A. E.; Ruppitsch, W. Identification and prevalence of tetracycline resistance in enterococci isolated from poultry in Ilishan, Ogun State, Nigeria. Journal of pharmacy & bioallied sciences 2016, 8(1), 69. [Google Scholar]
- Smith, M. V. Therapeutics. In Textbook of Rabbit Medicine, 3rd ed.; Smith, M. V., Ed.; Elsevier: Poland, 2023; pp. 100–137. [Google Scholar]
- Ngbede, E. O.; Raji, M. A.; Kwanashie, C. N.; Kwaga, J. K. P. Antimicrobial resistance and virulence profile of enterococci isolated from poultry and cattle sources in Nigeria. Tropical animal health and production 2017, 49, 451–458. [Google Scholar] [CrossRef]
- Barlow, R. S.; McMillan, K. E.; Duffy, L. L.; Fegan, N.; Jordan, D.; Mellor, G. E. Antimicrobial resistance status of Enterococcus from Australian cattle populations at slaughter. PLoS One 2017, 12, e0177728. [Google Scholar] [CrossRef]
- Xuan, H.; Yao, X.; Pan, R.; Gao, Y.; Wei, J.; Shao, D.; Liu, K.; Li, Z.; Qiu, Y.; Ma, Z.; Li, B.; Xia, L. Antimicrobial resistance in Enterococcus faecium and Enterococcus faecalis isolates of swine origin from eighteen provinces in China. Journal of Veterinary Medical Science 2021, 83, 1952–1958. [Google Scholar] [CrossRef]
- Yu, L.; Liu, Y.; Liu, M.; Li, Z.; Li, L.; Wang, F. Research Note: Molecular characterization of antimicrobial resistance and virulence gene analysis of Enterococcus faecalis in poultry in Tai'an, China. Poultry Science 2022, 101, 101763. [Google Scholar] [CrossRef]
- Hollenbeck, B. L.; Rice, L. B. Intrinsic and acquired resistance mechanisms in Enterococcus. Virulence 2012, 3(5), 421–569. [Google Scholar] [CrossRef]
- Jian, Z. , Zeng, L., Xu, T., Sun, S., Yan, S., Yang, L., Huang, Y.; Jia, J.; Dou, T. Antibiotic resistance genes in bacteria: Occurrence, spread, and control. Journal of basic microbiology 2021, 61, 1049–1070. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, J.; Wei, Q.; Hu, Q.; Lin, X.; Chen, M.; Ye, R.; Lv, H. Characterization of aminoglycoside resistance and virulence genes among Enterococcus spp. isolated from a hospital in China. International journal of environmental research and public health 2015, 12, 3014–3025. [Google Scholar] [CrossRef]
- Kristich, C. J.; Rice, L. B.; Arias, C. A. Enterococcal infection—treatment and antibiotic resistance. In Enterococci: from commensals to leading causes of drug resistant infection [Internet]; Gilmore, M. S., Ed.; Massachusetts Eye and Ear Infirmary: Boston, USA, 2014; pp. 1–47. [Google Scholar]
- Channaiah, L. H.; Subramanyam, B.; Zurek, L. Molecular characterization of antibiotic resistant and potentially virulent enterococci isolated from swine farms and feed mills. Journal of Stored Products Research 2018, 77, 189–196. [Google Scholar] [CrossRef]
- Hernando-Amado, S.; Blanco, P.; Alcalde-Rico, M.; Corona, F.; Reales-Calderón, J. A.; Sánchez, M. B.; Martínez, J. L. Multidrug efflux pumps as main players in intrinsic and acquired resistance to antimicrobials. Drug Resistance Updates 2016, 28, 13–27. [Google Scholar] [CrossRef]
- Sanchez Valenzuela, A.; Lavilla Lerma, L.; Benomar, N.; Gálvez, A.; Perez Pulido, R.; Abriouel, H. Phenotypic and molecular antibiotic resistance profile of Enterococcus faecalis and Enterococcus faecium isolated from different traditional fermented foods. Foodborne pathogens and disease 2013, 10, 143–149. [Google Scholar] [CrossRef]
- Rehman, M. A.; Yin, X. , Zaheer; R., Goji; N., Amoako; K. K., McAllister, T., Pritchard, J.; Diarra, M. S. Genotypes and phenotypes of Enterococci isolated from broiler chickens. Frontiers in Sustainable Food Systems 2018, 2, 83. [Google Scholar] [CrossRef]
| Target gene | Primer sequence (5'-3') | Fragment size (pb) |
|---|---|---|
| Enterococcus spp. (16S rRNA) | F: AGCGCAGGCGGTTTCTTAA R: CTCGTTGTACTTCCCATTGT |
941 |
| Enterococcus faecalis | F: ATCAAGTACAGTTAGTCTTTATTAG R:ACGATTCAAAGCTAACTGAATCAGT |
658 |
| Enterococcus faecium | F: TTGAGGCAGACCAGATTGACG R: GCTGCTAAAGCTGCGCTT |
822 |
| Enterococcus gallinarum | F: GGTATCAAGGAAACCTC R: CTTCCGCCATCATAGCT |
484 |
| Enterococcus casseliflavus | F: CGGGGAAGATGGCAGTAT R: CGCAGGGACGGTGATTTT |
521 |
| Enterococcus hirae | F: GCATATTTATCCAGCACTAG R: CTCTGGATCAAGTCCATAAGTGG |
639 |
| asa1 | F: CACGCTATTACGAACTATGA R: TAAGAAAGAACATCACCACGA |
375 |
| ace | F: GGAATGACCGAGAACGATGGC R: GCTTGATGTTGGCCTGCTTCCG |
616 |
| cylA | F: ACTCGGGGATTGATAGGC R: GCTGCTAAAGCTGCGCTT |
688 |
| efaA | F: CGTGAGAAAGAAATGGAGGA R: CTACTAACACGTCACGAATG |
499 |
| esp | F: AGATTTCATCTTTGATTCTTG R: AATTGATTCTTTAGCATCTGG |
510 |
| gelE | F: TATGACAATGCTTTTTGGGAT R: AGATGCACCCGAAATAATATA |
213 |
| hyl | F: ACAGAAGAGCTGCAGGAAATG R: GACTGACGTCCAAGTTTCCAA |
276 |
| Antimicrobial agent | Target gene | Primer sequence (5'-3') | Fragment size (pb) |
|---|---|---|---|
| Macrolides | ermA | F: TAACATCAGTACGGATATTG R: AGTCTACACTTGGCTTAGG |
200 |
| Macrolides | ermB | F: CCGAACACTAGGGTTGCTC R: ATCTGGAACATCTGTGGTATG |
139 |
| Macrolides | mef | F: AGTATCATTAATCACTAGTGC R: TTCTTCTGGTACTAAAAGTGG |
348 |
| Tetracyclines | tet(L) | F: ATAAATTGTTTCGGGTCGGTAAT R: AACCAGCCAACTAATGACAATGAT |
1077 |
| Tetracyclines | tet(M) | F: GTTAAATAGTGTTCTTGGAG R: CTAAGATATGGCTCTAACAA |
657 |
| Tetracyclines | tet(O) | F: GATGGCATACAGGCACAGAC R: CAATATCACCAGAGCAGGCT |
614 |
| Phenicols | cfr | F: TGAAGTATAAAGCAGGTTGGGAGTCA R: ACCATATAATTGACCACAAGCAGC |
746 |
| Phenicols | fexA | F: GTACTTGTAGGTGCAATTACGGCTGA R: CGCATCTGAGTAGGACATAGCGTC |
1272 |
| Phenicols | optrA | F: AGGTGGTCAGCGAACTAA R: ATCAACTGTTCCCATTCA |
1379 |
| Efflux pump | eme(A) | F: AGCCCAAGCGAAAAGCGGTTT R: CCATCGCTTTCGGACGTTCA |
123 |
| Efflux pump | efr(A) | F: GTCTGTTTCGTTTAATGGCAGCAGCC R: CGAATAGCTGGTTCATGTCTAAGGC |
258 |
| Efflux pump | lsa | F: GTGACTTCTTTTGAACAGTGGGA R: TTCAGCCACTTGTTGTCTGCC |
232 |
| Aminoglycoside modifying enzyme |
aac(6’)Ie-aph(2")-la | F: CAGAGCCTTGGGAAGATGAAG R: CCTCGTGTAATTCATGTTCTGGC |
348 |
| Aminoglycoside modifying enzyme |
aph(2")-Ib | F: CTTGGACGCTGAGATATATGAGCAC R: GTTTGTAGCAATTCAGAAACACCCTT |
867 |
| Aminoglycoside modifying enzyme |
aph(2")-Ic | F: CCACAATGATAATGACTCAGTTCCC R: CCACAGCTTCCGATAGCAAGAG |
641 |
| Aminoglycoside modifying enzyme |
aph(2")-Id | F: GTGGTTTTTACAGGAATGCCATC R: CCCTCTTCATACCAATCCATATAACC |
284 |
| Aminoglycoside modifying enzyme |
ant(3")-Ia | F: TGATTTGCTGGTTACGGTGAC R: CGCTATGTTCTCTTGCTTTTG |
284 |
| Aminoglycoside modifying enzyme |
aph(6)-Ia | F: ACTGGCTTAATCAATTTGGG R: GCCTTTCCGCCACCTCACCG |
596 |
| Glycopeptıdes | vanA | F: ATTGCTATTCAGCTGTACTC R: GGCTCGACTTCCTGATGAAT |
559 |
| Glycopeptıdes | vanB | F: AACGGCGTATGGAAGCTATG R: CCATCATATTGTCCTGCTGC |
467 |
| Glycopeptıdes | vanC1 | F: GGCATCGCACCAACAATGGA R: TCCTCTGCCAGTGCAATCAA |
902 |
| Glycopeptıdes | vanC2 | F: TTCAGCAACTAGCGCAATCG R: TCACAAGCACCGACAGTCAA |
663 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




