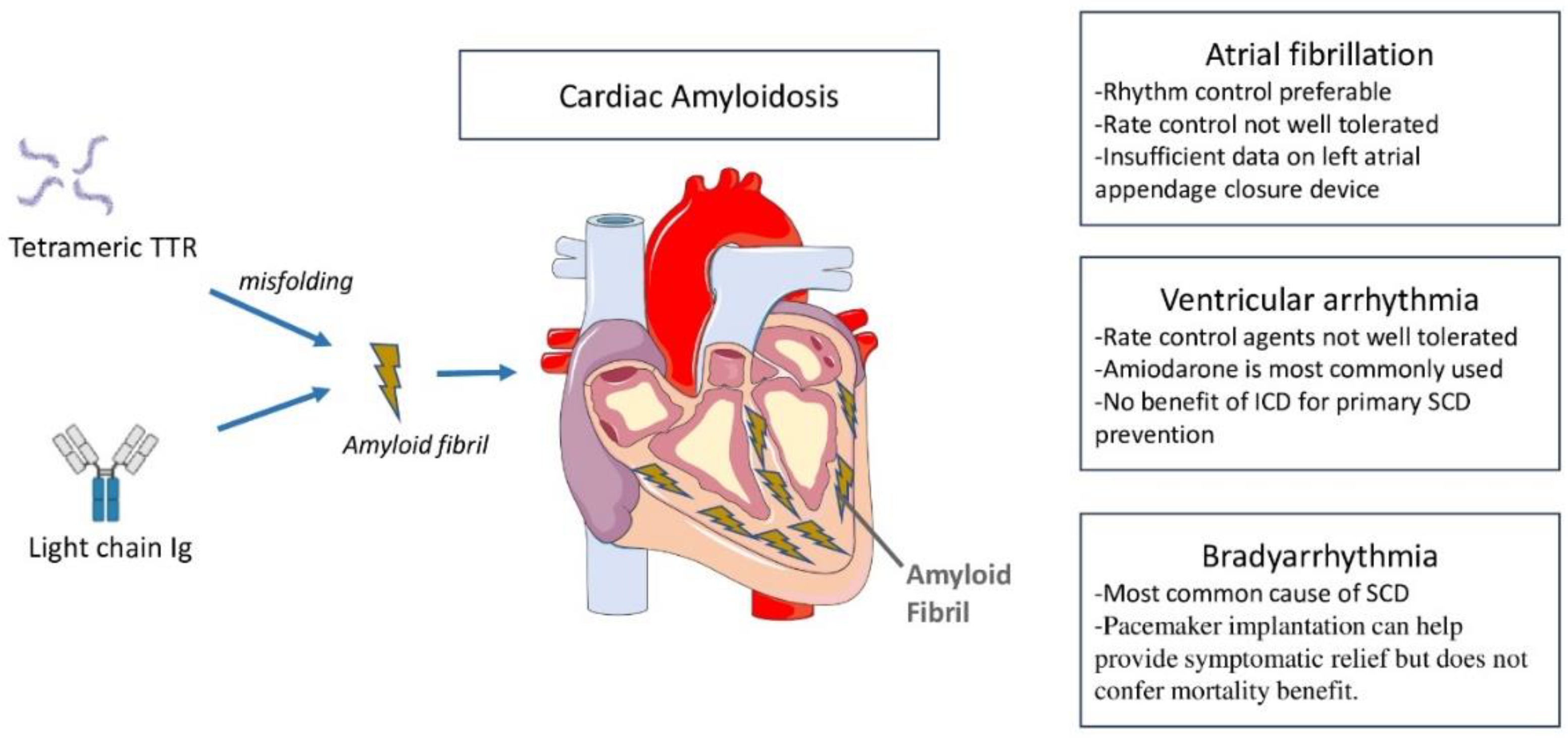
Highlights
Left atrial appendage closure device has shown benefit in small scale studies, although prospective studies are warranted in cardiac amyloidosis.
Pacemaker implantation in bradyarrhythmias can help provide symptomatic relief but does not confer mortality benefit.
Implantable cardioverter-defibrillator in ventricular tachyarrhythmias has not demonstrated benefit for primary prevention of sudden cardiac death.
Cardiac amyloidosis (CA) is an underdiagnosed etiology of heart failure, which can lead to death if untreated. It results from myocardial deposition of insoluble amyloid fibrils that originate either from hepatically synthesized transthyretin protein (ATTR) or plasma cell-derived immunoglobulin light chain (AL) [
1]. ATTR is further differentiated into wildtype (wtATTR) and hereditary (hATTR) based on the absence or presence of a mutation in the TTR gene sequence. While AL requires histological confirmation, ATTR can be diagnosed noninvasively with Tc-pyrophosphate scintigraphy in the majority of cases [
2,
3]. The clinical phenotype varies significantly between different types of CA, resulting in a spectrum of presentations.
AL is a hematological disorder caused by the proliferation of an abnormal plasma cell clone that overproduces lambda or kappa light chains. It affects multiple organs, but involvement of the heart determines prognosis. The age at diagnosis is about 60-70 years and has a more aggressive disease course compared with ATTR due to cardiotoxicity associated with amyloidogenic light chains [
4,
5]. wtATTR, previously known as senile CA has been increasingly recognized with the advent of refined imaging modalities and its prevalence ranges between 13% and 19% of patients hospitalized with diastolic heart failure [
6]. wtATTR typically affects elderly caucasian men, and cardiac involvement is always present [
7]. On the other hand, hATTR demonstrates variability in the age of onset, primary phenotype (cardiomyopathy, neuropathy, or mixed) and disease course depending on the mutation and fibril type. V122I mutation is the most common mutation in the US, affecting ~3 % of American-American patients, and clinically resembles wtATTR in phenotype. Conversely, V30M mutation may manifest as either a predominantly neuropathic phenotype in the early stages or a predominantly cardiomyopathic phenotype in the late-onset cases.
Rhythm disturbances are a common occurrence in CA (
Figure 1). The mechanisms are multifaceted, involving inflammatory trigger, structural changes with chamber dilatation, and neurohumoral abnormalities. While atrial tachyarrhythmias are most commonly seen in CA, ventricular tachyarrhythmias as well as bradyarrhythmias are also observed. The types of arrhythmias and their prevalence vary with CA subtypes. In this review, we discuss mechanisms and epidemiology of various types of arrhythmias in CA, explore the utility of device therapies, and highlight knowledge gaps and future directions.
1. Atrial arrhythmias in CA
Prevalence and mechanisms
Atrial arrhythmias are more prevalent in CA compared with the general population. The prevalence of atrial fibrillation (AF) in CA has been reported to range from ~ 40% to 88%, and is significantly higher in wtATTR compared with both AL and hATTR [
8,
9]. In a study of 238 patients examining all major CA subtypes (115 AL, 123 ATTR), the overall prevalence of AF was 44%, which is significantly higher than the prevalence of AF in the community [
10,
11,
12]. Bukhari et al. reported that the prevalence of AF in wtATTR was 2-fold higher compared to the age-matched non-amyloid heart failure group (88% versus 39%, p< 0.01) [
9].
The development of AF in CA involves different mechanisms. The intramyocardial amyloid accumulation leads to impaired ventricular relaxation and elevated filling pressures, ultimately resulting in left atrial (LA) enlargement. In addition, amyloid deposition also disrupts electrical conduction and myocyte contractility leading to biomechanical dysfunction which may provide an arrhythmogenic substrate for AF [
13]. There can be isolated atrial amyloidosis from direct deposition of amyloid in atria, particularly in the LA, leading to impaired atrial mechanics [
14,
15,
16]. Amyloid deposition in the atria disturbs myocyte contractility and disrupts homogenous electrical conduction, thereby providing an arrhythmogenic substrate for the development of AF by giving rise to functional re-entry [
17]. It is thought that AF, in turn, potentiates further amyloid deposition, thereby leading to a vicious cycle [
14].
Management of AF and role of left appendage closure device
Patients with CA often exhibit intolerance to rate control strategies, primarily due to restrictive pathophysiology. The restrictive mechanics render cardiac output dependent on heart rate and left ventricular (LV) diastolic filling [
18]. In addition, amyloid fibrils avidly bind to calcium channel blockers, which can exaggerate medication side effects including orthostatic hypotension and syncope in these patients [
19,
20]. Amyloid fibrils also have an affinity for digoxin, but studies have shown that cautious use and frequent monitoring of serum drug level can mitigate medication side effects [
21,
22,
23]. While rhythm control, which restores atrial contribution and improves ventricular filling, may potentially be a more favorable strategy in CA, it does not have any mortality benefit over a rate control strategy [
24]. However, data on the safety of antiarrhythmics is insufficient in this population. Many antiarrhythmic agents cannot be used in CA either due to cardiomyopathy (such as flecainide and propafenone) or due to concomitant advanced kidney disease (such as sotalol, dronedarone, and dofetilide) [
25]. Amiodarone is the preferred agent and most commonly used in this population. Direct current cardioversion (DCCV) provides an important alternative for restoration of sinus rhythm and possible avoidance of negative chronotropic agents. The data on the efficacy and safety of catheter ablation in CA is limited.
CA is associated with an increased risk of thromboembolism, and presence of AF heightens this risk. CHA
2DS
2VASc score has limited utility as a risk prediction tool. Anticoagulation is recommended in patients with CA and AF regardless of the CHA
2DS
2VASc score [
26,
27]. Both direct oral anticoagulants and warfarin are comparable in terms of efficacy and safety profile [
28,
29,
30]. Unfortunately, high bleeding risk is often a prohibitive factor for anticoagulation in CA [
31,
32,
33]. In patients who are on anticoagulation, LA appendage thrombus can persist despite receiving therapeutic doses, making anticoagulation strategy very challenging in CA [
34]. The reported LA appendage thrombus resolution in the general population is approximately 90% after a median of 4 weeks of adequate anticoagulation, but the LA appendage thrombus resolution rate in CA is lower (34).
In patients who are intolerant to anticoagulation, the potential option is LA appendage closure device, but it has not been studied extensively in CA yet. In Cardiac Amyloidosis and Left Atrial Appendage Closure (CAMYLAAC) study, there were no significant differences in mortality between patients with and without CA (20% vs 13.6%, p=0.248) at 2-year follow up. However, at 5-year follow-up, ATTR patients had higher mortality (40% vs 19.2%;
P < .001), but importantly this difference was unrelated to hemorrhagic complications or ischemic stroke [
35]. Hence, while LA appendage closure in CA could provide an exciting opportunity for stroke prophylaxis when anticoagulation cannot be tolerated, prospective studies are required to evaluate its efficacy in CA.
2. Ventricular tachyarrhythmias
Prevalence and mechanisms
Ventricular tachyarrhythmias (VT) in CA could present as ventricular ectopy, non-sustained VT, sustained VT, or a combination of the above. The prevalence of VT in CA is higher than those without amyloidosis, and among the CA subtypes it is higher in AL compared to ATTR. It has been reported to be 27% in AL as compared to 17% in ATTR patients. [
36,
37,
38,
39]. AL patients undergoing stem cell transplantation have a higher prevalence of VT, likely due to the extent of myocardial damage caused by the aggressive nature of AL [
38].
There are numerous mechanisms of pathogenesis of VT in CA, including electromechanical dysfunction and autonomic dysregulation that are uniquely related to systemic amyloid deposition [
40]. Amyloid deposition results in the activation of the inflammatory cascade and oxidative stress, leading to ventricular remodeling and fibrosis [
41,
42]. This, in addition to microvascular ischemia caused by amyloid infiltration, results in the development of anatomical re-entrant circuits causing VT. The pre-fibrillar amyloidogenic light chains are also more cytotoxic than ATTR, inducing a greater degree of apoptosis through oxidative stress, which potentially explains the higher prevalence of VT in AL compared with ATTR [
3].
Management of VT and role of Implantable Cardioverter-Defibrillator
CA patients poorly tolerate most of the medications that are used to control VT. Calcium channel blockers and beta-blockers often lead to a low-output state, worsening hemodynamics and cardiac decompensation, as discussed above. Amiodarone is most commonly used as an antiarrhythmic agent in CA. The role of catheter ablation has not been validated in large-scale studies.
CA, especially AL, is associated with an increased risk of SCD, accounting for approximately one-third of the mortality within the first 90 days of AL diagnosis [
43]. Despite the high risk of SCD in CA, consensus guidelines have demonstrated little enthusiasm for implantable cardioverter-defibrillator (ICD) placement for the prevention of SCD in CA due to multiple reasons. The prognosis of AL with heart failure has generally been poor, with survival lasting < 12 months after diagnosis, representing a relative contraindication to ICD implantation. More importantly, the most frequent etiology of SCD in CA is pulseless electrical activity or bradyarrhythmia due to electromechanical dissociation, rather than VT [
37,
43].
Data on the efficacy of ICD for primary or secondary prevention in CA is scant and primarily derived from case reports and small-scale observational studies (
Table 1) [
43,
44,
45,
46,
47,
48,
49]. There has been no mortality benefit demonstrated in these studies. In a study comprising of 19 histologically proven AL patients who were followed for a mean period of 811 ±151 days, two patients with sustained VT were successfully treated by the ICD. Seven (37%) patients died during the study period, and the majority (n=6) died due to electromechanical dissociation not amenable to ICD treatment. The authors deduced that while the majority of SCDs were caused by bradyarrhythmias that are not amenable to ICD, a select group of patients with life-threatening tachyarrhythmias could potentially benefit from ICD implantation.
ICD implantation specifically for primary prevention has no proven benefit and could even be harmful [
46,
47]. A single-centered study examined 53 CA patients who underwent ICD implantation for either primary prevention of SCD (n=41) or secondary prevention (n=12). The rate of appropriate ICD shocks was 32% at 1-year follow-up, observed almost exclusively in AL patients and those who had received an ICD for secondary prevention (p < 0.001); however, ICD therapy was not associated with improved mortality in follow-up.
Another study from Stanford University revealed that 26% of 19 patients received appropriate ICD shocks in follow-up, and all of them had received an ICD for secondary prevention [
44]. Additionally, the authors proposed criteria for appropriate implantation in patients with CA, including patients who have a high risk of SCD as well as a good quality of life and minimal heart failure symptoms as assessed by the New York Heart Association functional classification (NYHA FC), and excluding patients who have a life expectancy of < 1 year. The criteria proposed that CA patients who have a life expectancy of > 1 year or NYHA FC I-III could be candidates for ICD implantation if they have 1) non-postural exertional syncope and/or 2) evidence of NSVT or VT on telemetric monitoring. There is emerging data that extracellular volume on cardiac magnetic resonance can predict the incident ventricular arrhythmia, irrespective of the etiology of cardiomyopathy, thereby providing an exciting research avenue to identify CA patients who may benefit from ICD. The 2015 European Society of Cardiology guidelines state that there is insufficient data to provide recommendations for the use of ICDs for primary prevention of SCD in CA, and giving a Class IIa, Level of Evidence: C, for ICD implantation for secondary prevention [
51]. The guidelines recommend consideration of ICD implantation for secondary prevention for those with either AL or hATTR and “ventricular arrhythmia causing hemodynamic instability who are expected to survive >1 year with good functional status.” The 2017 American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines for management of patients with ventricular arrhythmias and the prevention of SCD recommend individualized decision making for both primary and secondary prevention with ICDs in CA [
52].
In summary, the decisions of ICD implantation should be cautiously considered and discussed between the patient and physician at an earlier stage, carefully weighing the risks and benefits of the procedure on life expectancy and quality of life.
Epidemiology of bradyarrhythmias and role of pacemaker in CA
CA is commonly associated with bradyarrhythmias. Atrioventricular conduction delay involving the His-Purkinje system is more common than pure sinus node disease and is associated with symptomatic AV block [
17]. A study comprising 16 consecutive patients with hATTR with polyneuropathy who underwent 24-hour ambulatory monitoring found evidence of sinus pauses (defined as >2 s) in 25% and conduction disturbances in 38% of the patients. During a follow-up period of 14 months, 5 patients received pacemaker implantation for either advanced AV nodal block or sinus node dysfunction [
53]. In another cohort of 18 CA patients (4 AL, 14 wtATTR) who were analyzed using electrophysiological studies and compared with age- and gender-matched non-CA patients, CA patients were found to have significant
AH interval (conduction time from the low-right atrium at the interatrial septum through the AV node to the His bundle) and
HV interval (conduction time from the proximal His bundle to the ventricular myocardium) prolongation with a lesser degree of QRS prolongation. Notably, the prolongation was more profound in wtATTR compared with AL [
17].
The prevalence of pacemaker implantation has been variably reported to range from 8.9% to ~40%, depending on the study size as well as the subtype of CA under investigation [
54,
55,
56]. wtATTR patients have higher rates of pacemaker implantation compared with both AL and hATTR, which is attributed to the older age and less aggressive disease course in these patients. History of AF, PR >200 ms, and QRS >120 ms on EKG are predictors of pacemaker placement in CA. While pacemaker implantation may provide symptomatic benefit, it does not impact mortality. In fact, the need for a pacemaker is a marker of advanced disease associated with a poor prognosis [
56,
57].
CA patients with pacemakers are noted to have progressive conduction disease and eventually become dependent on ventricular pacing. The use of cardiac implantable electronic devices (CIED) in CA patients has provided a window for the surveillance of rhythm disturbances and has given insight into the patterns of disease progression. In a single-centered retrospective study, longitudinal data was analyzed in 34 CA patients who had undergone CIED implantation for bradycardia or SCD. The interrogation of device data for 3.1 ± 4 years showed a progressive increase in the mean ventricular pacing, and the pacing burden increased from 56% at 1-year post-implantation to ~ 100% ventricular pacing at 5 years in the majority of patients [
56,
58].
The progressive increase in right ventricular pacing burden causes impairment of cardiac function and is associated with worse outcomes in CA. In a retrospective observational cohort study of 78 patients with ATTR and implantable devices, RV pacing was associated with worsening ejection fraction, mitral regurgitation, and heart failure symptoms [
59]. On the other hand, biventricular therapy resulted in improvement in ejection fraction, systemic congestion, and mitral regurgitation severity [
59]. In another retrospective study from the Cleveland Clinic, cardiac resynchronization therapy was associated with improved survival among patients with ATTR and also resulted in improvements in heart failure symptoms and left ventricular ejection fraction [
60].
In a study from UK National Amyloidosis Centre that used loop recorders to characterize arrhythmias in Mayo Stage III AL patients, it was discovered that marked bradyarrhythmia heralded terminal cardiac decompensation in the majority of patients [
61]. Over a median follow-up period of 308 days, 13 patients died, and the median survival in the whole cohort was 61 days from device insertion. In 8 evaluable cases, death was heralded by complete atrioventricular block, followed shortly thereafter by pulseless electrical activity. 3 out of 4 patients who received pacemakers had rapid cardiac decompensation and died. Despite 272 loop recordings, there was only one episode of non-sustained VT, which was preceded by severe bradycardia. The authors proposed that a study of prophylactic pacemaker implantation in this patient population is needed. While there are small-scale studies supporting use of prophylactic pacemakers in hATTR, large-scale and multi-centered studies are needed to assess the effectiveness of prophylactic pacemakers in all subtypes of CA [
62,
63].
Knowledge Gaps and Future Directions
The recognition of CA has grown significantly with the advent of noninvasive testing, contributing to an enhanced understanding of the disease and its associations, including arrhythmias [
64]. A comprehensive, multicenter collaborative effort is crucial to define arrhythmias associated with CA, allowing for a better understanding of the arrhythmia burden, its mechanisms, and the associated clinical outcomes in this population. The current landscape lacks prospective and large-scale studies to validate the efficacy of devices such as the Watchman's device, pacemakers, or ICDs in CA patients. It remains to be seen whether rhythm control with
antiarrhythmic agents, catheter ablation, or
DCCV is superior to rate control for atrial
tachyarrhythmias in these patients. Similarly, more studies are needed to assess the efficacy of early implantation of a pacemaker in patients with a high risk of heart block, which may also serve to monitor arrhythmias. Moreover, the utility of biventricular pacing and the role of ventricular synchrony in CA cardiomyopathy need further clarification. Future investigations should focus on identifying clinical factors and imaging biomarkers to predict fatal arrhythmias in CA patients, aiming to identify a subset of patients that may derive a mortality benefit from cardiac devices.
There is a need for development of validated risk assessment tools for thromboembolic and bleeding risk in CA. Mechanisms leading to exceptionally high thromboembolic risk in CA are unclear yet. In addition, no prospective, large-scale studies are available to compare the efficacy and safety profile of direct oral anticoagulants and warfarin for prevention of stroke and systemic embolism in CA patients with AF.
The disease-modifying drugs for CA act by preventing amyloid formation and deposition, but none targets removal of existing amyloid deposits from the affected organ. Removing preexisting amyloid deposits to potentially restore organ function remains a huge treatment gap. It also remains to be seen whether advancements in therapies for both ATTR and AL have a favorable impact on prevention of life-threatening arrhythmias, and also on mitigation of stroke risk in AF.
Conclusions
CA arises from the deposition of amyloid fibrils in the myocardial interstitium, triggering electromechanical, inflammatory, and autonomic changes that result in arrhythmias. A prevalent arrhythmia in this condition is atrial fibrillation, posing management challenges with insufficient data on left appendage closure devices. Ventricular arrhythmias are not uncommon, and the role of ICD in CA remains controversial, with no evidence of improved outcomes in primary prevention cases. The most common arrhythmias leading to sudden cardiac death are bradyarrhythmias and complete heart block. Although pacemaker implantation can provide symptomatic relief, it does not confer a mortality benefit.
References
- Maleszewski, JJ. Cardiac amyloidosis: pathology, nomenclature, and typing. Cardiovasc Pathol. 2015;24:343-50. [CrossRef]
- Falk RH, Alexander KM, Liao R, Dorbala S. AL (Light-Chain) Cardiac Amyloidosis: A Review of Diagnosis and Therapy. J Am Coll Cardiol. 2016;68:1323-41. [CrossRef]
- Masri A, Bukhari S, Eisele YS, Soman P. Molecular Imaging of Cardiac Amyloidosis. J Nucl Med. 2020;61:965-970. [CrossRef]
- Bukhari S, Khan SZ, Bashir Z. Atrial Fibrillation, Thromboembolic Risk, and Anticoagulation in Cardiac Amyloidosis: A Review. J Card Fail. 2023;29(:76-86. [CrossRef]
- Shi J, Guan J, Jiang B, Brenner DA, Del Monte F, Ward JE, Connors LH, Sawyer DB, Semigran MJ, Macgillivray TE, Seldin DC, Falk R, Liao R. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38alpha MAPK pathway. Proc Natl Acad Sci U S A. 2010;107:4188-93. [CrossRef]
- Bashir Z, Chen EW, Tori K, Ghosalkar D, Aurigemma GP, Dickey JB, Haines P. Insight into different phenotypic presentations of heart failure with preserved ejection fraction. Prog Cardiovasc Dis. 2023;79:80-88. [CrossRef]
- Bukhari, S., Barakat, A., Mulukutla, S., et al. Faster progression of left ventricular thickness in men compared to women in wild-type transthyretin cardiac amyloidosis. Journal of the American College of Cardiology, 75(11_Supplement_1), 812-812. Abstract. [CrossRef]
- Mints YY, Doros G, Berk JL, Connors LH, Ruberg FL. Features of atrial fibrillation in wild-type transthyretin cardiac amyloidosis: a systematic review and clinical experience. ESC Heart Fail. 2018;5:772-779. [CrossRef]
- Bukhari S, Barakat AF, Eisele YS, Nieves R, Jain S, Saba S, Follansbee WP, Brownell A, Soman P. Prevalence of Atrial Fibrillation and Thromboembolic Risk in Wild-Type Transthyretin Amyloid Cardiomyopathy. Circulation. 2021;143:1335-1337. [CrossRef]
- Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial Fibrillation: Epidemiology, Pathophysiology, and Clinical Outcomes. Circ Res. 2017;120:1501-1517. [CrossRef]
- Papathanasiou M, Jakstaite AM, Oubari S, Siebermair J, Wakili R, Hoffmann J, Carpinteiro A, Hagenacker T, Thimm A, Rischpler C, Kessler L, Rassaf T, Luedike P. Clinical features and predictors of atrial fibrillation in patients with light-chain or transthyretin cardiac amyloidosis. ESC Heart Fail. 2022;9:1740-1748. [CrossRef]
- Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370-5. [CrossRef]
- Rosenberg MA, Manning WJ. Diastolic dysfunction and risk of atrial fibrillation: a mechanistic appraisal. Circulation. 2012;126:2353-62. [CrossRef]
- Röcken C, Peters B, Juenemann G, Saeger W, Klein HU, Huth C, Roessner A, Goette A. Atrial amyloidosis: an arrhythmogenic substrate for persistent atrial fibrillation. Circulation. 2002;106:2091-7. [CrossRef]
- Kumar S, Bhaskaran A. Atrial Fibrillation in Transthyretin Cardiac Amyloidosis: The Growing Need to Look Forward. JACC Clin Electrophysiol. 2020;6:1128-1130. [CrossRef]
- Henein MY, Suhr OB, Arvidsson S, Pilebro B, Westermark P, Hörnsten R, Lindqvist P. Reduced left atrial myocardial deformation irrespective of cavity size: a potential cause for atrial arrhythmia in hereditary transthyretin amyloidosis. Amyloid. 2018;25:46-53. [CrossRef]
- Barbhaiya CR, Kumar S, Baldinger SH, Michaud GF, Stevenson WG, Falk R, John RM. Electrophysiologic assessment of conduction abnormalities and atrial arrhythmias associated with amyloid cardiomyopathy. Heart Rhythm. 2016;13:383-90. [CrossRef]
- Falk RH, Rubinow A, Cohen AS. Cardiac arrhythmias in systemic amyloidosis: correlation with echocardiographic abnormalities. J Am Coll Cardiol. 1984;3:107-13. [CrossRef]
- Gertz MA, Falk RH, Skinner M, Cohen AS, Kyle RA. Worsening of congestive heart failure in amyloid heart disease treated by calcium channel-blocking agents. Am J Cardiol. 1985;55:1645. [CrossRef]
- Itzhaki Ben Zadok O, Kornowski R. Cardiac Care of Patients with Cardiac Amyloidosis. Acta Haematol. 2020;143:343-351. [CrossRef]
- Rubinow A, Skinner M, Cohen AS. Digoxin sensitivity in amyloid cardiomyopathy. Circulation. 1981;63:1285-8. [CrossRef]
- CASSIDY JT. Cardiac amyloidosis. Two cases with digitalis sensitivity. Ann Intern Med. 1961;55:989-94. [CrossRef]
- Muchtar E, Gertz MA, Kumar SK et al.: Digoxin use in systemic light-chain (AL) amyloidosis: contra-indicated or cautious use? Amyloid. 2018;25:86-92. [CrossRef]
- Bukhari S, Oliveros E, Parekh H, et al. Epidemiology, Mechanisms, and Management of Atrial Fibrillation in Cardiac Amyloidosis. Curr Probl Cardiol. 2023;48:101571. [CrossRef]
- Basza M, Maciejewski C, Bojanowicz W, et al. Flecainide in clinical practice. Cardiol J. 2023;30(3):473-482. [CrossRef]
- Feng D, Edwards WD, Oh JK, et al.: Intracardiac thrombosis and embolism in patients with cardiac amyloidosis. Circulation. 2007;116:2420-6. [CrossRef]
- Feng D, Syed IS, Martinez M, et al.: Intracardiac thrombosis and anticoagulation therapy in cardiac amyloidosis. Circulation. 2009;119:2490-7. [CrossRef]
- Bukhari S. Cardiac amyloidosis: state-of-the-art review. J Geriatr Cardiol. 2023;20:361-375. [CrossRef]
- Mitrani LR, De Los Santos J, Driggin E, et al. Anticoagulation with warfarin compared to novel oral anticoagulants for atrial fibrillation in adults with transthyretin cardiac amyloidosis: comparison of thromboembolic events and major bleeding. Amyloid. 2021;28:30-34. [CrossRef]
- Di Lisi D, Di Caccamo L, Damerino G, et al.: Effectiveness and Safety of Oral Anticoagulants in Cardiac Amyloidosis: Lights and Shadows. Curr Probl Cardiol. 2022 Mar 25:101188. [CrossRef]
- Yood RA, Skinner M, Rubinow A, et al. Bleeding manifestations in 100 patients with amyloidosis. JAMA. 1983;249:1322-4. [CrossRef]
- Bukhari, S., Fatima, S., Nieves, R., et al. (2021). Bleeding risk associated with transthyretin cardiac amyloidosis. Journal of the American College of Cardiology, 77(18_Supplement_1), 530-530. [CrossRef]
- Mumford AD, O'Donnell J, Gillmore JD, et al. Bleeding symptoms and coagulation abnormalities in 337 patients with AL-amyloidosis. Br J Haematol. 2000;110:454-60. [CrossRef]
- El-Am EA, Grogan M, Ahmad A, et al. Persistence of Left Atrial Appendage Thrombus in Patients With Cardiac Amyloidosis. J Am Coll Cardiol. 2021 Jan 26;77(3):342-343. [CrossRef]
- Amat-Santos IJ, Delgado-Arana JR, Cruz-González I, et al. Cardiac amyloidosis and left atrial appendage closure. The CAMYLAAC study. Rev Esp Cardiol (Engl Ed). 2023;76:503-510. [CrossRef]
- Chen YY, Kuo MJ, Chung FP, et al. Risks of Ventricular Tachyarrhythmia and Mortality in Patients with Amyloidosis - A Long-Term Cohort Study. Acta Cardiol Sin. 2022;38: 464-474. [CrossRef]
- Dubrey SW, Cha K, Anderson J, et al. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM; 91: 141-57. [CrossRef]
- Goldsmith YB, Liu J, Chou J, et al. Frequencies and types of arrhythmias in patients with systemic light-chain amyloidosis with cardiac involvement undergoing stem cell transplantation on telemetry monitoring. Am J Cardiol. 2009;104:990-4. [CrossRef]
- Hörnsten R, Wiklund U, Olofsson BO, et al. Liver transplantation does not prevent the development of life-threatening arrhythmia in familial amyloidotic polyneuropathy, Portuguese-type (ATTR Val30Met) patients. Transplantation. 2004;78:112-6. [CrossRef]
- Bukhari S, Khan B. Prevalence of ventricular arrhythmias and role of implantable cardioverter-defibrillator in cardiac amyloidosis. J Cardiol. 2023;81:429-433. [CrossRef]
- Zampieri M, Allinovi M, Olivotto I, Antonioli E, Gabriele M, Argirò A, Fumagalli C, Nardi G, Di Mario C, Vannucchi AM, Perfetto F, Cappelli F. Ventricular tachyarrhythmias and sudden cardiac death in light-chain amyloidosis: a clash of cardio-toxicities? Br J Haematol. 2021 May;193(4):e27-e31. [CrossRef]
- Hashimura H, Ishibashi-Ueda H, Yonemoto Yet al. Late gadolinium enhancement in cardiac amyloidosis: attributable both to interstitial amyloid deposition and subendocardial fibrosis caused by ischemia. Heart Vessels. 2016;31:990-5. [CrossRef]
- Kristen AV, Dengler TJ, Hegenbart U, et al. Prophylactic implantation of cardioverter-defibrillator in patients with severe cardiac amyloidosis and high risk for sudden cardiac death. Heart Rhythm. 2008;5:235-40. [CrossRef]
- Varr BC, Zarafshar S, Coakley T, et al. Implantable cardioverter-defibrillator placement in patients with cardiac amyloidosis. Heart Rhythm. 2014;11:158-62. [CrossRef]
- Hamon D, Algalarrondo V, Gandjbakhch E, et al. Outcome and incidence of appropriate implantable cardioverter-defibrillator therapy in patients with cardiac amyloidosis. Int J Cardiol. 2016;222:562-568. [CrossRef]
- Lin G, Dispenzieri A, Kyle R, et al. Implantable cardioverter defibrillators in patients with cardiac amyloidosis. J Cardiovasc Electrophysiol. 2013;24:793-8. [CrossRef]
- Higgins AY, Annapureddy AR, Wang Y, et al. Survival Following Implantable Cardioverter-Defibrillator Implantation in Patients With Amyloid Cardiomyopathy. J Am Heart Assoc. 2020;9:e016038. [CrossRef]
- Donnellan E, Wazni OM, Hanna M, et al. Primary prevention implantable cardioverter-defibrillators in transthyretin cardiac amyloidosis. Pacing Clin Electrophysiol. 2020;43:1401-1403. [CrossRef]
- Brown MT, Yalamanchili S, Evans ST, et al. Ventricular arrhythmia burden and implantable cardioverter-defibrillator outcomes in transthyretin cardiac amyloidosis. Pacing Clin Electrophysiol. 2022;45:443-451. [CrossRef]
- Olausson E, Wertz J, Fridman Y, et al. Diffuse myocardial fibrosis associates with incident ventricular arrhythmia in implantable cardioverter defibrillator recipients. medRxiv [Preprint]. 2023 Feb 16:2023.02.15.23285925. [CrossRef]
- Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793-2867. [CrossRef]
- Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018;15:e73-e189. [CrossRef]
- Eriksson P, Karp K, Bjerle P, et al.: Disturbances of cardiac rhythm and conduction in familial amyloidosis with polyneuropathy. Br Heart J. 1984 Jun;51(6):658-62. [CrossRef]
- Porcari A, Rossi M, Cappelli F, et al.: Incidence and risk factors for pacemaker implantation in light-chain and transthyretin cardiac amyloidosis. Eur J Heart Fail. 2022;24:1227-1236. [CrossRef]
- Bukhari S, Kasi A, Khan B. Bradyarrhythmias in Cardiac Amyloidosis and Role of Pacemaker. Curr Probl Cardiol. 2023;48:101912. [CrossRef]
- Pinney JH, Whelan CJ, Petrie A, et al. 2013. Senile systemic amyloidosis: clinical features at presentation and outcome. J. Am. Heart Assoc. 2:e000098. [CrossRef]
- Ladefoged BT, Dybro A, Dahl Pedersen AL, Rasmussen TB, Vase HØ, Clemmensen TS, Gillmore J, Poulsen SH. Incidence and predictors of worsening heart failure in patients with wild-type transthyretin cardiac amyloidosis. ESC Heart Fail. 2022 Oct;9(5):2978-2987. [CrossRef]
- Rehorn MR, Loungani RS, Black-Maier E, et al.: Cardiac Implantable Electronic Devices: A Window Into the Evolution of Conduction Disease in Cardiac Amyloidosis. JACC Clin Electrophysiol. 2020;6:1144-1154. [CrossRef]
- Donnellan E, Wazni OM, Saliba WI, et al.: Cardiac devices in patients with transthyretin amyloidosis: Impact on functional class, left ventricular function, mitral regurgitation, and mortality. J Cardiovasc Electrophysiol. 2019;30:2427-2432. [CrossRef]
- Donnellan E, Wazni OM, Hanna M, et al.: Cardiac Resynchronization Therapy for Transthyretin Cardiac Amyloidosis. J Am Heart Assoc. 2020;9:e017335. [CrossRef]
- Sayed RH, Rogers D, Khan F, et al.: A study of implanted cardiac rhythm recorders in advanced cardiac AL amyloidosis. Eur Heart J. 2015;36:1098-105. [CrossRef]
- Slart RHJA, Glaudemans AWJM, Hazenberg BPC, Noordzij W. Imaging cardiac innervation in amyloidosis. J Nucl Cardiol. 2019 Feb;26(1):174-187. [CrossRef]
- Algalarrondo V, Dinanian S, Juin C, et al.: Prophylactic pacemaker implantation in familial amyloid polyneuropathy. Heart Rhythm. 2012;9:1069-75. [CrossRef] [PubMed]
- Masri A, Bukhari S, Ahmad S, et al. Efficient 1-Hour Technetium-99 m Pyrophosphate Imaging Protocol for the Diagnosis of Transthyretin Cardiac Amyloidosis. Circ Cardiovasc Imaging. 2020;13:e010249. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).