Submitted:
15 January 2024
Posted:
16 January 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
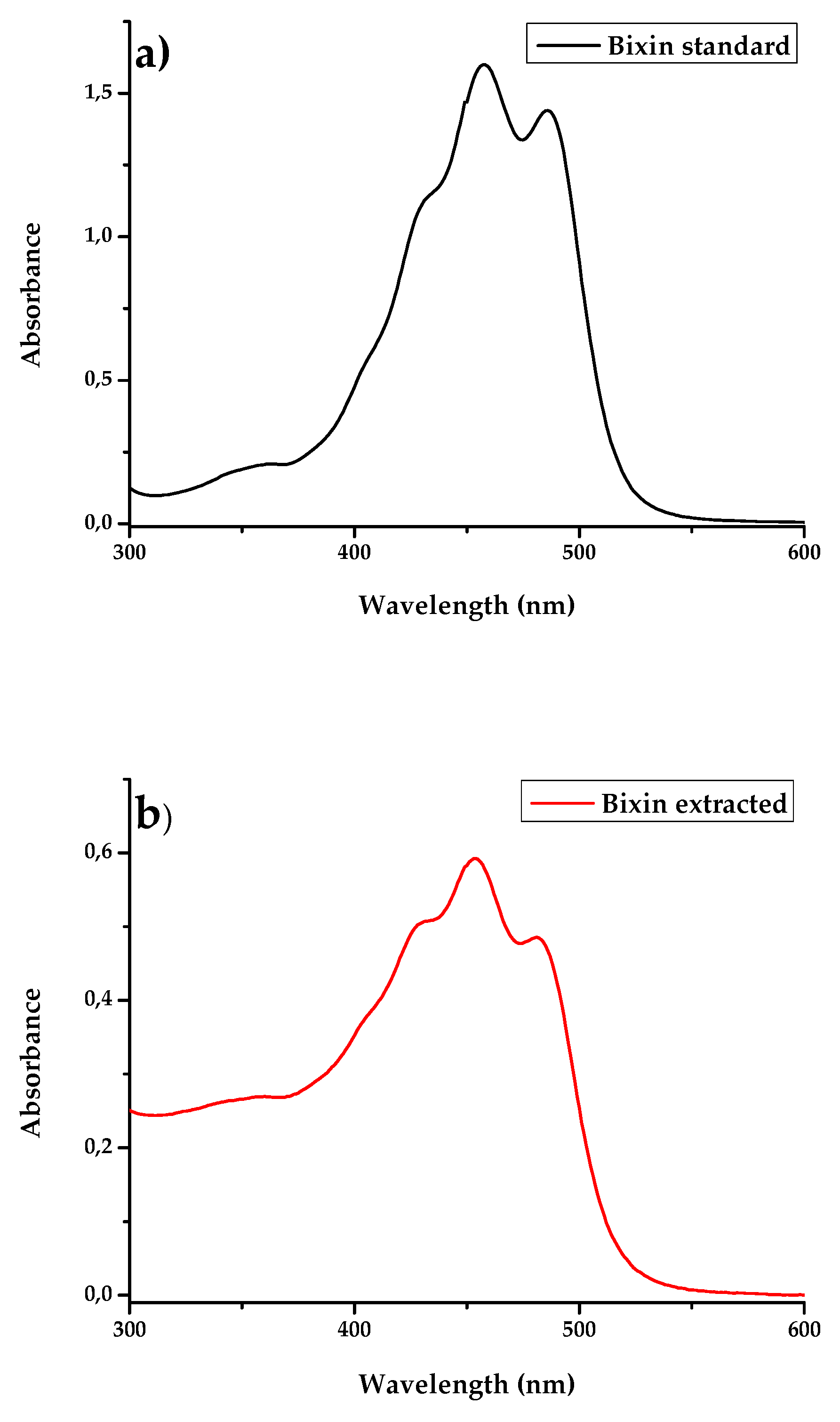
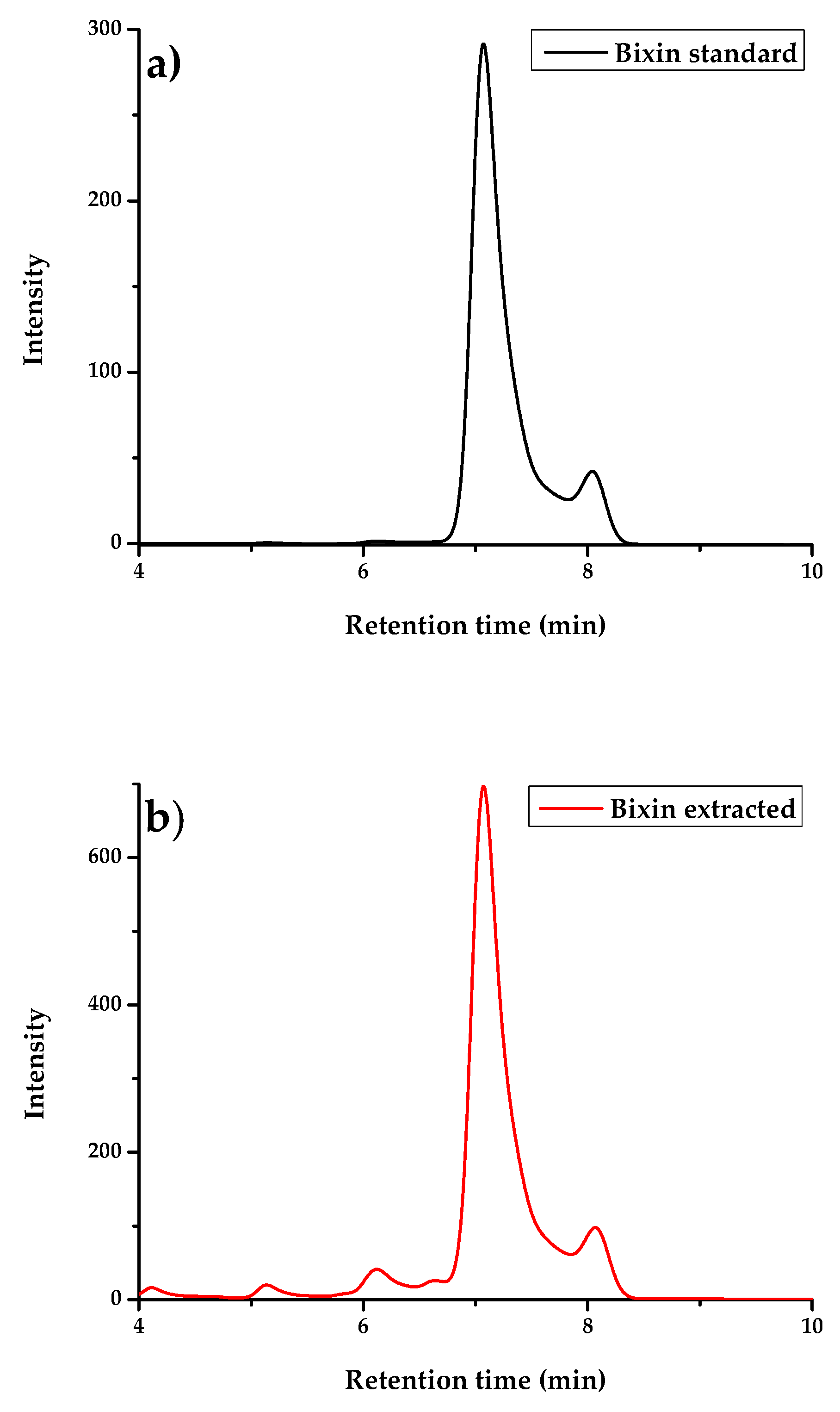
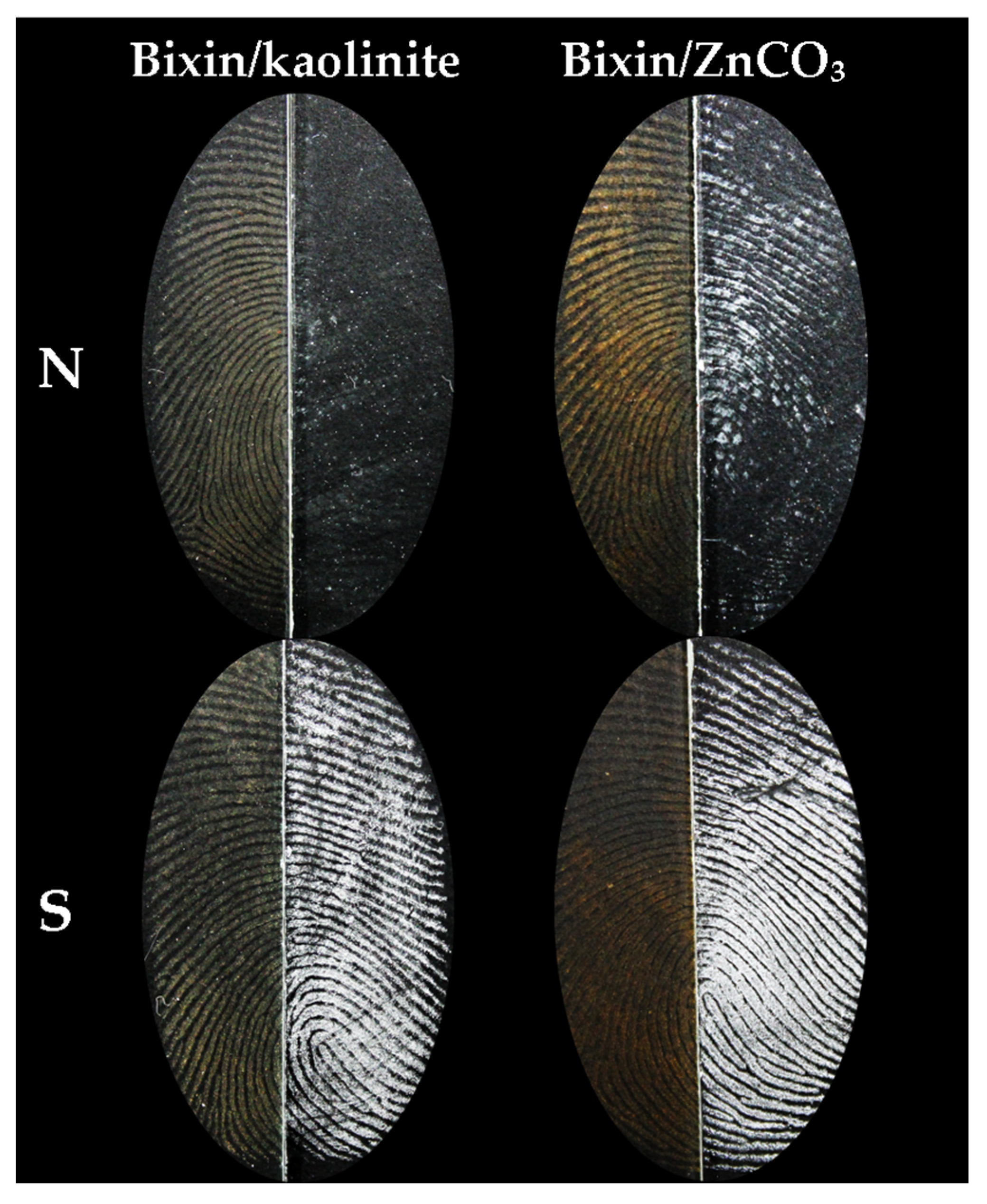
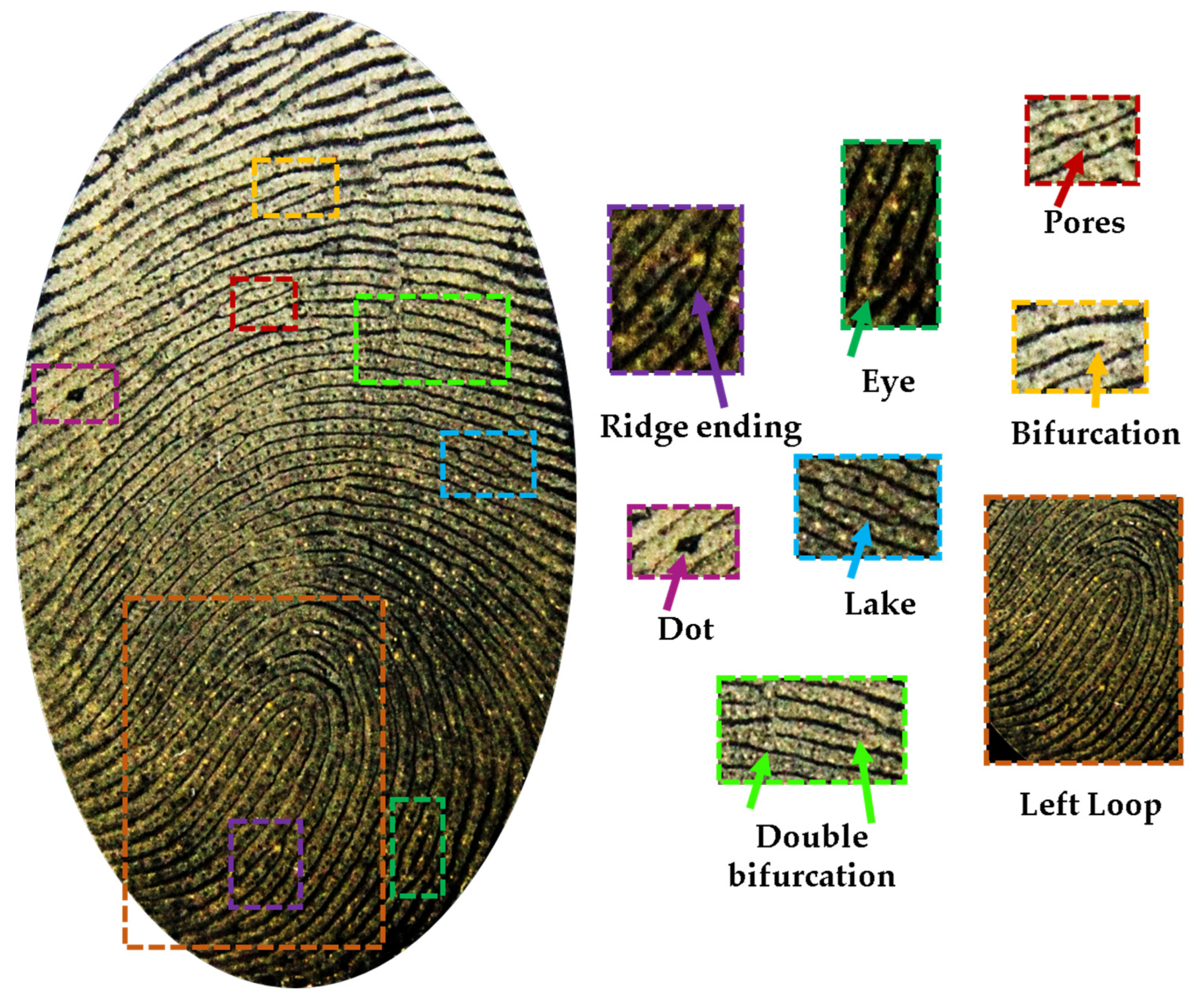
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MEZZOMO, N.; FERREIRA, S. R. Carotenoids functionality, sources, and processing by supercritical technology: a review. Journal of chemistry, v. 2016, p. 1-16, 2016. [CrossRef]
- MELÉNDEZ-MARTÍNEZ, A. J. An overview of carotenoids, apocarotenoids, and vitamin A in agro-food, nutrition, health, and disease. Molecular nutrition & food research, v. 63, n. 15, p. 1801045, 2019. [CrossRef]
- ENAYATI, A.; REZAEI, A.; FALSAFI, S.R.; ROSTAMABADI, H.; MALEKJANI, N.; AKHAVAN-MAHDAVI, S.; KHARAMI, M.S. and JAFARI, S.M. Bixin-loaded colloidal nanodelivery systems, techniques and applications. Food Chemistry, v. 412, n. January, p. 135479, 2023. [CrossRef]
- SHADISVAARAN, S.; CHIN, K. Y.; MOHD-SAID, S.; LEONG, X. F. Therapeutic potential of bixin on inflammation: a mini review. Frontiers in Nutrition, v. 10, p. 01-08, 2023. [CrossRef]
- ASHRAF, A.; IJAZ, M.U.; MUZAMMIL, S.; NAZIR, M.M.; ZAFAR, S.; ZIHAD, S.M.N.K.; UDDIN, S.J.; HASNAIN, M.S. and NAYAK, A.K. The role of bixin as antioxidant, anti-inflammatory, anticancer, and skin protecting natural product extracted from Bixa orellana L. Fitoterapia, v. 169, n. July, p. 105612, 2023. [CrossRef]
- MOREIRA, P. R.; MAIOLI, M.A.; MEDEIROS, H.C.D.; GUELFI, M.; PEREIRA, F.T.V. and MINGATTO, F.E. Protective effect of bixin on carbon tetrachloride-induced hepatotoxicity in rats. Biological Research, v. 47, n. 1, p. 1–7, 2014. [CrossRef]
- HANDAYANI, I., HARYANTI, P. and SULISTYO, S. B. Color and antibacterial activity of annatto extracts at various pH of distilled. Food Research, v. 5, n. December, p. 247–253, 2021. [CrossRef]
- SANTOS, G. C.; MENDONÇA, L.M.; ANTONUCCI, G.A.; DOS SANTOS, A.C.; ANTUNES, L.M.G. and BIANCHI, M.L.P. Protective effect of bixin on cisplatin-induced genotoxicity in PC12 cells. Food and Chemical Toxicology, v. 50, n. 2, p. 335–340, 2012. [CrossRef]
- PACHECO, S. D. G.; GASPARIN, A.T.; JESUS, C.H.A.; SOTOMAIOR, B.B.; VENTURA, A.C.S.B.; REDIVO, D.D.B.; CABRINI, D.A.; DIAS, J.F.G.; MIGUEL, M.D.; MIGUEL, O.G. and CUNHA, J.M. Antinociceptive and Anti-Inflammatory Effects of Bixin, a Carotenoid Extracted from the Seeds of Bixa orellana. Planta Medica, v. 85, n. 16, p. 1216–1224, 2019. [CrossRef]
- LI, J.; YANG, Y.; WEI, S.; CHEN, L.; XUE, L.; TIAN, H. and TAO, S. Bixin Protects Against Kidney Interstitial Fibrosis Through Promoting STAT6 Degradation. Frontiers in Cell and Developmental Biology, v. 8, n. November, p. 1–15, 2020. [CrossRef]
- TUPUNA, D. S., PAESE, K., GUTERRES, S. S., JABLONSKI, A., FLÔRES, S. H., & DE OLIVEIRA RIOS, A. Encapsulation efficiency and thermal stability of norbixin microencapsulated by spray-drying using different combinations of wall materials. Industrial crops and products, v. 111, p. 846-855, 2018. [CrossRef]
- NOPPE, H.; ABUIN MARTINEZ, S.; VERHEYDEN, K.; VAN LOCO, J.; COMPANYÓ BELTRAN, R.; DE BRABANDER, H. F. Determination of bixin and norbixin in meat using liquid chromatography and photodiode array detection. Food Additives and Contaminants, v. 26, n. 1, p. 17-24, 2009. [CrossRef]
- ZIA-UL-HAQ, M.; DEWANJEE, S.; RIAZ, M. AUTHOR 1, A.; Carotenoids: Structure and Function in the Human Body, Springer: Springer Nature Switzerland AG, 2021. [CrossRef]
- SILVA, B. D. S. Importância da Perícia Papiloscópica em Laboratório para a Investigação Policial em Casos do Estado de Goiás, no Brasil. Brazilian Journal of Forensic Sciences, Medical Law and Bioethics, v. 10, n. 2, p. 130–146, 2021. [CrossRef]
- WANG, M.; ZHU, Y.; MAO, C. Synthesis of NIR-Responsive NaYF4:Yb,Er Upconversion Fluorescent Nanoparticles Using an Optimized Solvothermal Method and Their Applications in Enhanced Development of Latent Fingerprints on Various Smooth Substrates. Langmuir, v. 31, n. 25, p. 7084–7090, 2015. [CrossRef]
- BARROS, H. L.; STEFANI, V. Micro-structured fluorescent powders for detecting latent fingerprints on different types of surfaces. Journal of Photochemistry and Photobiology A: Chemistry, v. 368, n. September 2018, p. 137–146, 2019. 20 September. [CrossRef]
- PASSOS, L. F.; BERNEIRA, L.M.; POLETTI, T.; MARIOTTI, K.C.; CARREÑO, N.L.V.; HARTWIG, C.A. and PEREIRA, C.M.P. Evaluation and characterization of algal biomass applied to the development of fingermarks on glass surfaces. Australian Journal of Forensic Sciences, v. 53, n. 3, p. 337–346, 2021. [CrossRef]
- POLETTI, T.; BERNEIRA, L.M.; BUENO, D.T.; SILVA, C.C.; SILVA, R.S. and PEREIRA, C.M.P. Talanta Open Chemical evaluation and application of cinnamaldehyde-derived curcumins as potential fingerprint development agents. Talanta Open, v. 6, n. July, p. 100133, 2022. [CrossRef]
- ARORA, K.K. and PASSEY, S. Use of a Fluorescent Schiff’S Base as Developing Agent for Latent Finger Prints. International Journal of Trend in Scientific Research and Development, v. 5, n. 1, p. 625–627, 2020.
- NETO, J.C.M.; NASCIMENTO, N.R.; BELLO, R.H.; VERÇOSA, L.A.; NETO, J.V.; COSTA, J.C.M. and DIAZ, F.R.V. Kaolinite Review: Intercalation and Production of Polymer Nanocomposites. Engineered Science, v. 17, p. 28–44, 2022. [CrossRef]
- LIANG, W.; BAI, J.; LI, Z.; MENG, Y.; LIU, K. and LI, L. Crystal growth, structure and thermal properties of anhydrous zinc carbonate (ZnCO3). Journal of Alloys and Compounds, v. 898, p. 162916, 2022. [CrossRef]
- KAPOOR S, GURVINDER S.S.; SANJIV, K. Visualization of Latent Fingermarks using Rhodamine B: A New Method. International Journal of Forensic Science & Pathology (IJFP) ISSN 2332-287X. v. 3, n. 5, p. 199–201, 2015. [CrossRef]
- PATTARITH, K.; BENCHAWATTANANON, R. Study on the Novel Photoluminescence Powder Synthesized from Zinc Carbonate Nanoparticles Associated with Fluorescein Dye for its Latent Fingerprint Detection. New Innovations in Chemistry and Biochemistry, v. 1, p. 14–25, 2021. [CrossRef]
- BUMBRAH, G. S. Small particle reagent (SPR) method for detection of latent fingermarks: A review. Egyptian Journal of Forensic Sciences, v. 6, n. 4, p. 328–332, 2016. [CrossRef]
- MIGUEL, K. B.; CARDOSO, V. L.; REIS, M. H. M. Concentration of Pigments and Health-Promoting Bioactive Compounds from Annatto Seeds by Ultrafiltration. Waste and Biomass Valorization, n. 0123456789, 2023. [CrossRef]
- CHISTÉ, R.C.; YAMASHITA, F.; GOZZO, F.C.; MERCADANTE, A. Z. Simultaneous extraction and analysis by high performance liquid chromatography coupled to diode array and mass spectrometric detectors of bixin and phenolic compounds from annatto seeds. Journal of Chromatography A, v. 1218, p. 57–63, 2011. [CrossRef]
- PACHECO, B. S.; SILVA, C.C.; DA ROSA, B.N.; MARIOTTI, K.C.; NICOLODI, C.; POLETTI, T.; SEGATTO, N.V.; COLLARES, T.; SEIXAS, F.K.; PANIZ, O.; CARREÑO, N.L.V. AND PEREIRA, C.M.P. Monofunctional curcumin analogues: evaluation of green and safe developers of latent fingerprints. Chemical Papers, v. 75, n. 7, p. 3119–3129, 2021. [CrossRef]
- VENTURINA, S.A.M.D.T.; COMUELO, J.Z.A.; SAMANIEGO, W.R.A. and, JOLITO, F.C. The utilization of methanolic Bixa orellana (Annatto) seed extract as substitute for safranin in Gram staining. Publiscience, v. 3, n. 1, p. 33–36, 2020.
- AZEVEDO-MELEIRO, C. H.; RODRIGUEZ-AMAYA, D. B. Confirmation of the identity of the carotenoids of tropical fruits by HPLC-DAD and HPLC-MS. Journal of food Composition and Analysis, v. 17, n. 3-4, p. 385-396, 2004. [CrossRef]
- ULLAH, Q.; MOHAMMAD, A. Vitamins determination by TLC/HPTLC—a mini-review. JPC – Journal of Planar Chromatography – Modern TLC, 2020. [CrossRef]
- MOHAMMAD, A.; MOHEMAN, A. TLC/HPTLC in biomedical applications. In: Srivastava MM (ed) High-performance thin-layer chromatography (HPTLC). Springer, Heidelberg, p. 151–178, 2011.
- CURI-BORDA, C.K.; TANNAIRA, V.; GENTILE, N.; ALVARADO, J-A. and BERGENSTAHL, B. Colloids and Surfaces A: Physicochemical and Engineering Aspects Model for measuring light stability of photolabile substances in powder beds using spray dried bixin microcapsules. Colloids and Surfaces A: Physicochemical and Engineering Aspects, v. 627, n. January, 2021. [CrossRef]
- BARROS, H. L.; TAVARES, L.; STEFANI, V. Dye-doped starch microparticles as a novel fluorescent agent for the visualization of latent fingermarks on porous and non-porous substrates. Forensic Chemistry, v. 20, n. June, p. 100264, 2020. [CrossRef]
- KHARE, V.; SINGLA, A. A review on the advancements in chemical examination of composition of latent fingerprint residues. Egyptian Journal of Forensic Sciences, v. 12, n. 1, 2022. [CrossRef]
- ROBSON, R.; GINIGE, T.; MANSOUR, S.; KHAN, I.; ASSI, S. Analysis of fingermark constituents: a systematic review of quantitative studies. Chemical Papers, v. 76, n. 8, p. 4645–4667, 2022. [CrossRef]
- LI, L.; LI, Q.; CHU, J.; XI, P.; WANG, C.; LIU, R.; WANG, X.; CHENG, B. Dual-mode luminescent multilayer core-shell UCNPs@SiO2@TEuTbB nanospheres for high-level anti-counterfeiting and recognition of latent fingerprints. Applied Surface Science, v. 581, n. January, 2022. [CrossRef]
- ANSARI, A. A.; ALDAJANI, K. M.; ALHAZAA, A. N.; ALBRITHEN, H. A. Recent progress of fluorescent materials for fingermarks detection in forensic science and anti-counterfeiting. Coordination Chemistry Reviews, v. 462, p. 214523, 2022. [CrossRef]
- ROHATGI, R.; SODHI, G. S.; KAPOOR, A. K. Small particle reagent based on crystal violet dye for developing latent fingerprints on non-porous wet surfaces. Egyptian Journal of Forensic Sciences, v. 5, n. 4, p. 162-165, 2015. [CrossRef]
- DHALL, J. K.; KAPOOR, A. K. Development of latent prints exposed to destructive crime scene conditions using wet powder suspensions. Egyptian Journal of Forensic Sciences, v. 6, n. 4, p. 396-404, 2016. [CrossRef]
- KAMBLE, D.; PANDEY, S.; KUMARI, A.; SHARMA, K. A new powder method for development of latent fingerprint. In: All India Forensic Science Conference, 2018.
- SODHI, G. S.; KAUR, J. Powder method for detecting latent fingerprints: a review. Forensic science international, v. 120, n. 3, p. 172-176, 2001. [CrossRef]
- GARG, R. K.; KUMARI, H.; KAUR, R. A new technique for visualization of latent fingerprints on various surfaces using powder from turmeric: a rhizomatous herbaceous plant (Curcuma longa). Egyptian Journal of Forensic Sciences, v. 1, n. 1, p. 53-57, 2011. [CrossRef]
- THAKUR, P.; GARG, R. K. New developing reagent for latent fingermark visualization: Fuller’s earth (Multani Mitti). Egyptian Journal of Forensic Sciences, v. 6, n. 4, p. 449-458, 2016. [CrossRef]
- SU, L.; ZENG, X.; HE, H.; TAO, Q.; KOMARNENI, S. Preparation of functionalized kaolinite/epoxy resin nanocomposites with enhanced thermal properties. Applied Clay Science, v. 148, p. 103-108, 2017. [CrossRef]
- ALCARAZ-FOSSOUL, J.; PATRIS C. M.; MUNTANER A. B.; FEIXAT, C. B.; GENÉ, B. M. Determination of latent fingerprint degradation patterns—a real fieldwork study. Int J Legal Med., v. 127, p. 857–870, 2013. [CrossRef]
- ANGELONI, M. D. A.; MARANA, A.N. Reconhecimento Automático de Impressões Digitais Baseado em Características Biométricas de Terceiro Nível. Researchgate, 2015.


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




