1. Introduction
Bipolar disorder is a mental disorder that contains three episodes, including mania, hypomania, and depression [
1]. The prevalence of bipolar disorder (BD) is about two to five percent of the worldwide population [
2]. Over 90% of patients with BD experience recurrence throughout their lives, and about 35 to 57% relapse in one year [
3]. The treatment goal for BD is to control acute episodes that, when severe, may lead to hospitalization and prevent future recurrence states[
4,
5]. Mood stabilizers (e.g., lithium, valproate acid, carbamazepine, and antipsychotics) are approved to suppress shifts between mania and depression. Monotherapy of mood stabilizers should be the primary choice, at least in mild and moderate mania. Although poly-therapy has proven to be more effective with the combinations of mood stabilizers plus atypical antipsychotics, poly-therapy should be reserved for severe mania or as a subsequent step in mild and moderate mania after unsuccessful monotherapy[
6]. Some Patients with BD need in-hospital care besides conventional pharmacotherapy. However, pharmacotherapy of lithium only improves the BD prognosis in one-third of patients [
7]. When their medications did not relieve manic or depressive symptoms, patients with BD tended to use complementary and alternative medicine (CAM) [
8]. Another reason patients with BD used CAM was that it cost less than psychiatric services[
9]. Worthy of attention, most patients with BD didn’t discuss their CAM usage with their physicians[
10]. Two papers identified the population of Patients with BD using CAM. Fifty percent of 435 Patients with BD took vitamins or herbs, and 44% of 50 elderly Patients with BD used CAM[
10,
11].
CAM is a broader term encompassing diverse healing practices from various cultural and medical traditions worldwide, such as traditional Chinese medicine (TCM), Ayurveda, homeopathy, naturopathy, etc. TCM has its roots in ancient Chinese philosophy, including principles such as Yin and Yang and the concept of Qi (vital energy), whose treatments include acupuncture, herbal medicine, Chinese traumatology medicine, and qigong. Research on CAM treatments for depression or bipolar depression is more than bipolar disorder. According to a review study, CAM therapy for BD includes fatty acids, St. John’s wort, herbal products, S-adenosyl-L-methionine, aromatherapy massage, therapeutic massage, yoga, Chinese oral medicine, and acupuncture. However, St. John’s wort and S-adenosyl-L-methionine can relieve mild to moderate depression but have the potential to induce mania. Evidence regarding the benefits of omega-3 fatty acids or acupuncture is inconsistent[
12].
TCM has been used for thousands of years in China.
Huang Di Nei Jing is an ancient Chinese medical text treated as a fundamental doctrinal source for Chinese medicine. Chapter 46 of
Huang Di Nei Jing Su Wan and Chapter 22 of
Ling Su describe symptoms and treatments associated with BD, such as fresh iron flakes and acupuncture on the spleen, bladder, stomach, lung, small intestine, and large intestine meridians[
13,
14]. Recent research about TCM on BD before April. 2022 is as follows. A clinical trial demonstrated that Patients with BD taking Free and Easy Wanderer Plus and carbamazepine had lower drop-out rates, side effects, and serum carbamazepine levels than the carbamazepine plus placebo group [
15]. A case report also inferred that acupuncture combined with mood stabilizers and antipsychotics as maintenance therapies would not aggravate bipolar disorder prognosis [
16].
Research about the population of TCM users and treatments of BD is insufficient. The probable reason is that the number of TCM users in patients with BD in published studies is ambiguous, which cannot support subsequent TCM clinical studies on BD. Therefore, investigating the number of people with BD using TCM is a priority research effort. In this study, we aimed to investigate usage patterns of TCM for patients with BD in Taiwan, including analyzing the characteristics of patients with BD who used TCM, the primary indication of patients with BD visiting TCM clinicians, and the TCM techniques and prescriptions they accept.
2. Materials and Methods
2.1. Data source
This study used data from the Longitudinal Health Insurance Database (LHID), which contains the claimed data of one million randomly selected patients in the National Health Insurance Research Database (NHIRD) of the single-payer healthcare system launched in Taiwan in 1995. The LHID data includes patients’ demographics, inpatient and outpatient visit records, prescriptions, and disease diagnoses, encoded based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). All patient data had been de-identified to preserve patient privacy. The structure of the NHIRD files is described in detail on the NHIRD website and in other publications[
17]. The National Health Insurance Research Database (NHIRD) also covers expenses related to TCM interventions, encompassing oral Chinese herbal medicines (single herbs and herbal formulas), acupuncture, and Chinese traumatology medicine. In the current study, we extracted all TCM users with BD but analyzed Chinese herbal medicine and acupuncture prescriptions in subsequent analysis. This study was approved by the Research Ethics Committee of China Medical University and Hospital [CMUH104-REC2-115(AR-4)], Taichung, Taiwan.
2.2. Study population
The inclusion criteria were as follows: both male and female patients with BD (ICD-9-CM: 296.0, 296.1, 296.4, 296.5, 296.6, 296.7, and 296.8) older than fifteen years old in the LHID 2000. Age at onset (AAO) is a critical variable in the prognoses of BD[
18]. There were several cut-off BD onset ages in the literature. For example, forty years old is a cut-off for early-onset and late-onset bipolar disorder (EOBD and LOBD)[
19]. Approximately 30-60% of BD patients onset between ages 15-19, called pediatric or juvenile BD[
20,
21]. Patients who suffered or maintained BD over 50 years old named older age bipolar disorder (OABD)[
22]. Bolton et al. 2021 systematically searched the databases. They proposed the trimodal age-at-onset distribution by average early-onset age of seventeen, mid-onset age of twenty-six, and late-onset age of forty-six[
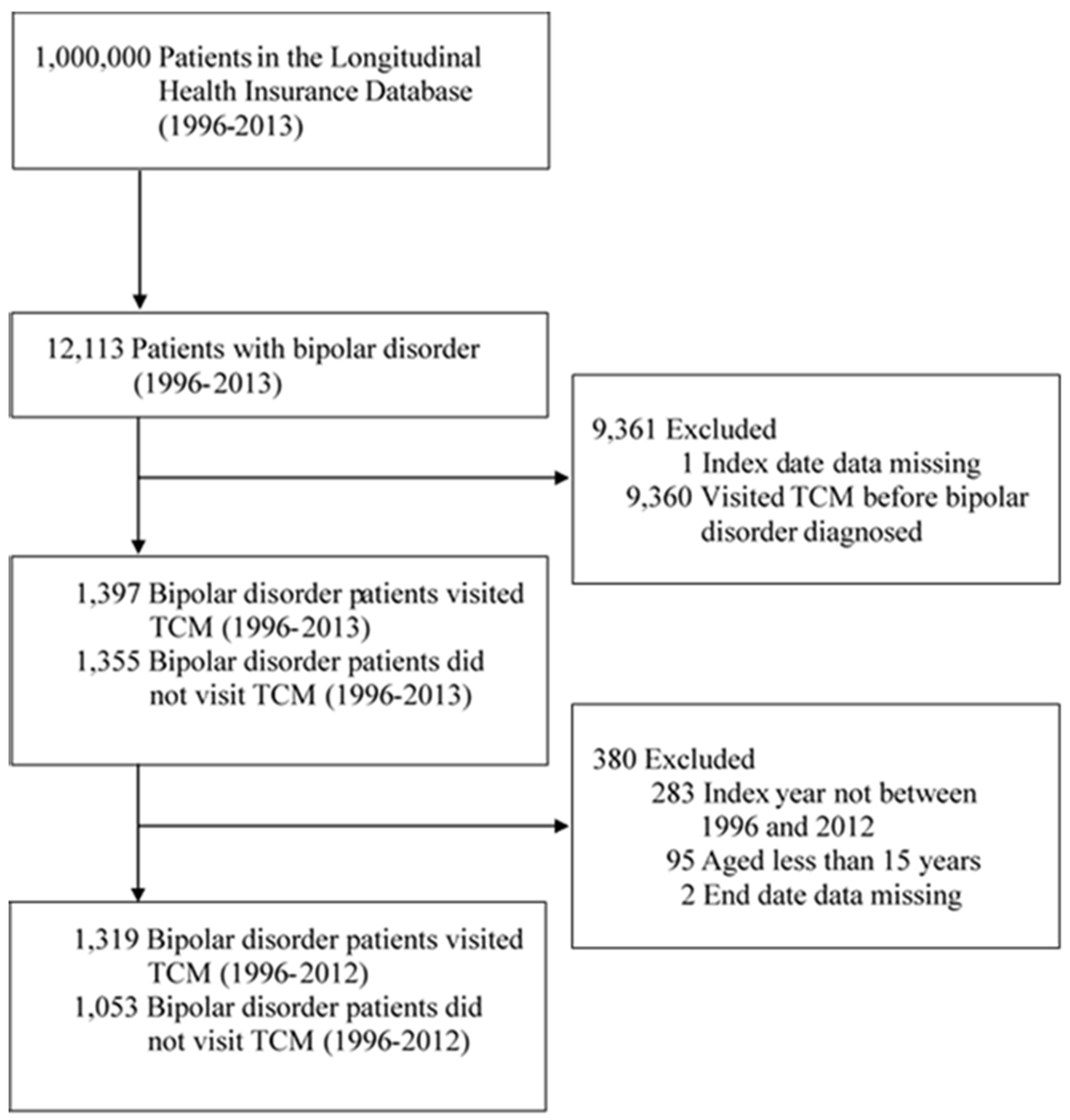
18]. Depending on the previous literature statements and the fact that citizens over 65 years old in Taiwan are elderly, we decided to include BD patients more than fifteen years old. In total, 12,113 patients diagnosed with BD from January 1, 1996, to December 31, 2013, were included in this study. The index date was the initial visit to a TCM clinic. Each patient in the cohort was followed until death, withdrawal from the National Health Insurance program, or until December 31, 2013. Of these patients, eighty-nine percent (n = 10,758) received TCM. Among TCM users with BD, eighty-seven percent (n = 9,361) visited TCM clinics before and 1,397 after BD diagnosis. To avoid potential TCM interference before the diagnosis of BD, we were more curious to explore the phenomenon that patients who never received TCM treatment before the diagnosis of BD changed to TCM treatment but still maintained psychiatric therapies. Therefore, all eligible participants in this study were excluded if they were recorded visiting a TCM clinic before their diagnosis of BD. Other exclusion criteria were index date not between the enrollment and withdrawal date, index year not between 1996 and 2012, age < 15 years, and missing end date data. After the diagnosis of BD, 1,319 of the included patients received TCM treatment, and 1,053 patients did not.
Figure 1 presents a visualization of the sample selection procedure.
2.3. Covariates of comorbidities and medications
Physical diseases are risk factors for psychiatric disorders[
23,
24,
25]; otherwise, psychiatric disorders also affect physical illnesses. When patients with physical conditions or psychiatric disorders do not control well, both will increase the disease prognoses bilaterally[
26]. Cognitive functions, pharmacotherapies, and psychiatric and medical comorbidities of patients with BD affect recurrence rates, increasing the odds of suicidality, disability, unemployment, and re-hospitalization[
27]. According to previous statements, we selected the following comorbidities in BD patients based on the literature review. Physical comorbidities in patients with BD included diabetes mellitus (ICD-9-CM: 250) [
28], hypertension (ICD-9-CM: 401–405) [
29], hyperlipidemia (ICD-9-CM: 272) [
30], chronic obstructive pulmonary disease (ICD-9-CM: 490–496)[
25,
31,
32], tobacco use (ICD-9-CM: 305.1) [
33], and obesity (ICD-9-CM: 278) [
34]. The previous comorbid diseases were risk factors for cardiovascular disease, such as ischemic heart disease (ICD-9-CM: 410–414)[
35], and one of the most common premature mortalities among BD patients [
36,
37].
Patients with BD also comorbid one to several psychiatric diseases. A meta-analysis study indicated that 40% of patients with BD suffer from any anxiety disorder[
38,
39]. Seventeen percent of BD patients had attention-deficit/hyperactivity disorder (ADHD)[
40]. McElroy et al. surveyed 875 patients about their eating disorder comorbidities, and 14.3% had at least one comorbid lifetime eating disorder, such as bulimia nervosa[
41]. Regier et al. found 43.6% of patients with BD had an alcohol diagnosis[
42]. Therefore, we also included anxiety (ICD-9-CM: 300.0, 300.2, and 300.3), attention deficit disorder (ICD-9-CM: 314.0), unspecified eating disorder (ICD-9-CM: 307.50), bulimia nervosa (ICD-9-CM: 307.51), alcohol use disorder (291, 303, 305.00, 305.01, 305.02, and 305.03) as psychiatric comorbidities in patients with BD.
All patients received" treatment-as-usual medications" like lithium carbonate, carbamazepine, valproic acid, lamotrigine, topiramate, aripiprazole, or loxapine between assessments with no interference with their prescribed course of BD treatment.
2.4. Statistical analysis
Descriptive statistics for categorical and continuous variables are presented as numbers or percentages and means and standard deviations, respectively. Chi-square and Student’s t-tests were respectively adopted to evaluate differences in categorical and continuous variables between the cohorts. A p-value less than 0.05 indicated significance in any hypothesis test. SAS 9.4 (SAS Institute Inc., Cary, NC, USA) was used for data analysis.
3. Results
3.1. Demographic characteristics
Table 1 summarizes all participants’ demographic and clinical characteristics (TCM and non-TCM groups). The mean age of patients in the TCM group was less than that of the non-TCM group (44.3 ± 16.8 vs. 48.2 ± 19.0; p < 0.0001). The non-TCM group included more men (63.3%) than women, whereas the TCM group included more women (53.4%) than men. The follow-up duration was 8.6 ± 4.8 years in the non-TCM group and 8.3 ± 4.3 years in the TCM group. Significant differences in the comorbidities of anxiety (p < 0.0001) and alcoholism (p = 0.0128) were noted between the TCM group (31.9% and 5.5% with anxiety and alcoholism, respectively) and the non-TCM group (20.7% and 3.3% with anxiety and alcoholism, respectively). Moreover, a significantly higher proportion of patients in the TCM group took medication, including lithium carbonate, carbamazepine, valproic acid, and aripiprazole.
3.2. TCM treatment interventions
From 1996 to 2013, 10,758 (88.8%) patients with BD visited TCM clinics. Among these patients, 9,361 received TCM treatment before the diagnosis of BD, and 1,319 received TCM treatment after the diagnosis of BD. In the latter group of 1,319 patients, 1,043 (79.1%) took single herbs, 1,155 (87.6%) took herbal formulae, and 120 (9.1%) received acupuncture treatment (
Table 1.).
3.3. Principal diagnostic codes used by TCM practitioners
Table 2 lists the top ten indications for Chinese herbal medicines and acupuncture among patients with BD. The indications identified by TCM doctors are arranged by diagnosis frequency in descending order. For patients who took Chinese herbal medication, indications were sleep disturbances, joint pain, acute nasopharyngitis, unspecified myalgia and myositis, lumbago, cough, headache, contusion of the ankle and foot (excluding toes), lumbar sprain, and constipation. For patients who received acupuncture, indications were joint pain, unspecified myalgia and myositis, lumbago, contusion of the knee and lower leg, ankle sprain, sprains and strains at unspecified sites of the shoulder and upper arm, contusion of the elbow and forearm, contusion of the wrist and hand(s) (except fingers), sprains and strains at unspecified sites of the knee and leg, and unspecified backache.
4. Discussion
4.1. Demographic characteristics
Compared with the non-TCM group, demographic characteristics of the TCM group were younger age, female, higher percentages of anxiety and alcohol use disorder, and higher medication usage rates. We highly suspect that patients with more severe illnesses of BD tended to receive TCM treatment. Patients with early-onset BD (age ≤ 18 years) exhibit more severe conditions, a shorter mood cycle, a higher likelihood of having comorbid anxiety, and more substance use[
43]. However, we could not determine whether the onset of BD was earlier among the patients in the TCM group than among those in the non-TCM group because NHIRD does not contain data on the age of BD onset. Screening for new diagnoses of BD in the NHIRD was possible, but the age at the time of diagnosis may differ from the onset age.
A systematic review calculated that the lifetime and current prevalence rate of any anxiety disorder comorbidities was 40.5% and 38.2% among patients with BD[
38]. In the present study, we figured out that 27% of patients with BD had anxiety states (ICD-9-CM: 300.0), phobic disorders (ICD-9-CM: 300.2), or obsessive-compulsive disorders (ICD-9-CM: 300.3), and more than two-thirds of them received TCM treatment. We found out that patients with BD visiting TCM clinicians may relate to anxiety. Lithium has an essential role in treating comorbid anxiety in BD in patients who were medication-free primarily and lithium-naïve at baseline[
44]. However, high anxiety scores had low lithium responsivity[
45]. In the current study, we could not determine whether the severity of anxiety disorders from the NHIRD data has a positive correlation with visiting TCM. BD and alcohol use disorder are often contemporary[
46]. A sizeable epidemiological study revealed that 43.6% of patients with BD had alcohol use disorder[
42]. In our study, only 4.5% of patients with BD had alcohol misuse, and more than 67% visited TCM clinics. However, because of the small sample size of this study, this percentage is not representative of all patients with BD in the real world of Taiwan. Anxiety was a predictor of a shorter time to recurrence, and alcohol abuse was a predictor of a long time to remission[
47]. Anxiety and alcohol misuse may be confounding factors in comparing BD severity between patients who did or did not receive TCM treatment.
Psychiatric physicians prescribe two or more medications based on the BD patients’ disease severity when the first-line monotherapy medicine fails to affect them[
6]. Our study indicates that significantly more patients with BD in the TCM group than in the non-TCM group took valproate, lithium, and carbamazepine to manage their illnesses. In particular, aripiprazole was prescribed only in the TCM group. However, we did not calculate the impact of combination/augmentation or adjunctive/add-on therapy, which needs future scrutiny.
4.2. Acupuncture treatment intervention
TCM has included acupuncture treatment for over three thousand years[
48]. Acupuncture amplified the American people’s interest in the early 1970s[
49]. A study analyzing 22,512 adults in the United States 2007 National Health Interview Survey revealed that 6.8% reported lifetime use of acupuncture and 1.5% reported use in the past 12 months[
50]. Taiwan’s NHIRD from 1996 to 2002 showed that 22.6% of total valid beneficiaries (n= 4,948,464) used acupuncture during the study period[
51]. Another comparative study analyzed the 2002 to 2011 data of Taiwan’s LHID 2000 and found that the one-year population of acupuncture users increased from 7.98% to 10.9%[
52]. Further calculations indicated that the average acupuncture population was 8.84% during the study period[
52]. Our analysis also used LHID 2000 and found that 9% of patients with BD received acupuncture treatment between 1996 and 2012, which seemed higher than the average but needed more comprehensive statistics to support our findings. In our study, patients with BD visiting TCM clinics tended to take herbal medicines rather than receive acupuncture. There are two possible reasons. First, patients with BD probably cannot overcome the fear of needles as acupuncture is a slightly painful invasive treatment that causes soreness in the treatment area[
53]. A clinical study indicated that the fear of pain induced by acupuncture needles increases patients’ pain rating scores and physiological responses [
54]. The amygdala controls fear reactions [
55], and acupuncture may alter the amygdala-specific brain network[
54]. Moreover, some abnormal structures were observed in BD patients’ amygdala [
56]. These findings may support the relatively low proportion of patients with BD who receive acupuncture treatment. Second, Chinese medicine physicians have expertise in treatment, such as acupuncture, TCM prescription, or traumatology. Some patients would say they did not want acupuncture when they came to TCM clinics. Some patients were even unwilling to take TCM. However, we cannot know the actual state of outpatients’ demand in the database study. A more suitable method is to design a questionnaire to ask patients what kind of assistance they want from TCM clinics.
It is essential to detect acupuncture’s positive effect and support its effectiveness in the comorbidities of patients with BD. However, we didn’t design corresponding research methods to find evidence of the impact of acupuncture on relative symptoms. The current study only showed the characteristics of BD patients who received TCM treatment, principal diagnosis codes, and prescriptions from TCM physicians. Future studies can use results from the current study to research other topics. For example, "myalgia and myositis" was one of the common diagnoses in patients with BD who accepted acupuncture. We can extract patients with BD and "myalgia and myositis" who did and didn’t use TCM, then compare their usage of corticosteroids and biomarkers of inflammation which didn’t present in Taiwan’s NHIRD.
4.3. Principal diagnostic codes used by TCM practitioners
In our study, pain-related diseases were the most frequent indication that patients with BD received acupuncture. Indeed, pain is one of the most common comorbidities in patients with BD. There exist high rates of pain (29%) and chronic pain (23%) among patients with BD, posing a 2.14 times higher risk of pain than healthy controls[
57]. Patients with BD are generally presented with various co-occurring pain-related conditions, including arthritis-related pain, rheumatic arthritis, polymyalgia rheumatica, myalgia and myositis, joint pain, back pain, lumbago, migraine, headache, psychogenic and neuropathic pain[
57,
58].
Numerous studies have shown that impaired neuroimmune function might be one of the common pathways for pain and mood symptoms in BD[
59]. The dysfunction of monoamine neurotransmitters[
60], mainly serotonin, noradrenaline, and dopamine, relative metabolites of neurotransmitters as mentioned above [
61], and the maladaptive alternations of their receptors in the CNS get involved in the pathogenesis of BD and endogenous pain[
62,
63]. Therefore, pharmacotherapies acting on monoamine neurotransmitter systems are considered to alleviate pain in affective diseases. Evidence from clinical research supports the ability of tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, and certain anticonvulsants to offer pain relief[
64].Clinical studies have proven the therapeutic efficacy of acupuncture for treating 28 conditions, including depression, headache, knee pain, low back pain, neck pain, periarthritis of the shoulder, rheumatoid arthritis, and sprain[
65]. Similarly, Chinese herbal medicine can alleviate musculoskeletal pain[
66], osteoarthritis[
67], fibromyalgia[
68], neuropathic pain[
69], low back pain[
70], and chronic pain[
71]. The data from our study demonstrated similar indications to prior studies, suggesting that oral Chinese herbal medicines and acupuncture can treat pain and BD effectively[
12,
72]. Still, future trials are needed to establish their efficacy further.
Short sleep duration is one of the BD diagnostic criteria, which probably explains why patients with BD are often diagnosed with sleep disturbances. Sleep disturbance may increase the risk of chronic pain[
73], which is mediated by cytokines, particularly interleukin (IL)-1β, IL-6, and tumor necrosis factor-alpha (TNF-α)[
74]. Elevated IL-6, IL-8, monocyte chemoattractant protein-1, interferon-gamma, and TNF-α levels in patients with BD were associated with inadequate response to antidepressants and short total sleep time[
63,
75]. Earlier findings indicated that patients with BD have elevated pro-inflammatory cytokine and decreased anti-inflammatory cytokine levels, such as IL-4 and IL-10[
76,
77]. The imbalance of pro-inflammatory and anti-inflammatory cytokines in patients with BD may worsen pain perception and sleep quality.
4.4. Top ten most commonly prescribed Chinese single herbs and herbal formulae
Our study’s most frequently prescribed single herb was Yanhusuo (Corydalis yanhusuo), which presented in myalgia and myositis, lumbago, cough, and headache prescriptions. Yanhusuo is one of the pain relief and blood activation TCM herbs[
78]. The alkaloids from Corydalis binding with GABA, dopamine, and benzodiazepine receptors can alleviate anxiety and depression symptoms[
79,
80]. A clinical study proved that Yanhusuo significantly decreased pain intensity and bothersomeness scores[
81]. Anxiety and depression can increase the sensitivity of pain[
82], and pain can also lead to anxiety and depression[
83], which may explain why Yanhusuo has anti-anxiety, anti-depression, and analgesic effects[
78]. Our finding also shows that Shu-Jing-Huo-Xue-Tang was the most frequently prescribed herbal formulae in treating lumbago, cough, and headache. It contains 15 herbs, including Dong Gui (Angelica sinensis), Chuan Xiong (Ligusticum wallichii), Di Huang (Rehmannia glutinosa), Fang Feng (Siler divaricatum), Bai Shao (Paeonia lactiflora), Long Dan Cao (Gentiana scabra), Tao Ren (Prunus persica), Huai Niu Xi (Achyranthes bidentata), Fu Ling (Poria cocos), Sheng Jiang (Zingiber officinale), Cang Zhu (Atractylodes lancea), Gan Cao (Glycyrrhiza glabra), Qiang Huo (Notopterygium incisium), Bai Zhi (Angelica anomala), and Chen Pi (Citrus reticulate). In neuropathic rats with chronic constriction injury (a model of neuropathic pain), Shu-Jing-Huo-Xue-Tang achieves anti-hypersensitivity effects through the regulation of α2 adrenoreceptors[
84]. Studies show that activation of α2 adrenoreceptors involves pain facilitation via the release of pro-nociceptive transmitters[
85]. These findings further support the efficacy of Shu-Jing-Huo-Xue-Tang in alleviating chronic and psychological pain.
Other Chinese herbal medicines with analgesic effects included Danshen (Salvia miltiorrhiza), Shao-Yao-Gan-Cao-Tang, and Xue-Fu-Zhu-Yu-Tang. In our study, Danshen (Salvia miltiorrhiza) was one of the herbals for myalgia and myositis, which has an analgesic effect because of its anti-inflammatory mechanism[
86]. Shao-Yao-Gan-Cao-Tang was prescribed for lumbago, headache, and contusion of ankle and foot excluding toes. Shao-Yao-Gan-Cao-Tang exerts a significant regulatory impact on neuropathic pain, which could increase an individual’s pain threshold and reduce surfactant protein, beta-endorphin, prostaglandin E2, and nitric oxide (NO) levels[
87]. In our study, Xue-Fu-Zhu-Yu-Tang was a formula used for headaches, which can relieve distending pain or the tingling sensation accompanying diseases such as ischemic heart disease and arthritis[
88].
The TCM herb and the formula with sedative and hypnotic effects were Suanzaoren (Ziziphus jujube Mill. var. spinosa) and Suan-Zao-Ren-Tang in our study. Suanzaoren is widely used to treat insomnia[
89] and possesses anxiolytic effects at low doses and sedative effects at high doses[
90]. Danshen (Salvia miltiorrhiza Bge.) and gegen (Pueraria lobata (Willd.) Ohwi) were also prescribed for sleep disturbances in the current study, which were associated with a lower risk of depression[
91]. The TCM formula Suan-Zao-Ren-Tang contains five herbs, including Suanzaoren (Semen Zizyphi spinosa), Fuling (Sclerotium Poriae Cocos), Chuanxiong (Radix Ligustici Chuanxiong), Zhimu (Rhizoma Anemarrhena), and Gancao (Radix Glycyrrhizae)[
92]. Suan-Zao-Ren-Tang ameliorates insomnia through the GABAergic and serotonergic systems, further suggested to regulate the immune system, particularly inflammation cytokines[
93,
94]. Other formulas for insomnia include Jia-Wei-Xiao-Yao-San, Chai-Hu-Jia-Long-Gu-Mu-Li-Tang, Gan-Mai-Da-Zao-Tang, and Tian-Wang-Bu-Xin-Dan[
95], which seem beneficial for reducing the time required for sleep onset. However, their potential to alleviate insomnia remains uncertain[
96].
4.5. Strengths and limitations of the study
This study is the first study using data from the NHIRD database to explore TCM usage after individuals diagnosed with BD. The major strength of our study is its use of a large population-based claim dataset of one ethnic group, which enabled the analysis of all cases of BD and TCM usage. The results reveal the rationale behind TCM usage in treating patients with BD, providing a new approach among clinicians and a comprehensive understanding of different levels of BD treatments.
We are aware that the present study has several limitations. First, we could not determine the BD severity of our study participants without further analysis. The Young Mania Rating Scale can evaluate manic symptoms at baseline and over time in individuals with mania[
97]. However, the NHIRD database doesn’t contain such information. We only noticed patients’ current relative ICD-9 code for BD. The possible solution is that we can assess the changes in BD patients’ outpatient, inpatient, emergency service records, and psychotropic medication usage before and after TCM interventions, which makes it possible to evaluate the prognoses and severities of BD. Second, the current approach could not estimate disease duration without information on the age of onset. Although patients with BD onset before eighteen years old exhibit more severe conditions[
43], physicians can try to diagnose BD as early as possible and provide suitable treatments that may relieve BD’s severity. Third, we didn’t check the prevalence and onset age of BD relative comorbidities or the timing of disease onset. Taking BD comorbid anxiety as an example, a paper published in 2012 indicated that females and early onset age were predominant in patients with bipolar one disorder comorbid anxiety[
98]. The prior literature, which had small subjects (n= 304), might not reveal the prevalence of anxiety in the real world. Still, it told us that women and younger patients had a higher risk of anxiety disorder. Fourth, we could not determine how long patients with BD received TCM treatment after taking standard medications. Therefore, we cannot detect adverse events for the drug-drug interaction between the prescribed medications and internal medicines of TCM directly from the NHIRD but only observe the relationships between each other. For example, whether the dosages or types of psychotropic medications for BD patients changed or not after TCM intervention under specific controls of NHIRD needs more studies to consolidate. Finally, we didn’t analyze the frequency of using single herbs, herbal formulae, and acupuncture together. The study proposal missed such a key comparison element. TCM clinicians tailor treatments based on their clinical judgment and abilities. We recognize that different TCM practitioners might suggest varied prescriptions for the same conditions, leading to potential differences in symptoms and adding a touch of unpredictability.
5. Conclusions
In this cohort study, we explored the feasibility of using TCM for patients with BD. Our study revealed the characteristics of Patients with BD receiving TCM treatments: a younger average age, a higher percentage of female individuals, more comorbidities of anxiety and alcohol use disorders, and higher mood stabilizer usage rates. Patients with BD visiting TCM clinics were diagnosed with sleep disturbances, joint pain, myalgia, and myositis related to inflammation and neuroimmune diseases by TCM physicians. Yanhusuo and Shu-Jing-Huo-Xue-Tang were the most used prescriptions, which had suggestive evidence of analgesic effects due to anti-inflammatory mechanisms and modulation of the monoamine neurotransmitter system in the central nervous system in Patients with BD by literature review.
The study’s findings offer valuable insights for clinical Chinese medicine practitioners, aiding them in selecting appropriate treatment plans when addressing patients with BD. This guidance extends to reflecting on the correlation between the symptoms presented by these patients during medical visits and their chief complaints. Moreover, an analysis of the current status of TCM utilization in BD treatment can serve as a foundational principle for academic and professional education by delving into the specifics of the patterns of single herbs and herbal formulas used by patients with BD since the NNHIRD initiation becomes crucial. This knowledge is a basis for prescribing medications and a foundation for planning additional basic or clinical experiments. Practically, mastering and predicting the development of patients with BD post-TCM treatment becomes instrumental in fostering patients’ adherence to medical advice. Furthermore, the adjustment of Western medicine dosage following TCM treatment is anticipated to mitigate toxic side effects.
In conclusion, our study provides new insight into TCM as a potential complementary treatment for BD. However, more evidence-based research is necessary to prove its efficacy in treating BD.
Author Contributions
Conceptualization, SPC, STY; methodology, SPC, KCH; software, KCH; Formal analysis, KCH; investigation, SPC, STY, KPS; writing – Original Draft, SPC, STY; writing – Review & Editing, SKS, KPS; visualization, SPC; supervision, STY, KPS; project administration, SPC; Funding acquisition, STY.
Funding
This research was funded by the Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW109-TDU-B-212-114004), MOST Clinical Trial Consortium for Stroke (MOST 109-2321-B-039-002), China Medical University Hospital (DMR-110-159, DMR-110-222, and CMU110-AWARD-02), Tseng-Lien Lin Foundation, Taichung, Taiwan.
Institutional Review Board Statement
This study was approved by the Research Ethics Committee of China Medical University and Hospital [CMUH104-REC2-115(AR-4)], Taichung, Taiwan.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used or analyzed during the current study are available
from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Grande, I., et al., Bipolar disorder. Lancet, 2016. 387(10027): p. 1561-1572.
- Ming T. Tsuang, M.T.a.P.B.J., Textbook in Psychiatric Epidemiology. 2011.
- Shim, I.H., et al., Predictors of a Shorter Time to Hospitalization in Patients with Bipolar Disorder: Medication during the Acute and Maintenance Phases and Other Clinical Factors. Clin Psychopharmacol Neurosci, 2017. 15(3): p. 248-255. [CrossRef]
-
Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry, 2002. 159(4 Suppl): p. 1-50.
- National Collaborating Centre for Mental, H., National Institute for Health and Care Excellence: Clinical Guidelines, in Bipolar Disorder: The NICE Guideline on the Assessment and Management of Bipolar Disorder in Adults, Children and Young People in Primary and Secondary Care. 2014, The British Psychological Society and The Royal College of Psychiatrists © The British Psychological Society & The Royal College of Psychiatrists, 2014.: London.
- Bai, Y.M., et al., Taiwan consensus of pharmacological treatment for bipolar disorder. J Chin Med Assoc, 2013. 76(10): p. 547-56. [CrossRef]
- Solomon, D.A., et al., Course of illness and maintenance treatments for patients with bipolar disorder. J Clin Psychiatry, 1995. 56(1): p. 5-13.
- Jarman, C.N., et al., Perceived treatment effectiveness, medication compliance, and complementary and alternative medicine use among veterans with bipolar disorder. J Altern Complement Med, 2010. 16(3): p. 251-5. [CrossRef]
- Perron, B.E., C.N. Jarman, and A.M. Kilbourne, Access to conventional mental health and medical care among users of complementary and alternative medicine with bipolar disorder. J Nerv Ment Dis, 2009. 197(4): p. 287-90. [CrossRef]
- Keaton, D., et al., Utilization of herbal and nutritional compounds among older adults with bipolar disorder and with major depression. Int J Geriatr Psychiatry, 2009. 24(10): p. 1087-93. [CrossRef]
- Kilbourne, A.M., et al., Determinants of complementary and alternative medicine use by patients with bipolar disorder. Psychopharmacol Bull, 2007. 40(3): p. 104-15.
- Andreescu, C., B.H. Mulsant, and J.E. Emanuel, Complementary and alternative medicine in the treatment of bipolar disorder--a review of the evidence. Journal of Affective Disorders, 2008. 110(1-2): p. 16-26. [CrossRef]
- Unschuld, P.U., Tessenow, H., & Jinsheng, Z. , Huang Di Nei Jing Su Wen: An Annotated Translation of Huang Di’s Inner Classic – Basic Questions: 2 volumes (1st ed.). 2011: University of California Press.
- Unschuld, P.U., Huang Di Nei Jing Ling Shu: The Ancient Classic on Needle Therapy (1st ed.). 2016: University of California Press.
- Zhang, Z.J., et al., The beneficial effects of the herbal medicine Free and Easy Wanderer Plus (FEWP) for mood disorders: double-blind, placebo-controlled studies. Journal of Psychiatric Research, 2007. 41(10): p. 828-36. [CrossRef]
- Kim, K.H., et al., Acupuncture for management of balance impairment in a patient with bipolar disorder. Jams Journal of Acupuncture & Meridian Studies, 2013. 6(1): p. 56-9. [CrossRef]
- Chang, L.C., et al., Utilization patterns of Chinese medicine and Western medicine under the National Health Insurance Program in Taiwan, a population-based study from 1997 to 2003. BMC Health Serv Res, 2008. 8: p. 170. [CrossRef]
- Bolton, S., et al., Bipolar disorder: Trimodal age-at-onset distribution. Bipolar Disord, 2021. 23(4): p. 341-356.
- Lavin, P., et al., Clinical correlates of late-onset versus early-onset bipolar disorder in a global sample of older adults. Int J Geriatr Psychiatry, 2022. 37(12). [CrossRef]
- Goetz, M., et al., Early stages of pediatric bipolar disorder: retrospective analysis of a Czech inpatient sample. Neuropsychiatr Dis Treat, 2015. 11: p. 2855-64. [CrossRef]
- Yee, C.S., et al., Maintenance Pharmacological Treatment of Juvenile Bipolar Disorder: Review and Meta-Analyses. Int J Neuropsychopharmacol, 2019. 22(8): p. 531-540. [CrossRef]
- Szmulewicz, A., M.P. Valerio, and D.J. Martino, Longitudinal analysis of cognitive performances in recent-onset and late-life Bipolar Disorder: A systematic review and meta-analysis. Bipolar Disord, 2020. 22(1): p. 28-37. [CrossRef]
- Chien, I.C., et al., Risk of hypertension in patients with bipolar disorder in Taiwan: a population-based study. Compr Psychiatry, 2013. 54(6): p. 687-93. [CrossRef]
- Hsu, J.H., I.C. Chien, and C.H. Lin, Increased risk of hyperlipidemia in patients with bipolar disorder: a population-based study. Gen Hosp Psychiatry, 2015. 37(4): p. 294-8. [CrossRef]
- Su, V.Y., et al., Chronic obstructive pulmonary disease associated with increased risk of bipolar disorder. Chron Respir Dis, 2017. 14(2): p. 151-160. [CrossRef]
- Penninx, B. and S.M.M. Lange, Metabolic syndrome in psychiatric patients: overview, mechanisms, and implications. Dialogues Clin Neurosci, 2018. 20(1): p. 63-73. [CrossRef]
- Peters, A.T., et al., The Burden of Repeated Mood Episodes in Bipolar I Disorder: Results From the National Epidemiological Survey on Alcohol and Related Conditions. J Nerv Ment Dis, 2016. 204(2): p. 87-94.
- Cassidy, F., E. Ahearn, and B.J. Carroll, Elevated frequency of diabetes mellitus in hospitalized manic-depressive patients. Am J Psychiatry, 1999. 156(9): p. 1417-20. [CrossRef]
- Goldstein, B.I., et al., Cardiovascular disease and hypertension among adults with bipolar I disorder in the United States. Bipolar Disord, 2009. 11(6): p. 657-62. [CrossRef]
- Kilbourne, A., et al., Burden of General Medical Conditions among Individuals with Bipolar Disorder. Bipolar disorders, 2004. 6: p. 368-73. [CrossRef]
- Chen, W., et al., Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med, 2015. 3(8): p. 631-9. [CrossRef]
- Hsu, J.H., I.C. Chien, and C.H. Lin, Increased risk of chronic obstructive pulmonary disease in patients with bipolar disorder: A population-based study. J Affect Disord, 2017. 220: p. 43-48. [CrossRef]
- Lasser, K., et al., Smoking and Mental IllnessA Population-Based Prevalence Study. JAMA, 2000. 284(20): p. 2606-2610.
- Fagiolini, A., et al., Prevalence of obesity and weight change during treatment in patients with bipolar I disorder. J Clin Psychiatry, 2002. 63(6): p. 528-33. [CrossRef]
- Hsu, J.H., I.C. Chien, and C.H. Lin, Increased risk of ischemic heart disease in patients with bipolar disorder: A population-based study. J Affect Disord, 2021. 281: p. 721-726. [CrossRef]
- Ösby, U., et al., Excess Mortality in Bipolar and Unipolar Disorder in Sweden. Archives of General Psychiatry, 2001. 58(9): p. 844-850.
- Chan, J.K.N., et al., Excess mortality and life-years lost in people with bipolar disorder: an 11-year population-based cohort study. Epidemiol Psychiatr Sci, 2021. 30: p. e39. [CrossRef]
- Yapici Eser, H., et al., Prevalence and Associated Features of Anxiety Disorder Comorbidity in Bipolar Disorder: A Meta-Analysis and Meta-Regression Study. Frontiers in Psychiatry, 2018. 9(229). [CrossRef]
- Merikangas, K.R., et al., Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Archives of general psychiatry, 2007. 64(5): p. 543-552. [CrossRef]
- Schiweck, C., et al., Comorbidity of ADHD and adult bipolar disorder: A systematic review and meta-analysis. Neurosci Biobehav Rev, 2021. 124: p. 100-123. [CrossRef]
- McElroy, S.L., et al., Prevalence and correlates of eating disorders in 875 patients with bipolar disorder. Journal of Affective Disorders, 2011. 128(3): p. 191-198. [CrossRef]
- Regier, D.A., et al., Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. Jama, 1990. 264(19): p. 2511-8. [CrossRef]
- Suominen, K., et al., Early age at onset of bipolar disorder is associated with more severe clinical features but delayed treatment seeking. Bipolar Disord, 2007. 9(7): p. 698-705. [CrossRef]
- Jones, G., et al., The role of lithium treatment on comorbid anxiety symptoms in patients with bipolar depression. J Affect Disord, 2022. 308: p. 71-75. [CrossRef]
- Trevor Young, L., et al., Anxious and non-anxious bipolar disorder. Journal of Affective Disorders, 1993. 29(1): p. 49-52. [CrossRef]
- Frye, M.A. and I.M. Salloum, Bipolar disorder and comorbid alcoholism: prevalence rate and treatment considerations. Bipolar Disord, 2006. 8(6): p. 677-85. [CrossRef]
- Pinto, J.V., et al., Remission and recurrence in bipolar disorder: The data from health outcomes and patient evaluations in bipolar disorder (HOPE-BD) study. J Affect Disord, 2020. 268: p. 150-157. [CrossRef]
- Zhuang, Y., et al., History of acupuncture research. Int Rev Neurobiol, 2013. 111: p. 1-23.
- Li, Y., Acupuncture journey to America: a turning point in 1971. Journal of Traditional Chinese Medical Sciences, 2014. 1(2): p. 81-83. [CrossRef]
- Upchurch, D.M. and B.W. Rainisch, A sociobehavioral wellness model of acupuncture use in the United States, 2007. J Altern Complement Med, 2014. 20(1): p. 32-9. [CrossRef]
- Chen, F.P., et al., Demographics and patterns of acupuncture use in the Chinese population: the Taiwan experience. J Altern Complement Med, 2006. 12(4): p. 379-87. [CrossRef]
- Wu, M.Y., et al., Trends in use of acupuncture among adults in Taiwan from 2002 to 2011: A nationwide population-based study. PLoS One, 2018. 13(4): p. e0195490. [CrossRef]
- Lee, I.S., et al., Fear of acupuncture enhances sympathetic activation to acupuncture stimulation. Acupunct Med, 2013. 31(3): p. 276-81. [CrossRef]
- Qin, W., et al., FMRI connectivity analysis of acupuncture effects on an amygdala-associated brain network. Mol Pain, 2008. 4: p. 55. [CrossRef]
- LeDoux, J., The emotional brain, fear, and the amygdala. Cell Mol Neurobiol, 2003. 23(4-5): p. 727-38.
- Strakowski, S.M., M.P. Delbello, and C.M. Adler, The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry, 2005. 10(1): p. 105-16. [CrossRef]
- Stubbs, B., et al., The prevalence of pain in bipolar disorder: a systematic review and large-scale meta-analysis. Acta Psychiatr Scand, 2015. 131(2): p. 75-88. [CrossRef]
- Leo, R.J. and J. Singh, Migraine headache and bipolar disorder comorbidity: A systematic review of the literature and clinical implications. Scand J Pain, 2016. 11: p. 136-145. [CrossRef]
- Goesling, J., L.A. Lin, and D.J. Clauw, Psychiatry and Pain Management: at the Intersection of Chronic Pain and Mental Health. Curr Psychiatry Rep, 2018. 20(2): p. 12. [CrossRef]
- Manji, H.K., et al., The underlying neurobiology of bipolar disorder. World Psychiatry, 2003. 2(3): p. 136-46.
- Kurita, M., Noradrenaline plays a critical role in the switch to a manic episode and treatment of a depressive episode. Neuropsychiatr Dis Treat, 2016. 12: p. 2373-2380. [CrossRef]
- Lee, M., C. Schwab, and P.L. McGeer, Astrocytes are GABAergic cells that modulate microglial activity. Glia, 2011. 59(1): p. 152-65. [CrossRef]
- Benedetti, F., et al., Higher Baseline Proinflammatory Cytokines Mark Poor Antidepressant Response in Bipolar Disorder. J Clin Psychiatry, 2017. 78(8): p. e986-e993. [CrossRef]
- Macone, A. and J.A.D. Otis, Neuropathic Pain. Semin Neurol, 2018. 38(6): p. 644-653.
- Orginazation, W.H., Acupuncture review and analysis of reports on controlled clinical trials. 2003.
- Arnold, M.D. and L.M. Thornbrough, Treatment of musculoskeletal pain with traditional Chinese herbal medicine. Phys Med Rehabil Clin N Am, 1999. 10(3): p. 663-71, ix-x. [CrossRef]
- Chrubasik, J.E., B.D. Roufogalis, and S. Chrubasik, Evidence of effectiveness of herbal antiinflammatory drugs in the treatment of painful osteoarthritis and chronic low back pain. Phytother Res, 2007. 21(7): p. 675-83. [CrossRef]
- Cao, H., J. Liu, and G.T. Lewith, Traditional Chinese Medicine for treatment of fibromyalgia: a systematic review of randomized controlled trials. J Altern Complement Med, 2010. 16(4): p. 397-409. [CrossRef]
- Forouzanfar, F. and H. Hosseinzadeh, Medicinal herbs in the treatment of neuropathic pain: a review. Iran J Basic Med Sci, 2018. 21(4): p. 347-358. [CrossRef]
- Gagnier, J.J., et al., Herbal Medicine for Low Back Pain: A Cochrane Review. Spine (Phila Pa 1976), 2016. 41(2): p. 116-33.
- Chen, L. and A. Michalsen, Management of chronic pain using complementary and integrative medicine. Bmj, 2017. 357: p. j1284. [CrossRef]
- Luo, Y., et al., Effects of Herbal Medicines on Pain Management. Am J Chin Med, 2020. 48(1): p. 1-16. [CrossRef]
- Finan, P.H., B.R. Goodin, and M.T. Smith, The association of sleep and pain: an update and a path forward. J Pain, 2013. 14(12): p. 1539-52. [CrossRef]
- Goldstein, B.I., et al., Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: a systematic review of the literature. J Clin Psychiatry, 2009. 70(8): p. 1078-90.
- Benedetti, F., et al., Neuroinflammation in Bipolar Depression. Front Psychiatry, 2020. 11: p. 71. [CrossRef]
- Kim, Y.-K., et al., Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. Journal of Affective Disorders, 2007. 104(1): p. 91-95. [CrossRef]
- Kunz, M., et al., Serum levels of IL-6, IL-10 and TNF-α in patients with bipolar disorder and schizophrenia: differences in pro- and anti-inflammatory balance. Braz J Psychiatry, 2011. 33(3): p. 268-74.
- Tian, B., M. Tian, and S.M. Huang, Advances in phytochemical and modern pharmacological research of Rhizoma Corydalis. Pharm Biol, 2020. 58(1): p. 265-275. [CrossRef]
- Wu, H., et al., A 1H-NMR-Based Metabonomic Study on the Anti-Depressive Effect of the Total Alkaloid of Corydalis Rhizoma. Molecules, 2015. 20(6): p. 10047-64. [CrossRef]
- Henkes, H., et al., Evaluation of the anxiolytic properties of tetrahydropalmatine, a Corydalis yanhusuo compound, in the male Sprague-Dawley rat. Aana j, 2011. 79(4 Suppl): p. S75-80.
- Yuan, C.S., et al., Effects of Corydalis yanhusuo and Angelicae dahuricae on cold pressor-induced pain in humans: a controlled trial. J Clin Pharmacol, 2004. 44(11): p. 1323-7. [CrossRef]
- Hermesdorf, M., et al., Pain Sensitivity in Patients With Major Depression: Differential Effect of Pain Sensitivity Measures, Somatic Cofactors, and Disease Characteristics. J Pain, 2016. 17(5): p. 606-16. [CrossRef]
- Michaelides, A. and P. Zis, Depression, anxiety and acute pain: links and management challenges. Postgrad Med, 2019. 131(7): p. 438-444. [CrossRef]
- Shu, H., et al., Anti-hypersensitivity effects of Shu-jing-huo-xue-tang, a Chinese herbal medicine, in CCI-neuropathic rats. Journal of Ethnopharmacology, 2010. 131(2): p. 464-470. [CrossRef]
- Sonohata, M., et al., Actions of noradrenaline on substantia gelatinosa neurones in the rat spinal cord revealed by in vivo patch recording. J Physiol, 2004. 555(Pt 2): p. 515-26.
- Imanshahidi, M. and H. Hosseinzadeh, The pharmacological effects of Salvia species on the central nervous system. Phytother Res, 2006. 20(6): p. 427-37. [CrossRef]
- Feng, L.-M., et al., An integrated strategy for discovering effective components of Shaoyao Gancao decoction for treating neuropathic pain by the combination of partial least-squares regression and multi-index comprehensive method. Journal of Ethnopharmacology, 2020. 260: p. 113050. [CrossRef]
- He, H., et al., Xue-Fu-Zhu-Yu capsule in the treatment of qi stagnation and blood stasis syndrome: a study protocol for a randomised controlled pilot and feasibility trial. Trials, 2018. 19(1): p. 515. [CrossRef]
- Shergis, J.L., et al., Ziziphus spinosa seeds for insomnia: A review of chemistry and psychopharmacology. Phytomedicine, 2017. 34: p. 38-43. [CrossRef]
- Peng, W.H., et al., Anxiolytic effect of seed of Ziziphus jujuba in mouse models of anxiety. J Ethnopharmacol, 2000. 72(3): p. 435-41. [CrossRef]
- Chiao, Y.W., et al., Use of Chinese Herbal Medicines Is Related to a Reduction in Depression Risk Among Patients With Insomnia: A Matched Cohort Study. Front Neurol, 2020. 11: p. 583485. [CrossRef]
- Yeh, C.H., et al., Suan zao ren tang as an original treatment for sleep difficulty in climacteric women: a prospective clinical observation. Evid Based Complement Alternat Med, 2011. 2011: p. 673813.
- Zhou, Q.H., et al., Suanzaoren Formulae for Insomnia: Updated Clinical Evidence and Possible Mechanisms. Front Pharmacol, 2018. 9: p. 76. [CrossRef]
- Gao, J., et al., In Silico Study of Anti-Insomnia Mechanism for Suanzaoren Prescription. Front Pharmacol, 2019. 10: p. 925. [CrossRef]
- Chen, F.P., et al., Prescriptions of Chinese Herbal Medicines for Insomnia in Taiwan during 2002. Evid Based Complement Alternat Med, 2011. 2011: p. 236341.
- Yang, X.Q., et al., Tian Wang Bu Xin Dan for Insomnia: A Systematic Review of Efficacy and Safety. Evid Based Complement Alternat Med, 2019. 2019: p. 4260801. [CrossRef]
- Zhang, Z.J., et al., Adjunctive herbal medicine with carbamazepine for bipolar disorders: A double-blind, randomized, placebo-controlled study. Journal of Psychiatric Research, 2007. 41(3-4): p. 360-9. [CrossRef]
- Tsai, H.C., et al., Empirically derived subgroups of bipolar I patients with different comorbidity patterns of anxiety and substance use disorders in Han Chinese population. J Affect Disord, 2012. 136(1-2): p. 81-89. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).