Submitted:
14 January 2024
Posted:
15 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Digestion and Mucosal Barrier
2.1. Digestion
2.2. Mucosal Barrier: Composition and Role
3. Microbiota and Food Allergies
4. Weaning and Its Role in Allergies
5. Methods
6. Discussion
7. Studies
7.1. Studies Performed in High-Risk Populations
7.1.1. Egg Proteins
7.1.2. Cow’s Milk, Peanut, Hard-Boiled Hen’s Egg, Sesame, Whitefish (cod), and Wheat
7.1.3. Rusk-like Biscuit Powder
7.1.4. Peanut
7.2. Studies Performed in Low-Risk Populations
7.2.1. Egg Proteins
7.2.2. Cow’s Milk, Peanut, Hard-Boiled Hen’s Egg, Sesame, Whitefish (Cod), and Wheat
7.3. Studies Performed in Both High-Risk and Low-Risk Populations
7.3.1. Cow’s Milk Proteins
| Author, Year, Country, Trial | Study Design | Sample Size | Population | Inclusion criteria | Allergen | Follow-up | Outcome |
|---|---|---|---|---|---|---|---|
| Snijders et al., 2008 Netherlands KOALA [64] |
Prospective birth cohort study | 2558 infants | General Population | Pregnant women with diverse lifestyles | Cow’s milk products and other solid products. | Questionnaires at 7,12, 24 months; Specific IgE > 0.3 UI/ml against eggs, cow’s milk at age 2 |
Delayed introduction of CMP and other food products associated with higher risk for eczema (P =.01 and .02 for trend, respectively); No association between delay introduction of CMP and AD; delay introduction of other food associated with higher risk for AD (P=.00 trend) and increased risk of atopy development at the age of 2 years |
| Palmer et al., 2013 Australia STAR [56] |
RDBPCT | 86 infants: 49 SG 37 CG |
High risk | 4 month of age singleton term infants with moderate-to-severe eczema no prior egg or solid food ingestion | Hen’s egg (0,9 g/day) |
OFC and SPT at 12 months | At 12 months 33% SG, 51% CG were diagnosed IgE-mediated egg allergy (relative risk, 0.65; 95% CI, 0.38-1.11; P=0.11) |
| Du Toit et al., 2015 England LEAP [61] |
RCT | 640 infants: 319 SG 321 CG |
Infants 4 to 11 months of age with severe eczema, egg allergy, or both | High risk | Peanut (6 g/week) |
Open OFC or DBPCFC at 12, 30, and 60 months |
In the intention to treat population: 13.7% in the CG and 1.9% in the SG who had negative SPT developed peanut allergy (P<0.001) |
| Perkin et al, 2016 England EAT [63] |
RCT | 1303 infants: 652 SG 651 CG |
Exclusively breastfed infants for ≥ months, regardless of atopic status or family history of allergy | General population |
Cow’s milk, peanut, hard-boiled hen’s egg, sesame, whitefish (cod), and wheat at 3 and 6 months of age (4 g/week) |
OFC at 1 and 3 years of age after allergenics food introduction | Among Infants with sensitization to 1 or more foods at enrollment, EIG infants developed significantly less FA than SIG infants SIG, 3+34.2%; EIG, 19.2%; P= 5 .03 |
| Bellach et al., 2017 Germany HEAP [62] |
RDBPCT | 383 infants: 184 SG 199 CG |
GA >/= 34 weeks and birth weight >/= 2.5 kg Specific IgE to egg <0.35 kU/L |
General Population 4-6 months |
Hen’s egg 2,5 g 3 times/week from 4-6 to 12 months |
OFC and specific IgE ≥0.35 KU/l at 12 months after hen’s egg introduction |
sensitized to hen’s egg at age 12 months : 5.6% (6/124) in SG 2.6% (4/152) in CG (P= 0.35) Allergy to hen’s egg 2.1% in SG 0.6% in CG (relative risk,3.30;95%CI, 0.35-31.32; P=0.35) No prevention in hen’s egg sensitization nor egg allergy |
| Tan et al, 2017 Australia BEAT [58]. |
RDBCT | 319 infants: SG 165 CG 154 |
Infants with at least 1 first-degree relative with allergic disease and SPT<2 mm | High risk | Hen’s egg 350 mg from4-8 months |
EW SPT response of 3 mm or greater OFC to whole egg at age 12 months. |
Sensitization to EW at 12 months: 20% in CG 11% in SG Allergy to EW at 12 months: 10,5% in CG 6,2% in SG (odds ratio, 0.46;95% CI, 0.22-0.95; P=0.03) |
| Natsume et al, 2017 Japan PETIT [55] |
RDBPCT | 147 infants | 4-5 months of age of age with eczema | High risk | Eggs 50 mg/die (3-9 months) 250 mg/die (9-12 months) |
Open OFC at 12 months of age |
Five (8%) of 60 participants had an egg allergy in the SG compared to 23 (38%) of 61 in the CG (risk ratio 0.221;95% CI,0.090-0.543; P=0.0001) |
| Palmer et al, 2017 Australia STEP [57] |
RCT | 820 infants: SG 165 CG 154 |
Singleton infants with atopic mothers, recruited before age 6.5 months No prior egg ingestion and allergic disease |
High risk | Hen’s egg pasteurized raw whole egg powder (SG = 407) or a rice powder (CG = 413) from 6 to 10 months; introduction egg at 10 months |
OFC to egg at 12 months and SPT positive | At 12 months: IgE mediated food allergy : SG 7.0% vs CG 10.3% (RR(95%CI) 0.75 (0.48-1,17) P=0.20) |
| Nishimura et al., 2022 Japan SEED [59] |
RCT | 163 children 83 SG 80 CG |
3-4 months old with atopic dermatitis | Egg, milk, wheat, soybean, buckwheat, and peanuts | Amount of powder increased at weeks 2, and 12 week. The occurrence of FA at 18 months old |
Incidence of FA episodes by 18 months: SG 7/83 vs CG 19/80; (risk ratio 0.301 [95% CI 0.116e0.784]; P = 0.0066). Egg allergies were reduced in the SG group |
|
| Kalb et al, 2022 German TEFFA [60]. |
RCT | 150 infants with atopic eczema at 4–8 months randomized in a 2:1 manner into an SG and CG | 4-8 months old infants with eczema | High risk | Rusk-like biscuit powder with HE, CM, PN, HN 2 mg for 6-8 months |
After 6 months of intervention, they will check sensitization against hen’s egg, cow’s milk, hazelnut, and peanut | At 12 months Egg allergy: SG 2.1% CG 06% (3.30;95% CI,0.31-3132 P=0.35) |
8. Limitations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Martinis M, Sirufo MM, Suppa M, Ginaldi L. New Perspectives in Food Allergy. Int J Mol Sci. 2020 Feb 21;21(4):1474. [CrossRef]
- Sicherer SH, Warren CM, Dant C, Gupta RS, Nadeau KC. Food Allergy from Infancy Through Adulthood. J Allergy Clin Immunol Pract. 2020 Jun;8(6):1854–64. [CrossRef]
- Reyes-Pavón D, Jiménez M, Salinas E. Physiopathology of food allergies. Rev Alerg Mex. 2020;67(1):34–53. [CrossRef]
- Michelet M, Balbino B, Guilleminault L, Reber LL. IgE in the pathophysiology and therapy of food allergy. Eur J Immunol. 2021 Mar;51(3):531–43. [CrossRef]
- Cianferoni, A. Non-IgE Mediated Food Allergy. Curr Pediatr Rev. 2020;16(2):95–105.
- Zhang S, Sicherer S, Berin MC, Agyemang A. Pathophysiology of Non-IgE-Mediated Food Allergy. Immunotargets Ther. 2021;10:431–46. [CrossRef]
- Chong KW, Ruiz-Garcia M, Patel N, Boyle RJ, Turner PJ. Reaction phenotypes in IgE-mediated food allergy and anaphylaxis. Ann Allergy Asthma Immunol. 2020 May;124(5):473–8. [CrossRef]
- Domínguez O, Plaza AM, Alvaro M. Relationship Between Atopic Dermatitis and Food Allergy. Curr Pediatr Rev. 2020;16(2):115–22. [CrossRef]
- Zubeldia-Varela E, Barker-Tejeda TC, Blanco-Pérez F, Infante S, Zubeldia JM, Pérez-Gordo M. Non-IgE-Mediated Gastrointestinal Food Protein-Induced Allergic Disorders. Clinical Perspectives and Analytical Approaches. Foods. 2021 Nov 2;10(11):2662. [CrossRef]
- Cafarotti A, Giovannini M, Begìn P, Brough HA, Arasi S. Management of IgE-mediated food allergy in the 21st century. Clin Exp Allergy. 2023 Jan;53(1):25–38. [CrossRef]
- Barni S, Liccioli G, Sarti L, Giovannini M, Novembre E, Mori F. Immunoglobulin E (IgE)-Mediated Food Allergy in Children: Epidemiology, Pathogenesis, Diagnosis, Prevention, and Management. Medicina (Kaunas). 2020 Mar 4;56(3):111. [CrossRef]
- Peters RL, Krawiec M, Koplin JJ, Santos AF. Update on food allergy. Pediatr Allergy Immunol. 2021 May;32(4):647–57. [CrossRef]
- Bøgh KL, Madsen CB. Food Allergens: Is There a Correlation between Stability to Digestion and Allergenicity? Crit Rev Food Sci Nutr. 2016 Jul 3;56(9):1545–67. [CrossRef]
- van Lieshout GAA, Lambers TT, Bragt MCE, Hettinga KA. How processing may affect milk protein digestion and overall physiological outcomes: A systematic review. Crit Rev Food Sci Nutr. 2020;60(14):2422–45. [CrossRef]
- Bhagavan NV, Ha CE. Chapter 11 - Gastrointestinal Digestion and Absorption. In: Bhagavan NV, Ha CE, editors. Essentials of Medical Biochemistry (Second Edition) [Internet]. San Diego: Academic Press; 2015 [cited 2023 Aug 14]. p. 137–64. Available from: https://www.sciencedirect.com/science/article/pii/B9780124166875000117. 9780.
- Nilius B, Gudermann T, Jahn R, Lill R, Petersen OH, De Tombe PP, editors. Reviews of Physiology, Biochemistry and Pharmacology [Internet]. Cham: Springer International Publishing; 2015 [cited 2023 Aug 14]. (Reviews of Physiology, Biochemistry and Pharmacology; vol. 168). Available from: https://link.springer.com/10.1007/978-3-319-22503-6.
- Verhoeckx K, Bøgh KL, Dupont D, Egger L, Gadermaier G, Larré C, Mackie A, Menard O, Adel-Patient K, Picariello G, Portmann R, Smit J, Turner P, Untersmayr E, Epstein MM. The relevance of a digestibility evaluation in the allergenicity risk assessment of novel proteins. Opinion of a joint initiative of COST action ImpARAS and COST action INFOGEST. Food Chem Toxicol. 2019 Jul;129:405–23. [CrossRef]
- Dallas DC, Sanctuary MR, Qu Y, Khajavi SH, Van Zandt AE, Dyandra M, Frese SA, Barile D, German JB. Personalizing protein nourishment. Crit Rev Food Sci Nutr. 2017 Oct 13;57(15):3313–31. [CrossRef]
- Ballegaard ASR, Bøgh KL. Intestinal protein uptake and IgE-mediated food allergy. Food Res Int. 2023 Jan;163:112150. [CrossRef]
- Umar, S. Intestinal stem cells. Curr Gastroenterol Rep. 2010 Oct;12(5):340–8.
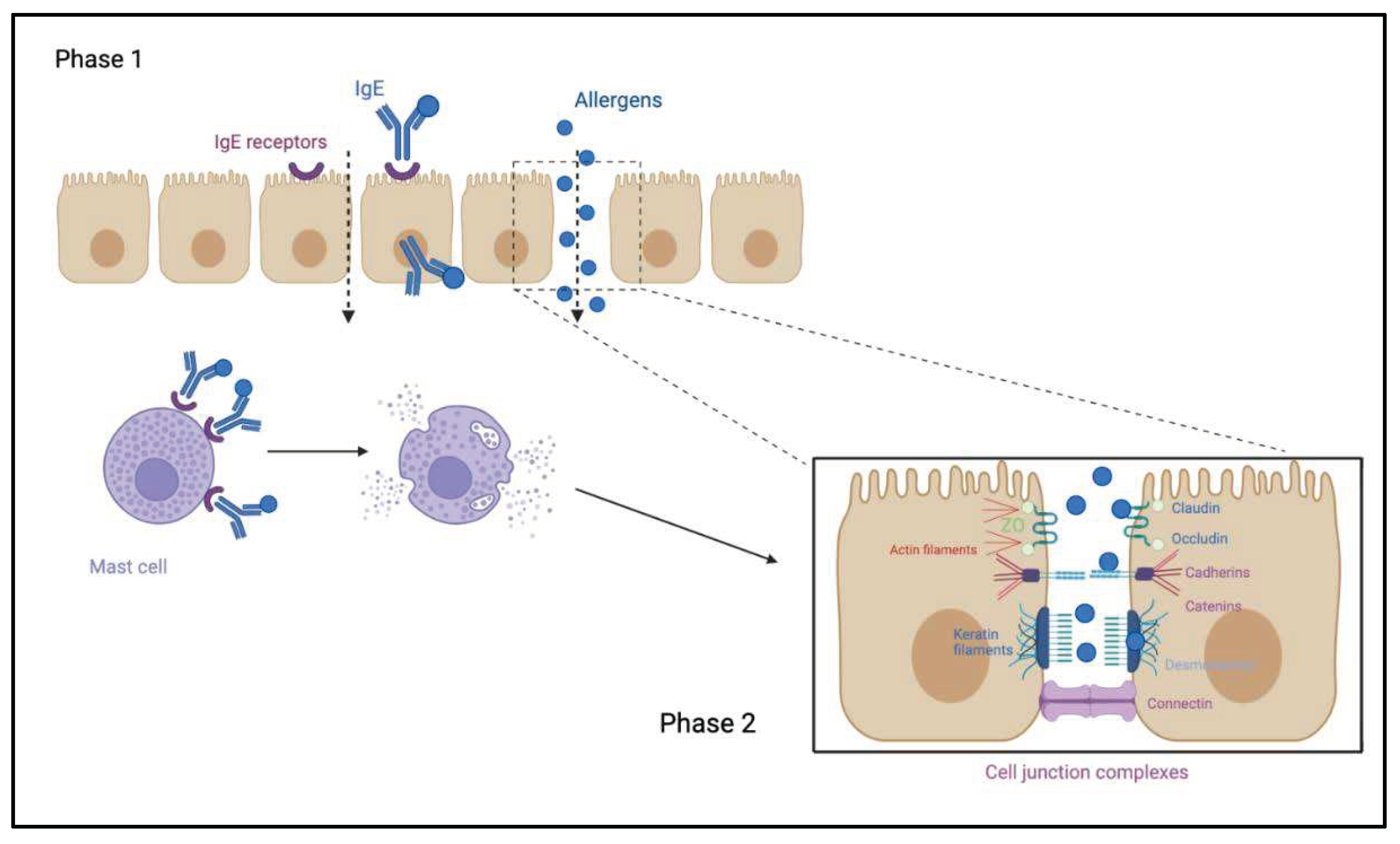
- Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends in Cell Biology. 2010 Mar 1;20(3):142–9. [CrossRef]
- Parrish A, Boudaud M, Kuehn A, Ollert M, Desai MS. Intestinal mucus barrier: a missing piece of the puzzle in food allergy. Trends Mol Med. 2022 Jan;28(1):36–50. [CrossRef]
- Ermund A, Schütte A, Johansson MEV, Gustafsson JK, Hansson GC. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2013 Sep;305(5):G341–7. [CrossRef]
- Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol. 2001 May;280(5):G922-929. [CrossRef]
- Santaolalla R, Abreu MT. Innate immunity in the small intestine. Current Opinion in Gastroenterology. 2012 Mar;28(2):124. [CrossRef]
- Brandtzaeg, P. The gut as communicator between environment and host: Immunological consequences. European Journal of Pharmacology. 2011 Sep 1;668:S16–32. [CrossRef]
- Perrier C, Corthésy B. Gut permeability and food allergies. Clin Exp Allergy. 2011 Jan;41(1):20–8. [CrossRef]
- Graversen KB, Ballegaard ASR, Kræmer LH, Hornslet SE, Sørensen LV, Christoffersen HF, Jacobsen LN, Untersmayr E, Smit JJ, Bøgh KL. Cow’s milk allergy prevention and treatment by heat-treated whey—A study in Brown Norway rats. Clinical & Experimental Allergy. 2020;50(6):708–21. [CrossRef]
- So AL, Pelton-Henrion K, Small G, Becker K, Oei E, Tyorkin M, Sperber K, Mayer L. Antigen Uptake and Trafficking in Human Intestinal Epithelial Cells. Dig Dis Sci. 2000 Jul 1;45(7):1451–61. [CrossRef]
- Yu LCH, Yang PC, Berin MC, Leo VD, Conrad DH, Mckay DM, Satoskar AR, Perdue MH. Enhanced transepithelial antigen transport in intestine of allergic mice is mediated by IgE/CD23 and regulated by interleukin-4. Gastroenterology. 2001 Aug 1;121(2):370–81. [CrossRef]
- Pickard JM, Zeng MY, Caruso R, Núñez G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. 2017 Sep;279(1):70–89. [CrossRef]
- Heintz-Buschart A, Wilmes P. Human Gut Microbiome: Function Matters. Trends Microbiol. 2018 Jul;26(7):563–74. [CrossRef]
- Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013 May;13(5):321–35. [CrossRef]
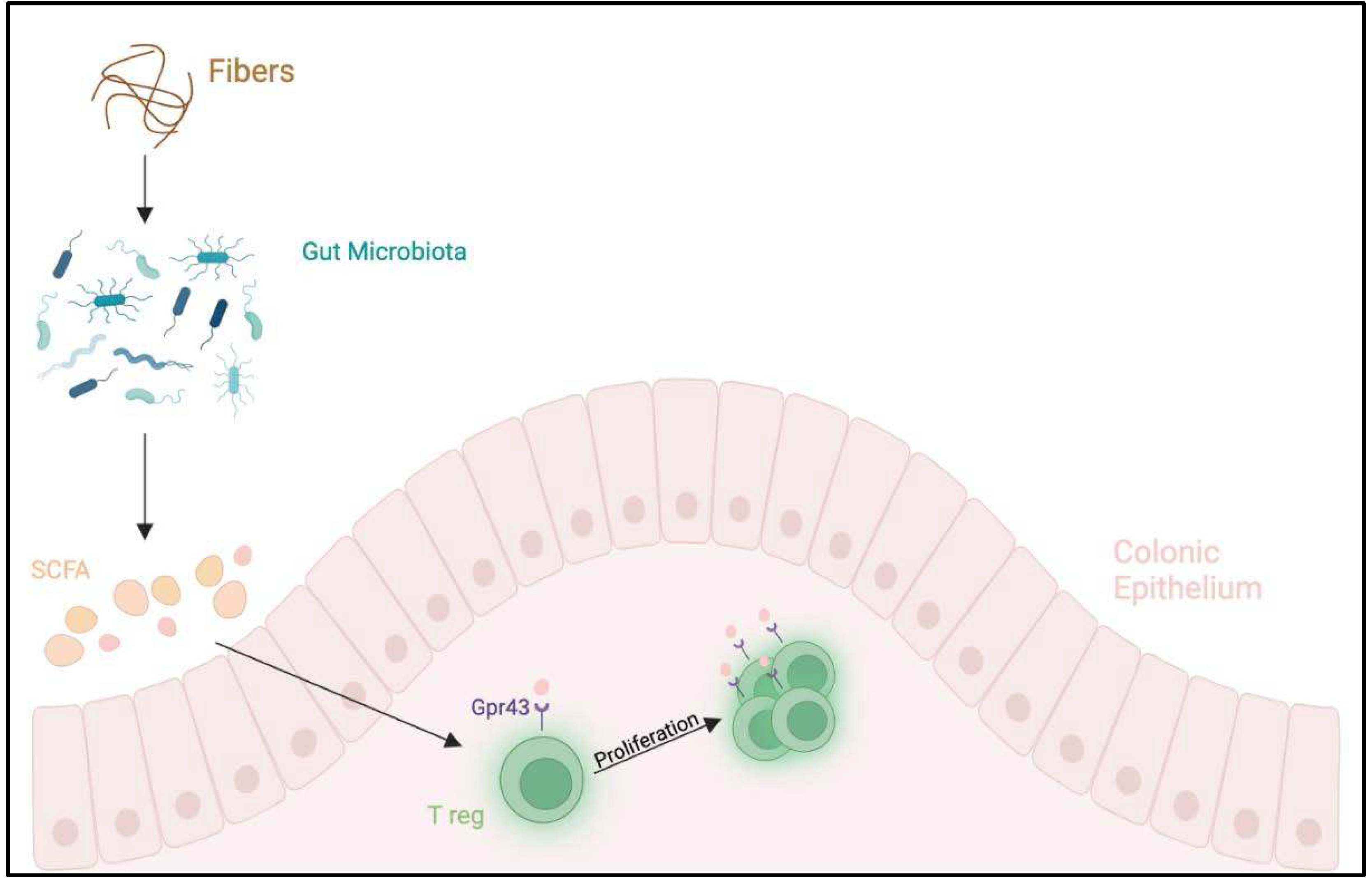
- Bibbò S, Ianiro G, Giorgio V, Scaldaferri F, Masucci L, Gasbarrini A, Cammarota G. The role of diet on gut microbiota composition. Eur Rev Med Pharmacol Sci. 2016 Nov;20(22):4742–9.
- Wopereis H, Oozeer R, Knipping K, Belzer C, Knol J. The first thousand days - intestinal microbiology of early life: establishing a symbiosis. Pediatr Allergy Immunol. 2014 Aug;25(5):428–38. [CrossRef]
- Wiertsema SP, van Bergenhenegouwen J, Garssen J, Knippels LMJ. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients. 2021 Mar 9;13(3):886. [CrossRef]
- West CE, Jenmalm MC, Prescott SL. The gut microbiota and its role in the development of allergic disease: a wider perspective. Clin Exp Allergy. 2015 Jan;45(1):43–53. [CrossRef]
- Stefka AT, Feehley T, Tripathi P, Qiu J, McCoy K, Mazmanian SK, Tjota MY, Seo GY, Cao S, Theriault BR, Antonopoulos DA, Zhou L, Chang EB, Fu YX, Nagler CR. Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci U S A. 2014 Sep 9;111(36):13145–50. [CrossRef]
- Prince BT, Mandel MJ, Nadeau K, Singh AM. Gut Microbiome and the Development of Food Allergy and Allergic Disease. Pediatr Clin North Am. 2015 Dec;62(6):1479–92. [CrossRef]
- Lee KH, Song Y, Wu W, Yu K, Zhang G. The gut microbiota, environmental factors, and links to the development of food allergy. Clin Mol Allergy. 2020;18:5. [CrossRef]
- Rachid R, Stephen-Victor E, Chatila TA. The microbial origins of food allergy. J Allergy Clin Immunol. 2021 Mar;147(3):808–13. [CrossRef]
- Shu SA, Yuen AWT, Woo E, Chu KH, Kwan HS, Yang GX, Yang Y, Leung PSC. Microbiota and Food Allergy. Clin Rev Allergy Immunol. 2019 Aug;57(1):83–97. [CrossRef]
- Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009 Oct 29;461(7268):1282–6. [CrossRef]
- Prescott S, Allen KJ. Food allergy: riding the second wave of the allergy epidemic. Pediatr Allergy Immunol. 2011 Mar;22(2):155–60. [CrossRef]
- Høst A, Koletzko B, Dreborg S, Muraro A, Wahn U, Aggett P, Bresson JL, Hernell O, Lafeber H, Michaelsen KF, Micheli JL, Rigo J, Weaver L, Heymans H, Strobel S, Vandenplas Y. Dietary products used in infants for treatment and prevention of food allergy. Joint Statement of the European Society for Paediatric Allergology and Clinical Immunology (ESPACI) Committee on Hypoallergenic Formulas and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition. Arch Dis Child. 1999 Jul;81(1):80–4. [CrossRef]
- American Academy of Pediatrics Committee on Nutrition: Hypoallergenic infant formulas. Pediatrics. 1989 Jun;83(6):1068–9.
- Bailey M, Haverson K, Inman C, Harris C, Jones P, Corfield G, Miller B, Stokes C. The development of the mucosal immune system pre- and post-weaning: balancing regulatory and effector function. Proc Nutr Soc. 2005 Nov;64(4):451–7. [CrossRef]
- Arshad SH, Bateman B, Sadeghnejad A, Gant C, Matthews SM. Prevention of allergic disease during childhood by allergen avoidance: the Isle of Wight prevention study. J Allergy Clin Immunol. 2007 Feb;119(2):307–13. [CrossRef]
- Zutavern A, Brockow I, Schaaf B, von Berg A, Diez U, Borte M, Kraemer U, Herbarth O, Behrendt H, Wichmann HE, Heinrich J, LISA Study Group. Timing of solid food introduction in relation to eczema, asthma, allergic rhinitis, and food and inhalant sensitization at the age of 6 years: results from the prospective birth cohort study LISA. Pediatrics. 2008 Jan;121(1):e44-52. [CrossRef]
- Lack G, Fox D, Northstone K, Golding J, Avon Longitudinal Study of Parents and Children Study Team. Factors associated with the development of peanut allergy in childhood. N Engl J Med. 2003 Mar 13;348(11):977–85.
- Anvari S, Chokshi NY, Kamili QUA, Davis CM. Evolution of Guidelines on Peanut Allergy and Peanut Introduction in Infants: A Review. JAMA Pediatr. 2017 Jan 1;171(1):77–82.
- Greer FR, Sicherer SH, Burks AW, American Academy of Pediatrics Committee on Nutrition, American Academy of Pediatrics Section on Allergy and Immunology. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008 Jan;121(1):183–91.
- Muraro A, Halken S, Arshad SH, Beyer K, Dubois AEJ, Du Toit G, Eigenmann PA, Grimshaw KEC, Hoest A, Lack G, O’Mahony L, Papadopoulos NG, Panesar S, Prescott S, Roberts G, de Silva D, Venter C, Verhasselt V, Akdis AC, Sheikh A, EAACI Food Allergy and Anaphylaxis Guidelines Group. EAACI food allergy and anaphylaxis guidelines. Primary prevention of food allergy. Allergy. 2014 May;69(5):590–601.
- Fewtrell M, Bronsky J, Campoy C, Domellöf M, Embleton N, Fidler Mis N, Hojsak I, Hulst JM, Indrio F, Lapillonne A, Molgaard C. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2017 Jan;64(1):119–32.
- Natsume O, Kabashima S, Nakazato J, Yamamoto-Hanada K, Narita M, Kondo M, Saito M, Kishino A, Takimoto T, Inoue E, Tang J, Kido H, Wong GWK, Matsumoto K, Saito H, Ohya Y, PETIT Study Team. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): a randomised, double-blind, placebo-controlled trial. Lancet. 2017 Jan 21;389(10066):276–86. [CrossRef]
- Palmer DJ, Metcalfe J, Makrides M, Gold MS, Quinn P, West CE, Loh R, Prescott SL. Early regular egg exposure in infants with eczema: A randomized controlled trial. J Allergy Clin Immunol. 2013 Aug;132(2):387-392.e1. [CrossRef]
- Palmer DJ, Sullivan TR, Gold MS, Prescott SL, Makrides M. Randomized controlled trial of early regular egg intake to prevent egg allergy. J Allergy Clin Immunol. 2017 May;139(5):1600-1607.e2. [CrossRef]
- Wei-Liang Tan J, Valerio C, Barnes EH, Turner PJ, Van Asperen PA, Kakakios AM, Campbell DE, Beating Egg Allergy Trial (BEAT) Study Group. A randomized trial of egg introduction from 4 months of age in infants at risk for egg allergy. J Allergy Clin Immunol. 2017 May;139(5):1621-1628.e8. [CrossRef]
- Nishimura T, Fukazawa M, Fukuoka K, Okasora T, Yamada S, Kyo S, Homan M, Miura T, Nomura Y, Tsuchida S, Yajima S, Aoki S, Nakamura Y, Hosaka T, Hidaka H, Yamamori H, Inoue A, Morimoto J. Early introduction of very small amounts of multiple foods to infants: A randomized trial. Allergol Int. 2022 Jul;71(3):345–53. [CrossRef]
- Kalb B, Meixner L, Trendelenburg V, Unterleider N, Dobbertin-Welsch J, Heller S, Dölle-Bierke S, Roll S, Lau S, Lee YA, Fauchère F, Braun J, Babina M, Altrichter S, Birkner T, Worm M, Beyer K. Tolerance induction through early feeding to prevent food allergy in infants with eczema (TEFFA): rationale, study design, and methods of a randomized controlled trial. Trials. 2022 Mar 12;23(1):210. [CrossRef]
- Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, Brough HA, Phippard D, Basting M, Feeney M, Turcanu V, Sever ML, Gomez Lorenzo M, Plaut M, Lack G, LEAP Study Team. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015 Feb 26;372(9):803–13. [CrossRef]
- Bellach J, Schwarz V, Ahrens B, Trendelenburg V, Aksünger Ö, Kalb B, Niggemann B, Keil T, Beyer K. Randomized placebo-controlled trial of hen’s egg consumption for primary prevention in infants. J Allergy Clin Immunol. 2017 May;139(5):1591-1599.e2. [CrossRef]
- Perkin MR, Logan K, Marrs T, Radulovic S, Craven J, Flohr C, Lack G, EAT Study Team. Enquiring About Tolerance (EAT) study: Feasibility of an early allergenic food introduction regimen. J Allergy Clin Immunol. 2016 May;137(5):1477-1486.e8. [CrossRef]
- Snijders BEP, Thijs C, van Ree R, van den Brandt PA. Age at first introduction of cow milk products and other food products in relation to infant atopic manifestations in the first 2 years of life: the KOALA Birth Cohort Study. Pediatrics. 2008 Jul;122(1):e115-122. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





