Submitted:
10 January 2024
Posted:
11 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
SGLT
Mechanism of Renoprotection in Non-Diabetic Kidney beyond Glucose Control (Table 1)
| -Antihyperglycemic -Anti-inflammatory→ Decreasing inflammatory and reactive oxygen species. -Antioxidant -Promote tubule-glomerular feedback→Decrease glomerular hyperfiltration -Activate adenosine mono-phosphate-activated protein kinase →Decrease glomerular and tubular injury -Hemodynamic changes→Decrease albuminuria -Improve lipid profile -Reduce body weight -Natriuresis→Mild decrease in systolic and diastolic blood pressure -Attenuate renal ischemia-reperfusion injury -Decrease serum uric acid |
2. SGLT2i and Non-Diabetic Kidney Dysfunction-Preclinical Experiments (Table 2)
Dapagliflozin
Canagliflozin
Empagliflozin
Ipragliflozin
| Drug | Animal | Effects of SGLT2i | Ref. |
|---|---|---|---|
| Dapagliflozin | C57BL/6 mice with renal-reperfusion injury - C57BL/6J mice with adenine (0.2% diet) -induced renal injury -Unilateral ureteral obstruction in C57BL/6J mice -Balb\c albino mice treated with cyclosporine A -C57BL/6N mice with protein-overload proteinuria induced by unilateral nephrectomy and injections of bovine serum albumin - Dahl salt sensitive rats with salt-induced hypertension and cardiorenal disease -Iron-overload rat model -Rats with doxorubicin induced glomerular atrophy -Spontaneously hypertensive rats -Rats with unilateral ureteral obstruction -Rats with 5/6 (subtotally) nephrectomized - Rats with subtotally nephrectomized |
-Attenuated renal ischemia-reperfusion injury, improved renal function, reduced apoptotic cell death and increased renal expression of hypoxia induced factor 1 -Improved renal function and ameliorated renal fibrosis, increased mitochondrial metabolism and fatty acid oxidation, reduction of inflammation and oxidative stress. -Improved renal function and renal fibrosis independent of direct blood glucose control -Reduced oxidative stress, apoptosis, and histopathological damage in renal tissue -Reduced proteinuria and ameliorated podocyte dysfunction and loss and provided renal protection -Reduced albuminuria and attenuated renal inflammation and oxidative stress- Preserved the glomerular and mesangial structure and reduced renal oxidative stress -Attenuated glomerular atrophy, renal fibrosis, and dysfunction -Reduced urinary albumin creatinine ratio but had no significant effect on serum creatinine levels or renal histological changes - Reduced renal fibrosis -Did not attenuate heavy proteinuria, declining glomerular filtration rate, the extent of glomerulosclerosis or tubulointerstitial fibrosis -Did not modify renal hemodynamic function nor attenuated proteinuria |
26 27 28 29 30 31 6 32 33 34 35 36 |
| Canagliflozin | -Rats with adenine-induced chronic kidney disease - Male C57BL/6 mice fed with 0.2% adenine Rats with adenine-induced chronic kidney disease Rats, isoprenaline-treated - Dahl salt-sensitive rats with high-salt diet-inducing hypertensive renal injury. - Mice with cisplatin-induced nephrotoxicity. - Mice with cisplatin-induced nephrotoxicity. -Mice with cisplatin induced nephrotoxicity. -Mice with unilateral ureteral occlusion and ischemia-reperfusion renal fibrosis -Rats with membranous nephropathy |
-Attenuated adenine induced chronic kidney disease, anti-inflammatory effect as well as reduction in oxidative stress -Did not ameliorate renal damage, however it reduced the accumulation of uremic toxins including p-cresyl sulfate -Failed to ameliorate the progressive loss of kidney function and there was no decreased interstitial area percentage, nor was there altered expression levels of genes related to fibrosis and inflammation -Improve kidney function by stimulating antioxidant, anti-inflammatory and anti-apoptotic signaling pathways -Attenuated the increase in blood pressure and ameliorated the associated hypertensive-induced renal injury, decreased epithelial-mesenchymal transition and oxidative stress and inhibited renal fibrosis -Reversed the biochemical and histopathological indices of possibly through its anti-inflammatory and antioxidant effects -Protected against cisplatin-induced acute kidney injury by activating adenosine monophosphate-activated protein kinase and autophagy in renal proximal tubular cells -Nephroprotective effect by Akt activation, reduced uptake of cisplatin in the kidneys. -Renal protection -Decreased proteinuria and improved the hyperplasia of glomerular mesangial cells and stroma, the thickening of basement membrane and spiky structure. |
13 37 38 39 40 41 42 43 44 45 |
| Empagliflozin | - Rats with 5/6 nephrectomy - Rats with 5/6 nephrectomy - Sprague Dawley rats with uni-nephrectomy and salt sensitive hypertension. -Spontaneously hypertensive rats -Rats with cyclosporine A induced nephropathy -Rats with angiotensin II induced hypertension -Rats with angiotensin II dependent kidney damage -Hypertensive and proteinuric renin-transgenic (mRen2)27 rats with additional administration of nitric oxide synthase inhibitor -Rats with renal ischemia-reperfusion injury - Rats with renal ischemia-reperfusion injury - C57/BL6 mice subjected to renal ischemia-reperfusion injury -Mouse model of Alport syndrome - Apo E−/− mice with vascular calcification and 5/6 nephrectomy - C57BL/6N mice with oxalate-related nephrocalcinosis -Fawn-hooded hypertensive rats -Uni-nephrectomized salt-loaded rats -Rats with Goldblatt hypertension |
- Reduced proteinuria, improved in creatinine clearance and renal interstitial fibrosis and glomerulosclerosis. - beneficial effect on kidney function and morphology due to an inhibition of CD206+CD68+ M2 macrophage polarization by targeting mammalian target of rapamycin (mTOR) and mitophagy pathways and attenuating inflammatory signals from CD8+ effector T cells. -Decreased blood pressure and ameliorated renal inflammation. -Beneficial renal protection by reducing renal lipid accumulation, inflammation and oxidative stress -Reduced blood pressure -Prevented the development of renal fibrosis by reducing inflammatory infiltrates -Reduced kidney damage by attenuating oxidative stress, proteinuria and glomerular filtration rate reduction associated with angiotensin II infusion -Reduced proteinuria and induced protection for renal vasculopathy, glomerulopathy, and tubular degeneration -Reduced renal tubular dilatation and necrosis -Attenuated renal injury with reduced oxidative stress, inflammation an apoptosis -Protected against renal injury, attenuated tubular damage, reduced inflammatory markers and inhibited apoptosis -Reduced podocyte lipotoxicity prevented renal lipid accumulation and improved renal function -Improved renal function -Did not affect chronic kidney disease progression in oxalate-related nephrocalcinosis -Did not provide renoprotection because it did not ameliorate proteinuria, elevated plasma urea and creatinine, oxidative stress or inflammation |
8 46 47 48 49 50 51 52 53 54 55 56 57 58 59 |
| Ipragliflozin | - C57BL/6JJcl mice with adenine induced chronic kidney disease -Mouse (FLS-ob/ob) model of non-alcoholic steatohepatitis - Dahl Salt sensitive rats |
- Renoprotective effect that was independent from plasma glucose levels and urinary glucose excretion - Improved the pathogenesis of chronic kidney disease by reducing ectopic lipid deposition in renal tubules, endoplasmic reticulum stress - Did not improve renal glomerulosclerosis or creatinine clearance |
60 61 62 |
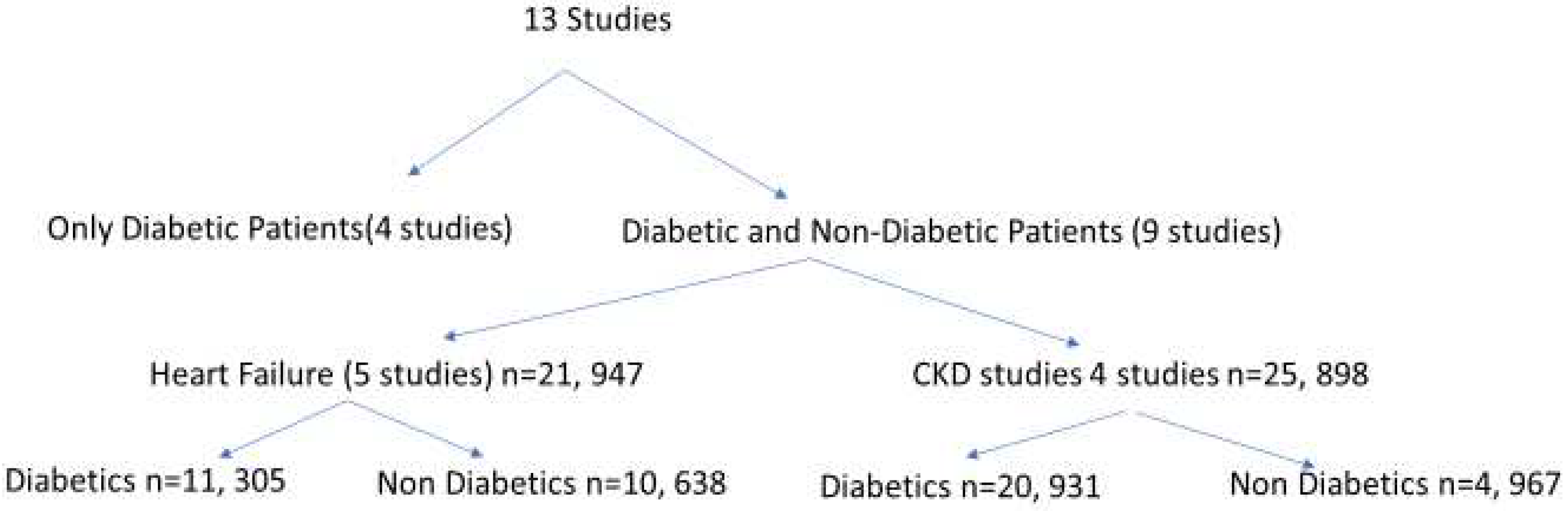
3. SGLT2 and Non-Diabetic Kidney Dysfunction-Clinical Trials (Table 3)
3.1. Effects of SGLT2i on CKD
3.2. Effects of SGLT2i on Glomerular Disorders
3.2.1. IgA Nephropathy (IgAN)
3.2.2. Focal Segmental Glomerulosclerosis (FSGS)
3.3. Effects of SGLT2i on Blood Pressure
3.4. Effects of SGLT2i on Nephrotic Proteinuria
4. Effects of SGLT2i on Survival
|
- Delay progression of CKD - Role in management of IgA Nephropathy - Potential role in the management of Focal segmental Glomerulosclerosis - Decrease Blood Pressure - Decrease Proteinuria - Improve renal-related survival |
5. SGLT2i Side Effects
6. Future Directives
7. Conclusions
References
- Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011). 2022 Apr;12(1):7-11. Epub 2022 Mar 18. PMID: 35529086; PMCID: PMC9073222. [CrossRef]
- https://usrds-adr.niddk.nih.gov/2022/end-stage-renal-disease/1-incidence-prevalence-patient-characteristics-and-treatment-modalities.Accessed 12/23/2023.
- Vallon V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu Rev Med. 2015;66:255-70. Epub 2014 Oct 17. PMID: 25341005. [CrossRef]
- Baker WL, Buckley LF, Kelly MS, Bucheit JD, Parod ED, Brown R, Carbone S, Abbate A, Dixon DL. Effects of Sodium-Glucose Cotransporter 2 Inhibitors on 24-Hour Ambulatory Blood Pressure: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2017 May 18;6(5):e005686. PMID: 28522675; PMCID: PMC5524106. [CrossRef]
- Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019 Jun 13;380(24):2295-2306. Epub 2019 Apr 14. PMID: 30990260. [CrossRef]
- Fırat SN, Kuşkonmaz ŞM, Çaydere M, Şeneş M, Hücümenoğlu S, Çulha C. Renoprotective effects of dapagliflozin in an iron overload non-diabetic rat model. Adv Med Sci. 2022 Sep;67(2):311-315. [CrossRef]
- Xuan MY, Piao SG, Ding J, Nan QY, Piao MH, Jiang YJ, Zheng HL, Jin JZ, Li C. Dapagliflozin alleviates renal fibrosis by inhibiting RIP1-RIP3-MLKL-mediated necroinflammation in unilateral ureteral obstruction. Front Pharmacol. 2022 Jan 7;12:798381. eCollection 2021. [CrossRef]
- Chen X, Delić D, Cao Y, Shen L, Shao Q, Zhang Z, Wu H, Hasan AA, Reichetzeder C, Gaballa MMS, Krämer BK, Klein T, Yin L, He B, Morgera S, Hocher B. Renoprotective effects of empagliflozin are linked to activation of the tubuloglomerular feedback mechanism and blunting of the complement system. Am J Physiol Cell Physiol. 2023 Apr 1;324(4):C951-C962. Epub 2023 Feb 13. [CrossRef]
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020 Oct 8;383(15):1436-1446. Epub 2020 Sep 24. PMID: 32970396. [CrossRef]
- The EMPA-KIDNEY Collaborative Group; Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, Emberson JR, Preiss D, Judge P, Mayne KJ, Ng SYA, Sammons E, Zhu D, Hill M, Stevens W, Wallendszus K, Brenner S, Cheung AK, Liu ZH, Li J, Hooi LS, Liu W, Kadowaki T, Nangaku M, Levin A, Cherney D, Maggioni AP, Pontremoli R, Deo R, Goto S, Rossello X, Tuttle KR, Steubl D, Petrini M, Massey D, Eilbracht J, Brueckmann M, Landray MJ, Baigent C, Haynes R. Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2023 Jan 12;388(2):117-127. Epub 2022 Nov 4. PMID: 36331190; PMCID: PMC7614055. [CrossRef]
- Miyata KN, Zhang SL, Chan JSD. The Rationale and Evidence for SGLT2 Inhibitors as a Treatment for Nondiabetic Glomerular Disease. Glomerular Dis. 2021 Mar 19;1(1):21-33. PMID: 36751486; PMCID: PMC9677741. [CrossRef]
- Lovshin JA, Gilbert RE (2015) Are SGLT2 inhibitors reasonable antihypertensive drugs and renoprotective. Curr Hypertens Rep 17(6):551. [CrossRef]
- Tang L, Wu Y, Tian M, Sjöström CD, Johansson U, Peng XR, Smith DM, Huang Y (2017) Dapagliflozin slows the progression of the renal and liver fibrosis associated with type 2 diabetes. Am J Physiol Endocrinol Metab 313:E563-E576.
- Ali BH, Al Salam S, Al Suleimani Y, Al Za'abi M, Abdelrahman AM, Ashique M, Manoj P, Adham SA, Hartmann C, Schupp N, Nemmar A. Effects of the SGLT-2 inhibitor canagliflozin on adenine-induced chronic kidney disease in rats. Cell Physiol Biochem. 2019;52(1):27-39. Epub 2019 Feb 18. [CrossRef]
- Rajasekeran, Harindraa,b; Cherney, David Z.a,b,*; Lovshin, Julie A.c,*. Do effects of sodium–glucose cotransporter-2 inhibitors in patients with diabetes give insight into potential use in non-diabetic kidney disease?. Current Opinion in Nephrology and Hypertension 26(5):p 358-367, September 2017. [CrossRef]
- Hawley SA, Ford RJ, Smith BK, Gowans GJ, Mancini SJ, Pitt RD, Day EA, Salt IP, Steinberg GR, Hardie DG. The Na+/Glucose Cotransporter Inhibitor Canagliflozin Activates AMPK by Inhibiting Mitochondrial Function and Increasing Cellular AMP Levels. Diabetes. 2016 Sep;65(9):2784-94. Epub 2016 Jul 5. PMID: 27381369; PMCID: PMC5689380. [CrossRef]
- Packer M. Interplay of adenosine monophosphate-activated protein kinase/sirtuin-1 activation and sodium influx inhibition mediates the renal benefits of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes: A novel conceptual framework. Diabetes Obes Metab. 2020 May;22(5):734-742. Epub 2020 Feb 20. PMID: 31916329. [CrossRef]
- van Bommel EJM, Muskiet MHA, van Baar MJB, Tonneijck L, Smits MM, Emanuel AL, Bozovic A, Danser AHJ, Geurts F, Hoorn EJ, Touw DJ, Larsen EL, Poulsen HE, Kramer MHH, Nieuwdorp M, Joles JA, van Raalte DH. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int. 2020 Jan;97(1):202-212. Epub 2019 Oct 10. Erratum in: Kidney Int. 2020 May;97(5):1061. PMID: 31791665. [CrossRef]
- Jongs N, Greene T, Chertow GM, McMurray JJV, Langkilde AM, Correa-Rotter R, Rossing P, Sjöström CD, Stefansson BV, Toto RD, Wheeler DC, Heerspink HJL; DAPA-CKD Trial Committees and Investigators. Effect of dapagliflozin on urinary albumin excretion in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021 Nov;9(11):755-766. Epub 2021 Oct 4. PMID: 34619106. [CrossRef]
- Fioretto P, Zambon A, Rossato M, Busetto L, Vettor R. SGLT2 Inhibitors and the Diabetic Kidney. Diabetes Care. 2016 Aug;39 Suppl 2:S165-71. PMID: 27440829. [CrossRef]
- Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013 Sep;15(9):853-62. Epub 2013 Jun 5. PMID: 23668478; PMCID: PMC3906841. [CrossRef]
- Storgaard H, Gluud LL, Bennett C, Grøndahl MF, Christensen MB, Knop FK, Vilsbøll T. Benefits and Harms of Sodium-Glucose Co-Transporter 2 Inhibitors in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. PLoS One. 2016 Nov 11;11(11):e0166125. PMID: 27835680; PMCID: PMC5106000. [CrossRef]
- Zhao Y, Xu L, Tian D, Xia P, Zheng H, Wang L, Chen L. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: A meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2018 Feb;20(2):458-462. Epub 2017 Sep 27. PMID: 28846182. [CrossRef]
- Yip ASY, Leong S, Teo YH, Teo YN, Syn NLX, See RM, Wee CF, Chong EY, Lee CH, Chan MY, Yeo TC, Wong RCC, Chai P, Sia CH. Effect of sodium-glucose cotransporter-2 (SGLT2) inhibitors on serum urate levels in patients with and without diabetes: a systematic review and meta-regression of 43 randomized controlled trials. Ther Adv Chronic Dis. 2022 Mar 23;13:20406223221083509 PMID: 35342538; PMCID: PMC8949773. [CrossRef]
- Rasaei N, Malekmakan L, Gholamabbas G, Mashayekh M, Hadianfard F, Torabi M. Sodium-Glucose Cotransporter-2 Inhibitors in Patients with Non-diabetic Chronic Kidney Disease: A Systematic Review. Iran J Kidney Dis. 2023 Jul;17(4):175-183. PMID: 37634243.
- Chang WT, Chang YK, Choi H, Jeong JY, Na KR, Lee KW, Lim BJ, Choi DE. Dapagliflozin, SGLT2 inhibitor, attenuates renal ischemia-reperfusion injury. PLoS One. 2016 Jul 8;11(7):e0158810. Erratum in: PLoS One. 2016;11(7):e0160478. PMID: 27391020; PMCID:PMC4938401. [CrossRef]
- Zeng J, Huang H, Zhang Y, Lv X, Cheng J, Zou SJ, Han Y, Wang S, Gong L, Peng Z. Dapagliflozin alleviates renal fibrosis in a mouse model of adenine-induced renal injury by inhibiting TGF-β1/MAPK mediated mitochondrial damage. Front Pharmacol. 2023 Mar 7;14:1095487. eCollection 2023. [CrossRef]
- Liu Y, Wang Y, Chen S, Bai L, Xie X, Zhang L, Wang X. Investigation into the effect and mechanism of dapagliflozin against renal interstitial fibrosis based on transcriptome and network pharmacology. Int Immunopharmacol. 2022 Nov;112:109195. Epub 2022 Sep 5. [CrossRef]
- Deger M, Kaya B, Akdogan N, Kaplan HM, Bagir E, Izol V, Aridogan IA. Protective effect of dapagliflozin against cyclosporine A-induced nephrotoxicity. Drug Chem Toxicol. 2022 Nov;45(6):2637-2643. Epub 2021 Sep 27. [CrossRef]
- Cassis P, Locatelli M, Cerullo D, Corna D, Buelli S, Zanchi C, Villa S, Morigi M, Remuzzi G, Benigni A, Zoja C. SGLT2 inhibitor dapagliflozin limits podocyte damage in proteinuric nondiabetic nephropathy. JCI Insight. 2018 Aug 9;3(15):e98720. eCollection 2018 Aug 9. [CrossRef]
- Urbanek K, Cappetta D, Bellocchio G, Coppola MA, Imbrici P, Telesca M, Donniacuo M, Riemma MA, Mele E, Cianflone E, Naviglio S, Conte E, Camerino GM, Mele M, Bucci M, Castaldo G, De Luca A, Rossi F, Berrino L, Liantonio A, De Angelis A. Dapagliflozin protects the kidney in a non-diabetic model of cardiorenal syndrome. harmacol Res. 2023 Feb;188:106659. Epub 2023 Jan 14. [CrossRef]
- Chang WT, Wu CC, Liao IC, Lin YW, Chen YC, Ho CH, Lee WC, Lin YC, Chen ZC, Shih JY, Wu NC, Kan WC. Dapagliflozin protects against doxorubicin-induced nephrotoxicity associated with nitric oxide pathway-A translational study. Free Radic Biol Med. 2023 Aug 5;208:103-111. [CrossRef]
- Wei J, Tan F, Long X, Fang Q, Wang Y, Wang J, He J, Yuan X, Du J. RNA-Seq transcriptome analysis of renal tissue from spontaneously hypertensive rats revealed renal protective effects of dapagliflozin, an inhibitor of sodium-glucose cotransporter 2. Eur J Pharm Sci. 2023 Oct 1;189:106531. Epub 2023 Jul 20. [CrossRef]
- Xuan MY, Piao SG, Ding J, Nan QY, Piao MH, Jiang YJ, Zheng HL, Jin JZ, Li C. Dapagliflozin alleviates renal fibrosis by inhibiting RIP1-RIP3-MLKL-mediated necroinflammation in unilateral ureteral obstruction. Front Pharmacol. 2022 Jan 7;12:798381. eCollection 2021. [CrossRef]
- Zhang Y, Thai K, Kepecs DM, Gilbert RE (2016) Sodium-glucose linked cotransporter-2 inhibition does not attenuate disease progression in the rat remnant kidney model of chronic kidney disease. PLoS ONE 11(1): e0144640. [CrossRef]
- Rajasekeran, H.; Reich, H.N.; Hladunewich, M.A.; Cattran, D.; Lovshin, J.A.; Lytvyn, Y.; Bjornstad, P.; Lai,V.; Tse, J.; Cham, L.; et al. Dapagliflozin in focal segmental glomerulosclerosis: A combined human-rodent pilot study. Am. J. Physiol. Renal Physiol. 2018, 314, F412–F422.
- Mishima E, Fukuda S, Kanemitsu Y, Saigusa D, Mukawa C, Asaji K, Matsumoto Y, Tsukamoto H, Tachikawa T, Tsukimi T, Fukuda NN, Ho HJ, Kikuchi K, Suzuki C, Nanto F, Suzuki T, Ito S, Soga T, Tomioka Y, Abe T. Canagliflozin reduces plasma uremic toxins and alters the intestinal microbiota composition in a chronic kidney disease mouse model. Am J Physiol Renal Physiol. 2018 Oct 1;315(4):F824-F833. Epub 2017 Nov 22. [CrossRef]
- Corremans R, Neven E, Maudsley S, Leysen H, De Broe ME, D'Haese PC, Vervaet BA, Verhulst A. Progression of established non-diabetic chronic kidney disease is halted by metformin treatment in rats. Kidney Int. 2022 May;101(5):929-944. Epub 2022 Mar 7. [CrossRef]
- Hasan R, Lasker S, Hasan A, Zerin F, Zamila M, Parvez F, Rahman MM, Khan F, Subhan N, Alam MA. Canagliflozin ameliorates renal oxidative stress and inflammation by stimulating AMPK-Akt-eNOS pathway in the isoprenaline-induced oxidative stress model. Sci Rep. 2020 Sep 4;10(1):14659. [CrossRef]
- Wang Z, Zhai J, Zhang T, He L, Ma S, Zuo Q, Zhang G, Wang Y, Guo Y. Canagliflozin ameliorates epithelial-mesenchymal transition in high-salt diet-induced hypertensive renal injury through restoration of sirtuin 3 expression and the reduction of oxidative stress. Biochem Biophys Res Commun. 2023 Apr 23;653:53-61. Epub 2023 Feb. [CrossRef]
- Abdelrahman AM, Al Suleimani Y, Shalaby A, Ashique M, Manoj P, Nemmar A, Ali BH. Effect of canagliflozin, a sodium glucose co-transporter 2 inhibitor, on cisplatin-induced nephrotoxicity in mice. Naunyn Schmiedebergs Arch Pharmacol. 2019 Jan;392(1):45-53. Epub 2018 Sep 11. [CrossRef]
- Park CH, Lee B, Han M, Rhee WJ, Kwak MS, Yoo TH, Shin JS. Canagliflozin protects against cisplatin-induced acute kidney injury by AMPK-mediated autophagy in renal proximal tubular cells. Cell Death Discov. 2022 Jan 10;8(1):12. [CrossRef]
- Song Z, Zhu J, Wei Q, Dong G, Dong Z. Canagliflozin reduces cisplatin uptake and activates Akt to protect against cisplatin-induced nephrotoxicity. Am J Physiol Renal Physiol. 2020 Apr 1;318(4):F1041-F1052. Epub 2020 Mar 9. [CrossRef]
- Yang Y, Li Q, Ling Y, Leng L, Ma Y, Xue L, Lu G, Ding Y, Li J, Tao S. m6A eraser FTO modulates autophagy by targeting SQSTM1/P62 in the prevention of canagliflozin against renal fibrosis. Front Immunol. 2023 Jan 4;13:1094556. eCollection 2022. [CrossRef]
- Lv X, Wang J, Zhang L, Shao X, Lin Y, Liu H, Ma G, Li J, Zhou S, Yu P. Canagliflozin reverses Th1/Th2 imbalance and promotes podocyte autophagy in rats with membranous nephropathy. Front Immunol. 2022 Dec 1;13:993869. eCollection 2022. [CrossRef]
- Lu YP, Wu HW, Zhu T, Li XT, Zuo J, Hasan AA, Reichetzeder C, Delic D, Yard B, Klein T, Krämer BK, Zhang ZY, Wang XH, Yin LH, Dai Y, Zheng ZH, Hocher B. Empagliflozin reduces kidney fibrosis and improves kidney function by alternative macrophage activation in rats with 5/6-nephrectomy. Biomed Pharmacother. 2022 Dec;156:113947. Epub 2022 Oct 31. [CrossRef]
- Kim S, Jo CH, Kim GH. Effects of empagliflozin on nondiabetic salt-sensitive hypertension in uninephrectomized rats. Hypertens Res. 2019 Dec;42(12):1905-1915. Epub 2019 Sep 19. [CrossRef]
- Malínská H, Hüttl M, Marková I, Miklánková D, Hojná S, Papoušek F, Šilhavý J, Mlejnek P, Zicha J, Hrdlička J, Pravenec M, Vaněčková I. Beneficial effects of empagliflozin are mediated by reduced renal inflammation and oxidative stress in spontaneously hypertensive rats expressing human C-reactive protein. Biomedicines. 2022 Aug 24;10(9):2066. [CrossRef]
- Castoldi G, Carletti R, Ippolito S, Colzani M, Barzaghi F, Stella A, Zerbini G, Perseghin G, Zatti G, di Gioia CRT. Sodium-glucose cotransporter 2 inhibition prevents renal fibrosis in cyclosporine nephropathy. Acta Diabetol. 2021 Aug;58(8):1059-1070. Epub 2021 Mar 24. [CrossRef]
- Castoldi G, Carletti R, Ippolito S, Colzani M, Barzaghi F, Stella A, Zerbini G, Perseghin G, di Gioia CRT. Renal anti-fibrotic effect of sodium glucose cotransporter 2 inhibition in angiotensin II-dependent hypertension. Am J Nephrol. 2020;51(2):119-129. Epub 2020 Jan 7. [CrossRef]
- Reyes-Pardo H, Bautista R, Vargas-Robles H, Rios A, Sánchez D, Escalante B. Role of sodium/glucose cotransporter inhibition on a rat model of angiotensin II-dependent kidney damage. BMC Nephrol. 2019 Aug 2;20(1):292. [CrossRef]
- Kolkhof P, Hartmann E, Freyberger A, Pavkovic M, Mathar I, Sandner P, Droebner K, Joseph A, Hüser J, Eitner F. Effects of finerenone combined with empagliflozin in a model of hypertension-induced end-organ damage. Am J Nephrol. 2021;52(8):642-652. Epub 2021 Jun 10. [CrossRef]
- Chu C, Delić D, Alber J, Feger M, Xiong Y, Luo T, Hasan AA, Zeng S, Gaballa MMS, Chen X, Yin L, Klein T, Elitok S, Krämer BK, Föller M, Hocher B. Head-to-head comparison of two SGLT-2 inhibitors on AKI outcomes in a rat ischemia-reperfusion model. Biomed Pharmacother. 2022 Sep;153:113357. Epub 2022 Jul 2. [CrossRef]
- Ala M, Khoshdel MRF, Dehpour AR. Empagliflozin enhances autophagy, mitochondrial biogenesis, and antioxidant defense and ameliorates renal ischemia/ reperfusion in nondiabetic rats. Oxid Med Cell Longev. 2022 Jan 28;2022:1197061. eCollection 2022. [CrossRef]
- Wang Q, Ju F, Li J, Liu T, Zuo Y, Abbott GW, Hu Z. Empagliflozin protects against renal ischemia/reperfusion injury in mice. Sci Rep. 2022 Nov 11;12(1):19323. [CrossRef]
- Ge M, Molina J, Kim JJ, Mallela SK, Ahmad A, Varona Santos J, Al-Ali H, Mitrofanova A, Sharma K, Fontanesi F, Merscher S, Fornoni A. Empagliflozin reduces podocyte lipotoxicity in experimental Alport syndrome. Elife. 2023 May 2;12:e83353. [CrossRef]
- Lu CW, Lee CJ, Hsieh YJ, Hsu BG. Empagliflozin attenuates vascular calcification in mice with chronic kidney disease by regulating the NFR2/HO-1 anti-inflammatory pathway through AMPK activation. Int J Mol Sci. 2023 Jun 12;24(12):10016. [CrossRef]
- Ma Q, Steiger S, Anders H-J (2017) Sodium glucose transporter-2 inhibition has norenoprotective effects on non-diabetic chronic kidney disease. Physiol Rep 5 (7), 2017, e13228. [CrossRef]
- Hojná S, Kotsaridou Z, Vaňourková Z, Rauchová H, Behuliak M, Kujal P, Kadlecová M, Zicha J, Vaněčková I. Empagliflozin Is Not Renoprotective in Non-Diabetic Rat Models of Chronic Kidney Disease. Biomedicines. 2022 Oct 7;10(10):2509. [CrossRef]
- Yamato M, Kato N, Kakino A, Yamada KI, Inoguchi T. Low dose of sodium-glucose transporter 2 inhibitor ipragliflozin attenuated renal dysfunction and interstitial fibrosis in adenine-induced chronic kidney disease in mice without diabetes. Metabol Open. 2020 Aug 8;7:100049. eCollection 2020 Sep. [CrossRef]
- Hosokawa K, Takata T, Sugihara T, Matono T, Koda M, Kanda T, Taniguchi S, Ida A, Mae Y, Yamamoto M, Iyama T, Fukuda S, Isomoto H. Ipragliflozin ameliorates endoplasmic reticulum stress and apoptosis through preventing ectopic lipid deposition in renal tubules. Int J Mol Sci. 2019 Dec 26;21(1):190. [CrossRef]
- Ito H, Okamoto R, Ali Y, Zhe Y, Katayama K, Ito M, Dohi K. Cardiorenal protective effects of sodium-glucose cotransporter 2 inhibition in combination with angiotensin II type 1 receptor blockade in salt-sensitive Dahl rats. J Hypertens. 2022 May 1;40(5):956-968 Epub 2022 Mar 11. [CrossRef]
- Nuffield Department of Population Health Renal Studies Group; SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists' Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022 Nov 19;400(10365):1788-1801. Epub 2022 Nov 6. PMID: 36351458; PMCID: PMC7613836. [CrossRef]
- Heerspink HJL, Cherney D, Postmus D, Stefánsson BV, Chertow GM, Dwyer JP, Greene T, Kosiborod M, Langkilde AM, McMurray JJV, Correa-Rotter R, Rossing P, Sjöström CD, Toto RD, Wheeler DC; DAPA-CKD Trial Committees and Investigators. A pre-specified analysis of the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial on the incidence of abrupt declines in kidney function. Kidney Int. 2022 Jan;101(1):174-184. Epub 2021 Sep 22. PMID: 34560136. [CrossRef]
- Petrie MC, Verma S, Docherty KF, Inzucchi SE, Anand I, Belohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett J, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Vinh PN, Schou M, Tereshchenko S, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Ponikowski P, Sabatine MS, Sjöstrand M, Solomon SD, Johanson P, Greasley PJ, Boulton D, Bengtsson O, Jhund PS, McMurray JJV. Effect of Dapagliflozin on Worsening Heart Failure and Cardiovascular Death in Patients With Heart Failure With and Without Diabetes. JAMA. 2020 Apr 14;323(14):1353-1368. Erratum in: JAMA. 2021 Apr 6;325(13):1335. PMID: 32219386; PMCID: PMC7157181. [CrossRef]
- Anker SD, Butler J, Filippatos G, Khan MS, Marx N, Lam CSP, Schnaidt S, Ofstad AP, Brueckmann M, Jamal W, Bocchi EA, Ponikowski P, Perrone SV, Januzzi JL, Verma S, Böhm M, Ferreira JP, Pocock SJ, Zannad F, Packer M. Effect of Empagliflozin on Cardiovascular and Renal Outcomes in Patients With Heart Failure by Baseline Diabetes Status: Results From the EMPEROR-Reduced Trial. Circulation. 2021 Jan 26;143(4):337-349. Epub 2020 Nov 11. PMID: 33175585; PMCID: PMC7834911. [CrossRef]
- Packer M, Zannad F, Butler J, Filippatos G, Ferreira JP, Pocock SJ, Brueckmann M, Zeller C, Hauske S, Anker 69-SD; EMPEROR-Preserved Trial Study Group. Influence of endpoint definitions on the effect of empagliflozin on major renal outcomes in the EMPEROR-Preserved trial. Eur J Heart Fail. 2021 Oct;23(10):1798-1799. Epub 2021 Aug 30. PMID: 34459076; PMCID: PMC9291539. [CrossRef]
- Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Desai AS, Jhund PS, Belohlavek J, Chiang CE, Borleffs CJW, Comin-Colet J, Dobreanu D, Drozdz J, Fang JC, Alcocer-Gamba MA, Al Habeeb W, Han Y, Cabrera Honorio JW, Janssens SP, Katova T, Kitakaze M, Merkely B, O'Meara E, Saraiva JFK, Tereshchenko SN, Thierer J, Vaduganathan M, Vardeny O, Verma S, Pham VN, Wilderäng U, Zaozerska N, Bachus E, Lindholm D, Petersson M, Langkilde AM; DELIVER Trial Committees and Investigators. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med. 2022 Sep 22;387(12):1089-1098. Epub 2022 Aug 27. PMID: 36027570. [CrossRef]
- Rasaei N, Malekmakan L, Gholamabbas G, Mashayekh M, Hadianfard F, Torabi M. Sodium-Glucose Cotransporter-2 Inhibitors in Patients with Non-diabetic Chronic Kidney Disease: A Systematic Review. Iran J Kidney Dis. 2023 Jul;17(4):175-183. PMID: 37634243.
- Cherney DZI, Dekkers CCJ, Barbour SJ, Cattran D, Abdul Gafor AH, Greasley PJ, et al. Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): a randomised, double-blind, cross-over trial. The lancet Diabetes & endocrinology. 2020;8(7):582-93.
- Wheeler DC, Toto RD, Stefánsson BV, Jongs N, Chertow GM, Greene T, et al. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 2021;100(1):215-24.
- van der van der Beek AB, Koomen JV, Dekkers CCJ, Barbour SJ, Boulton DW, Gansevoort RT, et al. Evaluation of the Pharmacokinetics and Exposure-Response Relationship of Dapagliflozin in Patients without Diabetes and with Chronic Kidney Disease. Clin Pharmacokinet. 202.
- Zannad F, Ferreira JP, Pocock SJ, Zeller C, Anker SD, Butler J, et al. Cardiac and Kidney Benefits of Empagliflozin in Heart Failure Across the Spectrum of Kidney Function Insights From EMPEROR-Reduced. Circulation. 2021;143(4):310-21.
- Vart P, Vaduganathan M, Jongs N, Remuzzi G, Wheeler DC, Hou FF, McCausland F, Chertow GM, Heerspink HJL. Estimated Lifetime Benefit of Combined RAAS and SGLT2 Inhibitor Therapy in Patients with Albuminuric CKD without Diabetes. Clin J Am Soc Nephrol. 2022 Dec;17(12):1754-1762. Epub 2022 Nov 22. PMID: 36414316; PMCID: PMC9718016. [CrossRef]
- Podestà MA, Sabiu G, Galassi A, Ciceri P, Cozzolino M. SGLT2 Inhibitors in Diabetic and Non-Diabetic Chronic Kidney Disease. Biomedicines. 2023 Jan 19;11(2):279. PMID: 36830815; PMCID: PMC9953060. [CrossRef]
- Dong Y, Shi S, Liu L, Zhou X, Lv J, Zhang H. Effect of SGLT2 inhibitors on the proteinuria reduction in patients with IgA nephropathy. Front Med (Lausanne). 2023 Sep 6;10:1242241. PMID: 37736600; PMCID: PMC10509766. [CrossRef]
- Wheeler DC, Jongs N, Stefansson BV, Chertow GM, Greene T, Hou FF, Langkilde AM, McMurray JJV, Rossing P, Nowicki M, Wittmann I, Correa-Rotter R, Sjöström CD, Toto RD, Heerspink HJL; DAPA-CKD Trial Committees and Investigators. Safety and efficacy of dapagliflozin in patients with focal segmental glomerulosclerosis: a prespecified analysis of the dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial. Nephrol Dial Transplant. 2022 Aug 22;37(9):1647-1656. PMID: 34850160; PMCID: PMC9395378. [CrossRef]
- Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation. 2016 Sep 6;134(10):752-72. Epub 2016 Jul 28. PMID: 27470878. [CrossRef]
- Mazidi M, Rezaie P, Gao HK, Kengne AP. Effect of sodium-glucose cotransport-2 inhibitors on blood pressure in people with type 2 diabetes mellitus: a systematic review and meta-analysis of 43 randomized control trials with 22,528 patients. J Am Heart Assoc. 2017; 6(6):e004007.
- Zanchi A, Pruijm M, Muller ME, Ghajarzadeh-Wurzner A, Maillard M, Dufour N, Bonny O, Wuerzner G, Burnier M. Twenty-Four Hour Blood Pressure Response to Empagliflozin and Its Determinants in Normotensive Non-diabetic Subjects. Front Cardiovasc Med. 2022 Mar 22;9:854230. PMID: 35391843; PMCID: PMC8981729. [CrossRef]
- Bays HE, Weinstein R, Law G, Canovatchel W. Canagliflozin: effects in overweight and obese subjects without diabetes mellitus. Obesity (Silver Spring). 2014 Apr;22(4):1042-9. Epub 2013 Dec 9. PMID: 24227660; PMCID: PMC4285787. [CrossRef]
- Díaz-Cruz C, González-Ortiz M, Rosales-Rivera LY, Patiño-Laguna AJ, Ramírez-Rodríguez ZG, Díaz-Cruz K, Martínez-Abundis E. Effects of dapagliflozin on blood pressure variability in patients with prediabetes and prehypertension without pharmacological treatment: a randomized trial. Blood Press Monit. 2020 Dec;25(6):346-350. PMID: 32815921. [CrossRef]
- Kalay Z, Sahin OE, Copur S, Danacı S, Ortiz A, Yau K, Cherney DZI, Kanbay M. SGLT-2 inhibitors in nephrotic-range proteinuria: emerging clinical evidence. Clin Kidney J. 2022 Aug 24;16(1):52-60. PMID: 36726436; PMCID: PMC9871839. [CrossRef]
- Boeckhaus J, Gross O. Sodium-Glucose Cotransporter-2 Inhibitors in Patients with Hereditary Podocytopathies, Alport Syndrome, and FSGS: A Case Series to Better Plan a Large-Scale Study. Cells. 2021 Jul 18;10(7):1815. PMID: 34359984; PMCID: PMC8303219. [CrossRef]
- Sjuls S, Jensen U, Littmann K, Bruchfeld A, Brinck J. Effective cholesterol lowering after myocardial infarction in patients with nephrotic syndrome may require a multi-pharmacological approach: a case report. Eur Heart J Case Rep. 2021 May 13;5(5):ytab151. PMID: 34124564; PMCID: PMC8189300. [CrossRef]
- A K Awad, M Tarek Hasan, M Shih, A N Attia, H Aboeldahab, M Bendary, A Bendary, Safety and efficacy of SGLT2 inhibitors in diabetic and non-diabetic heart failure patients, a meta-analysis of randomized controlled trials, European Heart Journal, Volume 43, Issue Supplement_2, October 2022, ehac544.941. [CrossRef]
- McEwan P, Boyce R, Sanchez JJG, Sjöström CD, Stefansson B, Nolan S, Correa-Rotter R, Rossing P, Chertow GM, McMurray JJV, Wheeler DC, Heerspink HJL. Extrapolated longer-term effects of the DAPA-CKD trial: a modelling analysis. Nephrol Dial Transplant. 2023 May 4;38(5):1260-1270. PMID: 36301617; PMCID: PMC10157747. [CrossRef]
- Halimi S, Verges B.: Adverse effects and safety of SGLT-2 inhibitors. Diabetes Metab 2014; 40: pp. S28-S34.
- Lega IC, Bronskill SE, Campitelli MA, Guan J, Stall NM, Lam K, McCarthy LM, Gruneir A, Rochon PA. Sodium glucose cotransporter 2 inhibitors and risk of genital mycotic and urinary tract infection: A population-based study of older women and men with diabetes. Diabetes Obes Metab. 2019 Nov;21(11):2394-2404. Epub 2019 Jul 21. PMID: 31264755. [CrossRef]
- Gupta R, Maitz T, Egeler D, Mehta A, Nyaeme M, Hajra A, Goel A, Sreenivasan J, Patel N, Aronow WS. SGLT2 inhibitors in hypertension: Role beyond diabetes and heart failure. Trends Cardiovasc Med. 2023 Nov;33(8):479-486. Epub 2022 May 18. PMID: 35597430. [CrossRef]
- Zhang Y, Jiang L, Wang J, Wang T, Chien C, Huang W, Fu X, Xiao Y, Fu Q, Wang S, Zhao J. Network meta-analysis on the effects of finerenone versus SGLT2 inhibitors and GLP-1 receptor agonists on cardiovascular and renal outcomes in patients with type 2 diabetes mellitus and chronic kidney disease. Cardiovasc Diabetol. 2022 Nov 5;21(1):232. PMID: 36335326; PMCID: PMC9637313. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





