1. Introduction
Carrot (D. carota var. sativa) is an apiaceous plant native to Afghanistan that is cultivated worldwide, including Europe, Asia, and North Africa, and has been cultivated in Korea since the 16th century (Kim et al., 2018). The main nutrients of carrots are vitamins A, C, and E, as well as carotenes such as α-carotene, β-carotene, and lutein, which are nutritionally important vegetables (Heinonen 1990). Carrots contain most of the vitamins and minerals needed by humans; therefore, eating carrots activates the body’s immune system, giving elasticity to the skin’s mucous membrane, maintaining the mucous membrane of the respiratory and digestive organs, and preventing the invasion of various bacteria (Park 2022).
Thermal processing of food is used to extend its shelf life and improve its quality. However, there are problems such as the destruction of nutrients and loss of bioactive substances during thermal processing. High-temperature and high-pressure (HTHP), a heat-treatment processing technique for food, differs from high-pressure processing, which uses hydrostatic pressure. Because food is processed through heat treatment, nutrient destruction occurs; however, various chemical changes occur during the heat treatment of fruits and vegetables, resulting in an increase in bioactive substances (Nicoil et al., 1999). High-temperature and high-pressure treatments improve the functional properties of fruits and vegetables such as ginseng (Yang et al, 2006), radish (Lee et al, 2009), garlic (Jo et al 2022), apples, melons, tomatoes, melons, and watermelons (Kim et al, 2008). However, there are no corresponding studies on carrots, one of the most consumed vegetables.
Therefore, this study aimed to evaluate the changes in the antioxidant and anti-inflammatory activities of carrots according to heating temperature and to evaluate the possibility of their development as a functional food material for the prevention and improvement of inflammatory diseases.
2. Materials and Methods
2.1. Materials
Carrots (Daucus carota L. var. sativa Dc.) was purchased from the Korean market in 2022. The carrot edible parts were ground with a mixer, 400 g each was placed in a 500 mL film bag, sealed, and steamed, and then stored at -20℃ to be used as an analysis sample.
2.2. High Temperature and Pressure Treatment
High temperature and pressure treatment device (Jasco, Seoul, Korea) is designed and manufactured to withstand pressure of 10 kg/cm2 or more. After being immersed in an internal container, a lid is sealed in an external container to which a certain amount of water is added, and then the external container is steamed according to a specified temperature and time to prevent carbonization by direct heat transfer (Hwang et al, 2011). The heating temperature was set to 110, 120, 130, 140, and 150℃, and heating time was set to 2 hours. Samples that was not heating was used as control, and all experiment was repeated three times. A high-temperature high-pressure treatment device (Jasco, Seoul, Korea) was designed and manufactured to withstand pressures of 10 kg/cm2 or higher. After immersion in an internal container, the lid was sealed in an external container to which a certain amount of water was added, and then the external container was steamed at a specified temperature and time to prevent carbonization by direct heat transfer (Hwang et al., 2011). The heating temperature was set to 110, 120, 130, 140, and 150℃, and heating time was set to 2 h. Unheated samples were used as controls, and all experiments were repeated three times.
2.3. Preparation of Steamed Carrot Extracts
Carrots heated to different temperatures were added to 70% ethanol 20 times (w/v) by weight and extracted twice for 1 h using an ultrasonic extraction device (frequency of 40 Hz, power of 300 W; SD-350H, Songdong, Seoul, Korea). After vacuum filtration, the sol-vent was completely removed at 40°C using a rotary vacuum concentrator (EYELA N-1000; Tokyo, Japan), and the sample was diluted to a certain concentration, freeze-dried, and used for the experiments. The extraction yield was calculated as a percentage.
2.4. Measurement of Total Polyphenol Content
The total polyphenol content of heated carrot extracts was measured using the method described by Blois et al. (1958). After diluting the sample, it was analyzed by fil-tration with a 0.45 µm syringe filter (Millipore, Billerica, MA, USA). Next, 2 mL of 2% Na2CO3 was added to 100 μL of the sample diluted at an appropriate ratio, the mixture was left at room temperature for 3 min, 100 μL of 50% Folin-Ciocalteu’s phenol reagent was added, and the reaction solution was incubated for 30 min. The absorbance was measured at 750 nm using a spectrophotometer (Epoch Microplate Spectrophotometer, BioTek Instruments, Winnski, VT, USA). Gallic acid (Sigma-Aldrich) was used as the standard, and the total polyphenol content was expressed as mg gallic acid per 1 mL of steamed carrot extract after the calibration curve was obtained.
2.5. Measurement of Total Flavonoid Content
The total flavonoid content of steamed carrot extracts was measured using the method described by Choi et al., (2003). After diluting the sample to an appropriate ratio, it was analyzed by filtration with a 0.45 µm syringe filter (Millipore, Billerica, MA, USA). Next, 1 mL of distilled water and 75 μL of 5% NaNO2 were added to 250 μL of the sample diluted at an appropriate ratio, and then 150 μL of 10% AlCl3·H2O was added 5 min later. After allowing the mixture to stand for 6 min, 500 μL of 1 M NaOH was added and left to stand for 11 min. The absorbance was measured at 510 nm using a spectrophotometer (Epoch Microplate Spectrophotometer, BioTek Instruments, Winooski, VT, USA). (+)-Catechin hydrate (Sigma-Aldrich Co.) was used as a standard, and the total flavonoid content was expressed as mg catechin per 1 mL of steamed carrot extract after a calibra-tion curve was obtained.
2.6. Measurement of ABTS and DPPH Radical Scavenging Activity
The DPPH radical scavenging activity of heated carrot extracts was measured by modifying the method described by Choi et al., (2006). DPPH radical scavenging activity was determined after dissolving 0.00788 g of 0.2 mM DPPH in 99.9% ethanol and adjust-ing the volume to 100 mL for 60 min. After adding 0.2 mL of the sample to 0.8 mL of the DPPH solution, the absorbance was measured at 520 nm using a spectrophotometer (Epoch microplate spectrophotometer, BioTek Instruments, Winooski, VT, USA). The ABTS radical scavenging activity of heated carrot extracts was measured by modifying the method described by Kim et al. (2007). One mL of 7.4 mM ABTS solution with 2.6 mM po-tassium persulfate was added to 20 µL of sample appropriately diluted and mixed. After 30 min in the dark, absorbance measured at 735 nm using a spectrophotometer (Epoch microplate spectrophotometer, BioTek Instruments, Winooski, VT, USA). L-ascorbic acid (Fluka, St. Louis, MO, USA) was used as the standard material for ABTS radical scaveng-ing activity analysis. The ABTS radical scavenging activity is expressed as the IC50 value. All samples were analyzed in triplicates.
2.7. Measurement of Reducing Power
The reducing power was measured as described by Jo et al. (2022), where 250 μL of 1% potassium ferricyanide [K3Fe(CN)6] and 250 μL of 0.2 M sodium phosphate buffer (pH 6.6) were mixed with 250 μL of extract and incubated at 50℃ for 20 min. Next, 1% trichloroa-cetic acid (CCl3COOH, w/v) was added, and the resulting solution was centrifuged at 1000 rpm for 10 min. Next, 500 μL of distilled water was mixed with 500 μL of the supernatant together with 100 μL of 0.1% ferric chloride (FeCl3·6H2O), and the absorbance of the solu-tion was determined at 700 nm.
2.8. Cell Culture and Cell Viability
The mouse macrophage cell line RAW 264.7, was purchased from the American Type Culture Collection (ATCC, Manassas, USA). For each cell line, 10% fetal bovine se-rum (FBS; Biowest, Kansas, USA) and 1% penicillin-streptomycin (GIBCO, Grand Island, NY, USA) were added to Dulbecco’s modified Eagle’s medium (DMEM; Biowest, Kansas, USA). Next, it was cultured in a 5% CO2, 37℃ incubator (Sanyo Electric, Osaka, Japan), and when the cell density increased, a subculture was performed by treatment with tryp-sin-EDTA for 5 min. The cytotoxicity of the heated carrot extract was measured using [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)] assay according to the method described by Ishiyama et al. (1996). After dispensing 100 μL of RAW 264.7 cells into 96-well plates at a density of 1×105 cells/well, they were incubated for 24 h in a 37℃, 5% CO2 incubator. The sample was diluted with DMEM media to a certain concen-tration (62.5, 125, 250, and 500 μg/mL), the medium was removed, and a sample diluted to a certain concentration was added to the medium, followed by incubation for 24 h. After completion of the culture, 20 μL of MTT reagent was added at 5 mg/mL per well, and the suspension was incubated for 4 h in a 37℃, 5% CO2 incubator. After removal of the me-dium containing the MTT reagent, 100 μL of DMSO was added at room temperature. Col-or was developed, and absorbance was measured at 540 nm using an enzyme-linked immunosorbent assay (ELISA) microplate reader (Shimadzu, UV-1650PC, Tokyo, Japan).
2.9. Nitric Oxide Determination
To confirm the NO production inhibitory activity and cytotoxicity, RAW 264.7 cells were seeded at 2 × 105 cells/mL in 24-well plates for 12 h, followed by treatment with heated carrot extract and lipopolysaccharide (LPS: Escherichia coli serotype 055:B5, Sigma- Aldrich, USA). The sample was then diluted with DMEM media to a certain concentration (62.5, 125, 250, 500 μg/mL). After 24 h, 50 μL of the supernatant from each well was added. NO production was measured using the Griess reagent system (Promega, Madison, WI, USA), according to the manufacturer’s instructions.
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
RAW 264.7 cells (2 × 105 cells/mL) were seeded in 24-well plates and incubated for 12 h at 37℃. Cells were treated with or without LPS (1 µg/mL) and the indicated concentra-tions of steamed carrot extracts (62.5, 125, 250, and 500 μg/mL) for 24 h. After 24 h of treatment, culture supernatants were collected. Tumor necrosis factor α (TNF-α), interleu-kin (IL)-1β, and interleukin (IL)-6 levels were measured using Quntikine Mouse ELISA kits (R&D systems, Minneapolis, MN, USA), according to the manufacturer’s instructions.
2.11. Statistical Analysis
All analyses were repeated three times and are expressed as mean ± SD. For statisti-cal analysis, the SPSS statistical program (Statistical Package for the Social Science, ver. 12.0, IBM SPSS Statistics, Chicago, IL, USA) was used to calculate the mean and standard deviation of each treatment group. After one-way ANOVA, Duncan’s multiple range test was used to evaluate significance.
3. Results and Discussion
3.1. Total Polyphenol and Flavonoid Content
The total polyphenol and flavonoid contents of heated carrots are listed in
Table 1. The total polyphenol content increased approximately six-fold with increasing heating temperature, from 69.50 mg/100 g in the control to 403.93 mg/100 g at 150°C. According to Kwon et al. (2006), in heated garlic, the total polyphenol content increased by approxi-mately seven times as the heating temperature increased, which is similar to a study that reported that the total polyphenol content increased as the heating temperature increased in various fruits and vegetables (Kim et al., 2008). The total polyphenol compound is more affected by the heating temperature than the heating time (Yu et al., 2019). This is because heat treatment converts the bound phenol component into a free form to facilitate dissolu-tion, or increase the content of phenolic compounds by decomposing the polymer phenol-ic compound into a low-molecular-weight phenolic compound (Koon et al., 2005). The to-tal flavonoid content increased with increasing heating temperature, which increased ap-proximately 13 times, from 4.00 mg/100 g in the control to 17.04 mg/100 g at 130°C and 53.14 mg/100 g at 150°C. This increase in total polyphenol content may have resulted from the destruction of the internal tissue of the heated carrot, and releasing bound phenolic compounds in their free form (Kang et al., 2013).
3.2. Antioxidant Activities
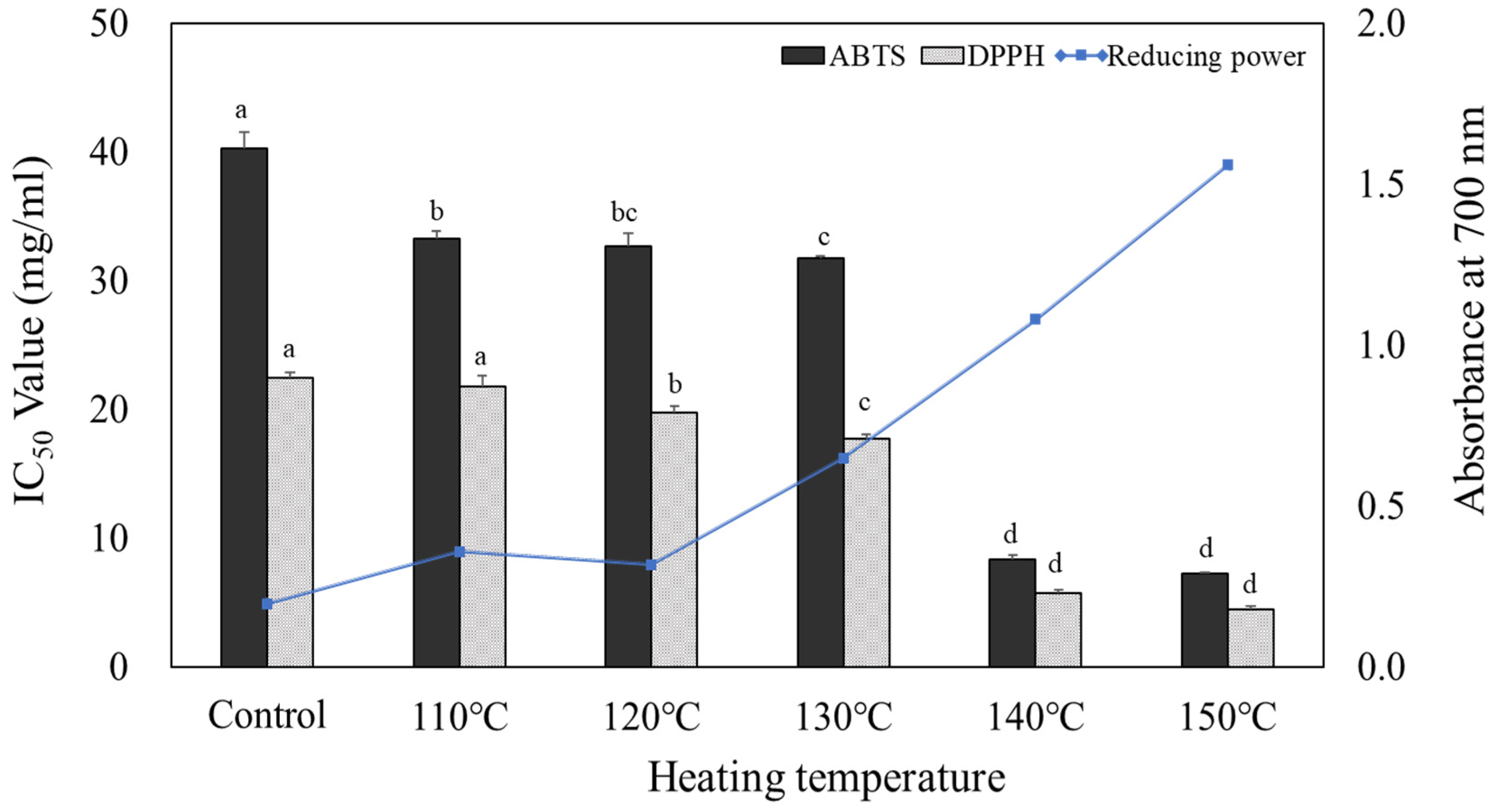
The antioxidant activities of heated carrot extracts are shown in
Figure 1. The IC50 values of the ABTS and DPPH radical scavenging abilities increased as the heating tem-perature increased. The ABTS radical scavenging ability of heated carrot extract ranged from 33.30 to 7.31 mg/mL depending on the temperature, and the lowest IC50 value was 7.31 mg/mL at 150°C. The DPPH radical scavenging ability also increased as the heating temperature increased. The IC50 value of the untreated control was 22.50 mg/mL, and it ranged from 21.87 to 4.51 mg/mL depending on heating temperature, and was the lowest at 150°C. When the antioxidant activity was high, the total polyphenol content was also high, which was considered to result from the increase in phenolic compounds exhibiting an antioxidant effect with the increasing heating temperature (Myeong et al., 2008). In ad-dition, the antioxidant effect increases as Maillard reaction products are formed, along with the antioxidant activity, during heat treatment (Manzocco et al., 2000). The antioxi-dant capacity of a compound is related to its reducing power and thus may be an im-portant indicator of potential antioxidant activity (Meir et al., 1995). The reducing power of heated carrot extract was 0.20 in the unheated control group at 5 mg/mL, and increased in the range of 0.36 to 1.56 as the heating temperature increased, showing the highest value at 150°C. These results were similar to those of the ABTS and DPPH radical scavenging assays.
Data are expressed as mean±SD (n=3). Different small letters(a~d) on the column indicate a significant difference among different heating temperature by Duncan’s range test (p<0.05). Treatment concentration of heated carrots extract for reducing power measurement was adjusted to 5 mg/mL.
3.3. Cytotoxicity
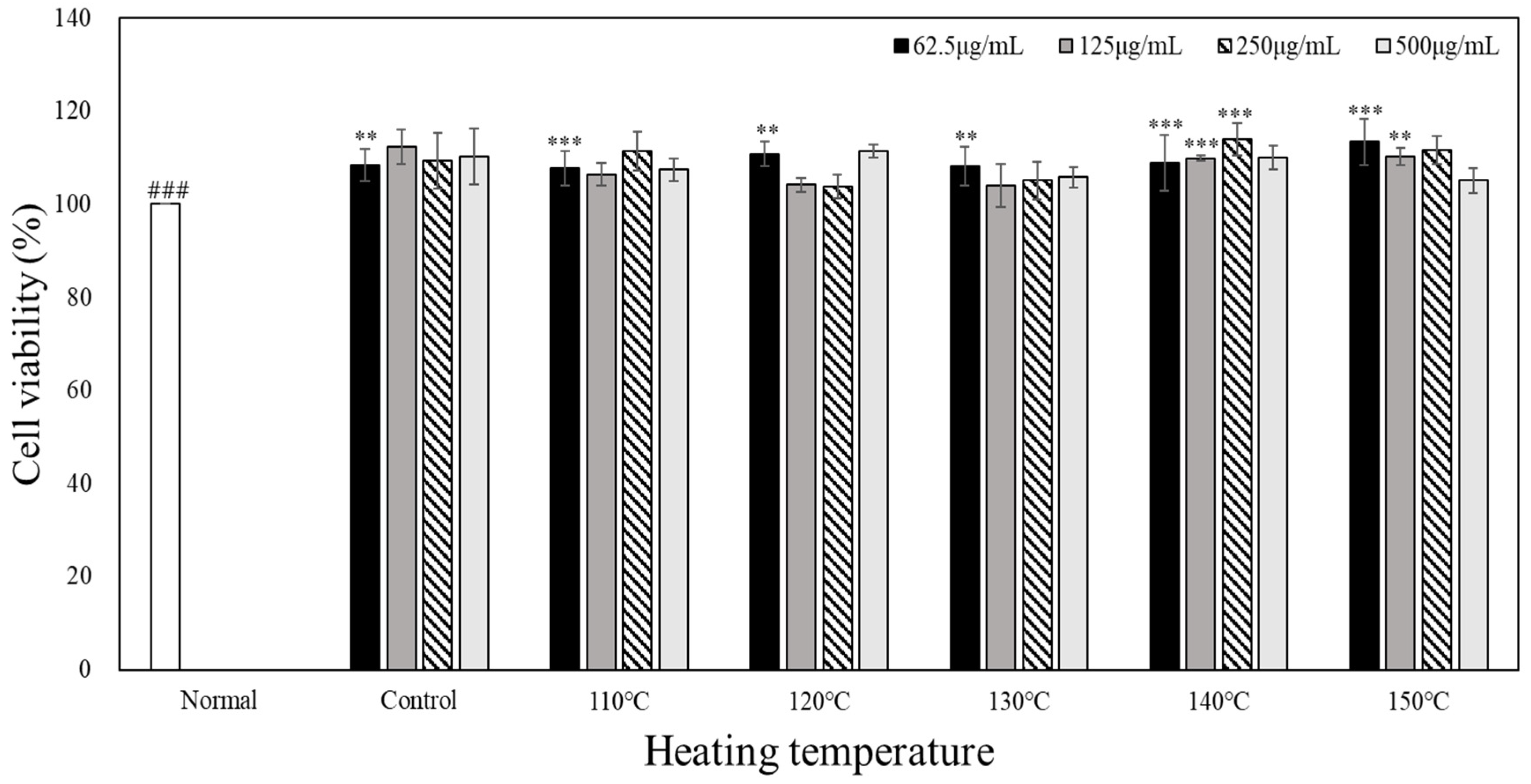
The results of the MTT assays with RAW 264.7 cells and heated carrot extract are shown in
Figure 2. To evaluate the cytotoxicity of heated carrot extract, RAW 264.7, cells were treated with different concentrations of extract (62.5, 125, 250, and 500 µg/mL). When the heated carrot extract was compared with 100% cell viability of the unheated control group, the survival rate did not change or slightly increased even at the low concentration of 62.5 µg/mL and the high concentration of 500 µg/mL according to heating temperature. The heated carrot extract did not show any significant differences with respect to the heating temperature or treatment concentration within the concentration range used in these experiments.
Heated carrots were treated at the concentration of 62.5, 125, 250, and 500 µg/mL and normal RAW264.7 used as a control. After 24 h, cell viability determined by MTT assay. Result is expressed as mean±D (n=3). Statistical analysis was performed using Tukey’s range test with a significant level of **p<0.01, ***p<0.001 compared to normal (###) group.
3.4. Nitric Oxide (NO) Inhibition Effects
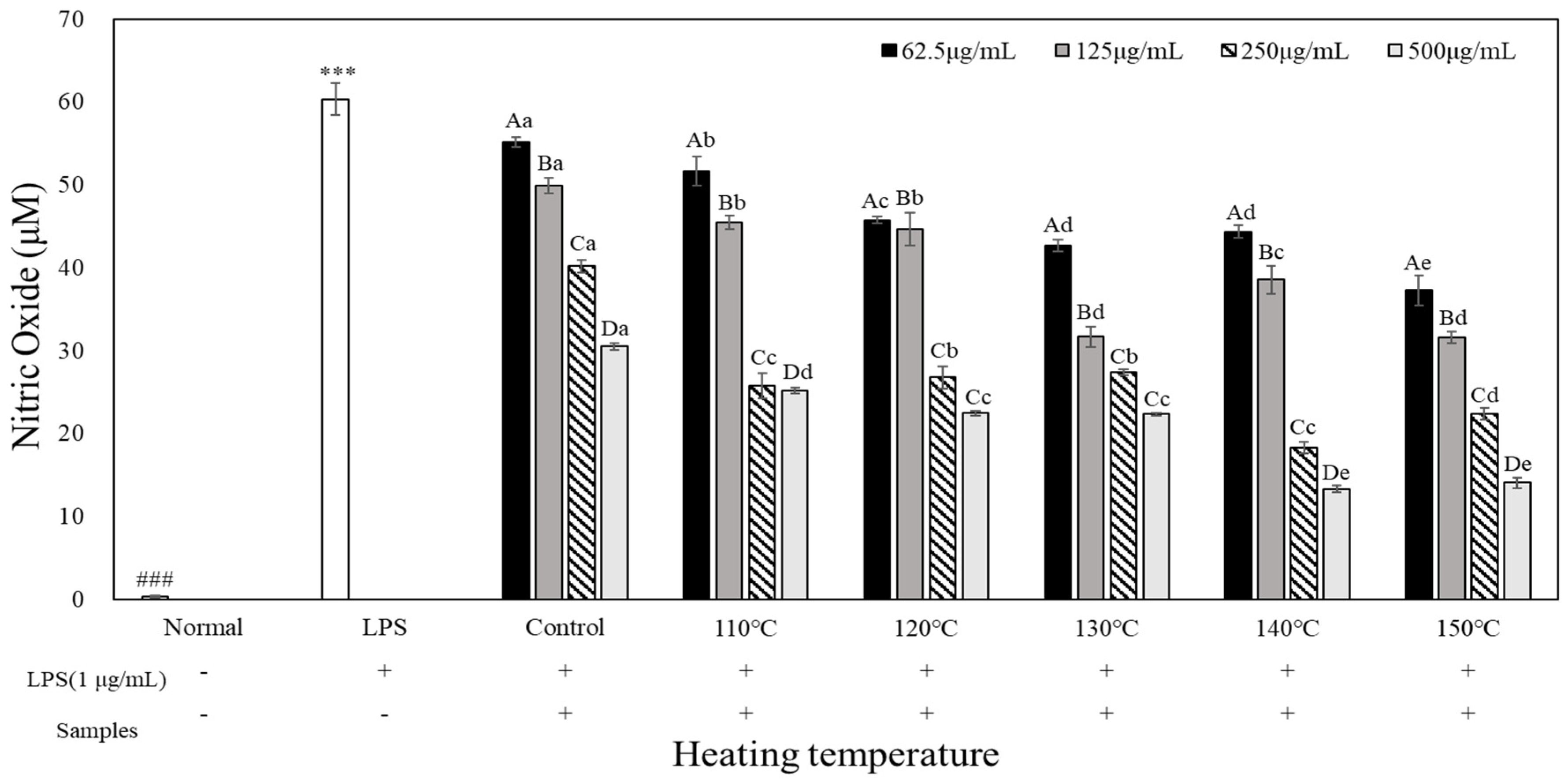
Figure 3 shows the effects of heated carrot extract on NO production in LPS-treated RAW264.7 cells. Control of NO in the inflammatory response is important, because exces-sive NO generation induces inflammatory factors and causes inflammatory disease (Ci-rino et al., 2006). When treated with LPS (1 µg/mL), NO production was significantly in-creased compared to that of the unheated control group, with 60.39 M. When heated car-rot extract was used after LPS treatment, NO production decreased as the heating temper-ature and treatment concentration increased. When treated with 500 µg/mL, NO produc-tion was low at all treatment temperatures, and NO production was 13.30 M and 14.11 M at 140°C and 150°C, respectively. Pan et al. (2023) and Metzger et al. (2008) confirmed that carrot and purple carrot extracts inhibit NO production in RAW264.7 cells. In the present study, we found that heated carrot extract was more effective in reducing NO production than unheated carrot extract.
Data are expressed as mean±SD (n=3). Different letters in the heat temperature indicate a significant difference among different concentrate (62.5, 125, 250, 500 µg/mL) by Duncan’s range test (p<0.05). Different small letters in the concentrate (62.5, 125, 250, 500 µg/mL) indicate a significant difference among different heat temperature by duncan’s range test(p<0.05). ***p<0.001, versus LPS alone compared to normal (###) group.
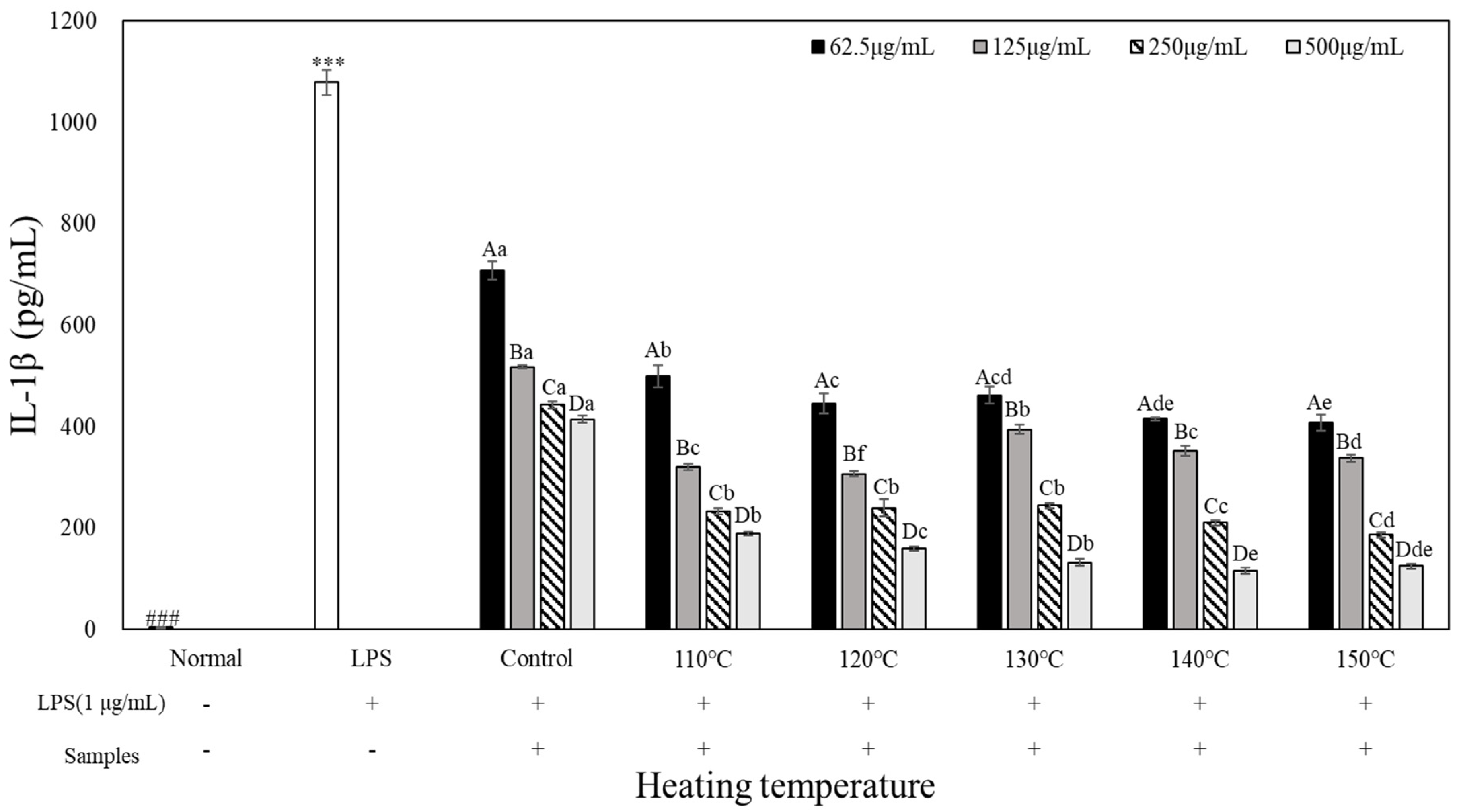
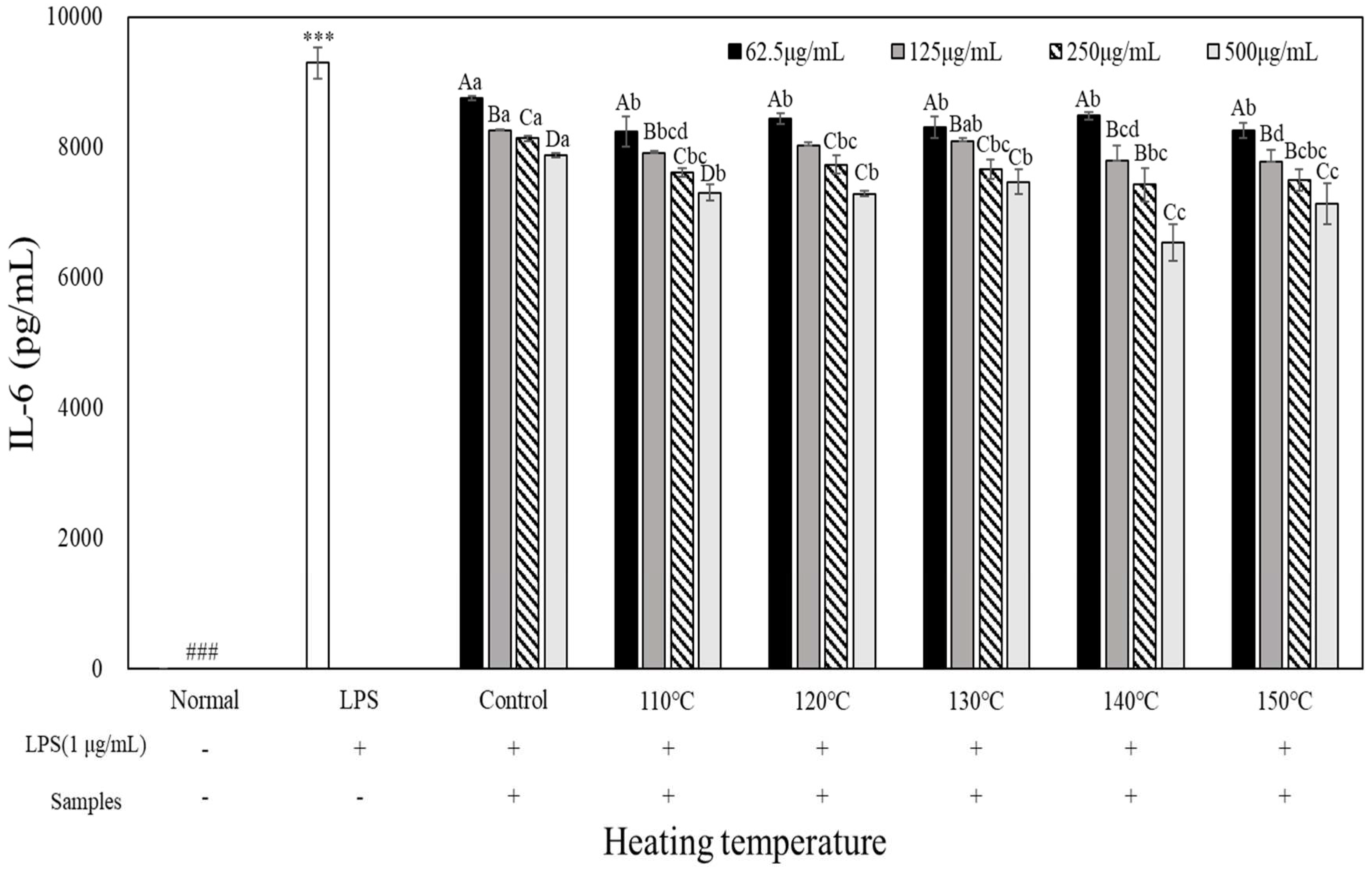
3.5. Pro-Inflammatory Cytokine Inhibition Effects
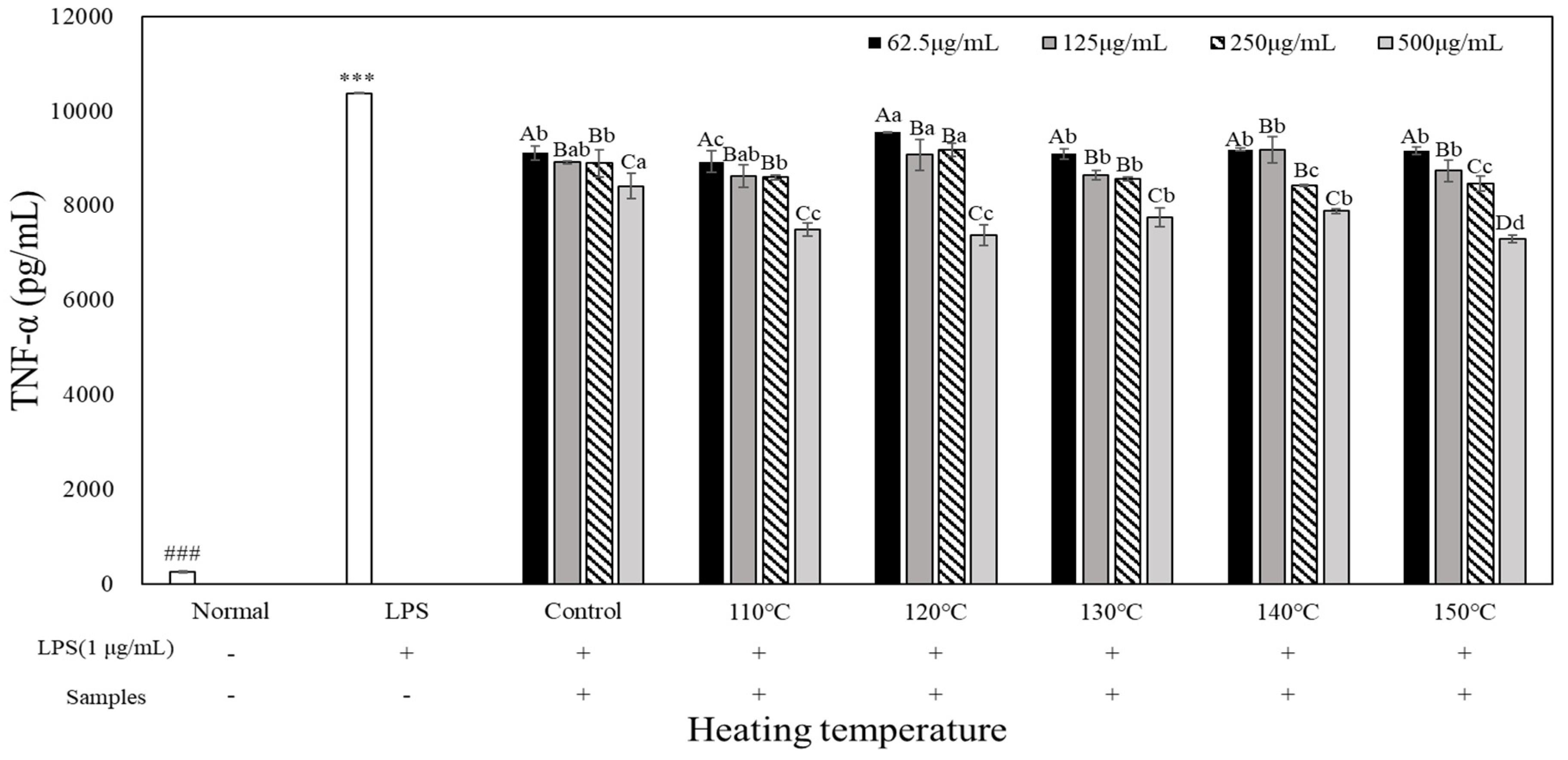
To evaluate the pro-inflammatory cytokine inhibitory activity of heated carrot extract, LPS-treated RAW 264.7 cells were treated with heated carrot extract for 24 h at each con-centration to measure its inhibitory activity on tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 production (
Figure 4,
Figure 5 and
Figure 6). When TNF-α, IL-1β, and IL-6 production of inflammatory cytokines were compared with those of the LPS-treated groups (10,385 pg/mL, 1,078.67 pg/mL, and 9,265.68 pg/mL, respectively), the concentration-dependent decrease was in the range of 9,192.29 to 7,302.28 pg/mL, 707.44 to 116.22 pg/mL, and 8,752.46 to 6,540.97 pg/mL, respectively. TNF-α, IL-1β, and IL-6 production in the heated carrot extract 500 µg/mL treatment group showed the greatest decrease, of 8,423.71 to 7,302.29 pg/mL, 4,14.56 to 116.22 pg/mL, and 7,878.81 to 6,540.97 pg/mL, respectively. It was most effective in inhibiting all inflammatory cytokines at 140 and 150°C. In particular, IL-1β showed the greatest decrease, with 415 to 116.22 pg/mL and 408.44 to 124.89 pg/mL, respectively, depending on the treatment concentration. However, in the case of TNF-α and IL-6, the decrease was not significant compared to IL-1β. LPS stimulates macrophages or monocytes to secrete inflammatory cytokines such as TNF-α, IL-1β, and IL-6 (Kim et al., 2009). The results of the present study confirmed that the heated carrot extract inhibit-ed the secretion of these inflammatory cytokines. In addition, the inhibitory effect on cyto-kine secretion increased with increasing heating temperature and treatment concentration.
RAW264.7 cells were treated with extracts temperature (Control, 110, 120, 130, 140 and 150℃) and LPS (1 µg/mL) for 24 h. The concentrations of TNF-α were measured by ELISA. The data represent the mean±SD (n=3). Different letters in the heat temperature indicate a significant difference among different concentrate (62.5, 125, 250, 500 µg/mL) by Duncan’s range test (p<0.05). Different small letters in the concentrate (62.5, 125, 250, 500 µg/mL) indicate a significant difference among different heat temperature by duncan’s range test(p<0.05). ***p<0.001, versus LPS alone compared to normal (###) group.
RAW264.7 cells were treated with extracts temperature (Control, 110, 120, 130, 140 and 150℃) and LPS (1 µg/mL) for 24 h. The concentrations of IL-1β is measured by ELISA. The data represent the Mean±SD (n=3). Different letters in the heat temperature indicate a significant difference among different concentrate (62.5, 125, 250, 500 µg/mL) by Duncan’s range test (p<0.05). Different small letters in the concentrate (62.5, 125, 250, 500 µg/mL) indicate a significant difference among different heat temperature by duncan’s range test(p<0.05). ***p<0.001, versus LPS alone compared to normal (###) group.
RAW264.7 cells were treated with extracts temperature (Control, 110, 120, 130, 140 and 150℃) and LPS (1 µg/mL) for 24 h. The concentrations of IL-6 were measured by ELISA. The data represent the mean±SD (n=3). Different letters in the heat temperature indicate a significant difference among different concentrate (62.5, 125, 250, 500 µg/mL) by Duncan’s range test (p<0.05). Different small letters in the concentrate (62.5, 125, 250, 500 µg/mL) indicate a significant difference among different heat temperature by duncan’s range test(p<0.05). ***p<0.001, versus LPS alone compared to normal (###) group.
4. Conclusions
In this study, the antioxidant and anti-inflammatory effects of carrot extracts were evaluated at different temperatures. The heat treatment temperatures were 110, 120, 130, 140, and 150℃ and were compared with the non-thermal treatment control group. The total polyphenol and flavonoid contents of carrot extract according to heating temperature ranged from 83.31 to 403.93 mg GAE/100 g and 11.09 to 53.14 mg CE/100 g, respectively, which were higher than those of the control group (69.50 mg GAE/100 g and 4.00 mg CE/100 g). The ABTS and DPPH radical scavenging activity (IC50 values) of heated carrot extract decreased from 33.30 to 7.31 mg/mL and 21.87 to 4.51 mg/mL, respectively, according to heating temperature, compared to 40.31 mg/mL and 22.50 mg/mL of the control group. To confirm the anti-inflammatory activity of heated carrot extract, the levels of NO and pro-inflammatory cytokine production in LPS-treated RAW264.7 macrophages were evaluated. NO production decreased in a concentration-dependent manner to 44.39-13.39 and 37.33-14.11 μM in the carrot extract treated at 140°C and 150°C, respectively. The levels of TNF-α, IL-1β, and IL-6, which are pro-inflammatory cytokines, decreased in a heating temperature- and treatment concentration-dependent manner, and showed high reduction rates at 500 μg/mL and 140°C and 150°C. From the above results, heat treatment increased the polyphenol and flavonoid contents of carrot, increasing antioxidant activity, and inhibiting the production of pro-inflammatory cytokines, thereby exhibiting an anti-inflammatory effect.