1. Introduction
Approximately 60,430 new diagnoses of pancreatic cancer (PC) were expected in the United States in 2021 [
1]. The incidence of PC is rising at a rate of 0.5–1.0% per year, and PC is projected to become the second leading cause of cancer-related deaths in the United States by 2030 [
1,
2]. Most of PC patients exhibit non-specific symptoms even at an advanced stage, which result in late diagnosis and not amenable to curative surgery [
1]. At the time of diagnosis, almost 50% of the patients have metastatic lesions, and 30% are diagnosed with locally advanced PC [
3]. Resectability, defined as the ability to completely remove cancer cells, is assessed to select a treatment strategy for localized PC [
4]. The American Joint Committee on Cancer (AJCC) classification of tumors, nodes, and metastases is used to assess prognosis [
5]. For the practical treatment planning, localized PCs are categorized as either resectable pancreatic cancer (R-PC), borderline resectable PC (BR-PC), or unresectable locally advanced pancreatic cancer (URLA-PC) based on the degree of tumor contact and invasion into major vessels such as superior mesenteric, hepatic artery, or celiac vasculature [
6].
There is growing interest in evaluating neoadjuvant systemic therapy for R-PC and BR-PC. Neoadjuvant or pre-operative therapy can eradicate occult metastatic disease and increase the number of eligible patients for surgery . Recent non-randomized prospective trials (Southwest Oncology Group S1505 trial) for R-PC demonstrated high completion rates of 84% in patients receiving total of 6 course neoadjuvant and adjuvant FOLFIRINOX,73% undergoing resection. Median overall survival (OS) was 23.2 months (95% CI, 17.6–45.9 months) and median disease-free survival after resection was 10.9 months [
7]. In a phase II trial, 34 of 43 (79%) patients with BR-PC received eight cycles preoperative FOLFIRINOX chemotherapy followed by chemoradiotherapy and the median progression-free survival (PFS) among all eligible patients was 14.7 months and the median OS was 37.7 months. R0 resection was achieved in 31 of the 48 eligible patients (65%; 95% CI, 49%-78%). Among patients who underwent resection, the median PFS was 48.6 months and the median OS was not reached [
8]. Another advantage is the potential downstaging before surgery, which facilitates margin-negative (R0) resection for BR-PC [
8,
9]. A multi-institutional trial demonstrated improved R0 resection rates in 58 patients with BR-PC receiving neoadjuvant therapy compared with initial surgery (82.4% vs. 33.3%; P=0.01) [
10].
However, URLA-PC is unable to remove tumor at the time of diagnosis owing to severe vessel invasion, thus, systemic treatments are mainly focused on survival benefits rather than conversion surgery. Surgical resection is subjected to in a small fraction of patients with a favorable response to chemotherapy or chemoradiotherapy after a long duration of the systematic treatment. Planning R0 surgical resection in patients with URLA-PC is challenging. For conversion surgery, the initial treatment typically consists of chemotherapy regimens, such as FOLFIRINOX or albumin-bound paclitaxel and gemcitabine [
11]. A systematic review of 11 studies and 315 patients with URLA-PC reported a pooled median OS of 24.2 months [
12]. An observational study including 101 patients with URLA-PC who were treated with FOLFIRINOX as induction therapy showed that 29% of the patients had a >30% reduction in tumor size, and half of the patients who experienced a reduction in tumor size underwent resection [
13]. Among patients who underwent resection, 55% achieved R0 resection.
The Clinical Practice Guidelines for Pancreatic Cancer, version 2 (2021), suggest that induction chemotherapy should initially be recommended for URLA-PC followed by radiotherapy or chemoradiotherapy. Conversion surgery is recommended for patients with a good performance status after treatment because favorable OS and/or relapse-free survival can be expected [
14].
However, there is no prospective study investigating surgical conversion rate after FOLFIRINOX chemotherapy in URLA-PC patients. Therefore, we conducted a prospective, single-arm, phase II trial of URLA-PC with FOLFIRINOX chemotherapy followed by surgical resection.
2. Materials and Methods
2.1. Study Design
The
Conversion therapy of
FOLFIRINOX in patients with unresectable
locally
advanced
pancreatic cancer (C-FLAP) study was an open-label, single-arm, phase II trial of the Yokohama Clinical Oncology Group (YCOG1403). The Institutional Review Board of Yokohama City University approved the study protocol (B140508029). This study was registered in the Japanese Clinical Trials Registry (UMIN-CTR) as UMIN000014039. (
http://www.umin.ac.jp/ctr/index.htm). Patients provided written informed consent before enrollment in the study. The primary endpoint was R0 resection rate, and the possibility of transfer to resection due to the therapeutic efficacy of FOLFIRINOX therapy was examined.
2.2. Inclusion Criteria
Inclusion criteria were as follows. Patients with histologically or cytologically confirmed pancreatic ductal adenocarcinoma from 2014 to 2022 at Yokohama City University Hospital. The absence of distant metastases confirmed by whole-body enhanced computed tomography, fluorodeoxyglucose-positron emission tomography, and laparoscopic examination. No previous treatment for PC. Aged ≥20 years and ≤75 years. Patients were expected to survive for at least eight weeks from the date of enrollment. Patients with measurable lesions (RECIST v1.1). Patients with at least one of the following URLA-PC criteria for primary lesions (NCCN Guidelines v1.2013): cancerous stenosis of more than half the circumference of the superior mesenteric artery or celiac artery, unreconstructable superior mesenteric vein occlusion, portal vein (PV) occlusion, aortic invasion, or cancerous stenosis of the aorta.
Included patient’s laboratory data showed adequate hematologic, liver, and renal functions (hemoglobin, >9.0 g/dL; neurocyte cell count, 1,500/mm3; platelet count, >100,000/mm3; aspartate aminotransferase and alanine transaminase, <2.5 times of the upper limit of each institutional data; total bilirubin, <2.5 times of the upper limit of institutional data; serum creatinine, <1.2 mg/dL; and C-reactive protein, <2.0 mg/dL). All laboratory tests were assessed within seven days prior to the start of treatment.
2.3. Exclusion Criteria
Exclusion criteria were as follows. Patients with history of severe drug hypersensitivity or serious drug allergies. Patients expected to have severe toxicity from irinotecan with two UGT1A1 genetic polymorphisms (UGT1A1*6 and UGT1A1*28), such as homozygotes (UGT1A1*6/*6 and UGT1A1*28/*28), or both heterozygotes (UGT1A1*6/* 28). Patients with active cancer which was either concurrent and metachronous cancer with a disease-free period of ≤ 5 years other than PC, however, carcinoma in situ and lesions equivalent to carcinoma in mucosal that were considered to be curable by local treatment was exception. Patients with serious complications that were difficult to control (heart disease, lung disease, severe diabetes, severe infection, intestinal paralysis, and intestinal obstruction). Pregnant, potentially pregnant, or breastfeeding women. Patients with psychosis or psychiatric symptoms who were considered to have difficulty participating in the study. Patients who received blood transfusions, blood products, or hematopoietic factor preparations, such as granulocyte colony-stimulating factor, within 7 days before enrollment of the study. Patients with large amounts of body cavity fluid (pleural fluid, ascites, and pericardial effusion). Patients with jaundice, experiencing diarrhea (watery stools) (however, in the case of colostomy, when diarrhea interfered with daily life). We also excluded vulnerable patients with poor general condition. Because the original regimen of FOLFIRINOX had relatively high toxicity, we had to exclude the patients who were predicted to have prolonged decreased oral intake and bone marrow function, making it difficult to continue treatment and causing severe adverse events. We also excluded patients were administered atazanavir sulfate.
2.4. Treatment Protocol
The FOLFIRINOX regimen used in our study comprised 85 mg/m2 oxaliplatin (L-OHP), 200 mg/m2 l-LV, 180 mg/m2 irinotecan (CPT-11), and 400 mg/m2 5-fluorouracil (FU) (bolus), followed by a continuous intravenous infusion of 2400 mg/m2 5-FU for 46 h. Treatment was administrated every 2 weeks. Bolus infusion of 5-FU was omitted, and the initial dose of CPT-11 was reduced to 150 mg/m2 after a protocol change in 2021.
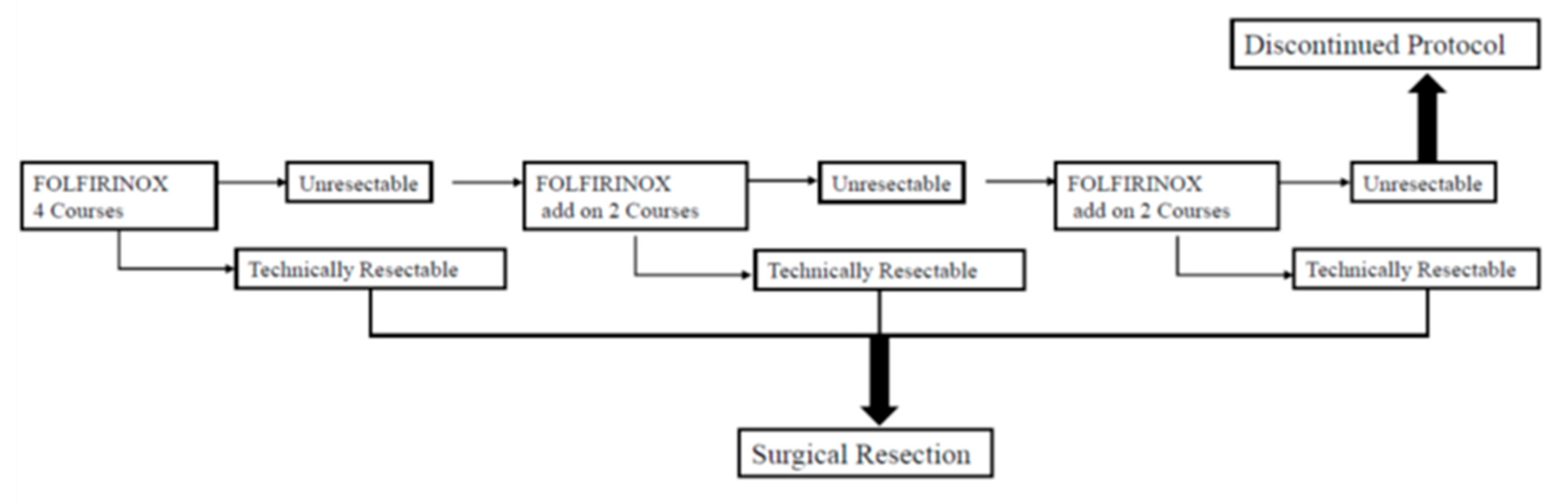
The clinical trial design is shown in
Figure 1. The decision for conversion to surgery was made at every two courses completion after four courses up to eight courses. If the tumor was determined to be unresectable after completion of the eight courses, the treatment protocol was discontinued. Patients judged to be technically resectable underwent surgery within four weeks, starting from the end date of FOLFIRINOX therapy. Radical resection and continued FOLFIRINOX therapy or discontinuation of the treatment protocol was performed. Although not prescribed in a treatment protocol, adjuvant chemotherapy with S-1 alone was recommended for six months after surgery. Treatment after discontinuation of FOLFIRINOX was not prescribed. However, continuation of FOLFIRINOX was recommended in cases where the tumor was judged to be unresectable but the efficacy was stable disease (SD) or higher persists after eight courses of FOLFIRINOX.
Depending on the severity of adverse events that occurred during the previous course, the dosages of L-OHP, CPT-11, and 5-FU were reduced. Dose reduction was required for one or more of the following events: (1) grade 4 neutropenia, (2) febrile neutropenia, (3) grade 4 thrombocytopenia, (4) any other grade 3 or 4 toxicity, or (5) delayed recovery from treatment-related toxicity for >2 weeks. Chemotherapy was discontinued in case of intestinal pneumonitis of any grade or grade 3 peripheral sensory neuropathy.
Dose reduction was performed simultaneously, according to the dosage variation standards for L-OHP, CPT-11, and 5-FU. The l-LV was not reduced. The initial dose of L-OHP was 85 mg/m2, -1 level was 65 mg/m2, and -2 levels was 50 mg/m2. The initial dose of CPT-11 was 180 mg/m2, -1 was 150 mg/m2, and -2 was 120 mg/m2. The initial 5-FU dose was 400 mg/m2 as a bolus injection and 2400 mg/m2 as a continuous injection. The -1 level was omitted as a bolus injection, and 2000 mg/m2 as a continuous injection. The -2 level was omitted as a bolus injection, and 1600 mg/m2 as a continuous injection. In patients who had been reduced to the -1 level according to the above criteria, if further toxicity was observed, L-OHP, CPT-11, and 5-FU may be further reduced to -2 levels. If further toxicity was observed in cases where 5-FU is reduced to -2 level, no further dose reduction was performed, and the treatment protocol was discontinued. In case where further toxicity was observed in patients whose L-OHP was reduced to -2 levels, no further reduction was performed, L-OHP was discontinued, and FOLFIRI treatment was continued. Similarly, in case where CPT-11 was reduced to -2 level and further toxicity was observed, FOLFOX therapy was continued. The treatment was discontinued in case both L-OHP and CPT-11 were discontinued. On the other hand, in case the adverse event resolved after a single-dose reduction, the dose was increased again. Dose reductions were not double counted for multiple reasons. For example, even if two toxicities, grade 3 diarrhea and grade 3 mucositis, were observed in the previous course, the reduction in the next course was at one level. All patients routinely received palonosetron, aprepitant, and dexamethasone as prophylaxis during each cycle.
2.5. Decision for conversion surgery
The feasibility of resection was judged for the patients whose FOLFIRINOX efficacy was SD or higher. It was decided based on anatomical standpoint in addition to the tumor shrinkage, i.e. the degree of the involvement of the tumor with critical adjacent vessels, such as celiac artery, superior mesenteric artery, common hepatic artery, superior mesenteric vein and portal vein. Thus, the decision to the surgery was made whether the finding of aforementioned criteria of URLA-PC was improved or not based on imaging study by the Diagnostic Imaging Review Committee, which consists of three surgical experts. Each committee member made an independent decision, and in case two or more judged the tumor to be resectable, the clinician proceeded with resection, in other cases, the tumor was considered as unresectable.
2.6. Statistical Analysis
We have set the target number of patients as follows. Prospective reports investigating resection rates after chemoradiotherapy or chemotherapy reported an R0 resection rate of 7–29% [
15,
16,
17,
18,
19], and the trial threshold R0 resection rate was 10%. In addition, reports on resection feasibility after FOLFIRINOX therapy have reported R0 resection rate of 8–36% [
20,
21,
22,
23]. This was a prospective study with the R0 resection rate as the primary endpoint. Therefore, the expected R0 resection rate in the present study was 25%. Using SWOG Statistical Tools: One Arm Binominal [
24] with a threshold R0 resection rate of 10%, an expected R0 resection rate of 25%, and a bilateral α = 0.05 and β = 0.2, the required number of cases was 49. Considering the small number of dropouts, the target number of patients included in this study was 50.
Survival analyses were performed by Kaplan-Meier curve with log-rank test. Waterfall plot was created based on the tumor size at the best response was observed. In case of the patient underwent surgery, the time of evaluation was right before the surgical resection, in other cases, it was at the end of either 4th, 6th or 8th course of the treatment where the best response was obtained. All statistical analyses were performed using SPSS version 28 software (IBM, Armonk, NY, USA).
3. Results
3.1. Patients
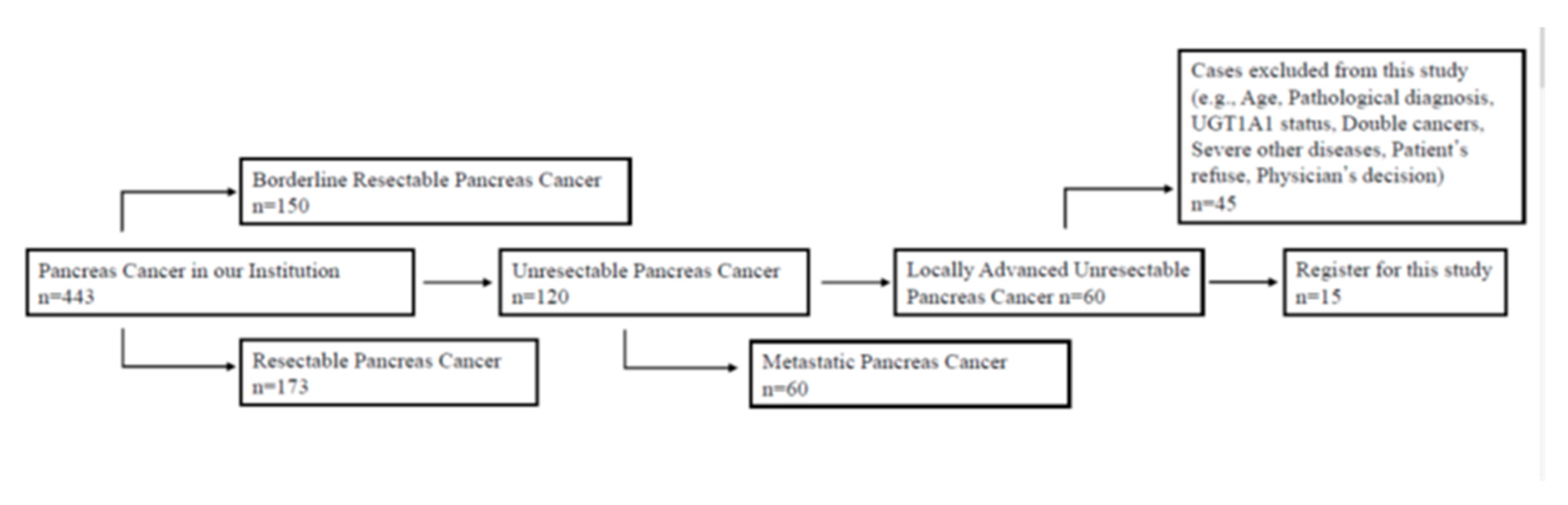
Among 443 PC patients from 2014 to 2022 at Yokohama City University hospital, 120 were diagnosed as unresectable PC (
Figure 2). Among them, 60 were diagnosed as URLA- CA. Because of aforementioned exclusion criteria, 45 were excluded, and fifteen patients were enrolled (
Figure 2). This enrollment did not meet the initial number of cases calculated in the power analysis for statistical significance.
The patient demgraphics at the enrollment were summarized in
Table 1. There are 12 male and three female patients and the median age was 63 years (range, 44–73 years). The ECOG Performance Status of all patients was 0. Five patients (33.3%) had diabetes mellitus. The median CA19-9 conentration was 44 mL/IU (range, 1.2–13549 mL/IU). The tumor location in the pancreatic head and body/tail was 9 and 6, respectively. Four patients (26.7%) underwent biliary stent placement. The median tumor diameter was 35 mm (range, 24–72 mm), and all tumors were defined as URLA-PCs based on arterial invasion. Twelve (80 %) tumors invaded the PV.
3.2. Efficacy of Chemotherapy
The efficacy of the chemotherapy was summarized in
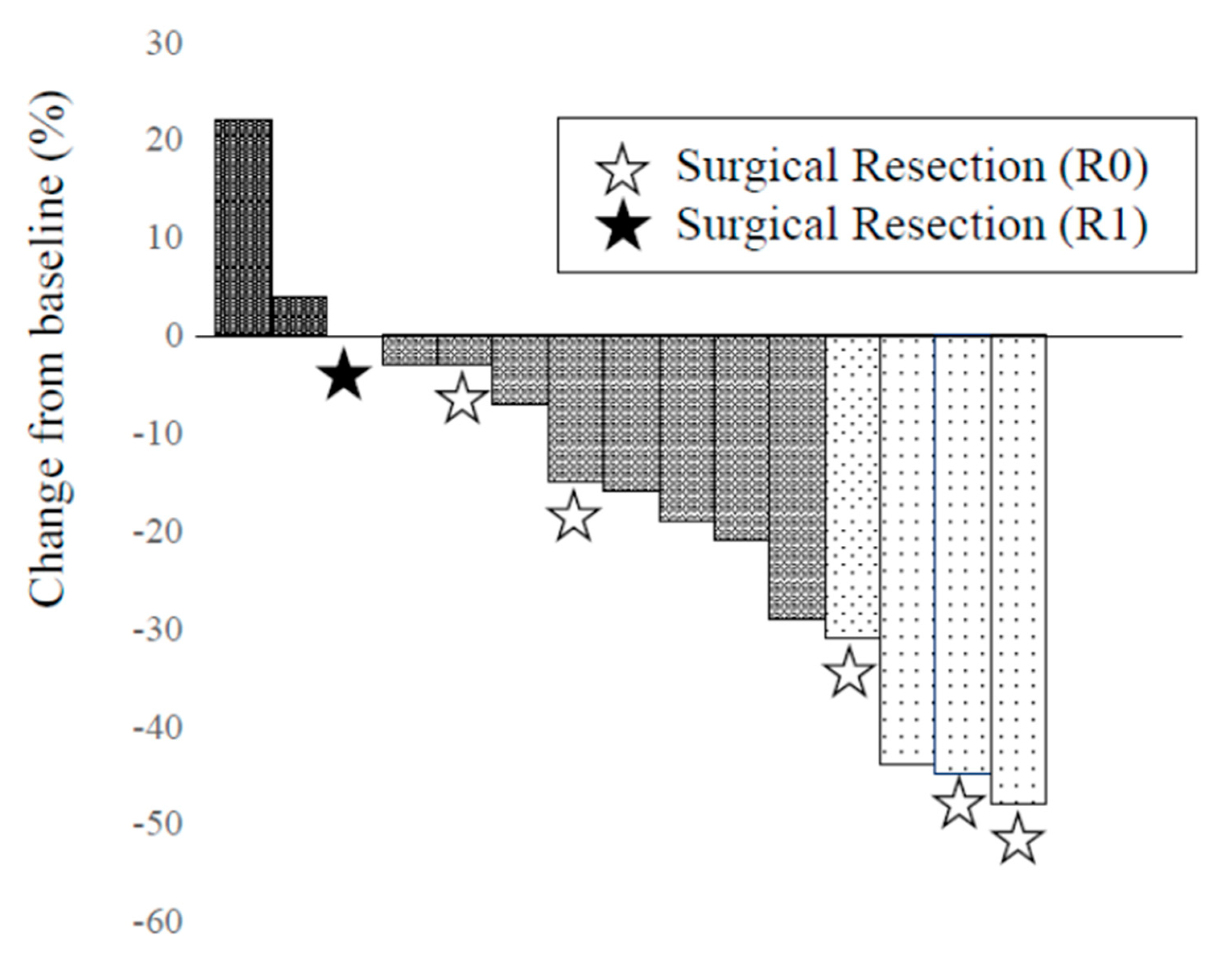
Table 2. FOLFIRINOX chemotherapy was administered for a median of six cycles (range, 3–8 cycles) in the designed protocol . Partial response (PR) was achieved in 26.7% (4/15) of the patients, SD in 66.7% (10/15), and progressive disease (PD) in 6.7% (1/15). The disease control rate was 93.3% (14/15). A waterfall plot was drawn based on the tumor size at the best response was observed in
Figure 3. The median tumor shrinkage ratio was 16% (range, -22–48%). The median CEA ratio decreased by -41.7% (range, -304–52.1%). The median decrease in the CA19-9 ratio was 38.6% (range, -425–92.3%). Dose intensity was 63.75 (range, 43–80) mg/m
2 (75%) for L-OHP, 129.47 (range, 84.7–158.8) mg/m
2 (71.9%) for CPT-11, 53.3 (range, 0–235.3) mg/m
2 (13.3%) for bolus 5-FU, and 1846.2 (range, 1411.8–2258.9) mg/m
2 (76.9%) for continuous 5-FU.
3.3. Toxicity of Chemotherapy
The toxicity of chemotherapy was summarized in
Table 3. Severe hematologic adverse events due to chemotherapy were observed in 66.7% of the patients (10/15; leukopenia, n = 3; neutropenia, n = 10; febrile neutropenia, n = 1). Severe non-hematologic adverse events due to chemotherapy were observed in 40.0% of the patients (6/15; liver dysfunction, n = 1; hyponatremia, n = 1; nausea, n = 2; diarrhea, n = 2; appetite loss, n = 2; sensory neuropathy, n = 1; acute cholangitis, n = 1; acute pancreatitis, n = 1; and pneumonitis, n = 1).
3.4. Surgery
Surgical procedure and outcomes was summarized in
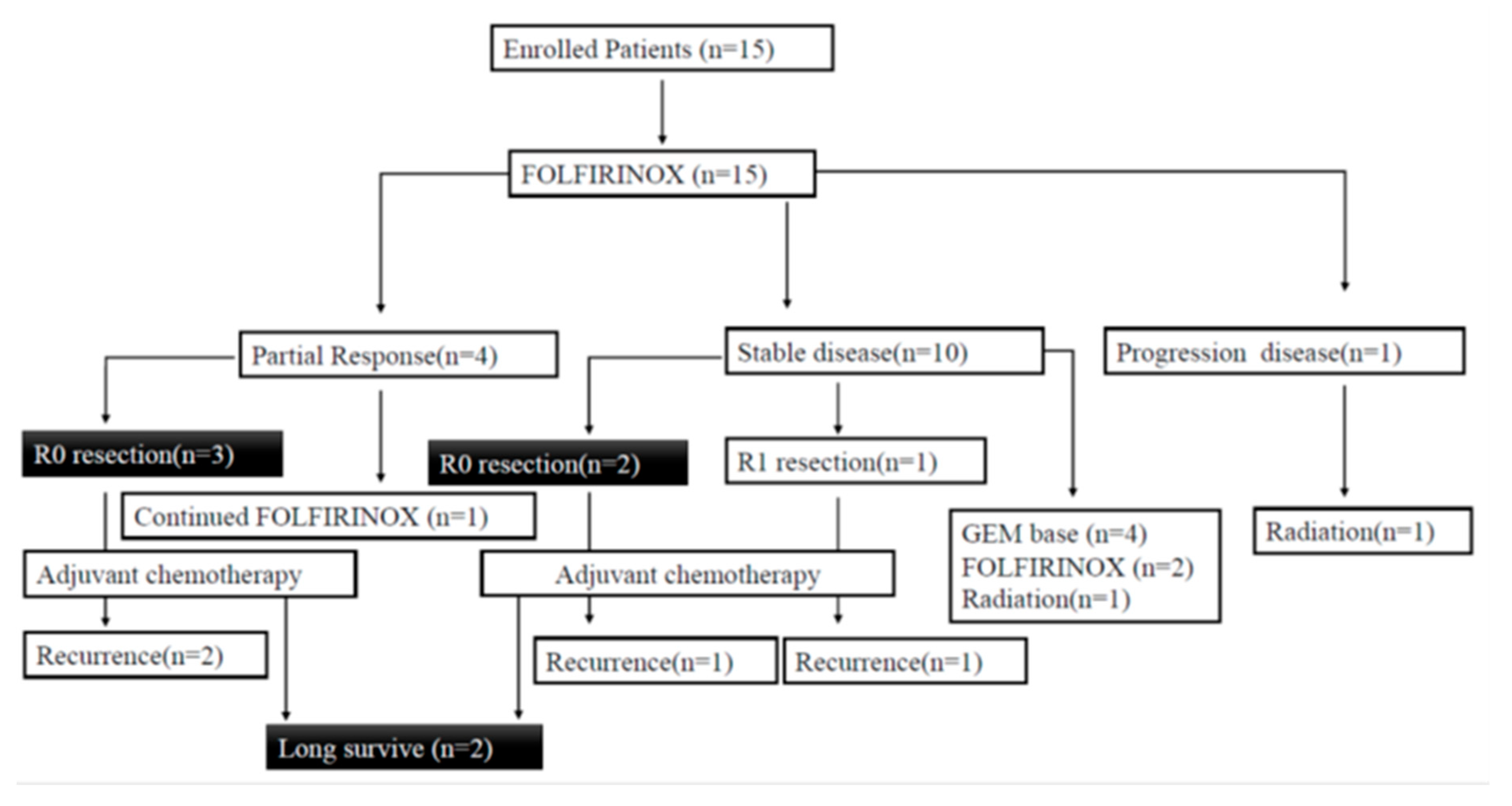
Table 4. Forty percent (6/15) of patients underwent surgery, and R0 resection was achieved in 33.3% (5/15) of patients and 81.3% (5/6) of the patients who underwent surgery (
Figure 4). Surgical procedures, including pancreaticoduodenectomy and distal pancreatectomy, were performed in five and one cases, respectively. The median operative time was 646 min (range, 534–1033 min). Arterial resection was performed in three cases (20%), and portal vein resection was performed in four cases (26.7%). The median blood loss was 1600 mL (range, 400–8853 mL). The pathological types of well and moderately differentiated were 2 and 4, respectively. Evans Grade 1, 2 and 3 was observed in three, two and one cases, respectively. The most severe complication after surgical resection was a pancreatic fistula (n = 1). Diarrhea (n = 5), delayed gastric emptying (n = 3), abdominal abscesses (n = 1), and ascites (n = 1) were also developed after surgical resection. Five patients received S-1 chemotherapy and one patient received gemcitabine (GEM) and albumin-bound paclitaxel combination chemotherapy after the surgical resection. Four patients had recurrent disease, and two patients did not have a recurrent at the last follow-up time with long survival. The breakdown of four patients’ recurrence sites was three of local recurrence and one of peritoneal disseminated recurrence. Relapse-free survival time for R0 resection cases was 25.7 months (95% CI, 11.3–40.1).
3.5. Overall Survival
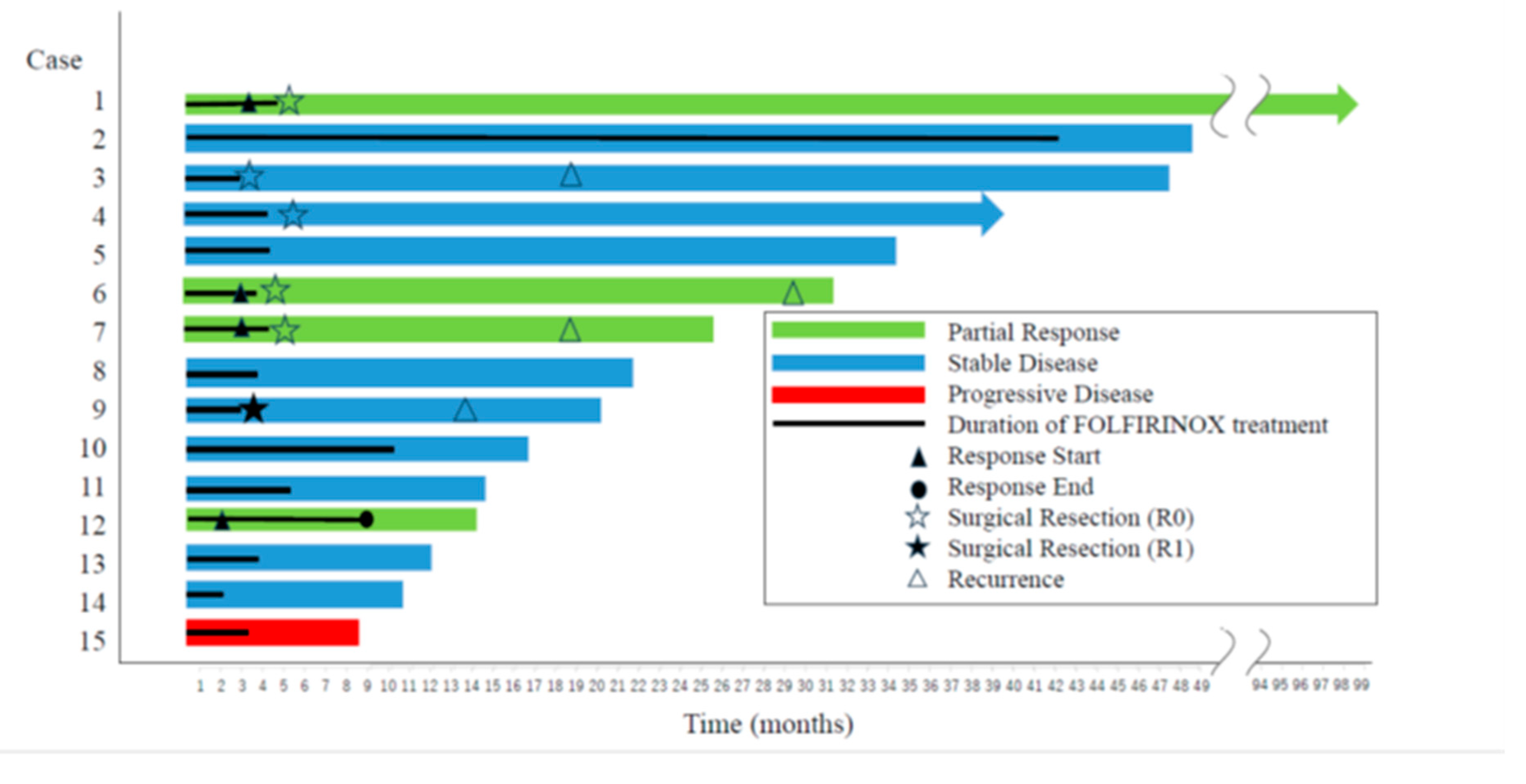
Swimmers plot data for all FOLFIRINOX treatment cases was shown in
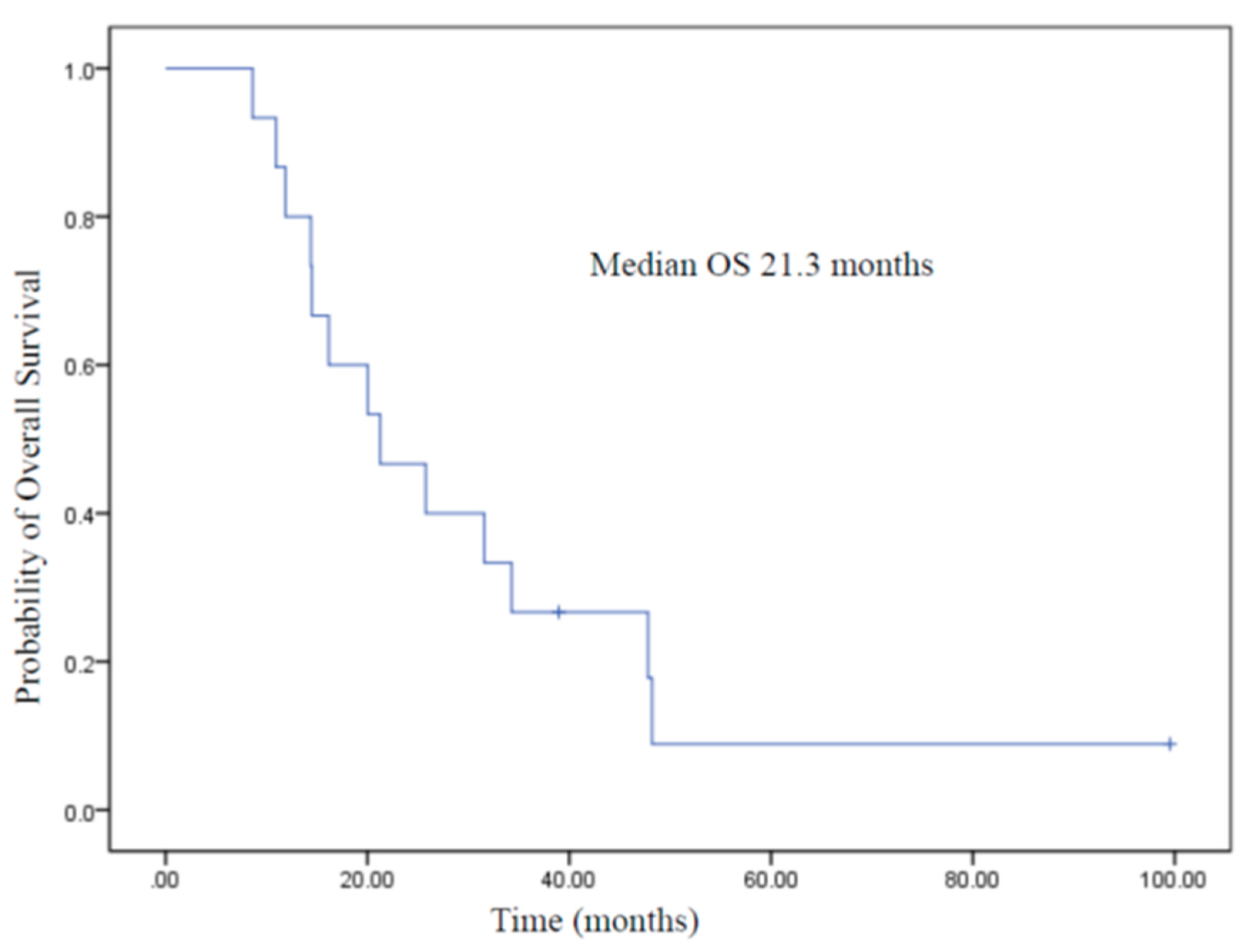
Figure 5. The median OS of all cases was 21.3 months (95% CI, 9.15–33.4 months) (
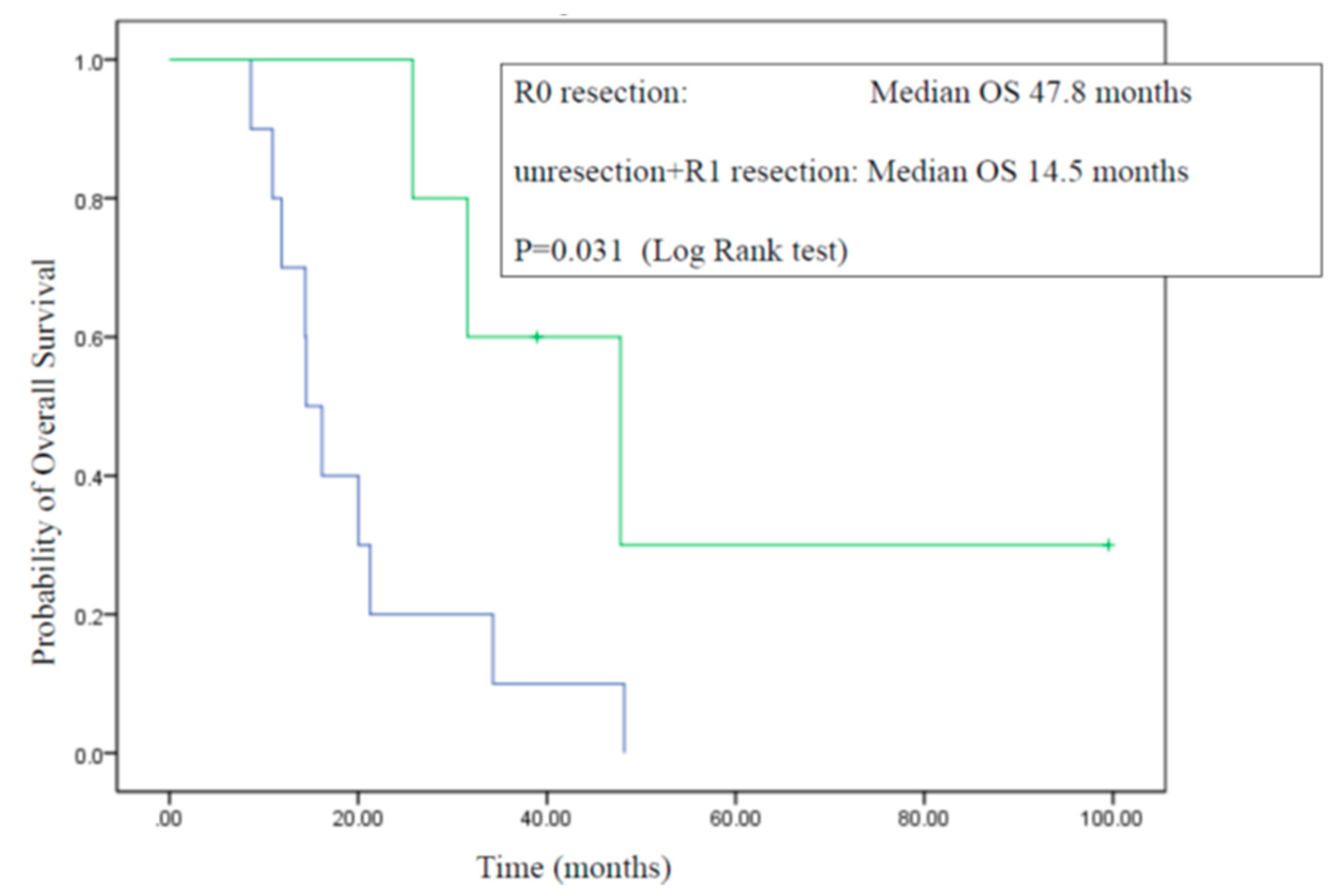
Figure 6). OS of R0 resection cases was significantly longer than that of other cases (47.8 months [95% CI, 22.5–73.1 months] vs. 14.5 months [95% CI, 11.8–17.2 months]; log-rank test, P = 0.031) (
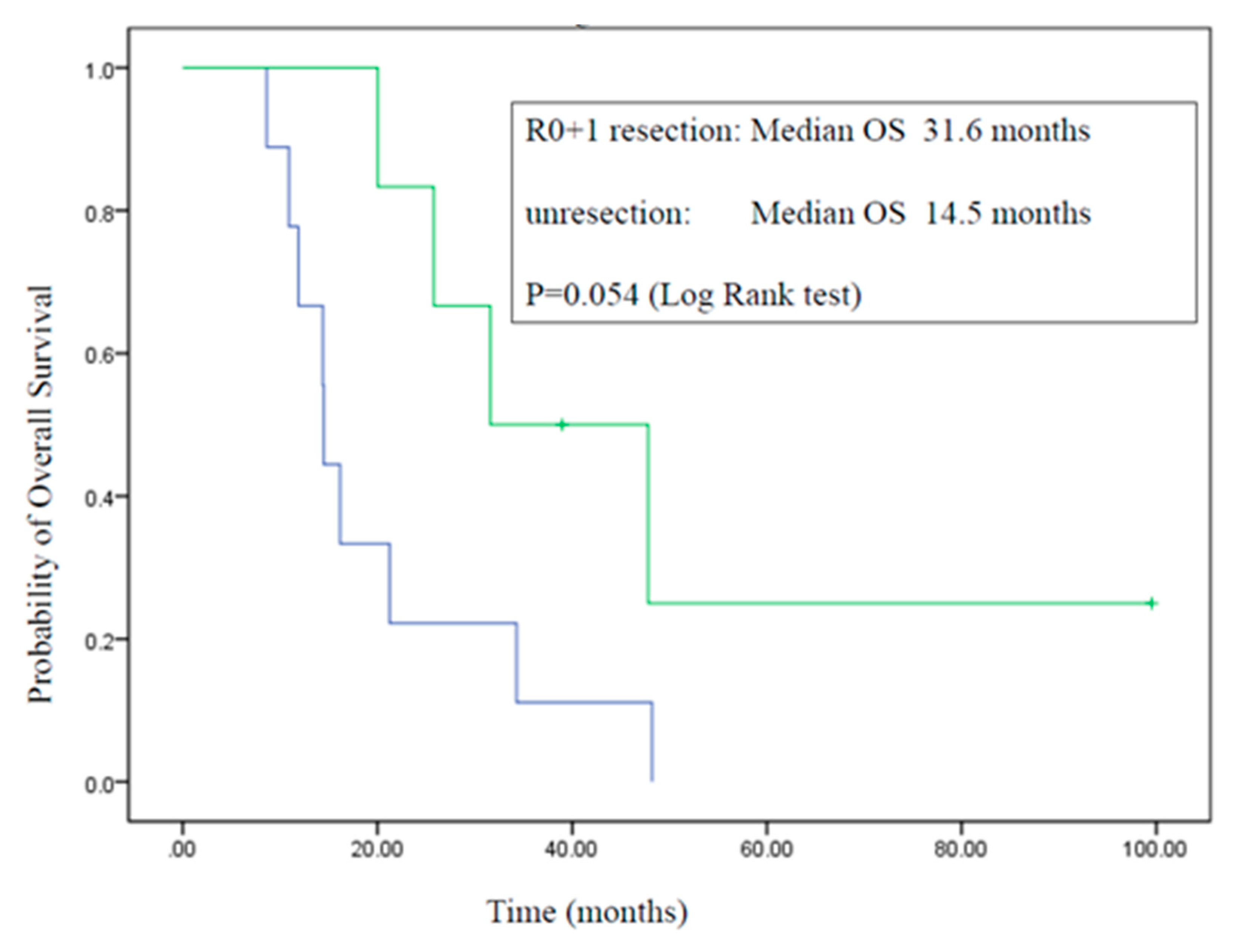
Figure 7). OS of R0 and R1 resection cases was longer than that of other cases (31.6 months [95% CI, 10.4–52.7 months] vs. 14.5 months [95% CI, 14.2–14.8 months]; log-rank test, P= 0.054) (
Figure 8).
4. Discussion
We conducted a prospective single-arm study on URLA-PCs to study conversion rate for surgery after FOLFIRINOX. R0 resection was achieved in 33.3% of the patients with URLA-PC. The OS of patients who underwent R0 resection was 47.8 months, which is extremely long among URLA-PC patients. There are many prospective studies of neoadjuvant chemotherapies, including FOLFIRINOX, and chemoradiotherapy for R-PC or BR-PC; however, there have been no prospective study for patients with URLA-PC to evaluate the resectability rate with pre-operative chemotherapy using FOLFIRINOX. To the best of our knowledge, this is the first study to evaluate the R0 resection rate of pre-operative chemotherapy using the FOLFIRINOX regimen in patients with URLA-PC.
The R0 resection rate in this study was 33%, which is similar to the previous retrospective studies and meta-analysis [
12,
25,
26,
27]. In the retrospective study by Blazer et al., R0 resection was performed in 10 of 25 (40%) patients with URLA-PC [
25]. The meta-analysis of 13 studies involving 253 patients by Petrelli et al. described that R0 resection was performed in 22.5% (95% CI, 13.3–35.4) of patients with URLA-PC [
26]. According to another meta-analysis, 91 of 325 patients (28.0%) underwent resection after FOLFIRINOX for URLA-PC. The pooled percentage of resection in a random-effects model was 25.9% (95% CI, 20.2%–31.9%; I
2 = 24%) [
12].
OS rate was also similar to the previous retrospective studies and meta-analyses [
12,
27]. Pooled analysis of 315 patients revealed a follow-up duration of 12.3 (8.0–20.5) months, progression-free survival (PFS) of 15.0 months and OS of 24.2 months [
12]. Among the 437 patients with URLA-PC who underwent radical pancreatic surgery, median OS was 33.4 months (95% CI, 29.0–36.3 months).
The number of chemotherapy cycles before conversion surgery varies across studies; however, a previous meta-analysis demonstrated that this may not have a major impact [
28]. In a retrospective multicenter study involving 97 patients with URLA-PC in Japan, conversion surgery was more beneficial for patients with >8 months of pre-operative therapy than those with <8 months of pre-operative therapy [
29]. Compared to this retrospective study, our study had a shorter duration of chemotherapy. However, this retrospective study mainly used GEM-based chemotherapy (91.4%), and albumin-bound paclitaxel was not approved for insurance support at the time of that study in Japan. It may be partially explained the difference of the treatment duration that the intensity of the GEM-based chemotherapy was lower than that of FOLFIRINOX.
Some studies showed survival benefit of additional radiotherapy after FOLFIRINOX treatment [
30]. However, the biologically effective dose and fractionation of administration of radiotherapy are not standardized, the method of administration varies depending on the combination with chemotherapy, and there is no rigor evidence of superiority of the additional radiotherapy compared to chemotherapy alone; therefore, we conducted this study with chemotherapy alone.
The difficulty of conversion surgery is that the decision of surgical resection is not necessarily made corresponding to the treatment efficacy. In our study, there were cases in which resection was performed despite the treatment response did not reach PR. Conversely, there was a case which was not performed resection after PR response. These are the results of comprehensive judgment by the experienced experts such as reducing the length and circumference of contact between the tumor and important arteries rather than shrinking the tumor, the increase in CT value of the adipose tissue around the tumor and the normalization of tumor markers.
In terms of statistical significance, this may be a controversial study as it did not meet the initial statistically set number of cases. The number of patients were originally set based on the power calculation, however, we noticed that it takes too long time to enroll the ideal number of patients because there were only 15 eligible patients for eight years. The major reason was a small proportion of the unresectable locally advanced pancreatic cancer (URLA-PC), which accounts for 13.5% of all PC cases in our study. Further, we had to exclude vulnerable patients with poor general condition because the original regimen of FOLFIRINOX had relatively high toxicity, which made the number of enrolled patients smaller. In addition, because of this exclusion, selection bias exists in this study.
However, this was a prospective study conducted in a homogeneous population, not conducted after radiotherapy, and allowed pure evaluation of the role of FOLFIRINOX therapy. In addition, the R0 resection rate was relatively high, the OS, especially in patients who underwent resection, was very long, and the side effects of chemotherapy were considered acceptable.
5. Conclusions
To the best of our knowledge, this is the first study to evaluate the R0 resection rate of pre-operative chemotherapy using the FOLFIRINOX regimen in patients with URLA-PC. R0 resection was achieved in 33.3% of the patients with URLA-PC. Long-term survival can be achieved by surgical resection in some cases of unresectable URLA-PC; even in cases where resection is not achieved, chemotherapy, such as FOLFIRINOX, is considered extremely important for extending the survival period. Further large-scale study is needed to confirm FOLFIRINOX utility as a preoperative therapy for URLA-PC.
Author Contributions
Conceptualization, Y.Y., R.M., T.T., K.M., Y.S., Y.H., R.M., N.O., E.K., K.K., S.Y., and Y. I.; methodology, R.M. and Y.Y.; validation, R.M, Y.Y., and R.M.; formal analysis, Y.Y. and N.K.; investigation, N.K.; data curation, Y.Y.; writing—original draft preparation, N.K.; writing—review and editing, K.K.; supervision, Y.I. and I.E.; project administration, I.E.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Yokohama City University Hospital (protocol code B140508029; date of approval: June 1st, 2014).
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available in this article (and Supplementary Materials).
Acknowledgments
The authors are grateful to T. Ishibashi from Y-NEXT for assistance with this study.
Conflicts of Interest
Noritoshi Kobayashi received an honorarium from Novartis,Teijin Pharma, Teijin Healthcare, EA Pharma, Ono Pharmaceutical, Taiho Pharmaceutical, Yakuruto Honsha, MSD, and PD Radiopharma (successor to the radiopharmaceutical business of Fujifilm Toyama Chemical Co., Ltd.). The authors declare no conflicts of interest. The funder had no role in the design of the study, collection, analyses, or interpretation of data, writing of the manuscript, or the decision to publish the results.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J Clin 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Gillen, A.S.; Schuster, T.; Meyer Zum Büschenfelde, C.; Friess, H.; Kleeff, J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med, 2010; 7, e1000267. [Google Scholar]
- Vauthey, J.N.; Ahpba, D.E.; SSO/SSAT. AHPBA/SSO/SSAT consensus conference on research and borderline research pancreatic cancer: A rationale and overview of the conference. Ann Surg Oncol Consensus Conference on Research and Borderline Research Pancreatic Cancer: Rationale and Overview of the Conference. Ann Surg Oncol 2009, 16, 1725–1726. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.J.; Kuk, D.; Castillo, C. F-D.; Basturk, O.; Wolfgang, C.L.; Cameron, J.L.; Lillemoe, K.D.; Ferrone, C.R.; Morales-Oyarvide, V.; He, J.; Weiss, M.J.; Hruban, R.H.; Gönen, M.; Klimstra, D.S.; Mino-Kenudson, M. Multi-institutional Validation Study of the American Joint Commission on Cancer (8th edition): Changes for T and N Staging in Patients With Pancreatic Adenocarcinoma. Ann Surg 2017, 265, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J. W.; Wolpin, B.; Clancy, T.; Wang, J.; Mamon, H.; Shinagare, A. B.; Jagannathan, J.; Rosenthal, M. Borderline resectable pancreatic cancer: conceptual evolution and current approach to image-based classification. Ann Oncol 2017, 28, 2067–2076. [Google Scholar] [CrossRef] [PubMed]
- Sohal, D.P.S.; Duong, M.; Ahmad, S.A.; Gandhi, N.S.; Beg, M.S.; Wang-Gillam, A.; Wade, J.L.; Chiorean, E.G.; Guthrie, K.A.; Lowy, A.M.; Philip, P.A.; Hochster, H.S. Efficacy of perioperative chemotherapy for resectable pancreatic adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol 2021, 7, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.E.; Wo, J.Y.; Ryan, D.P.; Jiang, W.; Yeap, B.Y.; Drapek, L.C.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W.; Faris, J.E.; Zhu, A.X.; Goyal, L.; Lillemoe, K.D.; DeLaney, T.F.; Castillo, C. F-D.; Ferrone, C.R.; Hong, T.S. Total neoadjuvant therapy with folfirinox followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma: A phase 2 clinical trial. JAMA Oncol 2018, 4, 963–969. [Google Scholar] [CrossRef]
- Chawla, A.; Molina, G.; Pak, L.M.; Rosenthal, M.; Mancias, J.D.; Clancy, T.E.; Wolpin, B.M.; Wang, J. Neoadjuvant therapy is associated with improved survival in borderline resectable pancreatic cancer. Ann Surg Oncol 2020, 27, 1191–1200. [Google Scholar] [CrossRef]
- Jang, J.Y.; Han, Y.; Lee, H.; Kim, S.W.; Kwon, W.; Lee, K.H.; Oh, D.Y.; Chie, E.K.; Lee, J.M.; Heo, J.S.; Park, J.O.; Lim, D.H.; Kim, S.H.; Park, S.J.; Lee, W.J.; Koh, Y.H.; Park, J.S.; Yoon, D.S.; Lee, I.J.; Choi, S.H. Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a prospective, randomized, open-label, multicenter phase 2/3 trial. Ann Surg 2018, 268, 215–222. [Google Scholar] [CrossRef]
- Sultana, A.; Tudur Smith, C.; Cunningham, D.; Starling, N.; Tait, D.; Neoptolemos, J.P.; Ghaneh, P. Systematic review, including meta-analyses on the management of locally advanced pancreatic cancer using radiation/combined modality therapy. Br J Cancer 2007, 96, 1183–1190. [Google Scholar] [CrossRef]
- Suker, M.; Beumer, B.R.; Sadot, E.; Marthey, L.; Faris, J.E.; Mellon, E.A.; El-Rayes, B.F.; Wang-Gillam, A.; Lacy, J.; Hosein, P.J.; Moorcraft, S.Y.; Conroy, T.; Hohla, F.; Allen, P.; Taieb, J.; Hong, T.S.; Shridhar, R.; Chau, I.; van Eijck, C.H.; Koerkamp, B.G. FOLFIRINOX therapy for locally advanced pancreatic cancer: A systematic review and patient-level meta-analysis. Lancet Oncol 2016, 17, 801–810. [Google Scholar] [CrossRef]
- Sadot, E.; Doussot, A.; O’Reilly, E.M.; Lowery, M.A.; Goodman, K.A.; Do, R.K.; Tang, L.H.; Gönen, M.; D’Angelica, M.I.; DeMatteo, R.P.; Kingham, T.P.; Jarnagin, W.R.; Allen, P.J. FOLFIRINOX induction therapy for stage 3 pancreatic adenocarcinoma. Ann Surg Oncol 2015, 22, 3512–3521. [Google Scholar] [CrossRef]
- NCCN Clinical Guideline, 2021.
- Cardenes, H.R.; Moore, A.M.; Johnson, C.S.; Yu, M.; Helft, P.; Chiorean, E.G.; Vinson, J.; Howard, T.J.; Stephens, A.W.; Tai, D.F.; Loehrer Sr, P.J. Phase II study of gemcitabine in combination with radiation therapy in patients with localized, unresectable pancreatic cancer: A Hoosier Oncology Group study. Am J Clin Oncol 2011, 34, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.; Kim, S.C.; Kim, J.H.; Lee, S.S.; Kim, T.W.; Park, D.H.; Seo, D.W.; Lee, S.K.; Kim, M.H.; Kim, J.H.; Park, J.H.; Shin, S.H.; Han, D.J. Prospective efficacy and safety of neoadjuvant gemcitabine and capecitabine combination chemotherapy for borderline resectable or unresectable locally advanced pancreatic adenocarcinoma. Surgery 2012, 152, 851–862. [Google Scholar] [CrossRef]
- Strobel, O.; Berens, V.; Hinz, U.; Hartwig, W.; Hackert, T.; Bergmann, F.; Debus, J.; Jäger, D.; Büchler, M.W.; Werner, J. Resection after neoadjuvant therapy for locally advanced, “unresectable” pancreatic cancer. Surgery 2012, 152 (suppl 1), S33–42. [Google Scholar] [CrossRef] [PubMed]
- Arvold, N.D.; Ryan, D.P.; Niemierko, A.; Blaszkowsky, L.S.; Kwak, E.L.; Wo, J.Y.; Allen, J.N.; Clark, J.W.; Wadlow, R.C.; Zhu, A.X.; Castillo, C. F-D.; Hong, T.S. Long-term outcomes of neoadjuvant chemotherapy before chemoradiation for locally advanced pancreatic cancer. Cancer 2012, 118, 3026–3035. [Google Scholar] [CrossRef]
- Leone, F.; Gatti, M.; Massucco, P.; Colombi, F.; Sperti, E.; Campanella, D.; Regge, D.; Gabriele, P.; Capussotti, L.; Aglietta, M. Induction gemcitabine and oxaliplatin therapy followed by a twice-weekly infusion of gemcitabine and concurrent external-beam radiation for neoadjuvant treatment of locally advanced pancreatic cancer: a single institutional experience. Cancer 2013, 119, 277–284. [Google Scholar] [CrossRef]
- Hosein, P.J.; Macintyre, J.; Kawamura, C.; Maldonado, J.C.; Ernani, V.; Loaiza-Bonilla, A.; Narayanan, G.; Ribeiro, A.; Portelance, L.; Merchan, J.R.; Levi, J.U.; Rocha-Lima, C.M. Neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma: A retrospective study BMC Cancer 2012, 12, 199. [CrossRef]
- Boone, B.A.; Steve, J.; Krasinskas, A.M.; Zureikat, A.H.; Lembersky, B.C.; Gibson, M.K.; Stoller, R.G.; Zeh, H.J.; Bahary, N. Outcome with FOLFIRINOX for borderline resectable and locally unresectable pancreatic cancer. J Surg Oncol 2013, 108, 236–241. [Google Scholar] [CrossRef]
- Faris, J.E.; Blaszkowsky, L.S.; Mcdermott, S.; Guimaraes, A.R.; Szymonifka, J.; Huynh, M.A.; Ferrone, C.R.; Wargo, J.A.; Allen, J.N.; Dias, L.E.; Kwak, E.L.; Lillemoe, K.D.; Thayer, S.P.; Murphy, J.E.; Zhu, A.X.; Sahani, D.V.; Wo, J.Y.; Clark, J.W.; Castillo, C.F-D.; Ryan, D.P.; Hong, T.S. FOLFIRINOX in locally advanced pancreatic cancer: The Massachusetts General Hospital Cancer Center experience. Oncologist 2013, 18, 543–548. [CrossRef] [PubMed]
- Blazer, M.; Wu, C.; Goldberg, R.M.; et al. Modified FOLFIRINOX is tolerable and effective in patients with borderline resectable (BRPC) and locally advanced unresectable (LAURPC) pancreatic cancer. Gastrointestinal Cancer Symposium. 2014; Vol. B48, abstr. 275.
- SWOG statistical Tools: one arm binominal. Available online: http://www.swogstat.org/stat/public/one_binomial.htm.
- Blazer, M.; Wu, C.; Goldberg, R.M.; Phillips, G.; Schmidt, C.; Muscarella, P.; Wuthrick, E.; Williams, T.M.; Reardon, J.; Ellison, E.C.; Bloomston, M.; Bekaii-Saab, T. Neoadjuvant-modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma Ann Surg Oncol 2015, 22, 1153–1159. [CrossRef]
- Petrelli, F.; Coinu, A.; Borgonovo, K.; Cabiddu, M.; Ghilardi, M.; Lonati, V.; Aitini, E.; Barni, S.; Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato Digerente (GISCAD). Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato Digerente (GISCAD). FOLFIRINOX-based neoadjuvant therapy in borderline resectable or unresectable pancreatic cancer: a meta-analytical review of published studies. Pancreas 2015, 44, 515–521. [Google Scholar] [CrossRef]
- Eshmuminov, D.; Aminjonov, B.; Palm, R.F.; Malleo, G.; Schmocker, R.K.; Abdallah, R.; Yoo, C.; Shaib, W.L.; Schneider, M.A.; Rangelova, E.; Choi, Y.J.; Kim, H.; Rose, J.B.; Patel, S.; Wilson, G.C.; Maloney, S.; Timmermann, L.; Sahora, K.; Rössler, F.; Lopez-Lopez, V.; Boyer, E.; Maggino, L.; Malinka, T.; Park, J.Y.; Katz, M.H.G.; Prakash, L.; Ahmad, S.A.; Helton, S.; Jang, J-Y. Hoffe, S.E.; Salvia, R.; Taieb, J.; He, J.; Clavien, P-A.; Held, U.; Lehmann, K. FOLFIRINOX or gemcitabine-based chemotherapy for borderline resectable and locally advanced pancreatic cancer: A multi-institutional, patient-level, meta-analysis and systematic review. Ann Surg Oncol 2023, 30, 4417–4428. [Google Scholar] [CrossRef] [PubMed]
- Janssen, Q.P.; Buettner, S.; Suker, M.; Beumer, B.R.; Addeo, P.; Bachellier, P.; Bahary, N.; Bekaii-Saab, T.; Bali, M.A.; Besselink, M.G.; Boone, B.A.; Chau, I.; Clarke, S.; Dillhoff, M.; El-Rayes, B.F.; Frakes, J.M.; Grose, D.; Hosein, P.J.; Jamieson, N.B.; Javed, A.A.; Khan, K.; Kim, K-P. Kim, S.C.; Kim, S.S.; Ko, A.H.; Lacy, J.; Margonis, G.A.; McCarter, M.D.; McKay, C.J.; Mellon, E.A.; Moorcraft, S.Y.; Okada, K-I.; Paniccia, A.; Parikh, P.J.; Peters, N.A.; Rabl, H.; Samra, J.; Tinchon, C.; van Tienhoven, G.; van Veldhuisen, E.; Wang-Gillam, A.; Weiss, M.J.; Wilmink, J.W.; Yamaue, H.; Homs, M.Y.V.; van Eijck, C.H.J.; Katz, M.H.G.; Koerkamp, B.G. Neoadjuvant FOLFIRINOX in patients with borderline resectable pancreatic cancer: A systematic review and patient-level meta-analysis. J Natl Cancer Inst 2019, 111, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Satoi, S.; Yamaue, H.; Kato, K.; Takahashi, S.; Hirono, S.; Takeda, S.; Eguchi, H.; Sho, M.; Wada, K.; Shinchi, H.; Kwon, A.H.; Hirano, S.; Kinoshita, T.; Nakao, A.; Nagano, H.; Nakajima, Y.; Sano, K.; Miyazaki, M.; Takada, T. Role of adjuvant surgery for patients with initially unresectable pancreatic cancer with a long-term favorable response to nonsurgical anticancer treatments: results of a project study for pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 2013, 20, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Teriaca, M.A.; Loi, M.; Suker, M.; Eskens, F.A.L.M.; van Eijck, C.H.J.; Nuyttens, J.J. A phase II study of stereotactic radiotherapy after FOLFIRINOX for locally advanced pancreatic cancer (LAPC-1 trial): long-term outcome. Radiother Oncol 2021, 155, 232–236. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).