Submitted:
10 January 2024
Posted:
11 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Plant microbial fuel cells: functioning and factors affecting the electrochemical characteristics of the system
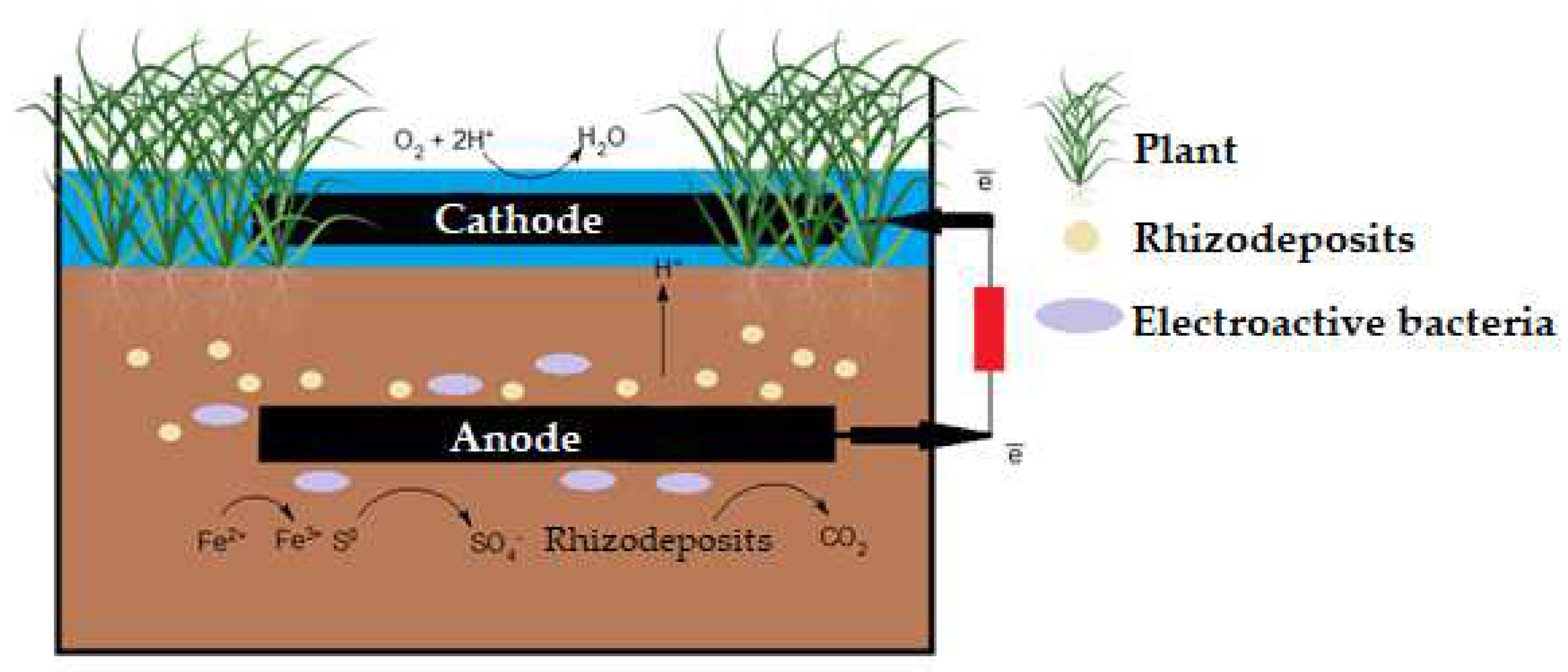
2.1 The principle of PMFC operation
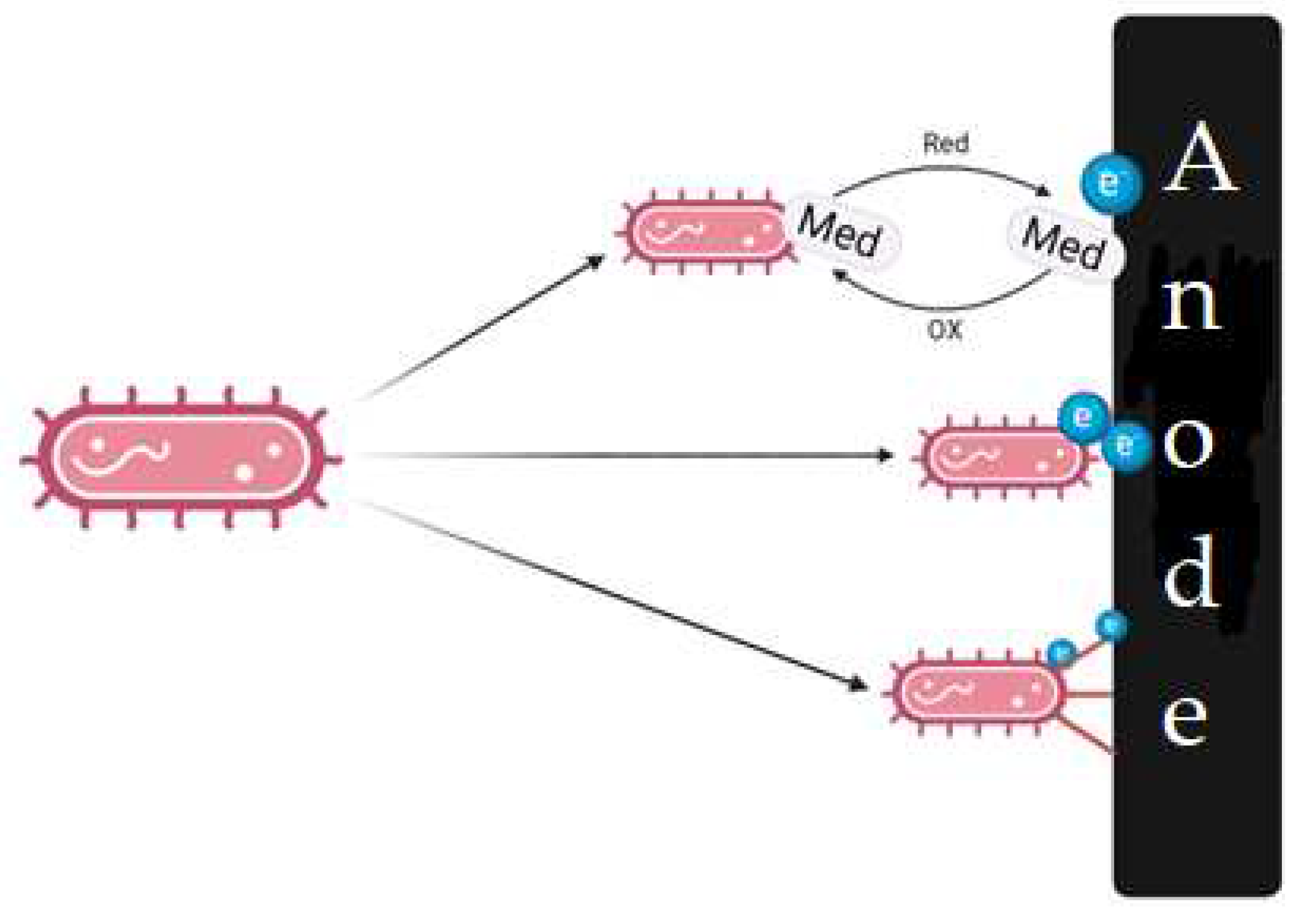
2.2. Electrodes in PMFC
2.3. Application of proton exchange membranes in PMFC system
2.3. The influence of environmental factors on the electricity generation in the PMFC
3. PMFC technology to utilize anthropogenic pollutants in aquatic and soil ecosystems
3.1. PMFC to purify water and soil ecosystems from organic compounds and biogenic elements
3.2. PMFC application for removal of heavy metals from soil and aquatic ecosystems
4. Further development of PMFC for the ecosystem purification from surfactants
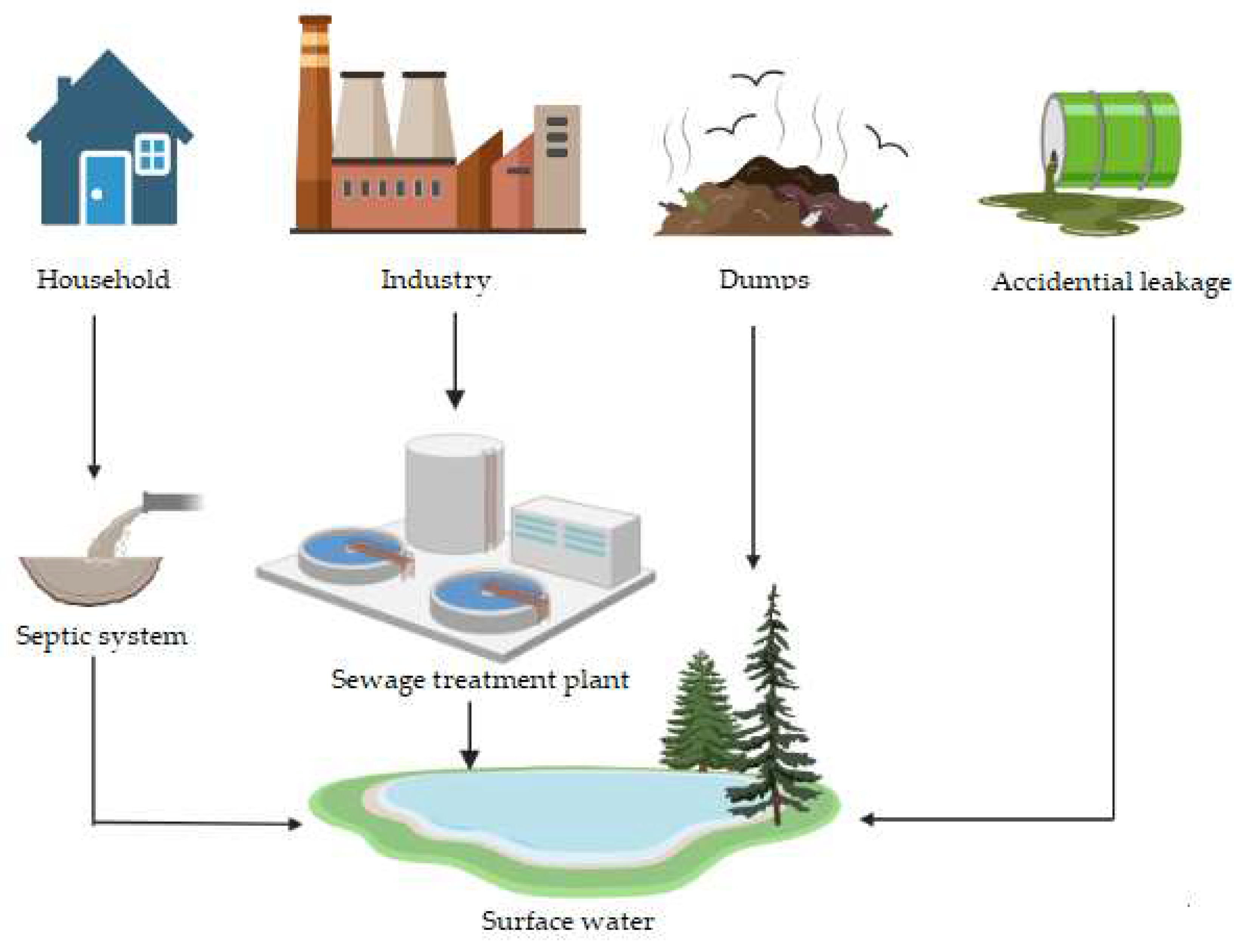
4.1. Ways of surfactant entry into surface waters as an actual pollutant and potential substrate for microorganisms in the PMFC system
4.2. Microorganisms-destructors of surfactants
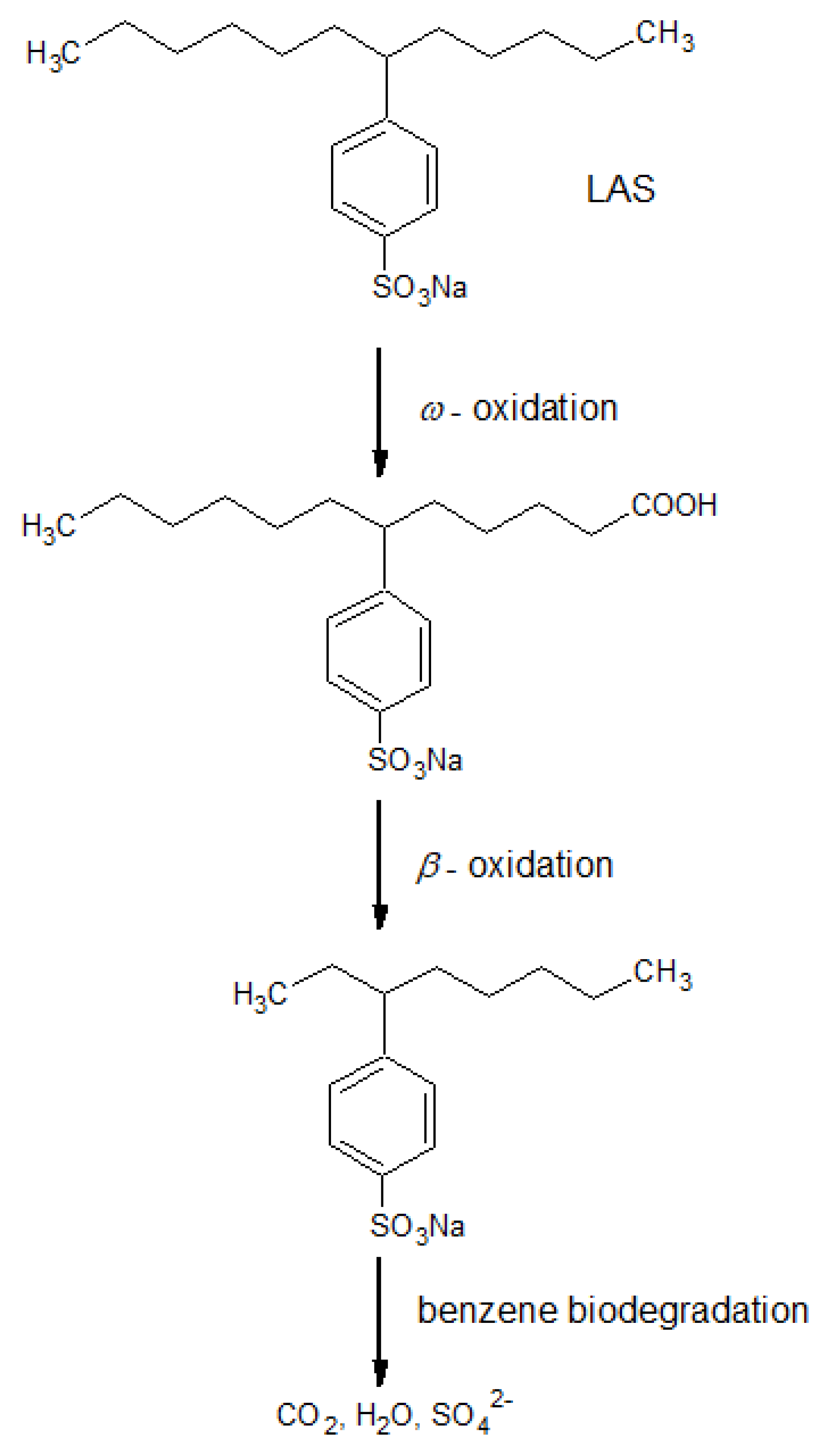
4.3. Bioelectric systems based on microorganisms for wastewater treatment from surfactants
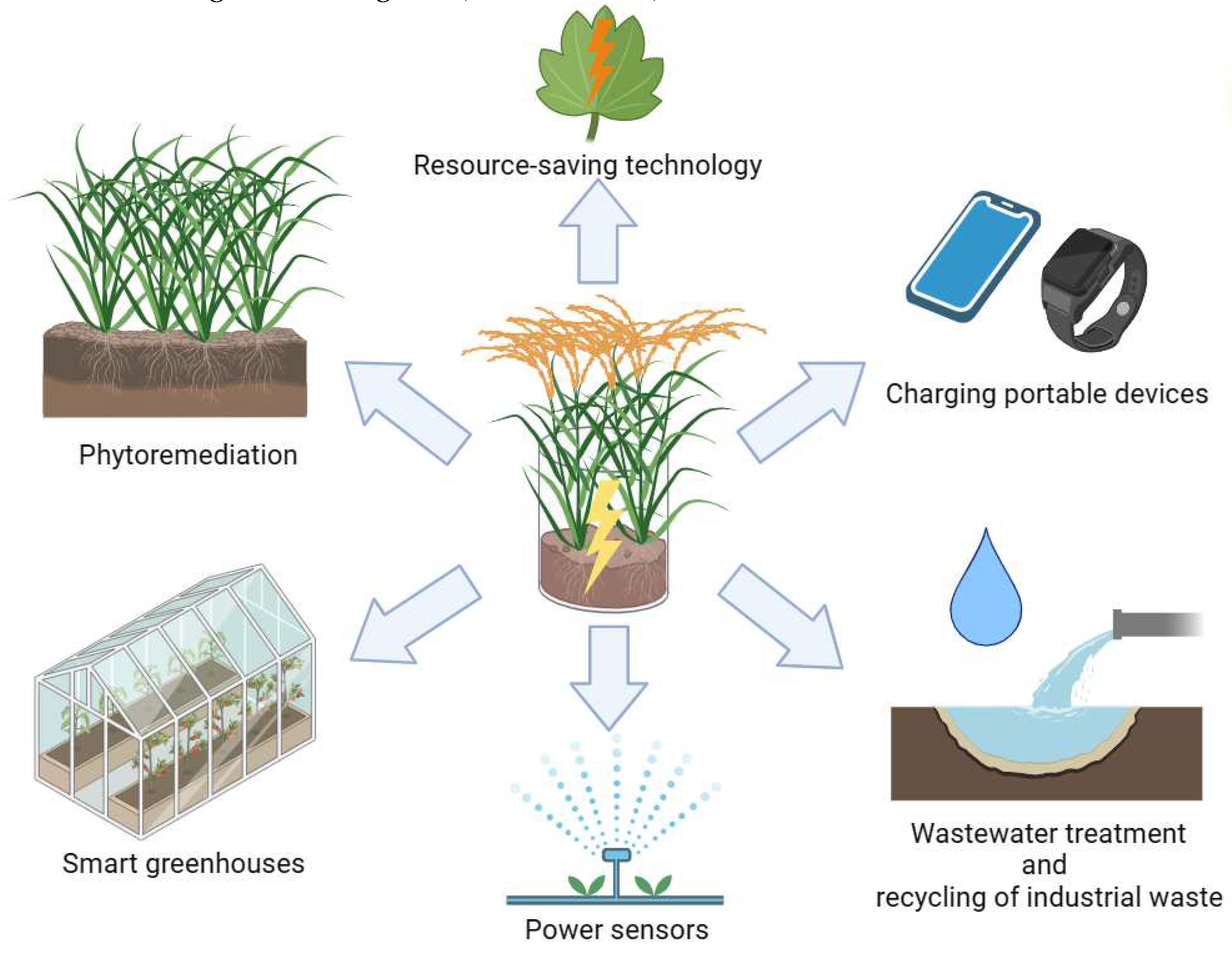
4.5. Integration of PMFC into hydrobotanical sites for wastewater treatment from surfactants as a prospect for further development of bioelectric systems
5. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A Review on Conventional and Novel Materials towards Heavy Metal Adsorption in Wastewater Treatment Application. Journal of Cleaner Production 2021, 296, 126589. [Google Scholar] [CrossRef]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of Soil and Water Contaminated with Petroleum Hydrocarbon: A Review. Environmental Technology & Innovation 2020, 17, 100526. [Google Scholar] [CrossRef]
- Vieno, N.M.; Tuhkanen, T.; Kronberg, L. Seasonal Variation in the Occurrence of Pharmaceuticals in Effluents from a Sewage Treatment Plant and in the Recipient Water. Environ. Sci. Technol. 2005, 39, 8220–8226. [Google Scholar] [CrossRef]
- Bhandari, G.; Atreya, K.; Scheepers, P.T.J.; Geissen, V. Concentration and Distribution of Pesticide Residues in Soil: Non-Dietary Human Health Risk Assessment. Chemosphere 2020, 253, 126594. [Google Scholar] [CrossRef]
- UNEP Harnessing Opportunity: Wastewater as a Managed Resource. 2017; 2. Group, T.I.-D.W.; Programme, U.N.E. Freshwater Strategic Priorities 2022-2025. 2022.
- Praveen, A.; Pandey, V.C. Pteridophytes in Phytoremediation. Environ Geochem Health 2020, 42, 2399–2411. [Google Scholar] [CrossRef]
- Tanwir, K.; Amna, *!!! REPLACE !!!*; Javed, M.T.; Shahid, M.; Akram, M.S.; Ali, Q. Antioxidant Defense Systems in Bioremediation of Organic Pollutants. In Handbook of Bioremediation; Elsevier, 2021; pp. 505–521. ISBN 978-0-12-819382-2. [Google Scholar]
- Moubasher, H.A.; Hegazy, A.K.; Mohamed, N.H.; Moustafa, Y.M.; Kabiel, H.F.; Hamad, A.A. Phytoremediation of Soils Polluted with Crude Petroleum Oil Using Bassia Scoparia and Its Associated Rhizosphere Microorganisms. International Biodeterioration & Biodegradation 2015, 98, 113–120. [Google Scholar] [CrossRef]
- Tiodar, E.D.; Văcar, C.L.; Podar, D. Phytoremediation and Microorganisms-Assisted Phytoremediation of Mercury-Contaminated Soils: Challenges and Perspectives. IJERPH 2021, 18, 2435. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation Strategies for Soils Contaminated with Heavy Metals: Modifications and Future Perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Shalaeva, D.S.; Kukartseva, O.I.; Tynchenko, V.S.; Kukartsev, V.V.; Aponasenko, S.V.; Stepanova, E.V. Analysis of the Development of Global Energy Production and Consumption by Fuel Type in Various Regions of the World. IOP Conf. Ser.: Mater. Sci. Eng. 2020, 952, 012025. [Google Scholar] [CrossRef]
- UNEP Harnessing Opportunity: Ensure Access to Affordable, Reliable, Sustainable and Modern Energy for All, 2017.
- Song, F.; Mehedi, H.; Liang, C.; Meng, J.; Chen, Z.; Shi, F. Review of Transition Paths for Coal-Fired Power Plants. Global Energy Interconnection 2021, 4, 354–370. [Google Scholar] [CrossRef]
- EnerData: Global Energy & CO2 Data. Available online: www.Enerdata.Net (accessed on 20 September 2022).
- Chowdhury, Md.S.; Rahman, K.S.; Chowdhury, T.; Nuthammachot, N.; Techato, K.; Akhtaruzzaman, Md.; Tiong, S.K.; Sopian, K.; Amin, N. An Overview of Solar Photovoltaic Panels’ End-of-Life Material Recycling. Energy Strategy Reviews 2020, 27, 100431. [Google Scholar] [CrossRef]
- Vezhenkova, I.; Semenova, M.; Kovalevskaya, A.; Gryaznov, A.; Rodríguez-Barroso, M.R.; Jimenez Castañeda, R. Chemical Composition Determination of Impurities and Effect on the Toxicity Degree of Solar Panel Components. E3S Web Conf. 2020, 220, 01057. [Google Scholar] [CrossRef]
- Korniejenko, K.; Kozub, B.; Bąk, A.; Balamurugan, P.; Uthayakumar, M.; Furtos, G. Tackling the Circular Economy Challenges—Composites Recycling: Used Tyres, Wind Turbine Blades, and Solar Panels. J. Compos. Sci. 2021, 5, 243. [Google Scholar] [CrossRef]
- Bandaru, S.H.; Becerra, V.; Khanna, S.; Radulovic, J.; Hutchinson, D.; Khusainov, R. A Review of Photovoltaic Thermal (PVT) Technology for Residential Applications: Performance Indicators, Progress, and Opportunities. Energies 2021, 14, 3853. [Google Scholar] [CrossRef]
- Riech, I.; Castro-Montalvo, C.; Wittersheim, L.; Giácoman-Vallejos, G.; González-Sánchez, A.; Gamboa-Loira, C.; Acosta, M.; Méndez-Gamboa, J. Experimental Methodology for the Separation Materials in the Recycling Process of Silicon Photovoltaic Panels. Materials 2021, 14, 581. [Google Scholar] [CrossRef] [PubMed]
- Prasad, J.; Tripathi, R.K. Scale-up and Control the Voltage of Sediment Microbial Fuel Cell for Charging a Cell Phone. Biosensors and Bioelectronics 2021, 172, 112767. [Google Scholar] [CrossRef]
- Osorio de la Rosa, E.; Vázquez Castillo, J.; Carmona Campos, M.; Barbosa Pool, G.; Becerra Nuñez, G.; Castillo Atoche, A.; Ortegón Aguilar, J. Plant Microbial Fuel Cells–Based Energy Harvester System for Self-Powered IoT Applications. Sensors 2019, 19, 1378. [Google Scholar] [CrossRef]
- Brunelli, D.; Tosato, P.; Rossi, M. Flora Health Wireless Monitoring with Plant-Microbial Fuel Cell. Procedia Engineering 2016, 168, 1646–1650. [Google Scholar] [CrossRef]
- Wang, X.; Tian, Y.; Liu, H.; Zhao, X.; Peng, S. The Influence of Incorporating Microbial Fuel Cells on Greenhouse Gas Emissions from Constructed Wetlands. Science of The Total Environment 2019, 656, 270–279. [Google Scholar] [CrossRef]
- Narayana Prasad, P.; Kalla, S. Plant-Microbial Fuel Cells - A Bibliometric Analysis. Process Biochemistry 2021, 111, 250–260. [Google Scholar] [CrossRef]
- Kuleshova, T.; Rao, A.; Bhadra, S.; Garlapati, V.K.; Sharma, S.; Kaushik, A.; Goswami, P.; Sreekirshnan, T.R.; Sevda, S. Plant Microbial Fuel Cells as an Innovative, Versatile Agro-Technology for Green Energy Generation Combined with Wastewater Treatment and Food Production. Biomass and Bioenergy 2022, 167, 106629. [Google Scholar] [CrossRef]
- Meshalkin, V.P.; Chetyrbotskii, V.A.; Chetyrbotskii, A.N.; Pelii, A.F. Computer Modeling of Chemical-Microbiological Processes in Rhizomicrobiophytospheric System. Dokl Phys Chem 2020, 495, 171–175. [Google Scholar] [CrossRef]
- Escolà Casas, M.; Matamoros, V. Linking Plant-Root Exudate Changes to Micropollutant Exposure in Aquatic Plants (Lemna Minor and Salvinia Natans). A Prospective Metabolomic Study. Chemosphere 2022, 287, 132056. [Google Scholar] [CrossRef] [PubMed]
- Kabutey, F.T.; Zhao, Q.; Wei, L.; Ding, J.; Antwi, P.; Quashie, F.K.; Wang, W. An Overview of Plant Microbial Fuel Cells (PMFCs): Configurations and Applications. Renewable and Sustainable Energy Reviews 2019, 110, 402–414. [Google Scholar] [CrossRef]
- Khilari, S.; Pandit, S.; Das, D.; Pradhan, D. Manganese Cobaltite/Polypyrrole Nanocomposite-Based Air-Cathode for Sustainable Power Generation in the Single-Chambered Microbial Fuel Cells. Biosensors and Bioelectronics 2014, 54, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Timmusk, S.; Behers, L.; Muthoni, J.; Muraya, A.; Aronsson, A.-C. Perspectives and Challenges of Microbial Application for Crop Improvement. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Raghavulu, S.V.; Babu, P.S.; Goud, R.K.; Subhash, G.V.; Srikanth, S.; Mohan, S.V. Bioaugmentation of an Electrochemically Active Strain to Enhance the Electron Discharge of Mixed Culture: Process Evaluation through Electro-Kinetic Analysis. RSC Adv. 2012, 2, 677–688. [Google Scholar] [CrossRef]
- El-Naggar, M.Y.; Wanger, G.; Leung, K.M.; Yuzvinsky, T.D.; Southam, G.; Yang, J.; Lau, W.M.; Nealson, K.H.; Gorby, Y.A. Electrical Transport along Bacterial Nanowires from Shewanella Oneidensis MR-1. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 18127–18131. [Google Scholar] [CrossRef]
- Schröder, U. Anodic Electron Transfer Mechanisms in Microbial Fuel Cells and Their Energy Efficiency. Phys. Chem. Chem. Phys. 2007, 9, 2619–2629. [Google Scholar] [CrossRef]
- Lovley, D.R. Microbial Fuel Cells: Novel Microbial Physiologies and Engineering Approaches. Current Opinion in Biotechnology 2006, 17, 327–332. [Google Scholar] [CrossRef]
- Reguera, G. Microbial Nanowires and Electroactive Biofilms. FEMS Microbiology Ecology 2018, 94. [Google Scholar] [CrossRef]
- Chong, G.W.; Karbelkar, A.A.; El-Naggar, M.Y. Nature’s Conductors: What Can Microbial Multi-Heme Cytochromes Teach Us about Electron Transport and Biological Energy Conversion? Current Opinion in Chemical Biology 2018, 47, 7–17. [Google Scholar] [CrossRef]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular Electron Transfer via Microbial Nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef]
- Reguera, G.; Nevin, K.P.; Nicoll, J.S.; Covalla, S.F.; Woodard, T.L.; Lovley, D.R. Biofilm and Nanowire Production Leads to Increased Current in Geobacter Sulfurreducens Fuel Cells. Appl Environ Microbiol 2006, 72, 7345–7348. [Google Scholar] [CrossRef]
- Liu, X.; Shi, L.; Gu, J.-D. Microbial Electrocatalysis: Redox Mediators Responsible for Extracellular Electron Transfer. Biotechnology Advances 2018, 36, 1815–1827. [Google Scholar] [CrossRef]
- Wu, S.; Xiao, Y.; Song, P.; Wang, C.; Yang, Z.; Slade, R.C.T.; Zhao, F. Riboflavin-Mediated Extracellular Electron Transfer Process Involving Pachysolen Tannophilus. Electrochimica Acta 2016, 210, 117–121. [Google Scholar] [CrossRef]
- Luo, J.; Li, X.; Zhang, J.; Feng, A.; Xia, M.; Zhou, M. Global Regulator Engineering Enhances Bioelectricity Generation in Pseudomonas Aeruginosa-Inoculated MFCs. Biosensors and Bioelectronics 2020, 163, 112269. [Google Scholar] [CrossRef] [PubMed]
- Ilamathi, R.; Merline Sheela, A.; Nagendra Gandhi, N. Comparative Evaluation of Pseudomonas Species in Single Chamber Microbial Fuel Cell with Manganese Coated Cathode for Reactive Azo Dye Removal. International Biodeterioration & Biodegradation 2019, 144, 104744. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, E.; Jiang, C.; Jia, R.; Liu, S.; Xu, D.; Gu, T.; Wang, F. Endogenous Phenazine-1-Carboxamide Encoding Gene PhzH Regulated the Extracellular Electron Transfer in Biocorrosion of Stainless Steel by Marine Pseudomonas Aeruginosa. Electrochemistry Communications 2018, 94, 9–13. [Google Scholar] [CrossRef]
- Zani, A.C.B.; Almeida, É.J.R.D.; Furlan, J.P.R.; Pedrino, M.; Guazzaroni, M.-E.; Stehling, E.G.; Andrade, A.R.D.; Reginatto, V. Electrobiochemical Skills of Pseudomonas Aeruginosa Species That Produce Pyocyanin or Pyoverdine for Glycerol Oxidation in a Microbial Fuel Cell. Chemosphere 2023, 335, 139073. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Zhang, X.; Pastorella, G.; Connolly, J.O.; Barry, N.; Woolley, R.; Krishnamurthy, S.; Marsili, E. Electron Transfer Mechanism in Shewanella Loihica PV-4 Biofilms Formed at Graphite Electrode. Bioelectrochemistry 2012, 87, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Long, X.; Okamoto, A. Enhancement of Microbial Current Production by Riboflavin Requires the Reduced Heme Centers in Outer Membrane Cytochromes in Shewanella Oneidensis MR-1. Electrochimica Acta 2023, 464, 142860. [Google Scholar] [CrossRef]
- De Schamphelaire, L.; Cabezas, A.; Marzorati, M.; Friedrich, M.W.; Boon, N.; Verstraete, W. Microbial Community Analysis of Anodes from Sediment Microbial Fuel Cells Powered by Rhizodeposits of Living Rice Plants. Appl Environ Microbiol 2010, 76, 2002–2008. [Google Scholar] [CrossRef] [PubMed]
- Nosek, D.; Cydzik-Kwiatkowska, A. Microbial Structure and Energy Generation in Microbial Fuel Cells Powered with Waste Anaerobic Digestate. Energies 2020, 13, 4712. [Google Scholar] [CrossRef]
- Lovley, D.R.; Ueki, T.; Zhang, T.; Malvankar, N.S.; Shrestha, P.M.; Flanagan, K.A.; Aklujkar, M.; Butler, J.E.; Giloteaux, L.; Rotaru, A.-E.; et al. Geobacter. In Advances in Microbial Physiology; Elsevier, 2011; Volume 59, pp. 1–100. ISBN 978-0-12-387661-4. [Google Scholar]
- Coates, J.D.; Ellis, D.J.; Gaw, C.V.; Lovley, D.R. Geothrix Fermentans Gen. Nov., Sp. Nov., a Novel Fe(III)-Reducing Bacterium from a Hydrocarbon-Contaminated Aquifer. International Journal of Systematic and Evolutionary Microbiology 1999, 49, 1615–1622. [Google Scholar] [CrossRef]
- Mehta-Kolte, M.G.; Bond, D.R. Geothrix Fermentans Secretes Two Different Redox-Active Compounds To Utilize Electron Acceptors across a Wide Range of Redox Potentials. Appl Environ Microbiol 2012, 78, 6987–6995. [Google Scholar] [CrossRef]
- Finneran, K.T. Rhodoferax Ferrireducens Sp. Nov., a Psychrotolerant, Facultatively Anaerobic Bacterium That Oxidizes Acetate with the Reduction of Fe(III). INTERNATIONAL JOURNAL OF SYSTEMATIC AND EVOLUTIONARY MICROBIOLOGY 2003, 53, 669–673. [Google Scholar] [CrossRef]
- von Canstein, H.; Ogawa, J.; Shimizu, S.; Lloyd, J.R. Secretion of Flavins by Shewanella Species and Their Role in Extracellular Electron Transfer. Appl Environ Microbiol 2008, 74, 615–623. [Google Scholar] [CrossRef]
- Wu, D.; Xing, D.; Mei, X.; Liu, B.; Guo, C.; Ren, N. Electricity Generation by Shewanella Sp. HN-41 in Microbial Fuel Cells. International Journal of Hydrogen Energy 2013, 38, 15568–15573. [Google Scholar] [CrossRef]
- Niessen, J.; Schroder, U.; Scholz, F. Exploiting Complex Carbohydrates for Microbial Electricity Generation ? A Bacterial Fuel Cell Operating on Starch. Electrochemistry Communications 2004, 6, 955–958. [Google Scholar] [CrossRef]
- Das, S.; Calay, R.K. Experimental Study of Power Generation and COD Removal Efficiency by Air Cathode Microbial Fuel Cell Using Shewanella Baltica 20. Energies 2022, 15, 4152. [Google Scholar] [CrossRef]
- Liu, S.; Feng, Y.; Li, H. RETRACTED: Effect of Geobacter metallireducens Nanowire on Electron Transfer Efficiency in Microbial Fuel Cell. J of Chemical Tech & Biotech 2023, 98, 473–482. [Google Scholar] [CrossRef]
- Liu, Z.D.; Du, Z.W.; Lian, J.; Zhu, X.Y.; Li, S.H.; Li, H.R. Improving Energy Accumulation of Microbial Fuel Cells by Metabolism Regulation Using Rhodoferax Ferrireducens as Biocatalyst. Lett Appl Microbiol 2007, 44, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, M.; Shanthi Sravan, J.; Venkata Mohan, S. Self-Induced Bioelectro-Potential Influence on Sulfate Removal and Desalination in Microbial Fuel Cell. Bioresource Technology 2020, 309, 123326. [Google Scholar] [CrossRef]
- Sun, M.; Tong, Z.-H.; Sheng, G.-P.; Chen, Y.-Z.; Zhang, F.; Mu, Z.-X.; Wang, H.-L.; Zeng, R.J.; Liu, X.-W.; Yu, H.-Q.; et al. Microbial Communities Involved in Electricity Generation from Sulfide Oxidation in a Microbial Fuel Cell. Biosensors and Bioelectronics 2010, 26, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Miran, W.; Jang, J.; Nawaz, M.; Shahzad, A.; Jeong, S.E.; Jeon, C.O.; Lee, D.S. Mixed Sulfate-Reducing Bacteria-Enriched Microbial Fuel Cells for the Treatment of Wastewater Containing Copper. Chemosphere 2017, 189, 134–142. [Google Scholar] [CrossRef]
- Treesubsuntorn, C.; Chaiworn, W.; Surareungchai, W.; Thiravetyan, P. Increasing of Electricity Production from Echinodosus Cordifolius-Microbial Fuel Cell by Inoculating Bacillus Thuringiensis. Science of The Total Environment 2019, 686, 538–545. [Google Scholar] [CrossRef]
- Anokhina, T.O.; Siunova, T.B.; Sizova, O.I.; Zakharchenko, N.S.; Kochetkov, V.V. Rhizospheric Bacteria of the Genus Pseudomonas in Modern Agrobiotechnology. Agriculture Chemistry 2018, 10, 54–66, [In Russian]. [Google Scholar]
- Icgen, B.; Salik, S.B.; Goksu, L.; Ulusoy, H.; Yilmaz, F. Higher Alkyl Sulfatase Activity Required by Microbial Inhabitants to Remove Anionic Surfactants in the Contaminated Surface Waters. Water Science and Technology 2017, 76, 2357–2366. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Kumar, A. Diversity of Culturable Sodium Dodecyl Sulfate (SDS) Degrading Bacteria Isolated from Detergent Contaminated Ponds Situated in Varanasi City, India. International Biodeterioration & Biodegradation 2011, 65, 961–971. [Google Scholar] [CrossRef]
- Arulmani, S.R.B.; Gnanamuthu, H.L.; Kandasamy, S.; Govindarajan, G.; Alsehli, M.; Elfasakhany, A.; Pugazhendhi, A.; Zhang, H. Sustainable Bioelectricity Production from Amaranthus Viridis and Triticum Aestivum Mediated Plant Microbial Fuel Cells with Efficient Electrogenic Bacteria Selections. Process Biochemistry 2021, 107, 27–37. [Google Scholar] [CrossRef]
- Raio, A.; Reveglia, P.; Puopolo, G.; Cimmino, A.; Danti, R.; Evidente, A. Involvement of Phenazine-1-Carboxylic Acid in the Interaction between Pseudomonas Chlororaphis Subsp. Aureofaciens Strain M71 and Seiridium Cardinale in Vivo. Microbiological Research 2017, 199, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Pan, H.; Boak, E.N.; Pierson, L.S.; Pierson, E.A. Phenazine-Producing Rhizobacteria Promote Plant Growth and Reduce Redox and Osmotic Stress in Wheat Seedlings Under Saline Conditions. Front. Plant Sci. 2020, 11, 575314. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Boon, N.; Höfte, M.; Verstraete, W. Microbial Phenazine Production Enhances Electron Transfer in Biofuel Cells. Environ. Sci. Technol. 2005, 39, 3401–3408. [Google Scholar] [CrossRef] [PubMed]
- Timmers, R.A.; Rothballer, M.; Strik, D.P.B.T.B.; Engel, M.; Schulz, S.; Schloter, M.; Hartmann, A.; Hamelers, B.; Buisman, C. Microbial Community Structure Elucidates Performance of Glyceria Maxima Plant Microbial Fuel Cell. Appl Microbiol Biotechnol 2012, 94, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Chiranjeevi, P.; Yeruva, D.K.; Kumar, A.K.; Mohan, S.V.; Varjani, S. Plant-Microbial Fuel Cell Technology. In Microbial Electrochemical Technology; Elsevier, 2019; pp. 549–564. ISBN 978-0-444-64052-9. [Google Scholar]
- Guo, K.; Donose, B.C.; Soeriyadi, A.H.; Prévoteau, A.; Patil, S.A.; Freguia, S.; Gooding, J.J.; Rabaey, K. Flame Oxidation of Stainless Steel Felt Enhances Anodic Biofilm Formation and Current Output in Bioelectrochemical Systems. Environ. Sci. Technol. 2014, 48, 7151–7156. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Ruiz, D.; Castillo Atoche, A.; Ruiz-Ibarra, E.; Osorio De La Rosa, E.; Vázquez Castillo, J. A Self-Powered PMFC-Based Wireless Sensor Node for Smart City Applications. Wireless Communications and Mobile Computing 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Azri, Y.M.; Tou, I.; Sadi, M. Electrodes Materials Evaluation in Plant Microbial Fuel Cells: A Comparison of Graphite and Stainless Steels. Biofuels 2023, 1–10. [Google Scholar] [CrossRef]
- Oodally, A.; Gulamhussein, M.; Randall, D.G. Investigating the Performance of Constructed Wetland Microbial Fuel Cells Using Three Indigenous South African Wetland Plants. Journal of Water Process Engineering 2019, 32, 100930. [Google Scholar] [CrossRef]
- Pamintuan, K.R.S.; Sanchez, K.M. Power Generation in a Plant-Microbial Fuel Cell Assembly with Graphite and Stainless Steel Electrodes Growing Vigna Radiata. IOP Conf. Ser.: Mater. Sci. Eng. 2019, 703, 012037. [Google Scholar] [CrossRef]
- Lin, F.-Y.; Lin, Y.-Y.; Li, H.-T.; Ni, C.-S.; Liu, C.-I.; Guan, C.-Y.; Chang, C.-C.; Yu, C.-P.; Chen, W.-S.; Liu, T.-Y.; et al. Trapa Natans Husk-Derived Carbon as a Sustainable Electrode Material for Plant Microbial Fuel Cells. Applied Energy 2022, 325, 119807. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Pan, Z.-B.; Chen, C.-R.; Wei, M.-X.; Chen, C.-A.; Lin, H.-P.; Hsu, C.-H. Synthesis of Multiporous Carbons from the Water Caltrop Shell for High-Performance Supercapacitors. ACS Omega 2020, 5, 10626–10632. [Google Scholar] [CrossRef]
- Huang, X.; Duan, C.; Duan, W.; Sun, F.; Cui, H.; Zhang, S.; Chen, X. Role of Electrode Materials on Performance and Microbial Characteristics in the Constructed Wetland Coupled Microbial Fuel Cell (CW-MFC): A Review. Journal of Cleaner Production 2021, 301, 126951. [Google Scholar] [CrossRef]
- Apollon, W.; Luna-Maldonado, A.I.; Kamaraj, S.-K.; Vidales-Contreras, J.A.; Rodríguez-Fuentes, H.; Gómez-Leyva, J.F.; Aranda-Ruíz, J. Progress and Recent Trends in Photosynthetic Assisted Microbial Fuel Cells: A Review. Biomass and Bioenergy 2021, 148, 106028. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, P.; Jiang, Y.; Huang, X. Enhanced Power Generation of Microbial Fuel Cell Using Manganese Dioxide-Coated Anode in Flow-through Mode. Journal of Power Sources 2015, 273, 580–583. [Google Scholar] [CrossRef]
- Lv, F.; Zhao, X.; Pan, S.; Cao, W.; Zuo, X.; Li, Y. Electrodeposition of FeOOH Nanosheets on Carbon Felt for Enhanced Sulfamerazine Removal via Visible Light-Assisted Electro-Fenton Process. Journal of Water Process Engineering 2022, 48, 102883. [Google Scholar] [CrossRef]
- Satar, I.; Daud, W.R.W.; Kim, B.H.; Somalu, M.R.; Ghasemi, M.; Bakar, M.H.A.; Jafary, T.; Timmiati, S.N. Performance of Titanium–Nickel (Ti/Ni) and Graphite Felt-Nickel (GF/Ni) Electrodeposited by Ni as Alternative Cathodes for Microbial Fuel Cells. Journal of the Taiwan Institute of Chemical Engineers 2018, 89, 67–76. [Google Scholar] [CrossRef]
- Türker, O.C.; Baran, T.; Yakar, A.; Türe, C.; Saz, Ç. Novel Chitosan Based Smart Cathode Electrocatalysts for High Power Generation in Plant Based-Sediment Microbial Fuel Cells. Carbohydrate Polymers 2020, 239, 116235. [Google Scholar] [CrossRef]
- Deng, H.; Wu, Y.-C.; Zhang, F.; Huang, Z.-C.; Chen, Z.; Xu, H.-J.; Zhao, F. Factors Affecting the Performance of Single-Chamber Soil Microbial Fuel Cells for Power Generation. Pedosphere 2014, 24, 330–338. [Google Scholar] [CrossRef]
- Srinophakun, P.; Thanapimmetha, A.; Plangsri, S.; Vetchayakunchai, S.; Saisriyoot, M. Application of Modified Chitosan Membrane for Microbial Fuel Cell: Roles of Proton Carrier Site and Positive Charge. Journal of Cleaner Production 2017, 142, 1274–1282. [Google Scholar] [CrossRef]
- Sabina-Delgado, A.; Kamaraj, S.K.; Hernández-Montoya, V.; Cervantes, F.J. Novel Carbon-Ceramic Composite Membranes with High Cation Exchange Properties for Use in Microbial Fuel Cell and Electricity Generation. International Journal of Hydrogen Energy 2023, 48, 25512–25526. [Google Scholar] [CrossRef]
- Fan, L.; Shi, J.; Gao, T. Comparative Study on the Effects of Three Membrane Modification Methods on the Performance of Microbial Fuel Cell. Energies 2020, 13, 1383. [Google Scholar] [CrossRef]
- Surti, P.; Kailasa, S.K.; Mungray, A.; Park, T.J.; Mungray, A.K. Vermiculite Nanosheet Augmented Novel Proton Exchange Membrane for Microbial Fuel Cell. Fuel 2024, 357, 130046. [Google Scholar] [CrossRef]
- Sarma, P.J.; Mohanty, K. Development and Comprehensive Characterization of Low-Cost Hybrid Clay Based Ceramic Membrane for Power Enhancement in Plant Based Microbial Fuel Cells (PMFCs). Materials Chemistry and Physics 2023, 296, 127337. [Google Scholar] [CrossRef]
- Saran, C.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Romanholo Ferreira, L.F.; Bilal, M.; Iqbal, H.M.N.; Hussain, C.M.; Mulla, S.I.; Bharagava, R.N. Microbial Fuel Cell: A Green Eco-Friendly Agent for Tannery Wastewater Treatment and Simultaneous Bioelectricity/Power Generation. Chemosphere 2023, 312, 137072. [Google Scholar] [CrossRef] [PubMed]
- Behera, M.; Ghangrekar, M.M. Performance of Microbial Fuel Cell in Response to Change in Sludge Loading Rate at Different Anodic Feed pH. Bioresource Technology 2009, 100, 5114–5121. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.B.; Simões, M.; Melo, L.F.; Pinto, A.M.F.R. Overview on the Developments of Microbial Fuel Cells. Biochemical Engineering Journal 2013, 73, 53–64. [Google Scholar] [CrossRef]
- Kuleshova, Т.E. , Galushko, A.S., Panova G.G., Volkova, E.N., Appolon, W., Shuang, Ch., Sevda, S. Bioelectrochemical systems based on the electroactivity of plants and microorganisms in the root environment (review). S-h. biol. 2022, 57, 425–440. [Google Scholar] [CrossRef]
- Borker, M.; Suchithra, T.V. Rice Paddy as a Source of Sustainable Energy in India. In Proceedings of the 7th International Conference on Advances in Energy Research; Bose, M., Modi, A., Eds.; Springer Proceedings in Energy; Springer Singapore: Singapore, 2021; pp. 383–392. [Google Scholar]
- Letcher, T.M. Introduction With a Focus on Atmospheric Carbon Dioxide and Climate Change. In Future Energy; Elsevier, 2020; pp. 3–17. ISBN 978-0-08-102886-5. [Google Scholar]
- Rae, J.W.B.; Zhang, Y.G.; Liu, X.; Foster, G.L.; Stoll, H.M.; Whiteford, R.D.M. Atmospheric CO2 over the Past 66 Million Years from Marine Archives. Annu. Rev. Earth Planet. Sci. 2021, 49, 609–641. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Ju, W.; Chen, J.M.; Ciais, P.; Cescatti, A.; Sardans, J.; Janssens, I.A.; Wu, M.; Berry, J.A.; et al. Recent Global Decline of CO2 Fertilization Effects on Vegetation Photosynthesis. Science 2020, 370, 1295–1300. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.-P.; Zeng, Y.; Li, D.-M.; Guo, D.-J.; Rajput, V.D.; Chen, G.-L.; Barakhov, A.; Minkina, T.M.; Li, Y.-R. Characteristics of Leaf Stomata and Their Relationship with Photosynthesis in Saccharum Officinarum Under Drought and Silicon Application. ACS Omega 2020, 5, 24145–24153. [Google Scholar] [CrossRef]
- Gowik, U.; Westhoff, P. The Path from C3 to C4 Photosynthesis. Plant Physiology 2011, 155, 56–63. [Google Scholar] [CrossRef]
- Rusyn, I.; Hamkalo, К. Electro-Biosystems with Mosses on the Green Roofs. EREM 2020, 76, 20–31. [Google Scholar] [CrossRef]
- Akavitaya, A., Gud, S. Biological Oxygen Demand Is One of the Most Important Pollution Characteristics of Natural and Domestic Waters. In Sakharov Readings 2018: Environmental Issues of the 21st Century 2018, 72-74 [In Russian].
- Borisova, E.A.; Krasnoperova, S.A. The Development of the Recultivation’s Method of the Ponds Sludge Collectors. Oil Province [In Russian].. 2019, 1, 238–250. [Google Scholar] [CrossRef]
- Kumar, A.; Samadder, S.R. A Review on Technological Options of Waste to Energy for Effective Management of Municipal Solid Waste. Waste Management 2017, 69, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Michael-Igolima, U.; Abbey, S.J.; Ifelebuegu, A.O. A Systematic Review on the Effectiveness of Remediation Methods for Oil Contaminated Soils. Environmental Advances 2022, 9, 100319. [Google Scholar] [CrossRef]
- Molognoni, D.; Chiarolla, S.; Cecconet, D.; Callegari, A.; Capodaglio, A.G. Industrial Wastewater Treatment with a Bioelectrochemical Process: Assessment of Depuration Efficiency and Energy Production. Water Science and Technology 2018, 77, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, R.; Stefanova, A.; Angelov, A. Treatment of Water Contamined by Petroleum Products through Constructed Wetlands with Integrated Plant Sediment Microbial Fuel Cells //Journal of Mining and Geological Sciences. – 2019. – Т. 62. – №. 2.
- Zhao, L.; Deng, J.; Hou, H.; Li, J.; Yang, Y. Investigation of PAH and Oil Degradation along with Electricity Generation in Soil Using an Enhanced Plant-Microbial Fuel Cell. Journal of Cleaner Production 2019, 221, 678–683. [Google Scholar] [CrossRef]
- Apollon, W.; Vidales-Contreras, J.; Rodríguez-Fuentes, H.; Gómez-Leyva, J.; Olivares-Sáenz, E.; Maldonado-Ruelas, V.; Ortiz-Medina, R.; Kamaraj, S.-K.; Luna-Maldonado, A. Livestock’s Urine-Based Plant Microbial Fuel Cells Improve Plant Growth and Power Generation. Energies 2022, 15, 6985. [Google Scholar] [CrossRef]
- Moqsud, M.A.; Yoshitake, J.; Bushra, Q.S.; Hyodo, M.; Omine, K.; Strik, D. Compost in Plant Microbial Fuel Cell for Bioelectricity Generation. Waste Management 2015, 36, 63–69. [Google Scholar] [CrossRef]
- Patel, D.; Bapodra, S.L.; Madamwar, D.; Desai, C. Electroactive Bacterial Community Augmentation Enhances the Performance of a Pilot Scale Constructed Wetland Microbial Fuel Cell for Treatment of Textile Dye Wastewater. Bioresource Technology 2021, 332, 125088. [Google Scholar] [CrossRef] [PubMed]
- Colares, G.S.; Dell’Osbel, N.; Barbosa, C.V.; Lutterbeck, C.; Oliveira, G.A.; Rodrigues, L.R.; Bergmann, C.P.; Lopez, D.R.; Rodriguez, A.L.; Vymazal, J.; et al. Floating Treatment Wetlands Integrated with Microbial Fuel Cell for the Treatment of Urban Wastewaters and Bioenergy Generation. Science of The Total Environment 2021, 766, 142474. [Google Scholar] [CrossRef]
- Sharma, A.; Gajbhiye, S.; Chauhan, S.; Chhabra, M. Effect of Cathodic Culture on Wastewater Treatment and Power Generation in a Photosynthetic Sediment Microbial Fuel Cell (SMFC): Canna Indica v/s Chlorella Vulgaris. Bioresource Technology 2021, 340, 125645. [Google Scholar] [CrossRef] [PubMed]
- González, T.; Puigagut, J.; Vidal, G. Organic Matter Removal and Nitrogen Transformation by a Constructed Wetland-Microbial Fuel Cell System with Simultaneous Bioelectricity Generation. Science of The Total Environment 2021, 753, 142075. [Google Scholar] [CrossRef]
- Wen, H.; Zhu, H.; Yan, B.; Xu, Y.; Shutes, B. Treatment of Typical Antibiotics in Constructed Wetlands Integrated with Microbial Fuel Cells: Roles of Plant and Circuit Operation Mode. Chemosphere 2020, 250, 126252. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, X.; Wang, M.; Zhang, C.; Li, J.; Xue, M.; Xia, W.; Xie, H. Degradation and Transformation of Linear Alkyl-Benzene Sulfonates (LAS) in Integrated Constructed Wetland–Microbial Fuel Cell Systems. Chemosphere 2023, 321, 138135. [Google Scholar] [CrossRef] [PubMed]
- Kaczorek, E.; Pacholak, A.; Zdarta, A.; Smułek, W. The Impact of Biosurfactants on Microbial Cell Properties Leading to Hydrocarbon Bioavailability Increase. Colloids and Interfaces 2018, 2, 35. [Google Scholar] [CrossRef]
- Rathour, R.; Patel, D.; Shaikh, S.; Desai, C. Eco-Electrogenic Treatment of Dyestuff Wastewater Using Constructed Wetland-Microbial Fuel Cell System with an Evaluation of Electrode-Enriched Microbial Community Structures. Bioresource Technology 2019, 285, 121349. [Google Scholar] [CrossRef]
- Kong, Y.; Li, W.; Wang, Z.; Yao, C.; Tao, Y. Electrosorption Behavior of Copper Ions with Poly(m-Phenylenediamine) Paper Electrode. Electrochemistry Communications 2013, 26, 59–62. [Google Scholar] [CrossRef]
- Timmers, R.A.; Strik, D.P.B.T.B.; Hamelers, H.V.M.; Buisman, C.J.N. Characterization of the Internal Resistance of a Plant Microbial Fuel Cell. Electrochimica Acta 2012, 72, 165–171. [Google Scholar] [CrossRef]
- Anwar, J.; Shafique, U.; Salman, M.; Waheed-uz-Zaman, *!!! REPLACE !!!*; Anwar, S.; Anzano, J.M. Removal of Chromium (III) by Using Coal as Adsorbent. Journal of Hazardous Materials 2009, 171, 797–801. [Google Scholar] [CrossRef]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of Heavy Metals: A Promising Tool for Clean-Up of Polluted Environment? Front. Plant Sci. 2018, 9, 1476. [Google Scholar] [CrossRef]
- Shah, V.; Daverey, A. Phytoremediation: A Multidisciplinary Approach to Clean up Heavy Metal Contaminated Soil. Environmental Technology & Innovation 2020, 18, 100774. [Google Scholar] [CrossRef]
- Sarma, P.J.; Mohanty, K. An Insight into Plant Microbial Fuel Cells. In Bioelectrochemical Interface Engineering; Krishnaraj, R.N., Sani, R.K., Eds.; Wiley, 2019; pp. 137–148. ISBN 978-1-119-53854-7. [Google Scholar]
- Habibul, N.; Hu, Y.; Wang, Y.-K.; Chen, W.; Yu, H.-Q.; Sheng, G.-P. Bioelectrochemical Chromium(VI) Removal in Plant-Microbial Fuel Cells. Environ. Sci. Technol. 2016, 50, 3882–3889. [Google Scholar] [CrossRef] [PubMed]
- Gustave, W.; Yuan, Z.-F.; Ren, Y.-X.; Sekar, R.; Zhang, J.; Chen, Z. Arsenic Alleviation in Rice by Using Paddy Soil Microbial Fuel Cells. Plant Soil 2019, 441, 111–127. [Google Scholar] [CrossRef]
- Tongphanpharn, N.; Chou, C.-H.; Guan, C.-Y.; Yu, C.-P. Plant Microbial Fuel Cells with Oryza Rufipogon and Typha Orientalis for Remediation of Cadmium Contaminated Soil. Environmental Technology & Innovation 2021, 24, 102030. [Google Scholar] [CrossRef]
- Pamintuan, K.R.S.; Gonzales, A.J.S.; Estefanio, B.M.M.; Bartolo, B.L.S. Simultaneous Phytoremediation of Ni 2+ and Bioelectricity Generation in a Plant-Microbial Fuel Cell Assembly Using Water Hyacinth ( Eichhornia Crassipes ). IOP Conf. Ser.: Earth Environ. Sci. 2018, 191, 012093. [Google Scholar] [CrossRef]
- Gustave, W.; Yuan, Z.-F.; Li, X.; Ren, Y.-X.; Feng, W.-J.; Shen, H.; Chen, Z. Mitigation Effects of the Microbial Fuel Cells on Heavy Metal Accumulation in Rice (Oryza Sativa L.). Environmental Pollution 2020, 260, 113989. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, T.; Zhu, N.; Feng, C.; Zhou, S.; Dahlgren, R.A. Bioelectricity Generation by Wetland Plant-Sediment Microbial Fuel Cells (P-SMFC) and Effects on the Transformation and Mobility of Arsenic and Heavy Metals in Sediment. Environ Geochem Health 2019, 41, 2157–2168. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Chen, T. Cost–Benefit Calculation of Phytoremediation Technology for Heavy-Metal-Contaminated Soil. Science of The Total Environment 2016, 563–564, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of Synthetic Surfactants on the Environment and the Potential for Substitution by Biosurfactants. Advances in Colloid and Interface Science 2021, 288, 102340. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.; Bhattacharyya, A. Quest for an Eco-Friendly Alternative Surfactant: Surface and Foam Characteristics of Natural Surfactants. Journal of Cleaner Production 2017, 150, 127–134. [Google Scholar] [CrossRef]
- Jangkorn, S.; Kuhakaew, S.; Theantanoo, S.; Klinla-or, H.; Sriwiriyarat, T. Evaluation of Reusing Alum Sludge for the Coagulation of Industrial Wastewater Containing Mixed Anionic Surfactants. Journal of Environmental Sciences 2011, 23, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Iribarnegaray, M.A.; Rodriguez-Alvarez, M.S.; Moraña, L.B.; Tejerina, W.A.; Seghezzo, L. Management Challenges for a More Decentralized Treatment and Reuse of Domestic Wastewater in Metropolitan Areas. Journal of Water, Sanitation and Hygiene for Development 2018, 8, 113–122. [Google Scholar] [CrossRef]
- Massoud, M.A.; Tarhini, A.; Nasr, J.A. Decentralized Approaches to Wastewater Treatment and Management: Applicability in Developing Countries. Journal of Environmental Management 2009, 90, 652–659. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Ademollo, N.; Cardoni, M.; Di Giulio, A.; Grenni, P.; Pescatore, T.; Rauseo, J.; Patrolecco, L. Assessment of Biodegradation of the Anionic Surfactant Sodium Lauryl Ether Sulphate Used in Two Foaming Agents for Mechanized Tunnelling Excavation. Journal of Hazardous Materials 2019, 365, 538–545. [Google Scholar] [CrossRef]
- Palmer, M.; Hatley, H. The Role of Surfactants in Wastewater Treatment: Impact, Removal and Future Techniques: A Critical Review. Water Research 2018, 147, 60–72. [Google Scholar] [CrossRef]
- Di̇ri̇lgen, N.; İnce, N. Inhibition Effect of the Anionic Surfactant SDS on Duckweed, LEMNA Minor with Considerations of Growth and Accumulation. Chemosphere 1995, 31, 4185–4196. [Google Scholar] [CrossRef]
- Tkalin, A.V. Background Pollution Characteristics of the N.E. Sakhalin Island Shelf. Marine Pollution Bulletin 1993, 26, 704–705. [Google Scholar] [CrossRef]
- Singer, M.M.; George, S.; Jacobson, S.; Lee, I.; Tjeerdema, R.S.; Sowby, M.L. Comparative Effects of Oil Dispersants to the Early Life Stages of Topsmelt ( Atherinops Affinis ) and Kelp ( Macrocystis Pyrifera ). Environ Toxicol Chem 1994, 13, 649–655. [Google Scholar] [CrossRef]
- Pavlić, Ž.; Vidaković-Cifrek, Ž.; Puntarić, D. Toxicity of Surfactants to Green Microalgae Pseudokirchneriella Subcapitata and Scenedesmus Subspicatus and to Marine Diatoms Phaeodactylum Tricornutum and Skeletonema Costatum. Chemosphere 2005, 61, 1061–1068. [Google Scholar] [CrossRef]
- Baderna, D.; Lomazzi, E.; Passoni, A.; Pogliaghi, A.; Petoumenou, M.I.; Bagnati, R.; Lodi, M.; Viarengo, A.; Sforzini, S.; Benfenati, E.; et al. Chemical Characterization and Ecotoxicity of Three Soil Foaming Agents Used in Mechanized Tunneling. Journal of Hazardous Materials 2015, 296, 210–220. [Google Scholar] [CrossRef]
- Mustapha, D.S.; Bawa-Allah, K.A. Differential Toxicities of Anionic and Nonionic Surfactants in Fish. Environ Sci Pollut Res 2020, 27, 16754–16762. [Google Scholar] [CrossRef]
- Ogeleka, D.F.; Ezemonye, L.I.; Okieimen, F.E. The Toxicity of a Synthetic Industrial Detergent and a Corrosion Inhibitor to Brackish Water Fish (Tilapia Guineensis). Turkish Journal of Biology 2011. [Google Scholar] [CrossRef]
- Kierkegaard, A.; Chen, C.; Armitage, J.M.; Arnot, J.A.; Droge, S.; McLachlan, M.S. Tissue Distribution of Several Series of Cationic Surfactants in Rainbow Trout ( Oncorhynchus Mykiss ) Following Exposure via Water. Environ. Sci. Technol. 2020, 54, 4190–4199. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the Biological and Chemical Treatment Technologies for Emerging Contaminant Removal from Wastewater: A Critical Review. Journal of Hazardous Materials 2017, 323, 274–298. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.V.; Gogate, P.R.; Bhat, A.P.; Ghosh, P.K. Treatment of Laundry Wastewater Containing Residual Surfactants Using Combined Approaches Based on Ozone, Catalyst and Cavitation. Separation and Purification Technology 2020, 239, 116594. [Google Scholar] [CrossRef]
- Hashim, M.A.; Kulandai, J.; Hassan, R.S. Biodegradability of Branched Alkylbenzene Sulphonates. J. Chem. Technol. Biotechnol. 2007, 54, 207–214. [Google Scholar] [CrossRef]
- Khleifat, K.M. Biodegradation of Linear Alkylbenzene Sulfonate by a Two-Member Facultative Anaerobic Bacterial Consortium. Enzyme and Microbial Technology 2006, 39, 1030–1035. [Google Scholar] [CrossRef]
- Corada-Fernández, C.; González-Mazo, E.; Lara-Martín, P.A. Evaluation of the Anaerobic Biodegradation of Linear Alkylbenzene Sulfonates (LAS) Using OECD 308 Water/Sediment Systems. Journal of Hazardous Materials 2018, 360, 24–31. [Google Scholar] [CrossRef]
- Scott, M.J.; Jones, M.N. The Biodegradation of Surfactants in the Environment. Biochimica et Biophysica Acta (BBA) - Biomembranes 2000, 1508, 235–251. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, L.; Song, R.; Ma, A. Research Progress of Surfactant Biodegradation. IOP Conf. Ser.: Earth Environ. Sci. 2019, 227, 052023. [Google Scholar] [CrossRef]
- Merrettig-Bruns, U.; Jelen, E. Anaerobic Biodegradation of Detergent Surfactants. Materials 2009, 2, 181–206. [Google Scholar] [CrossRef]
- Løbner, T.; Toräng, L.; Batstone, D.J.; Schmidt, J.E.; Angelidaki, I. Effects of Process Stability on Anaerobic Biodegradation of LAS in UASB Reactors: Process Stability on Anaerobic Biodegradation of LAS in UASB Reactors. Biotechnol. Bioeng. 2005, 89, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, A.S.; Ahring, B.K. Formation of Metabolites during Biodegradation of Linear Alkylbenzene Sulfonate in an Upflow Anaerobic Sludge Bed Reactor under Thermophilic Conditions. Biotechnol. Bioeng. 2002, 77, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Lara-Martín, P.A.; Gómez-Parra, A.; Köchling, T.; Sanz, J.L.; Amils, R.; González-Mazo, E. Anaerobic Degradation of Linear Alkylbenzene Sulfonates in Coastal Marine Sediments. Environ. Sci. Technol. 2007, 41, 3573–3579. [Google Scholar] [CrossRef] [PubMed]
- Feitkenhauer, H.; Meyer, U. Anaerobic Digestion of Alcohol Sulfate (Anionic Surfactant) Rich Wastewater – Batch Experiments. Part I: Influence of the Surfactant Concentration. Bioresource Technology 2002, 82, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Dhouib, A.; Hamad, N.; Hassaı̈ri, I.; Sayadi, S. Degradation of Anionic Surfactants by Citrobacter Braakii. Process Biochemistry 2003, 38, 1245–1250. [Google Scholar] [CrossRef]
- Ambily, P.S.; Jisha, M.S. Biodegradation of Anionic Surfactant, Sodium Dodecyl Sulphate by Pseudomonas Aeruginosa MTCC 1031. Journal of Environmental Biology 2012, 33, 717–20. [Google Scholar] [PubMed]
- Chen, H.-J.; Tseng, D.-H.; Huang, S.-L. Biodegradation of Octylphenol Polyethoxylate Surfactant Triton X-100 by Selected Microorganisms. Bioresource Technology 2005, 96, 1483–1491. [Google Scholar] [CrossRef]
- Chakraborty, I.; Bhowmick, G.D.; Nath, D.; Khuman, C.N.; Dubey, B.K.; Ghangrekar, M.M. Removal of Sodium Dodecyl Sulphate from Wastewater and Its Effect on Anodic Biofilm and Performance of Microbial Fuel Cell. International Biodeterioration & Biodegradation 2021, 156, 105108. [Google Scholar] [CrossRef]
- Sathe, S.M.; Bhowmick, G.D.; Dubey, B.K.; Ghangrekar, M.M. Surfactant Removal from Wastewater Using Photo-Cathode Microbial Fuel Cell and Laterite-Based Hybrid Treatment System. Bioprocess Biosyst Eng 2020, 43, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Askari, A.; Vahabzadeh, F.; Mardanpour, M.M. Quantitative Determination of Linear Alkylbenzene Sulfonate (LAS) Concentration and Simultaneous Power Generation in a Microbial Fuel Cell-Based Biosensor. Journal of Cleaner Production 2021, 294, 126349. [Google Scholar] [CrossRef]
- Askari, A.; Vahabzadeh, F.; Mardanpour, M.M. The Identification and Performance Assessment of Dominant Bacterial Species during Linear Alkylbenzene Sulfonate (LAS)-Biodegradation in a Bioelectrochemical System. Bioprocess Biosyst Eng 2021, 44, 2579–2590. [Google Scholar] [CrossRef] [PubMed]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Titanium Dioxide Photocatalysis for Pharmaceutical Wastewater Treatment. Environ Chem Lett 2014, 12, 27–47. [Google Scholar] [CrossRef]
- Vymazal, J. Constructed Wetlands for Wastewater Treatment: Five Decades of Experience. Environ. Sci. Technol. 2011, 45, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Cold-Climate Constructed Wetlands. Water Science and Technology 1995, 32. [CrossRef]
- Huang, X.; Ye, G.; Yi, N.; Lu, L.; Zhang, L.; Yang, L.; Xiao, L.; Liu, J. Effect of Plant Physiological Characteristics on the Removal of Conventional and Emerging Pollutants from Aquaculture Wastewater by Constructed Wetlands. Ecological Engineering 2019, 135, 45–53. [Google Scholar] [CrossRef]
- Marangon, B.B.; Silva, T.A.; Calijuri, M.L.; Alves, S. do C.; dos Santos, V.J.; Oliveira, A.P. de S. Reuse of Treated Municipal Wastewater in Productive Activities in Brazil’s Semi-Arid Regions. Journal of Water Process Engineering 2020, 37, 101483. [Google Scholar] [CrossRef]
- Teixeira, D.L.; Souza, A.; Moura, G. de S.; Leite Júnior, M.C.R. Reuse of Aquaculture Wastewater Treated in Constructed Wetlands. Reveng 2021, 29, 347–354. [Google Scholar] [CrossRef]
- Nacar, T.; Uçar, D.; Sapci̇ Ayas, Z. Removal of Detergents in Car Wash Wastewater by Sub-Surface Flow Constructed Wetland. Bitlis Eren Üniversitesi Fen Bilimleri Dergisi 2022, 11, 820–827. [Google Scholar] [CrossRef]
- Ramprasad, C.; Philip, L. Contributions of Various Processes to the Removal of Surfactants and Personal Care Products in Constructed Wetland. Chemical Engineering Journal 2018, 334, 322–333. [Google Scholar] [CrossRef]
- Yadav, A.K.; Dash, P.; Mohanty, A.; Abbassi, R.; Mishra, B.K. Performance Assessment of Innovative Constructed Wetland-Microbial Fuel Cell for Electricity Production and Dye Removal. Ecological Engineering 2012, 47, 126–131. [Google Scholar] [CrossRef]
- Doherty, L.; Zhao, Y.; Zhao, X.; Hu, Y.; Hao, X.; Xu, L.; Liu, R. A Review of a Recently Emerged Technology: Constructed Wetland – Microbial Fuel Cells. Water Research 2015, 85, 38–45. [Google Scholar] [CrossRef]
- Ramírez-Vargas, C.; Prado, A.; Arias, C.; Carvalho, P.; Esteve-Núñez, A.; Brix, H. Microbial Electrochemical Technologies for Wastewater Treatment: Principles and Evolution from Microbial Fuel Cells to Bioelectrochemical-Based Constructed Wetlands. Water 2018, 10, 1128. [Google Scholar] [CrossRef]
- Nitisoravut, R.; Regmi, R. Plant Microbial Fuel Cells: A Promising Biosystems Engineering. Renewable and Sustainable Energy Reviews 2017, 76, 81–89. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, Y.; Wang, T.; Liu, R.; Gao, F. Energy Capture and Nutrients Removal Enhancement through a Stacked Constructed Wetland Incorporated with Microbial Fuel Cell. Water Science and Technology 2017, 76, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Kolajo, O.O.; Pandit, C.; Thapa, B.S.; Pandit, S.; Mathuriya, A.S.; Gupta, P.K.; Jadhav, D.A.; Lahiri, D.; Nag, M.; Upadhye, V.J. Impact of Cathode Biofouling in Microbial Fuel Cells and Mitigation Techniques. Biocatalysis and Agricultural Biotechnology 2022, 43, 102408. [Google Scholar] [CrossRef]
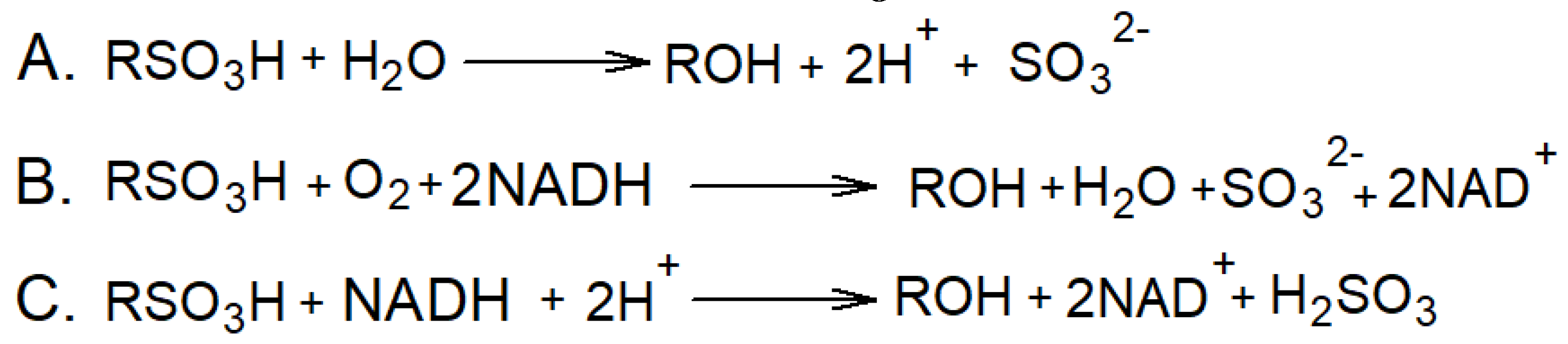
| Microorganism | Description | Consumable substrates | References |
|---|---|---|---|
| Desulfobulbus sp. | Obligate anaerobes capable of oxidizing sulfur to sulfate using an anode as an electron acceptor | Acetate, Propionate, butyrate, lactate, pyruvate | [35,48,49] |
| Geobacter sp. | Anaerobic metal-reducing bacteria. Fe (III) and Mn (IV) are used as electron acceptors. They can transmit electrons using pili – filamentous protein formations. | Benzoate, p-cresol, trichloroethane, benzene, lactate, acetate, starch | [50] |
| Geothrix fermentans | Anaerobic metal reducers, Fe (III) is used as an electron acceptor. They are capable of forming extracellular mediators of the quinone series and riboflavin, which makes it possible to transfer electrons to the electrode more efficiently | Acetate, Propionate, lactate, fumarate | [51,52] |
| Rhodoferax ferrireducens | Facultative metal reducing anaerobe with a wide temperature range of growth. Fe (III), Mn (IV), nitrate, fumarate and oxygen can be used as electron acceptors | Acetate, lactate, propionate, pyruvate, malate, succinate, benzoate | [53] |
| Shewanella sp. | Facultative anaerobic bacteria using Fe (III) and Mp (IV) as electron acceptors are capable of producing flavins that act as electronic transfer mediators | Lactate, formate | [54,55] |
| Clostridium butyricumC. beijerinckii | Obligate anaerobes can use an anode as an electron acceptor. Hydrogen, which is able to oxidize at the anode, is produced during the enzymatic fermentation of substrates. | Glucose, starch, sucrose, lactate | [56] |
| Plants | Microorganisms | Electrode material | Organic compound/ rate |
Purification rate, % | Max. output | Ref. |
|---|---|---|---|---|---|---|
| Spartina sp |
Pseudomonas veronii Ps. chlororaphis Ps. putida Ps. libanensis Azoarcus communis |
Cathode – stainless steel Аnode - stainless steel |
Oil | 99.6 | 11.56 mW/m2 | [108] |
| Aglaonema commutatum | Active sludge | Cathode – carbon felt Аnode – carbon felt |
Oil | Up to 82.3 | 382 mV | [109] |
| PAC | Up to 45.5 | 377 mV | ||||
| Steviare baudiana | Soil extraction | Cathode – stainless steel Аnode – carbon felt |
Urea | No data | 132 mW/m2 | [110] |
| Ozyra sp | Soil extraction | Cathode – carbon felt Аnode – carbon felt |
Compost | No data | 39.2 mW/m2 | [111] |
| Fimbristylis ferruginea | Association DC5 (Firmicutes Proteobacteria Bacteroidota Desulfobacterota Actinibacteriota Verrucomicrobiot) Soil extraction |
Cathode – glassy carbon fiber Аnode – glassy carbon fiber |
Textile wastewater | Up to 97.3 | Up to 197.9 mW/m2 | [112] |
|
Canna generalis, Chrysopogon zizanioides, Cyperus papyrus Hymenachne grumosa Equisetum hyemale |
Wastewater bacteria | Cathode – graphite Аnode - graphite |
BOD5 COD |
71 74 |
0.93 mW/m2 | [113] |
| Chlorella vulgaris | Anaerobic sludge | Cathode – carbon felt Аnode – carbon felt |
COD Nitrates Phosphates |
65.3 66.6 95.6 |
3.64 mW/m2 | [114] |
| Canna indica | Cathode – carbon felt А – carbon felt |
COD Nitrates Phosphates |
57.2 59.8 88.8 |
22.76 mW/m2 | ||
| Schoenoplectus californicus | Sludge | Cathode – activated carbon Аnode – activated carbon | COD Nitrogen |
Up to 87 Up to 98 |
8.6 mW/m2 | [115] |
| Canna indica | Anaerobic sludge | Cathode – stainless steel, activated carbon Аnode – stainless steel, activated carbon |
Tetracycline | 99.66 | Up to 124.89 mW/m2 | [116] |
| Sulfatotoxal | 100 | |||||
| Canna indica | Soil extraction | Cathode – graphite plate Anode – graphite rod |
Sodium dodecyl benzene sulfonate | Up to 56.8% | 4.01 mW/m2 | [117] |
| Plant | Microorganism | Electrode material | Metal | Purification rate, % | Max.generation | Ref. |
|---|---|---|---|---|---|---|
| Lolium perenne |
Proteobacteria Bacteroidetes Firmicutes |
Anode – graphite granules, carbon felt Cathode – carbon felt |
Cr2O72- | 90-99 | 55 mA/m2 | [126] |
| Oryza sativa L. |
Alphaproteobacteria Anaerolineae Clostridia Deltaproteobacteria Gammaproteobacteria Actinobacteria Bacteroidia Bacilli Thermoleophilia |
Anode – carbon felt Cathode – carbon felt |
As (V) | 25.2-41.8 | 22.2 mW/m2 | [127] |
| Oryza rufipogon |
Nocardioides Anaerolinea Geobacter Tumebacillus Azospirillum Bacillus |
Anode – carbon felt Cathode – carbon felt |
Cd (II) | Up to 31.7 | 351 mV | [128] |
| Typha orientalis | Up to 30.2 | 137 mV | ||||
| Eichhornia crassipes | No data | Anode – graphite rod Cathode – graphite rod |
Ni (II) | Up to ~10 | 0.86 mW/m2 | [129] |
| Oryza sativa L |
Proteobacteria Firmicutes Actinobacteria Chroroflexi |
Anode – carbon felt Cathode – carbon felt |
Cd Cu Cr Ni |
35.1 32.8 56.9 21.3 |
22.2 mW/m2 | [130] |
|
Cyperus alternifolius Cyperu smalaccensis |
River sludge | Anode – carbon felt Cathode – carbon felt |
As Zn Cd |
6.7 7.3 38.5 |
10.74 mW/m2 | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





