1. Introduction
Congenital optic nerve anomalies or cavitary anomalies are a rare, but important cause of visual impairment, with a visual prognosis ranging from asymptomatic to blindness [
1]. This spectrum of pathologies includes optic disc coloboma (ODC), optic disc pit (ODP), and morning glory syndrome (MGS). The clinical presentation is usually unilateral, isolated, or associated with both ocular abnormalities and as part of systemic disorders [
2].
Ophthalmologic examination with fundoscopy is usually sufficient to detect congenital optic nerve anomalies. However, these abnormalities can vary in disease severity and overlap clinically. Complementary imaging of the peripapillary region may increase the accuracy of diagnosis and potentially shed light on the pathophysiology of these disorders, which is still unclear [
3]. Therefore, multimodal imaging with non-invasive exams may help to define the diagnosis, identify potential complications, and avoid unnecessary additional tests.
The aim of this review is to summarise the current literature on the pathophysiology, clinical manifestations, multimodal imaging for diagnosis and possible treatments of complications associated with congenital optic nerve anomalies.
2. Materials and Methods
A literature search was conducted in several research databases, including PubMed, Scopus and Google Scholar, limiting the search to articles published in the last 15 years. Several keywords were used: “optic disc/nerve cavitary anomalies”, “congenital optic disc/nerve anomalies”, “optic nerve/disc coloboma”, “morning glory syndrome”, “morning glory disc anomaly”, “optic disc pit”. Exclusion criteria included non-English language articles, lack of access to full-text and lack of access to full-abstract. We included case reports if there were no other research studies on the same specific in the literature.
3. Optic Disc Coloboma
ODC is the second most common congenital malformation with a reported a prevalence of 8.9/100 000, which is not influenced by gender or race [
4]. It is characterized by a typical aspect of bowl-shaped excavation with sharp borders, usually localized inferonasally, with a normal vasculature, and equally affected unilaterally or bilaterally [
5]. When it occurs bilaterally, it can cause severe visual impairment in children with nystagmus; whereas, when it occurs unilaterally or in asymmetric cases, visual function may not be significantly affected. Visual acuity is influenced by possible involvement of the fovea if retinoschisis or retinal detachment is present. It occurs sporadically but can be inherited in autosomal dominant manner. It can be isolated or in association with other ocular abnormalities, such as iris and/or retinal coloboma, aniridia in patients with PAX6 mutation [
6]. Several associations with genetic disorders have been described: PAX2 mutation-related papillorenal syndrome [
7,
8], CHARGE syndrome, Aicardi syndrome [
5,
9].
Regarding the pathophysiology, it results from a defective closure of the proximal part of the embryonal fissure during the 6-7 weeks of gestation [
1]. There are several animal models, that have been used to explore the genetic basis of ODC development. In addition to the role of PAX2, Yan and colleagues investigated the role of BMPR1B-mediated signaling in optic nerve development in a mouse model. They demonstrated that a Bmpr1b mutation alters the expression pattern of PAX2 during embryonic development, leading to a delay in the closure of the optic fissure and, subsequently, to coloboma of the optic disc [
10].
3.1. Multimodal Imaging
There are only a few studies on OCT in ODC. Structurally, SD-OCT shows a retinochoroidal–scleral excavation in the area of ODC and the presence of sclera directly under the retina, when a choroidal coloboma is present. If the ODC is complicated with retinoschisis, this will be visible above the excavation. Moreover, SS-OCT can detect the presence of herniated retinal tissue into the colobomatous area and the subarachnoid space immediately behind the highly reflective tissue lining, which may be the pia mater [
11]. On OCTA, Cennamo and colleagues demonstrated the absence of a radial peripapillary microvascular network in ODC result of imperfect closure of the embryonic fessure [
3].
Fluorescein angiography is useful in ODC complicated by choroidal neovascularization as it shows the presence of late hyperfluorescence at the margin of the optic nerve [
12].
3.2. Complications
Less frequently than the other forms of optic disc anomalies, ODC is associated with retinal complications: retinoschisis, retinal detachment (RD), which occurs in 6%–43% of these patients, serous macular detachment (SMD) and, extremely rarely, choroidal neovascularization (CNV) [
4]. Both retinoschisis and RD are linked to the presence of persistent traction of the vitreous on a specific area, the intercalary membrane (ICM). The ICM corresponds to the continuation of the inner retina from the non-colobomatous area to the colobomatous area. Vitreous traction on the ICM and at its edges can lead to schisis- defects, micro or mini breaks, allowing fluid to enter the sub retinal space, resulting in RD [
13]. Pars plana vitrectomy is the main treatment to release vitreal traction. In addition, some adjuvant manoeuvres have been described to achieve the goal of retinal reattachment, especially in the case of recurrent RD: laser photocoagulation, applied at the temporal edge of the coloboma, ILM graft technique, use of an autologous platelet concentrate,
different tamponade agents such as sulfur hexafluoride gas, standard silicone oil, and heavy silicone oil [
14]
.
In presence of CNV, some case reports describe a good response to anti-VEGF treatment [
15,
16].
4. Optic Disc Pit
ODP is a rare cavitary congenital anomaly of the optic nerve head, with a prevalence of 1/11 000, unaffected by gender or race [
17]. It usually presents as a unilateral grayish oval or round depression, that tends to be located in the temporal segment of the optic disc and can sometimes be associated with ODC [
18]; it is bilateral in 15% of patients. It is commonly congenital, but can also be acquired and associated with glaucoma and myopia [
19]. Like ODC, congenital ODP can also be related to various systemic diseases (Aicardi, Alagille syndrome, neurological developmental malformations) and to specific gene mutations (such as PAX2). About the pathophysiology, Gass proposed that ODP is an abnormal development of the primordial optic nerve papilla and described it as a mild spectrum of ODC [
20]; some histologic reports, confirmed by structural OCT studies showed a defect in the lamina cribrosa and the presence of dysplastic retinal herniations into the subarachnoid space [
13,
21]. In addition, Theodossiadis et al. highlighted the anatomical association of ODPs with cilioretinal arteries in up to 64% of cases [
22].
At the time of diagnosis, patients often have a normal visual acuity unless they develop macular involvement with a visual acuity of 20/200 or worse in most untreated patients. As with the other congenital OD anomalies, the diagnosis of ODP is essentially clinical by fundus examination. Imaging techniques can be useful as an aid, particularly to investigate the possible pathophysiology of ODP and its complications.
4.1. Multimodal Imaging
In the case of ODP, structural OCT reveals an excavation in the optic nerve head with a hyporeflective area corresponding to the pit. As described by Maertz, OCT in the follow-up of ODPs may show signs of intrapapillary proliferation and the change of pit aspect over time [
23]. When macular involvement is present (ODP maculopathy, ODP-M), OCT is useful to assess the presence of fluid, its distribution and evolution. As Lincoff described, in most cases the fluid follows a specific distribution pattern. First, it Causes a schisis-like separation of the inner retina and then reaches the subretinal space, creating a macular neuroepithelial detachment [
24]. Currently, OCT helps to determine the distribution and development of retinal fluid and a possible correlation with visual prognosis. Iros et al. described the presence of outer retinal fluid (ORF) in most patients at diagnosis, indicating progression from outer retinal layers to other layers. In 40% of cases, multilayers fluid (MLF) is present, including both outer and intraretinal fluid. He also reported intraretinal fluid in about 78% of patients [
25]. Subretinal fluid (SRF) is described as less common than other fluid distribution but correlates with a decrease in vision and deterioration in functional prognosis, especially when associated with subretinal hyperreflective deposits, which are an indicator of chronicity of ODP-M [
19,
26]. Lamellar macular holes have been reported in association with ODPM [
25]. Fluorescein angiography shows early hypofluorescence at the ODP, followed by late hyperfluorescence when macular involvement is present [
27]. Although fluorescein angiography and indocyanine green angiography (if necessary) provide a valuable qualitative image of retinal vascular perfusion, they cannot provide a quantitative assessment of the microvasculature. OCTA can integrate these data. Cennamo et al. reported for the first time the absence of the radial peripapillary microvascular network in both ODP and ODC resulting in imperfect closure of the embryonic fessure [
3].
[
3]. This report was confirmed by Jiang who described a decrease in capillary perfusion density within the disc, especially in patients with low visual acuity [
28].
4.2. Complications
4.2.1. Optic Disc Pit Maculopathy
ODP maculopathy (ODP-M) may be present in 25-75% of patients between the second and fourth decade of life and consists mainly of a serous macular detachment or macular schisis. Spontaneous resolution occurs in up to 25% of cases. The origin of the fluid is not clear, but as Michalewski described, it may have a dual origin, both vitreal and cerebrospinal. Among current therapies, pars plana vitrectomy (PPV) is the most recommended method to achieve complete anatomical success, defined as the flattening of the macula without fluid. According to the “European VitreoRetinal society OPTIC PIT STUDY”, SRF responds better to surgical treatment than IRF, with SRF completely or partially regressing in 83% of cases (compared to 73%) 12 months after surgery; in addition, the recurrence rate for SRF is lower than IRF, at 7% and 23%, respectively. Moreover, the same study reported a retreatment rate of 15.6% after 14 months, with SRF completely disappearing in 89% of cases, compared to 67% with IRF. In these cases, the anatomical success and the functional outcomes are not related. After the first surgical time, both anatomical and functional results improve slowly; therefore, special attention must be paid before retreatment. In terms of surgical technique, this study emphasised the fundamental role of PPV with gas tamponade, with endolaser and ILM peeling providing no additional benefit [
25]. Two Italian study groups reported results on the use of human amniotic membrane to plug the disc pit during PPV, with improved visual acuity at 12 months and no fluid recurrence [
29,
30]. Gklava reported the possible use of autologous platelets, both as primary and rescue therapy, with improved functional and morphologic outcomes without relapse over a long follow-up period [
31]. A single case of paediatric CNV associated with ODP treated with anti-VEGF injection is reported in literature [
32].
5. Morning Glory Syndrome
Morning glory syndrome (MGS) or Morning glory disc anomaly (MGDA) is described as an enlarged optic disc with funnel-shaped excavation, peripapillary pigmentation, radiating distribution of retinal blood vessels and a central white tuft of glial tissue, with a prevalence of 3,6 / 100000 children. It is more common in females (2:1 compared to males), is usually unilateral, begins early in childhood and is associated with extremely poor visual function and prognosis, which is generally less than 0.1 decimal visual acuity [
33]. The pathogenesis is unclear: some authors suggest poor development of the posterior sclera and lacrima cribrosa during gestation [
34]; recent OCT-based studies have demonstrated the presence of abnormal communication between the subarachnoid and subretinal spaces. In agreement with these data, an abnormality in primary neuroectodermal development with dilatation of the terminal optic stalk has been suggested, followed by a secondary postnatal mesenchymal abnormality [
1,
35]. Moreover, Cennamo et all, suggesting that MGS arises from mesenchymal abmormality due to an increase in the peripapillary network [
3].. Extremely rare cases of contractile MGDA have been reported. Two main theories have been proposed: the muscle contraction theory, which attributes the contractions to a heterotopic smooth muscle in posterior sclera, and the pressure balance theory, which suggests a fluid flux through a communication between the subarachnoid space and the juxtapapillary subretinal space [
36]. Ocular tissues and cerebral vessels are also frequently affected in patients diagnosed with MGDA. Persistent fetal vasculature (PVF), cataracts, microphthalmia, retinal detachment and retrobulbar cysts have been described as ocular complications [
37,
38,
39]. She et al. reported a high prevalence of nonperfused peripheral retina in pediatric MGDS and suggested that more attention should be paid to this aspect [
40]. About 45% of patients diagnosed with MGS have associated cerebrovascular anomalies that support the hypothesis of a primary mesenchymal defect [
41]: basal encephalocele, hypopituitarism, Moya Moya disease, midline cranial defects, and agenesis of the corpus callosum [
42]. Moreover, Poillon described a case series of patients with MDGA, who also presented an aspect of optic pathway enlargement, which might be a malformation associated with MGDA. Therefore, patients found to have MGD should always undergo neuroimaging [
43]. Initial clinical manifestation of MGS can be various: low visual acuity with posterior segment involvement, leucocoria, microphthalmia, retinal detachment, less commonly strabismus. The diagnosis is clinical and supported by retinal imaging.
5.1. Multimodal Imaging
SD- OCT shows increased cup diameter with deep excavation, a raised hyperreflective area corresponding to the presence of glial tissue overlying the optic disc. Cennamo et al. reported OCT angiography of the peripapillary retina showing a dense microvascular network in the radial peripapillary capillary (RPC) with no vascular difference between the superficial and deep vascular plexus around the optic nerve [
44]. Fluorescein angiography shows hypofluorescence in the center of the disc, numerous radial vessels and possible peripapillary changes around the optic disc as mottled fluorescence. Ocular echography is a useful tool in patients with PVF associated with MGDA and for preoperative planning in case of RD surgery [
45].
5.2. Complications
5.2.1. Retinal Detachment
Retinal detachment (RD) is the most common and serious complication in patients with MGS, occurring in approximately one third of patients. It can take an unpredictable clinical course with spontaneous attachment and re-detachment, and can be rhegmatogenous, tractional, and exudative [
46,
47]. Currently, the pathogenesis is still unclear: according to Ho et al. an abnormal communication between subretinal and subarachnoid or vitreous compartments seems to be the causative factor. A careful preoperative OCT examination should help the surgeon to detect possible retinal breaks, which have been frequently described along the margin of excavation [
48]. Zou et al. defined some possible risk factors for the development of RRD, such as a deeper excavation with a sharper curve angle at the edge of the excavation, which could result in increased vitreoretinal traction and increased retinal breaks [
45]. Considering the poor prognosis after surgery and the need for multiple procedures, only a small number of eyes will undergo surgery, while the remaining eyes should be recommended for regular follow-up. In fact, RD-related MGDA is a challenge for surgeons in several aspects: a high rate of recurrence, poor visual outcomes, the difficulty to induce posterior vitreous detachment and to perform a complete vitreous removal, the possibility to perform the epiretinal membrane (ERM) and the inner limiting membrane (ILM) peeling, the iatrogenic induction of retinal tears during fibroglial tissue removal, the use of perfluorocarbon fluids (PFCLs) that can migrate into both the subretinal and the subarachnoid space, the high rate of proliferative vitreoretinopathy (PVR) [
49,
50,
51]. Some adjuvant treatments can be performed during pars plana vitrectomy (PPV), but there are no clear results in the literature. For example, laser photocoagulation at the optic disc margin in absence of obvious retinal breaks is controversial because it could damage the nerve fibers at papillomacular bundle; therefore, diode laser may be preferable to preserve the nerve fiber layer. Some procedures have been described as possible treatments for recurrent RD to close peripapillary holes: packaging with ILM, the use of a human amniotic patch placed directly on the optic nerve [
52,
53]. There has been some concerns about the potential communication between the subretinal space or vitreous cavity and the subarachnoid space when using silicone oil as a tamponade because of the risk of migration of emulsified bubbles [
54]. Preoperative surgical planning with OCT, especially the three-dimensional remodelling scan, is fundamental for the correct selection of patients to undergo surgery and to achieve a better outcome. Some reports have described spontaneous resolution of RD in patients with only serous components, without retinal breaks [
55]. Prakash et al. described the use of oral acetazolamide as a possible treatment for serous macular detachments [
56].
5.2.2. Choroidal Neovascularization
A rare complication of MGD is the development of choroidal neovascularization (CNV), both in adults and children. It typically occurs at the edge of the staphyloma and is associated with sudden painless visual decrease. The diagnosis is made clinically and supported by imaging examinations: OCT, fluorescein and indocyanine green angiography [
57]. Cennamo et al. emphasised the usefulness of OCT angiography for CNV-related MGS diagnosis, especially in patients with possible misdiagnosis [
58]. Although there is no established regimen, anti-VEGF therapy is indicated for treatment [
59].
6. Conclusions
This comprehensive review highlights the current state of knowledge of congenital cavitary optic nerve anomalies in terms of pathophysiology, clinical assessment, potential treatment and associated complications.
Despite being a spectrum of rare disorders, these congenital anomalies are associated with a significant impact on the medical history of the patient, who is diagnosed with them. Indeed, in ophthalmology, these conditions lead to significant visual impairment, long follow-up periods (from childhood to adulthood) and the need for repeated medical and especially surgical treatments. In addition, the diagnosis of MGDA or ODC, compared to the associated systemic diseases, requires the involvement of the patient in a multidisciplinary approach, with several medical experts and complementary examinations, such as neurological imaging.
Congenital anomalies are a challenge both in research and in clinical practice. At present, we still have imprecise data on the pathophysiology and researchers are trying to elucidate the pathological mechanisms using animal models. Several gene mutations have been described, but the pathways of optic nerve head abnormalities are still not clear. Regarding clinical aspect, non-invasive exams such as OCT and OCTA have been used to study morphological aspect of the optic nerve, characterize these abnormalities and try to define the basis for the development of the associated complications. These data may be fundamental for the development of an improved treatment approach, i.e. for the selection of patients to be treated in case of complications and for the definition of the best treatment approach, especially in case of RD.
In conclusion, congenital anomalies of the optic nerve represent a major challenge for ophthalmologists and medical professionals. Further research and collaboration between scientists and clinicians can clarify the pathophysiology of these diseases and define a correct and specific treatment approach.
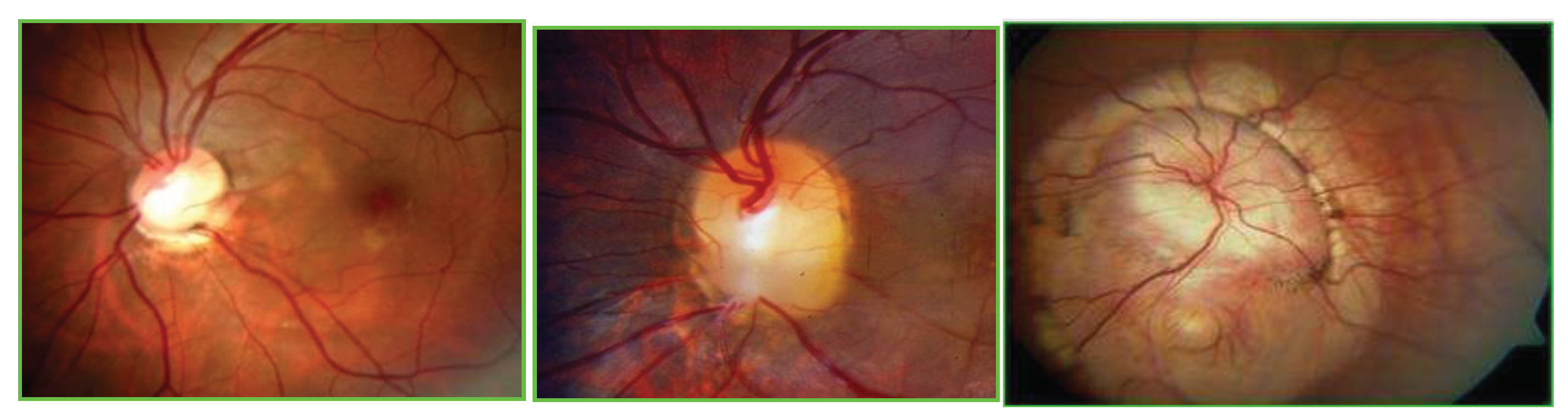
Figure legend.
Color fundus photography of optic disc coloboma (ODC), optic disc pit (ODP), and morning glory syndrome (MGS).( from the right to left line).
Figure legend.
Color fundus photography of optic disc coloboma (ODC), optic disc pit (ODP), and morning glory syndrome (MGS).( from the right to left line).
Author Contributions
Conceptualization, G.C. and M.C.; methodology, writing—original draft preparation, editing; C.C., M.R. writing—review, visualization, supervision, validation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jeng-Miller KW, Cestari DM, Gaier ED. Congenital anomalies of the optic disc: insights from optical coherence tomography imaging. Curr Opin Ophthalmol. 2017, 28, 579-586. [CrossRef]
- Mansoori, T. Radial peripapillary capillary network in congenital cavitary optic disk anomalies. Indian J Ophthalmol. 2023, 71, 1058. [Google Scholar] [CrossRef] [PubMed]
- Cennamo G, Rossi C, Ruggiero P, de Crecchio G, Cennamo G. Study of the Radial Peripapillary Capillary Network in Congenital Optic Disc Anomalies With Optical Coherence Tomography Angiography. Am J Ophthalmol. 2017, 176:1-8. [CrossRef]
- Skriapa Manta A, Olsson M, Ek U, Wickström R, Teär Fahnehjelm K. Optic Disc Coloboma in children - prevalence, clinical characteristics and associated morbidity. Acta Ophthalmol. 2019, 97, 478-485. [CrossRef]
- Heidary, G. Congenital optic nerve anomalies and hereditary optic neuropathies. J Pediatr Genet. 2014, 3, 271–80. [Google Scholar] [CrossRef] [PubMed]
- Lee B, Choi DG, Chun BY, Oh EH, Lee YJ, Kim UK, Park JS. A family with a mild form of congenital nystagmus and optic disc coloboma caused by a novel PAX6 mutation. Gene. 2019, 705, 177-180. [CrossRef]
- Schimmenti, L.A. Renal coloboma syndrome. Eur J Hum Genet. 2011, 19, 1207–12. [Google Scholar] [CrossRef] [PubMed]
- Okumura T, Furuichi K, Higashide T, Sakurai M, Hashimoto S, Shinozaki Y, Hara A, Iwata Y, Sakai N, Sugiyama K, Kaneko S, Wada T. Association of PAX2 and Other Gene Mutations with the Clinical Manifestations of Renal Coloboma Syndrome. PLoS One. 2015, 10, e0142843. [CrossRef]
- Shirley K, O'Keefe M, McKee S, McLoone E. A clinical study of Aicardi syndrome in Northern Ireland: the spectrum of ophthalmic findings. Eye (Lond). 2016, 30, 1011-6. [CrossRef]
- Yan X, Atorf J, Ramos D, Thiele F, Weber S, Dalke C, Sun M, Puk O, Michel D, Fuchs H, Klaften M, Przemeck GKH, Sabrautzki S, Favor J, Ruberte J, Kremers J, de Angelis MH, Graw J; German Mouse Clinic Consortium. Mutation in Bmpr1b Leads to Optic Disc Coloboma and Ventral Retinal Gliosis in Mice. Invest Ophthalmol Vis Sci. 2020, 61, 44. [CrossRef]
- Inoue M. Retinal complications associated with congenital optic disc anomalies determined by swept source optical coherence tomography. Taiwan J Ophthalmol. 2016, 6, 8-14. [CrossRef]
- Naithani P, Vashisht N, Mandal S, et al. Intravitreal bevacizumab in choroidal neovascularization associated with congenital choroidal and optic nerve coloboma in children: long-term improvement in visual acuity. J Aapos 2010, 14, 288–90.
- Prabhu V, Mangla R, Acharya I, Handa A, Thadani A, Parmar Y, Yadav NK, Chhablani J, Venkatesh R. Evaluation of baseline optic disc pit and optic disc coloboma maculopathy features by spectral domain optical coherence tomography. Int J Retina Vitreous. 2023, 9, 46. [CrossRef]
- Temmerman IM, Mahmoud TH, Veckeneer MAH. AUTOLOGOUS NEUROSENSORY RETINAL TRANSPLANT TO TREAT REFRACTORY SEROUS RETINAL DETACHMENT SECONDARY TO OPTIC DISK COLOBOMA. Retin Cases Brief Rep. 2022, 16, 606-609. [CrossRef]
- Schimansky S, Wu XN, Egan C, Mohamed Q. Intravitreal ranibizumab for the management of serous maculopathy secondary to optic disc coloboma-associated choroidal neovascularisation. BMJ Case Rep. 2021, 14, e235452. [CrossRef]
- Grewal DS, Tran-Viet D, Vajzovic L, Mruthyunjaya P, Toth CA. Association of Pediatric Choroidal Neovascular Membranes at the Temporal Edge of Optic Nerve and Retinochoroidal Coloboma. Am J Ophthalmol. 2017, 174:104-112. [CrossRef]
- Meng L, Zhao X, Zhang W, Wang D, Chen Y. The characteristics of optic disc pit maculopathy and the efficacy of vitrectomy: a systematic review and meta-analysis. Acta Ophthalmol. 2021, 99, e1176-e1189. [CrossRef]
- Abe RY, Iguma CI, Wen LC. A hybrid coloboma and optic disc pit associated with macular retinoschisis. BMC Ophthalmol. 2019, 19, 212. [CrossRef]
- Taslipinar Uzel AG, Gelisken F, Bartz-Schmidt KU, Neubauer J. Natural Course of Optic Disc Pit Maculopathy: An Optical Coherence Tomography Study. Ophthalmologica. 2022, 245, 563-569. [CrossRef]
- Gass JDM. Serous detachment of the macula. Secondary to congenital pit of the optic nerve head. Am J Ophthalmol. 1969, 67, 821–841.
- Maertz J, Kolb JP, Klein T, Mohler KJ, Eibl M, Wieser W, Huber R, Priglinger S, Wolf A. Combined in-depth, 3D, en face imaging of the optic disc, optic disc pits and optic disc pit maculopathy using swept-source megahertz OCT at 1050 nm. Graefes Arch Clin Exp Ophthalmol. 2018, 256, 289-298. [CrossRef]
- Theodossiadis GP, Kollia AK, Theodossiadis PG. Cilioretinal arteries in conjunction with a pit of the optic disc. Ophthalmologica 1992, 204, 115–121.
- Maertz J, Mohler KJ, Kolb JP, Kein T, Neubauer A, Kampik A, Priglinger S, Wieser W, Huber R, Wolf A. Intrapapillary proliferation in optic disk pits: Clinical Findings and Time-Related Changes. Retina. 2017, 37, 906-914. [CrossRef]
- Lincoff H, Kreissig I. Optical coherence tomography of pneumatic displacement of optic disc pit maculopathy. Br J Ophthalmol. 1998, 82, 367-72. [CrossRef]
- Iros M, Parolini B, Ozdek S, Gini G, Nawrocka ZA, Ellabban AA, Faramawi MF, Adelman R, Sallam AB; EVRS Study Group. Management of optic disc pit maculopathy: the European VitreoRetinal society optic pit study. Acta Ophthalmol. 2022, 100, e1264-e1271. [CrossRef]
- Steel DHW, Suleman J, Murphy DC, Song A, Dodds S, Rees J. Optic Disc Pit Maculopathy: A Two-Year Nationwide Prospective Population-based Study. Ophthalmology. 2018, 125, 1757-1764. [CrossRef]
- Shah SD, Yee KK, Fortun JA, Albini T. Optic disc pit maculopathy: a review and update on imaging and treatment. Int Ophthalmol Clin 2014, 54, 61–78.
- Jiang S, Turco B, Choudhry N. VASCULAR PERFUSION DENSITY MAPPING USING OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY COMPARING NORMAL AND OPTIC DISK PIT EYES. Retin Cases Brief Rep. 2022, 16, 126-132. [CrossRef]
- Rizzo S, Caporossi T, Pacini B, De Angelis L, De Vitto ML, Gainsanti F. Management of optic disk pit-associated macular detachment with human amniotic membrane patch. Retina. (2023) 43:144–7. [CrossRef]
- Caporossi T, D’Amico G, Tartaro R, Governatori L, Scampoli A, Amorelli G, et al. Optic disk pit maculopathy treatment using a human amniotic membrane patch: one-year results. Am J Ophthalmol. (2022) 240:30–6. [CrossRef]
- Gklavas K, Athanasiou A, Neubauer J, Lilou E, Pohl L, Bartz-Schmidt KU, Dimopoulos S. Long-term outcomes of autologous platelet treatment for optic disc pit maculopathy. Graefes Arch Clin Exp Ophthalmol. 2023, 261, 3177-3185. [CrossRef]
- Alshammari A, Alabduljalil T. Choroidal neo-vascular membrane in a paediatric optic disc pit: A case report. Am J Ophthalmol Case Rep. 2022, 28:101751. [CrossRef]
- Ceynowa DJ, Wickström R, Olsson M, Ek U, Eriksson U, Wiberg MK, Fahnehjelm KT. Morning glory disc anomaly in childhood - a population-based study. Acta Ophthalmol. 2015, 93, 626-34. [CrossRef]
- Lee BJ, Traboulsi EI. Update on the morning glory disc anomaly. Ophthalmic Genet. 2008, 29, 47-52. [CrossRef]
- Cennamo G, de Crecchio G, Iaccarino G, Forte R, Cennamo G. Evaluation of morning glory syndrome with spectral optical coherence tomography and echography. Ophthalmology. 2010, 117, 1269-73. [CrossRef]
- Sevgi DD, Orge FH. Contractile morning glory disk anomaly: analysis of the cyclic contractions and literature review. J AAPOS. 2020, 24, 99.e1-99.e6. [CrossRef]
- Fei P, Zhang Q, Li J, Zhao P. Clinical characteristics and treatment of 22 eyes of morning glory syndrome associated with persistent hyperplastic primary vitreous. Br J Ophthalmol. (2013) 97:1262–7. [CrossRef]
- Zheng S, Cao JF, Wang XY, Wu B, Wang ZJ, Xiao B, Duan JL, Hu P. Multimodal imaging of morning glory syndrome with persistent hyperplastic primary vitreous. J Clin Ultrasound. 2023, 51, 1364-1365. [CrossRef]
- Chen YN, Patel CK, Kertes PJ, Devenyi RG, Blaser S, Lam WC. RETINAL DETACHMENT AND RETROBULBAR CYSTS IN A LARGE COHORT OF OPTIC NERVE COLOBOMA. Retina. 2018, 38, 692-697. [CrossRef]
- She K, Zhang Q, Fei P, Peng J, Lyu J, Li Y, Huang Q, Zhao P. Peripheral Retinal Nonperfusion in Pediatric Patients With Morning Glory Syndrome. Ophthalmic Surg Lasers Imaging Retina. 2018, 49, 674-679. [CrossRef]
- Duvall J, Miller SL, Cheatle E, Tso MO. Histopathologic study of ocular changes in a syndrome of multiple congenital anomalies. Am J Ophthalmol 1987, 103, 701–705.
- Wang YY, Zhou KY, Ye Y, Song F, Yu J, Chen JC, Yao K. Moyamoya Disease Associated With Morning Glory Disc Anomaly and Other Ophthalmic Findings: A Mini-Review. Front Neurol. 2020, 11, 338. [CrossRef]
- Poillon G, Henry A, Bergès O, Bourdeaut F, Chouklati K, Kuchcinski G, Caputo G, Lecler A; Morning Glory Disc Anomaly Study Group. Optic Pathways Enlargement on Magnetic Resonance Imaging in Patients with Morning Glory Disc Anomaly. Ophthalmology. 2021, 128, 172-174. [CrossRef]
- Cennamo G, Montorio D, Breve MA, Morra VB, Cennamo G. Optical coherence tomography angiography in contractile morning glory syndrome. Eur J Ophthalmol. 2021, 31, NP13-NP16. [CrossRef]
- Zou Y, She K, Hu Y, Ren J, Fei P, Xu Y, Peng J, Zhao P. Clinical and Echographic Features of Morning Glory Disc Anomaly in Children: A Retrospective Study of 249 Chinese Patients. Front Med (Lausanne). 2022, 8, 800623. [CrossRef]
- Zou Y, Wang X, Li J, Peng J, Zhao P. Natural Course for Retinal Detachment in Morning Glory Disc Anomaly Based on a Grading System. Asia Pac J Ophthalmol (Phila). 2022 Oct 17. [CrossRef]
- Ho CL, Wei LC. Rhegmatogenous retinal detachment in morning glory syndrome pathogenesis and treatment. Int Ophthalmol. 2001, 24, 21-4. [CrossRef]
- Ho TC, Tsai PC, Chen MS, Lin LL. Optical coherence tomography in the detection of retinal break and management of retinal detachment in morning glory syndrome. Acta Ophthalmol Scand. 2006, 84, 225-7. [CrossRef]
- Chang S, Gregory-Roberts E, Chen R. Retinal detachment associated with optic disc colobomas and morning glory syndrome. Eye (Lond). 2012, 26, 494-500. [CrossRef]
- Naseripour M, Ghasempour A, Falavarjani KG, Sanjari MS, Yousefi M. Perfluorocarbon liquid migration into the subarachnoid space in a patient with morning glory syndrome.J CurrOphthalmol 2015, 27, 60-2. [CrossRef]
- Babu N, Kohli P. Surgical challenges in the management of morning glory disc anomaly-associated retinal detachment. Indian J Ophthalmol. 2021, 69, 2540-2541. [CrossRef]
- Shen J, Chen X, Gong X, Wu Z. Internal limiting membrane packing for treatment of morning glory syndrome with rhegmatogenous retinal detachment. Am J Ophthalmol Case Rep. 2022, 26, 101454. [CrossRef]
- Caporossi T, Ferrara S, Savastano A, Gambini G, De Vico U, Savastano MC, Rizzo S. Management of retinal detachment associated with morning glory syndrome using human amniotic membrane. Retin Cases Brief Rep. 2022 Aug 16. [CrossRef]
- Sen P, Maitra P, Vaidya H, Bhende P, Das K. Outcomes of vitreoretinal surgery in retinal detachment associated with morning glory disc anomaly. Indian J Ophthalmol. 2021, 69, 2116-2121. [CrossRef]
- Choudhry N, Ramasubramanian A, Shields CL, Brown G, Shields JA. Spontaneous resolution of retinal detachment in morning glory disk anomaly. J AAPOS. 2009, 13, 499-500. [CrossRef]
- Prakash P, De Salvo G, Lotery AJ. Morning glory with serous macular detachment responds to oral acetazolamide. Eye (Lond). 2010, 24, 1732-3. [CrossRef]
- Cennamo G, Rossi C, Velotti N, de Crecchio G. Ranibizumab in the treatment of choroidal neovascularization associated with morning glory syndrome. Acta Ophthalmol. 2015, 93, e516-7. [CrossRef]
- ennamo G, Montorio D, Brescia Morra V, Costagliola C. Multimodal imaging in differential diagnosis between papilledema and choroidal neovascularization associated with morning glory syndrome. Eur J Ophthalmol. 2023, 33, NP100-NP102. [CrossRef]
- Özkaya A, Yilmaz I, Alkin Z, Karakucuk Y, Yazici AT. Intravitreal ranibizumab in the treatment of choroidal neovascularization secondary to morning glory syndrome in a child. Saudi J Ophthalmol. 2016, 30, 140-3. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).