Submitted:
12 July 2025
Posted:
16 July 2025
Read the latest preprint version here
Abstract
Keywords:
Introduction
Melanocytic Nevi Considerations
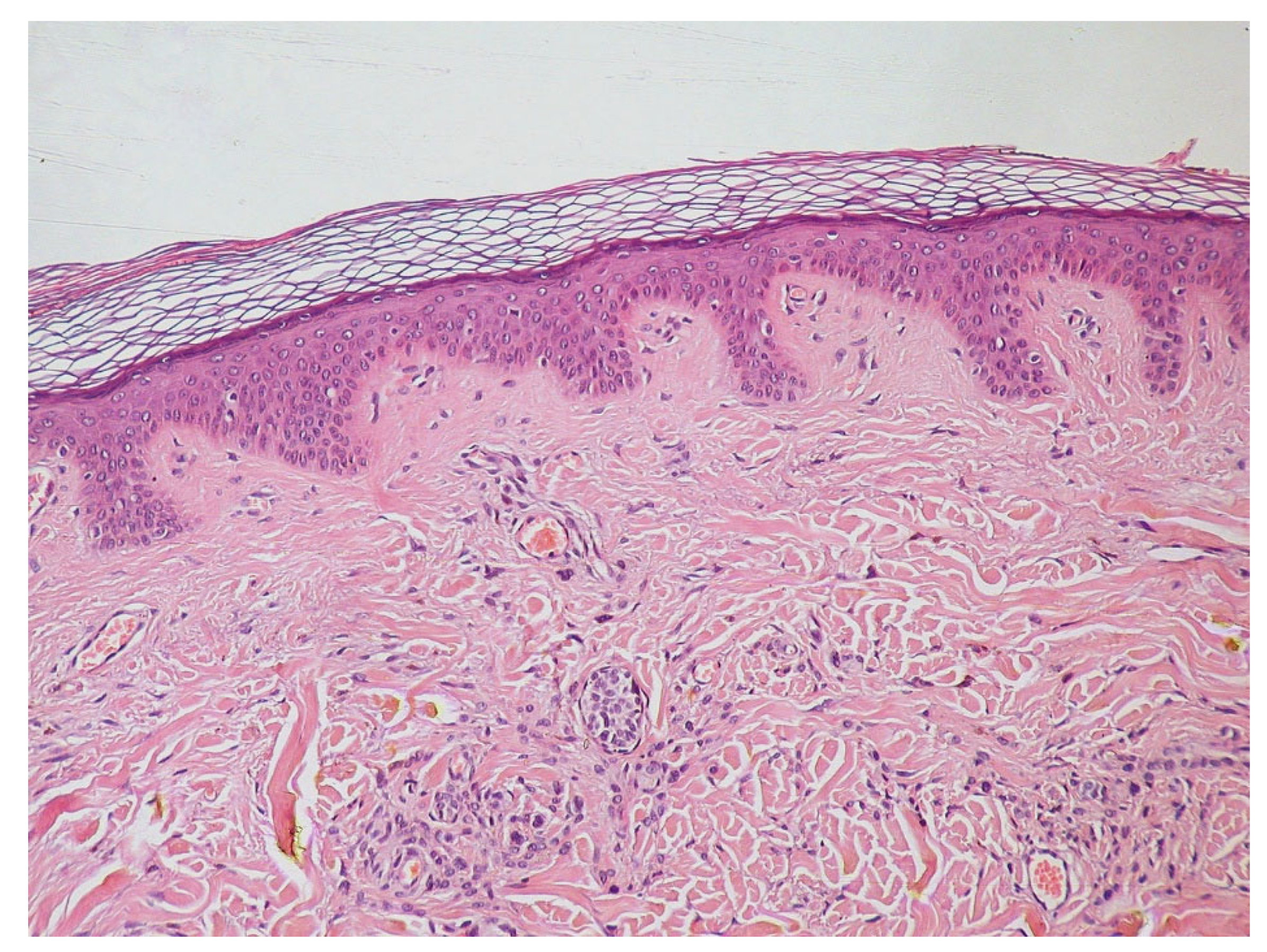
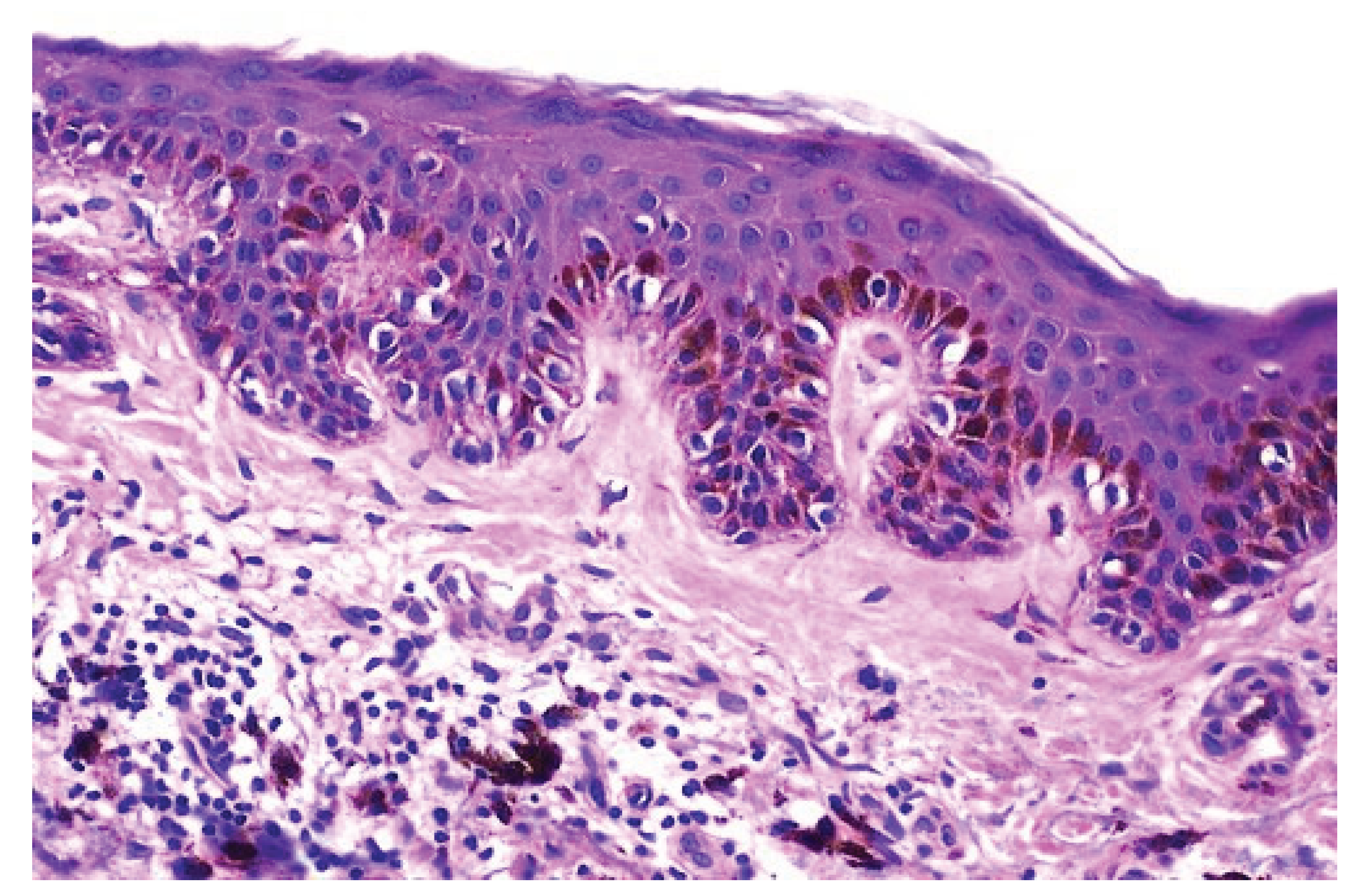
Histologycally and Pathologycally Key Points of Epiderm
Material and Methods
Results
Education Activity Outcomes and Management Strategies
Discussions
Conclusions
References
- Salzer M.C., Lafzi A., Berenguer-Llergo A., Youssif C., Castellanos A., Solanas G., Peixoto F.O., Stephan-Otto Attolini C., Prats N., Aguilera M., Martín-Caballero J., Heyn H., Benitah S.A. Identity noise and adipogenic traits characterize dermal fibroblast aging. Cell. 2018;175:1575–1590.e22.
- Palicka GA, Rhodes AR. Acral melanocytic nevi: prevalence and distribution of gross morphologic features in white and black adults. Arch Dermatol. 2010 Oct;146(10):1085-94.
- Wang M, Xu Y, Wang J, Cui L, Wang J, Hu XB, Jiang HQ, Hong ZJ, Yuan SM. Surgical Management of Plantar Melanoma: A Retrospective Study in One Center. J Foot Ankle Surg. 2018 Jul-Aug;57(4):689-693.
- Richtig, E. ASCO Congress 2018: melanoma treatment. Memo. 2018;11(4):261-265.
- Wang B, Qu XL, Chen Y. Identification of the potential prognostic genes of human melanoma. J Cell Physiol. 2019 Jun;234(6):9810-9815.
- Bristow I, Bower C. Melanoma of the Foot. Clin Podiatr Med Surg. 2016 Jul;33(3):409-22.
- Lallas A, Kyrgidis A, Koga H, Moscarella E, Tschandl P, Apalla Z, Di Stefani A, Ioannides D, Kittler H, Kobayashi K, Lazaridou E, Longo C, Phan A, Saida T, Tanaka M, Thomas L, Zalaudek I, Argenziano G. The BRAAFF checklist: a new dermoscopic algorithm for diagnosing acral melanoma. Br J Dermatol. 2015 Oct;173(4):1041-9.
- Tronnier M. Melanotische Flecke und melanozytäre Nävi. In: Plewig G, Ruzicka T, Kaufmann R, Hertl M. Braun-Falcos Dermatol. Venerol. Allergol. Springer Berlin Heidelberg, 7. Auflage, 2016.
- Tolleson WH. Human Melanocyte Biology, Toxicology, and Pathology. J Environ Sci Health Part C 2005; 23: 105–61.
- Thomas AJ, Erickson CA. The making of a melanocyte: the specification of melanoblasts from the neural crest. Pigment Cell Melanoma Res 2008; 21: 598–610.
- Colebatch AJ, Ferguson P, Newell F et al. Molecular genomic profiling of melanocytic nevi. J Invest Dermatol 2019; 139: 1762–8.
- Kinsler VA, Thomas AC, Ishida M et al. Multiple congenital melanocytic nevi and neurocutaneous melanosis are caused by postzygotic mutations in codon 61 of NRAS. J Invest Dermatol 2013; 133: 2229–36.
- Bandyopadhyay D. Halo nevus. Indian Pediatr 2014; 51: 850.
- Fernandez-Flores A. Eponyms, Morphology, and Pathogenesis of some less mentioned types of melanocytic nevi. Am J Dermatopathol 2012; 34: 607–18.
- Kim JJ (Department of Dermatology, Henry Ford Health System, Detroit, MI 48202, USA), Chang MW, Shwayder T. Topical tre-tinoin and 5-fluorouracil in the treatment of linear verrucous epi dermal nevus. J Am Acad Dermatol. 2000 Jul;43(1 Pt 1):129–132.
- Brown HM, Gorlin RJ. Oral mucosal involvement in nevus unius lateris (Icthyosis Hysterix). Arch Dermatol. 1960 Apr;81:509–515.
- Zvulunov A (Soroka Medical Center, Ben-Gurion University, Beer-Sheva Israel), Grunwald MH, Halvy S. Topical calcipotriol for treatment of infammatory linear verrucous epidermal nevus. Arch Dermatol. 1997 May;133(5):567–568.
- Boyce S (Washington Institute of Dermatologic Laser Surgery, Washington, DC 20037, USA), Alster T. CO2 laser treatment of epidermal nevi: Long-term success. Dermatol Surg. 2002 Jul;28(7):611–614.
- Arad Ehud, Zuker M. Ronald, The shifting paradigm in the management of giant congenital melanocytic nevi: review and clinical applications, Plast Reconstr Surg., 2014 Feb;133(2):367-376.
- Mologousis MA, Tsai SY, Tissera KA, Levin YS, Hawryluk EB., Updates in the Management of Congenital Melanocytic Nevi., Children (Basel). 2024 Jan 2;11(1):62.
- Bristow I, Bower C. Melanoma of the Foot. Clin Podiatr Med Surg. 2016 Jul;33(3):409-22.
- Lallas A, Kyrgidis A, Koga H, Moscarella E, Tschandl P, Apalla Z, Di Stefani A, Ioannides D, Kittler H, Kobayashi K, Lazaridou E, Longo C, Phan A, Saida T, Tanaka M, Thomas L, Zalaudek I, Argenziano G. The BRAAFF checklist: a new dermoscopic algorithm for diagnosing acral melanoma. Br J Dermatol. 2015 Oct;173(4):1041-9.
- Bartoš V, Kullová M. Malignant Melanomas of the Skin Arising on the Feet. Klin Onkol. 2018 Summer;31(4):289-292.
- Palicka GA, Rhodes AR. Acral melanocytic nevi: prevalence and distribution of gross morphologic features in white and black adults. Arch Dermatol. 2010 Oct;146(10):1085-94.
- Bristow IR, de Berker DA, Acland KM, Turner RJ, Bowling J. Clinical guidelines for the recognition of melanoma of the foot and nail unit. J Foot Ankle Res. 2010 Nov 01;3:25.
- Gershenwald JE, Ross MI. Sentinel-lymph-node biopsy for cutaneous melanoma. N Engl J Med. (2011) 364:1738–45. 10.1056/NEJMct1002967.
- Eggermont AM, Spatz A, Robert C. Cutaneous melanoma. Lancet. (2014) 383:816–27.
- Elder DE. Precursors to melanoma and their mimics: nevi of special sites. Mod Pathol. (2006) 19(Suppl. 2):S4–20.
- Gershenwald JE, Ross MI. Sentinel-lymph-node biopsy for cutaneous melanoma. N Engl J Med. (2011) 364:1738–45.
- Eggermont AM, Spatz A, Robert C. Cutaneous melanoma. Lancet. (2014) 383:816–27.
- van Krieken JH, Boom BW, Scheffer E. Malignant transformation in a naevus of Ito. A case report. Histopathology 1988; 12: 100–2.
- Wise SR, Capra G, Martin P et al. Malignant melanoma transformation within a nevus of Ito. J Am Acad Dermatol 2010; 62: 869–74.
- Pérez ME, Bley C, Cárdenas C. Nevus of Ota, a classic presentation. Med Clin (Barc) 2019; 153: 92.
- Agarwal P, Patel BC. Nevus of Ota and Ito. StatPearls 2020.
- Kang HY, Kang WH. Bilateral type of nevus of Ota presenting as agminated lentigines. Eur J Dermatol EJD 2003; 13: 205–6.
- Turnbull JR, Assaf C, Zouboulis C, Tebbe B. Bilateral naevus of Ota: a rare manifestation in a Caucasian. J Eur Acad Dermatol Venereol JEADV 2004; 18: 353–5.
- Hori Y, Kawashima M, Oohara K, Kukita A. Acquired, bilateral nevus of Ota-like macules. J Am Acad Dermatol 1984; 10: 961–4.
- Que SKT, Weston G, Suchecki J, Ricketts J. Pigmentary disorders of the eyes and skin. Clin Dermatol 2015; 33: 147–58.
- Martínez-Peñuela A, Iglesias ME, Mercado MR et al. Malignant transformation of a nevus of Ito: description of a rare case. Actas Dermosifiliogr 2011; 102: 817–2.
- Wise SR, Capra G, Martin P et al. Malignant melanoma transformation within a nevus of Ito. J Am Acad Dermatol 2010; 62: 869–74.
- Martínez-Peñuela A, Iglesias ME, Mercado MR et al. Malignant transformation of a nevus of Ito: description of a rare case. Actas Dermosifiliogr 2011; 102: 817–20.
- Zayour M, Lazova R. Congenital melanocytic nevi. Clin Lab Med. 2011 Jun;31(2):267-80.
- Schaffer, JV. Update on melanocytic nevi in children. Clin Dermatol. 2015 May-Jun;33(3):368-86.
- Kinsler VA, O'Hare P, Bulstrode N, Calonje JE, Chong WK, Hargrave D, Jacques T, Lomas D, Sebire NJ, Slater O. Melanoma in congenital melanocytic naevi. Br J Dermatol. 2017 May;176(5):1131-1143.
- Price, HN. Congenital melanocytic nevi: update in genetics and management. Curr Opin Pediatr. 2016 Aug;28(4):476-82.
- Vergier B, Laharanne E, Prochazkova-Carlotti M, de la Fouchardière A, Merlio JP, Kadlub N, Avril MF, Bodemer C, Lacoste C, Boralevi F, Taieb A, Ezzedine K, Fraitag S. Proliferative Nodules vs Melanoma Arising in Giant Congenital Melanocytic Nevi During Childhood. JAMA Dermatol. 2016 Oct 01;152(10):1147-1151.
- Flores-Sarnat, L. Neurocutaneous melanocytosis. Handb Clin Neurol. 2013;111:369-88.
- Bandyopadhyay D. Halo nevus. Indian Pediatr 2014; 51: 850.
- Bristow IR, de Berker DA, Acland KM, Turner RJ, Bowling J. Clinical guidelines for the recognition of melanoma of the foot and nail unit. J Foot Ankle Res. 2010 Nov 01;3:25.
- Richtig, E. ASCO Congress 2018: melanoma treatment. Memo. 2018;11(4):261-265.
- Crompton JG, Gilbert E, Brady MS. Clinical implications of the eighth edition of the American Joint Committee on Cancer melanoma staging. J Surg Oncol. 2019 Jan;119(2):168-174.
- Richtig, E. ASCO Congress 2018: melanoma treatment. Memo. 2018;11(4):261-265.
- Bergstresser PR, Taylor JR. Epidermal 'turnover time'--a new examination. Br J Dermatol. 1977 May;96(5):503-9.
- Hoath SB, Leahy DG. The organization of human epidermis: functional epidermal units and phi proportionality. J Invest Dermatol. 2003 Dec;121(6):1440-6.
- Delevoye, C. Melanin transfer: the keratinocytes are more than gluttons. J Invest Dermatol. 2014 Apr;134(4):877-879.
- Pasparakis M, Haase I, Nestle FO. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol. 2014 May;14(5):289-301.
- Van Keymeulen A, Mascre G, Youseff KK, Harel I, Michaux C, De Geest N, Szpalski C, Achouri Y, Bloch W, Hassan BA, Blanpain C. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J Cell Biol. 2009 Oct 05;187(1):91-100.
- Patel P, Hussain K. Merkel cell carcinoma. Clin Exp Dermatol. 2021 Jul;46(5):814-819.
- Colebatch AJ, Ferguson P, Newell F et al. Molecular genomic profiling of melanocytic nevi. J Invest Dermatol 2019; 139: 1762–8.
- Kinsler VA, Thomas AC, Ishida M et al. Multiple congenital melanocytic nevi and neurocutaneous melanosis are caused by postzygotic mutations in codon 61 of NRAS. J Invest Dermatol 2013; 133: 2229–36.
| Immunohistochemistry (IHC) | a process that involves using of selective antibodies to target and stain antigens |
|---|---|
| Immunohistochemistry (IHC) | known to be specific to a particular condition. |
| Immunohistochemistry (IHC) | a process that creates a color signal that can be visualized on light microscopy. |
| Light microscopy | The 100x magnification usually requires a medium between the lens and the slide, such as oil immersion |
| Light microscopy | Most microscopes used in dermatopathology are compound microscopes. |
| Light microscopy | Is the primary means for the magnified examination of tissue blocks. |
| Light microscopy | Generally shows cells as colorless, necessitating the need for various stains. Most tissue is initially stained with hematoxylin and eosin (H&E). |
| Review the management options available for melanocytic nevi. | Describe the presentation of a patient with melanocytic nevi. |
| Identify the risk factors for melanocytic nevi. | Outline interprofessional team strategies for improving care coordination and communication to advance the management of melanocytic nevi and improve outcomes. |
| Atypical mole | Basal cell carcinoma | Café au lait spots |
|---|---|---|
| Cutaneous melanoma |
Nevi of Ota and Ito | Nevus spilus |
| Cockade nevus | Nodular lesions | Pyogenic granuloma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




