1. Introduction
Kiribati faces severe leprosy control challenges because of its widely dispersed population spread across 33 small atolls in the Pacific Ocean. The leprosy new case detection rate in Kiribati is one of the highest in the world with 11.5 cases per 10,000 population reported in 2022 [
1]. The WHO target for leprosy elimination of <1 case per 10,000 people per year was reached briefly in 2000 following a mass population screening and chemoprophylaxis programme (single dose rifampicin, ofloxacin and minocycline) in 1997 and 1998 [
2]. However, the impact of this intervention was not sustained, and the numbers of new cases and child cases increased during a period of passive surveillance between 1999-2009. Because of increasing numbers of cases, an intensified awareness programme was implemented in 2011, and an active case-finding programme was commenced in 2016 [
2].
Single dose rifampicin (SDR) post exposure chemoprophylaxis (SDR-PEP) has been shown to reduce the risk of newly incident leprosy amongst leprosy contacts by 57% at 2 years, with added benefit in those who have previously received BCG vaccination [
3]. Those most at risk of leprosy are household contacts although neighbours and social contacts are also at increased risk compared with the general population. Mathematical modelling has been used to estimate the possible benefit of various chemoprophylaxis approaches in Kiribati [
4]. This predicted that the introduction of household contact (HHC) chemoprophylaxis would lead to a gradual but sustained reduction in the number of new leprosy cases. More than 80% of i-Kiribati people receive the BCG vaccination at birth and therefore the efficacy of SDR-PEP in Kiribati was predicted to be strong.
After considering this evidence, the Ministry of Health and Medical Services (MH&MS) of Kiribati adopted SDR for household contacts as policy in 2017 and partnered with the Pacific Leprosy Foundation (PLF) to implement the chemoprophylaxis programme alongside pre-existing active case-finding activities. After careful consideration of both the potential intensity of contacts’ leprosy exposure and privacy of the index patient, a HHC was defined as a nuclear or extended family member who used the same kitchen as the index patient. Due to the high population density and the complexity of social networks in Kiribati (for example due to frequent use of village 'maneaba' (community buildings) for meetings, ceremonies and religious and educational activities), chemoprophylaxis of social contacts was not feasible as part of this intervention but was planned as part of a population-wide mass chemoprophylaxis programme when resources allowed. The chemoprophylaxis programme began in 2018 and consisted of retrospective (catch-up) screening and SDR chemoprophylaxis of HHCs of new leprosy cases diagnosed between 2010 and 2017 (retrospective cohort) as well as ongoing prospective screening and chemoprophylaxis of HHCs of new leprosy cases diagnosed from 2018 onwards (prospective cohort). The screening and case-finding was integrated into routine community clinical services and coordinated centrally by the National Leprosy Programme.
Since initiation of the chemoprophylaxis programme in Kiribati, further studies have demonstrated the feasibility and acceptability of contact tracing and SDR chemoprophylaxis [
5] and the approach has been endorsed by the World Health Organization (WHO) [
6]. Pillar two of the Global Leprosy Strategy 2021-2030 focuses on scaling-up leprosy prevention alongside integrated active case detection to break the chain of transmission [
7].
The objective of this study was to evaluate the completeness and timeliness of contact tracing and SDR chemoprophylaxis administration as well as the acceptability of the SDR chemoprophylaxis program across all health districts in Kiribati - a geographically widely dispersed and resource poor setting. A target of 80 percent coverage was regarded as minimum to justify the programme [
4].
2. Materials and Methods
This was a retrospective audit using routinely collected data on leprosy patients and contacts recorded in the electronic database of the National Leprosy Programme (NLP) in Kiribati.
2.1. Setting
The National Leprosy Programme is located in the skin clinic at the base hospital in Nawerewere in South Tarawa, the most populous island in Kiribati (population approximately 63,439) [
8]. The staff includes a doctor with postgraduate qualification in dermatology, a medical assistant and four specialist nurses. All suspected leprosy cases from South Tarawa and the Outer Islands of Kiribati are referred to the NLP for validation, complex case management and maintenance of clinical records. Leprosy cases are classified according to WHO criteria [
6]. In brief, paucibacillary (PB) disease is defined as a case with fewer than 5 skin lesions and multibacillary (MB) disease as a case with 5 or more lesions.
All primary care following case confirmation is delivered through village clinics located on the inhabited atolls. Community health services are divided into geographically related districts that each include multiple inhabited atolls. Routine care is integrated into the primary care clinical services run by medical assistants and nurses who are responsible for passive case detection, screening of HHCs, implementation of the SDR-PEP programme, provision of multidrug treatment packs and referral of patients with complications to the NLP.
An experienced leprologist (AC) visits South Tarawa regularly to conduct education sessions for medical assistants and nurses at the NLP and contact tracers. He also reviews and validates leprosy cases on South Tarawa. The specialist nurses from the NLP visit the outer islands regularly to conduct education of local nurses, validate cases and review the SDR-PEP programme.
2.1. Data collection
The NLP records the following data on physical data collection forms: patient registration number for both cases and contacts, name, address, current location of residence, date of diagnosis, treatment history, clinical data and date and dose of SDR, if received. The hard copy data entry forms are scanned and sent monthly to the PLF office in Christchurch, New Zealand where the information is entered into a secure Microsoft Access database ®. The database is used to generate lists of household contacts by index case and village to facilitate planning of contact tracing activities and workforce allocation.
2.2. SDR chemoprophylaxis program
The SDR-PEP programme was integrated into the services provided by general community clinics in 2018. After validation or consultation, the medical clinics are notified of a case and the staff are responsible for following up the cases, enumeration of the households, administering SDR-PEP and reporting data back via a standardised report form to the NLP. Index cases diagnosed from 2010-2017 were identified from the database for the retrospective component and notified to the appropriate clinic. New cases identified prospectively from 2018-2022 were notified to clinics at the time of diagnosis.
Each village medical clinic was supported to perform promotional activities to raise awareness of the programme, complimented by nationwide initiatives including use of national radio and social media. Clinic nurses were trained in the diagnosis of leprosy and with support from the NLP, set up contact tracing teams in each location. Nursing staff visited the residence of the index case, verified the accuracy of the leprosy diagnosis and sought consent to trace and screen all current and previous members and enumerated the household contacts. Household contacts (HHCs) of index patients were traced and screened at their household. Contacts were examined for signs of leprosy and any suspected cases were referred to the NLP for confirmation or exclusion of the diagnosis by a leprosy specialist. The remainder were given SDR immediately, except contacts of newly diagnosed patients for whom SDR administration was postponed for 1 month after MDT initiation.
2.3. Inclusion criteria
Household contacts were defined as all those family members sharing the same kitchen facilities as the index case. This included all those living in the household for more than 30 days at any time in the past 2 years. For the prospective component, HHCs were identified at the time of diagnosis of a new leprosy case.
2.4. Exclusion criteria for SDR PEP
The following rendered HHCs ineligible for SDR-PEP: current TB or leprosy treatment, pregnancy, age < 2 years, history of serious liver or kidney disease, severe medical illness requiring hospitalisation, terminal illness, known allergy to rifampicin or prior severe adverse effect with rifampicin use.
2.5. Chemoprophylaxis regimen
All contacts of leprosy cases meeting eligibility criteria were offered SDR as chemoprophylaxis. Single dose rifampicin dosing was based on age and weight and dosing regimens are outlined in the supplementary material (table S1).
2.6. Audit procedures
A systematic audit of electronic records for both the retrospective and prospective SDR-PEP cohorts held in the NLP database was performed. Index cases were cross-referenced with household contact information to ensure removal of duplicates and to identify new cases who had previously received SDR-PEP. The primary outcome measures included the proportion of retrospective and prospective contacts traced, SDR coverage in both cohorts, time to SDR administration and SDR refusal rate . The time to delivery of SDR-PEP for the retrospective component was defined as the interval between introduction of the programme in 2018 (1 January) and administration of the SDR-PEP dose, and in the prospective cohort, the time interval from diagnosis of the index case to administration of the SDR-PEP dose to contacts.
2.7. Data analysis
Analyses presented are descriptive. Continuous variables are summarised using means, medians, standard deviations, ranges and interquartile ranges as appropriate. Categorical variables are summarised with frequencies and percentages. Categorical variables were compared using Chi-squared tests. A p-value less than 0.05 was considered statistically significant. Analyses were done in Microsoft Excel® 2010.
2.8. Ethical committee approval
Ethical approval was obtained from the Kiribati Ministry of Health and Medical services, the University of Otago (H22/111) and the University of Sydney (project no. 2021/127).
3. Results
3.1. Results
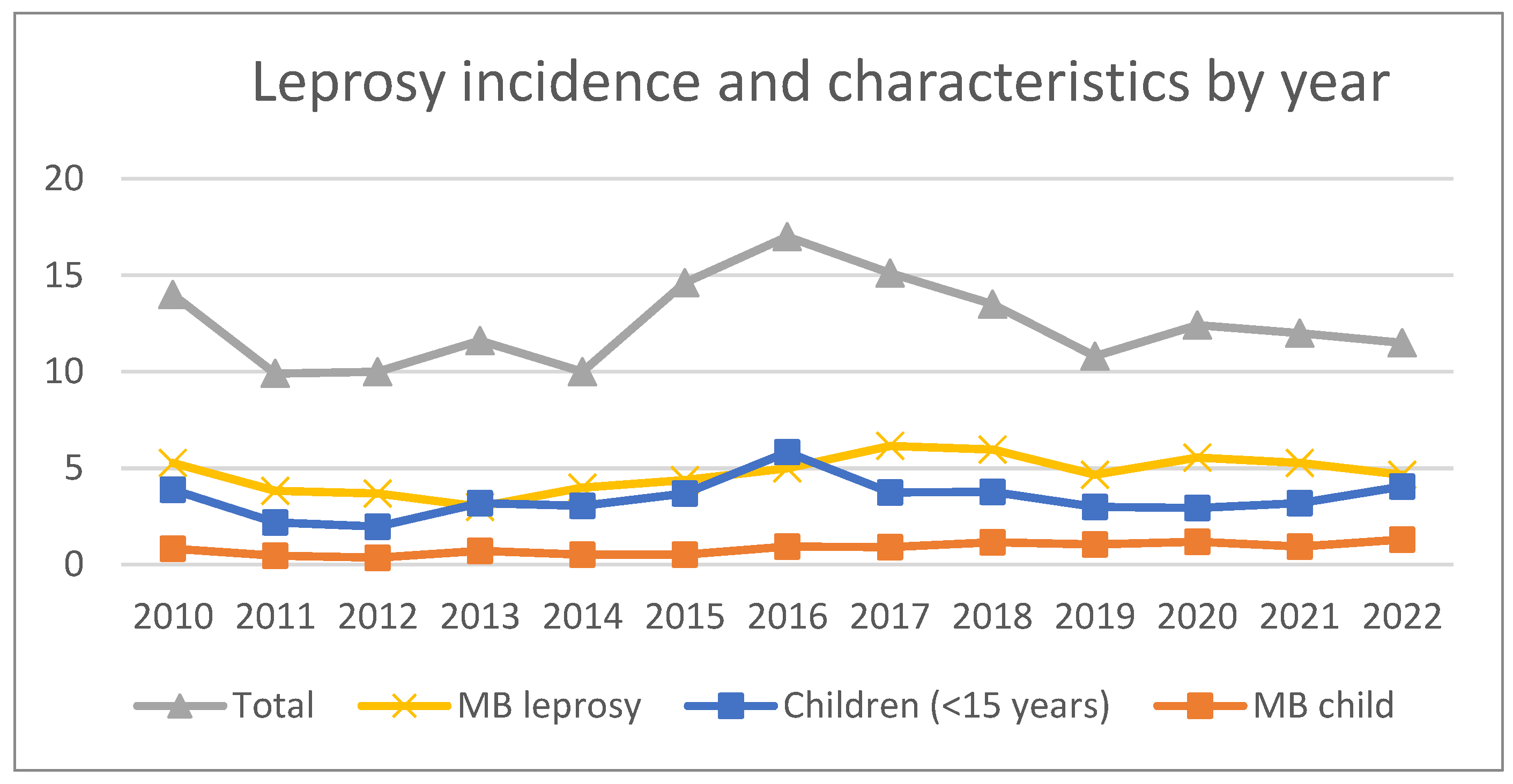
The annual incidence of new leprosy cases and disease characteristics over the study period are presented in
Table 1. The case detection rate peaked in 2016 at 202 cases per 10,000 population per year with a gradual decline in overall incidence in subsequent years. The rates of MB leprosy and childhood cases remained high at the end of the study period, accounting for 40% and 35% of cases in 2022 respectively (
Figure 1.). The characteristics of index patients from the five main health districts for both study cohorts are presented in
Table 2. There were 1,173 cases identified in the retrospective cohort, of whom 34% were classified as having MB leprosy. Four percent of patients had grade 2 disability at presentation and 27% were children under the age of 15 years. In the prospective cohort, 762 index cases were identified, of whom 43% were classified as having MB leprosy. Three percent had grade 2 disability and 28% were children under the age of 15 years.
Overall, there were 13,643 household contacts of index patients identified during the study period (9,791 in the retrospective and 3,850 in the prospective cohort) (Figure 2). 1,044 HHCs were either absent or unable to be traced in the retrospective cohort and 164 in the prospective cohort leaving 8,747 contacts who were screened in the retrospective and 3,688 in the prospective cohort. The number of HHCs in each health district, their screening status and reasons for exclusion from SDR-PEP are presented in
Table 3.
In the retrospective cohort, 8,297 received SDR-PEP, representing 95% of contacts screened and 84.7% overall SDR coverage of all contacts. The most common reasons for exclusion from chemoprophylaxis in this group were active or former leprosy (40%) and being less than 2 years of age (32%). Four patients (1%) refused chemoprophylaxis. There were 42 cases of leprosy diagnosed as a result of household contact screening in the retrospective cohort, representing 0.5% of those screened. Of these, 36% were classified as MB and 36% were children less than 15 years of age. There were no reported cases of grade 2 disability in this group (
Table 3).
In the prospective cohort, 3,392 contacts received SDR-PEP, representing 92% of contacts screened and 88.1% overall SDR coverage of all enumerated contacts. The most common reasons for exclusion from SDR-PEP were being less than 2 years of age (50%) followed by pregnancy (15%) and active or former leprosy (14%). Nineteen patients (8%) in the prospective cohort refused chemoprophylaxis. There were 23 new diagnoses of leprosy as a result of screening of household contacts in the prospective cohort (0.6% of those screened). Of these, 7 (30%) were classified as MB, 2 (9%) had grade 2 disability and 6 (26%) were children under the age of 15 (
Table 3).
The median time to administration of SDR-PEP for each of the health districts over the study period is presented in table 3. In the retrospective cohort, the median time between the intervention start date and receiving SDR-PEP was 220 days (interquartile range [IQR] 162-468 days). Those closer to larger population centres received prophylaxis sooner than more geographically isolated contacts, such as those in the outer islands. In the prospective cohort, the median time to receiving SDR-PEP was 120 days (IQR 36-283 days). The percentage of cases of PB in children decreased after the introduction of the SDR-PEP programme (2018-2022) compared with the retrospective study period (2010-2017), (mean 81% v 69%, p=0.0002).
4. Discussion
The aim of this audit was to determine whether it was practicable to integrate an active screening and chemoprophylaxis programme into routine clinical leprosy services in a resource-poor setting with a widely dispersed population in the Pacific. This programme was adopted as policy in 2017 and implemented in 2018 by the MH&MS of Kiribati as a tool to improve leprosy control in response to the very high new case detection rate. Overall, we found that >85% of HHCs were traced in both the retrospective and prospective cohorts, a significant number of new cases were identified on screening and a very high acceptance rate of SDR-PEP was observed. However, there were significant delays to SDR administration, largely because of the widely dispersed population.
Retrospective SDR-PEP to our knowledge has only been reported previously in Cambodia, a country with a much greater population and significantly lower leprosy endemicity than Kiribati [
10]. The average number of contacts per index case screened in the Cambodian study was higher than in Kiribati (19 vs 7) but the proportion of contacts screened was lower (72% vs 91%) and number of exclusions significantly higher (17.4% vs 5%) in Cambodia compared to Kiribati. The number of new cases detected amongst HHCs in Cambodia (0.4% of those screened, 1/3 of whom were neighbours) was similar to that in Kiribati (0.5%). These results demonstrate that the retrospective approach provides useful gains in terms of case detection and can be used as part of an enhanced control strategy in both low and high endemic settings.
Overall, the programme in Kiribati screened 12,435 contacts which represents 10% of the total population. After exclusions, SDR-PEP was administered to 8,297 and 3,392 contacts in these cohorts respectively. The proportion of contacts identified and screened was lower in the retrospective cohort than the prospective one. The primary reason for this was the high number of people who moved away from the area where they had been a HHC in the retrospective cohort. Most of these HHCs had moved within Kiribati but it was often not possible to identify their new location as there is no street address system in Kiribati and it was not practicable to conduct house-to-house enquiries. A smaller group had moved to other countries and were also not traced. Contacts were often not available during daylight hours when people were unavailable because of work, fishing or visiting neighboring islands.
The very high rate of acceptance of SDR-PEP in both groups is consistent with previous studies that have indicated that people with leprosy are very keen to prevent their relatives and contacts from getting leprosy [
12]. Other factors that may have contributed to this success are the effectiveness of the initial communication and awareness programmes that were conducted by local community health workers who were known to, and trusted by the populace, and the effective communication skills of the nurses doing the screening. Unfortunately, as access to the internet is becoming available in Kiribati, misinformation about leprosy is surfacing.
The number of HHCs enumerated and traced per index case was lower than expected particularly in the prospective cohort. The definition used in this programme was much more limited than that proposed by WHO and used in other studies as it did not include neighbours or social contacts. (1,5,12) This was done because of concerns about the confidentiality of including extended contacts given the stigma associated with leprosy [
13]. Certain areas of Kiribati are densely populated (for example in the most populous part of South Tarawa, Betio, approximately 20,000 people live in 1.5 square kilometers). Defining the limits of significant contact exposure in such environments is very difficult. For this reason, a mass SDR-PEP programme was seen as a necessary in addition to the contact tracing and such a programme has now been commenced as part of a large implementation study combining leprosy treatment and mass chemoprophylaxis with enhanced tuberculosis detection, treatment and chemoprophylaxis [
14].
The wide geographic distribution of inhabited islands across millions of square kilometers of ocean posed a significant barrier to implementation of the SDR-PEP programme in Kiribati. There were significant delays in implementation of the policy in some health districts. This is related to the very high costs of maintaining education, competence and enthusiasm of local staff in remote areas. To improve performance, staff from the NLP visited the outer islands as part of multidisciplinary teams to both support local staff, help with screening contacts and administration of SDR-PEP. Some delays were also attributable to the COVID control measures in 2019-21 that included lockdowns, interruption of supply of rifampicin, and reallocation of staff to other duties.
The roll-out of the project had additional indirect benefits as previously reported by others [
5]. The SDR-PEP project put renewed attention on the high rates of leprosy at the political level, increased leprosy awareness amongst the general population and improved leprosy knowledge of staff at the NLP as well as medical assistants and nurses at the community clinics.
In conclusion, the SDR-PEP programme integrated into routine community services produced high coverage and acceptability in Kiribati. A centralised database managed through the NLP was pivotal and additional human resource from community clinics was needed to support contact tracing activities and ensure adequate SDR-PEP coverage. Both the retrospective and prospective components identified a significant number of new cases. This makes an important contribution to leprosy control but needs to be supported with mass chemoprophylaxis given the high new case detection rate and population density of Kiribati.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Table S1: Single dose rifampicin (SDR) chemoprophylaxis dosing.
Author Contributions
Conceptualization, S.C., T.B., A.C., E.T. and E.R..; methodology, S.C., P.C., and T.B.; formal analysis, PC, SC and ND; investigation, TB, ER, CB and SC; data curation, P.C., T.B., C.B., N.D. and S.C.; writing—original draft preparation, P.C. and S.C.; writing—review and editing, S.C., N.D., A.C., N.I., P.C., C.B., E.R., E.T. and T.B.; supervision, S.C., A.C. and N.D.; project administration, E.R.; All authors have read and agreed to the published version of the manuscript.
Funding
CB received Health Research Council of New Zealand funding for a summer studentship. This research received no other external grant funding.
Institutional Review Board Statement
Ethical approval was obtained from the Kiribati Ministry of Health and Medical services, the University of Otago (H22/111) and the University of Sydney (project no. 2021/127).
Informed Consent Statement
Informed consent was obtained from all subjects prior to tracing of household contacts. Patient consent for accessing records was not sought specifically for this project as it is an audit of health information collected routinely as part of the management of the condition for which they are being treated and did not require any further information for completion.
Data Availability Statement
Available upon reasonable request
Acknowledgments
The authors would like to acknowledge the support of the Ministry of Health and Medical Services in Kiribati and the Pacific Leprosy Foundation for the programmatic and administrative support provided for the project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. World Health Organization. Global leprosy (Hansen disease) update, 2022: New paradigm - control to elimination. 2023.
- Chambers ST IN, Timeon E, Rimon E, Murdoch H, Green J et al. . Surveillance of Leprosy in Kiribati, 1935-2017. Emerg Infect Dis 2020, 26, 833–840.
- Moet FJ PD, Oskam L, Richardus JH. ; COLEP study group. Effectiveness of single dose rifampicin in preventing leprosy in close contacts of patients with newly diagnosed leprosy: Cluster randomised controlled trial. BMJ 2008, 336, 761–764.
- Gilkison C CS, Blok DJ, Richardus JH, Timeon E. , Rimon E et al. Predicting the impact of household contact and mass chemoprophylaxis on future new leprosy cases in South Tarawa, Kiribati: A modelling study. PLoS Negl Trop Dis 2019, 13, e0007646.
- Richardus JH TA, Barth-Jaeggi T, Arif MA, Banstola NL, Baskota R et al. Leprosy post-exposure prophylaxis with single-dose rifampicin (LPEP): An international feasibility programme. Lancet Glob Health 2021, 9, e81–e90.
- Guidelines for the diagnosis, treatment and prevention of leprosy: World Health Organization; 2018 [Available from: https://apps.who.int/iris/bitstream/handle/10665/274127/9789290226383-eng.pdf.
- WHO. Towards zero leprosy. Global leprosy (Hansen’s Disease) strategy 2021–2030. 2021.
- Kiribati National Statistics Office [Available from: https://nso.gov.ki/census-surveys/.
- World bank population estimates and projections [Available from: https://databank.worldbank.org/source/population-estimates-and-projections.
- Cavaliero A AS, Aerts A, Lay S, So V, Robijn J, Steinmann P. . Preventing leprosy with retrospective active case finding combined with single-dose rifampicin for contacts in a low endemic setting: Results of the Leprosy Post-Exposure Prophylaxis program in Cambodia.. Acta Trop 2021, 224, 106138.
- Faatoese A SS, Priest P, Chambers S. Knowledge and attitudes to leprosy of Pacific People living in New Zealand. Lepr Rev 2016, 87, 368–377.
- Fürst T CA, Lay S, Dayer C, Chan S. , Smrekar A et al. Retrospective active case finding in Cambodia: An innovative approach to leprosy control in a low-endemic country. Acta Trop 2018, 180, 26–32.
- Thompson L, Ioteba N, Chambers S. Leprosy in Kiribati: The lived experience. Leprosy Review 2020, 91, 353–366.
- Coleman M. HJ, Timeon E; et al. Effectiveness of population-wide screening and mass drug administration for leprosy control in Kiribati: The COMBINE protocol. BMJ Open 2023, 13, e065369. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).