3. Results
In
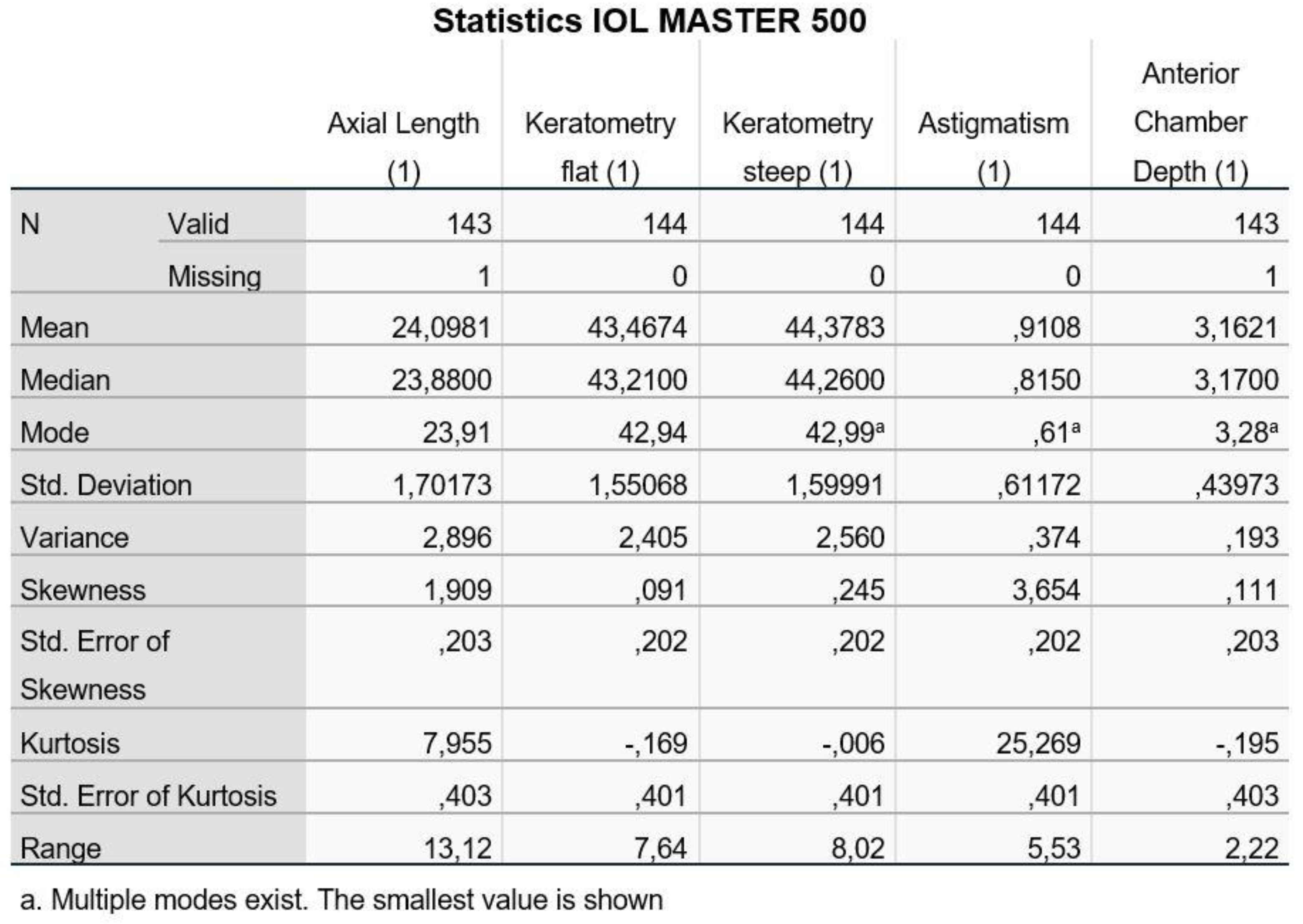
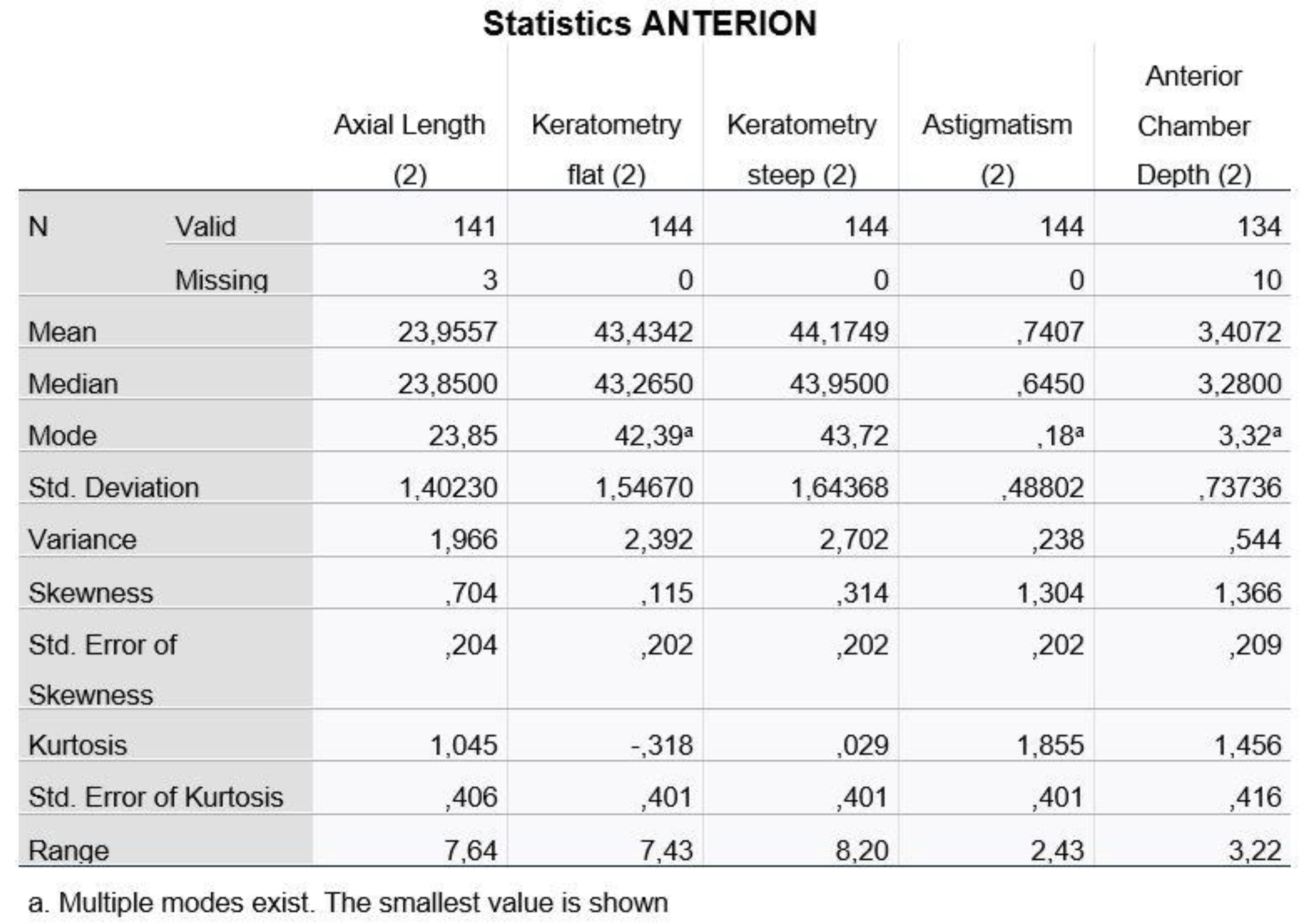
Figure 1 and
Figure 2, we present the descriptive data for the five variables as recorded with the two machines (1: IOL Master 500, 2: ANTERION).
We notice that for the Axial Length and the Anterior Chamber Depth, we have values that we did not measure (missing values) and, therefore, the statistical analysis program recognizes them as incomplete.
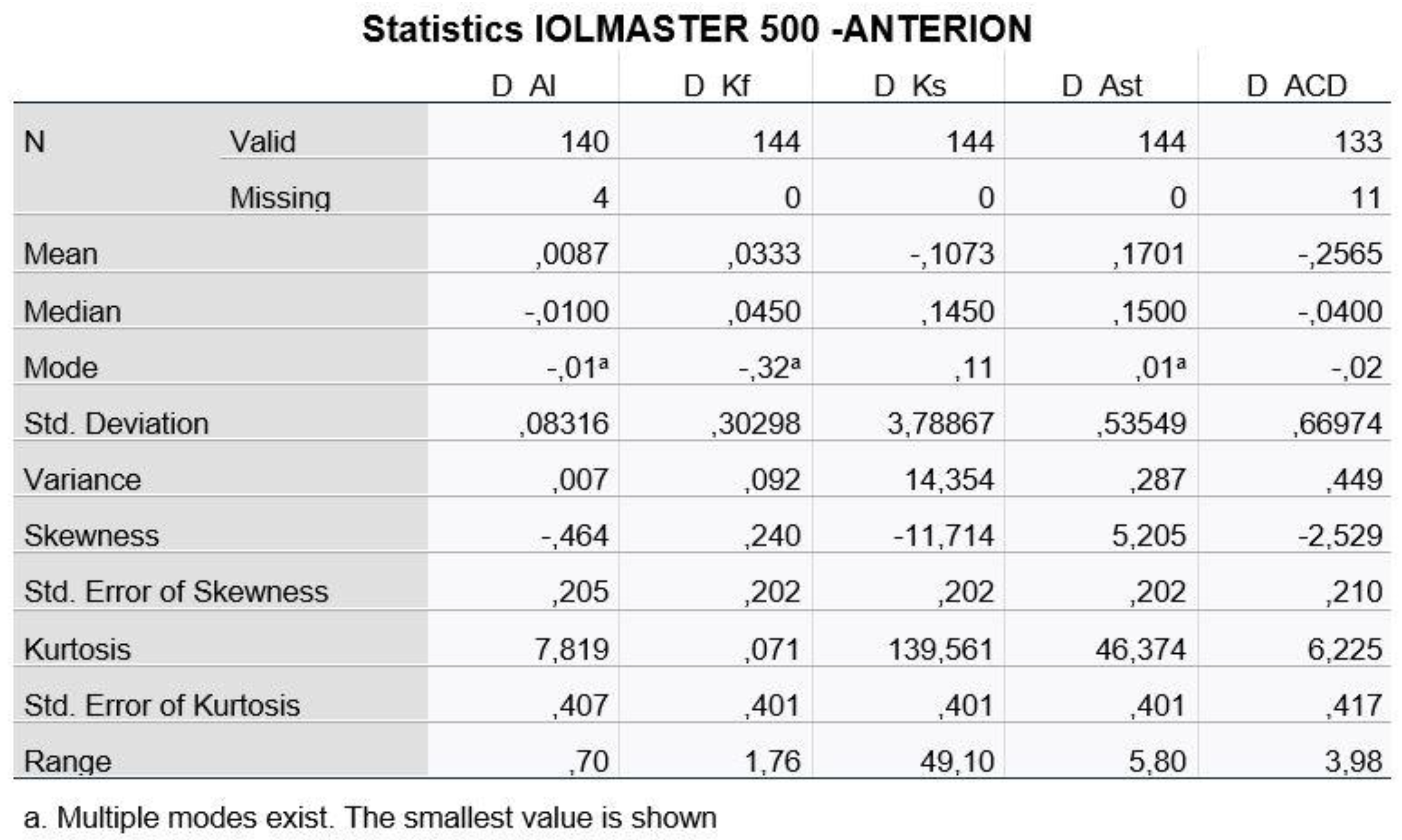
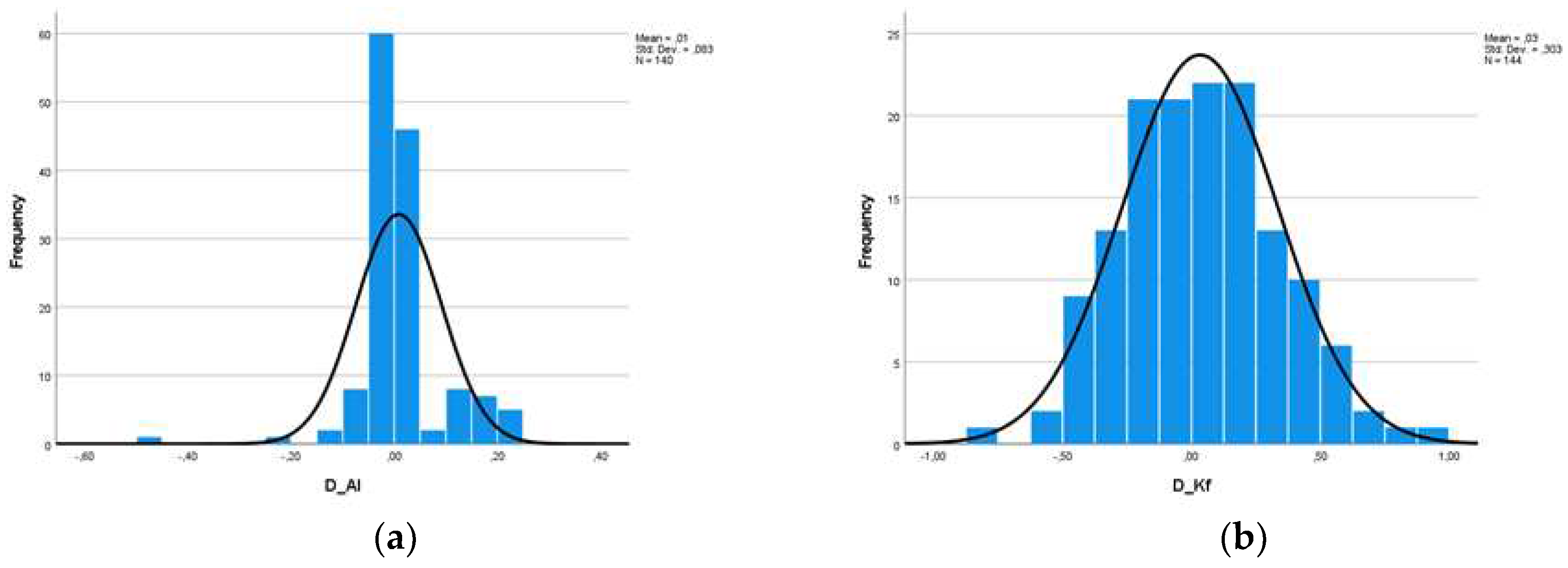
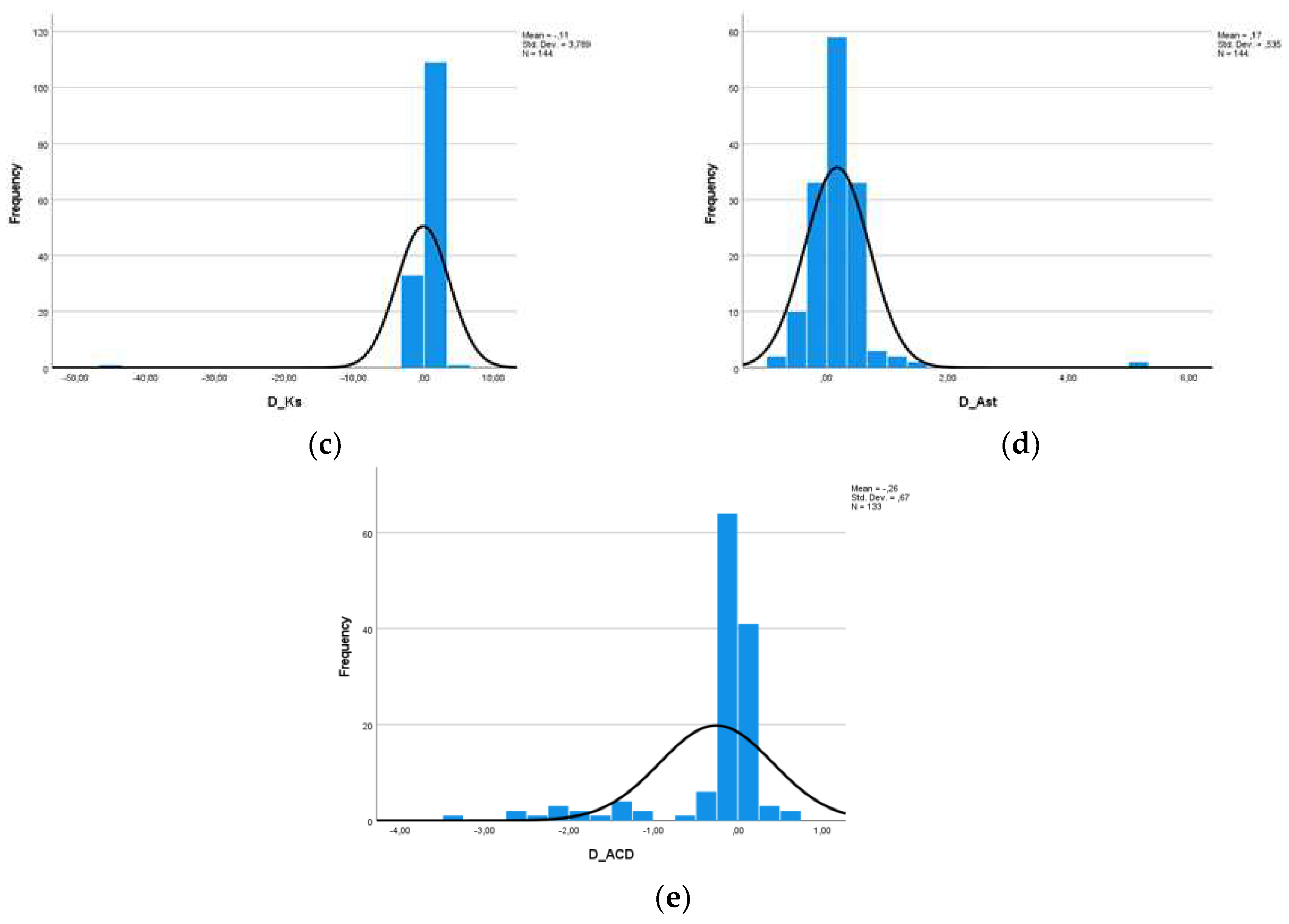
At this point, we should mention, however, that the measurements of the same person from two different machines cannot be considered as two independent populations, a condition which is necessary in order to carry out a statistical study. For this reason, we defined five new variables, which are the difference of the measurements of each variable for the two machines (D_AL, D_Kf, D_Ks, D_Ast and D_ACD). Descriptive data as well as histograms of the new variables are shown in
Figure 3 and
Figure 4.
Based on the descriptive data obtained as well as the diagrams shown, we assumed from the early beginning that the variables probably do not follow the Normal Distribution, maybe only the difference of Kf. Nevertheless, in adherence to the rules of statistical analysis, we performed hypothesis testing (1):
H0 : The data comes from a Normal Distribution, vs
H1 : The data does not come from a Normal Distribution.
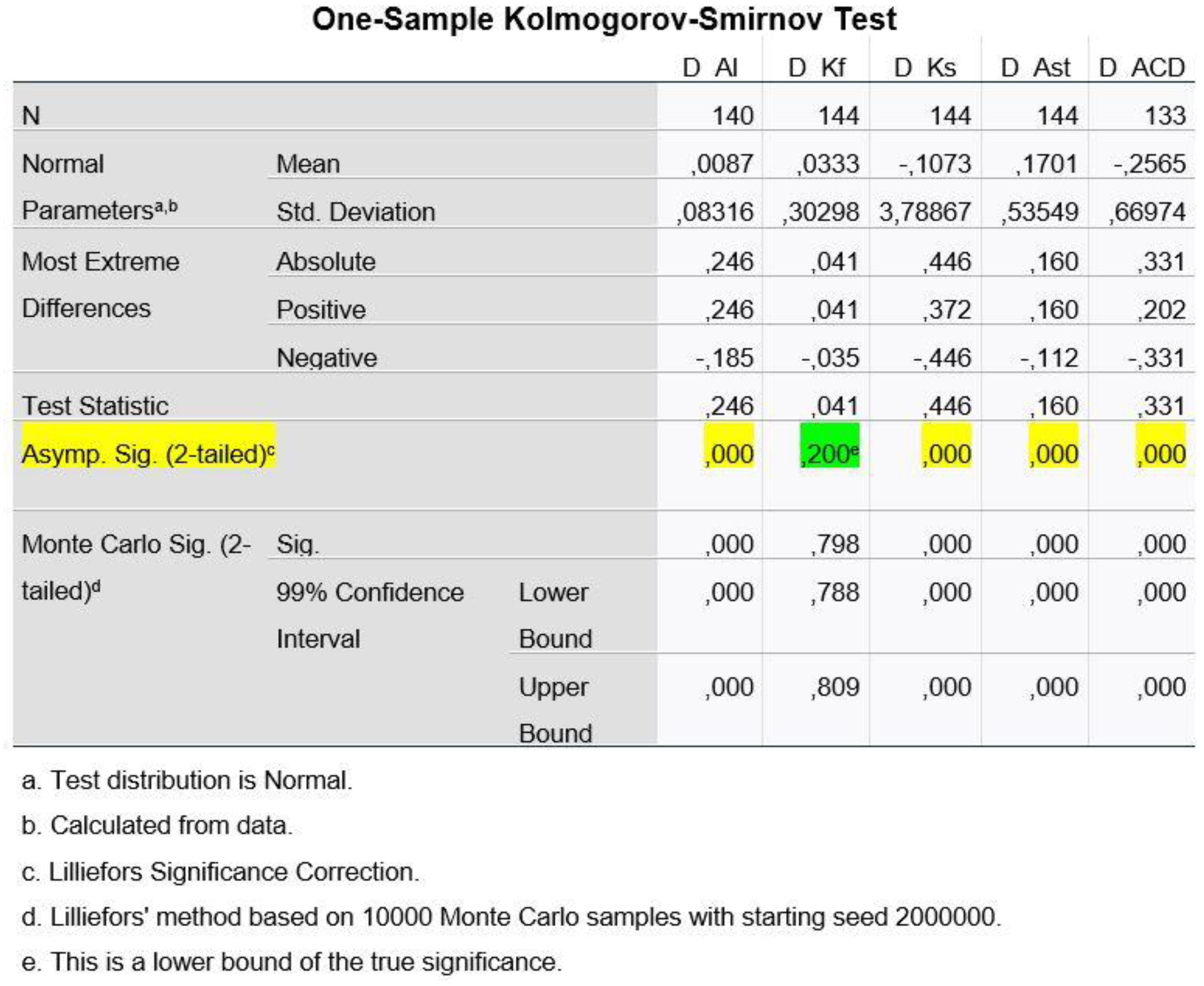
To test the hypothesis (1) about Normal Distribution of our variables, we used the Kolmogorov-Smirnov Test, for each of the five variables and, depending on the result we got, we chose the method with which we performed the rest statistical procedure, as to say to test hypothesis (2), i.e.:
H0 : The means of the two populations do not differ, vs
H1 : The means of the two populations differ statistically significantly,
which is the main objective of this research. In other words, the aim was to ascertain if the measurements of the two machines regarding the variables under consideration differ statistically significantly.
Figure 5 shows that, at a statistical significance level of 1%, the differences of the variables AL, Kst, Ast and ACD have a p-value < 0.01 and therefore we reject H0 from hypothesis (1) (H0: The data come from Normal Distribution). Only for Kf can we claim that the differences follow the Normal Distribution.
Hence, to test hypothesis (2) (H0: The means of the two populations do not differ), we cannot use the usual method of Paired Samples T-test for paired observations, since Normal Distribution is not ensured, except for the variable D_Kf.
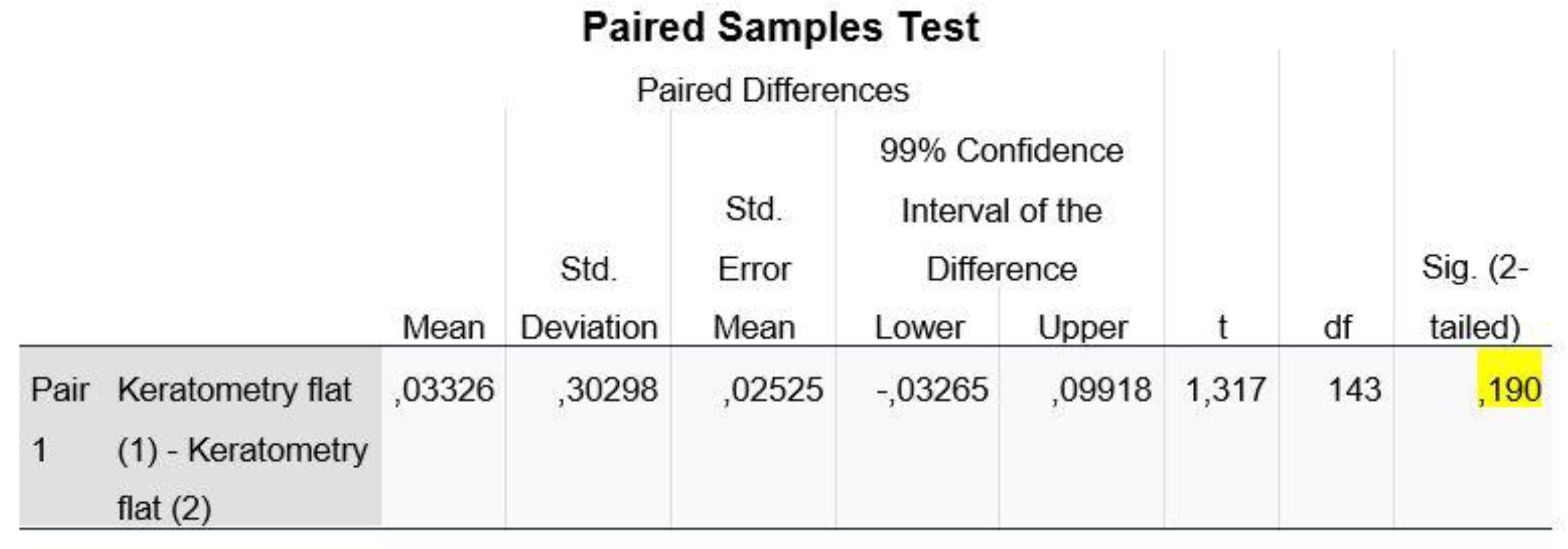
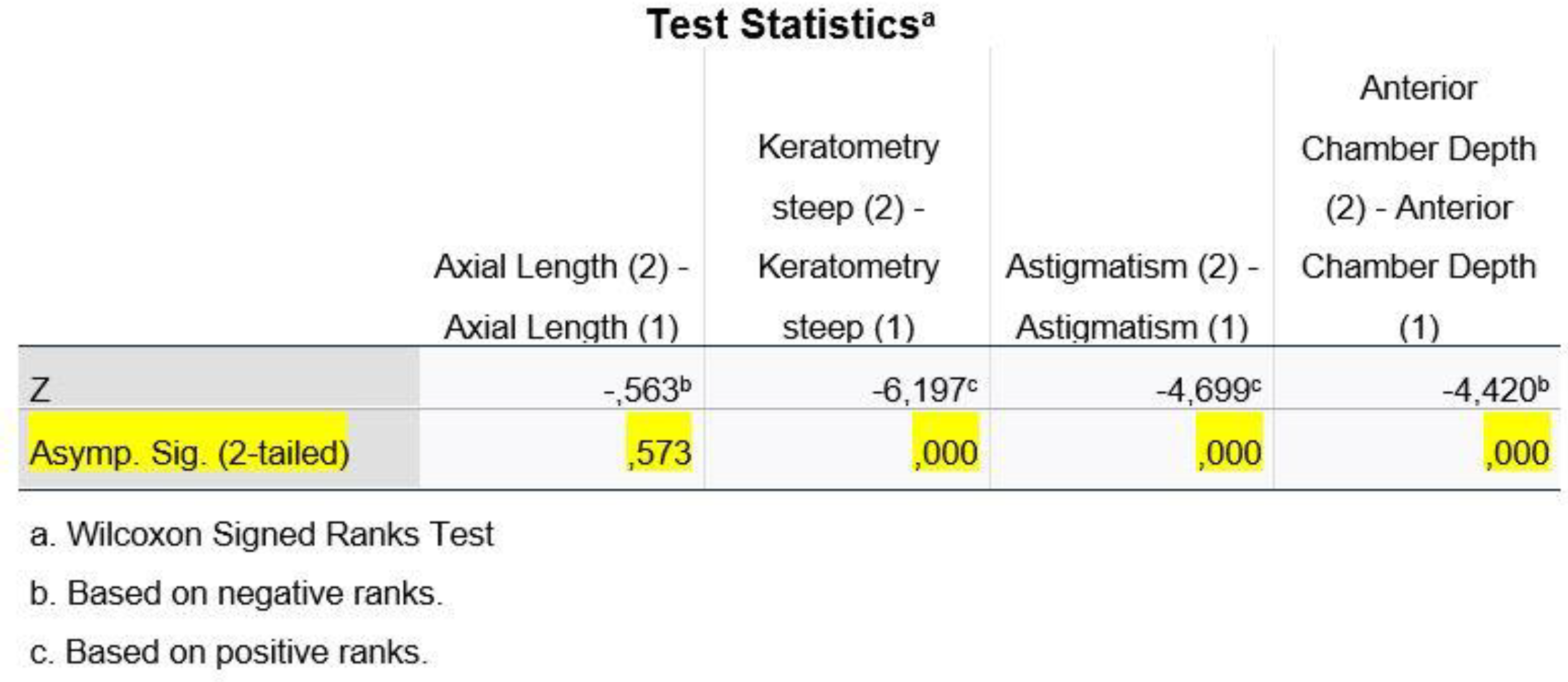
Regarding the variable of D_Kf, we ran the Paired Samples Test, whereas for the remaining variables (D_AL, D_Ks, D_Ast and D_ACD), we used the non-parametric Wilcoxon Signed Ranks Test method.
Figure 6 shows that, at a statistical significance level of 1%, the p-value of the control is 0.19 >0.01 and thus we do not reject our H0 from hypothesis (2) (H0: The means of the two populations do not differ). Therefore, we cannot claim that the corneal meridian Kf is statistically significantly different in the two machines.
In
Figure 7, we observe that, at a statistical significance level of 1%, the variables D_Ks, D_Ast and D_ACD are statistically significantly different, as the p-value<0.01 and therefore we reject the H0 from hypothesis (2), while for the variable AL we have a p-value >0.01 and therefore we do not reject the H0 from hypothesis (2) (H0: The means of the two populations do not differ).
In conclusion, we could say that the IOL Master 500 and ANTERION machines do not appear to be statistically significantly different in terms of axial length (AL) and flat corneal meridian (Kf) measurements, while they appear to be statistically significantly different in terms of steep corneal meridian (Ks), astigmatism and anterior chamber depth measurements.
4. Discussion
The global prevalence and incidence of age-related cataract has been a field of study by many researchers in the last fifteen years. According to the World Health Organization (WHO), 18,000,000 people worldwide suffer from blindness due to bilateral cataract. Other studies have shown that 36,000,000 people are blind worldwide and over 13,500,000 of them are due to cataract [
1]. The highest percentage for any type of cataract is recorded in the region of South-East Asia with 36.55% as well as for the posterior subcapsular subtype, while the highest rates for cortical and nuclear cataract are recorded in the Western Pacific. On the other hand, the lowest percentage of 9.08%, is detected in the region of America [
2].
Cataract prevalence is higher in less developed societies due to low financial status, high levels of illiteracy, a high rate of outdoor activity that leads to longer sun exposure, and of course more limited access to cataract surgery services. More specifically, nuclear cataract has been linked to poorer nutrition, lower socio-economic status, lower educational and occupational attainment. The risk of nuclear cataract also increases with the amount and duration of smoking, as well as with the exposure to smoke produced by household fuels during cooking. Concerning cortical cataract, positive risk factors have been reported to be high exposure to sunlight, heredity, lens size (an increased incidence of cortical opacity is associated with smaller lens size, in contrast with nuclear cataract), increasing age (according to AREDS: Age-Related Eye Disease Studies), diabetes mellitus and smoking, while male sex, white race and high educational level are considered as negative risk factors [
2,
4]. Finally, studies have shown that patients with diabetes mellitus have an increased risk of developing posterior subcapsular cataract, as well as cortical cataract, although those with well-controlled blood sugar have a similar risk as non-diabetic people [
2]. Other risk factors for posterior subcapsular cataract are high myopia, exposure to therapeutic doses of steroids and ionizing radiation, male sex, excessive weight change, and use of thyroid hormones, while hyperopia appears to be associated with a reduced risk of posterior subcapsular cataract [
2,
5].
The main cause of cataract development and progression seems to be age (age-related cataract), with an age over 60 years old being considered an important threshold for its appearance [
1,
2,
3,
4,
5]. The most common type of this cataract etiology appears to be nuclear, followed by cortical and finally posterior subcapsular [
1,
2,
3,
4]. At the same time, due to the continuous increase in the average age globally, and especially on the European continent, an increase in the prevalence of age-related cataract is expected in future [
2,
5]. It becomes apparent that age is directly related to cataract, as it is considered a normal part of the aging process. Nonetheless, some scholars disagree with this age-cataract causal theory and believe that this is a cumulative effect of certain risk factors, such as UV radiation or oxidative damage [
1,
4]. In addition, certain genetic and environmental factors, such as smoking, alcohol consumption, high body mass index, certain diseases, such as diabetes mellitus, hypertriglyceridemia, asthma, or chronic bronchitis, cardiovascular disease, pterygium, uveitis, high myopia, eye trauma, IOP-lowering drugs and surgery, extensive steroid use (systemic, inhaled, and topical), estrogen intake, and certain occupations increase the risk of cataract appearance [
1,
2,
3,
4]. As a result, it seems that a balanced diet, a rational use of antioxidants and the avoidance of oxidizing agents in our lifestyle such as smoking, excessive alcohol consumption and excessive exposure to ultraviolet radiation, still remain, for the time being, the best prevention measures in delaying the onset and progression of cataract [
3,
4].
Regarding gender, according to the available data, there is still no clear agreement. Some studies suggest female gender as a risk factor for the development of cataract, attributing this view to hormonal disturbances especially at older ages [
2,
4], to greater exposure to fuel and household waste, to insufficient access to reproductive health services as well as to genetic variations. However, some other studies support exactly the opposite, suggesting male gender as a risk factor due to higher UV exposure and higher smoking rates [
2].
Based on the above, we understand the high incidence of cataract, which affects the quality of everyday life of a huge number of people, the rapid increase of its prevalence due to the increase in average life expectancy and finally the necessity to correct it, due to the financial issues that it creates, since cataract constitutes one of the main problems of vision loss [
6].
Adequate cataract removal by phacoemulsification and intraocular lens insertion is now recognized as the most effective approach to cataract correction worldwide [
5,
7]. The accurate calculation of the power of the intraocular lens (IOL) is one of the most vital points of the preoperative check of cataract patients, especially nowadays, where the widespread use of premium intraocular lenses has rendered phacoemulsification a refractive surgery technique, with patients of particularly high demands and expectations for their visual outcome [
5,
6]. To accurately measure the power of IOL, depending on the selected type of calculation formula, we use the values of axial length (AL), keratometry (Κs-K steep and Kf-Kflat), anterior chamber depth (ACD), thickness of the crystalline lens (LT), as well as the diameter of the cornea (WTW:White-To-White distance) [
8,
9]. Among these factors, it has been shown that axial length (AL) is the main factor that affects and determines mostly any deviation from the desired refractive result that we target according to willingness of each patient [
7,
10,
11,
12,
13].
Over the years, due to the continuous development of technology in the field of cataract surgery, the way we calculate the appropriate power of the desired IOL has progressively changed. In the beginning, cataract surgeons were limited to the use of a simple mathematical formula, that took into consideration only the preoperative refractive error of the eye (IOL power =18 + 1.25 x refractive error), whereas, later in the 1970s, formulas that take into account values of Axial Length (measured by ultrasound) and Corneal Curvature were available [
6]. Biometry performed by using A-scan ultrasonography, which first appeared in the 1950s, uses echo delay time to measure the distances of intraocular structures. It features a resolution of 200μm and an accuracy of 100–120μm in axial length measurement. However, studies have shown that an error of 100 μm in this measurement could lead to 0.28 D of postoperative refractive error [
5]. In addition, the ultrasound technique requires contact with the eye and has the disadvantage of corneal pressure during measurement, which can be uncomfortable for the patient and often lead to incorrect subjective measurements, depending on the pressure exerted on the eye by each examiner. These disadvantages have led the scientific community to search for newer, more objective and more accurate formulas to calculate IOL power [
5,
6].
In recent years, these formulas have evolved and therefore check more parameters in order to increase the precision in the selection of the suitable IOL and finally achieve the desired postoperative refractive result, giving space to optical biometry and leaving aside the ultrasound one. The acoustic waves used in ultrasound biometry are being replaced by light in optical biometry, which has become popular because it is an easy, non-invasive procedure without eye contact, with good repeatability and high accuracy [
6,
14].
Therefore, nowadays, optical biometry is considered to be the gold standard for measuring ocular parameters and calculating IOL power in cataract patients [
7,
10,
11,
12,
13,
14]. Modern optical biometry tools are based on different technologies, including Partial Coherence Interferometry, Optical Low Coherence Reflectometry, Optical Low Coherence Interferometry, and recent biometers that use Swept-Source Optical Coherence Tomography technology [
6,
9,
14,
15,
16].
The IOLMaster 500 (version 5.5, Carl Zeiss Meditec, Germany) is the major representative of the Partial Coherence Laser Interferometry technology. This optical biometry technique is a non-contact method, thus it does not require local anesthesia of the cornea, which provides acquisition of values of keratometry, anterior chamber depth and of course axial length in a single measurement with high resolution (12 μm) and accuracy (0.3 –10 μm) 10 times greater than that achieved by the conventional ultrasound [
7,
17,
18]. It can achieve reliable AL measurements in pseudophakic eyes, a clinically important feature especially in eyes that may require a piggyback IOL [
7,
19,
20,
21]. Finally, it shows an advantage over traditional ultrasound biometry in measuring AL of eyes with silicone oil or posterior staphyloma [
7]. On the other hand, since PCI relies on adequate fixation through the fovea, eyes with corneal scars, dense cataract, posterior opacity, macular degeneration, and eccentric fixation, fail to achieve reliable results [
7,
21,
22,
23]. It should be emphasized that there is a tendency for hyperopic shift in eyes undergoing PCI, probably because the axial length is measured about 100 μm longer than using ultrasonography (distant and no-touch approach) [
7,
19]. In the IOLMaster 500, the ACD is measured along the optical axis from the corneal epithelium to the anterior crystalline lens, so it includes the thickness of the cornea. It should be noted that the ACD is defined as the distance between the corneal epithelium and the anterior surface of the crystalline lens (external ACD), and as the distance between the corneal endothelium and the lens (internal ACD) [
7,
17,
24].
For years, Partial Coherence Interferometry (PCI) had been the first choice for optical biometry since its introduction in 1999 [
7,
11,
12,
13,
14,
17,
20,
21]. However, traditional devices based on the PCI technique could not provide data about the posterior surface and pachymetry of the cornea as well as the thickness of the crystalline lens, while at the same time they presented difficulties in measuring very dense cataracts due to the use of infrared light of wavelength 780 nm [
7,
21,
22,
23,
25]. As a result, over the years, new machines and new technologies of optical biometry have been developed in order to overcome the above weaknesses, giving more information about the status of the eye, in an effort to draw more secure conclusions in biometrics, aiming at the most accurate calculation of the intraocular lens. Hence, new third-generation optical biometers based on Swept Source Optical Coherence Tomography were developed, which use longer wavelengths, ranging from 1050 to 1300 nm, thus allowing less scattering and deeper penetration into cloudy media [
25].
ANTERION is based on Swept Source Optical Coherence Tomography, a new imaging technology (Fourier-Domain Optical Coherence Tomography) [
26] that uses light waves, rather than sound waves, to produce images and is the successor of the previous versions such as the TD-OCT (Time-Domain OCT) and SD-OCT (Spectral-Domain OCT) [
19]. It is a non-invasive and extremely quick method which uses a longer wavelength light source that allows deeper light penetration and thus achieves simultaneously OCT-imaging of structures of the posterior part of the eye [
19,
23,
27]. The soft light source of the SS-OCT Laser allows the patient to be seated without any discomfort during the examination. SS-OCT was introduced in 2012, and since then its popularity has grown exponentially due to its remarkable advantages, resulting in the production of images of complex internal structures, such as those of the human eye, in a very high resolution. [
7,
10,
11,
12,
19,
20,
21,
24,
27,
28,
29]. SS-OCT allows imaging of the vitreous, choroid, and retina, tissues that are small in size and therefore difficult to image, which is an advantage of primary importance in cataract surgery [
19,
20,
21,
25,
28,
29,
30]. ANTERION uses a 1300 nm wavelength light source with a scan rate of 50,000 scans/sec, a scan depth of 14 mm and an axial resolution of 10 mm. It is known that the higher the number of pixels, the higher the resolution. In addition, it allows simultaneous imaging of multiple structures with a single measurement and does not require image realignment [
19,
28,
29]. At the same time, it has the “Cataract Application” (Heyex Software, Version 2.4.3, Heidelberg Engineering), which combines important measurements, such as the Aqueous Depth (AQD), the thickness of the Crystalline Lens (LT), the Corneal Diameter (WTW- White to White distance) and Axial Length (AL), for the preoperative planning of the IOL [
7,
8,
19,
20,
21,
22,
23,
27,
28,
29]. It is emphasized that AQD is defined as the distance from the posterior surface of the cornea to the anterior surface of the lens, and thus ACD is calculated by adding CCT to AQD [
19,
25]. The overall evaluation of the cornea is achieved by measuring both the anterior and posterior cornea and is calculated with the help of multiple maps (anterior and posterior axial curvature maps, tangential curvature and elevation maps, corneal power map, anterior and total corneal wavefront and pachymetry maps). Theoretically, determination of total corneal power, by calculating both anterior and posterior corneal curvatures, could increase accuracy in predicting the IOL power suitable for each patient. [
19,
24,
28,
29,
30,
31].
As a result of our present research, comparing IOL Master 500 and ANTERION machines, we found statistically significant differences for the variables of "Ksteep", "Astigmatism" and "Anterior Chamber Depth", while, as far as the variables of "Axial Length" and "Kflat" are concerned, no statistically significant differences were found.
It is particularly interesting to point out that the results of our research are not in complete alignment with the results of other research. For example, Szalai et al. 2022 proved that there are no statistically significant differences in the various measurements between the two machines [
19]. Moreover, Yang et al. 2019 did not observe any statistically significant difference in AL values between them [
25]. On the other hand, Kim et al 2020, in the first published study comparing IOL MASTER 500 and ANTERION, demonstrated that there was a statistically significant difference in the axial length measurement between them, but this was not able to affect the clinical outcome of the intraocular lens power [
20]. Furthermore, Moon et al 2022, observed significant differences in ocular biometers, except for axial length [
21] and Chan et al 2021 found significant disagreements concerning keratometric values and axial length, considering the two devices non-interchangeable [
22]. Overall, we noticed that in the international literature, there is a controversy without any clear answer about the existence of clinically significant differences between the two machines (some studies show differences, while others do not), and at the same time we were surprised by the fact that there are clearer and more specific conclusions in comparisons made between the IOL MASTER 500 and other SS-OCT biometers (OA, IOL MASTER 700, ARGOS) something that, we assume, is due to the very short follow-up period of ANTERION [
5,
18,
25,
32,
33,
34,
35].
At this point, we should mention the limitations of our study, starting with the exclusive enrollment of eyes with age-related cataract, excluding patients who had cataract of another etiology, such as post-traumatic, uveal or congenital. Additionally, our control sample was very small and the rate of missing values was relatively high for this data set. The limitations and difficulties we encountered from the small control group during our study are identical to the studies already published about ANTERION. This is due to the fact that ANTERION is an extremely modern machine, introduced to the ophthalmological community in recent years, a period of time in which, due to COVID-19, the number of cataract surgeries had been particularly decreased. At the same time, it is not widely available in Greece as is the case with other SS-OCT biometers. It is therefore considered necessary to continue recording data and comparing the two machines with a larger sample of patients over a longer period of time, in order to draw more secure conclusions about the superiority of one or the other, as well as to review our results. In case of a larger sample, we could have different age groups, examine other variables, for example, the repeatability of the different formulas for determining the power of the intraocular lens, evaluate differences of the variables in a divided sample of patients based on the axial length (hyperopic and myopic, very short and very long axial length) and of course make comparisons between more than two devices.