1. Introduction
Saltmarshes are ecosystems of vital importance due to the high biological productivity, hydrological flux regulation, biogeochemical cycling of metals and nutrients, as well as habitat provision for wildlife [
1]. Because of their proximity to major urban centers, these coastal areas commonly serve as sinks for a myriad of contaminants, concentrating potential toxic compounds in the sediment [
2]. As a result, saltmarsh plants are typically exposed to high amounts of trace elements, including mercury (Hg), which has become an environmental and legislative concern due to its widespread prevalence and high toxicity. In the Atlantic coast of Europe, halophytes, such as
Halimione portulacoides, dominate in these habitats in terms of primary production and biomass, while playing important ecological roles in nutrient and contaminants cycling. Previous research indicated a large accumulation of Hg in saltmarsh plants, as well as a significant rise of organic Hg forms in the rhizosphere environment [
3]. Recently, it was shown that elemental Hg can be emitted from the leaves of saltmarsh plants, indicating an efficient translocation process inside the plants, and resulting in a low accumulation of Hg in the aerial parts [
4,
5]. Despite that, there are still many gaps on the understanding of saltmarsh halophytes’ interactions with Hg forms. Considering the pivotal role of these plants in saltmarshes, overcoming those gaps will allow to predict Hg effects and fate in extremely sensitive ecosystems that are also severely under the impact of climate change.
Saltmarsh plants might had developed an ability to cope with the presence of high levels of Hg forms in the sediment, namely inorganic mercury (iHg) that is the most abundant Hg counterpart in this matrix [
6]. Halophytes inhabiting Hg-contaminated saltmarshes may present specific biochemical and physiological features, likely translating biological attributes to cope with the environmental disturbance. Only few studies were focused on the understanding of those effects in saltmarsh halophytes under iHg exposure, while most conclusions were taken from field works. Contrastingly, findings under laboratory-controlled conditions are still scarce and have mainly comprised long-term exposures to iHg, while conclusions provided from a short exposure time window remain elusive. Integrating data from different time scales creates a cohesive narrative about the biological effects, as each exposure duration provides a piece of a larger puzzle, elucidating the progression of responses to contaminants and environmental factors, from immediate reactions to potential long-term consequences. Considering all exposure durations provides an accurate and mechanistically based risk assessment, supporting the formulation of effective management strategies. It also helps to identify critical thresholds, vulnerable periods, and potential recovery phases following contamination events. Moreover, when examining the impact of a given environmental factor, it must be considered the nested nature of exposure durations,
i.e., each larger duration inherently encapsulates smaller time frames. Each level of exposure duration is interconnected, with smaller increments forming the larger units. This interconnectedness implies that observations made within shorter durations contribute to the understanding of the dynamics and effects observed in longer exposures. In the light of this perspective, it is evident, from the scientific literature analysis, that the focus of research on the effects of Hg forms is predominantly directed towards long-term effects, probably due to the recognized features of this metal like high bioaccumulation potential and persistence. Contrastingly, very short-term exposures (on a time scale of hours) to Hg have been neglected, devaluating the contribution of immediate responses to uncover organism’s adaptive mechanisms, as well as to the understanding of broader and longer-term consequences.
Halophytes can tolerate Hg stress via defense mechanisms afforded by antioxidant system (revised in [
7]). This is because Hg can trigger the production of reactive oxygen species (ROS), which may result in cellular damage, namely protein oxidation, lipid peroxidation and DNA damage. The role of various enzymatic (
e.g., catalase, ascorbate peroxidase, guaiacol peroxidase, superoxide dismutase) and non-enzymatic (glutathione, phytochelatins, proline, and ascorbic acid) antioxidants in plants has been elucidated with respect to enhanced generation of ROS and resulting oxidative stress. These key components interact in a sophisticated network, which main priority is the ROS detoxification and, consequently, the prevention of the cellular injuries referred above, pursuing redox homeostasis. There are several studies linking Hg exposure to the production of ROS in plants (revised in [
7]), but only a few focused in saltmarsh species being mostly developed in the field, lacking laboratorial evidence. Recently, a biochemical and lipidomic approach was used to assess the effects of Hg on
H. portulacoides occurring at two sites of an Hg historically contaminated area (Laranjo basin, Ria de Aveiro, Portugal) differing in the contamination extent [
8]. The enzymatic antioxidant mechanisms protecting membranes [glutathione peroxidase, glutathione S-transferases (GSTs) and dehydroascorbate redutase] were not induced in any of the three analyzed organs (leaves, stems and roots) [
8]. Differently, a decrease of GSTs activity was reported in roots and leaves of
H. portulacoides from the same ecosystem in line with Hg exposure levels [
9]. Predictably, the responses of
H. portulacoides to Hg exposure could vary depending on the environmental temperature or other abiotic factors, in line with what has been recorded in other plants [
10], and according to a seasonal fluctuation of accumulated levels [
11]. Despite that, no works are available on saltmarsh plants exploring the influence of abiotic factors, such as temperature and light intensity, on biological responses to Hg.
Although saltmarsh halophytes can tolerate trace metal contamination to some extent, excessive levels internalized in the plants can result in severe impairment of fundamental processes related to protein and energy metabolism [
12]. Both antagonistic and synergistic effects between Hg
2+ and salinity were confirmed by differential levels of proteins (magnesium-chelatase and ribulose-l,5-bisphosphate carboxylase/oxygenase) and metabolites (valine, malonate, asparagine, glycine, fructose and glucose) in the halophyte
Suaeda salsa [
12]. Moreover, metal overload has been proven to cause serious damage in the photosystem II (PS II) [
13,
14]. Photobiology parameters, as efficiency and photoprotection capability, were assessed in leaves of
H. portulacoides exposed to Hg forms [
5]. Few differences between control and exposed plants were observed, indicating high tolerance of this salt marsh plant to Hg forms [
5]. Pulse Modulated Amplitude (PAM) fluorescence examines the photonic energy capture mechanisms and transformation to electrical energy. Thus, any disturbance at the primary productivity level can be efficiently assessed by this technique.
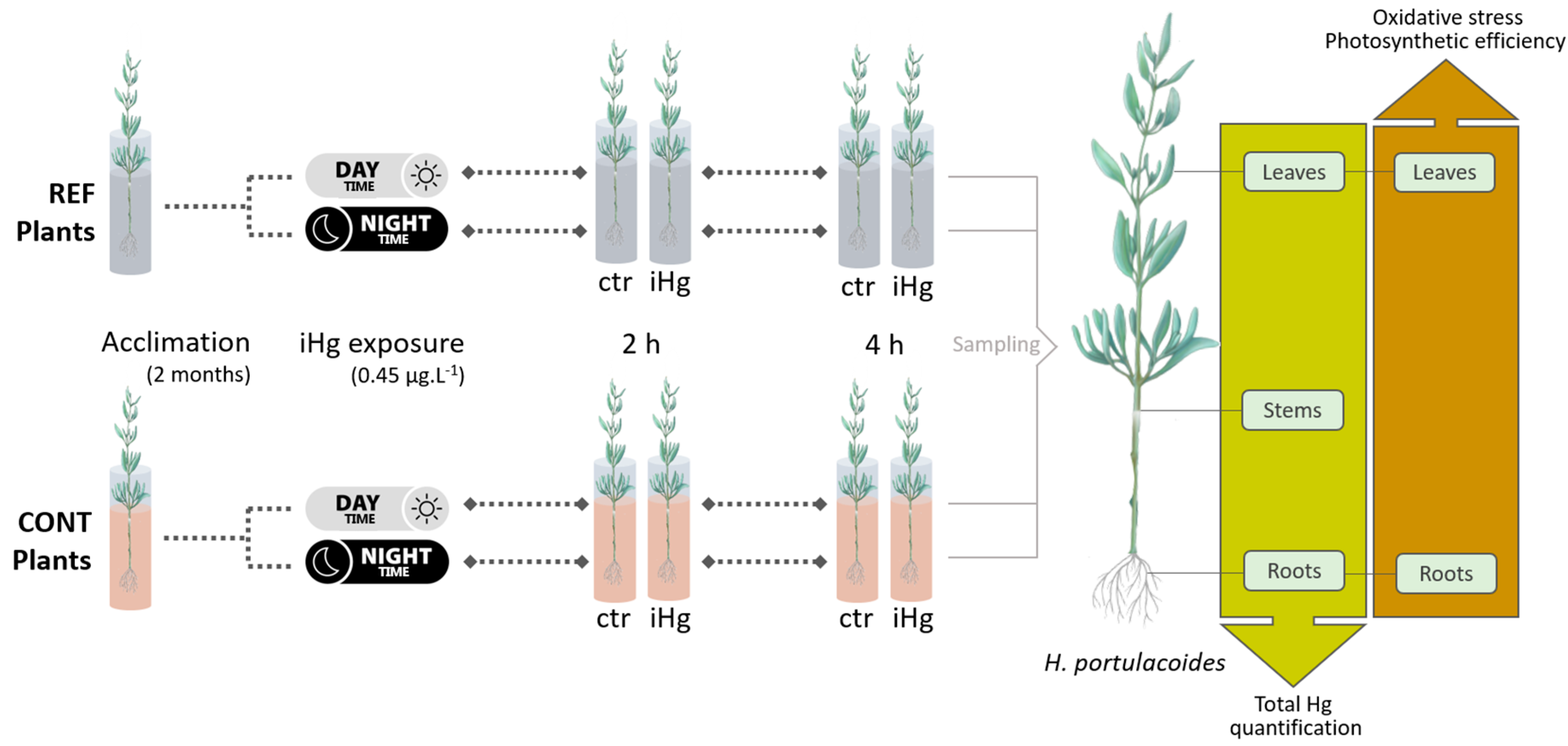
There are still many questions that need to be clarified in relation to the biological attributes of saltmarsh plants, namely H. portulacoides, to cope with iHg. Hence, the current research aims to fulfil major knowledge gaps related to the biochemical effects of iHg in a short time exposure window (2 and 4 hours), disclosing adaptative responses specific of the roots and leaves regarding redox homeostasis, together with physiological impairments and Hg bioaccumulation. The influence of major abiotic parameters (such as temperature and light) on those responses was also investigated, as well a possible role of plant’s historic contamination background. Thus, a short-time scale in vitro study was carried out, in hydroponic conditions, relying on the exposure of H. portulacoides to a realistic level of iHg and combining the assessment of root iHg uptake and partitioning with the evaluation of oxidative stress responses to iHg, keeping in view the redox homeostasis, and photosynthetic efficiency. This is a powerful approach for the elucidation of H. portulacoides plasticity in the presence of a specific environmental disturbance, as well as on the way this species is currently contributing to the iHg cycling.
4. Discussion
The decision to approach a (very) short-term context in the present study relied on the understanding that it can provide critical information about the iHg potential for acute toxicity, shedding light on the mechanisms through which this metal form may harm H. portulacoides, helping also to identify specific targets or pathways that make it vulnerable (or resistant). It should be noted that this may not capture the full range of potential effects and how they evolve over time, and thus, a comprehensive understanding of the dynamic nature of saltmarsh plant toxicological responses to iHg requires a complement with long-term toxicity studies. In this direction, the present research was run in parallel with a long-term study, whose results will be published shortly, allowing their integration with those now presented.
4.1. Mercury accumulation and its modulation by environmental factors and ecological traits
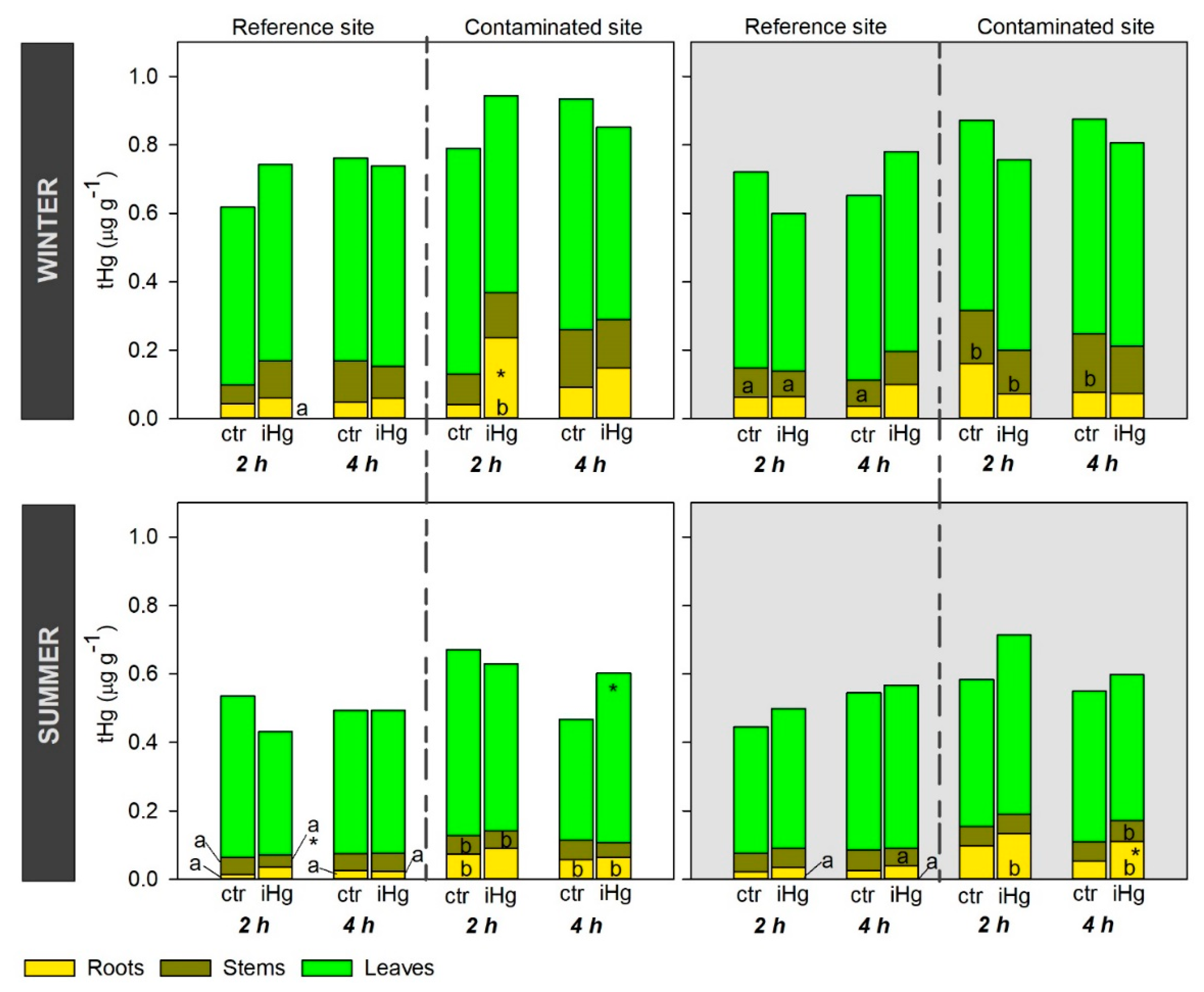
Accumulation of iHg was poorly noticed, but a significant enrichment of Hg in the roots of CONT plants exposed to iHg for 2 hours only, under hydroponic conditions simulating winter and daylight, indicates that this Hg form was available for roots uptake. This finding also indicates that iHg uptake by the saltmarsh plant
H. portulacoides is a short-term process (within a couple of hours), even under realistic waterborne exposure levels (0.45 μg L
-1). Comparable concentrations were found in the water of contaminated systems [
6]. Although not statistically different, higher Hg accumulation levels were also found in the roots of plants from the contaminated site that were exposed for 4 hours (mean levels in exposed and control plants were 0.15 and 0.09 μg g
-1, respectively). The uptake of Hg by saltmarsh plants has been widely described, but mostly in field studies that reported the accumulation of this element in the roots [
3,
11,
25], while short-term studies are scarce. Cabrita et al. [
5] pioneered in this context with plants from another system, by describing for the first time the uptake and transport of Hg isotopes in
H. portulacoides grown under hydroponic conditions. Findings of Cabrita et al. [
5] described a significant accumulation of Hg forms within a few hours of exposure (1-4 hours). Trace metals uptake by plants from the surrounding environment follows the same transport pathways as the ones used by micronutrient metal ions [
26]. Hg cations (as Hg
2+) have a high affinity for sulphydryl groups, facilitating its uptake through sequestration into cysteine-rich peptides, namely metallothioneins and phytochelatins, by binding to the organic sulphur groups [
5]. The main pathway of HgII entering the roots was recently confirmed, consisting in its binding to lower molecular organic matter (as cysteine) [
27].
Despite the uptake of iHg in roots of plants from the contaminated saltmarsh was found in winter at daylight exposure, no significant enhancements of Hg levels in the stems and leaves were recorded. A different pattern was described by Cabrita et al. [
5] upon
H. portulacoides short-term exposure to Hg isotopes, demonstrating Hg translocation to the stems and leaves within few hours. Environmentally realistic levels were used in the current experiment (0.45 μg L
-1) with
H. portulacoides, which combined with the short-term exposure could explain the lack of a detectable translocation for the aboveground organs. Findings discrepancy with those of Cabrita et al. [
5] could be related with the exposure levels, which were indeed doubled in that study for the iHg isotope (1.05 μg L
-1). Summer findings go in the same direction as those of winter simulated conditions, pointing out uptake in the roots of plants from the most contaminated area and poor translocation to the stems and leaves. Mercury accumulation was found in the roots of plants from the contaminated site upon iHg exposure for 4 hours, under darkness. Despite that, no significant enhancements of Hg in the stems were found, suggesting poor translocation to the aboveground organs, as recorded in winter conditions.
Previous studies have found that light at proper intensity, spectral quality, and photoperiod can fuel plants growth and increase the efficiency of soil nutrient absorption [
28]. Thus, a role of light on trace elements uptake in saltmarsh plants could be speculated, as the same uptake pathways are used for micronutrient metal ions and trace elements [
26]. The current results on
H. portulacoides have not evidenced the role of light on iHg uptake.
Current experiments simulated winter and summer conditions, regarding temperature and photoperiod. Different uptake patterns were found for
H. portulacoides exposed in winter and summer simulated conditions, but current data do not support conclusions on the season that is more favorable to iHg uptake under controlled laboratorial conditions. Despite that, winter plants from both saltmarshes had, in general, higher levels of Hg than those exposed in summer. This is probable related to a seasonal pattern of Hg accumulation in Aveiro lagoon saltmarshes. In fact, seasonal variations on Hg accumulation in
H. portulacoides at Aveiro lagoon were investigated, documenting slightly higher levels in the winter that summer, particularly in the stems and leaves [
11].
Inorganic Hg uptake was only found in the roots of plants from the contaminated saltmarsh, while plants from the REF area did not make an efficient uptake. This points out the role of
H. portulacoides background in terms of contamination on the uptake of iHg. CONT and REF plants have been subjected to distinct contamination levels, as pointed out by the total levels of Hg found in surface sediments collected in those sites during the same field winter campaign, namely: 1.93 ± 0.01 μg g
-1 at REF and 17.6 ± 6.5 μg g
-1 at CONT (average ± standard deviation) (J. Canário, unpublished data). The distinct provenance of the plants seems to promote distinct Hg accumulation levels, as widely reported in the field [
11]. Despite that, the two months of acclimation were enough to promote a reset on those differences by the growth of new radicular tissue. In fact, average levels in the roots of REF and CONT plants immediately before exposure in winter simulated conditions were 0.045 ± 0.020 μg g
-1 and 0.046 ± 0.008 μg g
-1, respectively. However, only the roots of CONT made an efficient uptake of iHg, as demonstrated by the significantly enhancement of accumulation levels in comparison to controls (after 2 hours of exposure in daylight/winter conditions and after 4 hours of exposure under darkness/summer conditions), supporting the discussion on the role of plant contamination background history on iHg uptake, which represents a new analytical perspective of this process.
4.2. Organ-specific oxidative stress responses to iHg as a function of light exposure, season and exposure history
The first note in this framework goes to the finding that responses at this level are detectable even when the plants under analysis do not show measurable variations in tHg accumulation, which demonstrates their great sensitivity and higher suitability to a (very) short time scale approach.
Redox homeostasis has been stated as the “Golden Mean” of healthy living [
29]. This was the foundational idea for the questions raised in the present study and for the interpretation of the results obtained, as redox homeostasis is regarded as a structuring guideline for the
H. portulacoides responses to the challenges (eliminated them and preventing damage) posed by iHg, therefore determining its toxicodynamics.
The root and leaf responses currently detected in terms of antioxidant modulation make it difficult to establish variation profiles as a function of iHg exposure (and internal concentrations detected) and exposure duration. While simplifying explanations is attractive and (misleadingly) more effective in science, it often does not reflect an approximation to reality, which has a paradigmatic example in the framework under study. In this direction, it must be brought to the fore that the maintenance of a physiological redox steady state through the intervention of the antioxidant system depends on signaling pathways (
e.g. electrophiles) and signal transduction that takes place through the fine adjustment of rheostat rather than by the flipping of an on–off switch, involving rapid feedback reactions [
29].
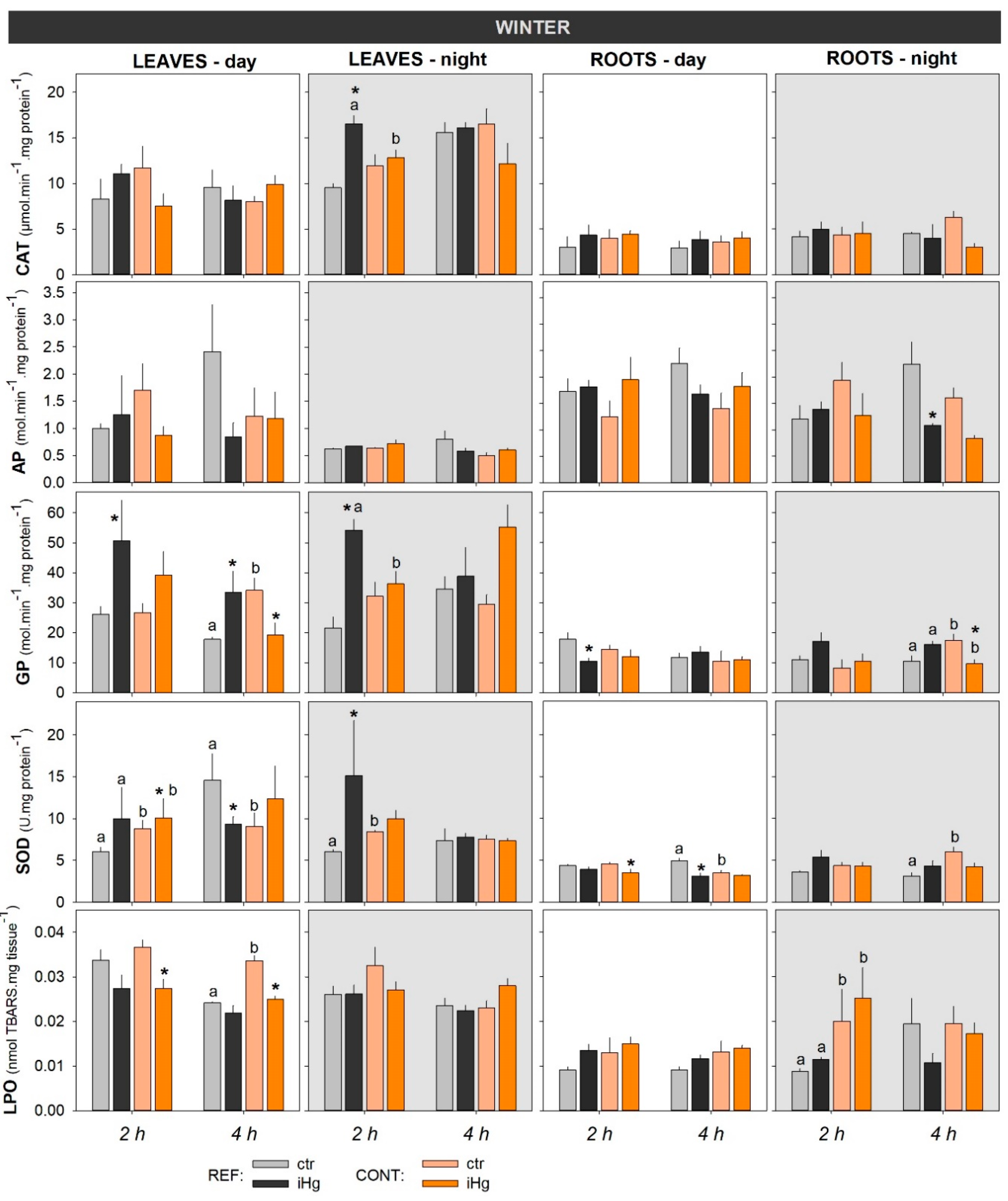
Anyway, it was discernible that, in winter, the leaves of plants sourced by the reference site (REF), when showing alterations on the assessed enzymatic antioxidants, displayed an activity increment as a pattern of response to iHg exposure (with a single exception for SOD following daytime exposure for 4 h). In the leaves of plants from the contaminated site (CONT) in the winter, as well as in the leaves of plants from both origins in the summer, the antioxidants were much less responsive and showed an irregularity in the direction of variation. Highlighting an organ-specific profile of response, in the roots, the alterations always reflected antioxidant activity decreases, with particular emphasis in the CONT groups (both seasons) and in REF groups in the winter.
Increases of antioxidant activities are easily explained as a self-correcting physiological response to iHg challenges. Differently, a reduction of those activities could suggest either an inhibition or a decreased expression/synthesis of the antioxidant enzymes. The hypothesis of activity inhibition would represent a clear sign of toxicity, with the consequence of increased risk of oxidative damage, which was not corroborated by the LPO results. Thus, the second explanation gains plausibility and reflects the operation of efficient feedback pathways on the antioxidant modulation, keeping in view the redox homeostasis. This instantaneous picture of part of the antioxidant system evidenced a low pro-oxidative pressure in H. portulacoides challenged by iHg, allowing to save endogenous resources via a lower expression/synthesis of enzymatic antioxidants.
Halophytes have developed a suite of traits, besides salt tolerance mechanisms, that gives them competitive advantages, and also included reinforced of antioxidant defenses [
30]. Thus, it can be hypothesized that, given the high constitutive levels of antioxidants (enzymatic and non-enzymatic) in
H. portulacoides, the responsiveness of antioxidant enzymes may be reduced, respecting the principle of proportionality of response to the oxidative insult. Biological systems must be able to react to challenges, but never too much [
29]. In this framework and paying particular attention to the results obtain in the winter experiment for the leaves, plants from the contaminated site (CONT) seems to have higher thresholds to the induction of antioxidant enzymes comparing with those from the reference site. This denotes that the former subset is better prepared to cope with iHg redox pressure.
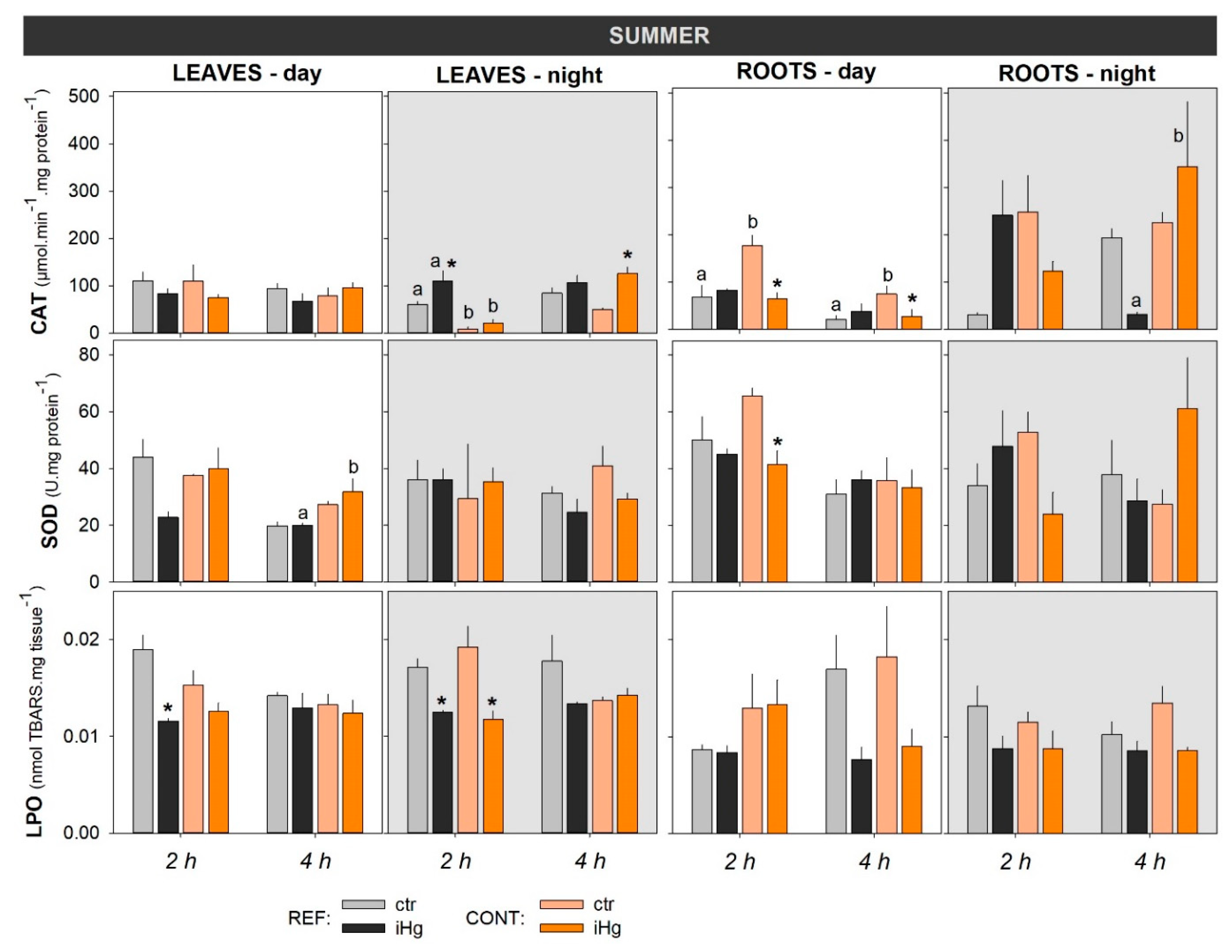
Lipid peroxidative damage results unveiled the most impressive identification of uncommon attributes of H. portulacoides, since both subsets of this halophyte population showed the capability of preventing peroxidative damage in both organs. This means that the species is properly equipped with an antioxidant shield that ensured that the boundary of the physiological redox steady state was breached in a short time scale. In the same direction, it is even more remarkable the detection of lower levels of peroxidative damage in the leaves following plant exposure to iHg. In the winter, this profile of response was restricted to the plants from the contaminated site (CONT) exposed at daytime (in both exposure lengths), which agrees with the suggestion presented above towards a better protection of this population subset. Nonetheless, it must be pointed out that this (apparent) beneficial response in CONT groups cannot be dissociated from the fact that they have higher LPO levels in the unexposed groups (ctr) when compared to REF groups.
In the summer experiment, this pattern of response as LPO was exhibited by the leaves of plants from both provenances (REF at daytime; REF and CONT at nighttime), following the shorter exposure.
The biological meaning of this paradoxical effect and its assumption as a toxicologically based mechanistic strategy is not consensual, but, in our perspective, it fits on the concept of “overcompensation hormesis” (OCSH). According to Calabrese and Baldwin [
31], OCSH is “an adaptive response to low levels of stress or damage resulting in enhanced fitness for some physiological systems for finite periods and, under specific defined circumstances (…)”. It can be regarded as a modest overcompensation to a disruption in homeostasis, generating its reestablishment and setting up a process of adaptive nature [
31]. In the light of this conceptualization, the adjustment features exhibited by
H. portulacoides fits into the paradigm of resistance/adaptation, involving the establishment new homeostatic setting and the corresponding phenotypic shift towards a permanent modification of a function.
The analysis of the literature on this topic makes clear the lack of research on the influence of abiotic factors, such as temperature and light, as well biological traits depending on the Hg exposure history, highlighting the novelty of the present study. In general, no clear patterns could be discerned on the effects of light on the iHg toxicodynamics in
H. portulacoides in this short-term exposure, as well as of season. However, a sole exception was found for CAT activity that was much higher in the summer experiment than in winter. This finding is in line with the investigation of season effect on the antioxidant activity in
Brassica vegetables, demonstrating its influence on the concentration of bioactive components of the plants and antioxidant activity [
32]. Regarding the historic contamination background, CONT plants seemed to have higher thresholds for the induction of antioxidant enzymes comparing to those from REF (put in evidence by leaves results), denoting that the former subset is better prepared to cope with iHg redox pressure, as previously discussed. Peroxidative damage findings pointed out in the same direction, as the leaves of CONT plants exposed to iHg had remarkably lower levels of LPO. This assumption based on the antioxidant protection and peroxidative damage corroborate that suggested by the Hg accumulation data, pointing out CONT plants as more prone to uptake iHg.
It was also demonstrated that biochemical markers related to oxidative stress are highly sensitive translating cellular disturbances/adjustments. In fact, effects at this level were detected even when significant increments on iHg accumulation were undetectable, in specific tissues, by conventional quantification methods. The early interactions of iHg with cellular components can disrupt cellular functions or activate biochemical pathways initiating adaptive processes before a substantial amount of the metal accumulates intracellularly.
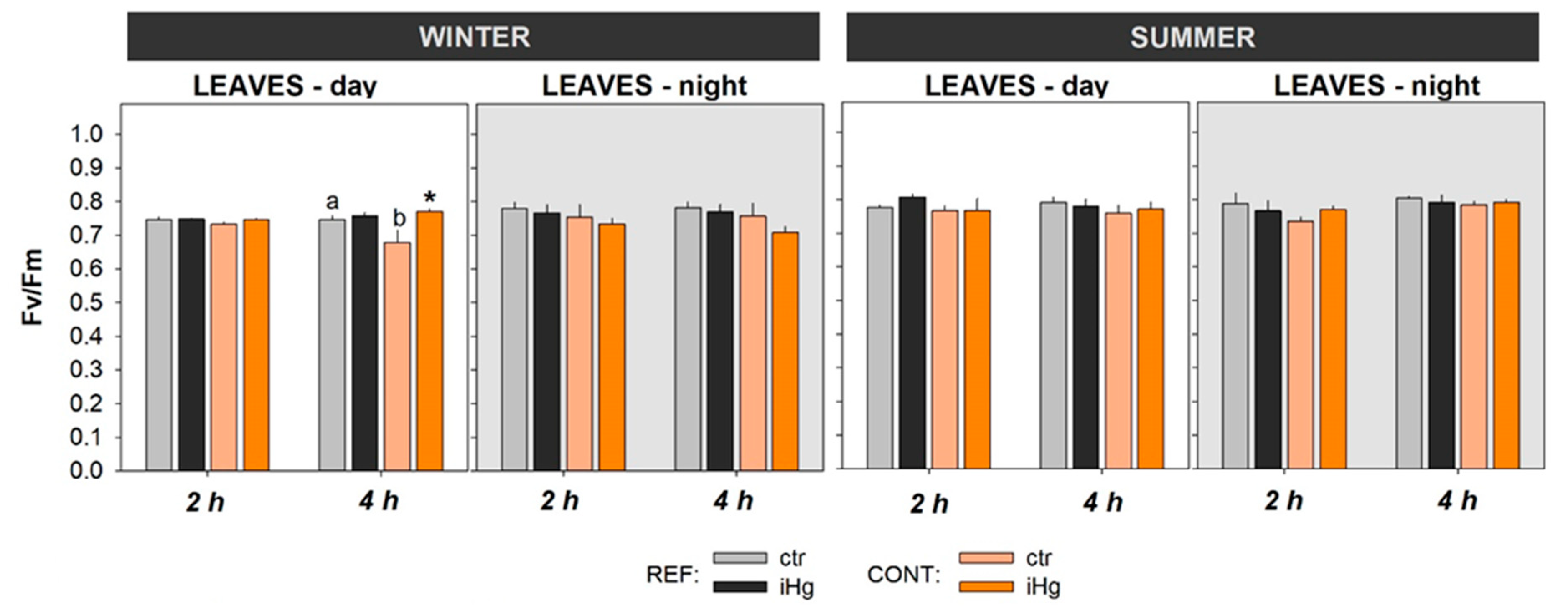
4.3. Modulation of photosynthetic efficiency by iHg exposure
It has been demonstrated that photosynthesis process is primarily affected under Hg treatments, hindering the functionality of PS II in different plant species [
33]. Though not so well-stablished, this vulnerability was also described in halophytes, where iHg deregulated several proteins that take part in the process of photosynthesis [
34].
In contrast, the present observations revealed that this critical physiological process was largely unaffected in the experimental plants following the exposure to iHg. This reinforces the interpretation made above on the H. portulacoides capacity to maintain a physiological balance. Furthermore, in the winter exposure (4 h) at daytime, the plants from the contaminated site (CONT) displayed an increase photosynthetic performance, which coincided in time with a reduction of LPO levels. Taking together, these two effects corroborate the assumption that an OCSH strategy is determining (dampening) impact of the plant interaction with iHg.
4.4. Overall interpretations and findings extrapolation
Homeostasis must be interpreted as a looked-for condition by the organism as a whole (or even by the population), so that, in theoretical terms, it cannot be excluded that a punctual imbalance in a given part of the body may occur, provided that the survival and efficiency of the whole is assured. Nonetheless, both roots and leaves of H. portulacoides were able to prevent, regionally, a stable alteration of redox homeostasis, that is to say, they were able to avoid oxidative stress. Though a short period was addressed, this sustains the assumption that the plant as a whole did not suffer critical variations in its health condition, which, interestingly, was confirmed by the current photobiology data.
At this point, it is important to reiterate the attempt to unravel whether the processes underlying the successful response of H. portulacoides to iHg reflect a phenomenon of tolerance or resistance/adaptation. Considering the long period of acclimatization to which the plants were subjected, likely to allow resetting the mechanisms and respective signaling pathways (as corroborated, in general, by the data on tHg accumulation), as well as the exhibition of an almost comparable capacity in the two subsets of H. portulacoides population (REF and CONT), the most plausible hypothesis is that of a resistance/adaptation phenomenon. Nevertheless, this assumption needs further validation.
The features described, likely encompassing toxicokinetic and toxicodynamic adjustments, should be interpreted as part of a complex net of mechanisms operating in H. portulacoides, probably depending on a genetic plasticity, that allows this species to smooth environmental shocks, including exposure to iHg in pore water.
Halophytes are the foundation of saltmarsh ecosystems, and thus, alterations in their physiology due to Hg exposure may have an impact on higher levels of ecological organization. Hence, it can be hypothesized that the individual feature above described has a favorable ecological impact, emerging from the species capacity to colonize Hg impacted areas, covering contaminated sediments, and thus, protecting the ecosystem against erosion. Interestingly, it seems that a scavenging capacity exhibited by H. portulacoides at a subcellular level in relation to free radicals can have a translation into an ecosystem’s ability to scavenge and chelate Hg in the sediments, limiting its remobilization and bioavailability.
5. Conclusions
Overall, the outputs of the present study allow the following conclusions:
i) Both subsets of the H. portulacoides population were able to keep redox homeostasis and photosynthesis efficiency under a very short-term exposure to a realistic concentration of iHg. However, plants from the site impacted by Hg (CONT) revealed to be better suited to cope with this environmental challenge, probably taking advantage of a strategy frameable in an overcompensation hormesis model.
ii) It was reinforced the knowledge on the (genetic/physiologic) plasticity of H. portulacoides and the ecological/biological attributes that determine the success of this species in saltmarshes historically contaminated by Hg.
iii) No clear effect of the factors light and season was discerned on iHg uptake and subsequent H. portulacoides responses.
iv) An inconsistency of Hg accumulation patterns was perceived (with no evidence of translocation to the stems and leaves) and explained by a combination of factors related with the exposure duration and sensitivity of detection methods; this points out the higher suitability of biochemical assay-based approaches in that time scale.