Submitted:
03 January 2024
Posted:
04 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture Conditions
2.3. Cell Viability
2.4. Flow Cytometry Analysis
2.4.1. Cell Cycle Arrest
2.4.2. DNA Damage Induction
2.4.4. Caspase 3 Activation
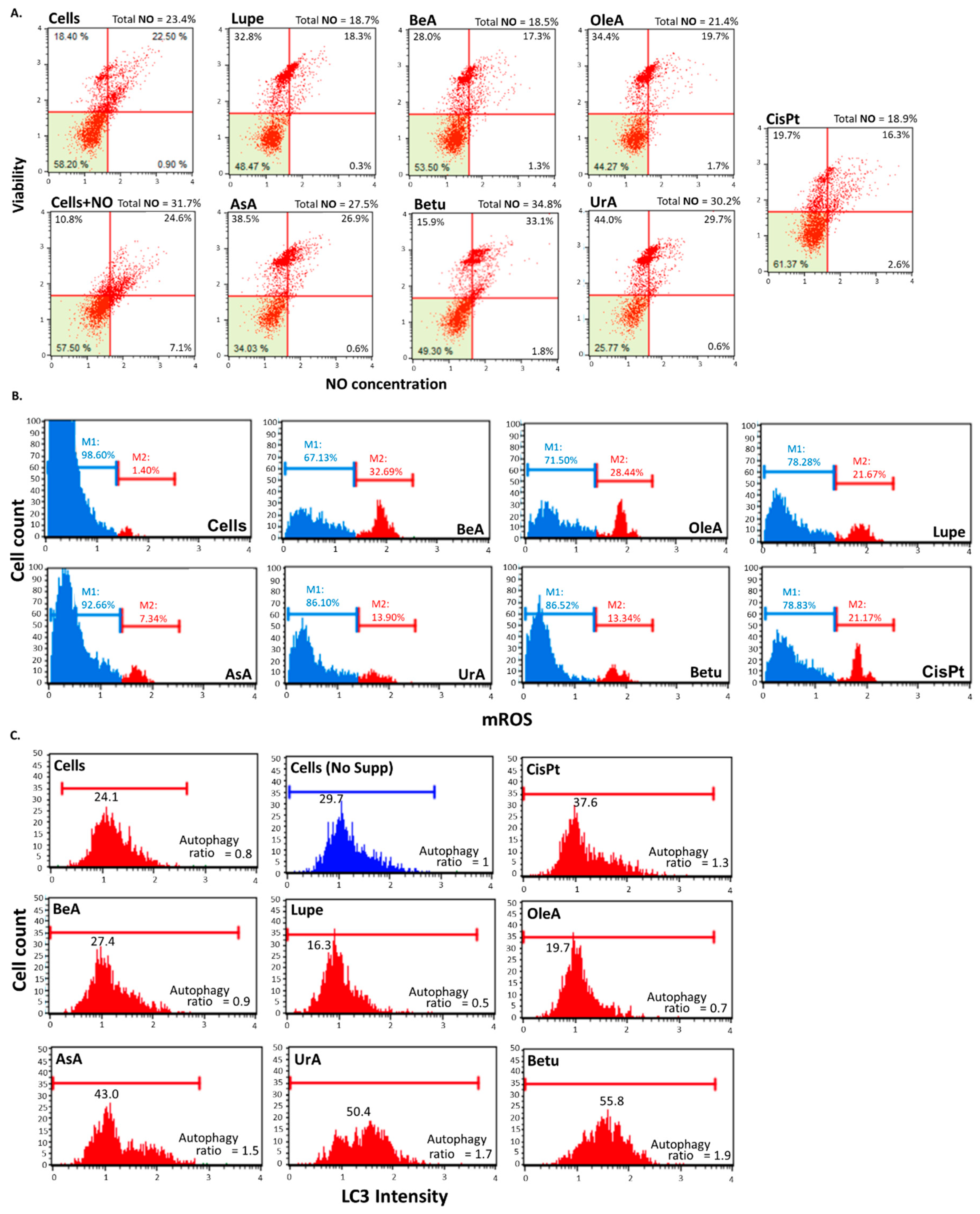
2.4.5. Oxidative Stress
2.4.6. Autophagy activation
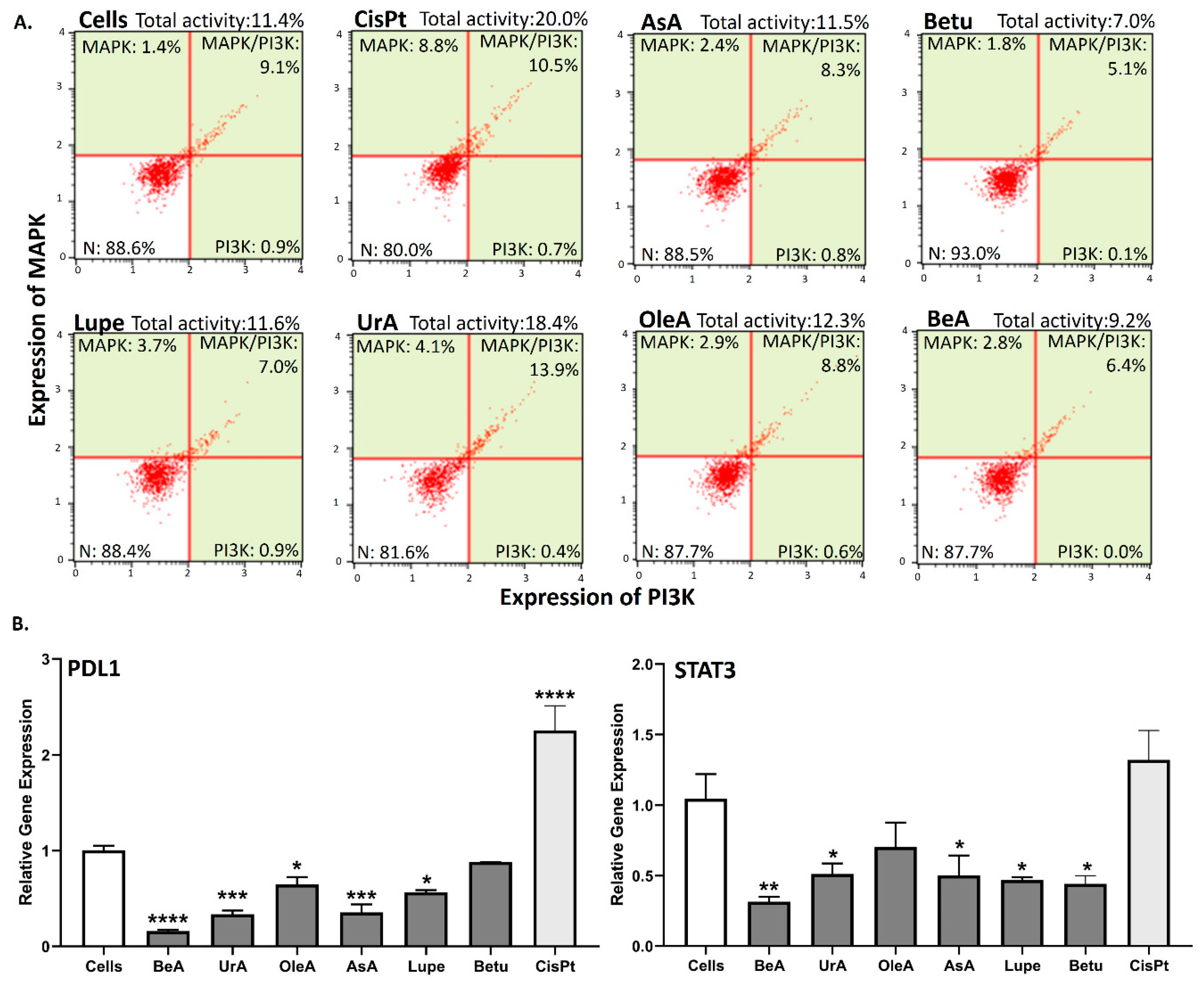
2.4.7. MAPK/PI3K Expression
2.5. RNA extraction and quantitative real-time PCR
2.6. Statistical Analysis
3. Results
3.1. Viability and IC50 in NSCLC and lung fibroblast cells
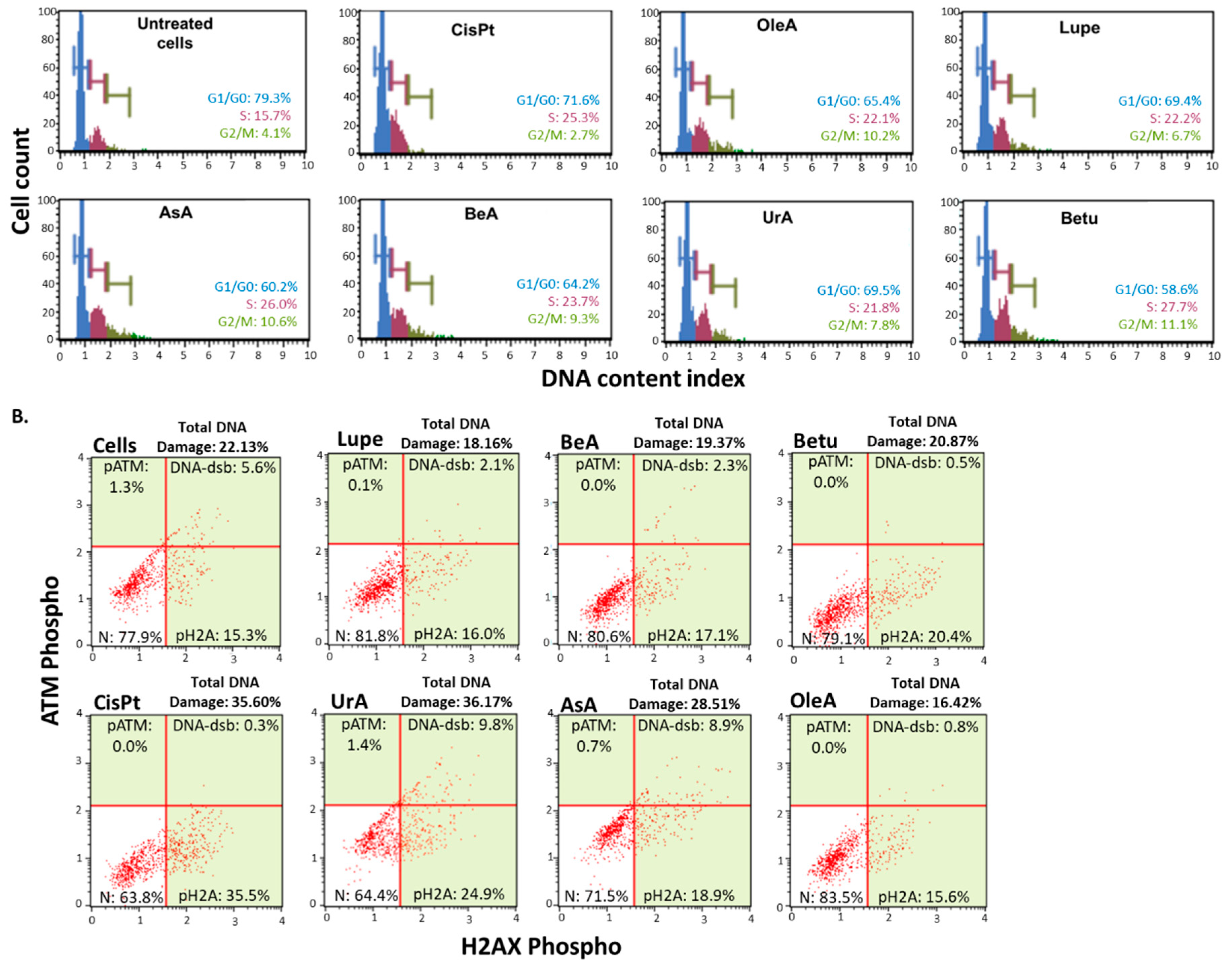
3.2. Cell cycle arrest and DNA damage
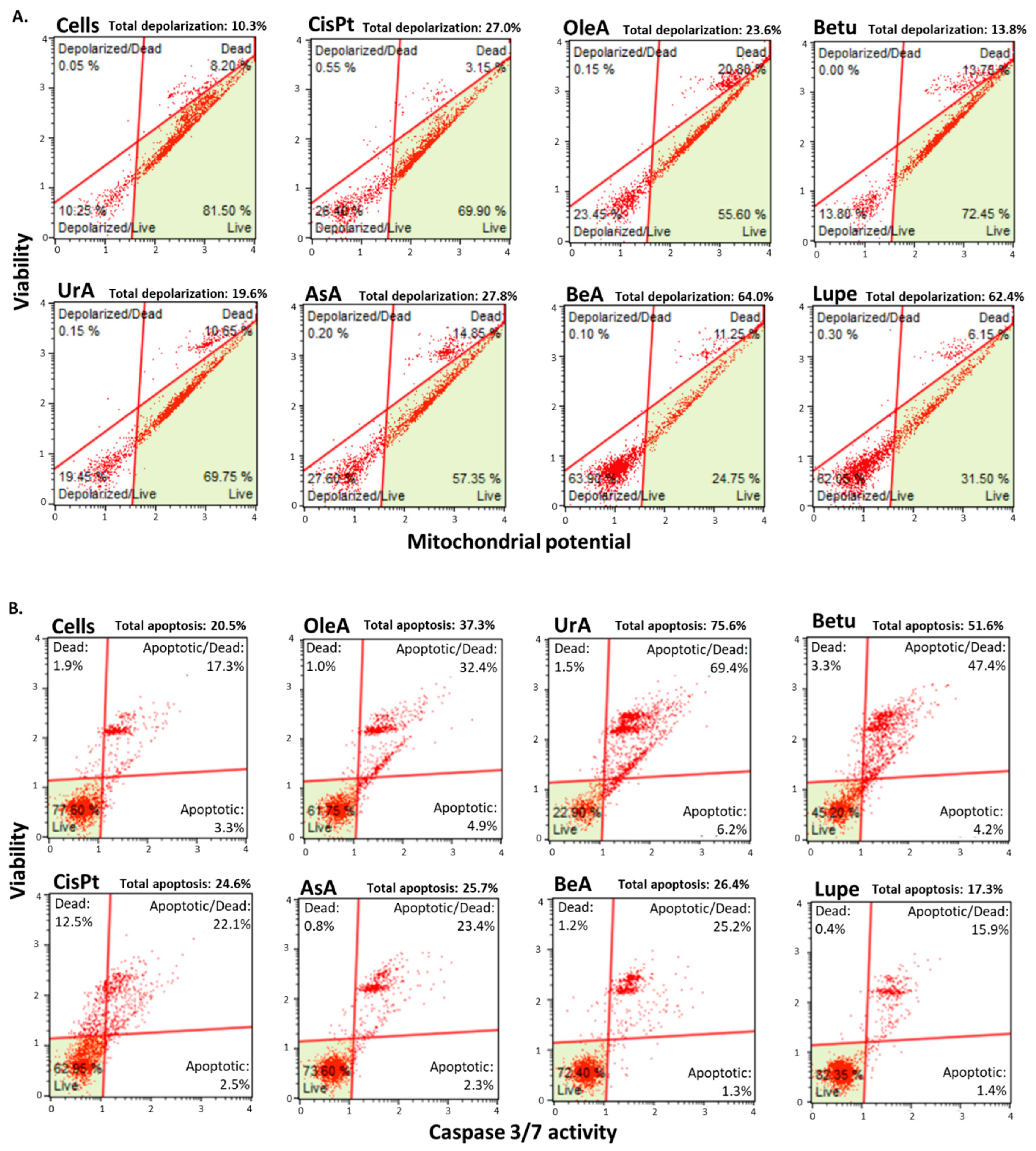
3.3. Mitochondrial membrane permeability and Caspase-3 activity
3.4. Autophagy, reactive nitrogen species (RNS), and mitochondrial ROS production (mROS)
3.5. MAPK/PI3K protein expression and PDL1 and STAT3 gene expression
3.7. Results Summary
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
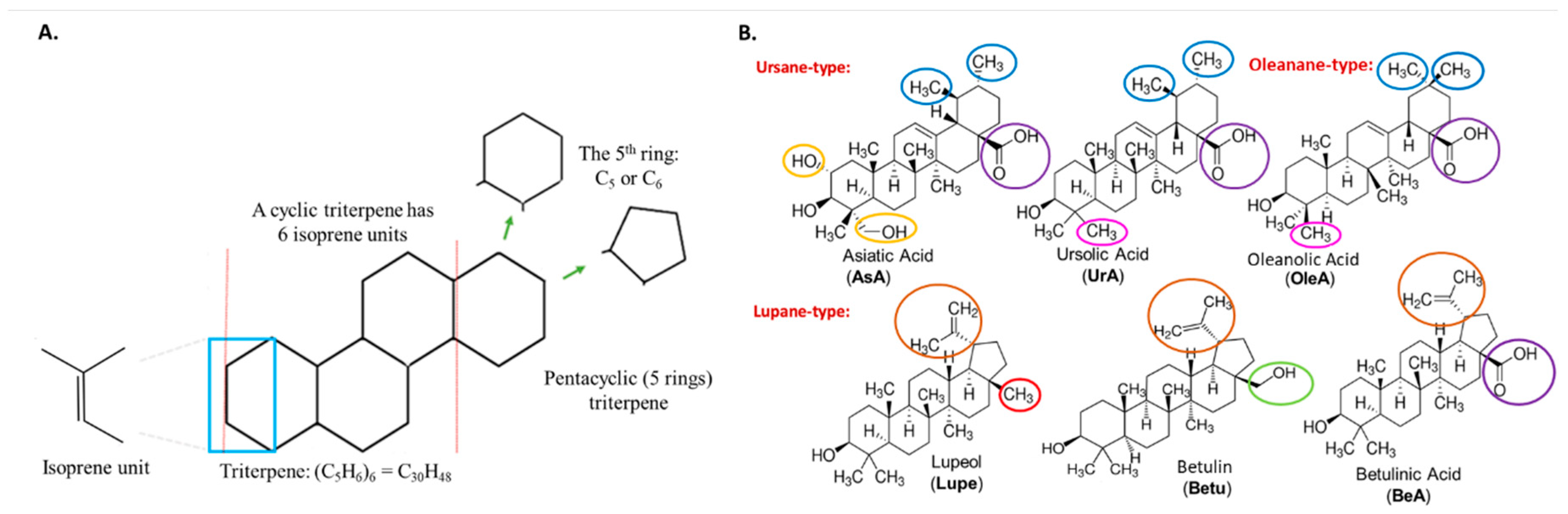
- Xu, R.; Fazio, G.C.; Matsuda, S.P.T. On the origins of triterpenoid skeletal diversity. Phytochem 2004, 65, 261–291. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Kinghorn, A.D. Natural product triterpenoids and their semi-synthetic derivatives with potential anticancer activity. Planta Med 2019, 85, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Ghosh, S.; Bothra, A.K.; Nanda, A.K.; Ghosh, P. Synthesis of friedelan triterpenoid analogs with DNA topoisomerase IIα inhibitory activity and their molecular docking studies. Eur J Med Chem 2012, 54, 137–143. [Google Scholar] [CrossRef]
- Vo, N.N.Q.; Nomura, Y.; Muranaka, T.; Fukushima, E.O. Structure-activity relationships of pentacyclic triterpenoids as inhibitors of cyclooxygenase and lipoxygenase enzymes. J Nat Prod 2019, 82, 3311–3320. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Baxevanos, P.; Mountzios, G. Novel chemotherapy regimens for advanced lung cancer: Have we reached a plateau? J Transl Med 2018, 6, 139. [Google Scholar] [CrossRef]
- Ranasinghe, R.; Mathai, M.L.; Zulli, A. Cisplatin for cancer therapy and overcoming chemoresistance. Heliyon 2022, 8, e10608. [Google Scholar] [CrossRef]
- Ghante, M.H.; Jamkhande, P.G. Role of pentacyclic triterpenoids in chemoprevention and anti-cancer treatment: An overview on targets and underling mechanisms. J Pharmacopuncture 2019, 22, 55. [Google Scholar] [CrossRef]
- Cheng, J.; Li, X.; Wang, S.; Han, Y.; Zhao, H.; Yang, X. Carrier-free triterpene prodrugs with glutathione response and biosafety for synergistically enhanced photochemotherapy. ACS Appl Mater Interfaces 2021, 13, 245–256. [Google Scholar] [CrossRef]
- Delgado, Y.; Torres, A.; Milian, M. Data on cytotoxic pattern of cholesterol analogs for lung adenocarcinoma cells. Data Brief 2019, 25, 104179. [Google Scholar] [CrossRef]
- Rufino-Palomares, E.E.; Pérez-Jiménez, A.; García-Salguero, L.; Mokhtari, K.; Reyes-Zurita, F.J.; Peragón-Sánchez, J.; Lupiáñez, J.A. Nutraceutical role of polyphenols and triterpenes present in the extracts of fruits and leaves of Olea europaea as antioxidants, anti-infectives and anti-cancer agents on healthy growth. Molecules 2022, 27, 2341. [Google Scholar] [CrossRef]
- Ding, S.M.; Lu, A.L.; Zhang, W.; Zhou, L.; Xie, H.Y.; Zheng, S.S; Li, Q.Y. The role of cancer-associated fibroblast MRC-5 in pancreatic cancer. J Cancer 2018, 9, 614–628. [Google Scholar] [CrossRef]
- Guo, W.; Xu, B.; Wang, X.; Zheng, B.; Du, J.; Liu, S. The analysis of the anti-tumor mechanism of ursolic acid using connectively map approach in breast cancer cells line MCF-7. Cancer Manag Res. 2020, 12, 3469–3476. [Google Scholar] [CrossRef]
- Wróblewska-Łuczka, P.; Cabaj, J.; Bąk, W.; Bargieł, J.; Grabarska, A.; Góralczyk, A.; Łuszczki, J.J. Additive interactions between betulinic acid and two taxanes in in vitro tests against four human malignant melanoma cell lines. Int J Mol Sci 2022, 23, 9641. [Google Scholar] [CrossRef]
- Pantia, S.; Kangsamaksin, T.; Janvilisri, T.; Komyod, W. Asiatic acid inhibits nasopharyngeal carcinoma cell viability and migration via suppressing STAT3 and Claudin-1. Pharmaceuticals 2023, 16, 902. [Google Scholar] [CrossRef]
- Gao, C.; Li, X.; Yu, S.; Liang, L. Inhibition of cancer cell growth by oleanolic acid in multidrug resistant liver carcinoma is mediated via suppression of cancer cell migration and invasion, mitochondrial apoptosis, G2/M cell cycle arrest and deactivation of JNK/p38 signalling pathway. J BUON 2019, 24, 1964–1969. [Google Scholar] [PubMed]
- Król, S. K.; Kiełbus, M.; Rivero-Müller, A.; Stepulak, A. Comprehensive review on betulin as a potent anticancer agent. Biomed Res Int 2015, 2015, 584189. [Google Scholar] [CrossRef] [PubMed]
- Pitchai, D.; Roy, A.; Ignatius, C. In vitro evaluation of anti-cancer potentials of lupeol isolated from Elephantopus scaber L. on MCF-7 cell line. J Adv Pharm Technol Res 2014, 5, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.M.; Kastan, M.B. 10 - DNA Damage Response Pathways and Cancer. In Abeloff's Clinical Oncology (Fifth Edition), Niederhuber, J.E., Armitage, J.O., Doroshow, J.H., Kastan, M.B., Tepper, J.E., Eds.; Churchill Livingstone: Philadelphia, 2014; pp. 142–153.e143. [Google Scholar]
- Aborehab, N.M.; Salama, M.M.; Ezzat, S.M. A novel lupene derivative from Thymus capitatus possesses an apoptosis-inducing effect via Let-7 miRNA/Cyclin D1/VEGF cascade in the A549 cell line. BMC Complement Med Ther 2023, 23, 365. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.K.; Li, J.L.; Zhang, S.; Xing, P.Y.; Xia, M.F. Betulinic acid exerts potent antitumor effects on paclitaxel-resistant human lung carcinoma cells (H460) via G2/M phase cell cycle arrest and induction of mitochondrial apoptosis. Oncol Lett 2018, 16, 3628–3634. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Hu, Y.; Yang, Y.; Wang, L.; Yang, X.; Wang, B.; Huang, M. Betulinic acid induces ROS-dependent apoptosis and S-phase arrest by inhibiting the NF-kappaB pathway in human multiple myeloma. Oxid Med Cell Longev 2019, 2019, 5083158. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.; Tan, Z.-J.; Hu, Y.-P.; Shu, Y.-J.; Bao, R.-F.; Jiang, L.; Wu, X.-S.; Li, M.-L.; Ding, Q.; Wang, X.-a.; et al. Ursolic acid induces cell cycle arrest and apoptosis of gallbladder carcinoma cells. Cancer Cell Int 2014, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, X.; Li, H.; Zhuang, J.; Feng, F.; Liu, L.; Liu, C.; Sun, C. Identifying the effect of ursolic acid against triple-negative breast cancer: Coupling network pharmacology with experiments verification. Front Pharmacol 2021, 12, 685773. [Google Scholar] [CrossRef]
- Kim, S.H.; Ryu, H.G.; Lee, J.; Shin, J.; Harikishore, A.; Jung, H.Y.; Kim, Y.S.; Lyu, H.N.; Oh, E.; Baek, N.I; Choi, K.Y. Ursolic acid exerts anti-cancer activity by suppressing vaccinia-related kinase 1-mediated damage repair in lung cancer cells. Sci Rep 2015, 5, 14570. [Google Scholar] [CrossRef]
- Park, C.; Jeong, J.W.; Han, M.H.; Lee, H.; Kim, G.Y.; Jin, S.; Park, J.H.; Kwon, H.J.; Kim, B.W.; Choi, Y.H. The anti-cancer effect of betulinic acid in u937 human leukemia cells is mediated through ROS-dependent cell cycle arrest and apoptosis. Anim Cells Syst (Seoul) 2021, 25, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Goswami, P.; Paul, S.; Banerjee, R.; Kundu, R.; Mukherjee, A. Betulinic acid induces DNA damage and apoptosis in SiHa cells. Mutat Res Genet Toxicol Environ Mutagen 2018, 828, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis. Annu Rev Genet 2009, 43, 95–118. [Google Scholar] [CrossRef]
- Wei, J.; Liu, M.; Liu, H.; Wang, H.; Wang, F.; Zhang, Y.; Han, L.; Lin, X. Oleanolic acid arrests cell cycle and induces apoptosis via ROS-mediated mitochondrial depolarization and lysosomal membrane permeabilization in human pancreatic cancer cells. J Appl Toxicol 2013, 33, 756–765. [Google Scholar] [CrossRef]
- Hearps, A.C.; Burrows, J.; Connor, C.E.; Woods, G.M.; Lowenthal, R.M.; Ragg, S.J. Mitochondrial cytochrome c release precedes transmembrane depolarisation and caspase-3 activation during ceramide-induced apoptosis of Jurkat T cells. Apoptosis 2002, 7, 387–394. [Google Scholar] [CrossRef]
- Sánchez-Alcázar, J.A.; Khodjakov, A.; Schneider, E. Anti-cancer drugs induce increased mitochondrial cytochrome c expression that precedes cell death. Cancer Res 2001, 61, 1038–1044. [Google Scholar]
- Bhadra, K. A mini review on molecules inducing caspase-independent cell death: A new route to cancer therapy. Molecules 2022, 27, 6401. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxid Med Cell Longev 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Interactions between reactive oxygen species and autophagy: Special issue: Death mechanisms in cellular homeostasis. Biochimica et Biophysica Acta (BBA) – Mol Cell Res 2021, 1868, 119041. [Google Scholar] [CrossRef]
- Ling, T.; Boyd, L.; Rivas, F. Triterpenoids as reactive oxygen species modulators of cell fate. Chem Res Toxicol 2022, 35, 569–584. [Google Scholar] [CrossRef]
- Liby, K.; Honda, T.; Williams, C.R.; Risingsong, R.; Royce, D.B.; Suh, N.; Dinkova-Kostova, A.T.; Stephenson, K.K.; Talalay, P.; Sundararajan, C.; et al. Novel semisynthetic analogues of betulinic acid with diverse cytoprotective, antiproliferative, and proapoptotic activities. Mol Cancer Ther 2007, 6, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, A.; Adamczyk-Grochala, J.; Kwasniewicz, E.; Deregowska, A.; Wnuk, M. Ursolic acid-mediated changes in glycolytic pathway promote cytotoxic autophagy and apoptosis in phenotypically different breast cancer cells. Apoptosis 2017, 22, 800–815. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Jung, J.; Jang, D.S.; Kim, J.; Kim, J.H. Induction of cell death by betulinic acid through induction of apoptosis and inhibition of autophagic flux in microglia BV-2 cells. Biomol & Ther 2017, 25, 618–624. [Google Scholar] [CrossRef]
- Zhang, Y.; He, N.; Zhou, X.; Wang, F.; Cai, H.; Huang, S.H.; Chen, X.; Hu, Z.; Jin, X. Betulinic acid induces autophagy-dependent apoptosis via Bmi-1/ROS/AMPK-mTOR-ULK1 axis in human bladder cancer cells. Aging (Albany NY) 2021, 13, 21251–21267. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Lee, S.H. The roles of autophagy in cancer. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, X.; Xu, L.; Li, Y.; Zeng, C. Current insight into the regulation of PD-L1 in cancer. Exp Hematol Oncol 2022, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.K.; Sung, B.; Aggarwal, B.B. Betulinic acid suppresses STAT3 activation pathway through induction of protein tyrosine phosphatase SHP-1 in human multiple myeloma cells. Int J Cancer 2010, 127, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Kutkowska, J.; Strzadala, L.; Rapak, A. Sorafenib in combination with betulinic acid synergistically induces cell cycle arrest and inhibits clonogenic activity in pancreatic ductal adenocarcinoma cells. Int J Mol Sci 2018, 19, 3234. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.Y.; Sp, N.; Lee, J.M.; Jang, K.J. Antitumor effects of ursolic acid through mediating the inhibition of STAT3/PD-L1 signaling in non-small cell lung cancer cells. Biomedicines 2021, 9. [Google Scholar] [CrossRef]
- Torres-Martinez, Z.; Pérez, D.; Torres, G.; Estrada, S.; Correa, C.; Mederos, N.; Velazquez, K.; Castillo, B.; Griebenow, K.; Delgado, Y. A synergistic pH-responsive serum albumin-based drug delivery system loaded with doxorubicin and pentacyclic triterpene betulinic acid for potential treatment of NSCLC. BioTech (Basel) 2023, 12. [Google Scholar] [CrossRef] [PubMed]
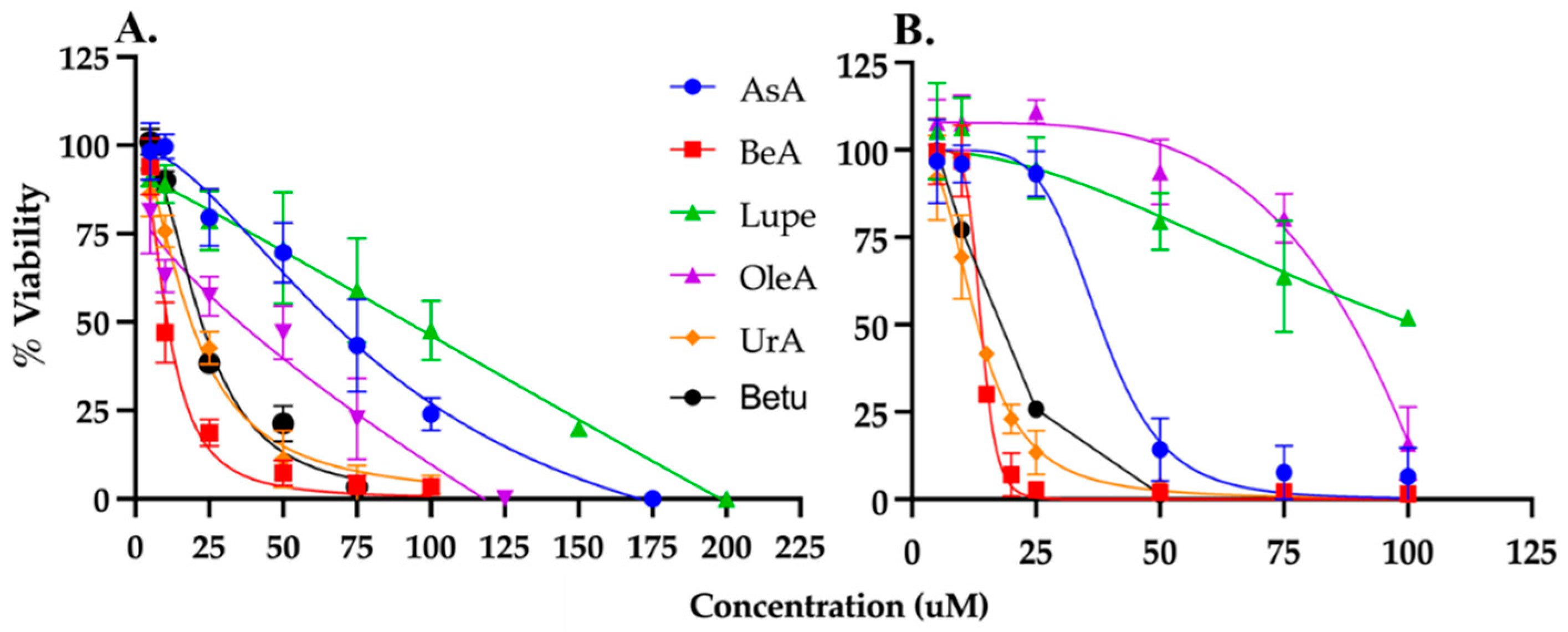
| Triterpenes | A549 IC50 (µM) | MRC5 IC50 (µM) | TI# |
|---|---|---|---|
| AsA | 59 ± 6* | 38 ± 2 | 0.64 |
| OleA | 43 ± 5* | 86 ± 2 | 2.00 |
| UrA | 23 ± 1* | 13 ± 1 | 0.57 |
| BeA | 15 ± 0.7* | 19 ± 0.4 | 1.27 |
| Lupe | 80 ± 6* | 101 ± 8 | 1.26 |
| Betu | 22 ± 1 | 16 ± 0.5 | 0.73 |
| Mechanism | UrA | AsA | OleA | Lupe | BeA | Betu | Highest | Lowest |
|---|---|---|---|---|---|---|---|---|
| TI > 1 | - | - | ✓ | ✓ | ✓ | - | OleA | UrA |
| Cell cycle arrest | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Betu | UrA |
| DNA damage | ✓ | ✓ | - | - | - | - | UrA | OleA |
| Mitochondrion depolarization | ✓ | ✓ | ✓ | ✓ | ✓ | - | BeA | Betu |
| Caspase 3 activation | ✓ | ✓ | ✓ | - | ✓ | ✓ | UrA | Lupe |
| RNS production | ✓ | ✓ | - | - | - | ✓ | Betu | BeA |
| mROS production | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | BeA | Betu |
| Autophagy inhibition | - | - | ✓ | ✓ | ✓ | - | Lupe | Betu |
| MAPK/PI3K inhibition | - | ✓ | ✓ | ✓ | ✓ | ✓ | Betu | UrA |
| PDL1 downregulation | ✓ | ✓ | ✓ | ✓ | ✓ | - | BeA | Betu |
| STAT3 downregulation | ✓ | ✓ | - | ✓ | ✓ | ✓ | BeA | OleA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





