Submitted:
03 January 2024
Posted:
04 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
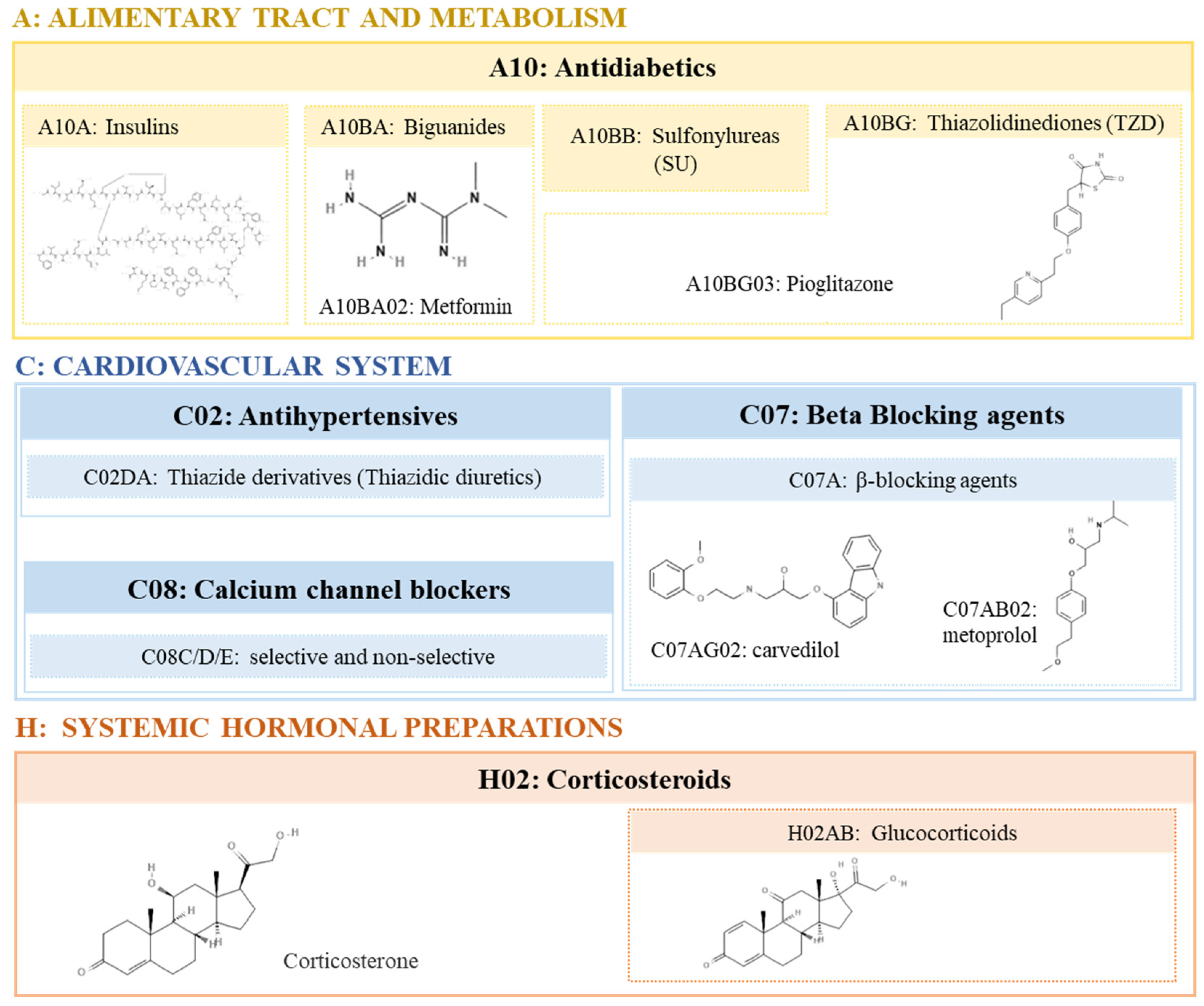
2. Pharmacological agents with obesogenic activity
2.1. Corticosteroids
2.2. Antidiabetics
2.3. Antihypertensive
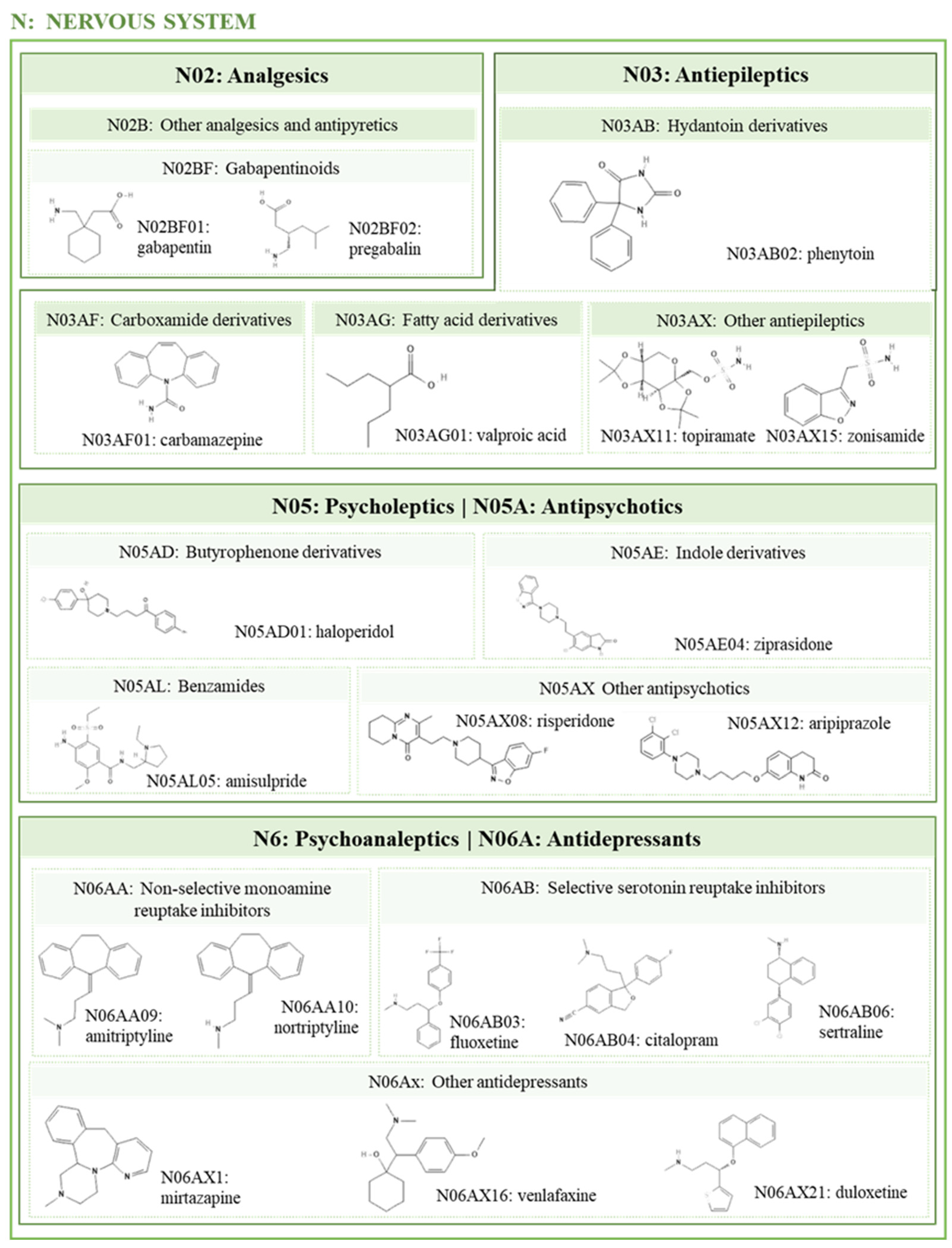
2.4. Psychotropic
2.4.1. Antidepressants
2.4.2. Lithium
2.4.3. Antipsychotics
2.4.4. Antiepileptics
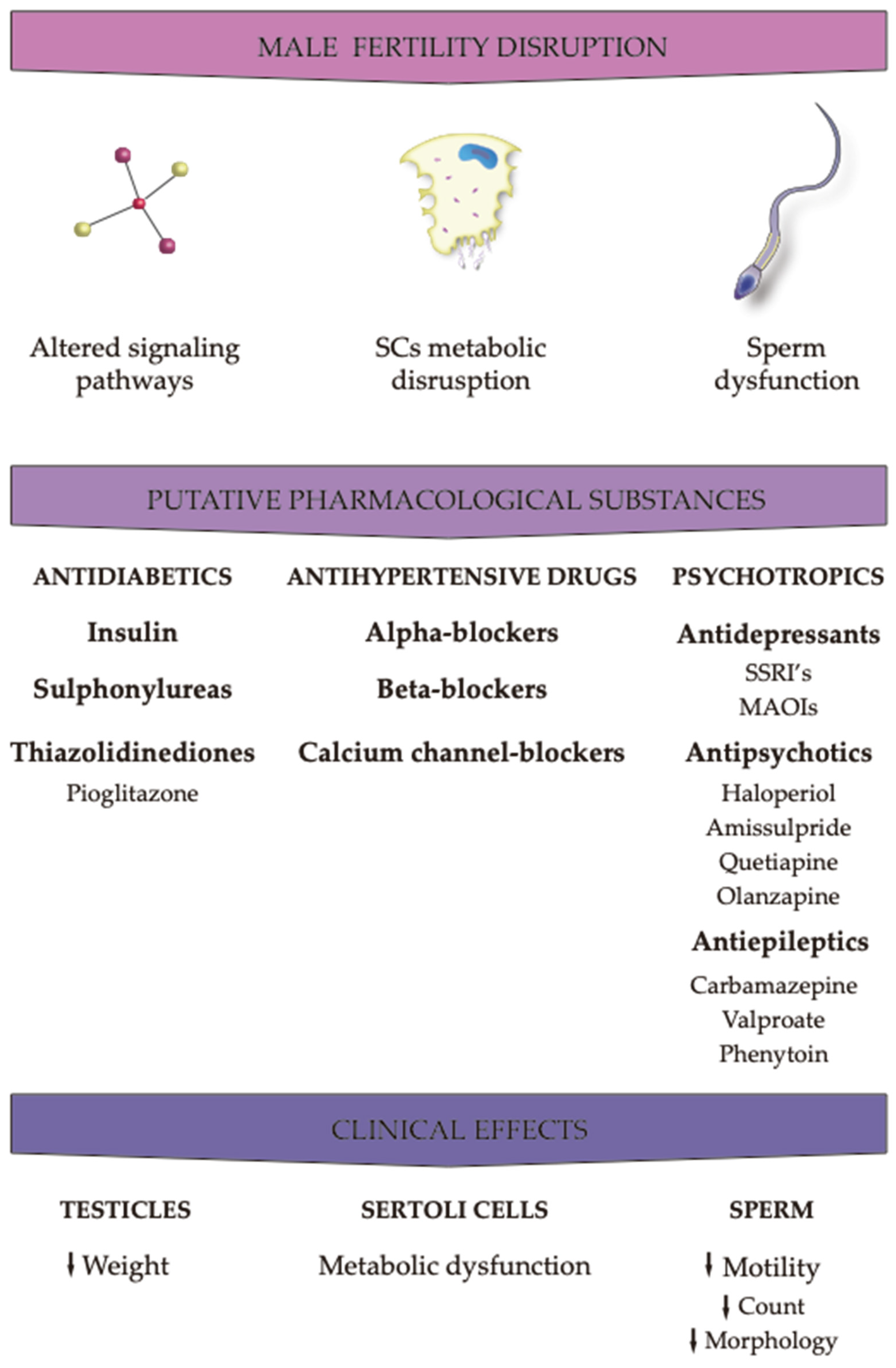
3. Effects of pharmacological agents on male fertility parameters
3.1. Antidiabetics
3.2. Antihypertensive drugs
3.3. Psychotropics
3.3.1. Antidepressants
3.3.2. Antipsychotics
3.3.3. Antiepileptics
4. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Heindel, J.; Schug, T., The Obesogen Hypothesis: Current Status and Implications for Human Health. Curr Envir Health Rpt 2014, 1, (4), 333-340.
- Lars Lind; P. Monica Lind; Margareta H. Lejonklou; Linda Dunder; Åke Bergman; Carlos Guerrero-Bosagna; Erik Lampa; Hong Kyu Lee; Juliette Legler; Angel Nadal; Youngmi Kim Pak; Richard P. Phipps; Laura N. Vandenberg; Daniel Zalko; Marlene Ågerstrand; Öberg, M.; Bruce Blumberg; Jerrold J. Heindel; Birnbaum, L. S., Uppsala Consensus Statement on Environmental Contaminants and the Global Obesity Epidemic. Envir. Health Perspect. 2016, 124, (5), A81-A83.
- De Coster, S.; Van Larebeke, N., Endocrine-disrupting chemicals: associated disorders and mechanisms of action. Journal of environmental and public health 2012, 2012.
- Janesick, A. S.; Blumberg, B., Obesogens: an emerging threat to public health. American journal of obstetrics and gynecology 2016, 214, (5), 559-565.
- Darbre, P. D., Endocrine disruptors and obesity. Current obesity reports 2017, 6, 18-27.
- Yang, O.; Kim, H. L.; Weon, J.-I.; Seo, Y. R., Endocrine-disrupting chemicals: review of toxicological mechanisms using molecular pathway analysis. Journal of cancer prevention 2015, 20, (1), 12.
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L. C.; Hauser, R.; Prins, G. S.; Soto, A. M.; Zoeller, R. T.; Gore, A. C., Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocrine reviews 2009, 30, (4), 293-342.
- Gore, A. C.; Chappell, V. A.; Fenton, S. E.; Flaws, J. A.; Nadal, A.; Prins, G. S.; Toppari, J.; Zoeller, R. T., EDC-2: the Endocrine Society's second scientific statement on endocrine-disrupting chemicals. Endocrine reviews 2015, 36, (6), E1-E150.
- Tontonoz, P.; Spiegelman, B. M., Fat and beyond: the diverse biology of PPARγ. Annu. Rev. Biochem. 2008, 77, 289-312.
- Lustig, R. H.; Collier, D.; Kassotis, C.; Roepke, T. A.; Ji Kim, M.; Blanc, E.; Barouki, R.; Bansal, A.; Cave, M. C.; Chatterjee, S.; Choudhury, M.; Gilbertson, M.; Lagadic-Gossmann, D.; Howard, S.; Lind, L.; Tomlinson, C. R.; Vondracek, J.; Heindel, J. J., Obesity I: Overview and molecular and biochemical mechanisms. Biochem. Pharmacol. 2022, 115012.
- Goldberg, E. D., TBT An environmental dilemma. Environment;(United States) 1986, 28, (8).
- Heindel, J. J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M. A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R., Metabolism disrupting chemicals and metabolic disorders. Reproductive toxicology 2017, 68, 3-33.
- Heindel, J. J.; Howard, S.; Agay-Shay, K.; Arrebola, J. P.; Audouze, K.; Babin, P. J.; Barouki, R.; Bansal, A.; Blanc, E.; Cave, M. C.; Chatterjee, S.; Chevalier, N.; Choudhury, M.; Collier, D.; Connolly, L.; Coumoul, X.; Garruti, G.; Gilbertson, M.; Hoepner, L. A.; Holloway, A. C.; Howell, G.; Kassotis, C.; Kay, M. K.; Ji Kim, M.; Lagadic-Gossmann, D.; Langouet, S.; Legrand, A.; Li, Z.; Le Mentec, H.; Lind, L.; Monica Lind, P.; Lustig, R. H.; Martin-Chouly, C.; Munic Kos, V.; Podechard, N.; Roepke, T. A.; Sargis, R. M.; Starling, A.; Tomlinson, C. R.; Touma, C.; Vondracek, J.; vom Saal, F.; Blumberg, B., Obesity II: Establishing Causal Links Between Chemical Exposures and Obesity. Biochem. Pharmacol. 2022, 115015.
- Casals-Casas, C.; Desvergne, B., Endocrine disruptors: from endocrine to metabolic disruption. Annual review of physiology 2011, 73, 135-162.
- Quagliariello, V.; Rossetti, S.; Cavaliere, C.; Di Palo, R.; Lamantia, E.; Castaldo, L.; Nocerino, F.; Ametrano, G.; Cappuccio, F.; Malzone, G., Metabolic syndrome, endocrine disruptors and prostate cancer associations: biochemical and pathophysiological evidences. Oncotarget 2017, 8, (18), 30606.
- Verhaegen, A. A.; Van Gaal, L. F., Drug-induced obesity and its metabolic consequences: a review with a focus on mechanisms and possible therapeutic options. Journal of endocrinological investigation 2017, 40, 1165-1174.
- Palmer, N. O.; Bakos, H. W.; Fullston, T.; Lane, M., Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis 2012, 2, (4), 253-263.
- Mínguez-Alarcón, L.; Gaskins, A. J.; Meeker, J. D.; Braun, J. M.; Chavarro, J. E., Endocrine disrupting chemicals and male reproductive health. Fertil. Steril. 2023.
- Thacharodi, A.; Hassan, S.; Acharya, G.; Vithlani, A.; Hoang Le, Q.; Pugazhendhi, A., Endocrine disrupting chemicals and their effects on the reproductive health in men. Environ. Res. 2023, 236, 116825.
- Sousa, A. C. A.; Alves, M. G.; Oliveira, P. F.; Silva, B. M.; Rato, L., Male Infertility in the XXI Century: Are Obesogens to Blame? International Journal of Molecular Sciences 2022, 23, (6), 3046.
- Curtis, J. R.; Westfall, A. O.; Allison, J.; Bijlsma, J. W.; Freeman, A.; George, V.; Kovac, S. H.; Spettell, C. M.; Saag, K. G., Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Care & Research 2006, 55, (3), 420-426.
- Bowles, N. P.; Karatsoreos, I. N.; Li, X.; Vemuri, V. K.; Wood, J.-A.; Li, Z.; Tamashiro, K. L.; Schwartz, G. J.; Makriyannis, A. M.; Kunos, G., A peripheral endocannabinoid mechanism contributes to glucocorticoid-mediated metabolic syndrome. Proceedings of the National Academy of Sciences 2015, 112, (1), 285-290.
- Russell-Jones, D.; Khan, R., Insulin-associated weight gain in diabetes–causes, effects and coping strategies. Diabetes, Obesity and Metabolism 2007, 9, (6), 799-812.
- Van Gaal, L.; Scheen, A., Weight management in type 2 diabetes: current and emerging approaches to treatment. Diabetes care 2015, 38, (6), 1161-1172.
- Mäkimattila, S.; Nikkilä, K.; Yki-Järvinen, H., Causes of weight gain during insulin therapy with and without metformin in patients with type II diabetes mellitus. Diabetologia 1999, 42, 406-412.
- Kahn, S. E.; Haffner, S. M.; Heise, M. A.; Herman, W. H.; Holman, R. R.; Jones, N. P.; Kravitz, B. G.; Lachin, J. M.; O'Neill, M. C.; Zinman, B., Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. New England Journal of Medicine 2006, 355, (23), 2427-2443.
- Turner, R. C., The UK prospective diabetes study: a review. Diabetes care 1998, 21, (Supplement_3), C35-C38.
- Inzucchi, S. E., Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA 2002, 287, (3), 360-72.
- Domecq, J. P.; Prutsky, G.; Leppin, A.; Sonbol, M. B.; Altayar, O.; Undavalli, C.; Wang, Z.; Elraiyah, T.; Brito, J. P.; Mauck, K. F., Drugs commonly associated with weight change: a systematic review and meta-analysis. The Journal of Clinical Endocrinology & Metabolism 2015, 100, (2), 363-370.
- Medici, V.; McClave, S. A.; Miller, K. R., Common medications which lead to unintended alterations in weight gain or organ lipotoxicity. Current gastroenterology reports 2016, 18, 1-12.
- Messerli, F. H.; Bell, D. S.; Fonseca, V.; Katholi, R. E.; McGill, J. B.; Phillips, R. A.; Raskin, P.; Wright Jr, J. T.; Bangalore, S.; Holdbrook, F. K., Body weight changes with β-blocker use: results from GEMINI. The American journal of medicine 2007, 120, (7), 610-615.
- Sharma, A. M.; Pischon, T.; Hardt, S.; Kunz, I.; Luft, F. C., Hypothesis: β-adrenergic receptor blockers and weight gain: a systematic analysis. Hypertension 2001, 37, (2), 250-254.
- Welle, S.; Schwartz, R. G.; Statt, M., Reduced metabolic rate during β-adrenergic blockade in humans. Metabolism 1991, 40, (6), 619-622.
- Koch, G.; Franz, I.-W.; Lohmann, F., Effects of Short-Term and Long-Term Treatment with Cardio-Selective and non-Selective β-Receptor Blockade on Carbohydrate and Lipid Metabolism and on Plasma Catecholamines at Rest and during Exercise. Clinical Science 1981, 61, (s7), 433s-435s.
- Bakris, G. L.; Fonseca, V.; Katholi, R. E.; McGill, J. B.; Messerli, F. H.; Phillips, R. A.; Raskin, P.; Wright, J. T., Jr.; Oakes, R.; Lukas, M. A.; Anderson, K. M.; Bell, D. S.; Investigators, G., Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA 2004, 292, (18), 2227-36.
- Ripley, T. L.; Saseen, J. J., β-blockers: a review of their pharmacological and physiological diversity in hypertension. Annals of Pharmacotherapy 2014, 48, (6), 723-733.
- Landsberg, L.; Aronne, L. J.; Beilin, L. J.; Burke, V.; Igel, L. I.; Lloyd-Jones, D.; Sowers, J., Obesity-related hypertension: Pathogenesis, cardiovascular risk, and treatment—A position paper of the The Obesity Society and the American Society of Hypertension. Obesity 2013, 21, (1), 8-24.
- Elliott, W. J.; Meyer, P. M., Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. The Lancet 2007, 369, (9557), 201-207.
- Ménard, C.; Pfau, M. L.; Hodes, G. E.; Russo, S. J., Immune and neuroendocrine mechanisms of stress vulnerability and resilience. Neuropsychopharmacology 2017, 42, (1), 62-80.
- Naughton, M.; Dinan, T. G.; Scott, L. V., Corticotropin-releasing hormone and the hypothalamic–pituitary–adrenal axis in psychiatric disease. Handbook of clinical neurology 2014, 124, 69-91.
- Capuron, L.; Lasselin, J.; Castanon, N., Role of adiposity-driven inflammation in depressive morbidity. Neuropsychopharmacology 2017, 42, (1), 115-128.
- Chuang, J.-C.; Krishnan, V.; Hana, G. Y.; Mason, B.; Cui, H.; Wilkinson, M. B.; Zigman, J. M.; Elmquist, J. K.; Nestler, E. J.; Lutter, M., A β3-adrenergic-leptin-melanocortin circuit regulates behavioral and metabolic changes induced by chronic stress. Biological psychiatry 2010, 67, (11), 1075-1082.
- Zimmermann, U.; Kraus, T.; Himmerich, H.; Schuld, A.; Pollmächer, T., Epidemiology, implications and mechanisms underlying drug-induced weight gain in psychiatric patients. Journal of psychiatric research 2003, 37, (3), 193-220.
- Vandenberghe, F.; Gholam-Rezaee, M.; Saigí-Morgui, N.; Delacretaz, A.; Choong, E.; Solida-Tozzi, A.; Kolly, S.; Thonney, J.; Gallo, S. F.; Hedjal, A., Importance of early weight changes to predict long-term weight gain during psychotropic drug treatment. The Journal of clinical psychiatry 2015, 76, (11), 8736.
- Himmerich, H.; Minkwitz, J.; C Kirkby, K., Weight gain and metabolic changes during treatment with antipsychotics and antidepressants. Endocrine, Metabolic & Immune Disorders-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders) 2015, 15, (4), 252-260.
- Casey, D. E.; Zorn, S. H., The pharmacology of weight gain with antipsychotics. Journal of Clinical Psychiatry 2001, 62, 4-10.
- Serretti, A.; Chiesa, A., A meta-analysis of sexual dysfunction in psychiatric patients taking antipsychotics. International clinical psychopharmacology 2011, 26, (3), 130-140.
- Livingstone, C.; Rampes, H., Lithium: a review of its metabolic adverse effects. Journal of Psychopharmacology 2006, 20, (3), 347-355.
- Spertus, J.; Horvitz-Lennon, M.; Abing, H.; Normand, S. L., Risk of weight gain for specific antipsychotic drugs: a meta-analysis. NPJ Schizophr 2018, 4, (1), 12.
- Allison, D. B.; Mentore, J. L.; Heo, M.; Chandler, L. P.; Cappelleri, J. C.; Infante, M. C.; Weiden, P. J., Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999, 156, (11), 1686-96.
- Balf, G.; Stewart, T. D.; Whitehead, R.; Baker, R. A., Metabolic adverse events in patients with mental illness treated with antipsychotics: a primary care perspective. Primary care companion to the Journal of clinical psychiatry 2008, 10, (1), 15.
- Ben-Menachem, E., Weight issues for people with epilepsy--a review. Epilepsia 2007, 48 Suppl 9, 42-5.
- Agró, L.; Demurtas, R.; Sander, J. W., Anticonvulsant Agents: Gabapentin and Pregabalin. In NeuroPsychopharmacotherapy, Riederer, P.; Laux, G.; Mulsant, B.; Le, W.; Nagatsu, T., Eds. Springer International Publishing: Cham, 2020; pp 1-14.
- Verrotti, A.; D'Egidio, C.; Mohn, A.; Coppola, G.; Chiarelli, F., Weight gain following treatment with valproic acid: pathogenetic mechanisms and clinical implications. Obes Rev 2011, 12, (5), e32-43.
- Belcastro, V.; D’Egidio, C.; Striano, P.; Verrotti, A., Metabolic and endocrine effects of valproic acid chronic treatment. Epilepsy research 2013, 107, (1-2), 1-8.
- Swann, A. C., Major system toxicities and side effects of anticonvulsants. Journal of Clinical Psychiatry 2001, 62, (14), 16-21.
- Antel, J.; Hebebrand, J., Weight-reducing side effects of the antiepileptic agents topiramate and zonisamide. Handb Exp Pharmacol 2012, (209), 433-66.
- Campion, S.; Catlin, N.; Heger, N.; McDonnell, E. V.; Pacheco, S. E.; Saffarini, C.; Sandrof, M. A.; Boekelheide, K., Male reprotoxicity and endocrine disruption. Molecular, Clinical and Environmental Toxicology: Volume 3: Environmental Toxicology 2012, 315-360.
- Creasy, D. M., Evaluation of testicular toxicity in safety evaluation studies: the appropriate use of spermatogenic staging. Toxicologic pathology 1997, 25, (2), 119-131.
- Cardoso, A. M.; Alves, M. G.; Mathur, P. P.; Oliveira, P. F.; Cavaco, J. E.; Rato, L., Obesogens and male fertility. Obes Rev 2017, 18, (1), 109-125.
- Rato, L.; Sousa, A. C. A., The Impact of Endocrine-Disrupting Chemicals in Male Fertility: Focus on the Action of Obesogens. J Xenobiot 2021, 11, (4), 163-196.
- Sousa, A. C. A.; Alves, M. G.; Oliveira, P. F.; Silva, B. M.; Rato, L., Male Infertility in the XXI Century: Are Obesogens to Blame? Int J Mol Sci 2022, 23, (6).
- Traggiai, C.; Stanhope, R., Disorders of pubertal development. Best practice & research Clinical obstetrics & gynaecology 2003, 17, (1), 41-56.
- Wilson, C.; Davies, D., The control of sexual differentiation of the reproductive system and brain. Reproduction 2007, 133, (2), 331-359.
- Rinaldi, S.; Moret, C.; Kaaks, R.; Biessy, C.; Kurzer, M.; Dechaud, H.; Peeters, P.; Van Noord, P., Reproducibility over time of measurements of androgens, estrogens and hydroxy estrogens in urine samples from post-menopausal women. European journal of epidemiology 2003, 18, 417-424.
- Viswanathan, V.; Eugster, E. A., Etiology and treatment of hypogonadism in adolescents. Pediatric Clinics 2011, 58, (5), 1181-1200.
- Semet, M.; Paci, M.; Saïas-Magnan, J.; Metzler-Guillemain, C.; Boissier, R.; Lejeune, H.; Perrin, J., The impact of drugs on male fertility: a review. Andrology 2017, 5, (4), 640-663.
- Kung, J.; Henry, R. R., Thiazolidinedione safety. Expert opinion on drug safety 2012, 11, (4), 565-579.
- Alves, M. G.; Martins, A. D.; Vaz, C. V.; Correia, S.; Moreira, P. I.; Oliveira, P. F.; Socorro, S., Metformin and male reproduction: effects on Sertoli cell metabolism. Br J Pharmacol 2014, 171, (4), 1033-42.
- Krentz, A. J.; Bailey, C. J., Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs 2005, 65, 385-411.
- Meneses, M. J.; Bernardino, R. L.; Sa, R.; Silva, J.; Barros, A.; Sousa, M.; Silva, B. M.; Oliveira, P. F.; Alves, M. G., Pioglitazone increases the glycolytic efficiency of human Sertoli cells with possible implications for spermatogenesis. Int J Biochem Cell Biol 2016, 79, 52-60.
- Ferrario, C. M.; Levy, P., Sexual dysfunction in patients with hypertension: implications for therapy. The journal of clinical hypertension 2002, 4, (6), 424-432.
- Grimm Jr, R. H.; Grandits, G. A.; Prineas, R. J.; McDonald, R. H.; Lewis, C. E.; Flack, J. M.; Yunis, C.; Svendsen, K.; Liebson, P. R.; Elmer, P. J., Long-term effects on sexual function of five antihypertensive drugs and nutritional hygienic treatment in hypertensive men and women: Treatment of Mild Hypertension Study (TOMHS). Hypertension 1997, 29, (1), 8-14.
- Manolis, A.; Doumas, M., Antihypertensive treatment and sexual dysfunction. Current hypertension reports 2012, 14, 285-292.
- Jones, D.; Norman, A.; Horwich, A.; Hendry, W., Ejaculatory dysfunction after retroperitoneal lymphadenectomy. European urology 1993, 23, (1), 169-171.
- Shindel, A.; Kishore, S.; Lue, T., Drugs designed to improve endothelial function: effects on erectile dysfunction. Current pharmaceutical design 2008, 14, (35), 3758-3767.
- Becker, A. J.; Ückert, S.; Stief, C. G.; Scheller, F.; Knapp, W. H.; Hartmann, U.; Jonas, U., Plasma levels of angiotensin II during different penile conditions in the cavernous and systemic blood of healthy men and patients with erectile dysfunction. Urology 2001, 58, (5), 805-810.
- Droupy, S. In Epidemiology and physiopathology of erectile dysfunction, Annales D'urologie, 2005; 2005; pp 71-84.
- Karavitakis, M.; Komninos, C.; Theodorakis, P. N.; Politis, V.; Lefakis, G.; Mitsios, K.; Koritsiadis, S.; Doumanis, G., Evaluation of sexual function in hypertensive men receiving treatment: a review of current guidelines recommendation. The Journal of Sexual Medicine 2011, 8, (9), 2405-2414.
- Shindel, A. W.; Althof, S. E.; Carrier, S.; Chou, R.; McMahon, C. G.; Mulhall, J. P.; Paduch, D. A.; Pastuszak, A. W.; Rowland, D.; Tapscott, A. H., Disorders of ejaculation: an AUA/SMSNA guideline. Journal of Urology 2022, 207, (3), 504-512.
- Gratzke, C.; Angulo, J.; Chitaley, K.; Dai, Y.-t.; Kim, N. N.; Paick, J.-S.; Simonsen, U.; Ückert, S.; Wespes, E.; Andersson, K. E., Anatomy, physiology, and pathophysiology of erectile dysfunction. The journal of sexual medicine 2010, 7, (1_Part_2), 445-475.
- Nudell, D. M.; Monoski, M. M.; Lipshultz, L. I., Common medications and drugs: how they affect male fertility. Urologic Clinics 2002, 29, (4), 965-973.
- Schlosser, J.; Nakib, I.; Carré-Pigeon, F.; Staerman, F. In Infertilité masculine: définition et physiopathologie, Annales d'urologie, 2007; Elsevier: 2007; pp 127-133.
- Benoff, S.; Cooper, G. W.; Hurley, I.; Mandel, F. S.; Rosenfeld, D. L.; Scholl, G. M.; Gilbert, B. R.; Hershlag, A., The effect of calcium ion channel blockers on sperm fertilization potential. Fertility and sterility 1994, 62, (3), 606-617.
- Brezina, P. R.; Yunus, F. N.; Zhao, Y., Effects of pharmaceutical medications on male fertility. Journal of reproduction & infertility 2012, 13, (1), 3.
- Kanwar, U.; Anand, R.; Sanyal, S., The effect of nifedipine, a calcium channel blocker, on human spermatozoal functions. Contraception 1993, 48, (5), 453-470.
- De Rosa, M.; Zarrilli, S.; Di Sarno, A.; Milano, N.; Gaccione, M.; Boggia, B.; Lombardi, G.; Colao, A., Hyperprolactinemia in men: clinical and biochemical features and response to treatment. Endocrine 2003, 20, 75-82.
- Tanrikut, C.; Schlegel, P. N., Antidepressant-associated changes in semen parameters. Urology 2007, 69, (1), 185. e5-185. e7.
- Baldwin, D.; Mayers, A., Sexual side-effects of antidepressant and antipsychotic drugs. Advances in Psychiatric Treatment 2003, 9, (3), 202-210.
- Dording, C. M.; Fisher, L.; Papakostas, G.; Farabaugh, A.; Sonawalla, S.; Fava, M.; Mischoulon, D., A double-blind, randomized, pilot dose-finding study of maca root (L. meyenii) for the management of SSRI-induced sexual dysfunction. CNS neuroscience & therapeutics 2008, 14, (3), 182-191.
- Montejo, A. L.; Llorca, G.; Izquierdo, J. A.; Rico-Villademoros, F., Incidence of sexual dysfunction associated with antidepressant agents: a prospective multicenter study of 1022 outpatients. Journal of Clinical Psychiatry 2001, 62, 10-21.
- Erdemir, F.; Atilgan, D.; Firat, F.; Markoc, F.; Parlaktas, B. S.; Sogut, E., The effect of sertraline, paroxetine, fluoxetine and escitalopram on testicular tissue and oxidative stress parameters in rats. International braz j urol 2014, 40, 100-108.
- Montejo, A. L.; Montejo, L.; Navarro-Cremades, F., Sexual side-effects of antidepressant and antipsychotic drugs. Current opinion in psychiatry 2015, 28, (6), 418-423.
- Safarinejad, M. R., Sperm DNA damage and semen quality impairment after treatment with selective serotonin reuptake inhibitors detected using semen analysis and sperm chromatin structure assay. The Journal of urology 2008, 180, (5), 2124-2128.
- Althof, S. E.; McMahon, C. G.; Waldinger, M. D.; Serefoglu, E. C.; Shindel, A. W.; Adaikan, P. G.; Becher, E.; Dean, J.; Giuliano, F.; Hellstrom, W. J.; Giraldi, A.; Glina, S.; Incrocci, L.; Jannini, E.; McCabe, M.; Parish, S.; Rowland, D.; Segraves, R. T.; Sharlip, I.; Torres, L. O., An Update of the International Society of Sexual Medicine's Guidelines for the Diagnosis and Treatment of Premature Ejaculation (PE). Sex Med 2014, 2, (2), 60-90.
- Taylor, M. J.; Rudkin, L.; Bullemor-Day, P.; Lubin, J.; Chukwujekwu, C.; Hawton, K., Strategies for managing sexual dysfunction induced by antidepressant medication. Cochrane Database of Systematic Reviews 2013, (5).
- Rosen, R. C.; Marin, H., Prevalence of antidepressant-associated erectile dysfunction. Journal of Clinical Psychiatry 2003, 64, 5-10.
- Panidis, D.; Rousso, D.; Skiadopoulos, S.; Panidou, E.; Mamopoulos, M., Evaluation of semen parameters in man with hyperprolactinemia induced by metoclopramide. Archives of andrology 1997, 39, (3), 237-242.
- Howes, O. D.; Wheeler, M. J.; Pilowsky, L. S.; Landau, S.; Murray, R. M.; Smith, S., Sexual function and gonadal hormones in patients taking antipsychotic treatment for schizophrenia or schizoaffective disorder. Journal of Clinical Psychiatry 2007, 68, (3), 361-367.
- Chen, S. S.; Shen, M. R.; Chen, T. J.; Lai, S. L., Effects of antiepileptic drugs on sperm motility of normal controls and epileptic patients with long-term therapy. Epilepsia 1992, 33, (1), 149-153.
- Isojärvi, J.; Löfgren, E.; Juntunen, K.; Pakarinen, A.; Päivänsalo, M.; Rautakorpi, I.; Tuomivaara, L., Effect of epilepsy and antiepileptic drugs on male reproductive health. Neurology 2004, 62, (2), 247-253.
- Herzog, A. G., Disorders of reproduction in patients with epilepsy: primary neurological mechanisms. Seizure 2008, 17, (2), 101-110.
- Lossius, M. I.; Taubøll, E.; Mowinckel, P.; Mørkrid, L.; Gjerstad, L., Reversible Effects of Antiepileptic Drugs on Reproductive Endocrine Function in Men and Women with Epilepsy—A Prospective Randomized Double-Blind Withdrawal Study. Epilepsia 2007, 48, (10), 1875-1882.
- Rättyä, J.; Turkka, J.; Pakarinen, A. J.; Knip, M.; Kotila, M.; Lukkarinen, O.; Myllylä, V.; Isojärvi, J., Reproductive effects of valproate, carbamazepine, and oxcarbazepine in men with epilepsy. Neurology 2001, 56, (1), 31-36.
- Webber, M. P.; Hauser, W. A.; Ottman, R.; Annegesr, J. F., Fertility in persons with epilepsy: 1935–1974. Epilepsia 1986, 27, (6), 746-752.
- Ocek, L.; Tarhan, H.; Uludag, F. I.; Sariteke, A.; Kose, C.; Colak, A.; Zorlu, F.; Zorlu, Y., Evaluation of sex hormones and sperm parameters in male epileptic patients. Acta Neurol Scand 2018, 137, (4), 409-416.
- Isojärvi, J. I.; Taubøll, E.; Herzog, A. G., Effect of antiepileptic drugs on reproductive endocrine function in individuals with epilepsy. CNS drugs 2005, 19, 207-223.
- Oliva, S. U.; Scarano, W. R.; Okada, F. K.; Miraglia, S. M., Harmful effects of carbamazepine on the postnatal development of the rat ventral prostate. Reproductive Biology and Endocrinology 2012, 10, 1-15.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




