Submitted:
02 January 2024
Posted:
04 January 2024
You are already at the latest version
Abstract
Keywords:
Introduction
The Early Years
The Rise and Subsequent Fall of a Metal Ion-Dependent Monooxygenase
The Fallow Years
New Insights—The Various Roles of Serendipity
Confirming the Role of 2,5-DKCMO as a Two-Component Monooxygenase
As the Pool of Light Expands, So Does the Surrounding Halo of Darkness
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres, M.E.S.; Sancedo-Vazquez, J.P.; Kroneck, P.M.H. The magic of dioxygen. In Sustsaining life on Planet Earth: metalloenzymes mastering dioxygen and other chewy gases: Kroneck, P.M.H.; Torres, M.E.S., Ed.; Springer International Publishing: Basle, Switzerland, 2015; pp. 1–12. [Google Scholar]
- Borden, W.T.; Hoffmann, R.; Stuyver, T.; Chen, B. Dioxygen: what makes this triplet diradical kinetically persistent? J. Amer. Chem. Soc. 2017, 139, 9010–9018. [Google Scholar] [CrossRef]
- Holtmann, D.; Hollmann, F. The oxygen dilemma: a severe challenge for the application of monooxygenases? ChemBioChem. 2016, 7, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Massey, V. Activation of molecular oxygen by flavins and flavoproteins. J. Biol. Chem. 1994, 269, 22459–22462. [Google Scholar] [CrossRef] [PubMed]
- Torres Pazmino, D.E.; Winkler, M.; Glieder, A.; Fraaije, M.W. Monooxygenases as biocatalysts: classification, mechanistic aspects and biotechnological applications. J. Biotechnol. 2010, 146, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Theorell, H. Preparation in pure state of the effect group of yellow enzyme. Biochimische Zeitschrift, 1935, 275, 344–346. [Google Scholar]
- Hayaishi, O.; Katagiri, M.; Rothberg, S. Mechanism of the pyrocatechase reaction. J. Amer. Chem. Soc. 1955, 77, 5450–5451. [Google Scholar] [CrossRef]
- Mason, H.S.; Fowlks, W.L.; Peterson, E. Oxygen transfer and electron transport by the phenolase complex. J. Amer. Chem. Soc. 1955, 77, 2914–2915. [Google Scholar] [CrossRef]
- Mason, H. Mechanisms of oxygen metabolism. Science 1957, 125, 1185–1188. [Google Scholar] [CrossRef]
- Hayaishi, O.; Katagiri, M.; Rothberg, S. Studies on oxygenases. J. Biol. Chem. 1957, 229, 376–386. [Google Scholar] [CrossRef]
- Hayaishi, O. An odyssey with oxygen. Biochem. Biophys. Res. Commun. 2005, 338, 2–6. [Google Scholar] [CrossRef]
- Chakrabarty, A.M.; Gunsalus, C.F.; Gunsalus, I.C. Transduction and clustering of genes in fluorescent pseudomonads. Proc. Nat. Acad. Sci. USA 1968, 60, 168–175. [Google Scholar] [CrossRef]
- Rheinwald, J.G.; Chakrabarty, A.M.; Gunsalus, I.C. A transmissible plasmid controlling camphor oxidation in Pseudomonas putida. Proc. Nat. Acad. Sci. USA 1973, 70, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Shaham, M.; Chakrabarty, A.M.; Gunsalus, I.C. Camphor plasmid-mediated chromosomal transfer in Pseudomonas putida. J. Bacteriol. 1973, 116, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, A.M. Plasmids in Pseudomonas. Ann. Rev. Genet. 1976, 10, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Willetts, A. Characterised flavin-dependent two-component monooxygenases from the CAM plasmid of Pseudomonas putida ATCC 17453 (NCIMB 10007). Ketolactonases by another name. Microorganisms 2019, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, W.H.; Conrad, H.E.; Corey, E.J.; Gunsalus, I.C.; Lederer, D. Microbial degradation of (+)-camphor. J. Amer. Chem. Soc. 1959, 81, 5507. [Google Scholar] [CrossRef]
- Conrad, H.E.; Corey, E.J.; Hedegaard, J.; Paisley, N.S.; Gunsalus, I.C. Terpenoid metabolism: oxidation and ring cleavage reactions. In 4th Int. Cong. Biochem; Moscow, Russia, 1961; vol. 29, p 284.
- Hedegaard, J.; Coinrad, H.E.; Gundsalus, I.C. Camphor oxidation: pathways and enzyme induction. Bacteriol. Proc. 1961, 183 Abstract P66.
- Baeyer, A.; Villiger, V. Einwirkung des caro’schen reagens auf ketone. Ber. Dtsch. Chem. Ges. 1899, 32, 3625–3633. [Google Scholar] [CrossRef]
- Fried, J.; Thoma, R.H.; Klingberg, A. Oxidation of steroids by microorganisms. III. Side chain degradation, ring D cleavage, and dehydrogenation of the A ring. J. Amer. Chem. Soc. 1953, 75, 5764–5765. [Google Scholar] [CrossRef]
- Peterson, D.H.; Eppstein, S.H.; Meister, P.D.; Murray, H.C.; Leigh, H.M.; Weintraub, A., M.; Reineke, L.M. Microbial transformation of steroids, IX. Degradation of C21 steroids to C19 lactones and testololactone. J. Amer. Chem. Soc. 1953, 75, 5768–5769. [Google Scholar] [CrossRef]
- Conrad, H. E.; DuBus, R.; Gunsalus, I.C. An enzyme system for cyclic lactonization. Biochem. Biophys. Res. Commun. 1961, 6, 293–297. [Google Scholar] [CrossRef]
- Klingenberg, M. Pigments of rat liver microsomes. Archiv. Biochem. Biophys. 1958, 75, 376–386. [Google Scholar] [CrossRef]
- Auld, D.S. Use of chelating agents to inhibit enzymes. Meth. Enzymol. 1988, 158, 110–114. [Google Scholar]
- Lipscomb, J.D. Life in a sea of oxygen. J. Biol. Chem. 2014, 289, 15141–15153. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Christian, W. Isolation of the prosthetic group of the amino acid oxidase. Biochemische Zeitschrift. 1938, 298, 150–168. [Google Scholar]
- Conrad, H.E.; DuBus, R.; Gunsalus, I.C. Enzymatic lactonization: a possible role for mixed function oxidase action. Fed. Proc. 1962, 21, 52 Abstract 031-038. [Google Scholar]
- Bertland, A.U.; Johnspon, S.; Gunsalus, I.C. Induced enzymes in terpene metabolism: an FMN-coupled DPNH oxidase in pseudomonads. Bacteriol. Proc. 1963, 105 Abstract P44.
- Conrad, H.E.; DuBus, R.; Lieb, I.C.; Gunsalus, I.C. Enzymatic keto-lactonization: inhibition of mixed function oxidation. In 6th Int. Cong. Biochem. New York, USA, 1964; 301 Abstract 32.
- Conrad, H.E.; Trudgill, P.W.; Gunsalus, I.C. Oxidative enzyme systems in the metabolism of camphor enantiomers. 148th Mtg. Am. Chem. Soc., Chigago, USA, 1964, Abstract Q3.
- Conrad, H.E.; DuBus, R. , Namtvedt, M. J., Gunsalus, I.C. Mixed function oxidation. II. Separation and properties of the enzymes catalysing camphor lactonization. J. Biol. Chem. 1965, 240, 495–503. [Google Scholar]
- Conrad, H.E. , Lieb, K.; Gunsalus, I.C. Mixed function oxidation. III. An electron transport complex in camphor lactonization. J. Biol. Chem. 1965, 240, 4029–4037. [Google Scholar] [CrossRef] [PubMed]
- Gunsalus, I.C.; Conrad, H.E.; Trudgill, P.W. Generation of active oxygen for mixed function oxidation. In Oxidases and Related Redox Systems.; King, T.E., Mason, H.S., Morrish, M., Eds.; Wiley: New York, NY, USA, 1965; pp 417-447. [Google Scholar]
- Gunsalus, I.C.; Trudgill, P.W.; Cushman, D.; Conrad, H.E. Stereospecific biological oxygenation of terpenoids. In Symposium on recent advances in the chemistry of terpenoids. National Chemistry Laboratory, Poona, India. 1965., 6 Abstract 3.
- Trudgill, P.W.; DuBus, R.; Gunsalus, I.C. Mixed function oxidation. V. Flavin interaction with reduced diphosphopyridine nucleotide dehydrogenase, one of the enzymes participating in camphor lactonization. J. Biol. Chem. 1966, 241, 1194–1205.
- Trudgill, P.W.; DuBus, R.; Gunsalus, I.C. Mixed function oxidation. VI. Purification of a tightly linked electron transport complex in camphor lactinization. J. Biol. Chem. 1966, 241, 4288–4290. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.A.; Gunsalus, I.C. Monooxygenases. VII. Camphor lactonase I and the role of the protein components. J. Biol. Chem. 1969, 244, 6149–6152. [Google Scholar] [CrossRef]
- Cooke, R.; Tsibris, J.C.M.; Debrunner, P.G.; Tsai, R.L.; Gunsalus, I.C.; Frauenfelder, H. Mossbauer studies on putidaredoxin. Proc. Natl. Acad. Sci. USA 1968, 59, 1045–1052. [Google Scholar] [CrossRef]
- Entsch, B.; Ballou, D.P.; Massey, V. Flavin-oxygen derivatives involved in hydroxylation by p-hydroxybenzoate hydroxylase. J. Biol. Chem. 1976, 251, 2550–2563. [Google Scholar] [CrossRef]
- Massey, V. Activation of molecular oxygen by flavins and flavoproteins. J. Biol. Chem. 1994, 269, 22459–22462. [Google Scholar] [CrossRef]
- Entsch, B.; Ballou, D.P.; Massey, V. The mechanism of action of xanthine oxidase. J. Biol. Chem. 1974, 249, 4363–4382. [Google Scholar]
- Vermilion, J.; Ballou, D.; Massey, V.; Coon, M.J. Separate roles for FMN and FAD in catalysis by liver microsomal NADPH-cytochrome P-450 reductase. J. Biol. Chem. 1981, 256, 266–277. [Google Scholar] [CrossRef]
- Ball, S.; Bruice, T.C. 4a-Hydroperoxyflavin N-oxidation of tertiary amines. J. Amer. Chem. Soc. 1979, 101, 4017–4023. [Google Scholar] [CrossRef]
- Bruice, T.C. Oxygen-flavin chemistry. Isr. J. Chem. 1984, 24, 54–61. [Google Scholar] [CrossRef]
- Robbins, J.M.; Ellis, H.R. Investigations of two-component flavin-dependent monooxygenase systems. Meth. Enzymol. 2019, 620, 399–422. [Google Scholar]
- Trudgill, P.W. Bacteria and the challenge of cyclic molecules. In Experiences in biochemical perception; Ornston, L.N.; Sligar, S.G. Eds; Academic Press; New Yory, USA, 1982; pp. 59–73.
- Trudgill, P.W. Microbial degradation of alicyclic hydrocarbons. In Developments in biodegradation of hydrocarbons – I; Watkinson, R.J. Ed; Applied Science Publishers; London, UK, 1982; pp. 47–84.
- Taylor, D.G.; Trudgill, P.W. Studies of 2,5-diketocamphane monooxygenase from Pseudomonas putida ATCC 17453. In Proceedings of the 8th Int. Symp. on Flavins and Flavoproteins; Bray, R.C.; Engel, P.C.; Mayhew, S.G. Eds; de Gruyter: Berlin, Germany, 1984; pp. 343–344. [Google Scholar]
- Trudgill, P.W. Microbial degradation of the alicyclic ring. In Microbial degradation of organic compounds; Gibson, D.T. Ed; Marcel Dekker; New York, USA, 1984; pp. 131-180.
- Taylor, D.G.; Trudgill, P.W. Camphor revisited: studies of 2,5-diketocamphane 1,2-monooxygenase from Pseudomonas putida ATCC 17453. J. Bacteriol. 1986, 165, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Trudgill, P.W. Terpenoid metabolism by Pseudomonas. In The Bacteria, Volume 10; Sokatch, J.R., Ed; Academic Press; Orlando, FL, USA, 1986; pp. 483-525.
- Jones, K.H.; Smith, R.T.; Trudgill, P.W. Diketocamphane enantiomer-specific ‘Baeyer-Villiger’ monooxygenases from camphor-grown Pseudomonas putida ATCC 17453. J. Gen. Microbiol. 1993, 139, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Grogan, G.; Roberts, S.M.; Willetts, A. Camphor-grown Pseudomonas putida, a multifunctional biocatalyst for oxygenations. Biotechnol. Lett. 1993, 15, 913–918. [Google Scholar] [CrossRef]
- Grogan, G.; Roberts, S.M.; Wan, P.; Willetts, A. Some Baeyer-Villiger oxygenations using monooxygenases from Pseudomonas putida. J. Chem. Soc., Chem. Commun, 1993, 699-701.
- Garnon, R.; Grogan, G.; Roberts, S.M.; Villa, R.; Willetts, A. Enzymatic Baeyer-Villiger oxidations of some bicyclic ketones using monooxygenases from Pseudomonas putida NCIMB 10007: enantioselective preparation a precursor of azadirachtin. J. Chem. Soc., Perkin Trans. I, 1995, 1505-1511.
- Beecher, J.E.; Grogan, G.; Roberts, S.M.; Wan, P.; Willetts, A. Oxidative biotransformations by microorganisms: enantioselective biooxidations by the enantiocomplementary diketocamphane monooxygenase isoenzymes from Pseudomonas putida NCIMB 10007. Biotechnol. Lett. 1996, 18, 571–576. [Google Scholar] [CrossRef]
- Alphand, V.; Furstoss, R.; Pedragosa-Moreau, S.; Roberts, S.M.; Willetts, A. Comparison of microbiological and enzymatically mediated Baeyer-Villiger oxidations: synthesis of optically active caprolactones. J. Chem. Soc., Perkin Trans. I, 1996, 1867-1871.
- Pasta, P.; Carrea, G.; Gagerro, N.; Grogan, G.; Willetts, A. Enantioselective oxidations catalysed by diketocamphane monooxygenase from Pseudomonas putida with macromolecular NAD in a membrane reactor. Biotechnol. Lett. 1996, 18, 1123–1127. [Google Scholar] [CrossRef]
- Chen, Y.; Peoples, O.; Walsh, C. Acinetobacter cyclohexanone monooxygenase: gene cloning and sequence determination. J. Bacteriol. 1988, 170, 781–789. [Google Scholar] [CrossRef]
- Villa, R.; Willetts, A. Oxidations by microbial NADH plus FMN-dependent luciferases from Photobacterium phospohoreum and Vibrio fischeri. J. Molec. Catal. B: Enzymatic, 1997, 2, 193-197.
- Willetts, A. Structural studies and synthetic applications of Baeyer-Villiger monooxygenases. Trends Biotechnol. 1998, 15, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ellis, H.R. The FMN-dependent two-component monooxygenase systems. Arch. Biochem. Biophys. 2010, 497, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Uetz, T.; Schneider, R.; Snozzi, M.; Egli, T. Purification and characterization of a two-component monooxygenase that hydroxylates nitrilotriacetate from ‘Chelobacter’ strain ATCC 29600. J.Bacteriol. 1992, 174, 1179–1188. [Google Scholar] [CrossRef]
- Priet, M.A.; Garcia, J.L. Molecular characterization of 4-hydroxyphenylacetate 3-hydroxylase of Escherichia coli. A two-protein component enzyme. J. Biol. Chem. 1994, 269, 22823–22829. [Google Scholar] [CrossRef]
- Oldfield,C. ; Pogrebinsky, O.; Simmonds, J.; Olson, E.S.; Kulpa, C.E. Elucidation of the metabolic pathway for dibenzothiophene desulphurization by Rhodococcus sp. strain IGTS8 (ATCC 53968). Microbiol. 1997, 143, 2961–2973. [Google Scholar]
- Payne, J.W.; Bolton, H.J.; Campbell, J.A.; Xun, J. Purification and characterization of the EDTA monooxygenase from the EDTA-degrading bacterium BNC1. J. Bacteriol. 1998, 180, 3823–3827. [Google Scholar] [CrossRef]
- Eichhorn, E.; van der Ploeg, J.R.; Leisinger, T. Characterization of a two-component alkanesulfonate monooxygenase from Escherichia coli. J. Biol. Chem. 1999, 274, 26639–26646. [Google Scholar] [CrossRef]
- Blanc, V.; Lagneaux, D.; Didier, P.; Gil, P.; Lacroix, P.; Crouzet, J. Cloning and analysis of the structural genes from Strepotomyces pristinaespiralis encoding enzymes involved in the conversion of pristinamycin IIB to pristinamycin A (PIIA): PHA synthase and NADH:riboflavin 5′-phosphate oxidereductase. J. Bacteriol. 1995, 177, 5206–5214. [Google Scholar] [CrossRef]
- Gisi, M.R.; Xun, L. Characterization of chlorophenol 4-monooxygenase (TftD) and NADH:flavin adenine dinucleotide oxidoreductase (TftC) of Burkholdaria cepacia ATCC 1100. J. Bacteriol. 2003, 185, 2786–2792. [Google Scholar] [CrossRef]
- Filisetti, L.; Fontecave, M.; Niviere, V. Mechanism and substrate specificity of the flavin reductase ActVB from Streptomyces coelicolor. J. Biol. Chem. 2003, 278, 296–303. [Google Scholar] [CrossRef]
- Valton, J.; Filisetti, L.; Fontecave, M.; Niviere, V. A two-component flavin-dependent monooxygenase involved in actinorhodin biosynthesis in Streptomyces coelicolor. J. Biol. Chem. 2004, 279, 44362–44369. [Google Scholar] [CrossRef]
- Coves, J.; Niviere, V.; Eschenbrenner, M.; Fontecave, M. NADPH-sulfite reductase from Escherichia coli – a flavin reductase participating in the generation of the free radical of ribonucleotide reductase. J. Biol. Chem. 1993, 268, 18604–18609. [Google Scholar] [CrossRef]
- Fieschi, F.; Niviere, V.; Frier, C. ; Decout, J-C; Fontecave, M. The mechanism and substrate specificity of the NADPH:flavin oxidoreductase from Escherichia coli. J. Biol. Chem. 1995, 270, 30392–30400. [Google Scholar]
- Louie, T.M.; Webster, C.M.; Xun, L. Genetics and biochemical characterization of 2,4,6-trichlorophenol degradation pathway in Ralstonia eutropha JMP134. J. Bacteriol. 2002, 184, 3492–3500. [Google Scholar] [CrossRef] [PubMed]
- Lee, J-K. ; Zhao, H. Identification and characterization of the flavin:NADH reductase (PrnF) involved in a novel two-component arylamine oxygenase. J. Bacteriol. 2007, 189, 8556–8563. [Google Scholar] [CrossRef] [PubMed]
- Galan, B.; Diaz, E.; Garcia, J.L. Enhancing desulphurization by engineering a flavin reductase-encoding gene cassette in recombinant biocatalysts. Environ. Microbiol. 2008, 2, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Yeom, J.; Jeon, C.; Madsen, E.L.; Park, W. Ferrodoxin-NADP+ reductase from Pseudomonas putida functions as a ferric reductase. J. Bacteriol. 2009, 191, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Gort, A.S.; Imlay, J.A. Balance between endogenous superoxide stress and antioxidant defences. J. Bacteriol. 1998, 180, 1402–1410. [Google Scholar] [CrossRef]
- Park, W.; Pena-Llopis, S.; Lee, Y.; Demple, B. Regulation of superoxide stress in Pseudomonas putida KT2440 is different from the SoxR paradigm in Escherichia coli. Biochem. Biophys. Res. Commun. 2006, 341, 51–56. [Google Scholar] [CrossRef]
- Kadow, M.; Sass, S.; Schmidt, M. Bornscheuer, U. Recombinant expression and purification of the 2,5-diketocamphane 1,2-monooxygenase from the camphor metabolising Pseudomonas putida strain NCIMB 10007. AMB Express, 2011, 1, 13. [CrossRef]
- Kadow, M.; Loschinski, K.; Sass, S.; Schmidt, M. Bornscheuer, U. Completing the series of BVMOs involved in camphor metabolism of Pseudomonas putida NCIMB 10007 by identification of the missing two genes, their functional expression in E. coli, and biochemical characterization. Appl. Microb. Biotechnol. 2012, 96, 419-429.
- Spyrou, G.; Haggard-Ljungquist, E.; Krook, M.; Jornvall, H.; Nilsson, E.; Reichard, P. Characterization of the flavin reductase gene (fre) of Escherichia coli and construction of plasmid for overproduction of the enzyme. J. Bacteriol. 1991, 173, 3673–3679. [Google Scholar] [CrossRef]
- Campbell, Z.; Baldwin, T.O. Fre is the major flavin reductase supporting bioluminescence from Vibrio harveyi luciferase in Escherichia coli. J. Biol. Chem. 2009, 284, 8322–8328. [Google Scholar] [CrossRef] [PubMed]
- Xun, L.; Sandvik, E.R. Characterization of 4-hydroxyphenylacetate 3-hydroxylase of Escherichia coli as a reduced flavin adenine dinucleotide-utilizing monooxygenase. Appl. Environ. Microbiol. 2000, 66, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Leisch, H.; Shi, R.; Grosse, S.; Morley, K.; Bergeron, H.; Cygler, M.; Iwaki, H.; Hasegawa, Y.; Lau, P.C. Clonin, Baeyer-Villiger biooxidations and structures of the camphor pathway monooxygenases of Pseudomonas putida ATCC 17453. Appl. Environ. Microbiol. 2012, 78, 2200–2212. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, H.; Grosse, S.; Bergeron, H.; Leisch, H.; Morley, K.; Hasegawa, Y.; Lau, P.C. Camphor pathway redox: functional recombinant expression of 2,5- and 3,6 diketocamphane monooxygenases in Pseudomonas putida ATCC 17453 with their cognate flavin reductase catalysing Baeyer-Villiger reactions. Appl. Environ. Microbiol. 2013, 79, 3282–3293. [Google Scholar] [CrossRef] [PubMed]
- Isupov, M.N.; Schroder, E.; Gibson, R.; Beecher, J.; Donadio, G.; Saneei, V.; Dcuncha, S.A.; McGhie, E.J.; Sayer, C.; Littlechild, J.A. The oxygenating constituent of 3,6-diketocamphane monooxygenase from the CAM plasmid of Pseudomonas putida: the first crystal structure of a type II Baeyer-Villiger monooxygenase. Acta Crystalog. Sect. D 2015, 71, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Isupov, M.N.; Schroder, E.; Gibson, R.; Beecher, J.; Donadio, G.; Saneei, V.; Dcuncha, S.A.; McGhie, E.J.; Sayer, C.; Littlechild, J.A. The oxygenating constituent of 3,6-diketocamphane monooxygenase from the CAM plasmid of Pseudomonas putida: the first crystal structure of a type II Baeyer-Villiger monooxygenase. Corrigendum. Acta Crystalog. Sect. D 2018, 74, 379. [Google Scholar] [CrossRef]
- Willetts, A.; Kelly, D.R. Multiple native flavin reductases in camphor-metabolising Pseudomonas putida NCIMB 10007 (ATCC 217453). Functional interaction with two-component diketocamphane monooxygenase isoenzymes. Microbiology 2014, 160, 1784–1794. [Google Scholar] [CrossRef]
- Willetts, A.; Kelly, D.R. Flavin-dependent redox transfers by two-component diketocamphane monooxygenases of camphor-grown Pseudomonas putida NCIMB 10007 (ATCC 17453). Microorganisms 2016, 4, 38. [Google Scholar] [CrossRef]
- Kendrew, S.G.; Harding, D.A.; Hopwood, E.N.; Marsh, E.N. Identification of a flavin:NADH oxidoreductase involved in the biosynthesis of actinorhodin. Purification and characterisation of the recombinant enzyme. J. Biol. Chem. 1995, 270, 17339–17343. [Google Scholar] [CrossRef]
- Thibaut, D.; Ratet, N.; Bisch, D.; Faucher, D.; Debussche, L.; Blanche, F. Purification of the two-enzyme system catalysing the oxidation of the D-proline residue of pristinamycin IIB during the last stage of pristinamycin IIA synthesis. J. Bacteriol. 1995, 177, 5199–5205. [Google Scholar] [CrossRef]
- Parry, R.J.; Li, W. An NADPH:FAD oxidoreductase from the valinimycin producer Streptromyces viridifaciens. J. Biol. Chem. 1997, 272, 23303–23311. [Google Scholar] [CrossRef]
- Hertwerk, C. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. 2009, 48, 4688–4716. [Google Scholar] [CrossRef]
- Begani, J.; Lakhani, J.; Harwani, D. Current strategies to induce secondary metabolites from microbial biosynthetic cryptic gene clusters. Annal. Microbiol. 2018, 68, 419–432. [Google Scholar] [CrossRef]
- Scott, T.A.; Piel, J. The hidden enzymology of bacterial natural product biosynthesis. Nat. Rev. Chem. 2019, 3, 404–425. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.H.; Truman, A.W. Genome mining strategies for ribosomally synthesised post-transcriptionally modified peptides. Comput. Struct. Biotechnol. J. 2020, 18, 1838–1851. [Google Scholar] [CrossRef] [PubMed]
- Scherlach, K.; Hertweck, C. Mining and unearthing hidden biosynthetic potential. Nature Commun. 2021, 12, 3864. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.T. Tailoring enzyme strategies and functional groups in biosynthetic pathways. Nat. Prod. Reports 2023, 40, 326–386. [Google Scholar] [CrossRef]
- Schroder, I.; Johnson, E.; de Vies, S. Microbial ferric iron reductases. FEMS Microbiol. Rev. 2003, 27, 427–447. [Google Scholar] [CrossRef] [PubMed]
- Cain, T.J.; Smith, A.T. Ferric ion reductases and their contribution to unicellular ferrous iron uptake. J. Inorg. Biochem. 2021, 218, 111407. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.E.; Weinel, C.; Paulsen, I.T.; Dodson, H. – and 38 others. Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ. Microbiol. 2002, 4, 799–808. [Google Scholar] [CrossRef]
- Lee, Y.; Pena-Llopis, S.; Kang, Y.S.; Shin, H.D.; Demple, B.; Madsen, E.L.; Jeon, C.O.; Park, W. Expression analysis of the fpr (ferrodoxin-NADP+ reductase) gene in Pseudomonas putida KT2440. Biochem. Biophys. Res. Commun. 2006, 339, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Yunho, L.; Yeom, J.; Kang, Y-S. ; Kim, J.; Sung, J-S.; Jeon, C.; Park, W. Molecular characterization of FprB (ferrodoxin-NADP+ reductase) in Pseudomonas putida KT2440. J. Microbiol. Biotechnol. 2007, 17, 1504–1512. [Google Scholar]
- Yeom, J.; Park, W. Biochemical characterisation of ferrodoxin-NADP+ reductase interaction with flavodoxin in Pseudomonas putida. Biochem. Molec. Biol. Rep, 2012, 476-431.
- Roome, P.W.; Philley, J.C.; Peterson, J.A. Purification and properties of putidaredoxin reductase. J. Biol. Chem. 1983, 258, 2593–2598. [Google Scholar] [CrossRef]
- Koga, H.; Yamaguchi, E.; Matsunaga, K.; Aramaki, H.; Horiuchi, T. Cloning and nucleotide sequences of NADH-putidaredoxin reductase gene (camA) and putidaredoxin gene (camB) involved in cytochrome P-450cam hydroxylase of Pseudomonas putida. J. Biochem. 1989, 106, 831–836. [Google Scholar] [CrossRef]
- Peterson, J.A.; Lorence, M.C.; Amameh, B. Putidaredoxin reductase and putidaredoxin. Cloning sequence determation, and heterologous expression of the proteins, J. Biol. Chem. 1990, 265, 6066–6076.
- Sevrioukova, I.F.; Poulos, T.L. Putidaredoxin reductase, a new function for an old protein. J. Biol. Chem. 2002, 277, 25831–25839. [Google Scholar] [CrossRef]
- Sevrioukova, I.F.; Li, H.; Poulos, T.L. Crystal structure of putidaredoxin reductase from Pseudomonas putida, the final structural component of the cytochrome P450cam monooxygenase. J. Molec. Biol. 2004, 336, 889–902. [Google Scholar] [CrossRef]
- Jensen, R.A. Enzyme recruitment in evolution of new function. Ann. Rev. Microbiol. 1976, 30, 409–425. [Google Scholar] [CrossRef]
- Aharoni, A.; Gaidokov, L.; Khersonsky, O.; Gould, S.; Roodveldt, C. The ‘evolvability’ of promiscuous protein functions. Nature Genetics 2005, 37, 73–76. [Google Scholar] [CrossRef]
- Hult, K.; Berglund, P. Enzyme promiscuity: mechanism and applications. Trends Biotechnol. 2007, 25, 231–238. [Google Scholar] [CrossRef]
- Khersonsky, O.; Tawfik, D.S. EWnzyme promiscuity: a mechanistic and evolutionary perspective. Ann. Rev. Biochem. 2010, 79, 471–505. [Google Scholar]
- Leveson-Gower, R.B.; Mayer, C.; Roelfes, G. The importance of catalytic promiscuity for enzyme design and evolution. Nat. Rev. Chem. 2019, 3, 687–705. [Google Scholar] [CrossRef]
- Gupta, M.N.; Uversky, V.N. The various facets of protein promiscuity: not just broad specificity of proteins. In Structure and intrinsic disorder in enzymology; Gupta, M.N.; Uversky, V. Eds; Academic Press, NY, USA, 2022: pp 241-277.
- Baghdianz, A. Role du zinc sur l’application de la composante du ‘pigment’ de Pseudomonas fluorescens. Arch. Sci. 1952, 5, 47–48. [Google Scholar]
- Chakrabarty, A.M.; Roy, S.C. Effects of trace elements on the production of pigments by a pseudomonad. Biochem. J. 1964, 93, 228–231. [Google Scholar] [CrossRef]
- Huyer, M.; Page, W.J. Zn2+ increases siderophore production in Azotobacter vinelandii. Appl. Environ. Microbiol. 1988, 54, 2626–2631. [Google Scholar] [CrossRef]
- Huyer, M.; Page, W.J. Ferric reductase activity in Azotobacter vinelandii and its inhibition by Zn2+. J. Bacteriol. 1989, 171, 4031–4037. [Google Scholar] [CrossRef]
- Willetts, A. Conferring the metabolic self-sufficiency of the CAM plasmid of Pseudomonas putida ATCC 17453. The key role of putidaredoxin reductase. Microorganisms 2019, 7, 395. [Google Scholar] [CrossRef]
- Coves, J.; Eschenbreener, M.; Montecave, M. Sulfite reductase of Escherichia coli is a ferrisiderophore reductase. Biochem. Biophys. Res. Commun. 1993, 192, 1403–140. [Google Scholar] [CrossRef]
- Coves, J.; Niviere, V.; Eschenbrenner, M.; Fontecave, M. NADPH-sulfite reductase from Escherichia coli. J. Biol. Chem. 1993, 268, 18604–18609. [Google Scholar] [CrossRef]
- Eschenbrenner, M.; Coves, J.; Fontecave, M. The flavin reductase activity of the flavoprotein component of sulfite reductase from Escherichia coli: a new model f the protein structure. J. Biol. Chem. 1995, 270, 20550–20555. [Google Scholar] [CrossRef]
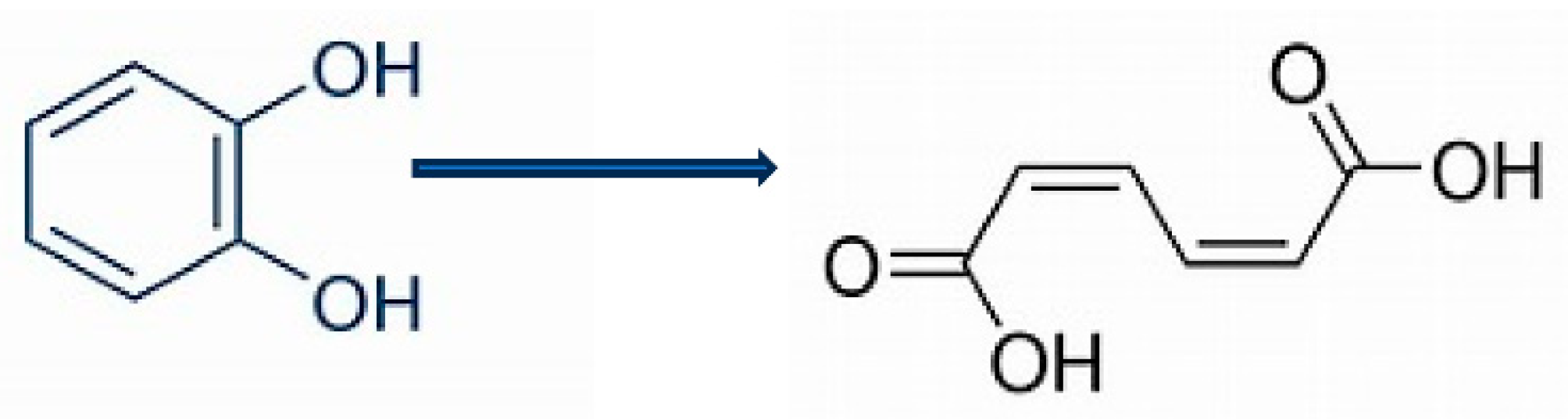
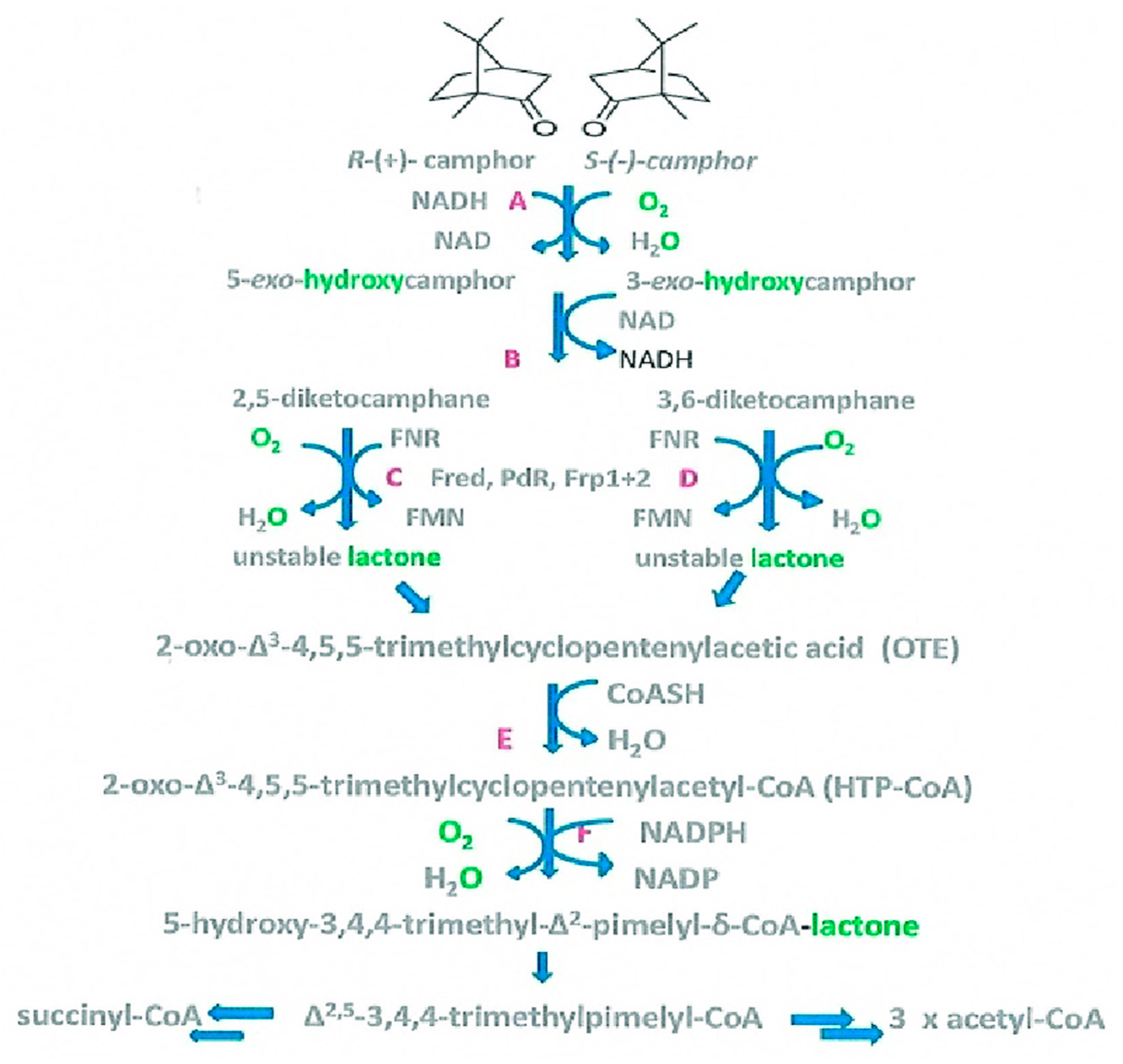
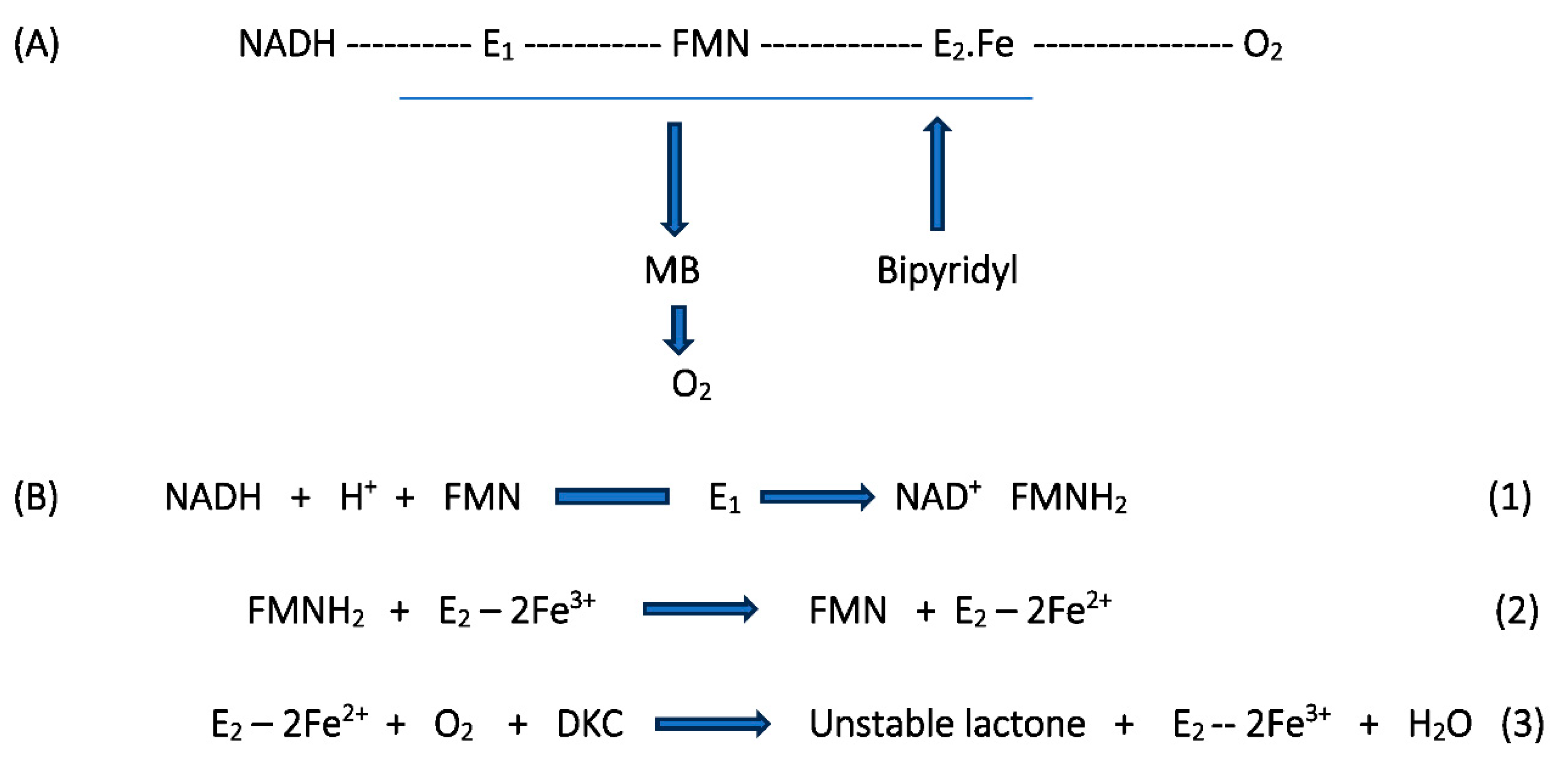
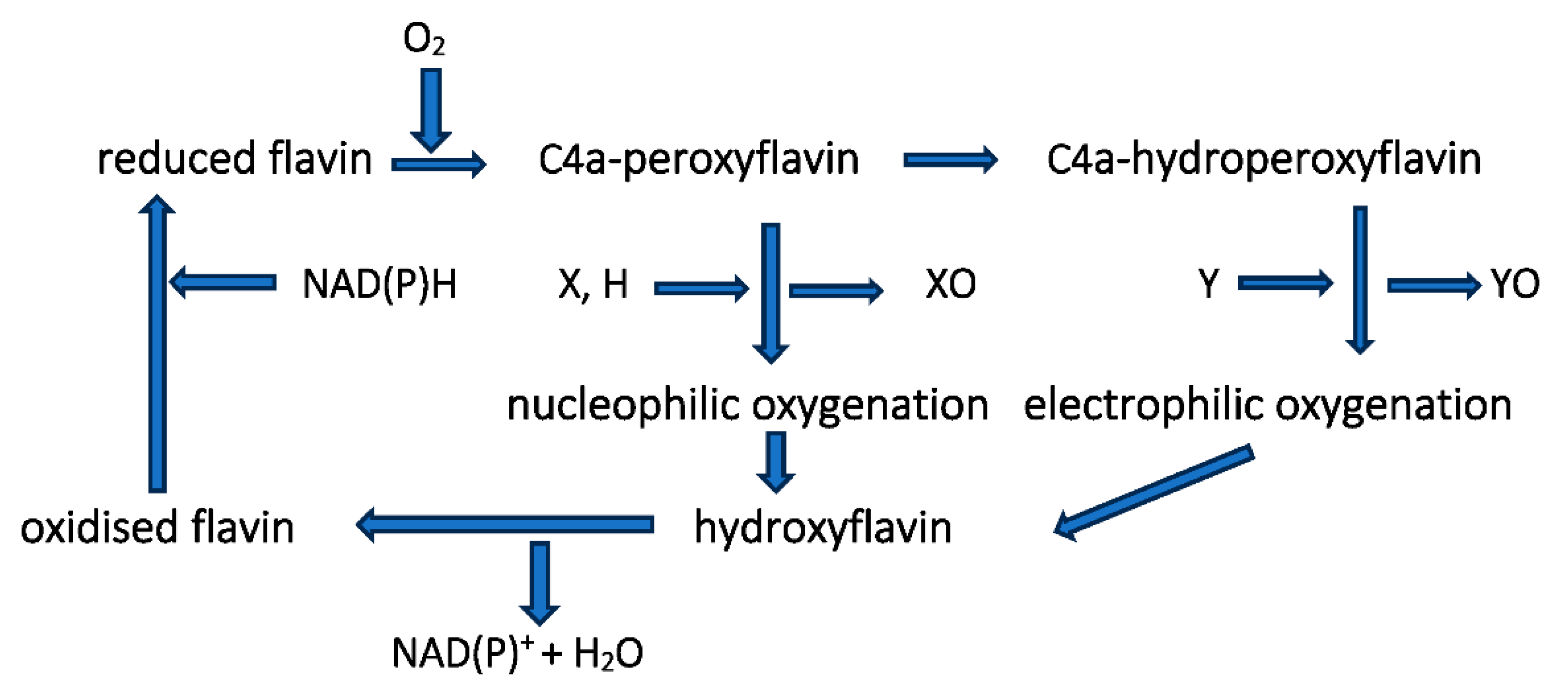
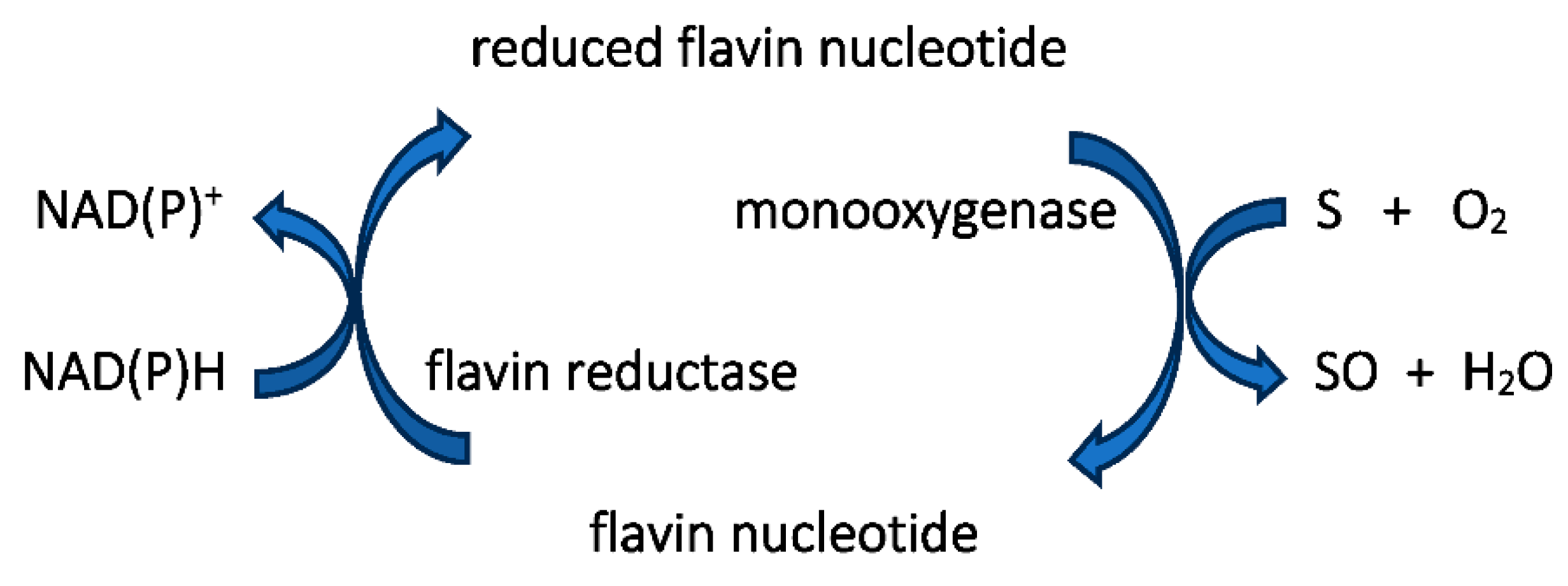
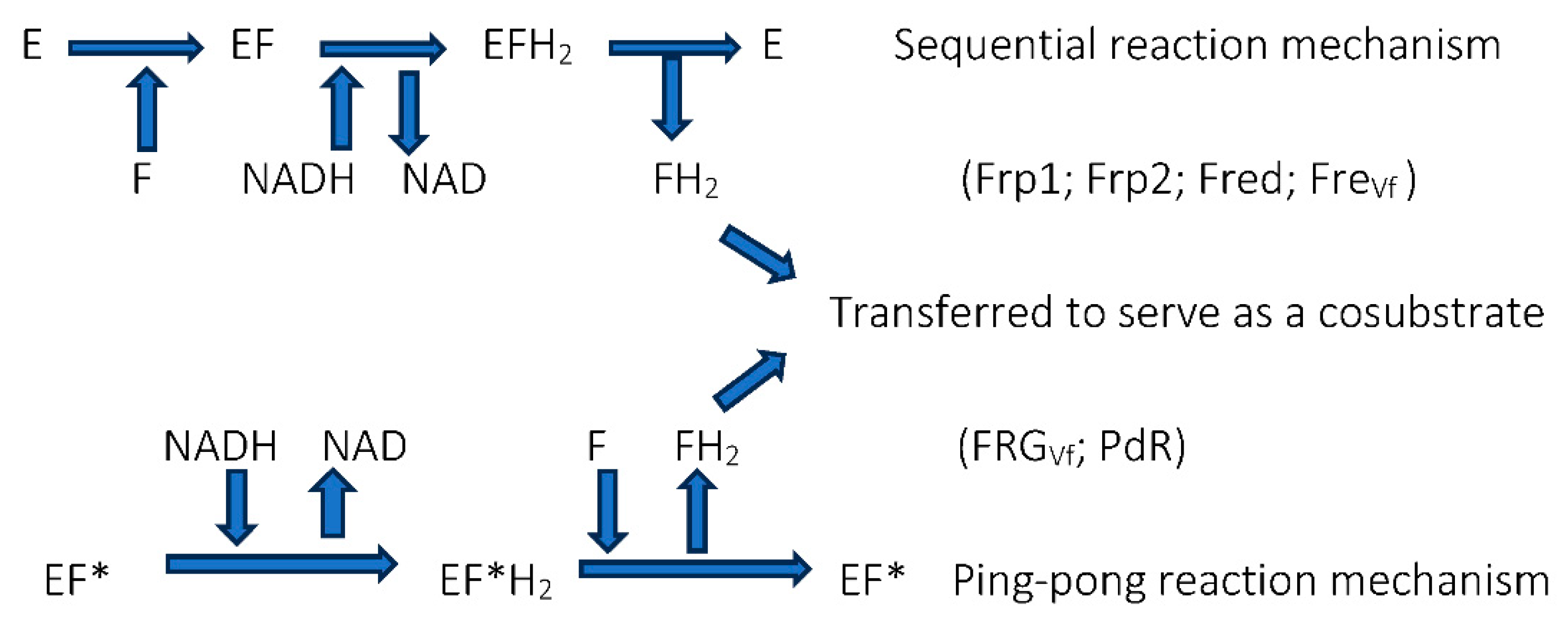
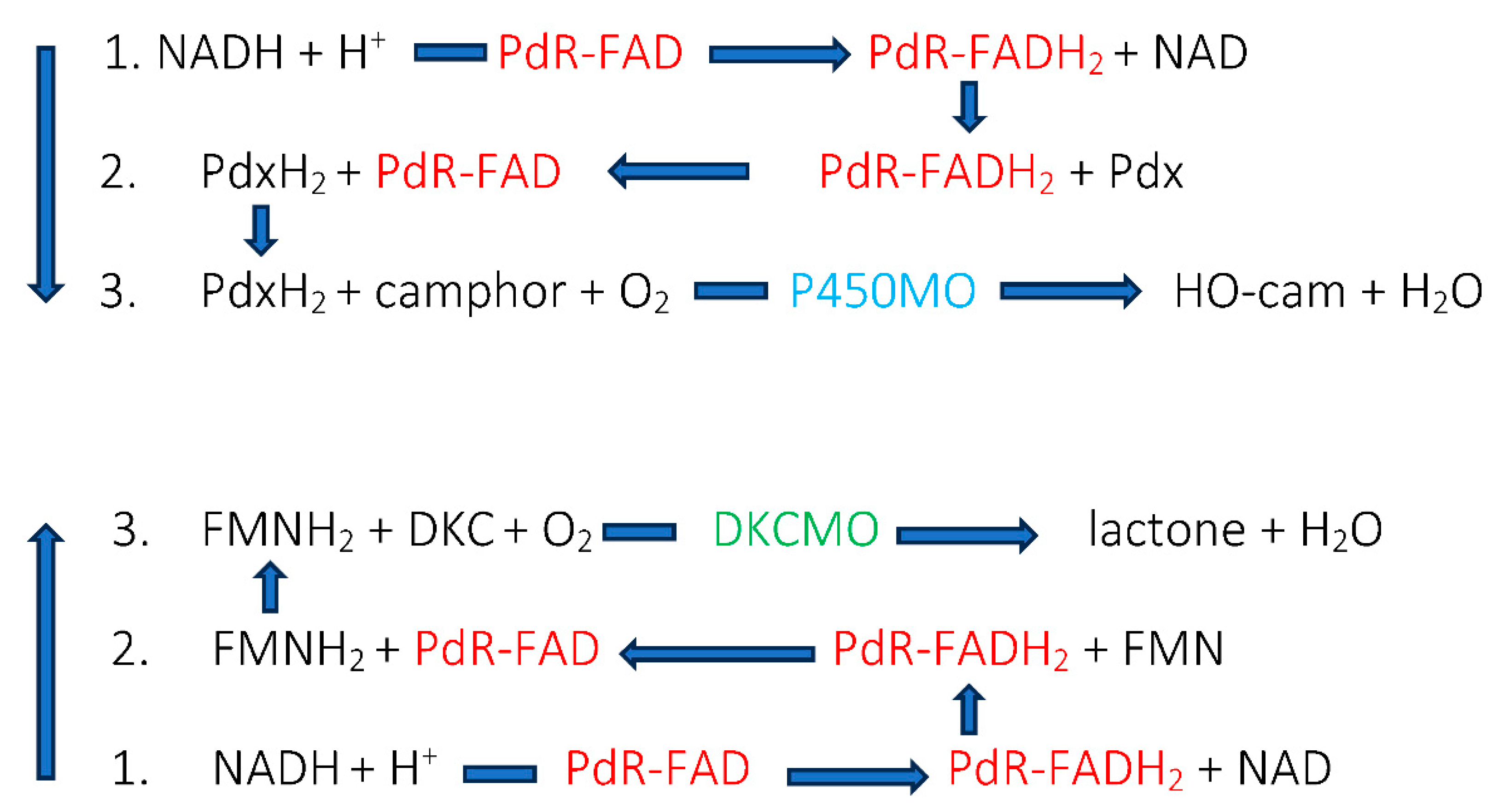
| Flavin nucleotides | Transition elements |
| Flavin mononucleotide FMN: Old Yellow Enzyme: Theorell 1935 [6] | Copper: 3,4-dimethylphenol monooxygenase: Mason et al. 1955 [8] |
| Flavin adenine dinucleotide FAD: D-amino acid oxidase: Warburg and Christian 1938 [27] | Nonheme.iron: catechol 1,2-dioxygenase: Hayashi et al. 1955 [7] |
| Heme-coordinated iron: Tryptophan 2 2,3-dioxygenase: Klingenberg 1958 [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





