Introduction
Extended orthodontic treatment can lead to various adverse effects such as decreased patient cooperation, compromised oral hygiene, increased risk of dental caries, periodontitis, and root resorption. Hence, accelerating orthodontic tooth movement (OTM) and reducing treatment duration might not only alleviate discomfort but also mitigate potential dental and periodontal complications in patients [
1,
2]. Some methods of accelerating OTM include surgical techniques such as corticotomy [
3], piezocision [
4,
5] and periodontally accelerated osteogenic orthodontics [
6,
7].
A technique known as micro-osteoperforations (MOPs) has emerged as a means to accelerating tooth movement. This method triggers alveolar bone remodeling with minimal surgical trauma induction. The most efficient procedures involve surgical techniques; however, they are rarely used owing to their invasive nature. Corticotomy is an acceptable method to accelerate tooth movement, whereas MOP is a less intrusive alternative to corticotomy. A study conducted by Alikahni et al. [
8] demonstrated that MOPs accelerated tooth movement approximately 2.3 times faster than traditional orthodontic methods.
Orthodontic root resorption (ORR) is an inevitable outcome of OTM. Various factors associated with orthodontic treatment and ORR have been identified: extended treatment [
9], heavy force [
10], and patient-related risk factors such as genetic predisposition [
11], age [
12], root irregularities [
10], previous tooth injuries [
13], and allergies [
14,
15]. In addition, it is believed that excessive tooth movement induce root resorption. Accelerating tooth movement due to strong load also causes root resorption [
16]. Chandorikar and Bhad [
17] reported that the overall root resorption after treating with MOPs was higher in the treated than the control group. Contrarily, a study by Dos Santos et al. conducted in 2020 [
18] demonstrated that MOPs did not affect root resorption. Furthermore, sporadic clinical observations have indicated that the use of MOPs does not worsen the progression of root resorption [
19]. Whether increasing tooth movement velocity with surgery procedures causes root resorption is not clear.
This study focused on the relationship between OTM acceleration by MOPs and root resorption. This study aimed to investigate the mechanism by which MOPs accelerate tooth movement without exacerbating the progression of root resorption by measureing the volume of resorbed root and performed the immunostaining for the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick-end-labelling method (TUNEL)-exposed MOPs during rat experimental tooth movement.
Materials and methods
Animals
The animal experimental protocol used in this study was approved by the Ethics Committee for Animal Experiments at the Nihon University School of Dentistry at Matsudo (approval No. AP20MAS011-2). A total of fifteen male 11-week-old Wistar rats (Sankyo Labo Service, Inc., Tokyo, Japan. body weight 300 ± 30 g) were used for the experiments. The animals were maintained in the animal center of Nihon University School of Dentistry at Matsudo in separate cages in an environment with 12-hour light/dark cycle and a constant temperature of 23 °C and were provided with food and water ad libitum. The health status of each rat was evaluated by daily body weight monitoring for 1 week before the start of the experiments. The rats were randomly divided into 3 groups according to the treatment given: 10-g orthodontic force applied to the maxillary first molar (OF group), 10-g force application plus 3 small perforations of the cortical plate (OF+MOP group), and 50-g orthodontic force applied to the maxillary first molar (HF group).
Application of orthodontic devices
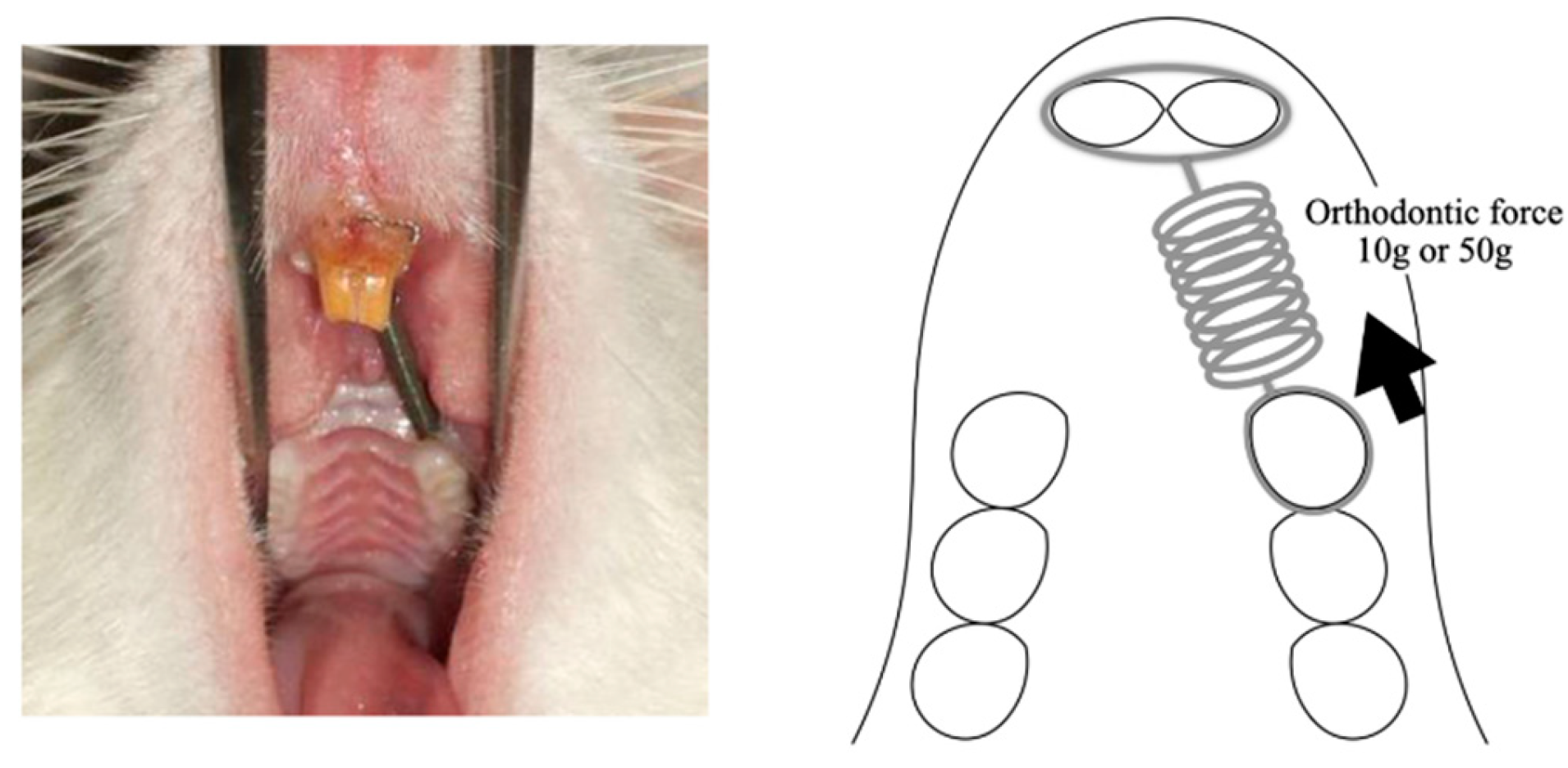
In each group, Pentobarbital sodium (40 mg/kg body weight) was used to anesthetize the animals prior to the application of orthodontic devices. Following the method outlined by Nakano et al. [
20], we employed a closed-coil spring (wire size: 0.005 in, diameter: 0.083 in; Accurate, Inc., Tokyo, Japan) secured to the maxillary first molar using a 0.008-in stainless-steel ligature wire (Tomy International, Inc., Tokyo, Japan). The opposite end of the coil spring was similarly secured, with the holes in the maxillary incisors drilled slightly above the gingival papilla using a 1⁄4 round bur and the same ligature wire. Movement of the upper first molar toward the front was achieved using a closed-coil spring with forces of either 10 or 50 g. The experiment spanned a period of 14 days (
Figure 1).
Surgical Procedure
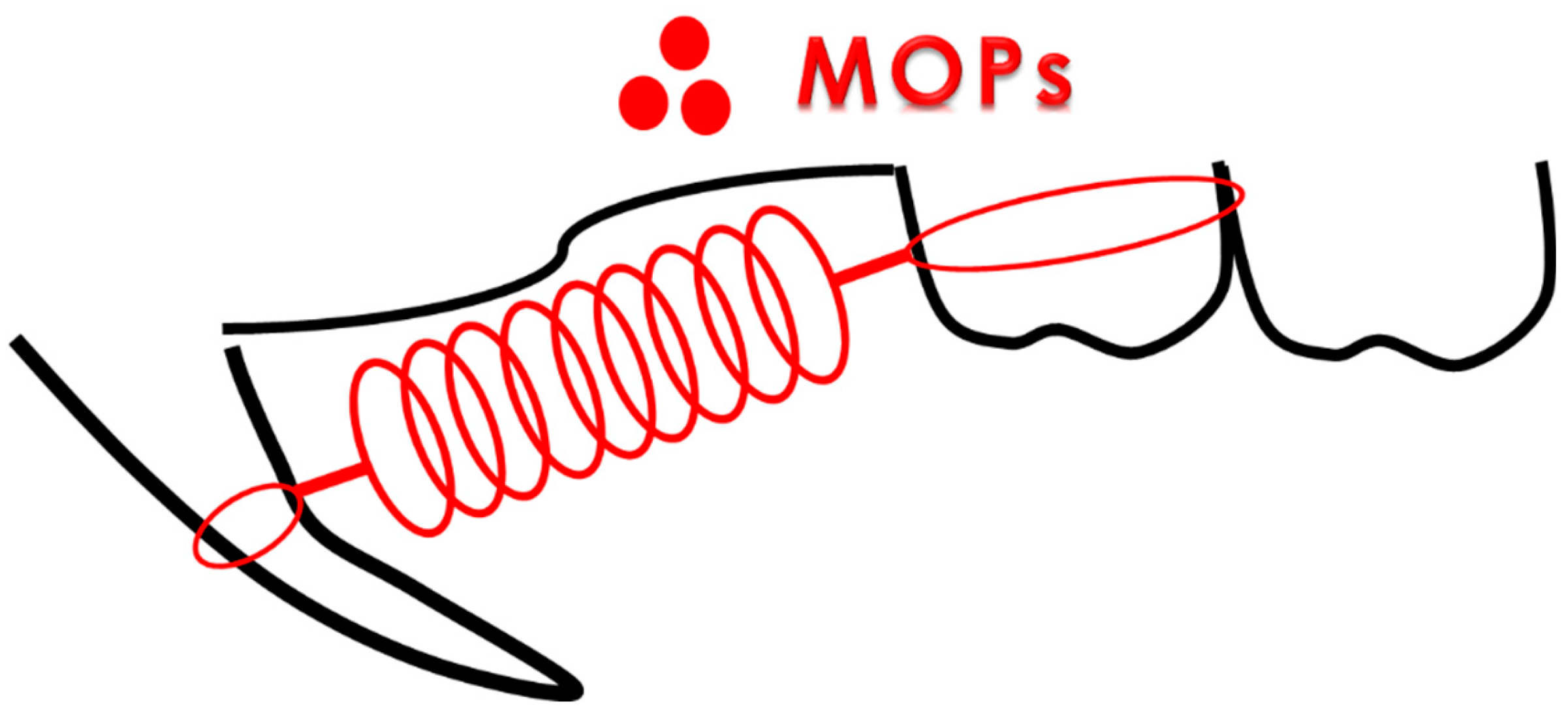
MOPs was induced using the method described by Teixeira et al. [
21]. In the OF+MOPs group, the animals received 3 shallow perforations, approximately 0.25 mm in diameter (0.25-mm depth), 5 mm mesial to the first molar using a
1/4 round bur and handpiece (
Figure 2).
Tissue preparation
The experiment lasted for 14 days following the initiation of tooth movement. The animals were deeply anesthetized using pentobarbital sodium and then underwent transcardial perfusion with a solution of 4% paraformaldehyde in 0.1 M phosphate buffer. Subsequently, the maxilla was promptly dissected and immersed in the same fixative for 18 hours at 4°C. To prepare the specimens, they were decalcified in a 10% solution of ethylenediaminetetraacetic acid disodium salt (pH 7.4) for 4 weeks. Afterward, the decalcified specimens underwent dehydration through an ethanol series and were embedded in paraffin using conventional methods. Each sample was continuously sliced into 4-μm sections horizontally and then processed for hematoxylin and eosin (HE) as well as TUNEL staining. Evaluation focused on the periodontal tissues in the mesial part of the distal buccal root of the first upper molar. Animals that did not display tooth movement were included in the control group (
Figure 3).
Tooth Movement Measurement
To measure tooth movement accurately, we used micro-CT to assess the distance between the enamel at the furthest point of the first molar and the closest point of the second molar in each animal. An in vivo micro-CT system (Rigaku-µCT®, Tokyo, Japan) was employed for quantitative image analysis of the tooth movement. Rat molars underwent scanning with a µ-CT using an X-ray source of 90kV/88µA on days 0, 1, 4, 7, 10, and 14. Rats, deeply anesthetized with intraperitoneally administered sodium pentobarbital (35mg/kg), were positioned on the stage, and imaging involved a full 360º rotation of the sample, each lasting 17 seconds. We selected an isotropic resolution of 30×30×30-µm voxel size to depict the microstructure of the interdental spaces between the first (M1) and second (M2) molars. The raw 3D images were visualized and analyzed using I-View® software (J. Morita, Kyoto, Japan).
Root resorption by micro-CT
The teeth were subsequently extracted from the images using software, and the bone was removed. The maxillary first molars of rats have five roots. Their distobuccal roots were analyzed. Custom programming of the software, based on a convex hull algorithm similar to that used by Harris [
22], was adopted to analyze the volume of the root resorption craters on the root surface.
The distobuccal roots were trimmed, which were considered to have the smallest individual differences, parallel to the occlusal surface of the molar, 0.5mm from the root bifurcation towards the root apex, and the volume of the tooth root was measured using the bone structure analysis software TRI/3D-BON (Ratoc System Engineering, Japan). The difference between the volumes on days 14 and 0 was calculated as the amount of root reduction.
TUNEL staining
Apoptosis Detection Kit (Trevigen, Gaithersburg, MD, USA). The 4-μm paraffin sections underwent an incubation step with proteinase K (Trevigen), diluted at a ratio of 1:200, at 37°C for 15 minutes. Following this, they were rinsed with deionized water, treated with 3% H2O2 for 5 minutes, and subsequently washed again with deionized water. For the positive control, sections were exposed to DNase I at 37°C for 30 minutes. All sections were then subjected to incubation with terminal deoxynucleotide transferase (TdT) and Biotin-dUTP at 37°C for 1 hour, except for the negative control sections that omitted the TdT enzyme. After a washing step, the sections were incubated in Strep–HRP solution at 37°C for 10 minutes. Following another wash, the apoptotic cells were marked using diaminobenzidine (DAB). These sections were counterstained with 1% methyl green, dehydrated, and finally mounted.
Statistical methods
The values are expressed as mean ± standard deviation (S.D.) for each group in each figure. Values are expressed as means ± S.D.. A Mann-Whitney U-test was employed to compare the means of the groups, and p <0.05 was considered to indicate statistical significance.
Discussion
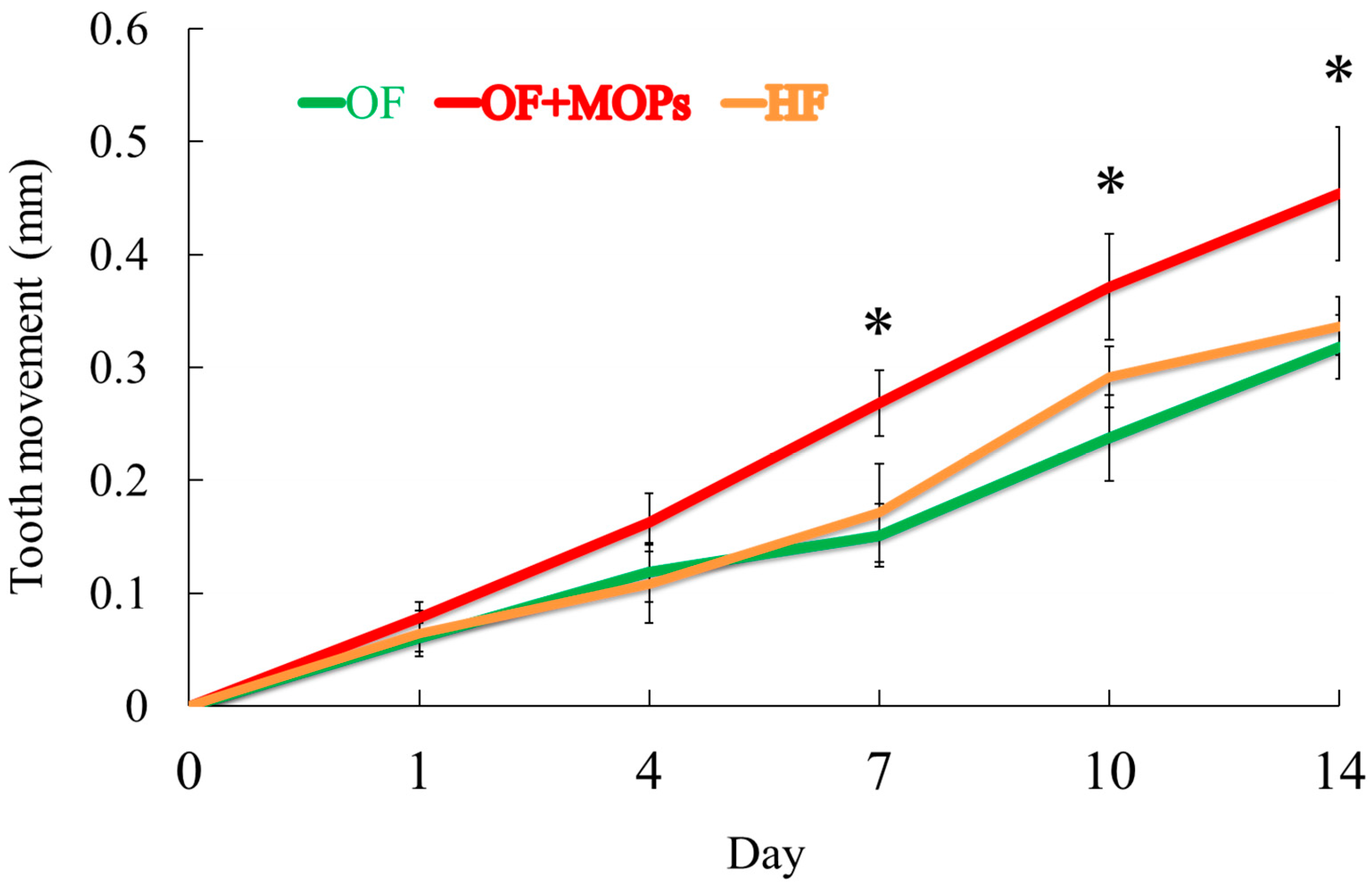
Figure 4 demonstrates that the OF + MOPs group exhibited a 1.8-fold increase in tooth movement compared with the OF group from days 7 to14 (
Figure 4). Cheung et al. [
23] showed how MOPs accelerated tooth movement in rats while simultaneously reducing both bone volume and density. They found that the maxillary first molar on the MOP-treated side moved almost twice the distance compared with the untreated side during the treatment period. This increased movement was caused by the demineralization of the bone on the pressure side induced by MOPs. Bone restructuring, triggered by force applied to the teeth, was investigated by Chang et al. [
24], who found that the direction of tooth movement correlated with a more pronounced decrease in alveolar bone density.
It is evident that the decrease in the bone volume to total volume ratio (BV/TV) and bone mineral density (BMD) on the MOP-treated side initiates regulatory processes that facilitate accelerated tooth movement. Our findings are consistent with those of Baloul et al. [
25], who reported a significant decrease in BV/TV and BMD after 7 days when tooth movement was combined with alveolar decortication. Consequently, our results further provide the MOPs enhance OTM by stimulating rapid bone remodeling.
Alikhani et al. reported that MOP significantly increased tooth movement and the levels of inflammatory markers [
8]. Furthermore, Frost et al. [
26] showcased that the initial injury prompted an acceleration of the typical localized healing mechanisms, termed the regional acceleratory phenomenon (RAP). Typically observed following a fracture, arthrodesis, osteotomy, or bone grafting, RAP potentially entails the mobilization and stimulation of precursor cells essential for wound healing, primarily concentrated at the site of injury. Therefore, inflammations by MOP activated bone remodeling around the tooth applied with orthodontic force. This report supported the results in this study.
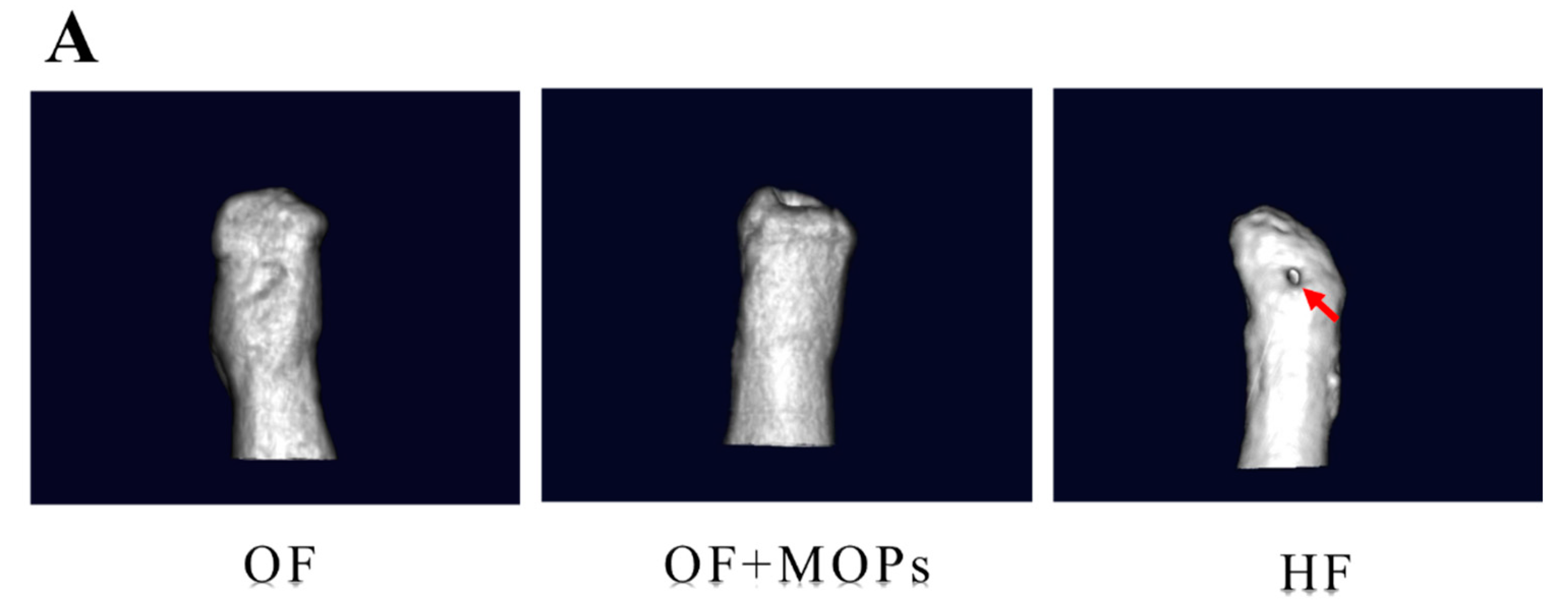
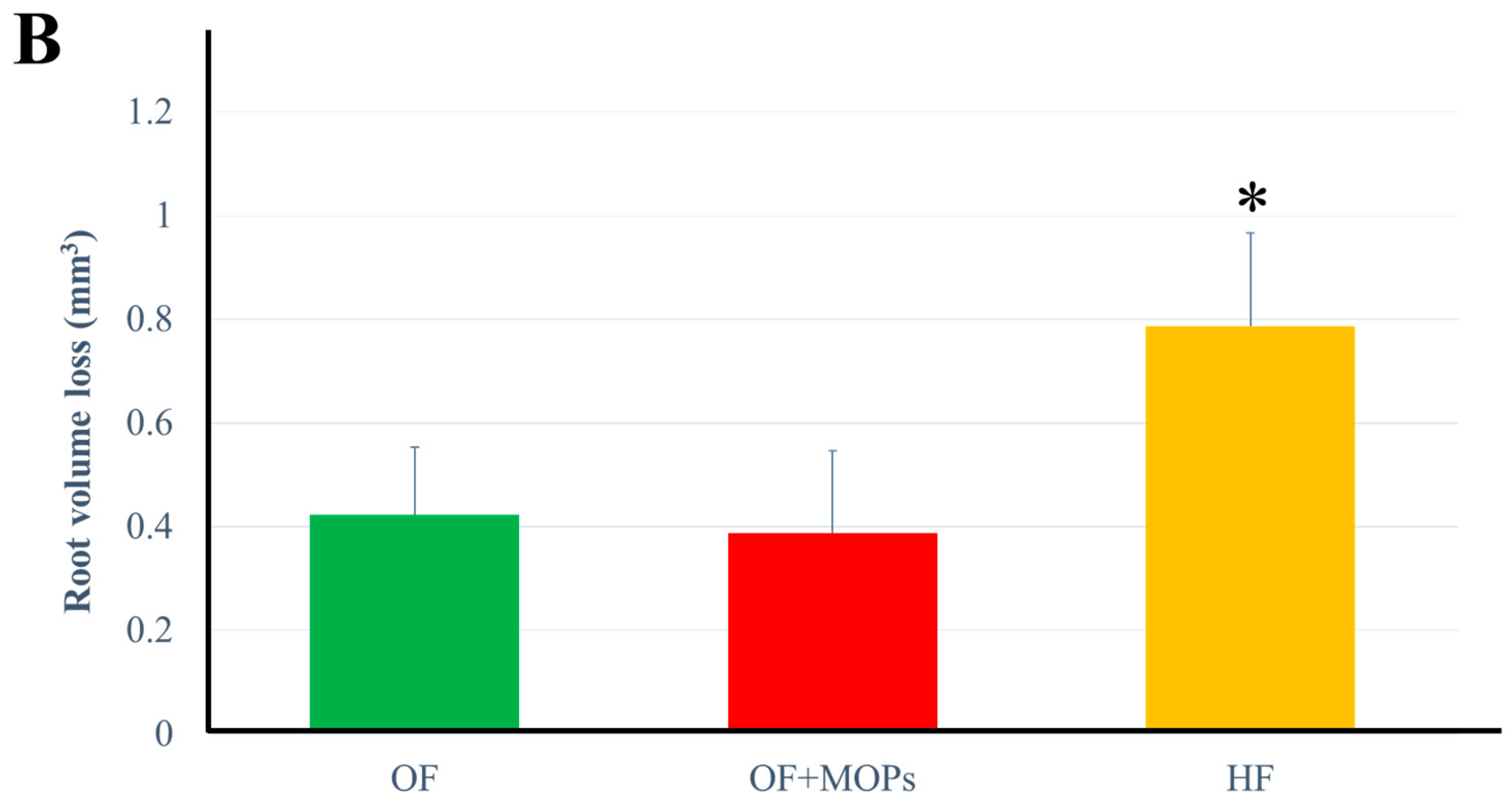
As regards the volume of root resorption, 3D images of the distobuccal roots were also reconstructed (
Figure 5A). There was no significant difference between the OF and OF + MOPs groups in terms of resorption volume. On day 14, the HF group had significantly greater total root resorption volumes than the OF and OF + MOPs groups (
Figure 5B). Ru et al. [
27] observed root resorption volume during orthodontic movement in three dimensions using
in vivo micro-CT and reported that the resorption volume of the mesial root significantly increased on day 7 of orthodontic loading (10 g). Furthermore, Gonzales et al. [
28] have demonstrated that force magnitude and duration affect tooth movement and root resorption in rat molars. The largest and deepest resorption craters were found in the distobuccal root in HF group. Interestingly, although the MOPs group had the greatest movement distance, it had a lower root resorption volume than the HF group (
Figure 5). Also,
Figure 6 demonstrated that resorption lacunae were observed on the root side of the PDL in the HF group, whereas in the OF and OF + MOPs groups, they were observed on the alveolar bone side of the PDL (
Figure 6). Recently, Tsai et al. [
29] revealed that MOP-facilitated tooth movement acceleration resulted in decreased root resorption in HE analysis. Similarly, Cheung et al. [
23] reported that volumetric analysis of all five roots of the maxillary first molar during rat experimental tooth movement revealed no significant increase in root resorption with MOPs. These results indicated that MOPs accelerate tooth movement without exerting adverse effects on the roots.
Cell death was initially thought to be the result of one of two distinct processes; apoptosis (also known as programmed cell death) or necrosis (uncontrolled cell death). Apoptosis is characterized by several characteristic morphological changes in the cell structure, together with some enzyme-dependent biochemical processes. Thus, the mechanism by which MOPs prevents root resorption was explored via apoptosis staining. Apoptosis staining using the TUNEL assay has been employed to detect DNA breakage in studies unrelated to cell death [
30,
31].
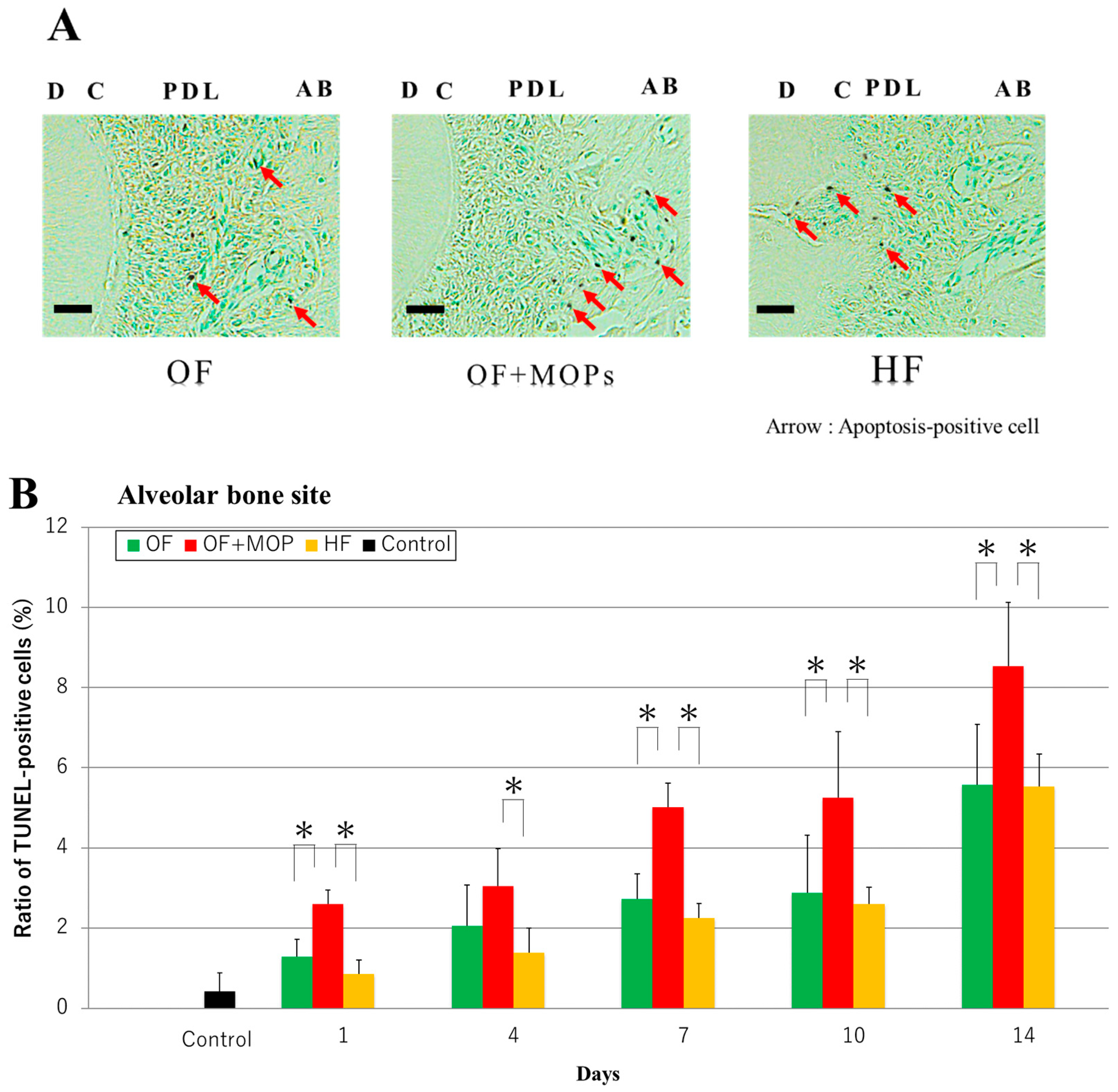
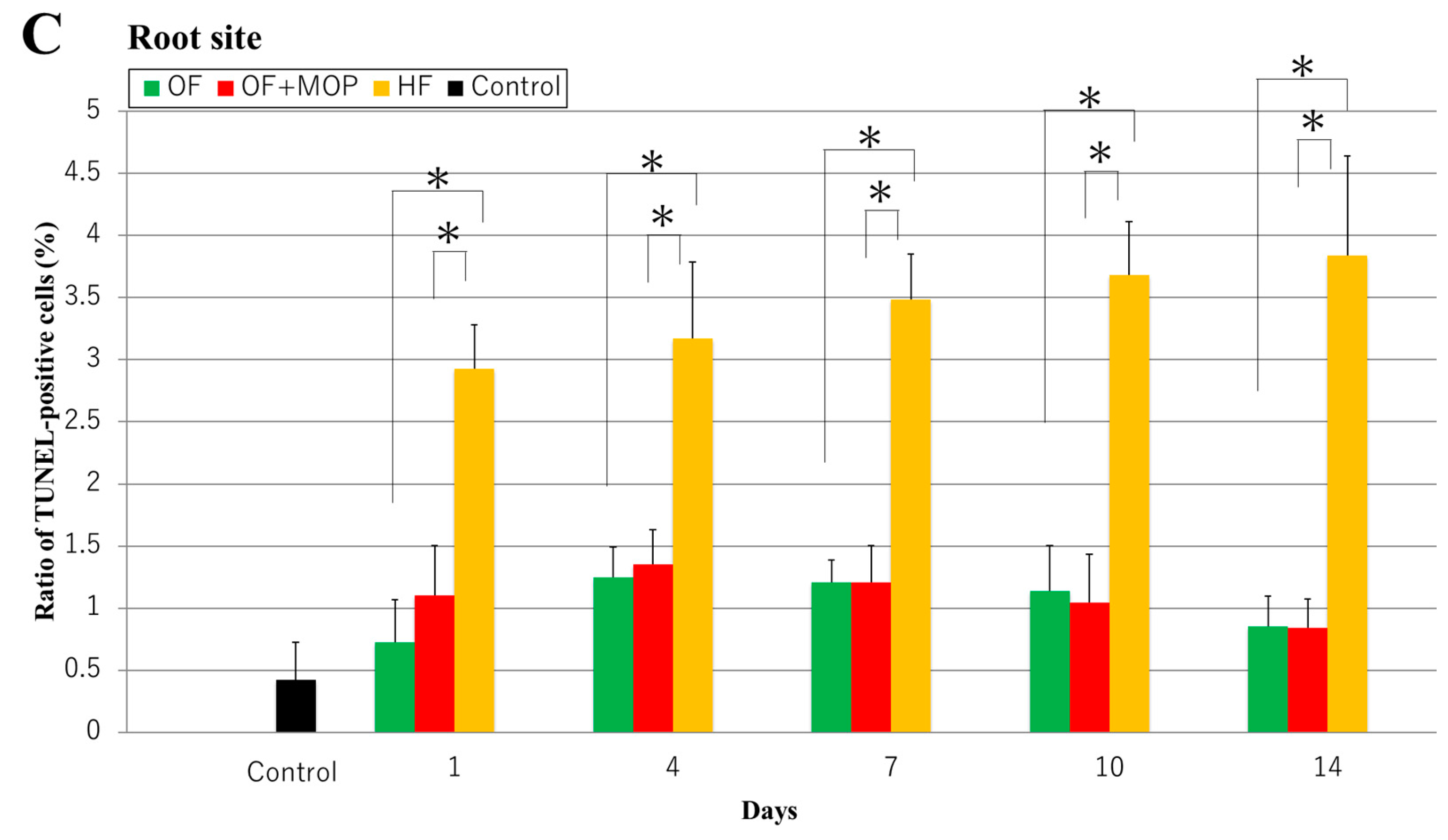
In TUNEL staining of this study, the apoptosis-positive cells were observed at the alveolar bone site of the OF and OF + MOPs groups on day 14, and the number of these cells increased in the former group compared with the latter group. In the HF group, however, apoptosis-positive cells were observed at the root (cementum) site. The number of apoptosis-positive cells in the alveolar bone site was significantly higher in the OF + MOPs groups than in the OF and HF groups from days 1 to 14. At the root site, the number of apoptosis-positive cells was significantly higher in the HF group than in the OF and HF groups from days 1 to 14. These results suggest that apoptosis of the PDL, cementum and osteocytes stimulate tooth root and alveolar bone resorption during tooth movement with MOPs (
Figure 7).
OTM has been shown to trigger apoptosis in periodontal tissues soon after force application. Several studies have reported that compressive force application leads to the death of various periodontal cells, including PDL cells [
32], osteocytes [
33,
34], and cementocytes (as observed in the work of Matsuzawa et al. [
35]), in experimental tooth movement.
Regarding the role of apoptosis in the occurrence of root resorption, Minato et al. [
36] found a significant increase in the number of caspase 3- and caspase 8-positive cells, as well as a receptor activator of nuclear factor-kappa B ligand (RANKL)-positive cells, in the HF (50 g) group compared with the OF (10 g) group. They observed that root resorption occurred after the induction of apoptosis in the cementum due to heavy force application. These observations strongly indicate the involvement of cementoblast apoptosis in root resorption. Furthermore, Wang et al. [
37] observed a significant increase in the expression levels of caspase-3 specifically within the PDL on the alveolar bone side following OTM using a light force (0.392 N) in their rat experimental model. In a similar model, Rana et al. [
38] observed TUNEL-positive staining in compressed PDL, indicating cellular apoptosis. In addition, Sugimori et al. [
39] reported that the number of TUNEL-positive cells in the OF (10 g) + MOPs group increased on days 1 and 7 compared with the OF (50 g) group. Consequently, apoptosis is identified as a significant contributor to OTM, potentially enhancing the pace of tooth movement and the root resorption process.
Regarding the association between OTM acceleration and root resorption, Shahrin et al. [
40] reported that OTM acceleration with MOPs during the alignment phase does not exacerbate root resorption in patients with moderate crowding of the upper labial segment.
Alqadasi et al. [
41]
also reported that MOPs accelerated orthodontic canine retraction and that this technique did not induce root resorption. Aksakalli et al. [
42] demonstrated that in a case report using a mini-screw, MOPs accelerated canine retraction without root resorption. Those reports supports the results of this experiment. However, there are also adverse reports indicating an exacerbation of root resorption. Chan et al. [
43] reported that MOPs resulted in greater root resorption on day 28 in a rat experimental model. Furthermore, Al-Attar et al. [
44] demonstrated that
MOPs exacerbated the progression of root resorption under the same conditions. These reports are data from day 28, and it is necessary to observe this experiment until day 28.
When optimal force was applied, apoptosis occurred in the PDL on the alveolar bone side, including bone resorption and tooth movement. Also, MOPs resulted in even more apoptosis in the PDL on the alveolar bone side, accelerating tooth movement without causing root resorption. Conversely, when heavy force was applied, apoptosis occurred in the PDL on the cementum side, leading to resorption of tooth root. Future studies are warranted to determine whether the site of apoptosis expression varies depending on the magnitude of the force.
Figure 1.
The rat model of orthodontic tooth movement used in the present study. Tooth movement is reproduced with a closed coil spring ligated to the maxillary first molar using a 0.008-inch stainless steel ligation wire (wire size, 0.005 inch; diameter, 1/12 inch). The other side of the coil spring is ligated using the same ligation wire to a hole in the maxillary incisor, which is drilled in the cleft just above the gingival papilla using a 1/4 round bur. The maxillary right first molar was moved in the mesial direction via the application of a force of 10 g or 50 g by the sealed coil spring.
Figure 1.
The rat model of orthodontic tooth movement used in the present study. Tooth movement is reproduced with a closed coil spring ligated to the maxillary first molar using a 0.008-inch stainless steel ligation wire (wire size, 0.005 inch; diameter, 1/12 inch). The other side of the coil spring is ligated using the same ligation wire to a hole in the maxillary incisor, which is drilled in the cleft just above the gingival papilla using a 1/4 round bur. The maxillary right first molar was moved in the mesial direction via the application of a force of 10 g or 50 g by the sealed coil spring.
Figure 2.
perforations of MOPs Rat in the MOPs group received 3 shallow perforations, approximately 0.25 mm in diameter (depth of 0.25 mm), on the buccal alveolar bone 5 mm mesial to the maxillary first molar.
Figure 2.
perforations of MOPs Rat in the MOPs group received 3 shallow perforations, approximately 0.25 mm in diameter (depth of 0.25 mm), on the buccal alveolar bone 5 mm mesial to the maxillary first molar.
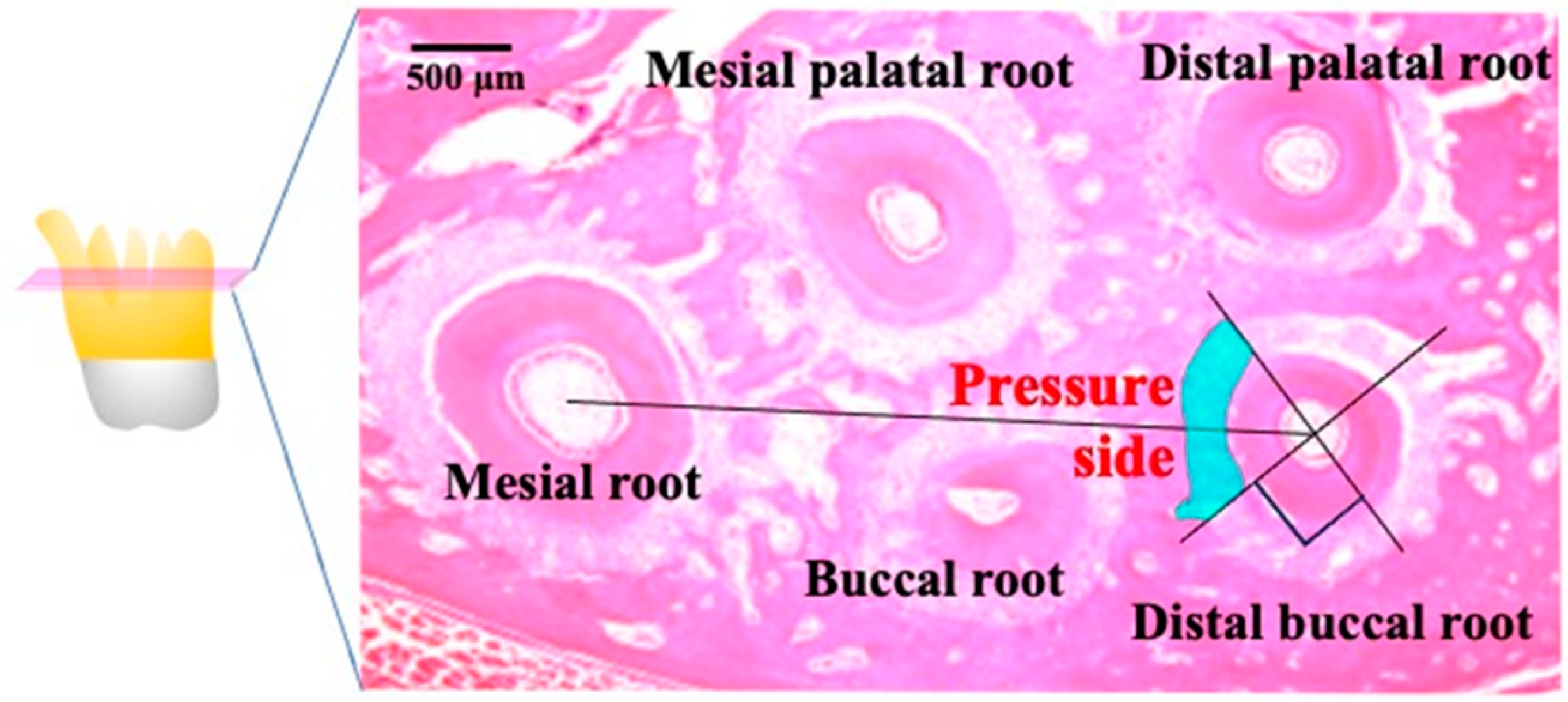
Figure 3.
A schematic diagram showing a survey area (shaded box) at the mesial center of the distal root of the maxillary first molar obtained from a rat model of orthodontic tooth movement. The periodontal tissue in the region of compression was defined as the tissue connecting the center of the distobuccal (DB) root and the center of the mesial root (M) of the maxillary first molar, and it comprises one-fourth of the mesial region facing the DB root. The large arrows indicate the direction of force. Root resorption is investigated in 300-µm sections (shaded box) from the area close to the furcation on the mesial surface of the DB root, which was the side of compression during tooth movement. MP, Mesial palatal root; DP, distal palatal root; MB, mesial buccal root.
Figure 3.
A schematic diagram showing a survey area (shaded box) at the mesial center of the distal root of the maxillary first molar obtained from a rat model of orthodontic tooth movement. The periodontal tissue in the region of compression was defined as the tissue connecting the center of the distobuccal (DB) root and the center of the mesial root (M) of the maxillary first molar, and it comprises one-fourth of the mesial region facing the DB root. The large arrows indicate the direction of force. Root resorption is investigated in 300-µm sections (shaded box) from the area close to the furcation on the mesial surface of the DB root, which was the side of compression during tooth movement. MP, Mesial palatal root; DP, distal palatal root; MB, mesial buccal root.
Figure 4.
Comparison of the tooth movement distance. The OF + MOPs group exhibited a 1.79-, 1.84-, 1.81-fold increase in tooth movement compared with the OF group at 7, 10 and 14 day, respectively. Data are expressed as means ± standard deviations. Tested by Mann–Whitney U test, at *p < 0.05.
Figure 4.
Comparison of the tooth movement distance. The OF + MOPs group exhibited a 1.79-, 1.84-, 1.81-fold increase in tooth movement compared with the OF group at 7, 10 and 14 day, respectively. Data are expressed as means ± standard deviations. Tested by Mann–Whitney U test, at *p < 0.05.
Figure 5.
Micro-CT analysis for root resorption. Crater volumes at the distobuccal root surfaces of maxillary 1st molar were compared among three groups on day 14. A: In the OF and OF + MOPs groups, there was slightly obvious root resorption. Both wide shallow and deep resorption craters were mostly observed in the HF group (red allow). B: Total root resorption volumes in the HF group were significantly greater than the OF and OF and OF + MOPs No significant difference was found between the OF and OF + MOPs groups in resorption volume. Data are expressed as means ± standard deviations. Tested by Mann–Whitney U test, at *p < 0.05.
Figure 5.
Micro-CT analysis for root resorption. Crater volumes at the distobuccal root surfaces of maxillary 1st molar were compared among three groups on day 14. A: In the OF and OF + MOPs groups, there was slightly obvious root resorption. Both wide shallow and deep resorption craters were mostly observed in the HF group (red allow). B: Total root resorption volumes in the HF group were significantly greater than the OF and OF and OF + MOPs No significant difference was found between the OF and OF + MOPs groups in resorption volume. Data are expressed as means ± standard deviations. Tested by Mann–Whitney U test, at *p < 0.05.
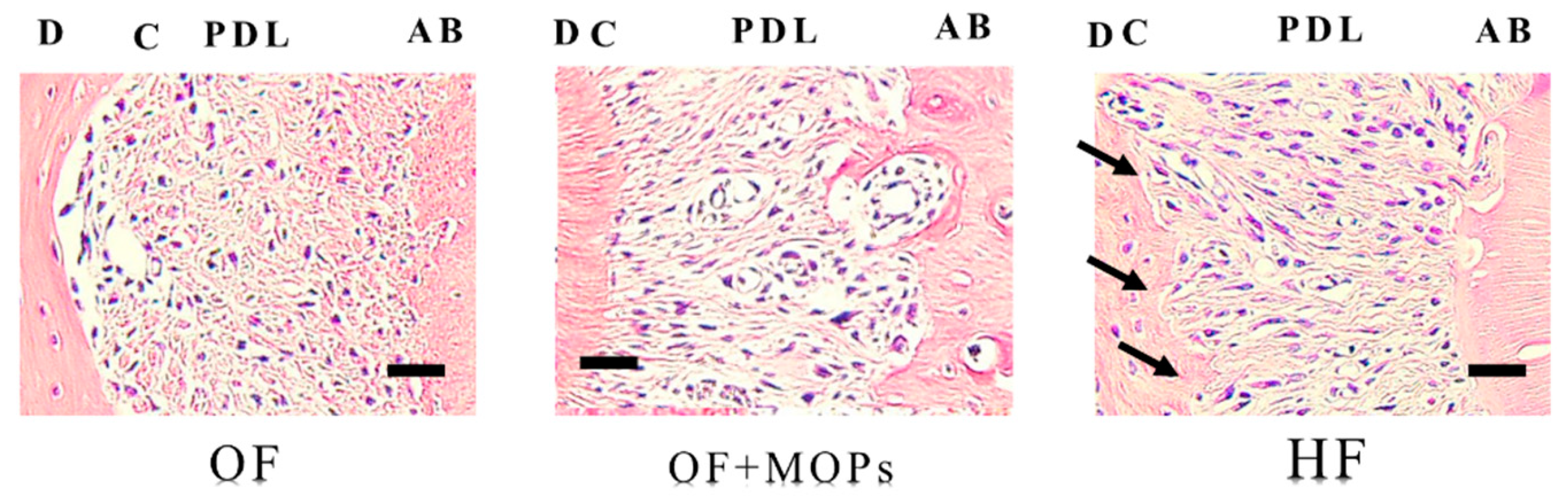
Figure 6.
Light microscopic images showing the effects of various orthodontic tooth movement (OF, OF + MOPs, HF) on the expression of multinuclear osteoclasts (hematoxylin and eosin staining, ×400) at 14 days after force application in a rat model of orthodontic tooth movement. The expression of odontoclasts (arrow) on the root surfaces in the HF (50 g) group is greater than that on the root surfaces in the OF (10 g) and OF (10 g) + MOPs groups on day 14. The arrows indicate the sites of root resorption. Scale bar = 50 µm., Periodontal ligament; C, cementum; D, dentin; AB, alveolar bone.
Figure 6.
Light microscopic images showing the effects of various orthodontic tooth movement (OF, OF + MOPs, HF) on the expression of multinuclear osteoclasts (hematoxylin and eosin staining, ×400) at 14 days after force application in a rat model of orthodontic tooth movement. The expression of odontoclasts (arrow) on the root surfaces in the HF (50 g) group is greater than that on the root surfaces in the OF (10 g) and OF (10 g) + MOPs groups on day 14. The arrows indicate the sites of root resorption. Scale bar = 50 µm., Periodontal ligament; C, cementum; D, dentin; AB, alveolar bone.
Figure 7.
Effects of different orthodontic tooth movement (OF, OF + MOPs, HF) on the expression of TUNEL-positive cells at 1, 3, 5, and 7 days after force application in a rat model of orthodontic tooth movement. A: The TUNEL-positive cells at 14 days after force application (OF, OF + MOPs, HF) in a rat model of orthodontic tooth movement. The apoptosis-positive cells of the OF and OF + MOPs groups were observed alveolar bone site, and that of the OF+MOP group increased compared with the OF group. On the other hand, that of the HF group were observed the apoptosis-positive cells at the root (cementum) site. PDL, Periodontal ligament; C, cementum; D, dentin; AB, alveolar bone. (×400). Scale bar = 50 µm. B: The quantitative evaluation of TUNEL-positive cells at 14 days after force application (OF, OF + MOPs, HF) in alveolar bone site in a rat model of orthodontic tooth movement. The number of apoptosis-positive cells of three groups increased in a time-dependent manner from days 1 to 14. Furthermore, the number of apoptosis-positive cells in the OF + MOPs groups was significantly higher than that in the OF and HF groups from day 1 to 14 (p < 0.05). C: The quantitative evaluation of TUNEL-positive cells at 14 days after force application (OF, OF + MOPs, HF) in root site in a rat model of orthodontic tooth movement. The number of apoptosis-positive cells in the HF group was significantly higher than that in the OF and HF group from day 1 to 14. No significant difference was found between the OF and OF + MOPs groups in the number of apoptosis -positive cells. *p < 0.05, significant difference when compared with the OF and HF groups.
Figure 7.
Effects of different orthodontic tooth movement (OF, OF + MOPs, HF) on the expression of TUNEL-positive cells at 1, 3, 5, and 7 days after force application in a rat model of orthodontic tooth movement. A: The TUNEL-positive cells at 14 days after force application (OF, OF + MOPs, HF) in a rat model of orthodontic tooth movement. The apoptosis-positive cells of the OF and OF + MOPs groups were observed alveolar bone site, and that of the OF+MOP group increased compared with the OF group. On the other hand, that of the HF group were observed the apoptosis-positive cells at the root (cementum) site. PDL, Periodontal ligament; C, cementum; D, dentin; AB, alveolar bone. (×400). Scale bar = 50 µm. B: The quantitative evaluation of TUNEL-positive cells at 14 days after force application (OF, OF + MOPs, HF) in alveolar bone site in a rat model of orthodontic tooth movement. The number of apoptosis-positive cells of three groups increased in a time-dependent manner from days 1 to 14. Furthermore, the number of apoptosis-positive cells in the OF + MOPs groups was significantly higher than that in the OF and HF groups from day 1 to 14 (p < 0.05). C: The quantitative evaluation of TUNEL-positive cells at 14 days after force application (OF, OF + MOPs, HF) in root site in a rat model of orthodontic tooth movement. The number of apoptosis-positive cells in the HF group was significantly higher than that in the OF and HF group from day 1 to 14. No significant difference was found between the OF and OF + MOPs groups in the number of apoptosis -positive cells. *p < 0.05, significant difference when compared with the OF and HF groups.